Abstract
Age-related alteration of the immune system with aging, or immunosenescence, plays a major role in several age-associated conditions, including loss of bone integrity. Studies over the past several years have clearly established the immune system is chronically activated with advanced aging, termed inflammaging, and is characterized by elevated levels of proinflammatory cytokines in response to physiological or environmental cues that essentially result in an arrested immune system that maintains a low-level state of activation. This age-associated inflammation impacts several biological systems including the innate immune system, where aging results in a skewing of the hematopoiesis towards the myeloid lineage, including the expansion of myeloid-derived suppressor cells (MDSCs). This heterogeneous population of myeloid cells classically displays immunosuppressive capacity but they also have the ability to directly differentiate into osteoclasts. This review explores the possibility of inflammaging to be involved in reduction of bone microarchitecture and loss of bone mass/strength through the expansion of MDSCs and the osteoclastogenic capacity and activity.
Keywords: aging, inflammation, bone, myeloid cells, osteoclasts
Introduction
With advancing age, the immune system undergoes dynamic changes characterized by both impairment of adaptive immunity and activation of low-grade chronic inflammation. This chronic activation of inflammation associated with aging has been coined ‘inflammaging’ (Franceschi and Campisi 2014; Franceschi et al. 2007; Franceschi et al. 2018). Although inflammation is an immunologically proper response to various pathogens and tissue damage, inflammaging results in excess production of cytokines (e.g. tumor necrosis factor (TNF)-α, interleukins, chemokines, and interferons) that ultimately affect several organs, including bone, where it manifests as an imbalance in bone remodeling. As a direct or indirect consequence, chronic inflammatory bone-related diseases increasingly develop with age, including osteoporosis, arthritis, and periodontal disease (Liu et al. 2015; Chalan et al. 2015; Billings et al. 2018). Myeloid-derived suppressor cells (MDSCs) represent an immature myeloid population of cells that can expand into osteoclasts and thus participate in inflammation-associated bone loss with age (See Figure 1). This review considers mechanisms of the aging immune system as they relate to osteoclasts and bone homeostasis with consideration of how inflammaging can contribute to bone fragility through the expansion of MDSCs.
Figure 1. MDSCs expand during aging to participate in reduction of bone mass.
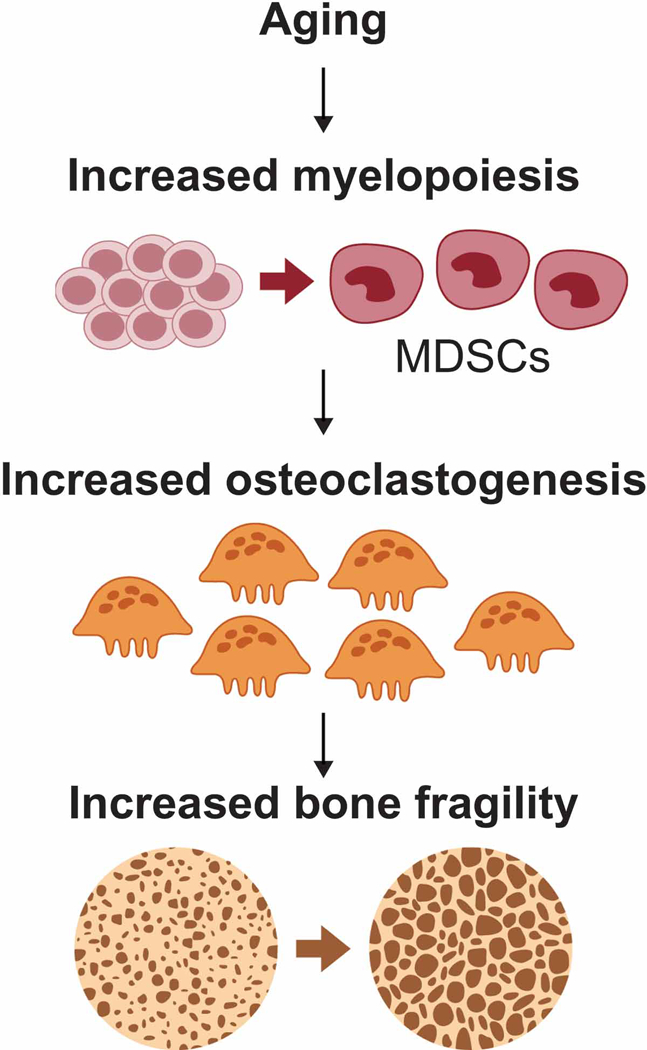
During the aging process, immunosenescence leads to myelopoiesis resulting in the expansion of myeloid-derived suppressor cell (MDSC) populations. This population is capable of differentiating directly into osteoclasts potentially contributing to loss of bone mass associated with aging.
