Abstract
The neonatal thymus generates Foxp3+ regulatory T (tTreg) cells that are critical in controlling immune homeostasis and preventing multi-organ autoimmunity. The role of antigen specificity on neonatal tTreg cell selection is unresolved. Here we identify seventeen self-peptides recognized by neonatal tTreg cells, and reveal ligand specificity patterns that include self-antigens presented in an age-dependent and inflammation-dependent manner. Fate mapping studies of neonatal Peptidyl arginine deiminase, type IV, (Padi4)-specific thymocytes reveal disparate fate choices. Neonatal thymocytes expressing TCRs that engage IAb-Padi4 with moderate dwell times within a conventional docking orientation are exported as tTreg cells. In contrast, Padi4-specific TCRs with short dwell time are expressed on CD4+ T cells, while long dwell times induce negative selection. Temporally, Padi4-specific thymocytes are subject to a developmental stage-specific change in negative selection, which precludes tTreg cell development. Thus, a temporal switch in negative selection and ligand binding kinetics constrains the neonatal tTreg selection window.
Introduction
αβ T cell development creates a repertoire of immature thymocytes expressing T cell receptors (TCRs) with a graded scale of reactivity for self-peptides presented by host-Major Histocompatibility Complex molecules (self-pMHC). The fate of these immature thymocytes is then guided by TCR signals emanating from the engagement of self-pMHC ligands. It has been well established that weak TCR signals are required for positive selection, thereby ensuring mature T cells are capable of recognizing MHC displayed ligands, while strong TCR signals often result in the clonal elimination of thymocytes, limiting the risk of autoimmunity1, 2. Despite these highly ordered molecular and cellular processes, some overtly self-reactive and tissue-specific antigen (TSA)-reactive T cells are exported from the thymus and are maintained within the mature conventional T (Tconv) cell repertoire. Limiting the autoimmune potential of self-reactive Tconv cells are several additional T cell lineages, including thymus-derived T regulatory cells that express the transcription factor Foxp3 (tTreg cells).
The neonatal exposure of thymocytes to self-antigens and the development of tTreg cells are critical for enforcing immune tolerance and preventing autoimmunity. Depletion of tTreg cells in mice, as well as mouse models that limit self-antigen display by mTECs results in multi-organ autoimmunity3–7. Further, tTreg complementation studies in NOD mice suggest that adult-derived tTreg cells are unable to fully limit autoimmunity when tTreg cells generated in the first 10 days of life are absent. This phenomenon correlated with the observation that distinct tTreg clonotypes are selected in the perinatal and neonatal thymus as compared to the adult thymus4.
How recognition of self-ligands by neonatal thymocytes influence lineage fate decisions remains incompletely understood. Following positive selection, thymocytes expressing MHC-II restricted TCRs upregulate TCR and chemokine receptor 7 (CCR7) expression, migrate to the medulla and differentiate into semi-mature and then mature CD4+ single positive (CD4SP) cells, eventually to be exported from the thymus8–10. During the CD4SP stage, thymocytes that engage self-pMHC presented by medullary epithelial cells (mTECs) or thymic dendritic cells (DC) can be diverted into the tTreg lineage, undergo a second wave of deletion, or continue along the CD4 Tconv cell differentiation process2, 9, 11–14. Self-tolerance and the development of a subset of tTreg cells generated in the first week of life requires Aire-dependent presentation of self-ligands4, 15, though the specific Aire-dependent and Aire-independent self-epitopes that regulate neonatal thymic development are largely unknown. Observations that perinate- and adult-derived tTreg cells express differing TCR clonotypes have suggested a role for age in the selection of particular antigen-specificities4. However, the dearth of identified tTreg self-ligands16–21, and minimal ability to monitor neonatal and adult thymic selection of antigen-specific tTreg cells within polyclonal repertoires has limited the ability to define the molecular basis for tTreg selection.
