the measurement of unidirectional rates of reaction, in vivo, using magnetization transfer techniques is a unique capability of nuclear magnetic resonance spectroscopy (NMRS). However, the complicated network of cytosolic reactions as well as the potential existence of small exchanging metabolic pools or enzyme-substrate complexes with poorly defined NMRS properties makes the interpretation of these data difficult. From and Ugurbil (14), in this issue, discuss using NMRS for analyzing the turnover of ATP in muscle and present the impact of cytosolic exchange networks on these measures along with approaches to ameliorate their effects. Herein, we discuss the potential influence of small metabolite pools or enzyme-substrate complexes on magnetization transfer measures, in vivo. Experimental approaches to evaluate their impact are also presented. A further understanding of the metabolic exchange networks and potential for examining the kinetics of enzyme substrate complexes, in vivo, may further the impact of NMRS in the understanding of cell physiology.
Since the original demonstration of the utility of 31P NMRS in biological tissues from Dr. Radda’s laboratory in skeletal muscle (18), numerous significant contributions to the understanding of tissue biochemistry have been extracted using this technology. The major advantage of NMRS is the noninvasive “on-line” monitoring of metabolite levels, especially those with rapid metabolic turnover including adenosine triphosphate (ATP), adenosine diphosphate (ADP), phosphocreatine (PCr), or inorganic phosphate (Pi). Measurement of enzyme kinetics noninvasively is truly one of the most unique attributes of NMR. The determination of steady-state unidirectional rates of enzymatic or chemical reactions using saturation transfer methods was originally described by Forsen and Hoffman (13) and extended to living cells by Gupta (16) and Brown et al. (9). From and Ugurbil do an excellent job of describing this approach of measuring unidirectional reaction rates. The most studied reaction in tissues has been creatine kinase, but there are a growing number of enzymes that can be studied by NMRS magnetization transfer techniques (41).
In complex biological systems the interpretation of magnetization transfer data can be challenging due to a number of factors that have been described for a number of years (2, 6, 8) From and Ugurbil review in this issue the specific challenges of interpreting saturation transfer data in the determination of ATP synthesis/hydrolysis rates in striated muscles. This reaction represents the key reaction for maintenance of energy metabolism and so the focus on this reaction for detailed study is appropriate. One of the overriding issues raised by these authors, as well as by Kemp (24) in a previous Letter to the Editor in American Journal of Physiology-Endocrinology and Metabolism, is the discrepancy between the measured ATP synthesis/hydrolysis rate and the estimated turnover of ATP using other methods, most notably the oxygen consumption. In contrast to the numerous control experiments conducted for the saturation transfer determination of creatine kinase reaction rates, including isolated enzyme and multiple magnetization transfer methods, no absolute validation studies using saturation transfer methods to measure ATP hydrolysis or synthesis have been performed in well-defined mammalian systems, such as isolated mitochondria. The only validation is that the exchange rate, under some conditions, is close to the predicted rate as determined from oxygen consumption. However, this correlation often fails, with the saturation transfer unidirectional rate for of ATP synthesis being much higher than the predicted rate from the oxygen consumption (see Ref. 24).
From and Ugurbil focus on using the saturation transfer technology and the role of competing equilibrium reactions that can catalyze exchange between Pi and γ-ATP on the general overestimate of the ATP synthesis rate determined in heart and skeletal muscle. We are convinced by the arguments made by From and Ugurbil and others in the field (1, 8, 34) that additional enzymes that can catalyze observed NMRS exchange such as the glycolytic enzyme pair GAPDH/PGK are a major problem in interpreting saturation transfer data. The authors do an excellent job of describing this complication and methods to compensate for these additional enzyme reactions. In the age of genomics/proteomics, a comprehensive list of ATPases that can catalyze forward and reverse exchange of Pi and γ-ATP would be very important to continue assessing contributions of other enzymes to the NMRS-measured rate in muscle as well as other tissues. Naturally, the concentration, activity, and whether the flux significantly exceeds the aerobic ATP production rate will all influence the contribution of these reactions to the ATP-Pi exchange. In addition to accounting for all enzymes that catalyze the measured exchange, we would like to suggest that other factors may also be contributing to the anomalous degree of magnetization transfer in the saturation transfer experiment between γ-ATP and Pi and offer experimental strategies to assess the importance of these other factors.