The Aging Immune System
Both innate and adaptive arms of the immune system undergo demonstrable changes with age, thus contributing to the concept known as immunosenescence (Boots et al. 2013; Chalan et al. 2015). In general, aging is associated with a decrease in the adaptive immune system, whereas innate mechanisms generally become more active. Immunosenescence is characterized by a thymic involution leading to a steady state decline in the production of naïve T-cells, a reduction of the T-cell repertoire and a chronic low-grade level of inflammation where there is an elevated level of proinflammatory cytokines, including TNF-α and IL-6 (Goronzy and Weyand 2003).
Although the underlying mechanisms are unclear regarding how age-related inflammation occurs, what it clear is that there is a progressive increase in circulating cytokines (Bowdish 2013). However, a recent study from this same group indicates that age-associated inflammation that drives macrophage dysfunction is derived from age-coupled gut microbial dysbiosis ((Thevaranjan et al. 2017) see Amsterdam and Ostrov review on the microbiome on immunosenescence in this thematic issue). As a direct consequence, leukocyte numbers, along with their phenotype and function, change with age. From an immunologic perspective, the most profound effect of hematopoietic stem cell aging in both mice (Sudo et al. 2000; Rossi et al. 2005) and humans (Pang et al. 2011) is a decreased capacity to produce lymphocytes and an increase in production of myeloid cells. This shift has been correlated with increased expression of myeloid lineage genes and downregulation of those specifying a lymphoid lineage fate (Rossi et al. 2005; Pang et al. 2011). This combination of increased inflammatory mediators and altered leukocyte phenotype ultimately impacts leukocyte functions including pathogen-associated molecular pattern (PAMP) signaling, phagocytic capacity, and immune cytokine secretion (Franceschi et al. 2007). Thus, it not too surprising that elderly populations suffer disproportionally higher rates of chronic inflammatory conditions where myeloid cells are greatly expanded including diabetes, arthritis, periodontal diseases and some cancers (Franceschi and Campisi 2014; Eke et al. 2016).
Aging and Bone Homeostasis
The maintenance of bone homeostasis is dependent on the balance of activity of bone-resorbing osteoclasts and bone-forming osteoblasts (Karsenty and Wagner 2002; Boyle, Simonet, and Lacey 2003). Abnormal bone resorption by osteoclasts results in bone destruction and is characteristic of bone-related diseases such as osteoporosis, rheumatoid arthritis, and periodontal disease (Kirkwood et al. 2007; Feng and McDonald 2011). Osteoclasts are derived from monocyte/macrophage lineage cells. Formation of functional osteoclasts is dependent on macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB (NF-κB) ligand (RANKL) (Liu and Zhang 2015; Troen 2003; Duque 2016). M-CSF permits abundance and survival of the osteoclasts precursor cells by acting through its receptor c-Fms to activate chiefly Akt and ERK½ (Gingery et al. 2003). RANKL promotes osteoclast differentiation, referred to as osteoclastogenesis, via its receptor RANK leading to recruitment of TNF receptor-associated factor 6 (TRAF6) and, in turn, activation of multiple downstream targets including mitogen-activated protein (MAP) kinases, activator protein-1 (AP-1) and NF-κB (Boyce 2013). However, negative regulators of MAPK activation, primarily MAP kinase phosphatase-1 (MKP-1) appear to be required for RANKL (physiological bone turnover) but inhibit pathological bone loss (Valerio et al. 2014; Valerio et al. 2015; Valerio and Kirkwood 2018). In addition, RANKL-driven osteoclastogenesis is also dependent on the generation of a calcium signal through the activation of the immunoreceptor tyrosine-based activation motifs (ITAMs) of DNAX-activation protein (DAP) 12 and Fc-receptor common γ subunit (FcRγ) (Boyce 2013). This enables TRAF6-mediated and ITAM-mediated signals to interact cooperatively in transcriptional upregulation of nuclear factor of activated T cells c1 (NFATc1, the master transcription factor).
