Abstract
Refractory hypertension (RfHTN) is a phenotype of antihypertensive treatment failure defined as uncontrolled BP despite the use of effective doses of ≥5 antihypertensive medications including a long-acting thiazide-like diuretic (chlorthalidone) and a mineralocorticoid receptor antagonist (MRA). The degree of medication non-adherence is unknown among patients with RfHTN.
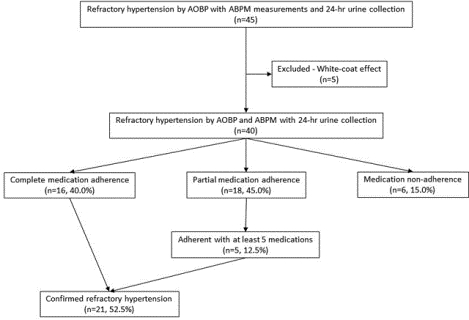
In this prospective evaluation, 54 patients with apparent RfHTN were recruited from the University of Alabama at Birmingham Hypertension Clinic after having uncontrolled BP at three or more clinic visits. All patients’ BP was evaluated by automated office BP (AOBP) and 24-hr ambulatory BP monitoring (ABPM; n=49). Antihypertensive medication adherence was determined by measuring 24-hr urine specimens for antihypertensive medications and their metabolites by high-performance liquid chromatography-tandem mass spectrometry (n=45). Of the 45 patients who completed 24-hr ABPM, 40 (88.9%) had confirmed RfHTN based on an elevated AOBP (≥130/80 mmHg), mean 24-hour ABP (≥125/75 mmHg) and mean awake (day-time) ABP (≥130/80 mmHg).
Out of the 40 fully evaluated patients with RfHTN, 16 (40.0%) were fully adherent with all prescribed medications. Eighteen (45.0%) patients were partially adherent and 6 (15.0%) had none of the prescribed agents detected in their urine. Of 18 patients who were partially adherent, 5 (12.5%) were adherent with at least 5 medications, including chlorthalidone and the MRA, consistent with true RfHTN.
Of patients identified as having apparent RfHTN, 52.5% were adherent with at least 5 antihypertensive medications, including chlorthalidone and a MRA, confirming true RfTHN. These findings validate RfHTN as a rare, but true phenotype of antihypertensive treatment failure.
Keywords: refractory hypertension, antihypertensive medication adherence, ambulatory blood pressure monitoring
Graphical Abstract
Summary
This study confirms the prevalence of true RfTHN based on adequate medication adherence. These findings validate RfHTN as a rare phenotype of true antihypertensive treatment failure.
Introduction
Refractory hypertension (RfHTN) is a phenotype of antihypertensive treatment failure defined as uncontrolled BP (≥ 130/80 mmHg), despite use of effective doses of 5 or more different classes of antihypertensive medications including a long-acting thiazide-like diuretic (chlorthalidone) and a mineralocorticoid receptor antagonist (MRA)1. Prior studies have indicated that RfHTN is rare, comprising only about 5% of patients referred to a hypertension specialty clinic for uncontrolled resistant hypertension (RHTN)2–4, which is defined as uncontrolled BP in spite of use of 3 or more antihypertensive agents, including a diuretic5. Compared with patients with controlled RHTN, patients with RfHTN are more likely to be female, African-American and have higher rates of cardiovascular complications, including stroke, left ventricular hypertrophy, and congestive heart failure2–4.
Patients may appear to be refractory to antihypertensive treatment based on the number of prescribed medications and having uncontrolled BP in clinic, i.e., apparent RfHTN, but in reality could have uncontrolled BP for other reasons, including inaccurate BP measurement, white-coat effect, inadequate or under treatment (inappropriate medication choice or dose of antihypertensive medications), and medication non-adherence. Multiple studies have shown these so-called pseudo-causes of treatment resistance to be common in patients with RHTN, and have to be fully ruled out before being able to confirm true RHTN.
