Abstract
Objective:
We aimed to understand the role of the tyrosine phosphatase PTPN14 –which in cancer cells modulates the Hippo pathway by retaining YAP in the cytosol- in fibroblast-like synoviocytes (FLS) from rheumatoid arthritis (RA) patients.
Methods:
Gene/protein expression levels were measured by qPCR and/or Western blotting. Gene knockdown in RA FLS was achieved using antisense oligonucleotides. The interaction between PTPN14 and YAP was assessed by immunoprecipitation. The cellular localization of YAP and SMAD3 was examined via immunofluorescence. SMAD reporter studies were carried out in HEK293T cells. The RA FLS/cartilage co-implantation and passive K/BxN models were used to examine the role of YAP in arthritis.
Results:
RA FLS displayed overexpression of PTPN14 when compared to FLS from osteoarthritis (OA) patients. PTPN14 knockdown in RA FLS impaired TGFβ-dependent expression of MMP13 and potentiation of TNF signaling. In RA FLS PTPN14 formed a complex with YAP. Expression of PTPN14 or nuclear YAP -but not of a non-YAP-interacting PTPN14 mutant- enhanced SMAD reporter activity. YAP promoted TGFβ-dependent SMAD3 nuclear localization in RA FLS. Differences in epigenetic marks within Hippo pathway genes, including YAP, were found between RA FLS and OA FLS. Inhibition of YAP reduced RA FLS pathogenic behavior and ameliorated arthritis severity.
Conclusion:
In RA FLS, PTPN14 and YAP promote nuclear localization of SMAD3. YAP enhances a range of RA FLS pathogenic behaviors which, together with epigenetic evidence, points to the Hippo pathway as an important regulator of RA FLS behavior.
Keywords: rheumatoid arthritis, fibroblast-like synoviocytes, PTPN14, YAP, TGFβ
INTRODUCTION
Fibroblast-like synoviocytes (FLS) in the synovial intimal lining of the joint play a pivotal role in the pathogenesis of rheumatoid arthritis (RA) [1–4] through invasion of extracellular matrix, secretion of pro-inflammation cytokines, and production of cartilage-degrading matrix metalloproteases (MMPs).
We previously surveyed the expression of protein tyrosine phosphatases (PTPs) in FLS from RA patients (RA FLS) and reported that PTPσ, PTPκ, and PTPα promote the aggressiveness of RA FLS [5–7]. In the same survey, non-receptor protein tyrosine phosphatase 14 (PTPN14, also known as PEZ) was found to be among the most highly expressed PTPs in RA FLS [8].
PTPN14 is a ubiquitous phosphatase with nuclear and cytosolic localization and is frequently mutated in various cancers [9–11]. Structurally PTPN14 includes an N-terminal “Band 4.1, ezrin, radixin, moesin homology” (FERM) domain, a linker region and a C-terminal catalytic PTP domain. The linker contains two PPxY motifs that drive the interaction between PTPN14 and Yes-associated protein (YAP), a tumor-promoting transcription co-activator that is a downstream effector of the Hippo pathway [12, 13]. In cancer cells, PTPN14 acts as a tumor suppressor via sequestration of YAP in the cytoplasm independent of phosphatase activity [14–17]. Among PTPN14 known substrates, protein kinase Cδ (PRKCD) and Ras and Rab interactor 1 (RIN1) are important regulators of endosome-related receptor trafficking, suggesting that PTPN14 activity can modulate surface receptor presenting and recycling [18].
PTPN14 has been previously implicated in promoting transforming growth factor β (TGFβ) signaling through the TGFβ receptor [9, 19], via an unknown mechanism of action. TGFβ is highly expressed in the RA synovium [20]. Although the overall role of TFGβ in RA pathogenesis remains incompletely understood, TGFβ potentiates the pro-inflammatory action of tumor necrosis factor α (TNF) and interleukin 1β (IL-1) on RA FLS [21].
Here we report that RA FLS display TGFβ-dependent overexpression of PTPN14 when compared to FLS derived from patients with osteoarthritis (OA FLS). We propose that in RA FLS, PTPN14 promotes TGFβ signaling via a YAP-mediated mechanism. In addition, we identify the Hippo pathway and YAP as molecules of interest in RA FLS pathogenic action.
MATERIALS AND METHODS
Further information is available in the online supplementary methods.
FLS lines.
FLS lines were obtained from arthroplasties (UC SAN DIEGO IRB#140175). Each line was derived from discarded synovial tissue from RA or OA patients undergoing synovectomy or total joint replacement, as previously described [22]. The diagnosis of RA conformed to the American College of Rheumatology 1987 revised criteria [23].
Antisense oligonucleotide (ASO) knockdown.
Human FLS were grown to 90% confluence and treated with 2.5 μM morpholino antisense oligonucleotides (ASO) (Gene Tools). ASO was replenished in fresh culture media after 3 days and in serum-starvation media after 6 days.
SMAD reporter Assays.
SMAD reporter assays were performed using the Qiagen’s Cignal SMAD Reporter (luc) kit.
Mice.
All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the La Jolla Institute (#AP140-NB4) and UC SAN DIEGO (#S16098). C57BL/6 KRN mice were provided by Dr. Christophe Benoist (Harvard Medical School, Boston, MA) and were crossed with NOD mice (Taconic Bioscience) to obtain arthritic offspring (K/BxN mice) whose serum was pooled for use in the K/BxN passive serum-transfer arthritis model.
Statistical Analysis.
Two tailed statistical analyses were performed as indicated in the figure legends using GraphPad Prism software. A comparison was considered significant if p value was <0.05.