Here we investigated the TCR:self-pMHC ligand recognition properties that regulate the neonatal window of T cell development. We observed that TCRs expressed on neonatal tTreg cells can recognize self-antigens whose presentation on antigen-presenting cells (APCs) is regulated by age and inflammation. A self-antigen discovery platform was created, and used to identify a panel of ligands recognized by neonate-derived tTreg cells, including Peptidyl arginine deiminase, type IV (Padi4). The development of Padi4-specific T cells is restricted to the neonatal thymus; alterations in the adult thymus lead to Padi4-specific thymocytes undergoing negative selection at the CD4+CD8+ (DP) CCR7neg to CCR7pos transition, thereby eliminating the precursor cells of Padi4-specific tTreg cells and CD4+ Tconv cells. Within the neonatal thymus, the CD4SP selection branch point correlated with TCR:pMHC dwell time of binding. Specifically, moderate TCR:IAb-Padi4 dwell times instruct neonatal tTreg cell development, whereas longer dwell times induce negative selection, and short dwell time TCRs are expressed on mature CD4+ Tconv cells. Our results provide insight into the ligand specificity of the neonatal tTreg repertoire, and reveal roles for age-dependent alterations in negative selection and TCR:pMHC binding kinetics in framing the neonatal T cell selection window.
RESULTS
Neonatal tTreg TCRs can recognize steady state and inflammation-dependent antigens
To test levels of self-reactivity carried within the neonatal tTreg repertoire, we cloned 66 TCRs expressed on Foxp3-GFP+ CD25+ CD4+ thymocytes isolated from 2-week old Tcra+/− C57BL/6, and 316 TCRs from 2-week old Tcra+/−Yae62β Tg mice (transgenic mice that express a fully rearranged TCRβ chain). Tcra+/− mice were used to ensure that the thymocytes expressed only a single TCRα chain paired with the expressed TCRβ chain. Cloned TCRs were re-expressed in a TCR-deficient hybridoma, 5KC TCRαβnull that also expresses mouse CD4, and tested in vitro for responses to syngeneic APCs. Analyses of C57BL/6-derived tTreg hybridomas revealed three self-reactivity categories: 14% were reactive to resting adult splenic APC, 9% either required, or were >3-fold more reactive to adult splenic APCs isolated from mice pretreated with lipopolysaccharide (LPS) plus anti-CD40 (LPS+αCD40) to induce inflammation, and 77% have self-reactivity that is below the detection of this assay (Fig. 1a,b). Yae62β+ tTreg hybridomas demonstrated an ~1.5-fold increase in frequencies of these self-reactivity categories (Fig. 1c,d).