One of the remarkable features of the saturation transfer experiment is that as long as the slow exchange condition is maintained and the resident time of the 31P in a given metabolite is long enough to be even partially saturated, the actual size of the pool being irradiated can be remarkably small, below the detectability of NMRS, and still detected via exchange with a larger, NMRS observable pool. This was first discussed in the context of enzyme-catalyzed exchange by Koretsky et al. (25) and was directly demonstrated by observing low concentrations of substrate and enzyme substrate complexes of creatine kinase (25, 27). The ability to amplify small pools via exchange with larger pools has been useful in a number areas of NMR and MRI including detecting magnetization transfer between small macromolecule proton pools and water (39), imaging regional perfusion where arterial water has been detected with magnetization transfer due to diffusional exchange with tissue water (12), and ammonia via exchange with water providing a 109 enhancement of the ammonia signal (40). These data demonstrate that the amplification of the saturation transfer experiment for detecting metabolites is remarkable. Recently, a number of enzymes have been detected using the idea to saturate where a small metabolite pool is located (41). Indeed, specific contrast agents based on exchange amplification (chemical exchange-dependent saturation transfer agents; CEST) are under active development (17, 38). Thus, small pools of metabolites including enzyme substrate complexes or substrates in compartments such as the protein dense and paramagnetic metal-rich mitochondrial matrix could contribute to the saturation transfer as well as the larger “free” metabolite pools detected by NMRS and usually attributed to the exchange processes.
Steady-state saturation transfer experiments are sensitive to small pools of metabolites, while other magnetization transfer techniques relying on short pulsed magnetization transfer techniques such as inversion transfer (11, 19, 22) or two-dimensional (2D) NOESY NMR (3, 25) experiments are not sensitive to small pools. This is due to the fact that the labeling of the pools only occurs over a very short period of time, making any transfer observed proportional to the size of the exchanging pools (19, 25). These pulsed magnetization transfer approaches can be used to compare and contrast with steady-state saturation transfer methods to evaluate the presence and role of these small exchanging pools. To date, such analysis has primarily focused on the creatine kinase reaction (11, 19, 25). This direct comparison of saturation and inversion recovery experiments in the same tissue reveals several troubling observations. First, to our knowledge the direct transfer between Pi and γ-ATP has never been observed with a pulsed magnetization transfer technique. Secondly, transfer between γ-ATP and other ATP phosphates observed with saturation transfer are not easily explained. We will use the data from Jucker et al. (23) for this example, but similar effects have been presented by numerous investigators (1, 11, 19, 23, 25, 26). As seen in Fig. 1, the irradiation of the γ-ATP phosphate results in the exchange of magnetization, or decrease in signal as marked by the bars, to PCr, putatively via creatine kinase, and inorganic phosphate, putatively via ATP synthesis/hydrolysis. However, large decreases in the γ-ATP and the α-ATP resonance occur with γ-ATP saturation that have not been fully explained. Again, measurement of these exchanges between ATP phosphates is not unique to this study and has been seen in almost every saturation transfer study performed in intact tissues. We selected this study since it was done on humans and is of very high quality. Confidence in the quantitative interpretation of the PCr-γ-ATP exchange via creatine kinase is much higher because when pulsed magnetization transfer and steady-state saturation transfer studies are performed, nearly identical data are obtained for the hydrolysis of PCr and this rate has been validated with in vitro studies (7, 11, 19, 27, 37). However, quite surprisingly, none of the other exchanges, Pi, γ-ATP, and α-ATP, have been observed using pulsed magnetization transfer techniques such as inversion transfer (11, 18, 22) or 2D NMR (4, 25). The exchange between γ-ATP and β-ATP detected by saturation transfer has been attributed either to two cycles through the adenylate cyclase (1, 16) or to saturation of the small β-ADP that resonates close to the γ-ATP and would be saturated and then exchange to β-ATP via creatine kinase or ATP synthesis/hydrolysis (1, 30). The lack of detection of this exchange by 2D and inversion transfer techniques argues for the involvement of the small ADP pool, or other exchanging pool, for this exchange (i.e., γ-ATP to β-ATP). The sensitivity of the pulsed magnetization transfer techniques is lower than the steady-state saturation transfer approaches even if large metabolite pools are exchanging; thus the lack of detection of these other exchanges from γ-ATP may be due to this lower sensitivity. Neeman et al. (30) assigned the γ-ATP peak to contain both the β-ADP and γ-ATP resonances, which is strictly correct at this level of spectral dispersion, and calculated the rate of exchange between the β-ADP and γ-ATP pool to the β-ATP pool to be ~2.0 mM/s in cultured cells, which is consistent with the large saturation transfer seen in many muscle preparations. If this reaction was simply the exchange of the γ-ATP to the β-ATP via two cycles of the adenylate kinase reaction, then this reaction occurring at 2 mM/s should be detected in the pulsed label experiments. However, to our knowledge this has never been seen, again suggesting a small pool contribution. The most bothersome of all of these transfers is the transfer to the α-ATP because there is no known enzymatic reaction pathway that can catalyze this exchange in the time frame of seconds necessary for saturation transfer effects. Thus, a more likely explanation of the saturation transfer to the β-ATP resonance, and almost certainly the α-ATP, would be the irradiation of small exchanging pools in the γ-ATP region that is not simply free bulk cytosolic γ-ATP. These pools might be responsible not only for the exchange to β- and α-ATP but also for PCr (19) and Pi contributing to the higher exchange rate discussed by Kemp (24) and From and Ugurbil.