RESULTS
TGFβ-dependent overexpression of PTPN14 in RA FLS
A comparison between FLS from 11 patients with RA and 10 patients with OA revealed that PTPN14 is significantly overexpressed in RA FLS than in OA FLS (p<0.01) (Figure 1A). We also detected significantly increased PTPN14 protein levels in five RA FLS lines compared to five OA FLS lines (p<0.01, Figure 1B,C). Immunofluorescence (IF) assessment of synovial specimens from patients with RA vs OA showed high expression of PTPN14 in RA (Figure 1D). Published data and a survey of ImmGen data suggest that PTPN14 is expressed prominently in stromal cells and poorly in immune cells [24, 25]. In line with this observation, a comparative assessment of PTPN14 mRNA expression in synovial biopsies from the Pathology of Early Arthritis Cohort (PEAC) -including 87 treatment-naïve RA patients - showed that PTPN14 was significantly more expressed in biopsies characterized by a prominent or exclusive FLS presence (fibroid) -which showed limited expression of CD3, CD20, CD138, and CD68 - markers of T cells, B cells, plasma cells and macrophages respectively (online Supplementary Figure 1)- versus biopsies characterized by prominent immune cell infiltration (non-fibroid) (p<0.0001, Figure 1E).
Figure 1. PTPN14 displays TGFβ-dependent overexpression in RA FLS.
A. PTPN14 mRNA expression was assessed by qPCR in 11 RA FLS lines and 10 OA FLS lines. Results were normalized to POL2A using 2−ΔCt method. Mean±SEM are shown. B. PTPN14 protein expression levels in 3 RA FLS and 3 OA FLS lines was assessed by Western blotting. C. PTPN14 protein expression was assessed by western blot in 5 RA FLS lines and 5 OA FLS lines. Results were normalized to GAPDH. Mean±SEM are shown. D. IF of synovial sections from OA or RA patients stained with anti-PTPN14 antibody (green signal) and DAPI (blue signal). Representative images are shown at 60X magnification. E. PTPN14 mRNA expression levels measured by RNAseq in 65 non-fibroid vs 17 fibroid RA synovium specimens. F. RA FLS (n=5) were stimulated with platelet-derived growth factor (PDGF, 50 ng/ml) or transforming growth factor β1 (TGFβ, 50 ng/ml) for 24 hours. PTPN14 expression was assessed by qPCR. Results were normalized to GAPDH using 2−ΔΔCt method. Mean±SEM are shown. G. The expression level of PTPN14, TGFBR1 and THBS1 was assessed by qPCR on 11 RA FLS lines and 11 OA FLS lines. Graphs show PTPN14 vs TGFBR1 expression or PTPN14 vs THBS1 expression for each line. H-I. PTPN14 mRNA expression was measured by qPCR performed in triplicate after RA FLS (n=4–5) treatment with 50 μM TGFβRI inhibitor SB505124 (H) or 1 μM RepSox (I) for 24 hours. Results were normalized to GAPDH using 2−ΔΔCt method. Box-and-whisker plots (E,H,I) depict median (line within box), 25th percentile and 75th percentile (bottom and top borders), and range of minimum to maximum values (whiskers). Data were analyzed using the two-tailed Mann-Whitney test (A,C,E,H,I), the Kruskal-Wallis test with two-tailed Mann-Whitney post-hoc test (F) or the Spearman correlation test (G). p-value was adjusted for multiple comparison in (F). LFS, fibroblast-like synoviocytes; IF, immunofluorescence; OA, osteoarthritis; qPCR, quantitative PCR; RA, rheumatoid arthritis.
We next examined the effect of growth factors on PTPN14 expression in RA FLS and found that TGFβ1 (TGFβ, 50 ng/ml), but not platelet-derived growth factor (PDGF, 50 ng/ml) stimulation enhances PTPN14 expression in serum-starved RA FLS (P<0.05) (Figure 1F). RA FLS exhibit an intrinsic up-regulation of the mRNAs for TGFβ (TGFB1), TGFβ receptor 1 (TGFBR1), and thrombospondin 1 (THBS1, encoding an activator of latent TGFβ [26]) when compared to OA FLS [27]. Since PTPN14 is induced by TGFβ, we assessed whether PTPN14 expression correlates with TGFB1, TGFB1R, and THBS1 in FLS. As shown in Figure 1G, the expression levels of PTPN14 positively correlated with TGFBR1 in RA (Spearman γ =0.8455, p<0.01) and OA FLS (Spearman γ =0.8364, p<0.01) and THBS1 in RA FLS (Spearman γ =0.6545, p<0.05) and OA FLS (Spearman γ =0.6727, p<0.05), while there was no correlation between the expression levels of PTPN14 and TGFB1 (data not shown). Inhibition of TGFβ signaling using two selective TGFβRI antagonists SB505124 [28] and RepSox [29], reduced PTPN14 expression in unstimulated RA FLS (p<0.05, p<0.01 respectively) (Figure 1H,I) suggesting that enhanced autocrine stimulation with TGFβ plays a role in the upregulation of PTPN14 in RA FLS. However, additional unknown pathways likely contribute to enhance PTPN14 mRNA and protein levels in RA FLS in vitro and in the rheumatoid joint.
PTPN14 promotes TNFα- and IL-1β-stimulated MMP production in RA FLS
We next tested whether PTPN14 regulates the response of RA FLS to TNF and IL-1β, critical pathogenic cytokines in RA [30]. For knockdown PTPN14 in RA FLS, we treated RA FLS with a cell-permeable antisense oligonucleotide (PTPN14 ASO) targeting exon 4 or a control scrambled ASO. Knockdown of PTPN14 in RA FLS after ASO treatment was confirmed by western blotting (Supplementary Figure 2). Knockdown of PTPN14 in RA FLS significantly inhibited TNFα-induced expression of MMP1(Figure 2A left panel) but did not affect expression of MMP3, VCAM1, or IL6 (data not shown). We also noticed that knockdown of PTPN14 significantly reduced the expression of MMP13 in RA FLS (Figure 2A right panel and data not shown). The effect of PTPN14 ASO on IL-1β-induced MMP1 induction in RA FLS was non-significant (data not shown). In order to rule out off target effects of PTPN14 ASO, we designed a second ASO targeting PTPN14 exon 2 (PTPN14 ASO 2). Treatment of RA FLS with PTPN14 ASO 2 led to inhibition of TNFα-induced MMP1 and inhibition of MMP13 expression identical to the ones obtained with PTPN14 ASO (Supplementary Figure 3). Flow cytometry assessment did not show any effect of PTPN14 deficiency on RA FLS survival (online Supplementary Figure 4). Knockdown of PTPN14 also did not significantly affect RA FLS migration or invasiveness in response to fetal bovine serum (FBS), or RA FLS attachment to cartilage (Figure 2B).