White-coat effect, defined as uncontrolled BP in clinic but controlled out-of-clinic in treated hypertensive patients, is a very common pseudo-cause of treatment resistance, present in 37–49% of patients with otherwise confirmed RHTN6,7,8. In contrast, we have recently reported that white coat effect is uncommon in patients with RfHTN, affecting only 6.5% of such patients9. Poor medication adherence is a common cause of treatment resistance, having been reported in 47–53% patients with RHTN.10,11,12. To what degree, RfHTN is attributable to poor medication adherence has not been determined. Given that medication adherence decreases with increasing numbers of prescribed agents and increasing complexity of dosing regimens, we postulated that medication non-adherence would be high in patients with apparent RfHTN, given that by definition patients with apparent RfHTN require use of at least 5 different antihypertensive class of medications. To test that hypothesis we carried out the present study to determine antihypertensive medication adherence in patients with apparent RfTHN by measuring urinary drug or drug metabolite levels with high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Methods
Study data will be available upon request 1 year after completion of the funding grant (April 2021).
Study Population
Patients referred to the UAB Hypertension Clinic for uncontrolled resistant hypertension were recruited between April 2014 and July 2019. Patients were evaluated for secondary causes of hypertension, including hyperaldosteronism, pheochromocytoma, and renal artery stenosis as clinically indicated. Patients were eligible for enrollment if their automated office BP (AOBP) remained elevated ≥130/80 mm Hg after having been seen by a hypertension specialist for a minimum of 3 follow-up visits and after having been prescribed at least 5 antihypertensive agents from different classes, including chlorthalidone and a mineralocorticoid receptor antagonist (MRA). Exclusions included patients with chronic kidney disease (CKD) stage 4 or 5 (eGFR <30 ml/min/1.73m2) or pregnancy. The study was approved by the UAB Institutional Review Board and written informed consent was obtained from all participants.
Blood Pressure Measurement
Clinic Automated Office BP Measurement
AOBP was measured after at least 5 minutes of quiet rest in a sitting position with the back supported and the arm supported at heart level13–15. The office BP was measured using the BpTRU device, which automatically obtains 6 serial BP readings, one minute apart, before displaying the average of the last 5 readings. All BpTRU assessments were unattended, i.e., unobserved in clinic14,16–19. An appropriate sized cuff was used with a cuff bladder encircling at least 80% of the arm14,20. A BP cutoff of ≥ 130/80 mmHg for hypertension was used1,15.
Out-of-clinic 24-hr Ambulatory Blood Pressure Monitoring (ABPM)
An automated, noninvasive, oscillometric device (Oscar 2; Suntech Medical Inc, Morrisville, NC) was used to perform 24-hr ABPM21,22. Recordings were made every 20 minutes during the awake (Day-time) and every 30 minutes during the nighttime (asleep) phases of the 24-hr period. Awake and asleep times were determined by patient self-report21,22. 24-hr ABPM was determined to be valid if ≥ 80% of measurements were successful23, including at least 20 awake (Day-time) and 7 asleep (Night-time) valid BP measurements15. Uncontrolled 24-hr ABPM was defined as mean 24-hr BP ≥125/75 mmHg and mean awake (Day-time) BP ≥130/80 mmHg1,15. All patients were counselled to take all antihypertensive medications during 24-hr ABPM period.
Biochemical analysis
Serum electrolytes, blood urea nitrogen and creatinine were measured according to routine laboratory methods.
24-hr urine high-performance liquid chromatography-tandem mass spectrometry of antihypertensive medications and metabolites
All study patients collected a 24-hour urine sample for research purposes. Participants were advised to be adherent with antihypertensive medications, but were not informed that medication adherence was being tested in the collected urine samples to avoid a Hawthorne effect (e.g., change in behavior when being observed)24. Permission was obtained from the UAB Institutional Review Board to have the previously collected urine samples assayed for medication levels. The urine samples were stored and shipped at a temperature of −80° C to the National Centre for Adherence Testing (NCAT) Department of Metabolic Diseases and Chemical Pathology, University Hospitals of Leicester NHS Trust, Leicester, UK; where they were analyzed by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) to detect antihypertensive medications and metabolites as previously described25. Briefly, the samples were analyzed in batches of 20. Each sample was run in dilution and after extraction. Separation was performed using Agilent technologies Zorbax Elipse column C18 2.1 × 50 mm. The samples were then introduced by electrospray ionization to an Agilent technologies 6140 tandem mass spectrometer. The analytes of interest were confirmed by their unique mass to charge ratios.
The assay provides a binary qualitative result for presence or absence of medications in the urine. Patients whose urine analysis confirmed the presence of all medications prescribed were classified as totally adherent and those with fewer medications detected than prescribed were classified as partially adherent. Patients with no detectable drug or metabolite levels were classified as totally non-adherent.
