Abstract
Increased cotton production in the Southwestern United States has increased the availability of cotton byproducts for use in cattle diets. The objective of this experiment was to evaluate the inclusion of cotton byproducts in feedlot finishing diets on the performance, carcass traits, fecal characteristics, and plasma metabolites of steers. Crossbred beef steers (n = 64; BW = 318 ± 12.3 kg) were assigned to 1 of 2 experimental treatments in a randomized complete block design (8 pens per treatment; 4 steers per pen). Treatments included a control (CON) diet, which included prairie hay, Sweet Bran, rolled corn, and a corn steep and molasses-based liquid fat supplement, and a cotton byproduct (CTN) diet, which included cotton gin trash, whole cottonseed, rolled corn, and water. Both diets contained urea and dry supplement. Over the entire feeding period, DMI (P = 0.04) was greater for CTN steers than CON steers with no difference in the gain to feed ratio (P = 0.86) between treatments. The CTN steers tended to have heavier final BW (P = 0.09) and greater overall average daily gain (P = 0.08). The CTN steers had heavier hot carcass weight (P = 0.02) and greater fat thickness (P = 0.03) than CON steers, but marbling score and rib eye area were not different between treatments (P ≥ 0.64). Steers fed the CON diet tended to have a lower yield grade (P = 0.07), less kidney, pelvic and heart fat (P = 0.09), and decreased dressing percentage (P = 0.10) than CTN steers. Liver scores did not differ (P ≥ 0.17) between treatments. Fecal consistency scores were decreased for CTN steers on day 56 (P = 0.03) and fecal pH tended to be greater for the CTN steers on day 28 (P = 0.09) compared with CON steers, but neither differed during other periods (P ≥ 0.18). A treatment × day interaction (P = 0.04) was detected for plasma urea nitrogen (PUN) concentrations, where PUN concentrations differed between treatments only on days 28 and 56. On both days 28 and 56, CTN steers had lower PUN concentrations (P = 0.03, P = 0.002, respectively). No treatment × day interaction was detected for plasma glucose or lactate concentrations. A day effect was observed for both metabolites (P < 0.01). Results from this experiment suggest that cotton byproducts can be effectively used as a source of fiber, fat, and protein in feedlot rations without adverse effects on performance or carcass characteristics.
Keywords: cotton byproducts, cotton gin trash, feedlot, finishing diet, whole cottonseed
Introduction
When considering economic inputs for beef cattle production, feed costs account for the majority of expenses (Ahola and Hill, 2012). Since 2015, cotton production has steadily increased in the Southwestern United States. The U.S. Department of Agriculture (USDA) predicts that cotton production will continue to increase in 2019 to 2020, producing approximately 4 million additional bales than 2018 to 2019 (Dohlman et al., 2019). This increase in cotton production has resulted in greater availability of byproducts such as cotton gin trash (CGT) and whole cottonseed (WCS) for use in beef cattle diets.
In feedlots, low- to medium-quality hay is commonly used as the primary roughage source in finishing diets; however, hay can be expensive when compared with other available low-quality plant byproducts. Cotton gin trash is a low-quality byproduct that consists of stems, burrs, lint, leaves, immature cottonseed, and dirt. Although CGT is low in protein and energy content, CGT is a source of physically effective fiber and has the potential to be a more economical option for producers than traditional roughages. Aside from the costs associated with hauling and transportation, CGT is often of no cost to purchase (Meyer, 2007). Because CGT is a waste product, CGT is readily available and has minimal competition as a feed commodity with other livestock species. It has been reported that when CGT is fed as part of the roughage in a diet, average daily gains were similar to steers fed silage (Erwin and Roubicek, 1958).
Whole cottonseed provides additional fiber to the diet and can also be used as a good source of fat and protein. Because WCS is a common ingredient in dairy diets (Kellog et al., 2001), there is a potential for price fluctuation and competition as more dairy operations move into the Southwestern region of the country. Based on current average prices reported by the Agricultural Marketing Service–USDA, corn byproducts such as wet corn gluten feed are less expensive than WCS. However, WCS still has potential to be included in feedlot diets because of the unique nutrient composition, as WCS may reduce the need for traditional roughages as well as additional protein and fat supplementation. It has been suggested that WCS can be added to finishing diets with little to no adverse effects on animal performance or carcass characteristics (Cranston et al., 2006).
A limited number of studies have been completed in the feedlot in which cotton byproducts have served as the major sources of protein, fat, and fiber in the diet. Therefore, the objective of this study was to determine the effects of including cotton byproducts in a finishing diet on the performance, carcass traits, fecal characteristics, and plasma metabolites of crossbred beef steers.
Materials and methods
All procedures were approved by the Institutional Animal Care and Use Committee at Oklahoma State University (Animal Care and Use Protocol Number: AG-17-13).
Cattle and Processing
Sixty-four crossbred steers (initial BW = 318 ± 12.3 kg) were transported approximately 589 km from the University of Arkansas Livestock and Forestry Research Station (Batesville, AR) to the Willard Sparks Beef Research Center (WSBRC) in Stillwater, Oklahoma. On arrival (day −1), steers were individually weighed and held in feedlot pens overnight with ad libitum access to prairie hay and water.
On day 0, steers were individually weighed, implanted (Revalor 200; Merck Animal Health, Madison, NJ), vaccinated against clostridial (Vision with SPUR; Merck Animal Health) and viral and bacterial respiratory (Titanium 5 + PH-M; Elanco Animal Health, Greenfield, IL) pathogens, administered an anthelmintic (Safeguard; Merck Animal Health, Madison, NJ), and a pour-on insecticide (StandGuard; Elanco Animal Health). Steers were blocked by BW at arrival and randomly allocated to pens within block. Steers were housed in sixteen 4.57 × 13.24 m partially covered feedlot pens with a shared 76-L concrete water tank between 2 adjacent pens (model J 360-F; Johnson Concrete, Hastings, NE).
Steers were monitored daily for health status as described by Wilson et al. (2015) and were treated according to standard WSBRC protocol, if necessary. Only 1 steer was treated for bovine respiratory disease symptoms with tildipirosin (Zuprevo; Merck Animal Health, Madison, NJ) according to label directions. Two steers, both consuming the cotton byproduct based (CTN) diet, were removed from the experiment due to animal well-being concerns not associated with the experimental treatments. One steer was removed due to a severe bone infection, and the other was removed due to complications associated with coccidiosis.
Diets and Feed Management
Within block, 4 cattle were randomly assigned to each pen, and treatment was randomly assigned to pens within block. A total of 16 pens were used for this experiment, with 8 pens per treatment. All steers were fed a common receiving diet (RCV; Table 1) for 8 d to allow steers to acclimate to the feedlot environment and stabilize feed intake before providing experimental diets. Steers were then transitioned to respective finishing diets over a 22-d period by increasing the amount of finishing diet delivered by 4% to 5% each day until each treatment received 100% of the respective finishing diet.
Table 1.
