Abstract
Anhedonia emerges in some people after psychological trauma, reflected by a loss of interest, diminished affect, and detachment. Structural abnormalities in specific neural pathways at the time of trauma may influence the development of these posttraumatic anhedonia (PTA) symptoms. In this prospective study, we determined whether white matter connectivity at around one month post-trauma predicts PTA and other PTSD symptoms at six months post-trauma. Thirty men and women aged 19–62 were recruited from the emergency department of a Level I trauma center. Participants received diffusion tensor imaging at approximately one month post-trauma and clinical assessments at one and six months post-trauma. Probabilistic tractography was used to examine connectivity of select pathways. A replication sample (N = 57) in an independent, cross-sectional dataset of traumatized women was similarly analyzed. Logistic regression results indicated that, after accounting for early PTSD symptoms (at one month) and other clinical risk factors, the integrity of the uncinate fasciculus (UF) uniquely predicted the presence of PTA at six months post-trauma (Beta = −225.6, p < .05). Together, these factors contributed to 76% of the variance in PTA. Integrity of the UF also predicted re-experiencing PTSD symptoms at six months post-trauma. These results were supported in our replication analyses. Our findings indicate that the integrity of the UF around 1 month post-trauma affects vulnerability for the development of anhedonic PTSD symptoms as well as re-experiencing symptoms. Connectivity of this amygdalaventromedial prefrontal pathway appears to be a salient predictor of anhedonia, above and beyond clinical risk factors.
1. Introduction
Anhedonia, or the inability to experience pleasure, emerges in some people in the aftermath of psychological trauma. Anhedonia is a transdiagnostic phenomenon that is observed across various psychiatric disorders, including depression, PTSD, and schizophrenia (Krynicki et al., 2018; Lambert et al., 2018; Vujanovic et al., 2017). Specific symptoms of post-traumatic stress disorder (PTSD) reflect anhedonia: feelings of detachment from others, diminished emotional responsiveness, and decreased interest in activities (American Psychiatric Association, 1994). They are highly correlated with anhedonic symptoms of depression (Kashdan et al., 2006), but are also distinct, and factor analytic studies indicate that they constitute a unique dimension of PTSD (Liu et al., 2014; Yang, H. et al., 2017). They are thus referred to here as posttraumatic anhedonia (PTA). These anhedonic symptoms differ from other symptoms of PTSD, given that they reflect abnormalities in appetitive response rather than fear or negative affect (Bryant et al., 2017). Although these symptoms overlap with anhedonic features of depression, a primary difference is the presence of numbness and social detachment. In prospective studies, the presence of these symptoms immediately post-trauma has been shown to be one of the most sensitive predictors of chronic PTSD (Feeny et al., 2000; Malta et al., 2009) at rates of ∼94% (North et al., 1999). PTA symptoms are more prevalent in people with childhood sexual and emotional abuse histories (Frewen et al., 2012) and have been associated with severe functional impairment (Breslau et al., 2005; Maguen et al., 2009; Miller et al., 2008; North et al., 1999; Solberg et al., 2015), self-reported distress (Kashdan et al., 2007), and suicide risk (Spitzer et al., 2017). People with more PTA also have a higher incidence of co-morbid psychosis (Kashdan et al., 2006) and major depressive disorder (Feeny et al., 2000; Malta et al., 2009) as well as problematic alcohol, nicotine or other substance use (Debell et al., 2014; Nickerson et al., 2014). As such, PTA is hypothesized to play a role in the development of substance use problems (Vujanovic et al., 2017).
The neural underpinnings of PTA symptom emergence after trauma are still unknown. Findings from studies of depressed populations suggest that anhedonia may result from structural abnormalities in neural pathways that subserve appetitive functions. A recent review of white matter associations with reward abnormalities in depression indicates that three tracts have consistently emerged in the literature: the cingulum bundle (CB), the uncinate fasciculus (UF), and the medial forebrain bundle (MFB) (Bracht et al., 2015). The MFB connects striatal regions, associated with reward, to regulatory prefrontal brain regions. The CB and UF tracts connect these prefrontal brain regions (including the ventromedial prefrontal cortex, vmPFC and dorsal anterior cingulate cortex, dACC) to limbic regions: the hippocampus and amygdala. In addition to its well-known role in fear learning, the amygdala modulates appetitive responses—damage to the amygdala-prefrontal pathway interferes with reward valuation as well as goal-directed behavior in the presence of rewarding stimuli (Baxter and Murray, 2002). Structural damage to the amygdala-vmPFC (UF) pathway has been linked to other disturbances in reward processing, such as apathy (Hollocks et al., 2015) and negative symptoms (Kitis et al., 2012; Sigmundsson et al., 2001). Thus, structural disruptions in these pathways may have functional consequences extending beyond anhedonia and PTSD symptoms, affecting emotional response and regulation more broadly.
Notably, few prospective studies have investigated structural biomarkers of overall PTSD symptom development, but the extant data suggests the relevance of disrupted limbic-prefrontal pathways. One study of motor vehicle accident survivors observed white matter changes (lower fractional anisotropy; FA) in the vmPFC, ACC, orbitofrontal cortex (OFC), midbrain and middle temporal cortex in those who developed PTSD compared to trauma-exposed controls at six months post-trauma (Sun et al., 2013). Our prior cross-sectional research suggests that the integrity of the CB (Fani et al., 2012) and UF (Almli et al., 2014) are uniquely associated with PTSD; the relevance of these pathways in PTSD have also been highlighted in meta-analyses (Daniels et al., 2013). Decrements in these particular tracts at the time of trauma may contribute to PTSD symptom development. A recent large-scale study highlights the utility of examining these white matter biomarkers in prospective studies of psychopathology; the length of the CB in childhood uniquely predicted nearly a third of the variance in behavioral and emotion dysregulation in adolescence (Bertocci et al., 2016). Genetic studies indicate that the integrity of these three tracts is highly heritable (Budisavljevic et al., 2016; Gatt et al., 2012; Jahanshad et al., 2013), and in combination with trauma, can be a substrate through which PTA develops. Taken together, these studies indicate that these pathways are highly promising candidate biomarkers for the emergence of anhedonia following trauma.