Early concepts of loss of bone mass and strength largely focused on loss of sex steroids. However, large epidemiological data supports the notion that bone loss starts as soon as the early 30’s, long before any change in sex steroid production (Riggs et al. 2008). Indeed, there is ample evidence for age-related, but sex-steroid independent, loss of bone mass that leads to compromised bone strength predisposing to increased bone fragility, loosely referred to as osteoporosis (reviewed in (Manolagas 2010)). One well supported theory of aging dictates that oxidative stress resulting from an increase in intracellular reactive oxygen species (ROS) is a major determinant of aging and lifespan (Russell and Kahn 2007; Lu and Finkel 2008; Manolagas 2010). Excessive accumulation of ROS due to aging occurs in many tissues and bone is no exception (Manolagas 2010). Formation of ROS, fueled by glucose, occurs primarily in the mitochondria from the escape of electrons passing through the electron transport chain during aerobic metabolism to generate ATP. ROS can also be generated following fatty acid oxidation or in response to external stimuli, including inflammatory cytokines (Manolagas 2010). Free electrons are added to oxygen generating superoxide, hydrogen peroxide, (H2O2), and free hydroxyl radical. Of all of these oxygen species, H2O2 has the highest stability and intracellular concentration.
Several studies indicate that intracellular ROS including superoxide anion and H2O2 play a significant intracellular signaling role in the receptor-mediated signaling cascades in various type of cells (Schieber and Chandel 2014). Activation of RANK by RANKL during osteoclastogenesis results in the generation of ROS which reinforces activation of the RANKL-mediated signaling (Park, Lee, and Lee 2017). Consequently, RANKL-induced ROS further stimulate OC formation and bone resorption. Therefore, a delicate regulation of ROS levels is critical in maintaining bone homeostasis. There are several other mechanisms, including telomere shortening, epigenetics, and alterations in cell signaling, including Wnt signaling, that also contribute to bone cell senescence in the aging skeleton (Marie 2014). For a more in-depth analysis on oxidative stress mechanisms and skeletal aging, the reader is referred to a relatively recent review paper on this topic (Callaway and Jiang 2015).
MDSC Expansion, Osteoclastogenesis, and Oxidative Stress
Myeloid lineage progenitors generated in the bone marrow classically differentiate into macrophages, dendritic cells (DC), and granulocytes. Myeloid-derived suppressor cells (MDSC) are not a separate lineage of cells, but are rather a heterogeneous population of activated myeloid cells with suppressive functions. Although suppressive myeloid cells were described more than three decades ago, the diverse phenotypes of MDSC and their biological roles have only recently begun to be characterized in detail (Parker, Beury, and Ostrand-Rosenberg 2015; Gabrilovich et al. 2007). These cells are defined by having myeloid markers, potent immunosuppressive activity, and for monocytic MDSC, the ability to differentiate into mature macrophages and DC. In mice, there are two relatively distinct subsets of MDSCs: monocytic MDSC (M-MDSC = CD11b+ LY6G− LY6Chigh) and granulocytic MDSC (G-MDSC = CD11b+ LY6G+ LY6Clow) (Bronte et al. 2016). Human MDSC are less easily categorized into monocytic vs. granulocytic because of the lack of a Ly-6G (Gr-1) gene homolog in humans. However, human MDSC have been defined as CD11b+ CD33+ HLA-DRlow/−, with monocytic MDSC being CD14± CD15low/− and granulocytic MDSC being CD14− CD15+ CD66b+, which appears consistent with hematologic morphology (Bronte et al. 2016). MDSC populations appear to be predominantly of the granulocytic type in the setting of cancer, as well as in early infancy. In chronic infectious diseases, the relative importance of monocytic and granulocytic MDSC is unclear (Youn and Gabrilovich 2010). These definitions and classifications are somewhat controversial, however, given the heterogeneity of MDSC populations and the variability in markers used by different groups, as well as the possibility of overlap between MDSC phenotypes (Youn and Gabrilovich 2010; Bronte et al. 2016).
Aging and metabolic disease have clearly been shown to enhance MDSC expansion (Verschoor et al. 2013; Jackaman and Nelson 2014; Bao et al. 2015). MDSCs expand and become activated in response to a variety of factors, including inflammatory cytokines (IL-6, VEGF), other pro-inflammatory factors (lipopolysaccharide (LPS), COX2 and prostaglandin E2 (PGE2), GM-CSF, M-CSF, stem cell factor (SCF)-1; see Figure 2 (Ugel et al. 2009). Although increased frequency of MDSCs has been widely reported in the context of cancer immunology, MDSCs have also been shown to contribute to chronic and acute inflammatory processes associated with aging (Gabrilovich and Nagaraj 2009; Jackaman and Nelson 2014; Jackaman et al. 2013) and are recurrently detected in different inflammatory-based pathological disorders. In acute infections, MDSCs may have a beneficial role when the inflammatory stimuli have been cleared by limiting tissue damage produced for a persistent immune response (Gabrilovich and Nagaraj 2009). In contrast, during chronic inflammation, expansion and activation of MDSCs contributes to immunosuppression and oxidative stress. However, differentiation and function of MDSCs are influenced by the inflammatory microenvironment generated, suggesting disease-specific function of MDSCs. For example, it has been reported in numerous autoimmune diseases that while MDSCs are increased they cannot suppress disease progression (Barnie et al. 2017). Hence, their contribution to pathological processes goes far beyond immune suppression.