Ingredient and nutrient composition of diets
| Diet | |||
|---|---|---|---|
| Ingredient, % of DM | RCV1 | CON2 | CTN3 |
| Rolled corn | 12.7 | 67.25 | 72.25 |
| Prairie hay | 22.5 | 7.0 | — |
| Cotton gin trash | — | — | 7.0 |
| Whole cottonseed | — | — | 15.0 |
| Sweet Bran4 | 60.7 | 15.0 | — |
| Liquid supplement5 | — | 5.0 | — |
| Dry supplement6 | 4.1 | 5.0 | 5.0 |
| Urea | — | 0.75 | 0.75 |
| Nutrient composition, DM basis | |||
| DM, % | 70.10 | 80.70 | 84.34 |
| CP, % | 16.70 | 14.16 | 14.13 |
| NDF, % | — 7 | 25.15 | 27.33 |
| ADF, % | 23.90 | 8.59 | 15.28 |
| peNDF8, % | — | 8.80 | 9.82 |
| TDN, % | 68.90 | 79.309 | 78.209 |
| Fat, % | — | 3.25 | 5.82 |
| NEm, Mcal/kg | 1.00 | 1.7210 | 1.6910 |
| NEg, Mcal/kg | 0.69 | 1.1010 | 1.0710 |
| Ca11, % | 0.53 | 0.62 | 0.85 |
| P, % | 0.53 | 0.57 | 0.46 |
| K, % | 0.93 | 1.00 | 0.84 |
| S, % | — | 0.22 | 0.19 |
| Na, % | — | 0.11 | 0.05 |
| Mg, % | — | 0.29 | 0.28 |
| Cu, mg/L | — | 20.00 | 24.60 |
| Fe, mg/L | — | 144.57 | 165.93 |
| Zn, mg/L | — | 161.03 | 145.97 |
| Mn, mg/L | — | 58.32 | 57.86 |
1Common receiving diet for all cattle. Diet was analyzed by Servi-Tech Laboratories, Dodge City, KS.
2Control diet (CON); representative of a typical finishing diet.
3Cotton diet (CTN); cotton byproducts used as the primary protein, fat, and fiber source in the diet.
4Sweet Bran (Cargill Inc., Dalhart, TX).
5Liquid supplement was formulated to contain (% DM basis) 45.86% corn steep, 36.17% cane molasses, 6% hydrolyzed vegetable oil, 5.46% 80/20 vegetable oil blend, 5.2 % water, 1.23% urea (55% solution), and 0.10% xanthan gum.
6Dry supplement was formulated to contain (% DM basis) 40.0% ground corn, 29.6% limestone, 20.0% wheat middlings, 7.0% urea, 1.0 % salt, 0.53% magnesium oxide, 0.51% zinc sulfate, 0.17% manganese oxide, 0.13% copper sulfate, 0.08% selenium premix (0.6%), 0.0037% cobalt carbonate, 0.32% vitamin A (30,000 IU/g), 0.10% vitamin E (500 IU/g), 0.009% vitamin D (30,000 IU/g), 0.20% tylosin (Tylan-40, Elanco Animal Health, Greenfield, IN), and 0.33% monensin (Rumensin-90; Elanco Animal Health).
7A “–” symbol under nutrient composition indicates nutrient not analyzed in the receiving ration.
8Physically effective fiber provided by the roughage and byproducts in the diet.
9Calculated according to Weiss et al. (1992).
10Calculated according to NASEM (2016).
11Minerals analyzed by the Soil, Water and Forage Analytical Laboratory (Stillwater, OK).
On a DM basis, treatment diets included a control (CON) diet (Table 1; 7% hay, 15% Sweet Bran, 67.25% rolled corn, 5% corn steep, and molasses-based liquid fat supplement) or a CTN diet (7% CGT, 15% WCS, 72.25% rolled corn, 5% water). Both diets contained 0.75% urea and 5% dry mineral supplement. Water was added to the CTN diet to reduce dust, act as a binder, and to improve palatability; no liquid supplement was included in the CTN diet due to the high fat content compared with the CON diet. Urea was weighed by hand separately from other ingredients, added to the mixer, and mixed into the complete ration. Ractopamine hydrochloride (Optaflexx 45; Elanco Animal Health, Greenfield, IL) was included in the diet (actual average ractopamine hydrochloride intake = 390 mg·steer−1·d−1) for 28 d before harvest.
At 0500 h each morning, feed bunks were visually evaluated to determine the amount of feed remaining from the previous day. The amount of feed to be delivered that day was adjusted based on this evaluation so that cattle left no more than 0.045 kg of feed in the bunk. Cattle were fed once daily at 1000 h. Feed was mixed and delivered using a trailer mounted feed mixer (274-12B feed mixer; Roto-mix, Dodge City, KS).
Diet samples were collected twice weekly and DM was calculated after samples were dried in a forced air oven at 60 °C for 48 h. A monthly composite was created after DM was calculated and stored in a freezer until nutrient analysis could be completed. Feed refusals were weighed back before feeding on days 0, 14, 28, 56, 84, 112, 140, and 168 or if excessive orts remained in the bunk. Refusal samples were dried to determine DM content and were subtracted from DM delivered to calculate DMI.
Data Collection and Calculations
Individual BW was recorded for all steers on days 0, 14, 28, 56, 84, 112 and before shipping for harvest. The BW was measured before morning feeding at approximately 0500 h with no withdrawal from feed or water. All BW were adjusted using a 4% pencil shrink (BW × 0.96). Steer BW was averaged within pen and used to calculate the following variables. Individual ADG was calculated by dividing individual shrunk BW gain in kg by days on feed for each period. Pen ADG was calculated as the average of the individual ADG for each steer in the pen for that period. Dry matter intake was calculated from total DMI for the pen for that period divided by the number of steers and the days on feed in that period. Gain to feed ratio was calculated by dividing the ADG for the pen by the average daily DMI for the pen for each respective period.
The data from the 2 steers removed from the experiment were excluded from all analyses (deads out data). Because feed intake was not measured on an individual animal basis, intake data were corrected by removing the average daily DMI for each steer removed from the pen until the respective steer ceased gaining BW. From the time the steer ceased gaining BW until the steer was physically removed from the pen and the experiment, DMI data were estimated and removed using the equation by National Academies of Sciences, Engineering and Medicine (NASEM) (2016) where NEm = 0.077 (SBW)0.75.
A fecal grab sample was obtained via rectal palpation on days 0, 14, 28, 56, 84, 112 and before shipping for harvest. The pH of the fecal sample was recorded using a portable pH meter (pH 6+ Meter; Oakton Instruments, Vernon Hills, IL). Fecal samples were also scored for consistency using the method adapted from Ireland-Perry and Stallings (1992) and Woolsoncroft et al. (2018). This method uses a 1 to 5 scale characterized by the following: 1 = firm, hard, and dry, 2 = slightly less firm and hard, 3 = relatively soft and moist, but not runny, 4 = loose, very moist and runny; consistency of pancake batter, 5 = very thin and watery, cannot be caught in hand. Samples were handled and visually appraised by the same evaluator at each collection. Changes in fecal score and fecal pH were calculated by subtracting the earlier date value from the later date value for each steer, then an average change for the pen was determined.