Statistical Analysis
Descriptive analyses were performed to summarize the demographic and biochemical characteristics, as well as comorbidities and antihypertensive medication classes adherence in RfHTN. All analyses were performed using SPSS version 25.
Results
Fifty-four patients were enrolled into the study based on uncontrolled AOBP. Of these, 49 patients had valid 24-hr ABPM readings and 45 patients completed 24-hr urine collections to determine antihypertensive medication adherence (Figure 1). Out of the 45 patients who completed 24-hr ABPM and 24-hr urine collections, 40 (88.9%) had confirmed RfHTN based on an elevated AOBP (≥130/80 mmHg), mean 24-hr ABP (≥125/75 mmHg) and mean awake (day-time) ABP (≥130/80 mmHg), while 5 patients had a white-coat effect (Figure 1).
Figure 1.
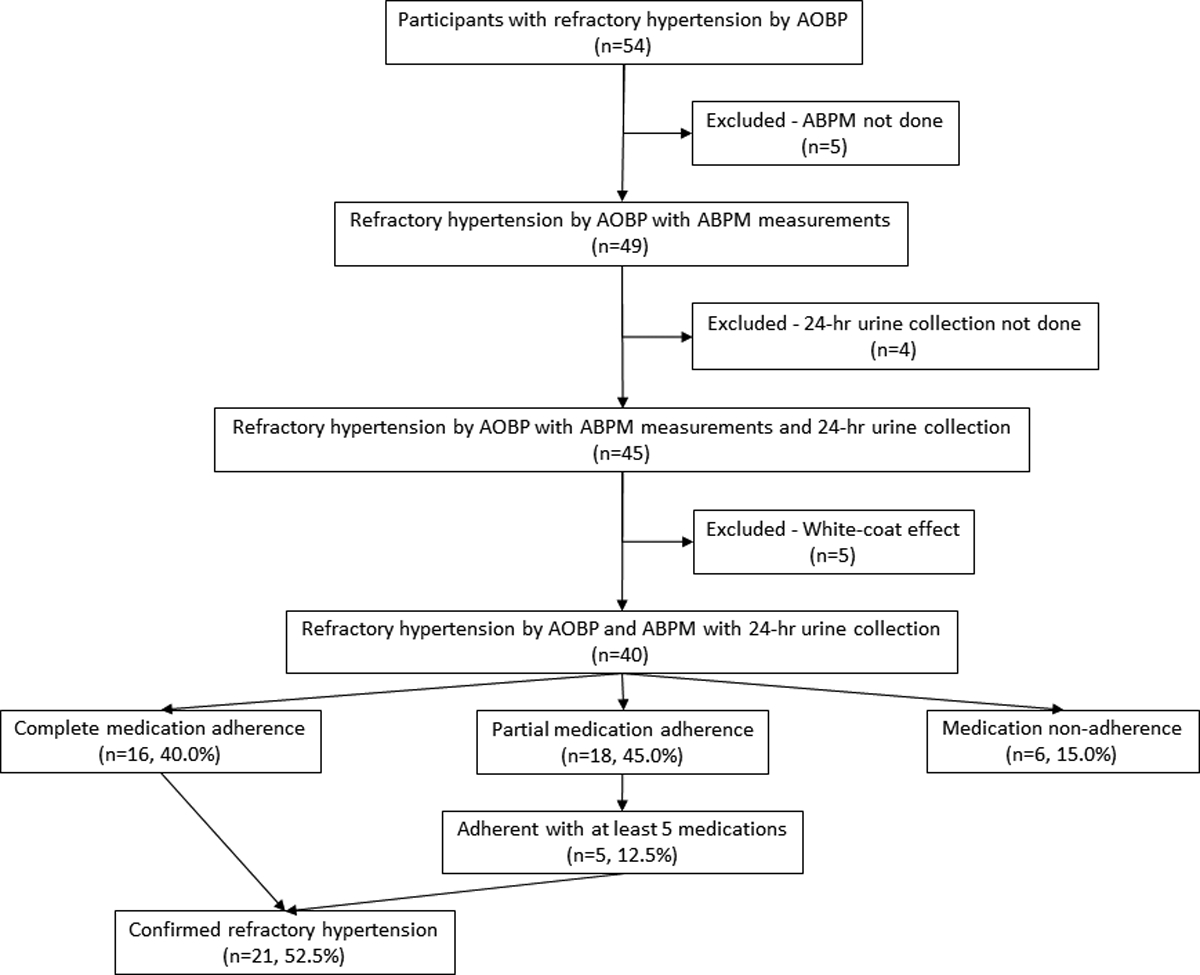
Schematic of enrolled study participants
The mean age of patients with RfHTN was 53.0±8.3 years, 65.0% were female and 85.0% were African American. The mean BMI was 36.0±6.4 kg/m2 (Table 1). The prevalence of dyslipidemia and diabetes was 52.5% and 50.0%, respectively (Table 1).