To our knowledge, no prospective studies have examined how the structural integrity of these pathways at around the time of trauma may contribute to the later development of anhedonic responses in traumatized people. The interaction of trauma with structural vulnerabilities in these tracts may give rise to PTA, but this has not yet been investigated. Thus, we assessed the integrity of the CB, UF and MFB in patients recruited from the emergency room, who had just experienced trauma. We examined whether the integrity of these tracts at one month post-trauma predicted PTA at six months post-trauma. Our objective was to test whether these paths are relevant biological markers of vulnerability for the development of PTA after accounting for variance associated with clinical risk factors, such as prior trauma exposure and PTSD symptoms, including anhedonia. We hypothesized that poorer structural connectivity of these pathways would predict future PTA symptoms. We also investigated how the integrity of these tracts predicted overall PTSD symptom severity and depressive anhedonia at six months post-trauma. Finally, we sought to replicate our findings in data collected from a larger, independent, cross-sectional study of traumatized people.
2. Methods and materials
2.1. Participants
A total of 38 men and women aged 19–62 years (Mean = 33.7, SD = 12.5) were recruited as part of a larger prospective study conducted in the emergency department (ED) of a Level 1 trauma center. Eligible patients were approached in the ED after initial medical evaluation, appropriate laboratory testing, and medical clearance had occurred. Once a patient signed informed consent, trained assessors collected demographic information and assessments that included information on prior trauma, substance abuse, current and past depression and PTSD symptoms, and details concerning the presenting trauma. Patients were queried about past and current medical conditions and medications. Participants who had experienced a DSM-IV criterion A trauma in the past 24 h were eligible for the study but were not included if they had current suicidal ideation or attempt in last 3 months, current intoxication, loss of consciousness as a result or the trauma, or a history of mania or psychotic disorder. Participants were also assessed for traumatic brain injury by ED physicians using the Glasgow Coma Scale, and people with scores of less than 15 were excluded from the study.
All MRI eligible participants were invited for an MRI scan one month post-trauma and were scanned within 2–3 weeks, on average 54 days after trauma exposure, as described previously (Stevens et al., 2013). Clinical risk factors associated with PTSD were assessed, including prior trauma exposure, peritraumatic dissociation, and childhood maltreatment (see Table 1). Diffusion tensor imaging (DTI) was conducted in 34 participants; 4 participants were lost to follow-up at 6 months, leaving full data for 30 participants. An independent sample of cross-sectional clinical and DTI data of 57 women aged 22–62 years (M = 39.4, SD = 10.6) from a large-scale study of trauma was used for replication analyses. Recruitment and data collection for this study, as well as sample characteristics, are described in the Supplement. The Institutional Review Board of Emory University approved all study procedures.
Table 1.
Demographic and clinical characteristics.
| No PTA at 6 months (n=21) | PTA (n=10) | F | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 33.1 (12.5) | 34.9 (13.1) | .1 |
| % (n) | % (n) | Chi square | |
| Males | 60 (12) | 50 (5) | .2 |
| Race | 3.9 | ||
| Black | 60 (12) | 90 (9) | |
| White/Caucasian | 25 (5) | 0 | |
| Mixed race | 10 (2) | 10 (1) | |
| Fisher’s Exact | |||
| Education | 3.1 | ||
| Some high school | 5 (1) | 0 | |
| High school graduate | 20 (4) | 40 (4) | |
| Associate’s degree/some college | 55 (11) | 60 (6) | |
| Bachelor’s degree | 15 (3) | 0 | |
| Master’s degree or higher | 5 (1) | 0 | |
| Clinical risk factors | Mean (SD) | Mean (SD) | F |
| Peritraumatic dissociation score | 2.4 (1.5) | 2.8 (1.5) | .5 |
| Fisher’s Exact | |||
| % endorsed (n) | % endorsed (n) | ||
| Childhood maltreatment | 35 (7) | 10 (1) | 2.1 |
| Prior trauma history | 55 (11) | 60 (6) | .1 |
PTA = Posttraumatic anhedonia.
2.2. Six month follow-up assessment
Participants returned at one and six months following their first ED visit for assessment sessions that required 60–90 min. In addition to receiving the aforementioned clinical assessments, they were asked about whether they had sought psychiatric treatment in the past six months; these data were available for 25 participants and out of these, only two endorsed having received treatment. Data from the six month timepoint were used as outcome variables in statistical analyses.
2.3. PTSD/PTA assessment
We examined PSS total score and re-experiencing, avoidance, and hyperarousal subscale scores at one and six month timepoints. The PTA subscale was created from the PSS, as detailed in the Supplement. For these items at six months post-trauma, a Cronbach’s alpha of .89 was observed, indicating high reliability. Anhedonia items of the BDI-II were also summed to create a depressive anhedonia subscale score in accordance with two factor models of the BDI (Kashdan et al., 2006).
At six months, 10 participants endorsed any PTA (Mean PTA score = 1.5, SD = 2.8, Mode = 0). Given the truncated range and distribution of PTA scores, we classified participants into two groups; those with PTA (n = 10) and without PTA (n = 20) at 6 months post-trauma. PTSD symptom scores between the two groups and in the group as a whole at one and 6 months post-trauma are detailed in Table 2. Anhedonia items of the BDI were endorsed with a similar frequency as PTA; a total of 13 participants endorsed any depressive anhedonic symptoms. As such, we also created groups of those with depressive anhedonia (n = 13) and no depressive anhedonia (n = 17) at 6 months post-trauma for logistic regression analyses. Given the significant overlap of depressive anhedonia with PTA, we also created a composite anhedonia score that represented the averaged summed score of both measures; half of the participants endorsed anhedonia at 6 months, whereas the other half did not. Thus, a similar, categorical anhedonia variable was entered into logistic regression analyses.