In addition, on days 0, 14, 28, 56, 84, 112 and before shipping to harvest, a 10-mL blood sample was collected via jugular venipuncture into a tube containing sodium heparin (BD Vacutainer, Franklin Lakes, NJ) and stored on ice. Blood was allowed to clot for an average of 1.5 h before centrifuging. Blood tubes were centrifuged at 1,294 × g for 10 min at 4 °C (Sorvall RC6; Thermo Scientific, Waltham, MA), and plasma was collected and stored in a −80 °C freezer until analysis for plasma urea nitrogen (PUN), lactate, and glucose concentrations.
Cattle were shipped approximately 522 km to Tyson Fresh Meats (Amarillo, TX) for harvest in 2 groups. The 4 heaviest blocks (8 pens) were shipped on day 140 of the experiment and the 4 lightest blocks (8 pens) were shipped on day 168. In further discussion, “final” will be representative of the data collected before shipping to harvest, which is either day 140 or day 168, depending on harvest group. Carcass data were collected by trained personnel from the West Texas A & M University Beef Carcass Research Center (Canyon, TX) at harvest.
Laboratory Analysis
For all rations, a single 400-g sample from the middle of the feed batch was collected from the mixer twice weekly. Within each month, the twice weekly samples were composited and stored until analysis. The composited RCV samples were sent to a commercial laboratory for analysis (Table 1; Servi-Tech, Dodge City, KS). To conduct proximate analysis on both treatment diets, samples of diets were composited, dried in a 60 °C oven for 48 h, and then ground through a 2-mm screen (Pulverisette 19, Fritsch, Pittsboro, NC). Laboratory DM was calculated by weight difference when samples were dried at 105 °C for 12 h. Acid detergent fiber and NDF were analyzed using an ANKOM 2000 automated fiber analyzer (ANKOM Technology, Macedon, NY) according to manufacturer’s instructions.
Particle size of prairie hay, Sweet Bran, WCS, and CGT was determined with a 3.8-L sample using a 3-sieve forage particle separator (Table 2; Nasco, Fort Atkinson, WI). The sieves were shaken in one direction 5 times, rotated one-quarter turn and repeated for a total of 8 sets or 40 shakes. The physically effective NDF (peNDF) for prairie hay, Sweet bran, WCS, and CGT was estimated by calculating the percent of the sample remaining in the top 3 sieves (all ≥4 mm) and multiplying by the NDF (DM basis) content of the feedstuff (NASEM, 2016). To determine the peNDF from the roughage and byproducts of each diet, the peNDF of each contributing ingredient was multiplied by the percent inclusion in the diet. The respective roughage and byproduct peNDF values were then added to create a total peNDF for the diet. The whole diets were not analyzed for peNDF, as rations containing whole grains and supplements can have particles become trapped on the 4-mm sieve, falsely inflating the physical effectiveness factor of the ration (NASEM, 2016). This was avoided by only calculating dietary peNDF values for whole ingredients that provided the greatest amount of effective fiber and omitting manufactured ingredients (NASEM, 2016).
Table 2.
Particle separation and estimated physically effective fiber of diet ingredients
| Ingredient | ||||
|---|---|---|---|---|
| Item | PH | SB | WCS | CGT |
| NDF, % DM | 66.0 | 35.7 | 40.0 | 65.7 |
| Sieve screen size, mm | Retained/screen % | |||
| 19.0 | 30.3 | 0 | 1.8 | 29.8 |
| 8.0 | 25.1 | 3.4 | 89.3 | 32.7 |
| 4.0 | 32.8 | 84.8 | 6.9 | 23.3 |
| Particles less than 4 mm | 11.8 | 11.8 | 2.0 | 14.3 |
| Particles greater than 4 mm | 88.2 | 88.2 | 98.0 | 85.7 |
| Estimated peNDF1, % DM | 58.2 | 31.5 | 39.2 | 56.3 |
1Percent of physically effective NDF was estimated by multiplying the percentage of sample larger than 4 mm in particle size by the percent NDF (as a decimal) of the ingredient before separation.
Percent N was determined using dry combustion analysis in a crude nitrogen analyzer (TruSpec CN, LECO, St. Joseph, MI). Crude protein was calculated by multiplying % N × 6.25. Fat was analyzed using an automated ether extractor (XT 15 Extractor; ANKOM) according to manufacturer’s instructions, with petroleum ether. Minerals were analyzed by the Soil, Water, and Forage Analytical Laboratory (Stillwater, OK) using wet digestion and an inductively coupled plasma spectrometer.
Plasma samples were thawed at room temperature immediately before PUN, glucose, and lactate analysis. Plasma urea nitrogen was analyzed according to the methods described by Marsh et al. (1965) adapted for a 96-well plate. Plasma glucose and l-lactate were analyzed using an immobilized enzyme system (YSI Model 2950 D; YSI Inc., Yellow Springs, OH).
Statistical Analysis
This experiment was organized in a randomized complete block design. For all data measurements, pen served as the experimental unit (n = 16). All data were analyzed using the MIXED procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). Treatment was included as the fixed effect and block as a random effect for performance and fecal characteristics. Plasma metabolite data were assessed for normality using the UNIVARIATE procedure of SAS 9.4. Based on results from the Shapiro–Wilk test, all data were normally distributed. Covariance structures (CS) within the model were compared. The autoregressive CS was the CS that best fit the data (the CS with the lowest Akaike information criterion) in the current experiment. The fixed effects of treatment, day, and treatment × day and block as a random effect were used in the model to analyze plasma metabolite data. Day was included as a repeated measure using autoregressive CS with pen as the subject. If there was a treatment × day interaction, the least square means were compared within day to determine on which days the treatments differed. All data from steers removed from the experiment were excluded from analysis. Significance was determined when P ≤ 0.05 and tendencies were considered when P > 0.05 and P ≤ 0.10.
Results and Discussion
Experimental Diets
When creating the experimental diets, it was not possible to balance the diets for equivalent NDF, ADF, or fat with the ingredients available (Table 1). Therefore, the objective of the experiment was not to create diets that had equivalent fat or fiber levels, but rather to determine if cotton byproducts could be used successfully to supply a majority of the protein, fat, and fiber (roughage) within a finishing diet. Thus, the primary sources of protein (Sweet Bran vs. WCS) and fiber (prairie hay vs. CGT) were included at equal percentages in both diets (DM basis), and diets were balanced to contain similar amounts of CP and calculated energy according to Weiss (1992). Sweet Bran and the liquid supplement served as the primary sources of protein and fat, respectively, in the CON diet, while WCS served as the primary source of both protein and fat in CTN diet. An additional 5% corn (67.25% vs. 72.25% of diet DM for CON and CTN, respectively) was included in the CTN diet. It was decided not to include additional WCS in the CTN diet (i.e., 20% diet DM) as then the experimental diets would have even greater differences in fat content. Urea was included at the same rate in both diets to ensure adequate degradable protein concentration.
