Abstract
Anucleate platelets are produced by fragmentation of megakaryocytes. Platelets circulate in the blood stream for a finite period: Upon vessel injury, they are activated to participate in hemostasis; upon senescence, unused platelets are cleared. Platelet hypofunction leads to bleeding. Conversely, pathogenic platelet activation leads to occlusive events that precipitate strokes and heart attacks. Recently, we and others have shown that autophagy occurs in platelets and is important for platelet production and normal functions including hemostasis and thrombosis. Due to the unique properties of platelets, such as their lack of nuclei and their propensity for activation, methods for studying platelet autophagy must be specifically tailored. Here, we describe useful methods for examining autophagy in both human and mouse platelets.
1. Introduction
1.1. Discovery of autophagy machinery and autophagic structures in platelets
Platelets are circulating first-responders which act upon blood vessel damage and facilitate hemostasis. Compromised platelet functions can cause bleeding diatheses. Conversely, inappropriate platelet activation can result in spurious thrombosis that causes acute vascular obstruction, precipitating strokes, heart attacks and other ischemic pathologies. Platelets are released under shear conditions from megakaryocytes as anucleate cellular fragments [1–3] and remain in circulation for about 4–5 (for mouse) or 7–10 days (for human) [3–5]. As their functions decay over time [6], the aged platelets are cleared by the liver and spleen (reviewed in [7]).
We and others [8, 9] have reported that as detected by immunoblotting, resting mouse and human platelets express numerous components of the major autophagy protein complexes. These include ULK1, FIP200, Beclin 1, VPS34, VPS15, ATG14, NRBF2, UVRAG, ATG7, the ATG12-ATG5 conjugate, ATG3, and LC3II (summarized in the Supplemental Table 1 in [9]). In addition to the protein data, microscopy clearly shows the presence of autophagy-related structures in platelets. Resting platelets, isolated from GFP-LC3/+ [10, 11], Becn1-EGFP/+ [12] and EGFP-Atg5/+ [9] transgenic mice, display distinct GFP-positive puncta by confocal fluorescence microscopy [9] or GFP-positive structures resembling incomplete rings by super-resolution microscopy (Fig. 3), demonstrating the presence of phagophores and autophagosomes. Endogenous LC3-positive puncta were also detected in human platelets by immunocytochemistry/immunofluorescence (ICC/IF) [8]. Further, we visualized phosphatidylinositol 3-phosphate (PI3P), the product of the class III phosphatidylinositol 3-kinase (PI3K) VPS34, as puncta in mouse platelets, using GST-2×FYVE-mediated ICC/IF (Fig. 1B). Autophagic structures, e.g., double-membraned phagophore-like structures wrapping around portions of cytosol, granules, and mitochondria, are also clearly visible by electron microscopy ([9] and unpublished data; representative micrographs shown in Fig. 2). Mitochondria-containing autophagosomes were observed in mouse platelets subjected to hypoxic conditions either ex vivo or in vivo [13]. Besides human and mouse, autophagosome-like structures were also seen in platelets from dogs with severe non-regenerative anemia [14].
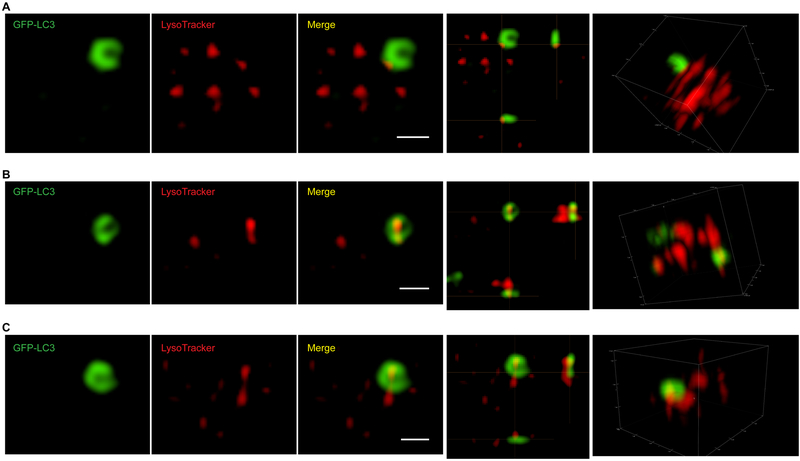
Figure 3. Super-resolution microscopy of platelet autophagy.
3D-Structured Illumination Microscopy (SIM) images of GFP-LC3 (GFP channel) and live-stained LysoTracker Blue (405 channel, pseudo-color in red) in GFP-LC3/+ mouse platelets show incomplete ring-like GFP-LC3 structures resembling isolation membranes/phagophores as observed by electron microscopy in Fig. 2. These 3D-SIM images show an interesting spatial relationship between the GFP-LC3-positive and lysoTracker-positive structures in platelets: The lysoTracker-positive structures appear to be associated with the incomplete, ring-shaped GFP-LC3-positive structures, i.e., the lysoTracker-stained, tubular structures are either on the outside (A) or inside (B–C) of the GFP-LC3-positive rings. Images were collected on a Nikon Ti-E N-STORM/N-SIM super-resolution microscope that was equipped with an Apo SR 100X/1.49 NA oil-objective and an A1R camera. Images were processed using Nikon NIS-Elements v3.2 N-SIM/STORM software and Adobe Photoshop. Scale bars: 1 μm.