Fig. 1.
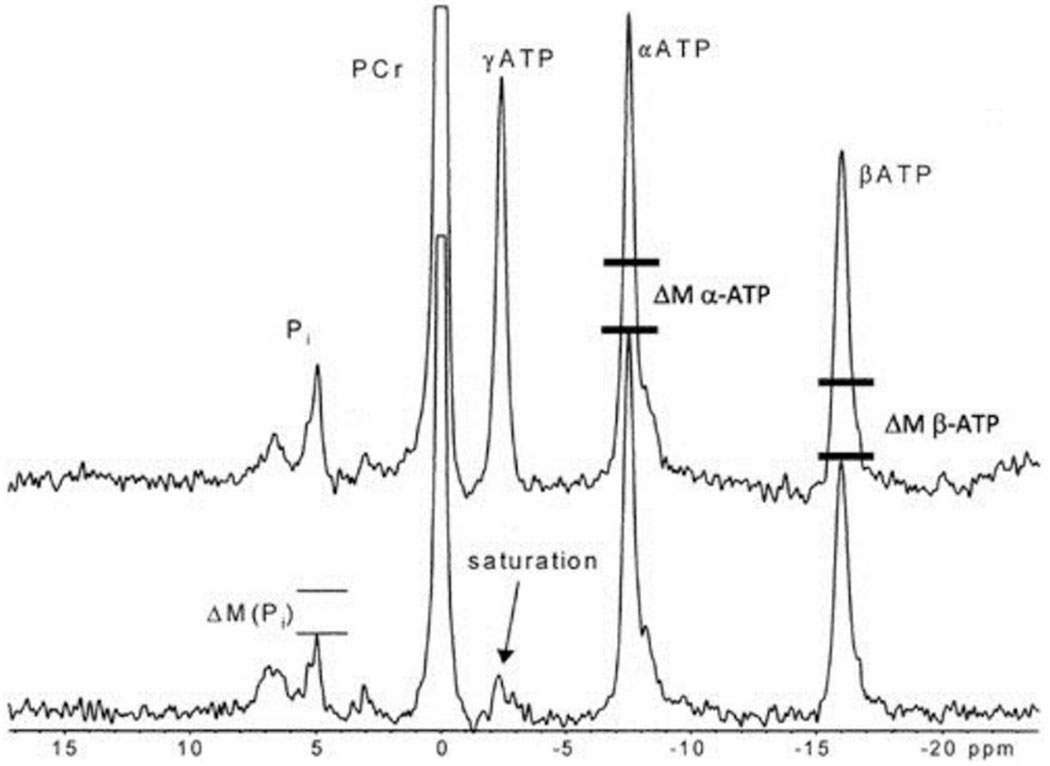
This is adapted from Jucker et al. (23; copyright 2000 National Academy of Sciences, U.S.A.). The top tracing is a 31P NMR spectrum from the human leg under control conditions.
The bottom spectrum is during the saturation of the γ-ATP phosphate where the signal is eliminated, or saturated, by the irradiation, as indicated by the arrow. The saturation of the γ-ATP results in a decrease in the Pi resonance, as shown by the two bars over the Pi peak placed by Jucker et al. This has been ascribed to the ATP hydrolysis rate. However, nearly equal decreases in signal are seen for the α-ATP (ΔM α-ATP) and β-ATP (ΔM β-ATP) resonance, as illustrated by the bars we have added. The reasons for these decreases in signal with the γ-ATP irradiation are discussed within the article. PCr, phosphocreatine; M, magnetization; ppm, parts per million.