Table 1:
Baseline characteristics in patients with refractory hypertension
| Demographics | |
| Age (years) | 53.0 ± 8.3 |
| Females | 26 (65.0%) |
| African Americans | 34 (85.0%) |
| Comorbidities | |
| Current smoker | 8 (20.0%) |
| Current alcohol | 18 (45.0%) |
| Dyslipidemia | 21 (52.5%) |
| Congestive heart failure | 7 (17.5%) |
| Coronary artery disease | 6 (15.0%) |
| Diabetes | 20 (50.0%) |
| Thyroid disorder | 7 (17.5%) |
| Prior stroke/transient ischemic attack | 6 (15.0%) |
| Chronic obstructive pulmonary disease | 10 (25.0%) |
| Body mass index (kg/m2) | 36.0 ± 6.4 |
| Biochemistry | |
| Sodium (mMol/L) | 138.3 ± 2.9 |
| Potassium (mMol/L) | 4.0 ± 0.5 |
| Bicarbonate (mMol/L) | 28.1 ± 2.8 |
| Blood urea nitrogen (mg/dL) | 17.4 ± 7.8 |
| Creatinine (mg/dL) | 1.1 ± 0.4 |
| Clinic Vitals | |
| AOBP systolic (mmHg) | 151.1 ± 23.5 |
| AOBP diastolic (mmHg) | 89.9 ± 13.8 |
| AOBP heart rate (beats/minute) | 76.7 ± 12.0 |
| ABPM Measurements | |
| 24-hour systolic BP (mmHg) | 157.5 ± 21.4 |
| 24-hour diastolic BP (mmHg) | 89.5 ± 13.0 |
| 24-hour mean arterial pressure (mmHg) | 112.6 ± 14.6 |
| 24-hour pulse pressure (mmHg) | 68.1 ± 14.7 |
| 24-hour heart rate (beats/minute) | 75.4 ± 11.3 |
| Awake (day-time) systolic BP (mmHg) | 161.0 ± 21.2 |
| Awake (day-time) diastolic BP (mmHg) | 92.4 ± 14.4 |
| Awake (day-time) mean arterial pressure (mmHg) | 115.6 ± 15.4 |
| Awake (day-time) pulse pressure (mmHg) | 68.8 ± 14.6 |
| Awake (day-time) heart rate (beats/minute) | 76.8 ± 11.4 |
| Asleep (night-time) systolic BP (mmHg) | 150.3 ± 23.1 |
| Asleep (night-time) diastolic BP (mmHg) | 83.7 ± 13.8 |
| Asleep (night-time) mean arterial pressure (mmHg) | 106.3 ± 15.5 |
| Asleep (night-time) pulse pressure (mmHg) | 66.8 ± 16.2 |
| Asleep (night-time) heart rate (beats/minute) | 71.9 ± 12.8 |
AOBP, automated office blood pressure; ABPM, ambulatory blood pressure monitoring
The mean serum sodium was 138.3±2.9 mMol/L, serum potassium was 4.0±0.5 mMol/L and serum creatinine was 1.1±0.4 mg/dL (Table 1).
Clinic AOBP measurement
The mean systolic and diastolic AOBP were 151.1±23.5 / 89.9±13.8 mmHg. The mean AOBP heart rate were 76.7±12.0 beats/minute (Table 1).
Out-of-Clinic BP measurements by ABPM
The mean 24-hour systolic and diastolic BP were 157.5±21.4 / 89.5±13.0 mmHg. The mean 24-hour heart rate was 75.4±11.3 beats/minute. The mean awake (day-time) systolic and diastolic BP were 161.0±21.2 / 92.4±14.4 mmHg. The mean awake (day-time) heart rate was 76.8±11.4 beats/minute. The mean asleep (night-time) systolic and diastolic BP were 150.3±23.1 / 83.7±13.8 mmHg. The mean asleep (night-time) heart rate was 71.9±12.8 beats/minute (Table 1).