Again, it is important to note that the objective was not to substitute one specific ingredient for another (i.e., WCS for Sweet Bran), but rather to provide the majority of the macronutrients in the finishing diet via cotton byproducts or the common ingredients at the facility. It should also be noted that some ingredients (i.e., Sweet Bran) may not have been fed at levels to promote optimal DMI and performance. For example, it has been suggested that wet corn gluten feed be included at a rate of 20% to 30% of diet DM to optimize cattle performance (Bremer et al., 2008; Loza et al., 2010).
Performance
As expected, no differences in BW were observed between treatment groups on day 0 (Table 3; P = 0.49). However, day 84 and final BW tended to be heavier for the CTN fed steers (P ≤ 0.09). The tendency toward a heavier final BW is a result of the tendency for CTN steers to have a greater ADG (P ≤ 0.08) from day 28 to final and over the course of the entire experiment. The tendency for greater overall ADG of the CTN steers (1.95 vs. 2.09 kg/d) was probably a result of the greater DMI of the CTN steers over the entire feeding period (P = 0.04).
Table 3.
Effect of including cotton byproducts in a finishing ration on growth performance and feed efficiency of crossbred steers
| Treatment1 | ||||
|---|---|---|---|---|
| Item | CON | CTN | SEM2 | P-value |
| BW,3 kg | ||||
| Day 0 | 312 | 313 | 11.8 | 0.49 |
| Day 14 | 349 | 350 | 11.5 | 0.87 |
| Day 28 | 382 | 380 | 11.9 | 0.54 |
| Day 56 | 446 | 453 | 14.4 | 0.18 |
| Day 84 | 496 | 509 | 17.4 | 0.07 |
| Day 112 | 542 | 559 | 17.6 | 0.50 |
| Final4 | 614 | 632 | 11.7 | 0.09 |
| ADG5, kg | ||||
| Days 0 to 14 | 2.69 | 2.68 | 0.203 | 0.98 |
| Days 14 to 28 | 2.36 | 2.12 | 0.115 | 0.14 |
| Days 28 to 56 | 2.28 | 2.61 | 0.161 | 0.15 |
| Days 56 to 84 | 1.78 | 2.02 | 0.132 | 0.10 |
| Days 84 to 112 | 1.63 | 1.79 | 0.063 | 0.04 |
| Days 112 to final | 1.73 | 1.80 | 0.060 | 0.29 |
| Days 0 to 28 | 2.53 | 2.40 | 0.107 | 0.43 |
| Days 28 to final | 1.84 | 2.03 | 0.085 | 0.06 |
| Days 0 to final | 1.95 | 2.09 | 0.080 | 0.08 |
| DMI6, kg/d | ||||
| Days 0 to 14 | 7.0 | 7.1 | 0.16 | 0.49 |
| Days 14 to 28 | 11.2 | 10.3 | 0.36 | 0.09 |
| Days 28 to 56 | 11.3 | 11.8 | 0.55 | 0.41 |
| Days 56 to 84 | 12.3 | 13.3 | 0.57 | 0.07 |
| Days 84 to 112 | 12.5 | 13.4 | 0.45 | 0.03 |
| Days 112 to final | 13.0 | 13.9 | 1.05 | 0.09 |
| Days 0 to 28 | 9.1 | 8.9 | 0.18 | 0.42 |
| Days 28 to final | 12.3 | 13.2 | 0.46 | 0.07 |
| Days 0 to final | 11.7 | 12.4 | 0.35 | 0.04 |
| G:F7 | ||||
| Days 0 to 14 | 0.391 | 0.379 | 0.0314 | 0.74 |
| Days 14 to 28 | 0.210 | 0.208 | 0.0091 | 0.83 |
| Days 28 to 56 | 0.204 | 0.220 | 0.0077 | 0.16 |
| Days 56 to 84 | 0.145 | 0.151 | 0.0060 | 0.40 |
| Days 84 to 112 | 0.131 | 0.135 | 0.0065 | 0.39 |
| Days 112 to final | 0.134 | 0.130 | 0.0032 | 0.30 |
| Days 0 to 28 | 0.279 | 0.272 | 0.0121 | 0.68 |
| Days 28 to final | 0.150 | 0.153 | 0.0029 | 0.32 |
| Days 0 to final | 0.167 | 0.167 | 0.0036 | 0.86 |
1Treatments included (DM basis): (CON) = 7% hay, 15% Sweet Bran, 67.25% rolled corn, 5% liquid supplement, or (CTN) = 7% cotton gin trash, 15% whole cottonseed, 72.75% rolled corn. Both rations contained 5% dry supplement and 0.75% urea.
2 n = 8 pens per treatment.
3Body weight adjusted by a 4% calculated pencil shrink.
4Cattle were harvested in 2 groups: day 140 (n = 4 pens per treatment) and day 168 (n = 4 pens per treatment).
5Pen average daily gain ADG calculated from individual shrunk BW gain, kg divided by days on feed for each period.
6Pen DMI calculated from total DMI for the pen for each period divided by the total steers and days on feed in each period.
7Gain to feed calculated by dividing the ADG for the pen by the average daily DMI for the pen for each respective period.
Although CTN steers tended to have a greater ADG and DMI during the finishing period and overall, the steers fed the CON ration had a numerically greater ADG during the transition period (days 0 to 28) of 0.13 kg/d (P = 0.43). This numerical difference in ADG during the transition period is also probably a result of the differences observed in DMI between treatments during this period, as the CON steers tended to have greater DMI from days 14 to 28 (P = 0.09). The numerically greater DMI (0.2 kg/d) by CON steers during the 28-d transition period may be attributed to palatability differences and ingredient recognition of the experimental diets. The steers had previously been consuming mature Bermuda grass pasture, and perhaps recognized and accepted diets containing prairie hay more readily compared with the unfamiliar (WCS) and less palatable (CGT) ingredients in the CTN diet. Intakes possibly remained lower throughout the transition period until steers were fully acclimated to the CTN diet. No differences in G:F were found during any period of the experiment (P ≥ 0.16).
The ADG results from day 28 to final in the current experiment are similar to those described by Huerta-Leidenz et al. (1990) who reported a numerically greater ADG (1.15 vs. 0.95 kg/d) when WCS was included in a finishing diet at 15% compared with a control diet that consisted of corn, cottonseed meal, cottonseed hulls, and molasses. In contrast, the ADG results presented in the current experiment differ from a study completed by Cranston et al. (2006), who reported that final BW and ADG were not affected by the inclusion of 15.4% WCS when fed in combination with steam-flaked corn in a finishing diet compared with a control diet that consisted of steam-flaked corn, cottonseed meal, cottonseed hulls, tallow, and alfalfa hay as the primary protein, fat, and fiber sources.
The difference in results between the current experiment and those reported by Cranston et al. (2006) could simply be due to a difference in the composition of the experimental diets. For example, the control diet used by Cranston et al. (2006) supplied fiber and protein in the form of cotton byproducts and alfalfa hay, whereas the control diet in the current experiment did not contain any cotton byproducts and utilized prairie hay and corn byproducts as the major fiber and protein sources. In addition, diets in the Cranston et al.’s (2006) experiment formulated contain similar amounts of protein, fat, and NDF supplied by the roughage, whereas the diets in the current experiment were formulated to contain similar amounts of CP and energy only.