Figure 2. Molecules involved in the induction and function of MDSCs.
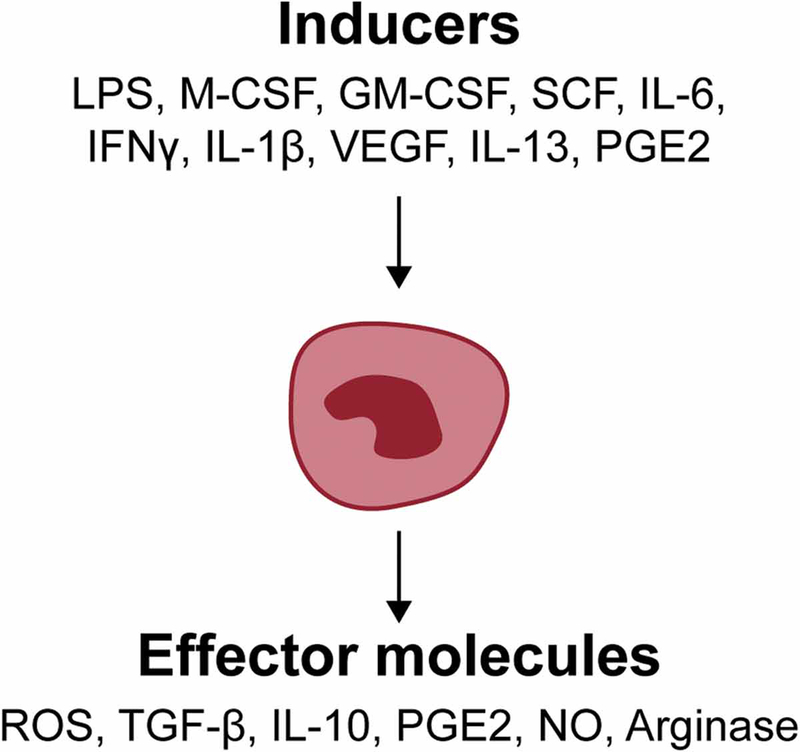
Numerous molecules can induce the development and differentiation of MDSCs. These factors include, but not limited to, lipopolysaccharide (LPS), macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), interleukin-6 (IL-6), interferon-gamma (IFNγ), IL-1β, Vascular endothelial growth factor (VEGF), IL-13, Prostaglandin E2 (PGE2). Known effector molecules include 1) Reactive Oxygen Species (ROS), Transforming growth factor beta (TGF-β), IL-10, PGE2, nitric oxide (NO), and Arginase. Refer to text for more details.
Besides MDSCs being macrophage progenitors, which can also become osteoclasts, we and others have suggested that MDSCs may directly become osteoclasts in the right bone microenvironment—either in the context of aging or cancer (Sawant and Ponnazhagan 2013; Steinkamp HM 2018). Indeed, we show in Figure 3, that MDSCs or circulating monocytes (CD11b+ LY6G− LY6Chigh) isolated from 12-week-old mice can differentiate directly into osteoclasts in response to RANKL/M-CSF. With respect to MDSC expansion with age, NF-κB was shown to be partially required to increase the percentage of MDSCs through genetic reduction of NF-κb-subunit p65 (RelA) in normally aged mice and in 2 different models of progeroid mouse models (Flores et al. 2017). Other mechanisms explored involve chemokines, but this has only been evaluated in bone metastasis (Sawant and Ponnazhagan 2013). The contribution of osteoclastogenesis from MDSC populations has not been well studied and remains an area of active investigation. Likewise, it is not known of the same mechanisms of MDSC differentiation into osteoclasts are operative compared to other osteoclastogenic populations. However, our group has recently shown that mice deficient in the RNA binding protein, tristetraprolin, where there is significant amounts of TNF-α being expressed, have an increased expansion of MDSC population and increased bone loss in the alveolar bone (Steinkamp HM 2018). Ongoing studies are addressing the potential mechanisms.
Figure 3. MDSC can directly differentiate into osteoclasts.