Table 2.
a. PTSD symptoms for anhedonic and non-anhedonic groups 6 months posttrauma. b. PTSD symptoms at 1 and 6 months post-trauma (all participants).
| (a) | |||
|---|---|---|---|
| PTSD Symptom Scale | No PTA at 6 months (n=20) | PTA (n=10) | F |
| Mean (SD) | Mean (SD) | ||
| Re-experiencing | 1.4 (2.4) | 6.1 (3.5) | 19.4** |
| Avoidance/numbing | 1 (1.7) | 9 (5.2) | 39.8** |
| Hyperarousal | 2.5 (2.5) | 8.9 (3.9) | 30.4** |
| Posttraumatic anhedonia | 0 | 4.7 (2.9) | 52.8** |
| Total Score | 4.8 (5.3) | 23.9 (10.8) | 42.8** |
| (b) | |||
| PTSD Symptom Scale | 1 month | 6 months | |
| Mean (SD) | Mean (SD) | ||
| Re-experiencing | 4.9 (4.5) | 2.9 (3.6) | |
| Avoidance/numbing | 6.5 (5.6) | 3.6 (4.9) | |
| Hyperarousal | 6.6 (4.3) | 4.6 (4.3) | |
| Posttraumatic anhedonia | 2.2 (2.6) | 1.6 (2.8) | |
| Total Score | 18 (13.3) | 11.2 (11.8) | |
p < .05.
p < .01.
2.4. DTI acquisition and processing
Scanning was conducted on a research-dedicated Siemens 3-T TIM-Trio scanner at Emory University Hospital. Acquisition parameters are detailed in the Supplement. Correction for head motion and eddy current distortion was performed for data from each participant using an automated affine registration algorithm. Both diffusion-weighted and T1 images were skull-stripped using the FSL brain extraction tool (Smith, 2002). FA maps were generated using the DTIfit in the FMRIB Diffusion Toolbox. Markov Chain Monte Carlo sampling was used to calculate within-voxel probability density functions of the principal diffusion direction using FSL’s BEDPOSTX tool, which also accounts for the possibility of crossing fibers within a voxel (Behrens et al., 2003).
2.5. Probabilistic tractography
To examine structural connectivity, probabilistic fiber tracking was conducted with PROBTRACKX implemented in FSL; this method repeatedly samples the distribution at each voxel to produce ‘streamlines’ that connect voxels from selected seed regions (5000 streamline samples, 0.5 mm step length, curvature threshold = 0.2). Masks of the CB and UF were created using the JHU White Matter Tractography Atlas (Mori et al., 2005) and used as an anatomical waypoint for these paths; a separate exclusion mask was created to eliminate the likelihood of pathways in irrelevant white matter tracts, gray matter regions and CSF. To create probabilistic streamlines for MFB pathways, we selected the nucleus accumbens and orbitofrontal cortex as seed regions using the Harvard-Oxford Subcortical Structural Atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html. The hippocampus and ACC, and the amygdala and vmPFC, were used as seed regions for CB and UF pathways, respectively, in accordance with our prior studies (Fani et al., 2015; Fani et al., 2016). The vmPFC was defined using a 6 mm sphere centered at Talairach coordinates x = 4, y = 42, z = −5, as described previously(Jovanovic et al., 2013). Only streamlines that passed through seed regions and the waypoint were retained. The resulting streamlines were transformed to Montreal Neurological Institute (MNI) space. All data were visually inspected for major artifacts before being included in analyses. Fractional anisotropy (FA) was used as our measure of tract integrity, given that earlier studies have indicated it to be a reliable assessment of microstructural integrity of white matter fibers (Fox et al., 2011). Individual FA maps were linearly aligned to a standard MNI brain using FLIRT; mean FA for all streamlines was then extracted and entered into statistical analyses. Example probabilistic paths for the CB, UF and MFB are illustrated in Fig. 1.
Fig. 1.

Example probabilistic tracts of the uncinate fasciculus (a), cingulum bundle (b), and medial forebrain bundle (c).
2.6. Data analysis
Using IBM SPSS version 24, hierarchical logistic regression using the enter method was conducted to examine the independent contributions of clinical factors and white matter integrity at the initial assessment to the presence/absence of PTA at six months. Childhood maltreatment, prior trauma history, and peritraumatic dissociation were entered into the first step, due to their relevance to PTA symptoms (DePierro et al., 2017; Dillon et al., 2009)(Frewen et al., 2015); we also included PTA at one month post-trauma in this step. We included overall PTSD symptoms at 1 month in a separate model, in order to reduce collinearity within each model while accounting for these effects. Similar variables were used in replication analyses, detailed in Supplemental Methods. Mean FA values of the CB, UF and MFB were entered into the second step; the left and right CB were entered separately into models, given the large size of this tract. A similar regression model was used to examine whether these clinical and neuroimaging variables were predictive of PTA at 1 month. Using effect sizes from our prior study (Fani et al., 2012) and 80% power, we calculated an a priori needed sample size of 10 per group. A threshold of p < .05, two-tailed, was used to define statistical significance.
Secondarily, we conducted logistic and linear regression analyses to examine whether these clinical risk factors and white matter integrity predicted other types of PTSD symptoms (re-experiencing, hyperarousal, avoidance, and total symptoms), a PTSD diagnosis, depression and depressive anhedonia at 6 months post-trauma. We also examined intercorrelations of white matter and clinical variables (Supplemental Table 3).
3. Results
Predictors of Posttraumatic Anhedonia at Six Months Post Trauma.
Results of logistic regression that included PTA at 6 months as the outcome variable (presence or absence), clinical risk factors (PTA at one month post-trauma, childhood maltreatment history, prior trauma history, peritraumatic dissociation) and integrity of the CB, MFB and UF as predictors indicated a significant overall model (X2 = 19.5, p = .01) that explained 66% of the variance in PTA outcome (Nagelkerke R2 = 0.66; see Table 3a). Integrity of the UF emerged as the only significant predictor of PTA (Beta = −110.4, p = .04), with lower integrity predicting the presence of PTA at 6 months (see Fig. 2); Cohen’s d= −1.04. The overall model (X2 = 19.5, p < .01) and variance associated with UF integrity (Beta = −225.6, p = .04) was relatively unchanged when age (Beta = −0.001, p = .99) was added to the analysis. A similar model that included our composite anhedonia score as the outcome variable was not significant (X2 = 0.13, p > .05, Nagelkerke R2 = 0.006). When analyses were repeated with total PTSD symptoms (at one month post-trauma) as a regressor (instead of PTA at one month post-trauma), the results remained significant (X2 = 23.9, p < .01, Nagelkerke R2 = 0.76); integrity of the UF was the only significant predictor of PTA (Beta = −225.6, p = .04).