What could these small metabolite pools, with a likely broad NMR line shape, be? The detection of metabolites using NMR is dependent on the mobility and environment that the metabolites are in. An extreme example of this is the effect of protein, albumin, binding on the hypersensitive quadrapole 14N resonance of urea. In this case, the urea 14N signal can be broadened to such a degree by rapid exchange with the relatively immobile protein that the 14N signal from urea could not be detected even though the free activity of the urea is unchanged (5). Similar effects occur with half-spin systems like 31P. Specifically, the association of ADP and ATP with enzyme complexes has been shown to broaden and shift the 31P resonance where the motion and magnetic environment is altered (10). These shifts and broadening have been observed in creatine kinase (27), pyruvate kinase (29), phosphoglycerate kinase (32), adenylate kinase (31, 32), and possibly most important for striated muscle, myosin ATPase (33, 35, 36). Likely, many more proteins generate similar effects. In addition, the mitochondrial matrix environment, in vitro, has been shown to broaden the 31P signal from ATP and Pi depending on the metal content of the matrix (21). The 31P metabolite line shapes from the mitochondria matrix in vivo are unknown. However, quantitative arguments of the total detection of metabolites suggest that matrix ATP is contributing to the total tissue NMR 31P ATP signal due to the mole fraction of ATP in the matrix and the total signal detected (15, 20). In addition, the mitochondria content of skeletal muscle is very low (1–5% vol/vol), making the contribution of this pool much lower than heart or other aerobic tissues. In any event, these data suggest that many pools of ATP, ADP, and Pi may exist in enzyme substrate complexes or local environments with different resonance frequencies and line shapes that could confuse the interpretation of saturation transfer experiments.
Thus, it is feasible that broad and chemically shifted ADP, Pi, and ATP bound to enzyme-active sites or in simple dynamic exchange on proteins could be contributing to the saturation transfer effect. The significant transfer from γ-ATP to the α-ATP is the strongest indication that small pool exchange is occurring. The α-ATP exchange likely occurs because of an exchanging enzyme or protein-bound ATP that shifts the α-ATP resonance to be near the bulk γ-ATP resonance signal. Upon irradiation of the γ-ATP position, the bound α-ATP resonance is also saturated, resulting in the observed transfer as the bound ATP leaves it site. This type of mechanism must be eliminated to interpret the other magnetization transfers observed as bulk metabolite pool exchanges alone. Furthermore, if the enzyme-bound species are contributing to the exchange, one must also be concerned about what step in the exchange reaction is rate limiting and dominating the magnetization transfer flux. Indeed, even creatine kinase can be placed under conditions such as low temperature and high [PCr] and low ADP where substrate release from the enzyme limits net flux rather than exchange of PCr and γ-ATP. Under these conditions, the NMRS observed rate does not measure the flux through the enzyme (7, 27). Rapid exchange processes could be occurring such as Pi or ATP binding that could be detected with saturation transfer methods if the enzyme-bound form is broadened and shifted as has been described in the literature above.
To test the role of these pools, we would suggest several experiments. First is to perform thorough comparisons of steady-state saturation transfer and pulsed magnetization transfer experiments of the Pi-γ-ATP exchange. Second is to perform an action spectrum of the exchange (25, 27, 39). This is done by varying the power and the frequency of the irradiating field to assure that only the free or narrow pool of ATP, or Pi, is contributing to the exchange. These experiments conducted on isolated enzyme systems reveal surprising broad and shifted resonances consistent with the protein-bound metabolite exchange pools (25, 27). Similar studies in more complex biological systems might be revealing. It might also be useful to vary the main magnetic field, which changes the absolute shift between peaks and the line-broadening characteristics of these bound pools. Finally, it is critical to verify the Pi-γ-ATP exchange in well-defined isolated mitochondrial preparations to validate the approach.