Figure 2. PTPN14 promotes TGFβ-dependent MMP production by RA FLS.
A. RA FLS (n=4) were treated with Ctrl ASO or PTPN14 ASO for 6 days, serum-starved in the presence of ASO for 24 hours and then stimulated with or without TNFα for 24 hours. MMP1, and MMP13 mRNA expression was measured by qPCR performed in triplicate. Results were normalized to GAPDH using 2−ΔΔCt method. B. RA FLS (n=4) were treated with Ctrl ASO or PTPN14 ASO for 6 days and serum starved for 24 hours in the presence of ASO. For migration assay, treated cells were allowed to migrate for 24 hours in transwell assay in response to 5% FBS. For invasion assay, treated cells were allowed to invade through matrix-coated transwells for 24 hours in response to 5% FBS. Cells were then fixed with 100% methanol and stained with 0.2% crystal violet. For each well, four non-overlapping area (top, bottom, left and right) were imaged and counted. For cartilage attachment assay, bovine cartilage fragments were pre-treated with IL-1β (2 ng/ml) for 24 hours. Cells were incubated in constant rotation with cartilage fragments for 2 hours and then incubated at 37°C overnight. C. After serum starvation for 24 hours, RA FLS (n=4) were incubated in the presence or absence of 25 μM SB505124 (SB) and stimulated with or without TNFα for 24 hours. MMP1 and MMP13 mRNA expression was analyzed by qPCR performed in triplicate. Results were normalized to GAPDH using 2−ΔΔCt method. D. RA FLS (n=5 or 6) were treated with Ctrl or PTPN14 ASO for 6 days, serum starved with the presence of ASO for 24 hours and then stimulated with TGFβ for 24 hours. MMP13 mRNA expression were analyzed by qPCR performed in triplicate. Results were normalized to GAPDH using 2−ΔΔCt method. (A-D) Box-and-whisker plots depict median (line within box), 25th percentile and 75th percentile (bottom and top borders), and range of minimum to maximum values (whiskers). Data were analyzed using the Kruskal-Wallis test with two-tailed Mann-Whitney post-hoc test (A-D) or the two-tailed Mann-Whitney test (right panels in A-C), NS = non-significant. ASO, antisense oligonucleotides; FBS, fetal bovine serum; FLS, fibroblast-like synoviocytes; qPCR, quantitative PCR; RA, rheumatoid arthritis
PTPN14 promotes TGFβ signaling in RA FLS
As shown in Figure 2A, the effect of PTPN14 knockdown was particularly significant on MMP13, an important collagenase in RA, whose expression is regulated by TGFβ signaling in RA FLS and other cell types [6, 31]. Due to the proposed role of PTPN14 in TGFβ signaling [9] and since TFGβ is known to potentiate the action of TNFα and IL-1β in RA FLS, we hypothesized that the phenotypes observed in RA FLS subjected to PTPN14 knockdown might be at least in part due to blockade of autocrine RA FLS TGFβ signaling. Consistent with this model, inhibition of TGFB1R using SB505124 reduced TNFα-stimulated MMP1 induction and abrogated MMP13 expression in RA FLS (Figure 2C). We then assessed whether PTPN14 regulates TGFβ-induced MMP1 and MMP13 expression in RA FLS and found that knockdown of PTPN14 reduced TGFβ-induced expression of MMP13 (Figure 2D), while no induction of MMP1 was observed after treatment of RA FLS with TGFβ alone (data not shown).