Table 3.
a. Predictors of posttraumatic anhedonia at six months post-trauma. b. Predictors of Re-experiencing PTSD symptoms at six months post-trauma.
| (a) | ||||
|---|---|---|---|---|
| B | SE | Wald | p | |
| Prior trauma history | 1.8 | 1.6 | 1.2 | 0.27 |
| Peritraumatic dissociation (severity) | −.1 | 0.6 | .0 | 0.91 |
| Childhood maltreatment | 2.8 | 1.9 | 2.1 | 0.15 |
| PTSD total symptoms at 1 month | 0.5 | 0.3 | 3.1 | 0.08 |
| Left cingulum (FA) | −29.1 | 30.9 | 0.9 | 0.34 |
| Right cingulum (FA) | 31.1 | 29.9 | 1.1 | 0.3 |
| Uncinate Fasciculus (FA) | −110.4 | 54.6 | 4.1 | 0.04* |
| Medial Forebrain Bundle (FA) | 46.5 | 58.4 | 0.6 | 0.43 |
| (b) | ||||
| B | SE | β | p | |
| Prior trauma history | .49 | .87 | .07 | .58 |
| Peritraumatic dissociation (severity) | −.02 | .32 | −.01 | .95 |
| Childhood maltreatment | −2.04 | 1.01 | −.26 | .06 |
| PTSD total symptoms at 1 month | .17 | .04 | .65 | <.000* |
| Left cingulum (FA) | −15.03 | 13.76 | −.18 | .29 |
| Right cingulum (FA) | 21.87 | 14.39 | .27 | .14 |
| Uncinate Fasciculus (FA) | −45.49 | 18.99 | −.34 | .03* |
| Medial Forebrain Bundle (FA) | −49.65 | 32.32 | −1.5 | .14 |
PTA = Post-trauma anhedonia, FA=fractional anisotropy.
p < .05.
Fig. 2.
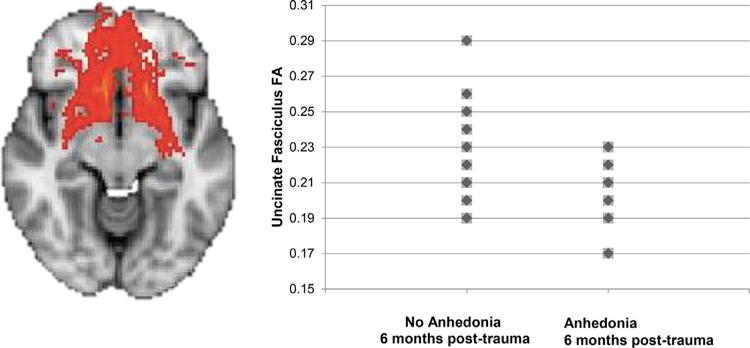
Uncinate fasciculus integrity (fractional anisotropy) around one month after trauma exposure is poorer in those who developed posttraumatic anhedonia symptoms at 6 months post-trauma, compared to those who did not develop posttraumatic anhedonia (four points overlap).
Predictors of Posttraumatic Anhedonia at One Month Post Trauma.
Analyses were repeated to examine whether clinical and white matter variables predicted PTA at one month post-trauma. Not surprisingly, non-anhedonic PTSD symptoms at one month significantly predicted PTA at 1 month (Beta = 0.16, p < .001), contributing to a significant overall model (R2 = 0.71; F8, 21 = 6.5; p < .001); however, other clinical and white matter variables were not significant predictors (all ps > .05).
Replication analyses.
Results of logistic regression that included PTA as the outcome variable (presence or absence), clinical risk factors (depressive symptoms, childhood maltreatment and adult trauma exposure, dissociation) and integrity of the CB, MFB and UF as predictors indicated a significant overall model (X2 = 14.2, p < .01) that explained 57% of the variance in PTA outcome (Nagelkerke R2 = 0.57, see Supplemental Table 2). Integrity of the UF (Beta = −72.04, p = .02) as well as the left CB (Beta = 40.18, p = .01) emerged as significant predictors of PTA; not surprisingly, depression symptoms were also significant predictors (Beta = 0.14, p = .01).
Predictors of PTSD at Six Months Post Trauma.
Results of linear and logistic regression analyses indicated that white matter integrity of these tracts did not significantly predict avoidance and hyperarousal symptoms (ps > .05). However, UF integrity marginally contributed to the presence of a PTSD diagnosis at 6 months (Beta = −142.9, p = .06), and no other factors approached significance in this model, which was statistically significant (X2 = 19.5, p = .01, Nagelkerke R2 = 0.7; see Table 3b). A similar linear regression that excluded PTA symptoms from the total PSS score at 6 months was significant (R2 = 0.75; F8, 21 = 5.9; p < .001), but the contribution of white matter, including UF integrity, was not significant (Beta = −72.7, p = .17). Linear regression analyses that included the same clinical risk factors in the first step and white matter integrity in the second step revealed that UF integrity significantly predicted re-experiencing symptoms (Beta = −45.5, p = .03; Table 3b), with the only other significant predictor being PTSD symptoms at one month (Beta = 0.17, p < .001), with this model predicting 75% of the variance in re-experiencing symptoms at 6 months (R2 = 0.75; F8, 21 = 7.7; p < .001).
4. Discussion
We examined whether the connectivity of three different white matter pathways, the UF, CB and MFB, predicted the development of anhedonia at six months post-trauma. Our findings showed that UF integrity robustly predicted the presence of PTA at 6 months, even after accounting for clinical risk factors, such as prior trauma exposure and PTSD symptoms. Prospective study findings were replicated in an independent cross-sectional study sample of traumatized people, indicating that these results are not likely to be attributable to chance, and are observable in a larger sample with chronic trauma and PTSD symptoms. The integrity of the UF also predicted re-experiencing symptoms at six months. These data collectively indicate that integrity of the UF is a potent predictor of vulnerability for PTSD symptom development, specifically, anhedonia and re-experiencing symptoms.