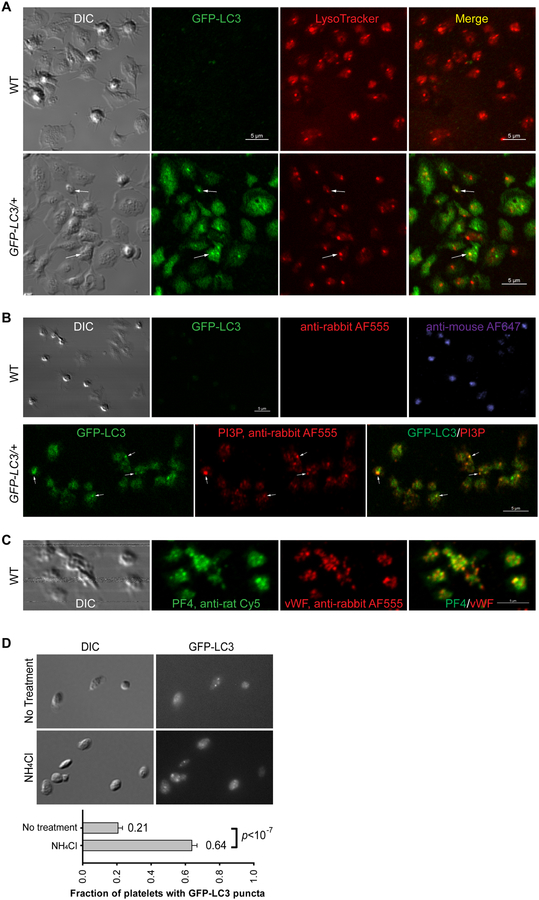
Figure 1. Imaging platelet autophagy using light microscopy.
(A) Confocal and DIC images of GFP-LC3 (GFP channel) in WT and GFP-LC3/+ mouse platelets stained live with 50 nM LysoTracker® Red DND-99 (Thermo Fisher Scientific, L7528) (Cy3 channel) at 37°C for 30 min. Images were collected on a Nikon A1R inverted confocal microscope. Arrows point to two puncta positive for both GFP-LC3 and LysoTracker. Scale bars: 5 μm.
(B) Confocal and DIC images of fixed WT and GFP-LC3/+ mouse platelets incubated with PI3P-binding GST-2×FYVE and then immunostained with GST antibody (Santa Cruz, sc-459). These images show punctate structures for both GFP-LC3 and PI3P, and colocalization of GFP-LC3 and PI3P on some (labelled by arrows), but not all puncta. Recombinantly purified GST-2×FYVE was incubated at 1 μg/mL in the presence of 10 μM ZnCl2 at 4°C overnight. Controls include WT (for GFP channel) immunostained with anti-rabbit Alexa Fluor® 555 (Thermo Fisher Scientific, A31572) or anti-mouse Alexa Fluor® 647 (Thermo Fisher Scientific, A21235), but without primary antibody. Images were collected on a Nikon A1R inverted confocal microscope. Images were acquired and processed using the same settings for GFP-LC3 and WT samples. Note that in the absence of primary antibody, immunostaining with anti-mouse Alexa Fluor® 647, but not with anti-rabbit Alexa Fluor® 555, shows punctate structures in WT mouse platelets. This non-specific anti-mouse Alexa Fluor® 647-staining is likely due to non-specific binding of the anti-mouse secondary antibody to endogenous mouse immunoglobulins that are naturally packaged in the platelet α-granules. Scale bars: 5 μm.
(C) Confocal and DIC images of WT and GFP-LC3/+ mouse platelets immunostained with rabbit anti-vWF (Dako, A0082) and rat anti-PF4 (R&D Systems, DY595). Note that PF4 and vWF, both of which are α-granule cargos, appear not perfectly colocalized, suggesting presence of sub-domains within the α-granules. Images were collected on a Nikon A1R inverted confocal microscope. Scale bar: 5 μm.
(D) Autophagic flux in mouse platelets as monitored by GFP-LC3 puncta in the presence and absence of NH4Cl (20 mM, 2 h) using an upright wide-field microscope. Samples were visualized for DIC and GFP fluorescence (GFP-LC3). Images were acquired using a Nikon Eclipse E600 microscope equipped with a 100×/1.40 numeric aperture DIC H oil objective lens and a Zeiss AxioCam MR camera. Ten images (17 and 74 platelets/field for no treatment versus NH4Cl treatment) were obtained at random and quantified for each condition. Platelets containing GFP-LC3-positive puncta are 21 ± 8% and 64 ± 10% without and with NH4Cl treatment, respectively. Statistical significance was evaluated with the Student’s t test. This panel is reproduced from Fig. 2C in [9] with publisher’s permission.