In summary, MRI was founded on the vision that imaging and function could be obtained noninvasively from humans in a manner that would help detect and intervene in human disease (28). The spectacular success of MRI as both an anatomical and functional imaging tool has lived up to these early expectations. The applications of NMRS and spectroscopic imaging are an important part of the functional tool kit available. The goal of assessing metabolic fluxes via magnetization transfer experiments remains an important measurement that has not yet translated to widespread biological and clinical application. A major hurdle is the ambiguous interpretation that still surrounds these measurements as clearly discussed in From’s and Ugurbil’s comments. With the great gains in sensitivity that new high field magnets and modern NMR detectors offer, it is time to perform the comprehensive experiments in vivo and in vitro that are required to remove these ambiguities. Indeed, the very issues that are complicating the saturation transfer measures, competing reactions and potential contribution of small metabolite pools or enzyme bound metabolites, may provide new insights into the operation of complex metabolic reaction sequences in vivo.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References:
- 1.Alger JR , den Hollander JA , Shulman RG. In vivo phosphorus-31 NMR saturation transfer studies of ATPase kinetics in Saccharomyces cerevisiae. Biochemistry 21: 2957–2963, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Alger JR , Shulman RG. NMR studies of enzymatic rates in vitro and in vivo by magnetization transfer. Q Rev Biophys 17: 83–124, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Balaban RS , Kantor HL , Ferretti JA. In vivo flux between phosphocreatine and adenosine triphosphate determined by two-dimensional phosphorous NMR. J Biol Chem 258: 12787–12789, 1983. [PubMed] [Google Scholar]
- 4.Balaban RS , Kantor HL , Ferretti JA. In vivo flux between phosphocreatine and adenosine triphosphate determined by two-dimensional phosphorous NMR. J Biol Chem 258: 12787–12789, 1983. [PubMed] [Google Scholar]
- 5.Balaban RS , Knepper MA. Nitrogen-14 nuclear magnetic resonance spectroscopy of mammalian tissues. Am J Physiol Cell Physiol 245: C439–C444, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Brindle KM , Campbell ID. NMR studies of kinetics in cells and tissues. Q Rev Biophys 19: 159–182, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Brindle KM , Radda GK. Measurements of exchange in the reaction catalysed by creatine kinase using 14C and 15N isotope labels and the NMR technique of saturation transfer. Biochim Biophys Acta 829: 188–201, 1985. [DOI] [PubMed] [Google Scholar]
- 8.Brindle KM , Radda GK. 31P-NMR saturation transfer measurements of exchange between Pi and ATP in the reactions catalysed by glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase in vitro. Biochim Biophys Acta 928: 45–55, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Brown TR , Ugurbil K , Shulman RG. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci USA 74: 5551–5553, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn M , Rao BDN. 31P studies of enzymatic reactions. Bull Magn Reson 1: 38–60, 1979. [Google Scholar]
- 11.Degani H , Laughlin M , Campbell S , Shulman RG. Kinetics of creatine kinases in the heart: A 31P NMR saturation- and inversion-transfer study. Biochemistry 24: 5510–5516, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Detre JA , Zhang W , Roberts DA , Silva AC , Williams DS , Grandis DJ , Koretsky AP , Leigh JS. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed 7: 75–82, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Forsen S , Hoffman RA. A new method for the study of moderately rapid chemical exchange rates employing nuclear magnetic double resonance. Acta Chem Scand 17: 1787–1788, 1963. [Google Scholar]
- 14.From AH , Ugurbil K. Standard magnetic resonance-based measurements of the Pi→ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles. Am J Physiol Cell Physiol (March 2, 2011). doi: 10.1152/ajpcell.00345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gard JK , Kichura GM , Ackerman JJH , Eisenberg JD , Billadello JJ , Sobel BE , Gross RW. Quantitative 31P nuclear magnetic resonance analysis of metabolite concentrations in Langendorff-perfused rabbit hearts. Biophys J 48: 803–813, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta RK. Saturation transfer 31P NMR studies of the intact human red blood cell. Biochim Biophys Acta 586: 189–195, 1979. [Google Scholar]