Anhedonic PTSD symptoms reflect abnormalities in appetitive functioning, a domain that has been largely neglected in PTSD research. Until now, little has been known about the neurobiological mechanisms that give rise to PTA—our findings represent the first study to examine these mechanisms using a prospective design. These data highlight the salience of fronto-limbic white matter in the development of abnormal appetitive responses in traumatized people. Compromised integrity of fronto-limbic networks, particularly the UF, has been repeatedly associated with trauma-related problems, including PTSD and depression, in cross-sectional studies (Admon et al., 2013; Daniels et al., 2013; de Diego-Adelino et al., 2014; Fani et al., 2012). As such, these pathways are emerging treatment targets for these disorders, including deep brain stimulation (Choi et al., 2015; Riva-Posse et al., 2014, 2018). Poorer integrity of white matter proximal to the vmPFC at two days post-trauma has been found to be predictive of PTSD symptoms at six months (Sun et al., 2013), and amygdala reactivity to aversive emotional cues has also shown promise as a salient biomarker of PTSD symptom development, as indicated by prospective studies in a civilian population (including our own)(McLaughlin et al., 2014; Stevens et al., 2017; Swartz et al., 2015). Our data extend earlier prospective study findings, indicating that structural integrity of the amygdala-vmPFC pathway may play a critical role in the development of trauma-related sequelae, including PTA. We found this structural disruption to be present around the time of trauma in the prospective study and in the cross-sectional study sample, which consisted largely of people who experienced chronic trauma. As such, we suspect that disruptions in prefrontal-limbic connectivity observed in the wake of trauma are predictors of susceptibility for the development of PTA, and the persistence of these structural disruptions can serve to maintain PTA symptoms.
Disruptions in amygdala-vmPFC white matter connections interfere with various social-emotional processes, as indicated by studies of brain injury (Johnson et al., 2011; Zappala et al., 2012) and fronto-temporal dementia (Galantucci et al., 2011; Hornberger et al., 2011)(Matsuo et al., 2008). Given that the UF serves as a primary limbic connection, disturbed connectivity in this pathway is likely to have consequences for a variety of emotional processes, including reward functioning. The UF is an association fiber connecting orbitofrontal cortical to anterior temporal regions, encompassing the vmPFC and amygdala. These two regions communicate in a bi-directional manner, and have been frequently highlighted in studies of appetitive processing (Scholl et al., 2015). Excitation of the central amygdala has been shown to increase motivation to pursue rewarding stimuli in animals (Robinson et al., 2014). In depressed populations, anhedonia severity has corresponded with both increased (Keedwell et al., 2005; Mitterschiffthaler et al., 2003) and decreased vmPFC activation to reward cues, whereas the amygdala, which is thought to be involved with evaluation of reward cues (Baxter and Murray, 2002), has shown a diminished response. Animal models show that the pathway between the vmPFC and central amygdala mediates reward-related behaviors via GABAergic connections; when this pathway, which originates in the amygdala, is activated, rodents show an increased preference for reward cues(Seo et al., 2016). The vmPFC is involved with various aspects of reward processing, including contingency learning (Knutson et al., 2001), and reward valuation during decision-making. The vmPFC engages during value computation of a reward (Hare et al., 2008), and some studies suggest its involvement in valuation of social goals, such as charitable donations (Hare et al., 2010). In terms of its relevance to other PTSD-related phenomena, the vmPFC-amygdala pathway is also involved in extinction learning (Phelps et al., 2004), and data from our own and other studies have shown that extinction deficits characterize many individuals with PTSD (Norrholm et al., 2011, 2015). Our results extend this prior work, indicating that compromised structural connectivity of the amygdala-vmPFC pathway disrupts reward processing, and predicts future anhedonic symptom development. Given the findings of both the prospective and cross-sectional study, we suspect that disruptions in amygdala-vmPFC connectivity that are present at the time of trauma can lead to the development of PTA, and these structural disruptions may be related to the course of these anhedonic symptoms.
We also found that UF integrity predicted re-experiencing PTSD symptoms at six months post-trauma. One possible explanation for these findings involves the role that the UF plays in the encoding and retrieval of emotional memories. Some researchers argue that this pathway plays a critical role in memory processing, and that UF decrements may lead to problems in the expression of particular memories (Von Der Heide et al., 2013). The vmPFC has a role in both integrating incoming emotional information with existing memories and monitoring memory retrieval, suppressing the interference of irrelevant memory traces (Nieuwenhuis and Takashima, 2011). Altered integrity of the amygdala-vmPFC pathway may disrupt these processes, such that exposure to related emotional stimuli (either endogenous or exogenous cues) may lead to disruptive, involuntary recollections of memory fragments. Greater amygdala-vmPFC resting-state functional connectivity after fear memory reactivation has been associated with extinction learning effects (Feng et al., 2016). Our data suggest that poorer structural connectivity between the amygdala and vmPFC disrupts the proper encoding and retrieval of trauma memories, which may lead to involuntary memory re-experiencing.
Our findings have implications not only for the detection of PTSD, but also for comorbid disorders that are related to appetitive functioning, such as substance use. Substance use disorders, which co-occur with PTSD at rates of up to 34%(Mills et al., 2006), have been tightly linked to anhedonia (Garfield et al., 2014); for example, lower hedonic tone has been shown to predict susceptibility to drug cravings (Cook et al., 2004). The findings from this study suggest that this UF predictor may not only detect those at high risk of developing PTSD, but also substance use disorders. These data similarly highlight the importance of examining abnormalities in appetitive processes in PTSD, as indicated by a recent review (Nawijn et al., 2015).