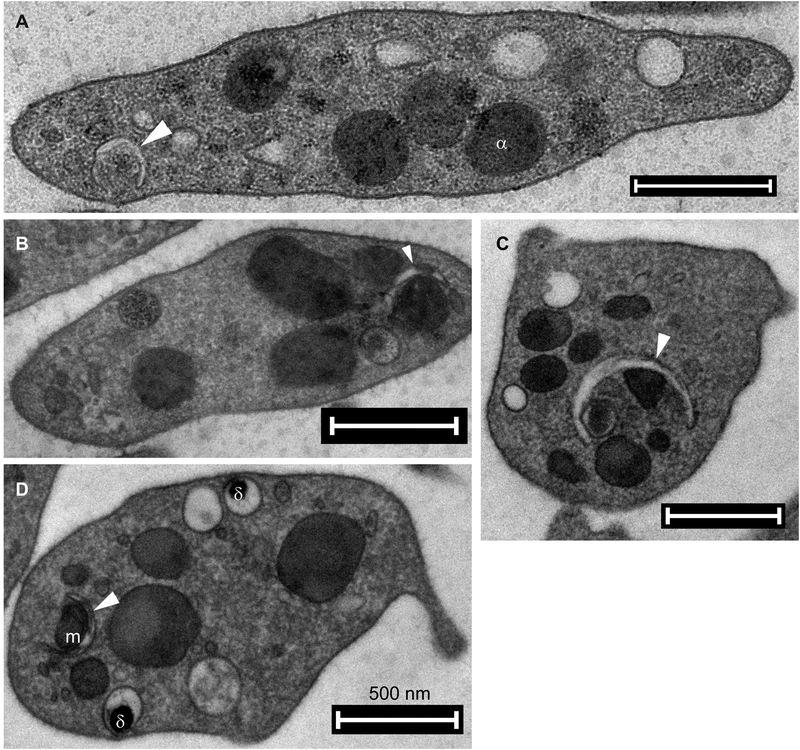
Figure 2. Electron micrographs of autophagosome-related structures in mouse platelets.
Double-membraned phagophore-like structures (arrow heads) wrapping bulk of cytosol and/or granules in (A–B) resting and (C–D) thrombin-stimulated (0.1 U/mL, 10–30 sec) mouse platelets. Labels: m, mitochondria; α, α granules; δ, dense granules. Scale bars: 500 nm. (A–B) are reproduced from [9] with publisher’s permission.
Taken as a whole, the above descriptive data clearly show that both the machinery and cellular structures associated with autophagy are readily detectible in resting platelets. These data were initially surprising since it was unclear why platelets, with their short life-span, would retain such an energy-requiring, degradative system.
1.2. Functional significance of autophagy in platelets
In nucleated eukaryotic cells, basal autophagy is constitutively active to maintain cellular homeostasis. Under stress (e.g., nutrient deprivation), autophagy can be further induced to meet increasing cellular needs for amino acids, nucleotides, sugars and fatty acids as metabolic fuels or anabolic building blocks. In addition to the presence of both the machinery and cellular structures associated with autophagy in resting platelets, we also demonstrated the occurrence of basal autophagy in resting platelets by monitoring autophagic flux, using both LC3II immunoblotting and an imaging assay that counts GFP-LC3 puncta ([9], reproduced in Fig. 1D). In resting platelets, autophagy can be induced by starvation or by treatment with the mTOR complex 1 (mTORC1) inhibitor, rapamycin [8]. Autophagy, particularly mitophagy (i.e., autophagy of mitochondria), can also be induced by hypoxia [13]. We further showed that autophagy can be induced during platelet activation by hemostatic agonists [9], suggesting an involvement of autophagy in platelet functions. We and others further showed that genetic impairment of autophagy, including mitophagy, leads to defective platelet functions ex vivo and in vivo in Atg7− [9], Atg5−[13], Becn1− (Ouseph and Huang et al., manuscript in preparation), Pik3c3/VPS34− ([15, 16], and Ouseph and Huang et al., manuscript in preparation), and FUNDC1− [13] deficient mice.
Together, both general and selective autophagy (e.g., mitophagy) occur in platelets and are essential for cellular functions of platelets as well as hemostasis and thrombosis. The presence and importance of autophagy in platelets warrants further investigation since autophagy could be a viable target for therapeutic intervention of occlusive thrombotic diseases. Here, we describe some useful methods that are specifically tailored for studying autophagy in both human and mouse platelets.
2. Materials
2.1. Mouse and human platelet preparation
Anticoagulant master mix: For each mouse, prepare 100 μl 3.8% (w/v) sodium citrate; add 1 μL apyrase (an ATP/ADP-diphosphohydrolase) stock; add 1 μL prostacyclin (PGI2) stock.
Apyrase stock: 200 U/mL in HEPES-Tyrode buffer (pH 7.4), stored at −20°C until use.
Protacyclin PGI2 stock: 10 μg/mL in 50 mM Tris/HCl pH 9.5, stored at −20°C until use.
Acid citrate dextrose (ACD) collection tubes.
HEPES-Tyrode buffers: 20 mM HEPES, 128 mM NaCl, 2.8 mM KCl, 1 mM MgCl2, 5 mM D-glucose, 12 mM NaHCO3 and 0.4 mM NaH2PO4, titrated to pH 7.4 and pH 6.5 using KOH and HCl, respectively. Store HEPES-Tyrode buffers at 4°C and pre-warm them to room temperature before use.
1× phosphate buffer saline (PBS): containing 8 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl and 2.7 mM KCl, with pH adjusted to 7.4 by HCl.
0.5 M EGTA.
500 mM CaCl2.
1 mL Tuberculin slip tip syringe.
26G 3 1/8 needle.
Euthanization chamber.
Compressed CO2 gas tank.
15 mL and 50 mL conical polypropylene or polyethylene tubes. Polystyrene or glass tubes will activate platelets and should be avoided.
Pasteur pipettes.
Particle count and size analyzer, for example Beckman Z2 Coulter Counter.
2.2. Visualizing autophagic structures in platelets by light microscopy using autophagy reporter mice
Glass-bottom 35-mm culture dishes.
Glass microscope slide.
1.5 mm thick coverslips.
0.1 mg/mL poly-D-Lysine.
1× PBS.
4% paraformaldehyde in 1× PBS. Make fresh.
Permeabilization buffer: 1× PBS containing 50 μg/mL digitonin, 2% BSA and 2% goat serum.