PTPN14-YAP interaction enhances nuclear YAP-mediated TGFβ-SMAD signaling
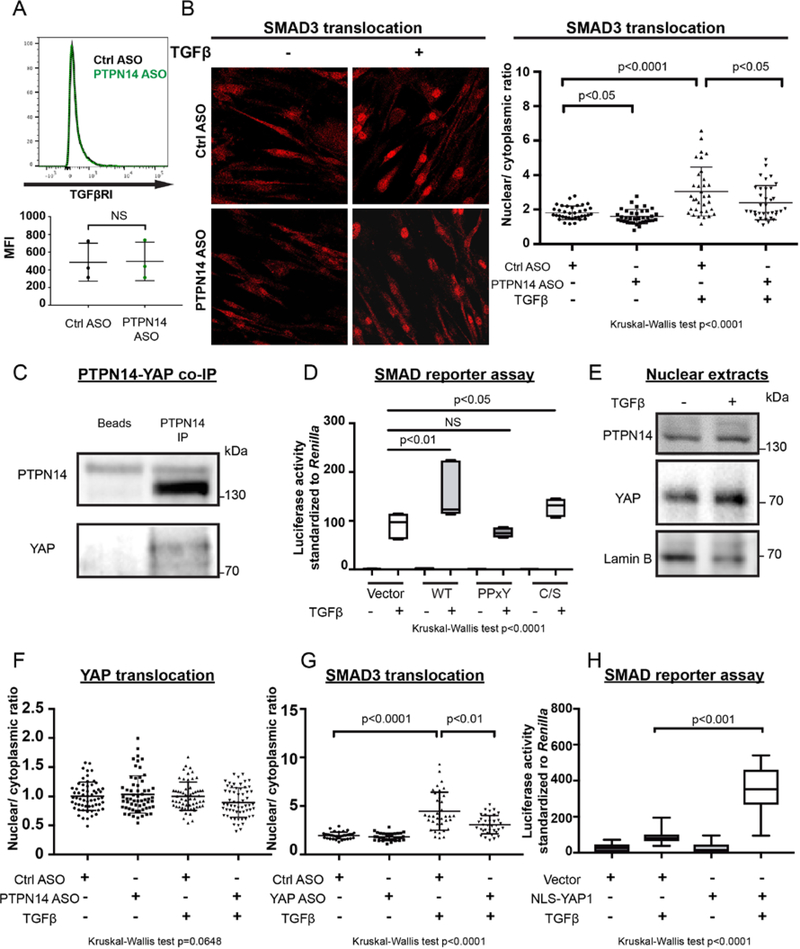
We next tried to assess the mechanism of action of PTPN14 in TGFβ signaling. We did not observe alterations in TGFβRI expression in cells treated with PTPN14 ASO (Figure 3A). Thus, we examined the role of PTPN14 in intracellular canonical TGFβ signaling, mediated by phosphorylated SMAD complexes, which translocate to the nucleus to regulate transcription of target genes [32]. We observed no difference in phospho-SMAD2 (pSer465/467) or phospho-SMAD3 (pSer42¾25) levels on TGFβ stimulation between RA FLS treated with control or PTPN14 ASO (data not shown). However, RA FLS treated with PTPN14 ASO showed significantly reduced basal and TGFβ-induced nuclear localization of SMAD3 -but not of SMAD2- when compared to cells treated with control ASO (p<0.05) (Figure 3B online Supplementary figure 5). MMP13 expression has been shown to be regulated by TGFβ through both canonical SMAD-dependent [33] and non-canonical mitogen-activated protein kinase (MAPK)-dependent pathways [31]. RA FLS subjected to knockdown of PTPN14 did not display significantly altered phosphorylation of extracellular-regulated kinase (Erk), C-Jun N terminal kinase (JNK), p38 MAPK, MAPK kinase 3 (MKK3), MKK4, MKK6 and MKK7 (data not shown), suggesting that non-canonical TGFβ signaling is unlikely to mediate PTPN14-driven expression of MMP13 in RA FLS.
Figure 3. PTPN14-YAP interaction and nuclear YAP enhance TGFβ-SMAD signaling.
A. Upper panel, representative flow cytometry analysis of TGFβRI on RA FLS (n=3) treated with Ctrl (shown in black) or PTPN14 ASO (shown in green) for 6 days. Lower panel, plot shows MFI for each line. B. RA FLS (n=3) were treated with Ctrl or PTPN14 ASO for 7 days, stimulated with TGFβ (50 ng/ml) for 30 min, then fixed and stained with an anti-SMAD3 antibody. Nuclear/cytoplasmic ratio of SMAD3 signal was calculated using image J for 12 cells from each RA FLS line. Representative images are shown in 60X magnification. Mean±SD is shown. C. Co-immunoprecipitation of PTPN14 with YAP. Western blotting with indicated antibodies is shown. Panel is representative of 3 experiments with similar results. D. TGFβ-induced SMAD activation was assessed via SMAD reporter assay in HEK293T cells. Cells were starved for 24 hours, transfected with empty vector, or vectors encoding WT PTPN14, or PTPN14 Y570F/Y732F (PPxY) or C1121S (C/S) mutants and then stimulated with TGFβ (50 ng/ml) for 24 hours. Graph shows ratio of firefly/Renilla luciferase signal. E. Western blotting of nuclear fraction of unstimulated or TGFβ-stimulated RA FLS using anti-PTPN14, anti-YAP or anti-lamin B (as a nuclear loading control). Panel is representative of 3 RA FLS lines with similar results. F. RA FLS (n=5) were treated with Ctrl or PTPN14 ASO for 7 days stimulated with TGFβ (50 ng/ml) for 30 min, then fixed and stained with an anti-YAP antibody. Nuclear/cytoplasmic ratio of YAP signal was calculated using image J for 12 cells from each RA FLS line. Representative images are shown in 60X magnification. Mean±SD is shown. G. RA FLS were treated with Ctrl or PTPN14 ASO for 7 days stimulated with TGFβ (50 ng/ml) for 30 min, then fixed and stained with an anti-SMAD3 antibody. Nuclear/cytoplasmic ratio of SMAD3 signal was calculated using image J for 12 cells from each RA FLS line. Representative images are shown in 60X magnification. Mean±SD is shown. H. TGFβ-induced SMAD activation was assessed via SMAD reporter assay in HEK293T cells. Cells were starved for 24 hours, transfected with empty vector, or vectors encoding NLS-YAP and then stimulated with TGFβ (50 ng/ml) for 24 hours. Graph shows ratio of firefly/renilla luciferase signal. (D,H) Box-and-whisker plots depict median (line within box), 25th percentile and 75th percentile (bottom and top borders), and range of minimum to maximum values (whiskers), 3 independent experiments performed in triplicate is shown. (A) Data were analyzed using the two-tailed paired t-test. (B,D,F-H) Data were analyzed using the Kruskal-Wallis test with two-tailed Mann-Whitney post-hoc test, NS = non-significant. p-value is adjusted for multiple comparison in (D). FLIS, fibroblast-like synoviocytes; MFI, mean fluorescence intensity; RA, rheumatoid arthritis.