The difference in overall DMI observed between treatments in this experiment may be related to the amount of physically effective fiber in the treatment diets. According to a review by Galyean and Defoor (2002), DMI = 1.858 + 0.0290 × eNDF % from roughage. Although eNDF was not directly measured in this experiment, eNDF and peNDF are highly correlated (NASEM, 2016). Given the correlation between eNDF and peNDF, the difference in DMI detected in the current experiment may be partially explained by the 11.6% more peNDF supplied by roughage and byproducts in the CTN diet compared to the CON diet.
Diets in this experiment were not balanced for peNDF, but rather formulated to include the primary roughage sources at the same rate between treatments. Prairie hay and CGT contributed similar amounts of peNDF to each diet, with 88.2% vs. 85.7% of the NDF classified as peNDF, respectively. However, it is also important to note that although WCS is a considered a concentrate, WCS also provides a substantial amount of fiber and peNDF that may reduce the amount of traditional roughage needed in the diet. The WCS in the CTN diet contained 98.0% of the NDF as peNDF, whereas the Sweet Bran in the CON diet contained 88.2% of the NDF as peNDF. The difference between the peNDF content of WCS and Sweet Bran in the diets was the primary source of the variation in overall peNDF between the diets. The inclusion of fibrous byproducts greatly increased the peNDF of the experimental diets; therefore, it may be important to consider fiber content from both the roughage and fibrous byproducts when using equations to predict differences in DMI.
Carcass Traits
The higher final BW was reflected in hot carcass weight (HCW), where the CTN steers had a 14-kg heavier HCW on average than CON steers (Table 4; P = 0.02). In addition, the CTN carcasses had greater fat content as demonstrated by a greater back fat thickness of 0.13 cm (P = 0.03), and a tendency to have a greater kidney, pelvic, and heart fat (KPH) percentage (P = 0.09), and yield grade (YG; P = 0.07). A similar result was reported by Huerta-Leidenz et al. (1990) who reported that YG was numerically greater (2.3 vs. 2.7) when 15% WCS was included in finishing diets compared with the control. Dressing percentage (DP) also tended to be higher for the CTN steers than the CON steers (62.7 and 62.2, respectively; P = 0.10).
Table 4.
Effect of including cotton byproducts in a finishing ration on the carcass characteristics of crossbred feedlot steers
| Treatment1 | ||||
|---|---|---|---|---|
| Item | CON | CTN | SEM2 | P-value |
| Hot carcass weight, kg | 382 | 396 | 7.0 | 0.02 |
| Rib eye area, cm2 | 96.0 | 95.1 | 1.93 | 0.64 |
| Fat thickness3, cm2 | 1.24 | 1.37 | 0.064 | 0.03 |
| KPH4, % | 1.81 | 1.91 | 0.063 | 0.09 |
| Dressing percentage | 62.2 | 62.7 | 0.28 | 0.10 |
| Calculated yield grade | 2.51 | 2.83 | 0.109 | 0.07 |
| Marbling score5 | 508 | 499 | 14.3 | 0.64 |
| Liver score6, % of pen | ||||
| O | 90.6 | 83.3 | 4.8 | 0.18 |
| A− | 3.13 | 9.38 | 3.917 | 0.17 |
| Contamination | 6.25 | 7.29 | 4.480 | 0.86 |
1Treatments included (DM basis): (CON) = 7% hay, 15% Sweet Bran, 67.25% rolled corn, 5% liquid supplement or (CTN) = 7% cotton gin trash, 15% whole cottonseed, 72.25% rolled corn. Both rations contained 5% dry supplement and 0.75% urea.
2 n = 8 pens per treatment.
3Fat measurement was taken between the 12th and 13th ribs.
4Kidney, pelvic, and heart fat.
5Small00 = 400; Modest00 = 500; Moderate00 = 600.
6Liver scores at harvest: O = normal, healthy liver, free of abscesses. A− = livers that displayed less than 2 abscesses which are generally less than 2.54 cm in diameter. Contaminated = contaminated with fecal material during harvest.
In contrast to our findings, Cranston et al. (2006) reported no differences in carcass characteristics when comparing finishing steers fed a diet containing 15% WCS to a control diet. The difference in fat thickness, KPH, and YG results between studies could be attributed the fact that diets used by Cranston et al. (2006) were formulated to contain equal percentages of fat, and diets in this current experiment were not. Increases in YG are not desirable and beef carcasses with a YG of 4 or 5 are often severely discounted. Although the CTN steers in this study had higher YG than CON, the average CTN YG was 2.83 and therefore discounts for YG 4 and 5 carcasses are probably of minimal concern when considering the inclusion of WCS at 15% in a finishing diet.
Cotton gin trash can have a highly variable nutrient composition depending on the region CGT is produced in Meyer (2007), which could be a source of variation in the results between this experiment and previous results reported. Although the energy values from the 2 diets in the current experiment were similar, the sources of energy varied between the 2 diets; thus, the CTN steers could have had greater carcass fat composition due to differences in metabolism of fat or other macro nutrients. Interestingly, there were no differences in marbling score between treatments, despite the greater back fat thickness, KPH, DP, and YG observed in the CTN steers. There were also no differences in rib eye area, further supporting the idea that the CTN cattle were gaining weight mostly as fat instead of lean tissue at the end of the finishing period. Liver scores were also assessed, and no differences were observed between treatments (Table 4; P ≤ 0.17).
Fecal Characteristics
Fecal grab samples were evaluated for consistency to estimate the extent of digestion of experimental diets. Although fecal consistency can be altered by various functions and factors, fecal consistency is thought to be indicative of the site and extent of digestion of feed. When hindgut fermentation increases as a result of an increased passage rate, fecal consistency can appear more “loose” (Kononoff et al., 2002; Hall, 2007). In addition, a loose fecal consistency can be a sign of less effective fiber in the diet (Woolsoncroft, 2018). Fecal scores were lowest, or more firm, on day 0 and increased after beginning the transition to the finishing rations. This was expected, as the steers had previously been consuming only mature Bermuda grass pasture and were transitioned to a high concentrate, low fiber diet, starting on day 8 of the experiment.
On day 56, CTN steers had lower fecal scores (2.93 vs. 3.19; P = 0.03) than CON steers, although this is probably of low biological significance. No differences in fecal scores were detected for any other period; however, steers consuming CTN diet were scored numerically lower during every collection period once consuming the finishing diet. This numerical difference in fecal consistency from day 28 to final possibly suggests that the CON diet resulted in a faster passage rate and greater extent of hindgut digestion than the CTN diet. Because the CTN diet contained more peNDF, mostly due to the inclusion of WCS, a lower fecal score was not surprising. There were no differences in fecal score change for either treatment within any collection period, therefore fecal scores did not change more dramatically for one treatment compared to the other between periods (P > 0.21).
Fecal pH was also taken as an indicator of site and extent of digestion. A decrease in fecal pH may suggest a decrease in extent of rumen fermentation and a subsequent increase in hindgut fermentation (Yang and Beauchemin, 2006). Although digestibility was not directly measured in this study, previous literature suggests that a higher fecal pH might be attributed to less starch present in the feces, indicating a further extent of starch digestion (Wheeler and Noller, 1977). Greater amounts of starch in the feces may be reflective of pH being too low for optimal amylase activity in the small intestine (Turgeon et al., 1981).