Blocking buffer: 1× PBS containing 2% bovine serum albumin (BSA) and 2% goat serum.
HEPES-Tyrode buffer, pH 7.4.
Lysotracker® Red DND-99.
Primary antibodies.
Fluorescently labeled secondary antibodies.
Anti-fade mounting medium.
Clear nail polish.
Inverted confocal microscope and upright bright field microscope.
2.3. Fixing platelets for electron microscopy
2× fixative stock A: 6% paraformaldehyde and 0.2% glutaraldehyde in 1× PBS. Make fresh.
ACD: 85 mM trisodium citrate, 83 mM dextrose, and 21 mM citric acid.
1 mL Tuberculin slip tip syringe.
26G 3 1/8 needle.
5% glutaraldehyde in 1× PBS.
HEPES-Tyrode buffer, pH 7.4.
2× fixative stock B: 2% paraformaldehyde and 5% glutaraldehyde in 1× PBS.
CaCl2.
Liquid nitrogen.
Cryovials.
2% agarose with ultralow gelling temperature in 1× PBS.
Acetone.
Acetone solution A: 2% OsO4, 0.1% glutaraldehyde and 1% H2O, in acetone.
Acetone solution B: 1% OsO4/1% H2O, in acetone.
1% tannic acid/1% H2O in acetone.
EMBED-resin 812.
Uranyl acetate.
Reynold’s lead citrate.
Ultramicrotome.
Electron microscope.
High pressure freezer with rapid transfer system.
Freeze substitution and low-temperature embedding system.
3. Methods
Anucleate platelets do not transcribe nuclear DNA, despite being capable of some level of cell division (i.e., increased cell number without DNA replication) [17]. Thus, for studying platelets, freshly isolated primary cells are generally used for in vitro experiments. Since washed platelets lose optimal functionality over time, one should strive to use them within 2–3 hours post-isolation. Moreover, as platelets can be easily activated and desensitized, caution is necessary to make good preparations for assays (see Note 1).
3.1. Preparing washed platelets from fresh mouse blood
From one adult mouse, ideally 0.6~1 mL of whole blood can be drawn and 0.6~1 × 109 washed platelets can be obtained. Factors affecting yield include mouse age and size, and importantly, whether platelets are activated during the preparation. All steps are performed at room temperature.
3.1.1. Blood harvesting from mice
Draw 100 μl of the anticoagulant master mix into a 1 mL Tuberculin slip tip syringe with a 26G 3 1/8 needle. In 1 mL of citrated blood, the final concentrations should be 0.38% sodium citrate, 0.2 U/mL apyrase, and 10 ng/mL PGI2.
Fill the euthanization chamber with CO2 from a compressed gas tank with continuous influx of CO2 at a rate of 20% chamber volumes per min. Transfer one mouse at a time into the euthanization chamber. Mouse will stop breathing within ~60 sec. Ensure that the mouse is not responsive by toe or tail pinching. Immediately harvest blood by cardiac puncture as described below. Minimal delay between losing toe/tail pinching response and blood harvesting is important for getting good quality platelets.
Pin the limbs loosely on a foam board. Cut through abdominal skin, muscle and diaphragm sequentially to expose the heart. Pin the xiphoid process loosely on the foam board.
Stick the 26G 3 1/8 needle into the exposed ventricle in the direction along the longitudinal axis of the heart and slowly draw blood. Blood drawing should be with little resistance while avoiding bubbles. To retain platelets in their resting state, complete the blood harvesting in one draw and do not re-stick the heart.
Take off the needle, gently expel the harvested blood into a 1.5 mL Eppendorf tube and mix.
3.1.2. Preparing platelet rich plasma (PRP) and washed platelets from mouse blood
(Optional) Pool blood from more than one mouse with the same genotype into 15 mL or 50 mL conical polypropylene or polyethylene tubes.
Dilute the blood 1:1 (v/v) with 1× PBS or HEPES-Tyrode buffer (pH 6.5). Supplement the diluted blood with 0.2 U/mL apyrase and 10 ng/mL PGI2, and incubate for 5 min to allow the platelet antagonists to take effect.
Balance the tubes and centrifuge at 215 × g in a swinging-bucket rotor (e.g., Thermo Sorvall LEGEND RT Centrifuge, with acceleration and deceleration set as 5 and 2, respectively) for 5–10 min (depending on the volumes of the samples, e.g., 6 min for 3 mL diluted blood). With an optimal centrifugation time, red blood cells and leukocytes will be in the bottom layers and platelet-rich-plasma (PRP) will be slightly yellowish platelet suspension in the top layer.
Without agitating the “buffy coat” interface layer of leukocytes, transfer the top PRP layer to new 15 mL or 50 mL tubes, starting from the top using large disposable transfer pipets followed by 200 μL pipet tips with tip cut.
(Optional) After adding 0.2 U/mL apyrase and 10 ng/mL PGI2 to the PRP layer collected in the new tubes, balance the new tubes and centrifuge again at 215 × g for 5–10 min to remove residual red blood cells and leukocytes. Transfer the supernatants to new 15 mL or 50 mL tubes using large disposable transfer pipets.
After adding 0.2 U/mL apyrase and 10 ng/mL PGI2 to the collected PRP, balance the new tubes and centrifuge at 675 × g for 7–10 min to pellet the platelets.
Gently re-suspend the platelet pellets in 1–2 mL HEPES-Tyrode buffer (pH 6.5) supplemented with 1 mM EGTA, 0.2 U/mL apyrase and 10 ng/mL PGI2, using large disposable transfer pipets.