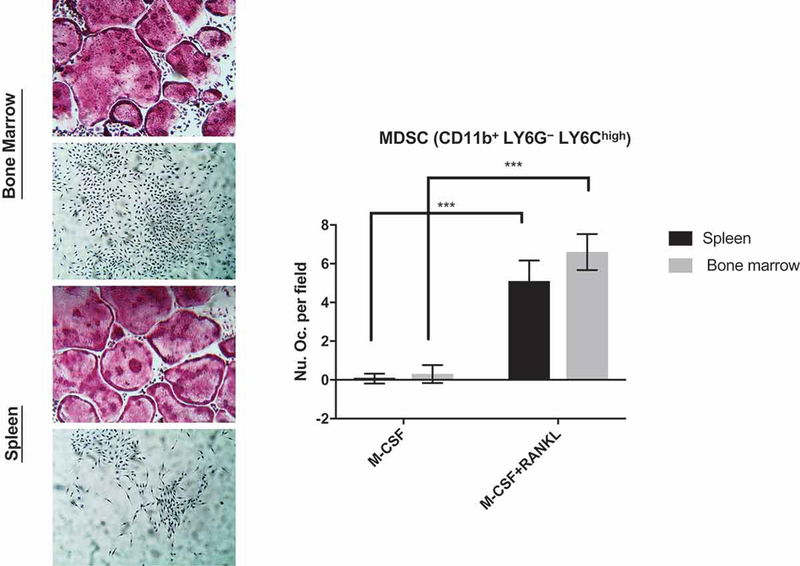
MDSC (CD11b+ LY6G− LY6Chigh) cell populations were isolated using magnetic cell separation from 12-week-old C57BL/6J mouse spleen and bone marrow and plate for osteoclast differentiation using RANKL/M-CSF to induce osteoclast maturation for 5 days. Note that CD markers for defining MDSC may also represent other monocytic populations and not exclusively MDSCs. Representative TRAP (tartrate resistant acid phosphatase) stained osteoclasts are shown in the left panels. Osteoclasts were enumerated using TRAP+ with ≥3 nuclei and presented in the right panel. (N=3; ***P<0.001).
A common mechanistic thread that may explain why MDSCs can directly differentiate into osteoclasts is oxidative stress. Studies have indicated that reactive oxygen species (ROS), including superoxide and hydrogen peroxide, are crucial components that regulate the differentiation process of osteoclasts (Nakashima and Takayanagi 2009). Both NO and ROS are well-known mediators of osteoclast differentiation, NO can induce osteoclast differentiation of macrophages. An inhibitor of inducible nitric oxide synthase (iNOS) and iNOS knock-out mice show reduced bone loss due to impaired osteoclast function. Stimulation of macrophages via RANKL transiently increases ROS production through TNF receptor-associated factor (TRAF) 6, Rac1 and NADPH oxidase (Nox) 1. Inhibitors that block Nox1 or a deficiency in TRAF6 inhibit response of macrophages to RANKL, thus resulting in reduced osteoclastogenesis (Sawant and Ponnazhagan 2013). In addition, NO synthesis was required for the generation of MDSC mediated osteoclastogenesis where inhibition of NO production reduced osteolysis both in vitro and in vivo. Since M-MDSCs are major producers of NO, M-MDSCs may be the major source of MDSC-generated osteoclasts. Indeed, we have observed many more osteoclasts formed from M-MDSC cultures compared to PMN-MDSC cultures (unpublished data).
MDSCs, Aging, and Bone Fragility
Bone fragility is a major health concern, as the increased risk of bone fractures has devastating outcomes in terms of mortality, decreased autonomy, and healthcare costs (Fonseca et al. 2014). Considerable therapeutic efforts made to address this problem have considerably increased our knowledge about the mechanisms that regulate bone formation and resorption. However, the burden of fragility fractures is increasing in absolute terms because longevity is increasing the proportion of the population over 65 years of age (Johnell and Kanis 2006). The term “osteoporosis” is often used synonymously with bone fragility, but osteopenia patients (those without pathological fractures) are at risk of fracture (Pasco et al. 2006). Indeed, most women and men sustaining fragility fractures have osteopenia (Sanders et al. 2006). More than half of women (Wainwright et al. 2005) and men (Thomas-John et al. 2009) who suffer fractures have a T-score better than –2.5. Patients with osteopenia at risk for fracture can be identified by determining microstructural changes but high-resolution imaging methods are not yet widely available (Bala et al. 2014). However, challenges remain with adherence to therapy, in part, because of serious concerns of long-term use (Khosla et al. 2007).
Antiresorptive therapeutics remain the first line and most commonly used treatments for prevention and treatment of bone fragility (Russell et al. 2008). Apart from denosumab (a humanized anti-RANKL antibody), which effectively abolishes bone remodeling, most anti-resorptives slow unbalanced remodeling so microstructural deterioration continues to occur albeit more slowly (Seeman et al. 2010). Since the basis of anti-resorptives stop, but not reverse, microstructural deterioration, fracture risk reduction with anti-resorptives is modest. Teriparatide, an anabolic form of parathyroid hormone, increases bone matrix quantity mainly through remodeling-based bone formation (Lindsay et al. 2006). Thus, there is a need to develop new therapeutic approaches to address aging and bone fragility. Towards this goal, we have recently shown that high intensity interval training (HIIT) exercise can have dramatic effects on muscle wasting (sarcopenia) and improve fragility assessments in mice without the same time commitments of other forms of exercise (Seldeen et al. 2018).