Fecal pH was highest on day 0, which was reflective of the cattle consuming mature Bermuda grass pasture before arrival to the feedlot. As expected, fecal pH decreased as concentrate levels increased through transition period. On day 28, fecal pH tended to be higher in the CTN steers (P = 0.09), but no differences in fecal pH were observed between treatments on any other collection day (Table 5). However, similar to fecal consistency scores, fecal pH was often numerically greater for the CTN steers compared with the CON steers. This numerical difference may support the suggestion that the CTN diet had further extent of digestion in the rumen, possibly due to a slower passage rate, which could result in less starch present in the feces. Because digestibility and fecal starch content were not directly measured in this experiment, further research is required to validate these suggestions. There were no differences in fecal pH change at any period in the study; therefore, fecal pH did not change more dramatically for one treatment compared with the other between periods (P ≥ 0.10).
Table 5.
Effects of including cotton byproducts in a finishing ration on the fecal score and fecal pH of crossbred feedlot steers
| Treatments1 | ||||
|---|---|---|---|---|
| Item | CON | CTN | SEM2 | P-value |
| Fecal score3 | ||||
| Day 0 | 2.33 | 2.50 | 0.110 | 0.30 |
| Day 14 | 3.03 | 3.06 | 0.182 | 0.91 |
| Day 28 | 2.78 | 2.76 | 0.115 | 0.89 |
| Day 56 | 3.19 | 2.93 | 0.087 | 0.03 |
| Day 84 | 3.00 | 2.83 | 0.161 | 0.43 |
| Day 112 | 3.06 | 2.88 | 0.103 | 0.18 |
| Final3 | 3.06 | 2.95 | 0.100 | 0.26 |
| Fecal score change5 | ||||
| Days 0 to 14 | 0.70 | 0.56 | 0.205 | 0.62 |
| Days 14 to 28 | −0.25 | −0.30 | 0.247 | 0.88 |
| Days 28 to 56 | 0.41 | 0.17 | 0.138 | 0.24 |
| Days 56 to 84 | −0.19 | −0.09 | 0.136 | 0.63 |
| Days 84 to 112 | 0.06 | 0.04 | 0.172 | 0.93 |
| Days 112 to final | 0.00 | 0.07 | 0.101 | 0.62 |
| Days 0 to 28 | 0.45 | 0.26 | 0.128 | 0.32 |
| Days 28 to final | 0.28 | 0.19 | 0.159 | 0.63 |
| Days 0 to final | 0.73 | 0.45 | 0.157 | 0.21 |
| Fecal pH | ||||
| Day 0 | 8.14 | 8.18 | 0.048 | 0.54 |
| Day 14 | 6.88 | 6.96 | 0.063 | 0.39 |
| Day 28 | 6.82 | 6.97 | 0.075 | 0.09 |
| Day 56 | 6.66 | 6.69 | 0.062 | 0.72 |
| Day 84 | 6.74 | 6.72 | 0.064 | 0.80 |
| Day 112 | 6.78 | 6.87 | 0.085 | 0.42 |
| Final | 6.80 | 6.88 | 0.083 | 0.48 |
| Fecal pH change5 | ||||
| Days 0 to 14 | −1.27 | −1.22 | 0.098 | 0.81 |
| Days 14 to 28 | −0.06 | −0.02 | 0.083 | 0.21 |
| Days 28 to 56 | −0.16 | −0.28 | 0.107 | 0.40 |
| Days 56 to 84 | 0.08 | 0.03 | 0.098 | 0.71 |
| Days 84 to 112 | 0.04 | 0.16 | 0.096 | 0.10 |
| Days 112 to final | 0.02 | 0.01 | 0.115 | 0.96 |
| Days 0 to 28 | −1.31 | −1.20 | 0.098 | 0.38 |
| Days 28 to final | −0.02 | 0.09 | 0.120 | 0.64 |
| Days 0 to final | −1.34 | −1.29 | 0.077 | 0.71 |
1Treatments included (DM basis): (CON) = 7% hay, 15% Sweet Bran, 67.25% rolled corn, 5% liquid supplement or (CTN) = 7% cotton gin trash, 15% whole cottonseed, 72.25% rolled corn. Both rations contained 5% dry supplement and 0.75% urea.
2 n = 8 pens per treatment.
3Fecal score adapted from Ireland-Perry and Stallings (1992) and Woolsoncroft et al. (2018), with a greater score indicating a looser fecal consistency on a scale of 1 to 5 with 1 representing a cow on dry hay and 5 being the consistency of water.
4Cattle were harvested in 2 groups: day 140 (n = 4 pens per treatment) and day 168 (n = 4 pens per treatment).
5The difference between collection periods; the later date was subtracted from the earlier date.
Plasma Metabolites
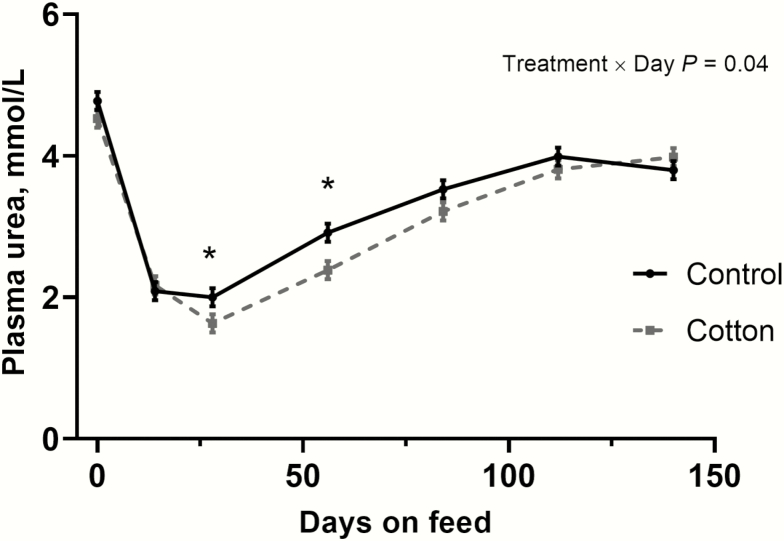
A treatment × day interaction (Fig. 1; P = 0.04), a treatment effect (P < 0.01), and a day effect (P < 0.001) were observed for PUN concentrations. Generally speaking, both treatments had the greatest PUN concentrations on day 0, decreased through the receiving and transition periods, and steadily increased from day 28 to harvest. There was no difference in PUN concentrations on day 0 or day 14. Although both treatments decreased in PUN concentration from days 14 to 28, the CTN steers had a greater decrease than the CON steers. From days 28 to 56, PUN concentrations for both treatments increased; however, the increase was greater in the CON steers than the CTN steers. On day 84, the CON steers tended to have greater PUN concentrations than the CTN steers. From day 112 to final, no differences were detected between treatments.
Figure 1.