We were surprised to find that the other selected white matter pathways were not associated with PTA development. Anhedonia severity in depression has been previously linked to decrements in UF and CB integrity (Yang, X.H. et al., 2017), and both of these pathways have also been implicated in treatment outcomes for depression (Korgaonkar et al., 2014). Our earlier cross-sectional research has demonstrated links between PTSD and CB integrity, particularly in the left hemisphere; this finding was observed in our replication analyses. Nonetheless, it is possible that the cingulum is more associated with particular aspects of anhedonia, such as reward learning (Burton et al., 2009), and a more fine-tuned assessment of reward abnormalities at six months post-trauma would have enhanced our ability to assess specific associations of tract integrity with reward processing, including motivational and consummatory aspects. It is also possible that the cross-sectional findings represent more chronic depression that may lead to different structural changes over time, compared to the relatively acute effects examined prospectively here in the aftermath of trauma. It was likewise notable that we did not observe associations of UF or other tract integrity with overall depression symptoms or depressive anhedonia in this population, which occurred with the same frequency as PTA. Thus, uncinate fasciculus integrity may serve as a more specific predictor for anhedonic features of PTSD, which are more social in nature as compared to depressive anhedonia.
Other study limitations are also worth noting. Given the relatively small sample size of the prospective study, we had insufficient statistical power to examine the effects of sex, which would be an important target for future large-scale prospective studies. Our a priori hypotheses led to analyses with three tracts of interest, and the results of primary analyses were supported in an independent sample using a similar approach—however, future studies with larger sample sizes using a whole-brain statistical approach could help confirm the specificity of our findings. We also did not sub-divide these white matter tracts and examine component associations with PTSD symptoms to reduce the number of statistical tests performed; this also merits future investigation with a larger study sample. Further, the distribution of PTA scores at 6 months led us to classify PTA categorically; in a study with a larger sample size (and likewise, one with a more expansive score range) would permit exploration of PTA as a continuous, dimensional construct, which is clinically meaningful. Microstructural integrity of the CB and UF were moderately correlated, as expected given their phylogenetic similarities; as such, their microstructure tends to covary (Wahl et al., 2010). However, this does not diminish the more subtle functional differences, which are not only made evident here but in our prior studies. Our neuroimaging data was collected after trauma occurred, thus we cannot preclude the possibility that pre-existing white matter vulnerabilities contributed to the findings. It is also important to note that our replication sample followed a different (cross-section) study design, which can only establish associations between the white matter and clinical variables. We also did not use clinician-administered measure of PTSD at the 6-month time point to establish symptoms, and as such, estimates of symptom severity may have been under- or over-reported. Finally, we did not examine changes in white matter over time, given the lack of scan data at 6 months post-trauma, but are currently collecting data to be able to perform these analyses in a large-scale, federally-funded study (AURORA; NIH U01 MH110925).
In conclusion, we found that poorer integrity of the UF in the weeks following trauma predicted the presence of PTA at six months post-trauma—notably, these findings were replicated in an independent study sample. Our data highlight, for the first time, the importance of this amygdala-vmPFC pathway for reward-related problems in PTSD, illustrating a potential biological mechanism for PTA development. These findings suggest that decrements in the UF in the wake of trauma may enhance vulnerability for the development of anhedonic PTSD symptoms, as well as re-experiencing phenomena. The integrity of this pathway was a powerful neurobiological marker of PTSD, and shows promise as a target for identifying those at highest risk for particular features of this disorder.
Supplementary Material
Funding and Acknowledgments
This work was primarily supported by National Institute of Mental Health R01 MH094757 (to KJR, BOR), R21 MH106902 (to TJ), U01 MH110925 (to KJR, TJ), K12 HD085850 (to VM), and MH101380 (to NF). We would like to thank Debra Houry, M.D. Lauren Hudak, M.D, MPH and Abigail Hankin-Wei, M.D., for their collaborative efforts on this study. We also thank Erin Renner, Alex O. Rothbaum, Thomas Crow, Heather Grinstead, Rebecca C. Hinrichs, Jessica Maples-Keller, Lydia Odenat, Loren M. Post, Liza C. Zwiebach, Devika Fiorillo, Kathryn Breazeale, Jessica Morgan, Natasha Mehta, Elicia D. Skelton, Taleesha S. Booker, Jonathan Zebrowski, Zachary Clifford, and Sterling Winters for their work in the emergency department recruiting and assessing participants.
Footnotes
Disclosures
The authors have no financial conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2019.05.009.