As described, PTPN14 is known to regulate signaling via phosphatase activity dependent and independent mechanisms. There are four known PTPN14 substrates identified in cancer cell lines: β-Catenin [34], p130Cas [35], PRKCD and RIN1 [18]. Substrate-trapping double mutated PTPN14 catalytic domain (D1079A/C1121S) was expressed and substrate trapping experiments [36, 37] were carried out with RA FLS lysates, but none of the identified substrates were pulled-down by PTPN14 in RA FLS (data not shown). We thus looked at phosphatase activity-independent regulatory mechanisms. It is well documented that PTPN14 regulates Hippo signaling by forming a complex with YAP [16, 17]. We confirmed the existence of a PTPN14-YAP physical complex in RA FLS by immunoprecipitation (Figure 3C). To assess the mechanism of action of PTPN14 in TGFβ-SMAD signaling, we next examined the effect of PTPN14, a catalytically inactive PTPN14 C1121S mutant and a PTPN14 PPxY Y570F/Y732F mutant –which is unable to bind to YAP [15, 17] -in a SMAD reporter assay in HEK293T cells. Consistent with the observations made in RA FLS, overexpression of PTPN14 in HEK293T cell enhanced SMAD reporter activity upon TGFβ stimulation (Figure 3D). Mutations of the PPxY domains significantly reduced the SMAD-enhancing activity of PTPN14 while catalytically inactive (C/S) PTPN14 was as effective as PTPN14 WT at promoting the SMAD reporter activity (Figure 3D). These data suggest that PTPN14-mediated promotion of TGFβ-induced SMAD activation depends on the ability of PTPN14 to interact with YAP rather than on the phosphatase activity.
The PTPN14-YAP complex enhances YAP cytosolic localization in cancer cells [15]. Therefore, we asked whether PTPN14 regulates the nuclear localization of YAP in RA FLS. Immunofluorescence of resting RA FLS showed that >80% YAP was localized to the nucleus in sub-confluent (~70% confluent) cells (data not shown). Figure 3E shows that both YAP and PTPN14 were found in resting and TGFβ-stimulated RA FLS nuclear lysates. Immunofluorescence analysis of RA FLS revealed no significant changes in nuclear localization of YAP in unstimulated vs TGF-β stimulated and in cells subjected to knockdown of PTPN14 vs cells treated with control ASO (Figure 3F, online Supplementary Figure 6A).
In the early embryo YAP is also known to control TGFβ-signaling by modulating SMAD nuclear/cytosolic distribution [38]. In RA FLS, we found that partial knockdown of YAP using an ASO directed against YAP exon 2 modulated TGFβ-induced SMAD3 nuclear translocation in RA FLS (Figure 3G, online Supplementary Figure 6B, 6C). To further demonstrate that nuclear YAP is important to sustain TGFβ-dependent SMAD signaling, we carried out SMAD reporter assays by expressing YAP in frame with a nuclear localization sequence (NLS-YAP), which results in exclusive overexpression of YAP in the nucleus. Figure 3H shows that NLS-YAP significantly enhanced TGFβ-induced SMAD reporter activity. We conclude that in RA FLS PTPN14 and YAP promote nuclear recruitment of SMAD3 during TGFβ signaling.
The Hippo pathway displays epigenetic alterations in RA FLS and modulates TNF signaling and invasiveness of RA FLS in vitro
The Hippo pathway has recently emerged as a critical regulator of cancer growth and survival and of multiple important basic cell functions, however no information is available yet on the role of this pathway in RA FLS. A recent highly integrated analysis of multiple epigenetics marks in RA FLS versus OA FLS has identified multiple pathways that are differentially marked and candidate players in the pathogenic behavior of rheumatoid FLS [39]. We thus interrogated the available RA-FLS and OA-FLS epigenetic database, inclusive of 9 marks -six histone modifications (H3K27ac, H3K4me1, H3K4me3, H3K36me3, H3K27me3 and H3K9me3), open chromatin, RNA-seq and DNA methylation- for epigenetic alterations in the Hippo pathway. Applying the same integrative method and pathway analysis described in Ref [39], we discovered that the “Hippo signaling” pathway was significantly enriched in differential epigenetic marks between RA FLS and OA FLS. As shown in Figure 4A, the vast majority of known genes belonging to the Hippo pathway (differentially modified genes are highlighted in magenta in the figure), displayed differences in one or more of 5 histone modification (detailed in Figure 4B), and/or open chromatin and/or DNA methylation marks. YAP1 (encoding YAP) was differentially modified in H3K4me1, H3K4me3 and open chromatin regions. Figure 4C shows a genome browser screenshot exemplifying the epigenetic landscape within 300kb of YAP1 for one representative couple of RA vs OA FLS lines with boxes identifying differentially marked regions.
Figure 4. The Hippo pathway displays epigenetic alterations in RA FLS and modulates RA FLS TNF signaling and invasiveness.
A. The ingenuity pathway of Hippo signaling. Differentially marked genes (DMG) between RA and OA FLS are indicated in magenta color. Legends: triangles for kinases; rectangles for G-protein coupled receptors; circles for transcription regulator; oval for other; double-sided shapes for complexes/groups. B. Map of differentially modified marks for DMG in the Hippo signaling pathway between RA and OA FLS. C. Representative epigenomic landscape of YAP1 including six histone modifications, open chromatin, RNA-seq and DNA methylation. The figure shows an example of relative signal intensity across a ± 300 kb region of YAP1 for each mark in one RA vs one OA FLS lines. Differentially marked regions between RA and OA are indicated by boxes. D. RA FLS (n=4) were serum-starved for 24 hours, and then treated with DMSO or 1μM verteporfin (VP) for 45 min. Cells were then stimulated for 24 hours with 50 ng/ml TNF in the presence of DMSO or VP. MMP1, and MMP13 mRNA expression levels were determined by qPCR performed in triplicate. Results were normalized to GAPDH using 2−ΔΔCt method. Box-and-whisker plots depict median (line within box), 25th percentile and 75th percentile (bottom and top borders), and range of minimum to maximum values (whiskers). E. After 24h serum starvation, RA FLS (n=5) were allowed to invade for 24h through a matrigel-coated transwell chamber in response to 5% FBS in the presence of DMSO or 1μM VP. For each well, four non-overlapping area (top, bottom, left and right) were imaged and counted. Plot shows mean±SD percent invading cells. Data were analyzed using the Kruskal-Wallis test with two-tailed Mann-Whitney post-hoc test (D,E) or the two-tailed Mann-Whitney test (right panel in D). DMG, differentially marked genes; DMSO, dimethyl sulfoxide; FLS, fibroblast-like synoviocytes; OA, osteoarthritis; qPCR, quantitative PCR; RA, rheumatoid arthritis; VP, verteporfin.