Concentration of plasma urea nitrogen (PUN) in finishing steers consuming a control (7% hay, 15% Sweet Bran, 67.25% rolled corn, 5% liquid supplement, 5% dry supplement, 0.75% urea) or cotton byproduct (7% cotton gin trash, 15% whole cottonseed, 72.25% rolled corn, 5% dry supplement, 0.75% urea) diet. A treatment × day interaction (P = 0.04), a treatment effect (P < 0.01), and a day effect (P < 0.001) were observed for PUN concentrations. An asterisk (*) represents a difference (P < 0.05) between treatments on that day. Cattle were harvested in 2 groups: day 140 (n = 4 pens per treatment) and day 168 (n = 4 pens per treatment). In this figure, 140 days on feed is representative of the final measurement, regardless of harvest date.
Although the reason for the differences between treatments on days 28 and 56 is unclear, we speculate that amino acid catabolism might have been decreased in the CTN steers, ultimately resulting in lower PUN concentrations. The increase in PUN concentrations from day 28 to final can be attributed to the fact that DMI was increasing in both treatments during that time, resulting in higher total protein consumption. Van Bibber-Krueger et al. (2017) reported PUN levels between 3.33 and 4.51 mMol/L in finishing heifers supplemented with ractopamine hydrochloride and Zn. These values are similar to the results seen in this experiment, which on average ranged from 3.80 to 3.99 mMol/L from day 112 to the end of the finishing period.
No treatment × day interaction was detected for plasma glucose or lactate concentrations (Table 6). A day effect (P < 0.0001) was observed for plasma glucose concentrations; however, there was no main effect (P = 0.67) of treatment. The day effect was observed on day 28 and final, when the lowest plasma glucose concentrations were detected for both treatments. Glucose values observed at any period averaged between 84.1 and 98.5 (± 3.1) mg/dL. These concentrations are within expected normal ranges; previous studies have reported plasma glucose levels ranging from 65.2 to 101.1 mg/dL in finishing feedlot steers (Evans et al., 1975; Hancock et al., 1988; Kolath et al., 2006). A day effect (P < 0.001) was also detected for plasma lactate concentrations, but no treatment effect (P = 0.91) was detected. Peak plasma lactate concentrations were observed on day 0 for both treatment groups, which is probably due to the stressors associated with shipping. Mitchell et al. (1998) also reported higher levels of plasma lactate in ruminants after transportation, with values averaging 0.42 ± 0.15 g/L, which are similar to day 0 results in the current experiment, 0.40 ± 0.03 g/L. After arrival, lactate concentrations decreased and remained steady regardless of treatment from day 14 to day 112 and were similar to those reported by Sako et al. (2007).
Table 6.
Effects of including cotton byproducts in a finishing ration on the plasma metabolite levels of crossbred feedlot steers
| Treatments1 | Days on feed | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | CON | CTN | SEM2 | P-Value | 0 | 14 | 28 | 56 | 84 | 112 | Final3 | SEM2 | P-value |
| Glucose, mg/dL | 93.4 | 92.7 | 1.30 | 0.67 | 96.3a | 95.0a | 86.9b | 96.3a | 96.7a | 95.6a | 84.9b | 3.10 | <0.0001 |
| Lactate, g/L | 0.258 | 0.256 | 0.013 | 0.91 | 0.373a | 0.253b | 0.254b | 0.246b | 0.257b | 0.233b | 0.182c | 0.0195 | <0.0001 |
1Treatments included (DM basis): (CON) = 7% hay, 15% Sweet Bran, 67.25% rolled corn, 5% liquid supplement or (CTN) = 7% cotton gin trash, 15% whole cottonseed, 72.25% rolled corn. Both rations contained 5% dry supplement and 0.75% urea.
2 n = 8 pens per treatment.
3Cattle were harvested in 2 groups: day 140 (n = 4 pens per treatment) and day 168 (n = 4 pens per treatment).
4Within row, values with unlike superscripts are different (P < 0.05).
At the final collection, lactate concentrations had further decreased. Because lactate is a product of glucose metabolism, the decrease in lactate at the final collection is probably related to the decrease in glucose concentration at the same time. When examined in combination, the minimal differences in plasma metabolite data between the 2 treatments indicate that observed differences in growth rates are not probably due to substantial alterations in glucose or protein metabolism. Additional metabolite measurements are needed to further support this conclusion, as only a small portion of overall metabolism was measured in this experiment.
Conclusion
This experiment suggests that WCS and CGT can be effectively used as protein, fat, and fiber sources in a finishing feedlot diet without compromising performance or carcass characteristics. Including cotton byproducts in the diet improved ADG and DMI without impacting G:F. Carcasses of steers fed the CTN diet were heavier with a greater DP, back fat, YGs, and KPH fat. The fecal consistency and pH data from this experiment combined with the limited research available investigating the digestibility of cotton byproducts warrants further investigation. A subsequent experiment will be conducted to evaluate the in situ digestibility of these diets and the individual ingredients. Overall, this experiment has implications for feedlots in the Southwestern United States to utilize cotton byproducts in finishing diets if cotton byproducts are available at an economical cost compared with other protein, fat, and fiber sources.
Acknowledgments
The authors thank the employees of the Willard Sparks Beef Research Center for assisting with this experiment. This experiment was funded in part by the USDA National Institute of Food and Agriculture Hatch project and the Oklahoma Agricultural Experiment Station of the Division of Agricultural Sciences and Natural Resources at Oklahoma State University. The authors declare no conflict of interest.