Balance the tubes and centrifuge again at 675 × g for 7 min to wash the platelets.
Gently re-suspend the platelet pellets in 1–2 mL HEPES-Tyrode buffer (pH 7.4), using large disposable transfer pipets.
Measure platelet concentrations using an appropriate Particle Count and Size Analyzer, following manufacturer’s protocols.
Adjust platelet concentration as needed using HEPES-Tyrode buffer (pH 7.4).
Incubate washed platelets at room temperature or 37°C for 30 min to dissipate the residual effects of PGI2 before assaying platelet functions. PGI2 is quickly degraded in aqueous buffers, with a t1/2 of ~3 min at 37°C/pH 7.4 [18].
At the end of incubation and right before experiments, add CaCl2 to a final concentration of 1 mM.
(Optional) A quick lumi-aggregometry measurement can be run to monitor the quality of the platelet preparations. This method can be found in [19].
3.2. Preparing washed platelets from banked human PRP and fresh human blood
Human platelets can be prepared from 1 unit of human PRP (40~50 mL) purchased from a local blood bank. Usually 2~6 × 109 platelets can be isolated from 100 mL of banked human PRP. Alternatively, fresh whole blood can be drawn from healthy volunteers, with appropriate local approvals. All steps are performed at room temperature.
3.2.1. Preparing washed platelets from banked human PRP
Divide banked human PRP into several 50 mL conical polypropylene or polyethylene centrifuge tubes (15~20 mL per tube). Supplement each tube with apyrase and PGI2 at the final concentrations of 0.2 U/mL and 10 ng/mL, respectively. Incubate samples for 5 min to allow the platelet antagonists to take effect.
Balance the tubes and centrifuge at 155 × g in a swinging-bucket rotor for 13 min to remove residual red blood cells and leukocytes. Transfer the PRP to new 50 mL tubes using large disposable transfer pipets.
After adding 0.2 U/mL apyrase and 10 ng/mL PGI2 to the PRP, balance the new tubes and centrifuge at 750 × g for 13 min to pellet the platelets.
Gently re-suspend the platelet pellets in 1–2 mL HEPES-Tyrode buffer (pH 6.5) containing1 mM EGTA, 0.2 U/mL apyrase and 10 ng/mL PGI2.
Balance the tubes and centrifuge again at 750 × g for 13 min to wash the platelets.
Gently re-suspend the platelet pellets in 1–2 mL HEPES-Tyrode buffer (pH 7.4).
Measure platelet concentrations using a Particle Count and Size analyzer.
Adjust platelet concentration as needed using HEPES-Tyrode buffer (pH 7.4).
Incubate washed platelets at room temperature or 37°C for 30 min before use to ensure that residual effects of PGI2 are dissipated.
At the end of incubation and right before experiments, add CaCl2 to a final concentration of 1 mM.
(Optional) A quick lumi-aggregometry measurement can be run to monitor the quality of the platelet preparations. This method can be found in [19].
3.2.2. Preparing washed platelets from fresh human blood
Draw fresh whole blood from healthy volunteers into ACD collection tubes.
Divide whole blood into several 50 mL conical polypropylene or polyethylene tubes (15~20 mL per tube). Dilute each tube in 1:1 (v/v) ratio with 1× PBS or HEPES-Tyrode buffer (pH 6.5). Supplement diluted blood with 0.2 U/mL apyrase and 10 ng/mL PGI2. Incubate the samples at room temperature for 5 min to allow the platelet antagonists to take effect.
Balance the tubes and centrifuge at 215 × g in a swinging-bucket rotor for 20 min.
Transfer the top layers (i.e., PRP) to new 50 mL tubes using large disposable transfer pipets.
(Optional) After adding 0.2 U/mL apyrase and 10 ng/mL PGI2 to the PRP, balance the new tubes and centrifuge again at 155 × g for 10 min to remove residual red blood cells and leukocytes. Transfer the new PRP fractions to new 50 mL tubes using large disposable transfer pipets.
After adding 0.2 U/mL apyrase and 10 ng/mL PGI2 to the new PRP fractions, balance the new tubes and centrifuge at 750 × g for 13 min to pellet the platelets.
Gently re-suspend the platelet pellets in 1–2 mL HEPES-Tyrode buffer (pH 6.5) supplemented with 1 mM EGTA, 0.2 U/mL apyrase and 10 ng/mL PGI2.
Balance the tubes and centrifuge again at 750 × g for 13 min to wash the platelets.
Gently re-suspend the platelet pellets in 1–2 mL HEPES-Tyrode buffer (pH 7.4).
Measure platelet concentrations using a Particle Count and Size analyzer.
Adjust platelet concentration as needed using HEPES-Tyrode buffer (pH 7.4).
Incubate washed platelets at room temperature or 37°C for 30 min before use to ensure that residual effects of PGI2 are dissipated.
At the end of incubation and right before experiments, add CaCl2 to a final concentration of 1 mM.
(Optional) A quick lumi-aggregometry measurement can be run to monitor the quality of the platelet preparations. This method can be found in [19].
3.3. Visualizing autophagic structures in platelets by light microscopy using autophagy reporter mice
GFP-LC3/+ transgenic mice (made by Dr. Noboru Mizushima, University of Tokyo, Japan) express GFP-LC3 transgene under an actin promoter [10, 11]. This mouse strain is currently available through Riken BioResource Center. Both Becn1-EGFP/+ [12] and EGFP-Atg5/+ [9] strains were made using Bacterial Artificial Chromosome (BAC) transgenics [20] to ensure that tagged genes are expressed under the endogenous Becn1 and Atg5 promoters and regulatory elements (see Note 2).