Since the above-mentioned findings point to YAP as an important pathogenic factor and a potential mediator of PTPN14 action in RA FLS, we next assessed whether inhibition of nuclear YAP functions alters RA FLS behavior and/or phenocopies the effect of PTPN14 knockdown in RA FLS. We treated RA FLS with the small molecule verteporfin, an FDA approved drug for photodynamic therapy that has been shown to inhibit YAP transcriptional activity in vitro and in vivo [40]. In line with the observed effect of PTPN14 knockdown, inhibition of YAP with 1 μM verteporfin in RA FLS inhibited TNFα-induced expression of MMP1, and reduced expression of MMP13. However, verteporfin also inhibited TNF-induced MMP3, VCAM1 and IL-6 expression compared to cells treated with vehicle (Figure 4D, and online Supplementary Figure 7). Moreover, treatment with verteporfin dramatically inhibited RA FLS invasiveness in response to FBS (Figure 4E), suggesting that YAP promotes RA FLS pathogenic action through transcriptional and potentially other mechanisms that only partially overlap with the mechanisms regulated by PTPN14.
YAP promotes RA FLS invasiveness in vivo and arthritis severity in mice
To further assess whether YAP promotes RA FLS pathogenic behavior in vivo, we employed the severe combined immunodeficiency (SCID) model of FLS cartilage engraftment [41]. In line with the in vitro observations reported in Figure 4, daily administration of verteporfin (15 mg/kg) to cartilage and RA FLS-engrafted SCID mice led to a significant (p<0.0001) reduction of in vivo cartilage invasion by RA FLS (Figure 5A). In order to further assess the role of YAP in a second synoviocyte-dependent model of RA, we also examined whether treatment with verteporfin affects disease development in the passive K/BxN serum-transfer arthritis model. Figure 5B shows that daily administration of 50 mg/kg verteporfin to K/BxN serum-transferred mice (n=28) led to significantly reduction of arthritis severity (p<0.0001) compared with control mice treated with DMSO (n=25). Histological assessment of affected joints showed that verteporfin treatment significantly protected mice from bone erosion, cartilage damage and inflammation (Figure 5C and online Supplementary Fig. 8).
Figure 5. YAP promotes RA FLS invasiveness and arthritis severity in mice.
A. Cartilage fragments were co-implanted with 5 X 105 RA FLS (n=2) subcutaneously in SCID mice. Mice (n=3–4) were injected intraperitoneally (i.p.) with 15 mg/kg VP or vehicle every other day for 34 days. Two days after the last administration, the cartilage was harvested and assessed for FLS invasion. Representative images of cartilage sections viewed at 20X magnification are shown. S = sponge, C = cartilage. Plot shows mean±SD of the depth of cartilage invasion by RA FLS assessed on a minimum of 4 fields/specimen. B. Eight-week-old female Balb/c mice were injected i.p. with 100μL of K/BxN serum to induce arthritis, and injected i.p. daily with 50 mg/kg verteporfin (n=28) or DMSO-containing vehicle (n=25) starting on the day of arthritis induction. Ankle thickness and clinical score were assessed every day. Graph shows mean±SEM of mouse ankle swelling (left graph) and clinical score (right graph). C. After 14 days, joints from mice in B were harvested and subjected to histological staining with H&E (for inflammation and bone erosion assessment) and safranin O (for cartilage damage assessment). Inflammation, bone erosion and cartilage damage of the talonavicular joint were scored blindly between 0 and 5 by two people. Plot shows mean±SEM of combined scores. Data were analyzed with two-tailed Mann-Whitney test (A,C) or two-tailed Mann-Whitney test using area under curve in (B). DMSO, dimethyl sulfoxide; FLS, fibroblast-like synoviocytes; RA, rheumatoid arthritis.
DISCUSSION
Here, we report that RA FLS display overexpression of PTPN14, which promotes TGFβ canonical signaling. We provide evidence that promotion of SMAD signaling by PTPN14 depends on the formation of a YAP-PTPN14 complex. Although the exact molecular mechanism through which PTPN14 regulates TGFβ-induced SMAD3 translocation in RA FLS remains to be clarified, we speculate that the PTPN14-YAP complex enhances the ability of nuclear YAP to recruit SMAD3 on TGFβ stimulation. YAP has been shown in other cells types to form a complex with SMAD2/3, which promotes nuclear translocation of SMAD complexes [38, 42]. Thus, it is possible that a trimolecular PTPN14-YAP-SMAD3 complex is formed in the nucleus of RA FLS. The observation that PTPN14 knockdown only partially recapitulates inhibition of YAP nuclear functions is in line with the fact (evident in Figure 3F) that PTPN14 is not necessary for YAP nuclear localization. The latter is a somehow unexpected finding since in many cancer cell types, the PTPN14-YAP complex prevents nuclear translocation of YAP[15] and RA FLS have been likened to tumor-like cells due to their peculiar aggressive features in vitro and in vivo [43]. However, the partial overlap between PTPN14-mediated and YAP-mediated signaling in RA FLS might also reflect unknown mechanisms of compensation in cells subjected to knockdown of PTPN14. A limitation of our studies of PTPN14 is that some of the changes observed after knockdown in FLS are modest and further studies -e.g. in animals with conditional deletion of PTPN14 in FLS when they become available- are needed to confirm that PTPN14 is involved in the pathogenesis of RA and its hierarchical dominance in disease pathogenesis.