Antihypertensive medication adherence
Of the 40 patients with RfHTN who were fully evaluated, 16 (40.0%) were completely adherent with all of their prescribed antihypertensive medications; 18 (45.0%) were partially adherent, taking less than the number of prescribed agents; and 6 (15.0%) were completely non-adherent with any of prescribed medications (Figure 1). Out of the 18 patients who were partially adherent, 5 (12.5%) were adherent with 5 or more antihypertensive medications, including an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, a calcium channel blocker, chlorthalidone and MRA. Accordingly, 52.5% of the evaluated patients were adherent with 5 or more antihypertensive medications, including chlorthalidone and an MRA, consistent with true RfHTN. Overall, adherence for the different antihypertensive medication classes or agents was 79.2% for angiotensin-converting enzyme inhibitors, 75.0% for angiotensin II receptor blockers, 72.5% for calcium channel blockers, 70.0% for chlorthalidone, 67.5% for MRA (spironolactone or eplerenone), 57.1% for α-β blockers, and 64.7% for alpha-2 agonists (Table 2).
Table 2:
Medication adherence by class in patients with refractory hypertension
| Average number of medications prescribed | 5.7 ± 0.7 |
| Average number of medications detected | 3.9 ± 2.1 |
| Antihypertensive medication classes adherence | |
| Angiotensin converting enzyme inhibitors | 19/24 (79.2%) |
| (benazepril, lisinopril, quinapril) | |
| Angiotensin II receptor blockers | 12/16 (75.0%) |
| (azilsartan, candesartan, irbesartan, losartan, olmesartan, valsartan) | |
| Calcium channel blockers | 29/40 (72.5%) |
| (amlodipine, nifedipine) | |
| Thiazide-like diuretics | 28/40 (70.0%) |
| (chlorthalidone) | |
| Loop diuretics | 4/4 (100.0%) |
| (furosemide) | |
| Mineralocorticoid receptor antagonists | 27/40 (67.5%) |
| (spironolactone, eplerenone) | |
| α blockers | 1/3 (33.3%) |
| (doxazosin) | |
| β blockers | 7/9 (77.8%) |
| (bisoprolol, metoprolol, nebivolol) | |
| αβ blockers | 12/21 (57.1%) |
| (carvedilol, labetalol) | |
| α2 agonists | 11/17 (64.7%) |
| (clonidine, guanfacine) | |
| Nitric oxide vasodilators | 5/6 (83.3%) |
| (hydralazine) | |
| Potassium channel openers | 2/8 (25.0%) |
| (minoxidil) |
We classified patients with refractory hypertension based on antihypertensive medication adherence into complete, partial and non-adherence. The mean number of antihypertensive medications prescribed was 5.5±0.6, 5.8±0.7 and 6.2±0.8 in the above groups, respectively, while the mean number of antihypertensive medications detected was 5.5±0.6, 3.8±1.3 and 0 in the above groups, respectively (Figure 2).
Figure 2.
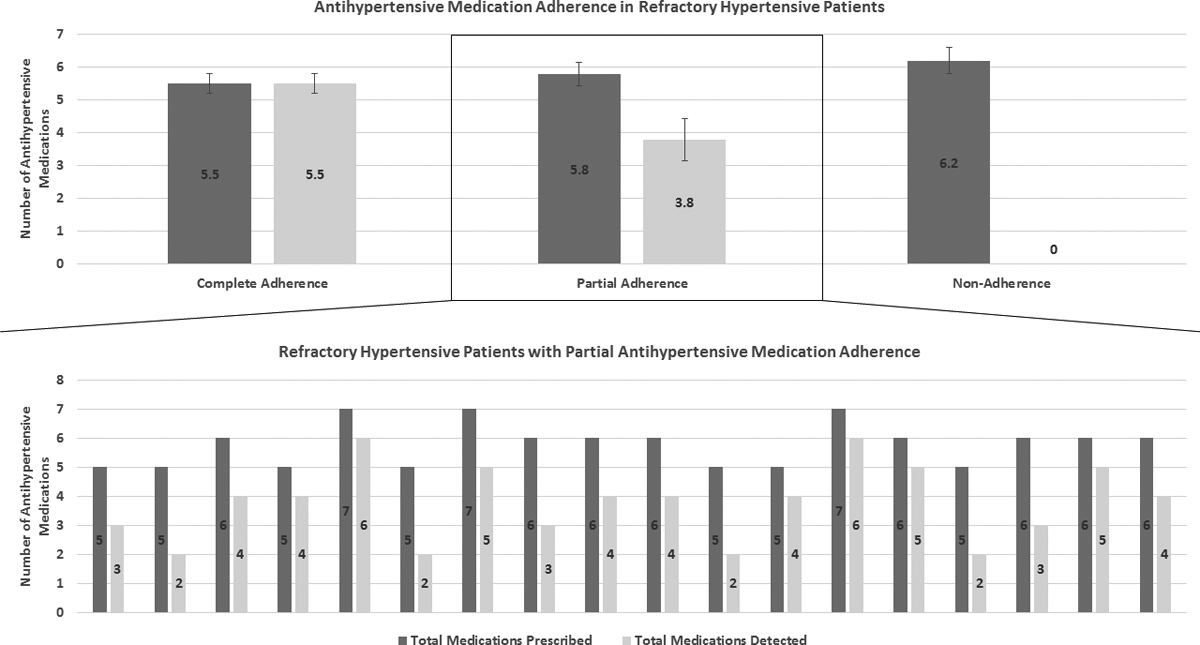
Antihypertensive medication adherence in refractory hypertensive patients
Discussion
This is the first study to prospectively determine antihypertensive medication adherence by 24-hr urine HP LC-MS/MS in patients with RfHTN confirmed by AOBP and 24-hr ABPM. We report that 40% of patients were fully adherent with all of their prescribed agents, with an additional 12% taking at least 5 of the agents, including chlorthalidone and MRA either spironolactone or eplerenone. This adherence rate is similar to the approximately 50% adherence rates observed in studies of patients with RHTN11,12,26, indicating that adherence was not markedly lower in patients with RfHTN in spite of a substantially larger number of prescribed agents.