3.3.1. Visualizing live platelets using autophagy reporter mice
3.3.1.1. Imaging live platelets stained with LysoTracker using an inverted confocal microscope
Prepare washed platelets from the autophagy reporter mice (e.g., GFP-LC3/+, Becn1-EGFP/+, EGFP-Atg5/+) and their wild type (WT) littermates.
Coat glass-bottom 35-mm culture dishes with 0.1 mg/mL poly-D-lysine at 4°C overnight (see Note 3); rinse dishes with 1 mL HEPES-Tyrode buffer (pH 7.4) twice.
Add 100 μL of 1×109/mL platelet suspension to the center of the poly-D-lysine-coated glass-bottom dishes; wait for at least 15 min for platelets to attach; aspirate un-attached cells.
Add 1 mL HEPES-Tyrode buffer containing 50 nM LysoTracker® Red DND-99 and incubate at 37°C for 30 min.
Aspirate LysoTracker-containing buffer and wash with 1 mL HEPES-Tyrode buffer (pH 7.4) two times; after the second wash, cover the platelets with 1 mL HEPES-Tyrode buffer for imaging.
Immediately image fluorescently-labeled autophagy protein (e.g., GFP-LC3), LysoTracker® Red DND-99 and differential interference contrast (DIC) using an inverted confocal microscope (see Fig. 1A for representative images).
3.3.1.2. Imaging live platelets stained with LysoTracker using an upright bright-field microscope
Prepare washed platelets from either WT or the autophagy reporter mice.
For LysoTracker staining, incubate resting washed platelets (100 μL of 1×109/mL) with LysoTracker® Red DND-99 (final concentration 50 nM) in a 1.5 mL Eppendorf tube at 37°C for 30 min.
Place 6 μL of platelets onto a glass microscope slide.
Overlay with a 1.5 mm thick coverslip.
Allow platelets to settle for 5–15 min.
Seal the coverslip to the slide with clear nail polish.
Using an upright miscroscope, for example, a Nikon Eclipse E600 microscope equipped with a 100×/1.40 numeric aperture DIC H oil objective lens and a Zeiss AxioCam MR camera, image DIC, fluorescently-labeled autophagy protein (e.g., GFP-LC3) (GFP channel) and LysoTracker® Red DND-99 (Cy3 channel) sequentially with appropriate exposure times. Representative images for live imaging of GFP-LC3/+ platelets using an upright bright-field microscope are shown in [9] (reproduced in Fig. 1D).
3.3.1.3. Monitoring autophagy flux in platelets using GFP-LC3 imaging
Prepare washed platelets from the GFP-LC3/+ autophagy reporter mice and their WT littermates.
Pre-treat un-stimulated GFP-LC3 platelets either with or without the lysosome inhibitor, NH4Cl (20 mM, 2 h) (see Note 4).
Acquire DIC and GFP fluorescence images of live platelets following either of the protocols described in Section 3.3.1.1 or 3.3.1.2.
Count the number of GFP-LC3 puncta in each platelet in each field.
Quantify fractions of platelets with different numbers of GFP-LC3 puncta to create a distribution histogram.
Average the results from multiple fields for each condition. Representative images and quantification are shown in [9] (reproduced in Fig. 1D).
3.3.2. Visualizing fixed platelets by ICC/IF and confocal microscopy
Prepare washed platelets from either WT or the autophagy reporter mice.
Coat glass-bottom 35-mm culture dishes with 0.1 mg/mL poly-D-lysine at 4°C overnight (see Note 3); rinse dishes with 1 mL HEPES-Tyrode buffer (pH 7.4) twice.
Add 100 μL of 1×109/mL platelet suspension to the center of the glass-bottom dishes; incubate at 37°C for 30 min; aspirate un-attached cells.
Fix with 500 μL 4% paraformaldehyde for 15 min at room temperature.
Wash with 200 μL 1× PBS three times.
Incubate cells in 200 μL permeabilization buffer at room temperature for 10–15 min.
Block with 200 μL blocking buffer at room temperature for 30 min.
Incubate cells with 150 μL primary antibodies in blocking buffer at 4°C overnight (see Note 5).
Wash with 500 μL 1× PBS, three times.
Incubate with 150 μL secondary antibodies in blocking buffer at room temperature for 40 min to 1 h (see Note 5).
Wash four times, each time with 500 μL 1× PBS. During the last wash, wait for 10 min.
After the last wash, aspirate liquid, add one drop of antifade mounting medium (warmed to room temperature before use) to the center of each glass-bottom dish, wait for 1–2 min until the mounting medium has spread throughout the glass bottom, slowly place a 1.5 mm cover glass on top of the mounting medium.
Cure for 24 h and seal all edges with a clear nail polish.
Image using an inverted confocal microscope (see Fig. 1B–C for representative images).
3.4. Visualizing autophagic structures in platelets by electron microscopy
3.4.1. Fixation of fresh mouse resting platelets
For each mouse, pre-fill a 1 mL Tuberculin slip tip syringe with 85 μL of ACD supplemented with 0.2 U/mL apyrase and 10 ng/mL PGI2, and 500 μL 2× fixative stock A.
Draw blood by cardiac puncture as described above in Section 3.1.1 into the fixative-prefilled syringe. Take off the needle, gently expel the harvested blood into a 1.5 mL Eppendorf tube and mix.