Our data also suggest for the first time that the Hippo pathway and nuclear YAP contribute to the aggressive phenotype of RA FLS. Although our re-analysis of the available dataset showed no differences in Hippo pathway transcript levels in resting RA vs OA FLS, the pathway carries an extensive epigenetic signature in RA FLS, which warrants mRNA and protein expression studies in RA and OA FLS subjected to RA- relevant stimulations and in RA synovium. Furthermore, our in vivo data also points to a potential benefit of YAP inhibition to reduce FLS pathogenesis in RA. Since verteporfin has been shown to ameliorate antigen-induced arthritis (AIA) in rabbits by inducing apoptosis of inflammatory cells [44], further investigations are warranted to elucidate whether YAP inhibition could also control the immune-mediated arm of RA pathogenesis, thus configuring YAP as a potentially unique target for dual immune and FLS-based RA therapy.
Supplementary Material
KEY MESSAGES.
What is already known about this subject?
Fibroblast-like synoviocytes (FLS) play an important role in the pathogenesis of rheumatoid arthritis (RA). Several signaling pathways are known to be dysregulated in rheumatoid FLS, however the contribution of the Hippo pathway remains unexplored.
What does this study add?
This study shows that the tyrosine phosphatase PTPN14 and the transcription coactivator YAP, known key players in the Hippo pathway, are dysregulated in RA FLS and can modulate the pathologic behavior of FLS in rheumatoid arthritis.
How might this impact on clinical practice?
The study suggests that YAP and potentially other members of the Hippo pathway, which is already being targeted for cancer therapy, could be leveraged as therapeutic targets for novel RA therapies.
FUNDING INFO
This study was funded in part by the National Institutes of Health (NIH) Grants R01 AR066053 and AI070544 and Department of Defense (DOD) grant # W81XWH-16–1-0751 (to N.B.). A.B. and D.J.W. were supported by NIH Training Grant T32 AR064194.
Footnotes
COMPETING INTERESTS
None declared.
PATIENT CONSENT
Not required.
ETHICAL APPROVAL INFORMATION
All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the La Jolla Institute (#AP140-NB4) and UC SAN DIEGO (#S16098). The generation and banking of FLS lines from arthroplasties was approved by the UC San Diego IRB (#140175). Ethical approval for the PEAC cohort was granted by the King’s College Hospital Research Ethics Committee (REC 05/Q0703/198).
DATA SHARING STATEMENT
There is no additional unpublished data.
REFERENCES
- 1.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013. January; 9(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller-Ladner U, Ospelt C, Gay S, Distler O, Pap T. Cells of the synovium in rheumatoid arthritis. Synovial fibroblasts. Arthritis Res Ther. 2007; 9(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley CD. Why does chronic inflammation persist: An unexpected role for fibroblasts. Immunol Lett. 2011. July; 138(1):12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmann M, Maini RN. Perspectives From Masters in Rheumatology and Autoimmunity: Can We Get Closer to a Cure for Rheumatoid Arthritis? Arthritis Rheumatol. 2015. September; 67(9):2283–2291. [DOI] [PubMed] [Google Scholar]
- 5.Doody KM, Stanford SM, Sacchetti C, Svensson MN, Coles CH, Mitakidis N, et al. Targeting phosphatase-dependent proteoglycan switch for rheumatoid arthritis therapy. Sci Transl Med. 2015. May 20; 7(288):288ra276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanford SM, Aleman Muench GR, Bartok B, Sacchetti C, Kiosses WB, Sharma J, et al. TGFbeta responsive tyrosine phosphatase promotes rheumatoid synovial fibroblast invasiveness. Ann Rheum Dis. 2016. January; 75(1):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanford SM, Svensson MN, Sacchetti C, Pilo CA, Wu DJ, Kiosses WB, et al. Receptor Protein Tyrosine Phosphatase alpha-Mediated Enhancement of Rheumatoid Synovial Fibroblast Signaling and Promotion of Arthritis in Mice. Arthritis Rheumatol. 2016. February; 68(2):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanford SM, Maestre MF, Campbell AM, Bartok B, Kiosses WB, Boyle DL, et al. Protein tyrosine phosphatase expression profile of rheumatoid arthritis fibroblast-like synoviocytes: a novel role of SH2 domain-containing phosphatase 2 as a modulator of invasion and survival. Arthritis Rheum. 2013. May; 65(5):1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyatt L, Wadham C, Crocker LA, Lardelli M, Khew-Goodall Y. The protein tyrosine phosphatase Pez regulates TGFbeta, epithelial-mesenchymal transition, and organ development. J Cell Biol. 2007. September 24; 178(7):1223–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson KE, Li YW, Yang N, Shen H, Orillion AR, Zhang J. PTPN14 forms a complex with Kibra and LATS1 proteins and negatively regulates the YAP oncogenic function. J Biol Chem. 2014. August 22; 289(34):23693–23700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004. May 21; 304(5674):1164–1166. [DOI] [PubMed] [Google Scholar]
- 12.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013. February 15; 27(4):355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JI, Poon CL, Harvey KF. The Hippo size control pathway--ever expanding. Sci Signal. 2013. January 22; 6(259):pe4. [DOI] [PubMed] [Google Scholar]