Assessment of adherence with use of LC-MS/MS has demonstrated that 25–65% of the general population of hypertensive patients are non-adherent with prescribed medications25,27. For example, among 1348 hypertensive patients, Gupta et. al. reported that 30–40% were non-adherent with all of their antihypertensive medications28. Among patients with RHTN, multiple studies have reported high rates of poor medication adherence. Jung et. al., Strauch et. al., and Lawson et. al., utilizing LC-MS/MS analysis, all found that approximately 50% of patients with RHTN are fully adherent with prescribed antihypertensive regimens11,12,26. In another study of 36 patients with resistant hypertension with use of ≥4 antihypertensive medications (mean number of medications were 5.3 ± 1.4), Florczak et. al. showed partial adherence of 72.2% and total non-adherence of 13.9%29.
The current results are consistent with those studies, finding that 40% of patients with suspected RfHTN were taking all of their prescribed antihypertensive medications, while 52% were talking 5 or more, including the chlorthalidone and an MRA. The findings highlight the role of poor adherence plays in controlling high BP and the need to advance strategies to improve medication adherence, including patient and clinician education, use of cost-efficient and effective adherence monitoring programs, and development of well-tolerated affordable simplified treatment regimens, including broader availability of dual and even triple combination pills.
A large number of studies have demonstrated that pseudo-causes of apparent RHTN are common. These include poor BP measuring technique, resulting in falsely high clinic BP readings; under treatment, including inappropriate medications and low doses; white coat effect, and poor medication adherence. Only by fully ruling out each of these factors can true RHTN be confirmed. The same is true for RfHTN, but given the difficulty of fully excluding each of the pseudo-causes of apparent treatment resistance, it is difficult to determine the prevalence of true RfHTN. The current protocol rigorously ruled out each of these pseudo-causes of lack of BP control, resulting in confirmation of a small number of patients who are truly refractory to maximal antihypertensive treatment. Specifically, in the current protocol 1) use of proper BP technique by hypertension specialists in the clinic was ensured by use of an automated BP device (i.e, BpTRU) recording unattended serial BP readings; 2) under treatment was excluded by the rigorous definition of RfHTN, requiring prescription of effective doses (at least 50% of the recommended maximum dose for treating HTN) of 5 or more antihypertensive agents from different classes, including chlorthalidone and MRA either spironolactone or eplerenone; 3) patients with white coat effect were identified by 24-hr ambulatory BP monitoring and excluded from study participation; and 4) medication adherence was determined by direct measurement of 24 hour urinary drug or drug metabolites.