References
- Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, Hendler T, 2013. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum. Brain Mapp 34 (11), 2808–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Srivastava A, Fani N, Kerley K, Mercer KB, Feng H, Bradley B, Ressler KJ, 2014. Follow-up and extension of a prior genome-wide association study of posttraumatic stress disorder: gene x environment associations and structural magnetic resonance imaging in a highly traumatized African-American civilian population. Biol. Psychiatry 76 (4), e3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 1994. Diagnostic and Statistical Manual of Mental Disorders, fourth ed. American Psychiatric Association, Washington, DC. [Google Scholar]
- Baxter MG, Murray EA, 2002. The amygdala and reward. Nat. Rev. Neurosci 3 (7), 563–573. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM, 2003. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med 50 (5), 1077–1088. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Bebko G, Versace A, Fournier JC, Iyengar S, Olino T, Bonar L, Almeida JR, Perlman SB, Schirda C, Travis MJ, Gill MK, Diwadkar VA, Forbes EE, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Frazier TW, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML, 2016. Predicting clinical outcome from reward circuitry function and white matter structure in behaviorally and emotionally dysregulated youth. Mol. Psychiatry 21 (9), 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T, Linden D, Keedwell P, 2015. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J. Affect. Disord 187, 45–53. [DOI] [PubMed] [Google Scholar]
- Breslau N, Reboussin BA, Anthony JC, Storr CL, 2005. The structure of posttraumatic stress disorder: latent class analysis in 2 community samples. Arch. Gen. Psychiatr 62 (12), 1343–1351. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Creamer M, O’Donnell M, Forbes D, McFarlane AC, Silove D, Hadzi-Pavlovic D, 2017. Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: a network analysis. JAMA psychiatry 74 (2), 135–142. [DOI] [PubMed] [Google Scholar]
- Budisavljevic S, Kawadler JM, Dell’Acqua F, Rijsdijk FV, Kane F, Picchioni M, McGuire P, Toulopoulou T, Georgiades A, Kalidindi S, Kravariti E, Murray RM, Murphy DG, Craig MC, Catani M, 2016. Heritability of the limbic networks. Soc. Cognit. Affect Neurosci 11 (5), 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton BG, Hok V, Save E, Poucet B, 2009. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav. Brain Res 199 (2), 222–234. [DOI] [PubMed] [Google Scholar]
- Choi KS, Riva-Posse P, Gross RE, Mayberg HS, 2015. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA neurology 72 (11), 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D, 2004. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob. Res. : Off. J. Soc. Res. Nicotine. Tobac 6 (1), 39–47. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Lamke JP, Gaebler M, Walter H, Scheel M, 2013. White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depress. Anxiety 30 (3), 207–216. [DOI] [PubMed] [Google Scholar]
- de Diego-Adelino J, Pires P, Gomez-Anson B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, Martin-Blanco A, Alvarez E, Perez V, Portella MJ, 2014. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol. Med 44 (6), 1171–1182. [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, Goodwin L, 2014. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc. Psychiatr. Psychiatr. Epidemiol 49 (9), 1401–1425. [DOI] [PubMed] [Google Scholar]
- DePierro J, D’Andrea W, Frewen P, Todman M, 2017. Alterations in Positive Affect: Relationship to Symptoms, Traumatic Experiences, and Affect Ratings. Psychological Trauma : Theory, Research, Practice and Policy [DOI] [PubMed]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA, 2009. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry 66 (3), 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Norrholm SD, Bradley B, Ressler KJ, Jovanovic T, 2015. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex; a journal devoted to the study of the nervous system and behavior 64, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ, 2012. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology : Off. Pub. Am. Col. Neuropsychopharmacol 37 (12), 2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, Bradley B, Ressler KJ, 2016. Structural and functional connectivity in posttraumatic stress disorder: associations with Fkbp5. Depress. Anxiety 33 (4), 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny NC, Zoellner LA, Fitzgibbons LA, Foa EB, 2000. Exploring the roles of emotional numbing, depression, and dissociation in PTSD. J. Trauma. Stress 13 (3), 489–498. [DOI] [PubMed] [Google Scholar]
- Feng P, Zheng Y, Feng T, 2016. Resting-state functional connectivity between amygdala and the ventromedial prefrontal cortex following fear reminder predicts fear extinction. Soc. Cognit. Affect Neurosci 11 (6), 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RJ, Sakaie K, Lee JC, Debbins JP, Liu Y, Arnold DL, Melhem ER, Smith CH, Philips MD, Lowe M, Fisher E, 2011. A validation study of multicenter diffusion tensor imaging: reliability of fractional anisotropy and diffusivity values. AJNR. Am. J. Neuroradiol [DOI] [PMC free article] [PubMed]
- Frewen PA, Brown MF, Steuwe C, Lanius RA, 2015. Latent profile analysis and principal axis factoring of the DSM-5 dissociative subtype. Eur. J. Psychotraumatol 6, 26406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dean JA, Lanius RA, 2012. Assessment of anhedonia in psychological trauma: development of the hedonic deficit and interference scale. Eur. J. Psychotraumatol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, Dronkers NF, Henry RG, Ogar JM, Miller BL, Gorno-Tempini ML, 2011. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain : J. Neurol 134 (Pt 10), 3011–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield JB, Lubman DI, Yucel M, 2014. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Aust. N. Z. J. Psychiatr 48 (1), 36–51. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Korgaonkar MS, Schofield PR, Harris A, Clark CR, Oakley KL, Ram K, Michaelson H, Yap S, Stanners M, Wise V, Williams LM, 2012. The TWIN-E project in emotional wellbeing: study protocol and preliminary heritability results across four MRI and DTI measures. Twin Res. Hum. Genet. : Off. J. Int. Soc. Twin. Stud 15 (3), 419–441. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A, 2010. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J. Neurosci. : Off. J. Soc. Neurosci 30 (2), 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A, 2008. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. : Off. J. Soc. Neurosci 28 (22), 5623–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks MJ, Lawrence AJ, Brookes RL, Barrick TR, Morris RG, Husain M, Markus HS, 2015. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain : J. Neurol 138 (Pt 12), 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Geng J, Hodges JR, 2011. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain : J. Neurol 134 (Pt 9), 2502–2512. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC, 2013. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 81, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Juranek J, Kramer LA, Prasad MR, Swank PR, Ewing-Cobbs L, 2011. Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J. Int. Neuropsychol. Soc. : JINS 17 (4), 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, Ressler KJ, 2013. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex; a journal devoted to the study of the nervous system and behavior 49 (7), 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC, 2006. Anhedonia and emotional numbing in combat veterans with PTSD. Behav. Res. Ther 44 (3), 457–467. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC, 2007. Anhedonia, emotional numbing, and symptom overreporting in male veterans with PTSD. Pers. Indiv. Differ 43 (4), 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML, 2005. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry 58 (11), 843–853. [DOI] [PubMed] [Google Scholar]