Furthermore, since MDSCs are expanded with aging and contribute to formation of osteoclasts and reduction of bone mineral density, it is conceivable that MDSCs may represent a new therapeutic target to treat and manage bone fragility. With the advent of immunotherapy in cancer therapeutics, the use of immune checkpoint receptor (ICR) inhibitors, which block specific proteins made by T cells and some cancer cells, has gained wide acceptance to treat many types of cancer. These proteins help keep immune responses in check and can keep T cells from killing cancer cells. When these proteins are blocked, the “brakes” on the immune system are released and T cells are able to kill cancer cells with greater proficiency. Examples of checkpoint proteins found on T cells or cancer cells include PD-1/PD-L1 and CTLA-4/B7–1/B7–2. One of the main limitations of using ICR blockade is the reduction of cancer cell specific T-cells through the expansion of MDSCs, thus there is a need to develop agents that can be used adjunctively with ICR that suppress MDSC expansion, deactivate MDSCs, inhibit MDSC development, or deplete MDSCs (Wesolowski, Markowitz, and Carson 2013). For example, MDSCs may be inhibited via the use of phosphodiesterase inhibitors, nitro-aspirins, synthetic triterpenoids, COX2 inhibitors, ARG1 inhibitors, anti-glycan antibodies, IL-17 inhibitors and histamine-based approaches. MDSC may be differentiated by using all-trans retinoic acid, vitamins A or D3 or IL-12. Agents that block the formation of MDSCs include bisphosphonates, modulators of tyrosine kinases, and STAT3 inhibitors. Consequently, future therapeutic strategies developed in the cancer biology arena maybe useful to treat the aging-associated expansion of MDSCs to treat and manage bone fragility.
Acknowledgments:
This work was supported by the National Institutes of Health (NIH) grant 1 R01 DE028258–01 and the Department of Defense Grant W81XWH-17-PRMRP-DA.
Footnotes
Disclosure of Interest:
There are no conflicts of interest to disclose.
References
- Bala Y, Zebaze R, Ghasem-Zadeh A, Atkinson EJ, Iuliano S, Peterson JM, Amin S, Bjornerem A, Melton LJ 3rd, Johansson H, Kanis JA, Khosla S, and Seeman E. 2014. ‘Cortical porosity identifies women with osteopenia at increased risk for forearm fractures’, J Bone Miner Res, 29: 1356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Mo J, Ruan L, and Li G. 2015. ‘Increased monocytic CD14(+)HLADRlow/- myeloid-derived suppressor cells in obesity’, Mol Med Rep, 11: 2322–8. [DOI] [PubMed] [Google Scholar]
- Barnie PA, Zhang P, Lv H, Wang D, Su X, Su Z, and Xu H. 2017. ‘Myeloid-derived suppressor cells and myeloid regulatory cells in cancer and autoimmune disorders’, Exp Ther Med, 13: 378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, and Dye BA. 2018. ‘Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012’, J Periodontol, 89 Suppl 1: S140–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots AM, Maier AB, Stinissen P, Masson P, Lories RJ, and De Keyser F. 2013. ‘The influence of ageing on the development and management of rheumatoid arthritis’, Nat Rev Rheumatol, 9: 604–13. [DOI] [PubMed] [Google Scholar]
- Bowdish DM 2013. ‘Myeloid-derived suppressor cells, age and cancer’, Oncoimmunology, 2: e24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce BF 2013. ‘Advances in the regulation of osteoclasts and osteoclast functions’, J Dent Res, 92: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, and Lacey DL. 2003. ‘Osteoclast differentiation and activation’, Nature, 423: 337–42. [DOI] [PubMed] [Google Scholar]
- Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, and Gabrilovich DI. 2016. ‘Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards’, Nat Commun, 7: 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway DA, and Jiang JX. 2015. ‘Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases’, J Bone Miner Metab, 33: 359–70. [DOI] [PubMed] [Google Scholar]
- Chalan P, van den Berg A, Kroesen BJ, Brouwer L, and Boots A. 2015. ‘Rheumatoid Arthritis, Immunosenescence and the Hallmarks of Aging’, Curr Aging Sci, 8: 131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque G and Demontiero O, and Troen BR 2016. Osteoporosis (McGraw-Hill: New York, NY: ). [Google Scholar]