Having ruled out each of these pseudo-causes of lack of BP control, we found that approximately 52% of patients enrolled into the protocol for suspected RfHTN based on having uncontrolled clinic BP in spite of prescription of 5 or more antihypertensive agents were confirmed to have true RfHTN. The most common pseudo-cause of RfHTN was inadequate medication adherence, occurring in 48% of the patients tested. These findings highlight the importance of standardized office BP measurement, maximization of effective and well tolerated combination treatment regimens, measurement of out-of-office BP to identify white coat effect, and perhaps, most challenging, confirmation of adequate medication adherence in diagnosing RfHTN.
The current study demonstrates that after eliminating pseudo-causes of apparent treatment resistance, there remains a small cohort of patients truly refractory to antihypertensive treatment. Such patients are extremely uncommon, but may be informative in identifying underlying causes of treatment failure. Prior studies from our laboratory suggest that patients with true RfHTN may be distinct from the larger population of patients with RHTN in that their treatment resistance is not volume dependent, that is, not attributable to persistent intravascular fluid retention, but instead, may be secondary to heightened sympathetic activation4,30. Additional studies are needed to confirm this mechanistic distinction and to develop effective pharmacological and device based treatments for this rare, but extremely high-risk population of hypertensive patients.
Strengths of the current study include its prospective design, rigorous confirmation of RfHTN status by AOBP and 24-hr ABPM and detection of antihypertensive medications by 24-hr urine LC-MS/MS, the current preferred method for detection of antihypertensive medications in patients. Weaknesses include the qualitative determination of drug and drug metabolite levels as opposed to a quantitative assessment, which may have precluded a more nuanced interpretation of drug exposure. Further assessment was limited to a single measurement uncoordinated with medication dosing history. Furthermore, varied half-lives of antihypertensive medications would make it difficult to assess when the last medication dose was taken.
In conclusion, we report, based on direct detection of urinary drug and drug metabolite levels, that 40.0% of patients with apparent RfHTN were fully adherent with all of their prescribed antihypertensive agents; 45.0% were partially adherent (with 12.5% being adherent with at least 5 antihypertensive medications, including chlorthalidone and a MRA); only 15.0% of patients presenting with apparent RfHTN had none of the prescribed agents detected. A total of, 52.5% of the patients with apparent RfHTN were adherent with 5 or more antihypertensive medications, including chlorthalidone and a MRA, consistent with having true RfHTN.
Perspectives
Of patients identified as having apparent RfHTN, 52.5% were adherent with at least 5 antihypertensive medications, including chlorthalidone and a MRA. The findings indicate that the degree medication non-adherence in this population is similar to that reported in RHTN in general. The findings both confirm that poor medication adherence is a common among patients with suspected RfHTN, but also, after ruling out all causes of possible pseudo-resistance to treatment, demonstrate that there remains a small number of patients truly refractory to maximal antihypertensive treatment. All patients with refractory hypertension should be evaluated for all pseudo causes of apparent refractory hypertension, most importantly medication adherence. The clinical management and therapeutic decisions in these patients should be focused on improving medication adherence by simplifying the antihypertensive drug regimen, in part by using fixed dose combinations and intradermal patch dosing.
Supplementary Material
Novelty and Significance.
What is new: This is the first study to evaluate prevalence of true refractory hypertension.
What is relevant: This study shows that 52.5% of patients with apparent refractory hypertension, were adherent with 5 antihypertensive medications, including a long-acting thiazide like diuretic and a mineralocorticoid receptor antagonist.
Sources of funding
The National Institutes of Health (NIH R01 HL113004 and 2T32HL007457-36A1) and the American Heart Association Strategically Focused Research Network (AHA 5SFRN2390002) supported this research.