- 17.Hancu I , Dixon WT , Woods M , Vinogradov E , Sherry AD , Lenkinski RE. CEST and PARACEST MR contrast agents. Acta Radiol 51: 910–923, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoult DI , Busby SJ , Gadian DG , Radda GK , Richards RE , Seeley PJ. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 252: 285–287, 1974. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh PS , Balaban RS. Saturation and inversion transfer studies of creatine kinase kinetics in rabbit skeletal muscle in vivo. Magn Reson Med 7: 56–64, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Humphrey SM , Garlick PB. NMR-visible ATP and Pi in normoxic and reperfused rat hearts: a quantitative study. Am J Physiol Heart Circ Physiol 260: H6–H12, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Hutson SM , Williams GD , Berkich DA , LaNoue KF , Briggs RW. A 31P NMR study of mitochondrial inorganic phosphate visibility: effects of Ca2+, Mn2+, and the pH gradient. Biochemistry 31: 1322–1330, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Joubert F , Gillet B , Mazet JL , Mateo P , Beloeil J , Hoerter JA. Evidence for myocardial ATP compartmentation from NMR inversion transfer analysis of creatine kinase fluxes. Biophys J 79: 1–13, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jucker BM , Dufour S , Ren J , Cao X , Previs SF , Underhill B , Cadman KS , Shulman GI. Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc Natl Acad Sci USA 97: 6880–6884, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp GJ. The interpretation of abnormal 31P magnetic resonance saturation transfer measurements of Pi/ATP exchange in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 294: E640–E642, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Koretsky AP , Basus VJ , James TL , Klein MP , Weiner MW. Detection of exchange reactions involving small metabolite pools using NMR magnetization transfer techniques: relevance to subcellular compartmentation of creatine kinase. Magn Reson Med 2: 586–594, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Koretsky AP , Wang S , Klein MP , James TL , Weiner MW. 31P NMR saturation transfer measurements of phosphorus exchange reactions in rat heart and kidney in situ. Biochemistry 25: 77–84, 1986. [DOI] [PubMed] [Google Scholar]
- 27.Kupriyanov VV , Balaban RS , Lyulina NV , Steinschneider AY , Saks VA. Combination of 31P-NMR magnetization transfer and radioisotope exchange methods for assessment of an enzyme reaction mechanism: rate-determining steps of the creatine kinase reaction. Biochim Biophys Acta 1020: 290–304, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 242: 190–191, 1973. [PubMed] [Google Scholar]
- 29.Nageswara Rao BD , Kayne FJ , Cohn M. 31P NMR studies of enzyme-bound substrates of rabbit muscle pyruvate kinase. Equilibrium constants, exchange rates, and NMR parameters. J Biol Chem 254: 2689–2696, 1979. [PubMed] [Google Scholar]
- 30.Neeman M , Rushkin E , Kaye AM , Degani H. 31P-NMR studies of phosphate transfer rates in T47D human breast cancer cells. Biochim Biophys Acta 930: 179–192, 1987. [DOI] [PubMed] [Google Scholar]
- 31.Price NC , Reed GH , Cohn M. Magnetic resonance studies of substrate and inhibitor binding to porcine muscle adenylate kinase. Biochemistry 12: 3322–3327, 1973. [DOI] [PubMed] [Google Scholar]
- 32.Rao BD , Cohn M , Scopes RK. 31P NMR study of bound reactants and products of yeast 3-phosphoglycerate kinase at equilibrium and the effect of sulfate ion. J Biol Chem 253: 8056–8060, 1978. [PubMed] [Google Scholar]
- 33.Shriver JW , Sykes BD. Energetics and kinetics of the interconversion of two myosin subfragment-1.adenosine 5′-diphosphate complexes as viewed by phosphorus-31 nuclear magnetic resonance. Biochemistry 20: 6357–6362, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Spencer RG , Balschi JA , Leigh JS , Ingwall JS. ATP synthesis and degradation rates in the perfused rat heart 31P-nuclear magnetic resonance double saturation transfer measurements. Biophys J 54: 921–929, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanokura M A phosphorus-31 nuclear magnetic resonance study on the complex of chicken gizzard myosin subfragment 1 with adenosine diphosphate. Mol Cell Biochem 190: 75–78, 1999. [PubMed] [Google Scholar]
- 36.Tanokura M , Ebashi S. Complexes of myosin subfragment 1 with pyrophosphate and with adenosine diphosphate as studied by phosphorus-31 nuclear magnetic resonance. J Biochem 113: 19–21, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Ugurbil K , Petein M , Maidan R , Michurski S , From AHL. Measurement of an individual rate constant in the presence of multiple exchanges: application to myocardial creatine kinase reaction. Biochemistry 25: 100–107, 1986. [DOI] [PubMed] [Google Scholar]
- 38.Ward KM , Aletras AH , Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 143: 79–87, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Wolff SD , Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation, in vivo. Magn Reson Med 10: 135–144, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Wolff SD , Balaban RS. NMR imaging of labile proton exchange. J Magn Reson 85: 164–169, 1989. [Google Scholar]
- 41.Xu S , Shen J. Studying enzymes by in vivo C magnetic resonance spectroscopy. Prog Nucl Magn Reson Spectrosc 55: 266–283, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