Glossary
Abbreviations
- CGT
cotton gin trash
- CON
control treatment diet
- CTN
cotton treatment diet
- CS
covariance structure
- DP
dressing percentage
- HCW
hot carcass weight
- KPH
kidney, pelvic, and heart fat
- peNDF
physically effective NDF
- PUN
plasma urea nitrogen
- RCV
receiving diet
- USDA
United States Department of Agriculture
- WCS
whole cottonseed
- WSBRC
Willard Sparks Beef Research Center
- YG
yield grade
Literature Cited
- Ahola, J. K., and Hill R. A.. . 2012. Input factors affecting profitability: A changing paradigm and a challenging time. In: Hill R. A., editor, Feed efficiency in the beef industry. John Wiley & Sons, Inc., Ames, IA: p. 7–19. doi: 10.1002/9781118392331.ch1 [DOI] [Google Scholar]
- Bremer, V. R., Erickson G. E., Klopfenstein T. J.. . 2008. Meta-analysis of UNL feedlot trials replacing corn with WCGF. Nebraska Beef Rep. Lincoln (NE):University of Nebraska MP88:37–38. [Google Scholar]
- Cranston, J. J., Rivera J. D., Galyean M. L., Brashears M. M., Brooks J. C., Markham C. E., McBeth L. J., and Krehbiel C. R.. . 2006. Effects of feeding whole cottonseed and cottonseed products on performance and carcass characteristics of finishing beef cattle. J. Anim. Sci. 84:2186–2199. doi: 10.2527/jas.2005-669 [DOI] [PubMed] [Google Scholar]
- Dohlman, E., Johnson J., MacDonald S., Meyer L., and Soley G.. . 2019. The world and united states cotton outlook. Presented at 95th Annual Agricultural Outlook Forum. Arlington (VA): United States Department of Agriculture. [Google Scholar]
- Erwin, E. S., and Roubicek C. B.. . 1958. The utilization of cotton gin trash by growing and fattening steers. J. Anim. Sci. 17:133–139. doi: 10.2527/jas1958.171133x [DOI] [Google Scholar]
- Evans, E., Buchanan-Smith J. G., and Macleod G. K.. . 1975. Postprandial patterns of plasma glucose, insulin and volatile fatty acids in ruminants fed low- and high-roughage diets. J. Anim. Sci. 41:1474–1479. doi: 10.2527/jas1975.4151474x [DOI] [PubMed] [Google Scholar]
- Galyean, M. L., and Defoor P. J.. . 2002. Effects of roughage source and level on intake by feedlot cattle. J. Anim. Sci. 81(ESuppl. 2):E8–E16. doi: 10.2527/2003.8114_suppl_2E8x [DOI] [PubMed] [Google Scholar]
- Hall, M 2007. Carbohydrate nutrition and manure scoring. Part II: Tools for monitoring rumen function in dairy cattle. In: Proc. Minnesota Dairy Health Conf.St. Paul, MN p. 81–86. [Google Scholar]
- Hancock, D. L., Williams J. E., Hedrick H. B., Beavera E. E., Hannaha S. M., Miller S. J., Ellerseick M. R., Garner G. B., Morrow R. E., and Gerrish J. R.. . 1988. Effects of previous forage systems on feedlot performance, body composition and plasma variables of finishing steers. J. Anim. Sci. 66:2272–2283. doi: 10.2527/jas1988.6692272x [DOI] [PubMed] [Google Scholar]
- Huerta-Leidenz, N. O., Cross H. R., Lunt D. K., L.Pelton S., Savell J. W., and Smith S. B.. . 1990. Growth, carcass traits, and fatty acid profiles of adipose tissues from steers fed whole cottonseed. J. Anim. Sci. 69:3665–3672. doi: 10.2527/1991.6993665x [DOI] [PubMed] [Google Scholar]
- Ireland-Perry, R. L., and Stallings C. C.. . 1992. Fecal consistency as related to dietary composition in lactating Holstein cows. J. Dairy. Sci. 76:1074–1082. doi: 10.3168/jds.S0022-0302(93)77436-6 [DOI] [PubMed] [Google Scholar]
- Kellog, D. W., Pennington J. A., Johnson Z. B., and Panivivat R.. . 2001. Survey of management practices used for the highest producing DHI herds in the Unites States. J. Dairy. Sci. 84:E120–E127. doi: 10.3168/jds.S0022-0302(01)70206-8 [DOI] [Google Scholar]
- Kolath, W. H., Kerley M. S., Golden J. W., and Keisler D. H.. . 2006. The relationship between mitochondrial function and residual feed intake in Angus steers. J. Anim. Sci. 84:861–865. doi: 10.2527/2006.844861x [DOI] [PubMed] [Google Scholar]
- Kononoff, P., Heinrichs J., and Varga G.. . 2002. Using manure evaluation to enhance dairy cattle nutrition. Penn. State Coop. Ext., Pennsylvania State Univ., University Park, PA. [Google Scholar]
- Loza, P. L., Buckner C. D., Vander Pol K. J., Erickson G. E., Klopfenstein T. J., and Stock R. A.. . 2010. Effect of feeding combinations of wet distillers grains and wet corn gluten feed to feedlot cattle. J. Anim. Sci. 88:1061–1072. doi: 10.2527/jas.2009-2190 [DOI] [PubMed] [Google Scholar]
- Marsh, W. H., Fingerhut B., and Miller H.. . 1965. Automated and manual direct methods for the determination of blood urea. Clin. Chem. 11:624–627. doi: 10.1093/clinchem/11.6.624 [DOI] [PubMed] [Google Scholar]
- Meyer, R. O 2007. Cotton gin trash: Alternative roughage feed for beef cattle. AN177. Gainesville (FL): University of Florida, IFAS Extension. [Google Scholar]
- Mitchell, G., Hattingh J., and Ganhao M.. . 1998. Stress in cattle assessed after handling, after transport and after slaughter. Vet. Rec. 123:201–205. doi: 10.1136/vr.123.8.201 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering and Medicine (NASEM). 2016. Nutrient requirements of beef cattle. 8th rev. ed Natl. Acad. Press, Washington, DC. doi: 10.17226/19014 [DOI] [Google Scholar]
- Sako, T., Urabe S., Kusaba A., Kimura N., Yoshimura I., Tazaki H., Imai S., Ono K., and Arai T.. . 2007. Comparison of plasma metabolite concentrations and lactate dehydrogenase activity in dogs, cats, horses, cattle and sheep. Vet. Res. Commun. 31:413–417. doi: 10.1007/s11259-006-3482-2 [DOI] [PubMed] [Google Scholar]
- Turgeon, O. A., Brink D. R. and Britton R. A.. . 1981. Corn particle size mixtures, roughage level, and starch utilization in finishing steer diets. J. Anim. Sci. 57:3. doi: 10.2527/jas1983.573739x [DOI] [Google Scholar]
- Van Bibber-Krueger, C. L., Amachawadi R. G., Scott H. M., Gonzales J. M., Drouillard J. S.. . 2017. Interactive effects of supplemental Zn sulfate and ractopamine hydrochloride on growth performance, carcass traits, and plasma urea nitrogen in feedlot heifers. J. Anim. Sci. 95:4638–4645. doi: 10.2527/jas2017.1764 [DOI] [PubMed] [Google Scholar]
- Weiss, W. P., Conrad H. R., and St. Pierre N. R.. . 1992. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed Sci. Technol. 39:95–110. doi: 10.1016/0377-8401(92)90034-4 [DOI] [Google Scholar]
- Wheeler, W. E., and Noller C. H.. . 1977. Gastrointestinal tract pH and starch in feces of ruminants. J. Anim. Sci. 44:131–135. doi: 10.2527/jas1977.441131x [DOI] [PubMed] [Google Scholar]
- Wilson, B. K., Step D. L., Maxwell C. L., Wagner J. J., Richards C. J., and Krehbiel C. R.. . 2015. Evaluation of multiple ancillary therapies used in combination with an antimicrobial in newly received high-risk calves treated for bovine respiratory disease. J. Anim. Sci. 93:3661–3674. doi: 10.2527/jas.2015-9023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsoncroft, M. A., Youngers M. E., McPhillips L. J., Lockard C. G., Haviland C. L., DeSocio E. S., Ryan W. R., Richards C. J., and Wilson B. K.. . 2018. Effects of exercise and roughage source on the health and performance of receiving beef calves. Prof. Anim. Sci. 34:183–191. doi: 10.15232/pas.2017-01673 [DOI] [Google Scholar]
- Yang, W. Z., and Beauchemin K. A.. . 2006. Increasing the physically effective fiber content of dairy cow diets may lower efficiency of feed use. J. Dairy. Sci. 89:2694–2704. doi: 10.3168/jds.S0022-0302(06)72345-1 [DOI] [PubMed] [Google Scholar]