- 14.Poernbacher I, Baumgartner R, Marada SK, Edwards K, Stocker H. Drosophila Pez acts in Hippo signaling to restrict intestinal stem cell proliferation. Curr Biol. 2012. March 06; 22(5):389–396. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, et al. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012. September 01; 26(17):1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A, et al. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013. April 25; 32(17):2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Yang N, Figel SA, Wilson KE, Morrison CD, Gelman IH, et al. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013. March 07; 32(10):1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belle L, Ali N, Lonic A, Li X, Paltridge JL, Roslan S, et al. The tyrosine phosphatase PTPN14 (Pez) inhibits metastasis by altering protein trafficking. Sci Signal. 2015. February 17; 8(364):ra18. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt L, Khew-Goodall Y. PTP-Pez: a novel regulator of TGFbeta signaling. Cell Cycle. 2008. August; 7(15):2290–2295. [DOI] [PubMed] [Google Scholar]
- 20.Taketazu F, Kato M, Gobl A, Ichijo H, ten Dijke P, Itoh J, et al. Enhanced expression of transforming growth factor-beta s and transforming growth factor-beta type II receptor in the synovial tissues of patients with rheumatoid arthritis. Lab Invest. 1994. May; 70(5):620–630. [PubMed] [Google Scholar]
- 21.Rosengren S, Corr M, Boyle DL. Platelet-derived growth factor and transforming growth factor beta synergistically potentiate inflammatory mediator synthesis by fibroblast-like synoviocytes. Arthritis Res Ther. 2010; 12(2):R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvaro-Gracia JM, Zvaifler NJ, Brown CB, Kaushansky K, Firestein GS. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991. May 15; 146(10):3365–3371. [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988. March; 31(3):315–324. [DOI] [PubMed] [Google Scholar]
- 24.Arimura Y, Yagi J. Comprehensive expression profiles of genes for protein tyrosine phosphatases in immune cells. Sci Signal. 2010. August 31; 3(137):rs1. [DOI] [PubMed] [Google Scholar]
- 25.Heng TS, Painter MW, Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008. October; 9(10):1091–1094. [DOI] [PubMed] [Google Scholar]
- 26.Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J Biol Chem. 1994. October 28; 269(43):26775–26782. [PubMed] [Google Scholar]
- 27.Pohlers D, Beyer A, Koczan D, Wilhelm T, Thiesen HJ, Kinne RW. Constitutive upregulation of the transforming growth factor-beta pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2007; 9(3):R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004. March; 65(3):744–752. [DOI] [PubMed] [Google Scholar]
- 29.Mei L, Sang W, Chen Z, Zheng L, Jin K, Lou C, et al. Small molecule inhibitor RepSox prevented ovariectomy-induced osteoporosis by suppressing osteoclast differentiation and bone resorption. J Cell Physiol. 2018. July 30. [DOI] [PubMed] [Google Scholar]
- 30.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007. June; 7(6):429–442. [DOI] [PubMed] [Google Scholar]
- 31.Leivonen SK, Chantry A, Hakkinen L, Han J, Kahari VM. Smad3 mediates transforming growth factor-beta-induced collagenase-3 (matrix metalloproteinase-13) expression in human gingival fibroblasts. Evidence for cross-talk between Smad3 and p38 signaling pathways. J Biol Chem. 2002. November 29; 277(48):46338–46346. [DOI] [PubMed] [Google Scholar]
- 32.Inman GJ, Nicolas FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol Cell. 2002. August; 10(2):283–294. [DOI] [PubMed] [Google Scholar]
- 33.Ravanti L, Hakkinen L, Larjava H, Saarialho-Kere U, Foschi M, Han J, et al. Transforming growth factor-beta induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J Biol Chem. 1999. December 24; 274(52):37292–37300. [DOI] [PubMed] [Google Scholar]
- 34.Wadham C, Gamble JR, Vadas MA, Khew-Goodall Y. The protein tyrosine phosphatase Pez is a major phosphatase of adherens junctions and dephosphorylates beta-catenin. Mol Biol Cell. 2003. June; 14(6):2520–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P, Guo A, Possemato A, Wang C, Beard L, Carlin C, et al. Identification and functional characterization of p130Cas as a substrate of protein tyrosine phosphatase nonreceptor 14. Oncogene. 2013. April 18; 32(16):2087–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barr AJ, Debreczeni JE, Eswaran J, Knapp S. Crystal structure of human protein tyrosine phosphatase 14 (PTPN14) at 1.65-A resolution. Proteins. 2006. June 1; 63(4):1132–1136. [DOI] [PubMed] [Google Scholar]
- 37.Blanchetot C, Chagnon M, Dube N, Halle M, Tremblay ML. Substrate-trapping techniques in the identification of cellular PTP targets. Methods. 2005. January; 35(1):44–53. [DOI] [PubMed] [Google Scholar]
- 38.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010. December 14; 19(6):831–844. [DOI] [PubMed] [Google Scholar]
- 39.Ai R, Laragione T, Hammaker D, Boyle DL, Wildberg A, Maeshima K, et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat Commun. 2018. May 15; 9(1):1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012. June 15; 26(12):1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geiler T, Kriegsmann J, Keyszer GM, Gay RE, Gay S. A new model for rheumatoid arthritis generated by engraftment of rheumatoid synovial tissue and normal human cartilage into SCID mice. Arthritis Rheum. 1994. November; 37(11):1664–1671. [DOI] [PubMed] [Google Scholar]
- 42.Fujii M, Toyoda T, Nakanishi H, Yatabe Y, Sato A, Matsudaira Y, et al. TGF-beta synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012. March 12; 209(3):479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Makarov SS. An essential role of NF-kappaB in the “tumor-like” phenotype of arthritic synoviocytes. Proc Natl Acad Sci U S A. 2006. November 14; 103(46):17432–17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratkay LG, Chowdhary RK, Iamaroon A, Richter AM, Neyndorff HC, Keystone EC, et al. Amelioration of antigen-induced arthritis in rabbits by induction of apoptosis of inflammatory cells with local application of transdermal photodynamic therapy. Arthritis Rheum. 1998. March; 41(3):525–534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.