- Eke PI, Wei L, Borgnakke WS, Thornton-Evans G, Zhang X, Lu H, McGuire LC, and Genco RJ. 2016. ‘Periodontitis prevalence in adults >/= 65 years of age, in the USA’, Periodontol 2000, 72: 76–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, and McDonald JM. 2011. ‘Disorders of bone remodeling’, Annu Rev Pathol, 6: 121–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RR, Clauson CL, Cho J, Lee BC, McGowan SJ, Baker DJ, Niedernhofer LJ, and Robbins PD. 2017. ‘Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a NF-kappaB-dependent mechanism’, Aging Cell, 16: 480–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca H, Moreira-Goncalves D, Coriolano HJ, and Duarte JA. 2014. ‘Bone quality: the determinants of bone strength and fragility’, Sports Med, 44: 37–53. [DOI] [PubMed] [Google Scholar]
- Franceschi C, and Campisi J. 2014. ‘Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases’, J Gerontol A Biol Sci Med Sci, 69 Suppl 1: S4–9. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, and Salvioli S. 2007. ‘Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans’, Mech Ageing Dev, 128: 92–105. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, and Santoro A. 2018. ‘Inflammaging: a new immune-metabolic viewpoint for age-related diseases’, Nat Rev Endocrinol, 14: 576–90. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, and Schreiber H. 2007. ‘The terminology issue for myeloid-derived suppressor cells’, Cancer Res, 67: 425; author reply 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, and Nagaraj S. 2009. ‘Myeloid-derived suppressor cells as regulators of the immune system’, Nat Rev Immunol, 9: 162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingery A, Bradley E, Shaw A, and Oursler MJ. 2003. ‘Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival’, J Cell Biochem, 89: 165–79. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, and Weyand CM. 2003. ‘Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation’, Arthritis Res Ther, 5: 225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackaman C, and Nelson DJ. 2014. ‘Are macrophages, myeloid derived suppressor cells and neutrophils mediators of local suppression in healthy and cancerous tissues in aging hosts?’, Exp Gerontol, 54: 53–7. [DOI] [PubMed] [Google Scholar]
- Jackaman C, Radley-Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, and Nelson DJ. 2013. ‘Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice’, Aging Cell, 12: 345–57. [DOI] [PubMed] [Google Scholar]
- Johnell O, and Kanis JA. 2006. ‘An estimate of the worldwide prevalence and disability associated with osteoporotic fractures’, Osteoporos Int, 17: 1726–33. [DOI] [PubMed] [Google Scholar]
- Karsenty G, and Wagner EF. 2002. ‘Reaching a genetic and molecular understanding of skeletal development’, Dev Cell, 2: 389–406. [DOI] [PubMed] [Google Scholar]
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E, Bone American Society for, and Research Mineral. 2007. ‘Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research’, J Bone Miner Res, 22: 1479–91. [DOI] [PubMed] [Google Scholar]
- Kirkwood KL, Cirelli JA, Rogers JE, and Giannobile WV. 2007. ‘Novel host response therapeutic approaches to treat periodontal diseases’, Periodontol 2000, 43: 294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, and Dempster DW. 2006. ‘A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide’, J Bone Miner Res, 21: 366–73. [DOI] [PubMed] [Google Scholar]
- Liu W, Yang LH, Kong XC, An LK, and Wang R. 2015. ‘Meta-analysis of osteoporosis: fracture risks, medication and treatment’, Minerva Med, 106: 203–14. [PubMed] [Google Scholar]
- Liu W, and Zhang X. 2015. ‘Receptor activator of nuclear factor-kappaB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review)’, Mol Med Rep, 11: 3212–8. [DOI] [PubMed] [Google Scholar]
- Lu T, and Finkel T. 2008. ‘Free radicals and senescence’, Exp Cell Res, 314: 1918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC 2010. ‘From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis’, Endocr Rev, 31: 266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie PJ 2014. ‘Bone cell senescence: mechanisms and perspectives’, J Bone Miner Res, 29: 1311–21. [DOI] [PubMed] [Google Scholar]
- Nakashima T, and Takayanagi H. 2009. ‘Osteoclasts and the immune system’, J Bone Miner Metab, 27: 519–29. [DOI] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, and Weissman IL. 2011. ‘Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age’, Proc Natl Acad Sci U S A, 108: 20012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee NK, and Lee SY. 2017. ‘Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation’, Mol Cells, 40: 706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KH, Beury DW, and Ostrand-Rosenberg S. 2015. ‘Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment’, Adv Cancer Res, 128: 95–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, and Kotowicz MA. 2006. ‘The population burden of fractures originates in women with osteopenia, not osteoporosis’, Osteoporos Int, 17: 1404–9. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, and Khosla S. 2008. ‘A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men’, J Bone Miner Res, 23: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, and Weissman IL. 2005. ‘Cell intrinsic alterations underlie hematopoietic stem cell aging’, Proc Natl Acad Sci U S A, 102: 9194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RG, Watts NB, Ebetino FH, and Rogers MJ. 2008. ‘Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy’, Osteoporos Int, 19: 733–59. [DOI] [PubMed] [Google Scholar]