Fix for 20 min at room temperature.
Pool the fixed blood from the animals with the same genotype; and prepare washed platelets as described in Section 3.1.2
Resuspend the platelet pellet in HEPES-Tyrode buffer (pH 7.4) and fix a second time by adding equal volume of 5% glutaraldehyde (final 2.5% glutaraldehyde) at room temperature for 20 min.
3.4.2. Fixation of washed mouse or human platelets
For analysis of agonist/inhibitor effects on platelets by electron microscopy, prepare washed platelets (2.5 × 108/mL) as described in Sections 3.1–3.2.
Supplement platelet suspension with 0.7 mM CaCl2 (final concentration) and incubate at room temperature for 5–30 min before stimulation with desired agonist/inhibitor.
Fix the platelet suspension with equal volume of 2× fixative stock B (final concentration 1% paraformaldehyde and 2.5% glutaraldehyde) at room temperature for 30 min.
Centrifuge at 1,000 × g and resuspend platelets in 1× PBS and wash three times to remove fixative.
3.4.3. High pressure freezing, freeze substitution dehydration, and low temperature embedding
This protocol is based on our published work [21] (see Note 6).
Resuspend platelets in 2% agarose with ultralow gelling temperature in 1× PBS.
Freeze cryoprotected platelets under high pressure (2,100 bar), for instance, in a Leica Microsystems EM PACT 2 high pressure freezer with rapid transfer system.
Transfer the frozen samples under liquid nitrogen to cryovials containing acetone solution A (2% OsO4, 0.1% glutaraldehyde and 1% H2O in acetone).
Carry out freeze substitution dehydration, for instance, in the Leica Microsystems EM AFS2 freeze substitution and low-temperature embedding system, following the schedule below: −90°C for 22 h, −60°C for 8 h, −30°C for 8 h, and 0°C, with transitions of 3°C/h between each temperature change.
Rinse samples with acetone three times.
Incubate samples in 1% tannic acid/1% H2O in acetone at 4°C for 1 h.
Rinse samples with acetone three times.
Incubate samples in acetone solution B (1% OsO4/1% H2O in acetone) at 4°C for 1 h.
Rinse samples with acetone three times.
Embed samples in EMBED-812 resin starting at a 25% concentration and proceeding through 50%, 76% and 100% resin steps.
3.4.4. Transmission electron microscopy (TEM)
Prepare ultrathin (50 nm) sections using an ultramicrotome.
Post-stain sections with uranyl acetate and Reynold’s lead citrate.
Acquire micrographs using, for instance, a FEI Tecnai TF20 FEG transmission electron microscope at 80 keV.
4. Notes
Platelets can be easily activated during isolation. Therefore, caution must be taken to minimize un-intended activation while handling platelets, particularly during blood harvesting and platelet preparation. For instance, the one-stick policy during cardiac puncture should be emphasized to avoid platelet activation by repeated piercing-induced vessel injury; polypropylene or polyethylene tubes, but not polystyrene or glass tubes, should be used to avoid contact activation. Whenever possible, centrifugation steps should be performed at low centrifugal forces to minimize shearing which activates platelets; centrifugation tubes containing platelets should be balanced within 0.1 g and acceleration and deceleration should be set at low settings to minimize vibrations that may activate platelets during acceleration and deceleration. Pipetting should be gentle and minimal; either pipet tips (1 mL or 200 μL) with the tips cut or large disposable transfer pipets should be used to minimize shearing during pipetting. Additionally, shaking of the sample tubes should be avoided at any time for handling blood and live platelets.
Mice either expressing autophagy reporter (GFP-LC3/+, Becn1-EGFP/+, EGFP-Atg5/+) or with genetically deficient autophagy genes have been utilized to study autophagy in platelets [8, 9, 13, 22]. GFP-LC3/+ mice have been extremely useful in imaging autophagy in platelets using both traditional wide-field/confocal microscopes [9] and super-resolution microscopes (Fig. 3, and Banerjee et al., manuscript in preparation). In Becn1-EGFP/+ and EGFP-Atg5/+ BAC transgenic mice, Beclin 1-EGFP and EGFP-ATG5 are expressed at low levels (thus giving out low GFP fluorescent signals), therefore limiting the usage of these autophagy reporter mice in live imaging of platelet autophagy (Ouseph and Huang et al., unpublished results). However, Becn1-EGFP/+ and EGFP-Atg5/+ mice are still valuable for immunofluorescence imaging or immuno-electron microscopic studies with anti-GFP antibodies. Of note, endogenous Becn1 and Atg5 genes are still expressed in Becn1-EGFP/+ and EGFP-Atg5/+ BAC transgenic mice.
Mice with their autophagy genes deleted should be used cautiously. First, as normal hemostasis relies on coordination of several cell types, e.g., platelets and endothelial cells, bleeding diatheses observed in whole body autophagy-deficient animals (e.g., Becn1+/− [8]) may result, in part, from deficiency in the endothelium. Second, platelets are originated from megakaryocytes, thus autophagy deficiency in megakaryocytes may lead to defects in megakaryopoiesis and thrombopoiesis, in addition to platelet functions.
Alternative to poly-D-lysine, fibrinogen coating (incubating with 50 μg/mL fibrinogen at room temperature for 2 h) can be used to facilitate platelet adhesion. However, to avoid any form of platelet activation, coating with poly-D-lysine is recommended.