Footnotes
Disclosures: None
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 2.Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, Cofield SS, Oparil S, Calhoun DA. Refractory hypertension: definition, prevalence, and patient characteristics. Journal of clinical hypertension. 2012;14(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoun DA, Booth JN 3rd Oparil S, Irvin MR, Shimbo D, Lackland DT, Howard G, Safford MM, Muntner P. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population-based cohort. Hypertension. 2014;63(3):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudenbostel T, Acelajado MC, Pisoni R, Li P, Oparil S, Calhoun DA. Refractory Hypertension: Evidence of Heightened Sympathetic Activity as a Cause of Antihypertensive Treatment Failure. Hypertension. 2015;66(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey RM, Calhoun DA, Bakris GL, et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72(5):e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902. [DOI] [PubMed] [Google Scholar]
- 7.Muxfeldt ES, Bloch KV, Nogueira Ada R, Salles GF. True resistant hypertension: is it possible to be recognized in the office? American journal of hypertension. 2005;18(12 Pt 1):1534–1540. [DOI] [PubMed] [Google Scholar]
- 8.Modolo R, Ruggeri Barbaro N, de Faria AP, Rodrigues Sabbatini A, Paganelli MO, Fontana V, Moreno H. The white-coat effect is an independent predictor of myocardial ischemia in resistant hypertension. Blood pressure. 2014;23(5):276–280. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui M, Judd EK, Oparil S, Calhoun DA. White-Coat Effect Is Uncommon in Patients With Refractory Hypertension. Hypertension. 2017;70(3):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grigoryan L, Pavlik VN, Hyman DJ. Characteristics, drug combinations and dosages of primary care patients with uncontrolled ambulatory blood pressure and high medication adherence. J Am Soc Hypertens. 2013;7(6):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. Journal of hypertension. 2013;31(4):766–774. [DOI] [PubMed] [Google Scholar]
- 12.Strauch B, Petrak O, Zelinka T, Rosa J, Somloova Z, Indra T, Chytil L, Maresova V, Kurcova I, Holaj R, Wichterle D, Widimsky J Jr. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. Journal of hypertension. 2013;31(12):2455–2461. [DOI] [PubMed] [Google Scholar]
- 13.Terent A, Breig-Asberg E. Epidemiological perspective of body position and arm level in blood pressure measurement. Blood pressure. 1994;3(3):156–163. [DOI] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. [DOI] [PubMed] [Google Scholar]
- 15.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT Jr. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension. 2019;73(5):e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC cardiovascular disorders. 2005;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JM, Mattu GS, Perry TL Jr, Gelferc ME, Strange KD, Zorn A, Chen Y. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood pressure monitoring. 2001;6(3):161–165. [DOI] [PubMed] [Google Scholar]
- 18.Mattu GS, Heran BS, Wright JM. Overall accuracy of the BpTRU--an automated electronic blood pressure device. Blood pressure monitoring. 2004;9(1):47–52. [DOI] [PubMed] [Google Scholar]
- 19.Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood pressure monitoring. 2006;11(1):37–42. [DOI] [PubMed] [Google Scholar]
- 20.Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation. 1983;68(4):763–766. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. Journal of hypertension. 2013;31(9):1731–1768. [DOI] [PubMed] [Google Scholar]
- 22.Pickering T Recommendations for the use of home (self) and ambulatory blood pressure monitoring. American Society of Hypertension Ad Hoc Panel. American journal of hypertension. 1996;9(1):1–11. [DOI] [PubMed] [Google Scholar]
- 23.de la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53(3):466–472. [DOI] [PubMed] [Google Scholar]
- 24.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, Samani NJ, Gupta P, Madira W, Stanley A, Williams B. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100(11):855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson AJ, Shipman KE, George S, Dasgupta I. A Novel ‘Dilute-and-Shoot’ Liquid Chromatography-Tandem Mass Spectrometry Method for the Screening of Antihypertensive Drugs in Urine. J Anal Toxicol. 2016;40(1):17–27. [DOI] [PubMed] [Google Scholar]
- 27.Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R, Solar M. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34(1):87–90. [DOI] [PubMed] [Google Scholar]
- 28.Gupta P, Patel P, Strauch B, et al. Risk Factors for Nonadherence to Antihypertensive Treatment. Hypertension. 2017;69(6):1113–1120. [DOI] [PubMed] [Google Scholar]
- 29.Florczak E, Tokarczyk B, Warchol-Celinska E, Szwench-Pietrasz E, Prejbisz A, Gosk M, Kabat M, Narkiewicz K, Januszewicz A, Kala M. Assessment of adherence to treatment in patients with resistant hypertension using toxicological serum analysis. A subgroup evaluation of the RESIST-POL study. Pol Arch Med Wewn. 2015;125(1–2):65–72. [DOI] [PubMed] [Google Scholar]
- 30.Velasco A, Siddiqui M, Kreps E, Kolakalapudi P, Dudenbostel T, Arora G, Judd EK, Prabhu SD, Lloyd SG, Oparil S, Calhoun DA. Refractory Hypertension Is not Attributable to Intravascular Fluid Retention as Determined by Intracardiac Volumes. Hypertension. 2018;72(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