- Russell SJ, and Kahn CR. 2007. ‘Endocrine regulation of ageing’, Nat Rev Mol Cell Biol, 8: 681–91. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Nicholson GC, Watts JJ, Pasco JA, Henry MJ, Kotowicz MA, and Seeman E. 2006. ‘Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective?’, Bone, 38: 694–700. [DOI] [PubMed] [Google Scholar]
- Sawant A, and Ponnazhagan S. 2013. ‘Myeloid-derived suppressor cells as osteoclast progenitors: a novel target for controlling osteolytic bone metastasis’, Cancer Res, 73: 4606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, and Chandel NS. 2014. ‘ROS function in redox signaling and oxidative stress’, Curr Biol, 24: R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, and Zanchetta J. 2010. ‘Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate’, J Bone Miner Res, 25: 1886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldeen KL, Lasky G, Leiker MM, Pang M, Personius KE, and Troen BR. 2018. ‘High Intensity Interval Training Improves Physical Performance and Frailty in Aged Mice’, J Gerontol A Biol Sci Med Sci, 73: 429–37. [DOI] [PubMed] [Google Scholar]
- Steinkamp HM, Hathaway-Schrader JD, Chavez MB, Aartun JD, Zhang L, Jensen T, Ahojaee Bakhtiari A, Helke KL, Stumpo D, Alekseyenko AV, Novince CM, Blackshear PJ, Kirkwood KL. 2018. ‘Tristetraprolin is Required for Alveolar Bone Homeostasis’, Journal of Dental Research, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, and Nakauchi H. 2000. ‘Age-associated characteristics of murine hematopoietic stem cells’, J Exp Med, 192: 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larche MJ, Davidson DJ, Verdu EF, Surette MG, and Bowdish DME. 2017. ‘Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction’, Cell Host Microbe, 21: 455–66 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-John M, Codd MB, Manne S, Watts NB, and Mongey AB. 2009. ‘Risk factors for the development of osteoporosis and osteoporotic fractures among older men’, J Rheumatol, 36: 1947–52. [DOI] [PubMed] [Google Scholar]
- Troen BR 2003. ‘Molecular mechanisms underlying osteoclast formation and activation’, Exp Gerontol, 38: 605–14. [DOI] [PubMed] [Google Scholar]
- Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, and Bronte V. 2009. ‘Therapeutic targeting of myeloid-derived suppressor cells’, Curr Opin Pharmacol, 9: 470–81. [DOI] [PubMed] [Google Scholar]
- Valerio MS, Herbert BA, Basilakos DS, Browne C, Yu H, and Kirkwood KL. 2015. ‘Critical role of MKP-1 in lipopolysaccharide-induced osteoclast formation through CXCL1 and CXCL2’, Cytokine, 71: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio MS, Herbert BA, Griffin AC 3rd, Wan Z, Hill EG, and Kirkwood KL. 2014. ‘MKP-1 signaling events are required for early osteoclastogenesis in lineage defined progenitor populations by disrupting RANKL-induced NFATc1 nuclear translocation’, Bone, 60: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio MS, and Kirkwood KL. 2018. ‘Sexual Dimorphism in Immunity to Oral Bacterial Diseases: Intersection of Neutrophil and Osteoclast Pathobiology’, J Dent Res: 22034518798825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor CP, Johnstone J, Millar J, Dorrington MG, Habibagahi M, Lelic A, Loeb M, Bramson JL, and Bowdish DM. 2013. ‘Blood CD33(+)HLA-DR(−) myeloid-derived suppressor cells are increased with age and a history of cancer’, J Leukoc Biol, 93: 633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, Hochberg MC, Vogt MT, Orwoll ES, and Group Study of Osteoporotic Fractures Research. 2005. ‘Hip fracture in women without osteoporosis’, J Clin Endocrinol Metab, 90: 2787–93. [DOI] [PubMed] [Google Scholar]
- Wesolowski R, Markowitz J, and Carson WE 3rd. 2013. ‘Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer’, J Immunother Cancer, 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, and Gabrilovich DI. 2010. ‘The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity’, Eur J Immunol, 40: 2969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