Caution is needed when using common autophagy inducers and inhibitors to study platelets, as these autophagy inducers and inhibitors may have additional targets in platelets besides the autophagy pathway. For example, as platelet secretion from α-granules, dense granules and lysosomes is essential for platelet activation and hemostasis, it is possible that lysosomal inhibitors, such as NH4Cl, chloroquine and bafilomycin A1, may alter lysosomal exocytosis in addition to autophagy.
Non-specific binding of primary antibodies on platelets usually has rarely been determined for commercial antibodies against autophagy proteins and must be tested before use. Moreover, platelets stained with secondary antibodies but without primary antibodies are needed as controls to monitor non-specific binding of secondary antibodies. In particular, anti-mouse secondary antibodies often non-specifically stain α-granules in mouse platelets (Fig. 1B), as immunoglobulins are α-granule cargos. In contrast, anti-rabbit, anti-rat, or anti-goat secondary antibodies have often been cross-adsorbed against human and mouse sera and thus do not stain human or mouse platelets non-specifically.
The high pressure freezing followed by freeze substitution is used to give a very gentle dehydration procedure that limits the formation of electron dense, α-granule nucleoids, an artifact of dehydration [23]. High pressure freezing is less practical for complex mixtures of cells as in freshly drawn blood or PRP. High pressure freezing could also be used as a rapid “fixation” technique to terminate in vitro experiments with highly purified platelets.
Acknowledgements
The authors thank the laboratory personnel and collaborators who conducted the research on platelet autophagy over the years. The authors thank Dr. Zhenyu Li for helpful discussion. The authors also thank Dr. Harry Chanzu and Laura Tichachek for their careful perusal of this manuscript. This work was supported by a New Scholar in Aging award from Ellison Medical Foundation (to Q.J.W.), Grant-in-Aid awards from the American Heart Association (AHA16GRNT31310020 to Q.J.W. and AHA16GRNT27620001 to S.W.W.), Predoctoral Fellowships from the American Heart Association (AHA 15PRE25550020 to S.J. and AHA 11PRE7500051 to Y.H.), National Institutes of Health (HL56652 and HL138179 to S.W.W., HL119393 to B.S.) and a Veterans Affairs Merit Award (to S.W.W.).
References
- 1.Pease DC, An electron microscopic study of red bone marrow. Blood, 1956. 11(6): p. 501–26. [PubMed] [Google Scholar]
- 2.Junt T, et al. , Dynamic visualization of thrombopoiesis within bone marrow. Science, 2007. 317(5845): p. 1767–70. [DOI] [PubMed] [Google Scholar]
- 3.Machlus KR and Italiano JE Jr., The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol, 2013. 201(6): p. 785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harker LA, The kinetics of platelet production and destruction in man. Clin Haematol, 1977. 6(3): p. 671–93. [PubMed] [Google Scholar]
- 5.Ault KA and Knowles C, In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp Hematol, 1995. 23(9): p. 996–1001. [PubMed] [Google Scholar]
- 6.Thompson CB, et al. , Platelet size and age determine platelet function independently. Blood, 1984. 63(6): p. 1372–5. [PubMed] [Google Scholar]
- 7.Grozovsky R, Hoffmeister KM, and Falet H, Novel clearance mechanisms of platelets. Curr Opin Hematol, 2010. 17(6): p. 585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng W, et al. , Dissection of autophagy in human platelets. Autophagy, 2014. 10(4): p. 642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouseph MM, et al. , Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood, 2015. 126(10): p. 1224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuma A and Mizushima N, Chromosomal mapping of the GFP-LC3 transgene in GFP-LC3 mice. Autophagy, 2008. 4(1): p. 61–2. [DOI] [PubMed] [Google Scholar]
- 11.Mizushima N, et al. , In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell, 2004. 15(3): p. 1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arsov I, et al. , BAC-mediated transgenic expression of fluorescent autophagic protein Beclin 1 reveals a role for Beclin 1 in lymphocyte development. Cell Death Differ, 2008. 15(9): p. 1385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, et al. , Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pieczarka EM, et al. , Platelet vacuoles in a dog with severe nonregenerative anemia: evidence of platelet autophagy. Vet Clin Pathol, 2014. 43(3): p. 326–9. [DOI] [PubMed] [Google Scholar]
- 15.Valet C, et al. , A dual role for the class III PI3K, Vps34, in platelet production and thrombus growth. Blood, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, et al. , Class III PI3K Positively Regulates Platelet Activation and Thrombosis via PI(3)P-Directed Function of NADPH Oxidase. Arterioscler Thromb Vasc Biol, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Schwertz H, et al. , Anucleate platelets generate progeny. Blood, 2010. 115(18): p. 3801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore PK, Prostaglandins, prostacyclin and thromboxanes. Biochemical Education, 1982. 10(3): p. 82–87. [Google Scholar]
- 19.Ren QS, et al. , Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Molecular Biology of the Cell, 2007. 18(1): p. 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heintz N, BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci, 2001. 2(12): p. 861–70. [DOI] [PubMed] [Google Scholar]
- 21.Pokrovskaya ID, et al. , STEM tomography reveals that the canalicular system and alpha-granules remain separate compartments during early secretion stages in blood platelets. J Thromb Haemost, 2016. 14(3): p. 572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y, et al. , Autophagy regulates the cell cycle of murine HSPCs in a nutrient-dependent manner. Exp Hematol, 2015. 43(3): p. 229–42. [DOI] [PubMed] [Google Scholar]
- 23.Yadav S and Storrie B, The cellular basis of platelet secretion: Emerging structure/function relationships. Platelets, 2017. 28(2): p. 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]