- Kitis O, Ozalay O, Zengin EB, Haznedaroglu D, Eker MC, Yalvac D, Oguz K, Coburn K, Gonul AS, 2012. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatr. Clin. Neurosci 66 (1), 34–43. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12 (17), 3683–3687. [DOI] [PubMed] [Google Scholar]
- Korgaonkar MS, Williams LM, Song YJ, Usherwood T, Grieve SM, 2014. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br. J. Psychiatry : J. Ment. Sci 205 (4), 321–328. [DOI] [PubMed] [Google Scholar]
- Krynicki CR, Upthegrove R, Deakin JFW, Barnes TRE, 2018. The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr. Scand 137 (5), 380–390. [DOI] [PubMed] [Google Scholar]
- Lambert C, Da Silva S, Ceniti AK, Rizvi SJ, Foussias G, Kennedy SH, 2018. Anhedonia in Depression and Schizophrenia: A Transdiagnostic Challenge. CNS neuroscience & therapeutics [DOI] [PMC free article] [PubMed]
- Liu P, Wang L, Cao C, Wang R, Zhang J, Zhang B, Wu Q, Zhang H, Zhao Z, Fan G, Elhai JD, 2014. The underlying dimensions of DSM-5 posttraumatic stress disorder symptoms in an epidemiological sample of Chinese earthquake survivors. J. Anxiety Disord 28 (4), 345–351. [DOI] [PubMed] [Google Scholar]
- Maguen S, Stalnaker M, McCaslin S, Litz BT, 2009. PTSD subclusters and functional impairment in Kosovo peacekeepers. Mil. Med 174 (8), 779–785. [DOI] [PubMed] [Google Scholar]
- Malta LS, Wyka KE, Giosan C, Jayasinghe N, Difede J, 2009. Numbing symptoms as predictors of unremitting posttraumatic stress disorder. J. Anxiety Disord 23 (2), 223–229. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Mizuno T, Yamada K, Akazawa K, Kasai T, Kondo M, Mori S, Nishimura T, Nakagawa M, 2008. Cerebral white matter damage in frontotemporal dementia assessed by diffusion tensor tractography. Neuroradiology 50 (7), 605–611. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA, 2014. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress. Anxiety 31 (10), 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Wolf EJ, Martin E, Kaloupek DG, Keane TM, 2008. Structural equation modeling of associations among combat exposure, PTSD symptom factors, and Global Assessment of Functioning. J. Rehabil. Res. Dev 45 (3), 359–369. [DOI] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Ross J, Peters L, 2006. Trauma, PTSD, and substance use disorders: findings from the Australian national survey of mental Health and well-being. Am. J. Psychiatry 163 (4), 652–658. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, Suckling J, Poon L, Simmons A, Andrew C, Sharma T, 2003. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport 14 (2), 177–182. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher L, Van Zijl PC, 2005. MRI Atlas of Human White Matter. Amsterdam, the Netherlands
- Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M, 2015. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav. Rev. 51, 189–204. [DOI] [PubMed] [Google Scholar]
- Nickerson A, Barnes JB, Creamer M, Forbes D, McFarlane AC, O’Donnell M, Silove D, Steel Z, Bryant RA, 2014. The temporal relationship between post-traumatic stress disorder and problem alcohol use following traumatic injury. J. Abnorm. Psychol 123 (4), 821–834. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A, 2011. The role of the ventromedial prefrontal cortex in memory consolidation. Behav. Brain Res 218 (2), 325–334. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, Ressler KJ, Jovanovic T, 2015. Fear load: the psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int. J. Psychophysiol. : Off. J. Int. Org. Psychophysiol 98 (2 Pt 2), 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ, 2011. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry 69 (6), 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North CS, Nixon SJ, Shariat S, Mallonee S, McMillen JC, Spitznagel EL, Smith EM, 1999. Psychiatric disorders among survivors of the Oklahoma City bombing. Jama 282 (8), 755–762. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE, 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43 (6), 897–905. [DOI] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, McIntyre CC, Gross RE, Mayberg HS, 2018. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psychiatry 23 (4), 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Crowell AL, Garlow SJ, Rajendra JK, Mayberg HS, 2014. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 76 (12), 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Warlow SM, Berridge KC, 2014. Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J. Neurosci. : Off. J. Soc. Neurosci 34 (50), 16567–16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl J, Kolling N, Nelissen N, Wittmann MK, Harmer CJ, Rushworth MF, 2015. The good, the bad, and the irrelevant: neural mechanisms of learning real and hypothetical rewards and effort. J. Neurosci. : Off. J. Soc. Neurosci 35 (32), 11233–11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, McCall JG, Krashes M, Sparta DR, Bruchas MR, 2016. A GABAergic projection from the centromedial nuclei of the amygdala to ventromedial prefrontal cortex modulates reward behavior. J. Neurosci. : Off. J. Soc. Neurosci 36 (42), 10831–10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B, 2001. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am. J. Psychiatry 158 (2), 234–243. [DOI] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum. Brain Mapp 17 (3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg O, Blix I, Heir T, 2015. The aftermath of terrorism: posttraumatic stress and functional impairment after the 2011 Oslo bombing. Front. Psychol 6, 1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer EG, Zuromski KL, Davis MT, Witte TK, Weathers F, 2017. Posttraumatic stress disorder symptom clusters and acquired capability for suicide: a reexamination using DSM-5 criteria. Suicide Life-Threatening Behav [DOI] [PubMed]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ, 2013. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res [DOI] [PMC free article] [PubMed]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, Hudak LA, Jovanovic T, Rothbaum BO, Ressler KJ, 2017. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol. Psychiatry 81 (12), 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang Z, Ding W, Wan J, Zhuang Z, Zhang Y, Liu Y, Zhou Y, Xu J, 2013. Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PLoS One 8 (12), e83473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR, 2015. A neural biomarker of psychological vulnerability to future life stress. Neuron 85 (3), 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR, 2013. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain : J. Neurol 136 (Pt 6), 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Wardle MC, Smith LJ, Berenz EC, 2017. Reward functioning in posttraumatic stress and substance use disorders. Current opinion in psychology 14, 49–55. [DOI] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P, 2010. Microstructural correlations of white matter tracts in the human brain. Neuroimage 51 (2), 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang L, Cao C, Cao X, Fang R, Zhang J, Elhai JD, 2017. The underlying dimensions of DSM-5 PTSD symptoms and their relations with anxiety and depression in a sample of adolescents exposed to an explosion accident. Eur. J. Psychotraumatol 8 (1), 1272789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XH, Wang Y, Wang DF, Tian K, Cheung EFC, Xie GR, Chan RCK, 2017. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatr. Res 264, 29–34. [DOI] [PubMed] [Google Scholar]
- Zappala G, Thiebaut de Schotten M, Eslinger PJ, 2012. Traumatic brain injury and the frontal lobes: what can we gain with diffusion tensor imaging? Cortex. a journal devoted to the study of the nervous system and behavior 48 (2), 156–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