Figure 1.
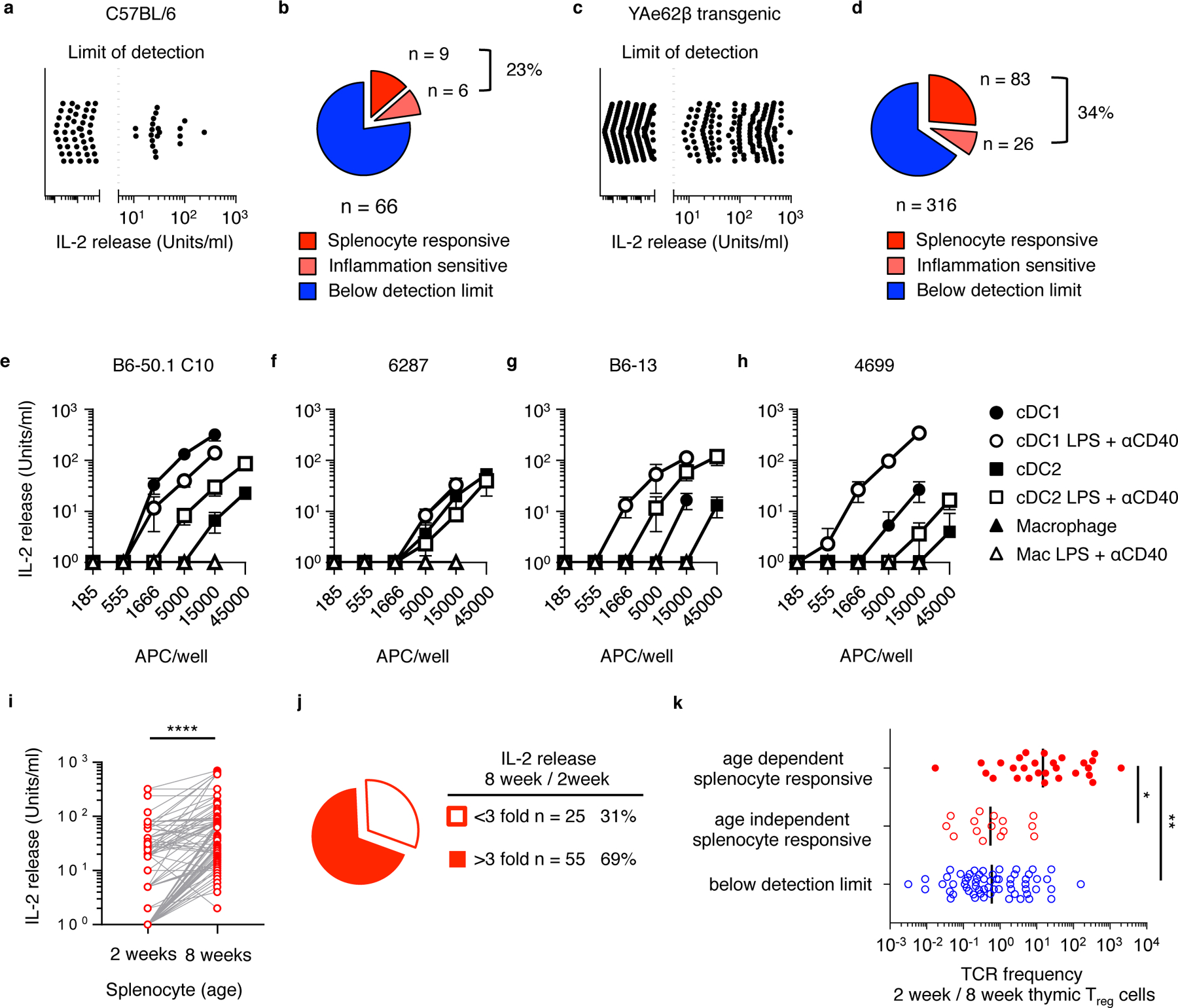
T cell receptors expressed on neonate-derived tTreg cells can recognize steady state, inflammation- and age-dependent self-antigens. (a) IL-2 release and (b) frequency at which 66 C57BL/6-derived tTreg hybridomas and (c, d) 316 Yae62β+ tTreg hybridomas react with splenocytes isolated from adult naïve mice (red) or mice pretreated with LPS and αCD40 (pink). (e) IL-2 response of B6–50.1C10, (f) 6287, (g) B6–13 and (h) 4699 tTreg hybridomas cultured with titrating numbers of cDC1, cDC2 and macrophages isolated from naïve mice (filled symbol) or mice pretreated with LPS and αCD40 (open symbol). Data are an example of three independent experiments giving similar results. Error bars show standard deviation. (i) IL-2 release by 80 in vitro splenocyte-reactive tTreg hybridomas cultured with spleen cells isolated from 2 week old and 8 week old C57BL/6 mice. Results are from two independent experiments with similar results. (j) Quantification of splenocyte-reactive hybridomas that increase IL-2 production in response to adult v. neonatal spleen cells. (k) The frequency of age-dependent (red, filled circles), age-independent in vitro splenocyte-responsive (red, open circles) and below detection limit (blue) TRAV14+ (Vα2+) tTreg TCRs carried in the thymic Foxp3+ tTreg pool at 2 weeks of age as compared to 8 weeks of age. Lines represent the data geometric mean. Data are from (i,j) 80 self-reactive and (k) 103 Va2+ clonotypes; 28 age-dependent, 12 age-independent and 63 below limit of detection. (i) ****P<0.0001 ratio paired 2-tailed t test, (k) *P<0.05, **P<0.01 one-way ANOVA Tukey multiple comparisons test.
Neonatal tTreg hybridomas were found to differentially recognize specific APC subsets. The B6–50.1 C10 and 6287 tTreg hybridomas recognize antigens similarly presented on resting and LPS+αCD40 activated DC populations (Fig. 1e,f), while the B6–13 and 4699 tTreg hybridomas showed increased reactivity to LPS+αCD40 activated DCs (Fig. 1g,h). Additional tTreg hybridomas were found to specifically recognize B cell presented antigens (Supplementary Fig. 1). ~70% of the in vitro self-reactive neonatal tTreg hybridomas are more reactive to adult as compared to neonatal splenocytes (Fig. 1i,j). The characterization of neonatal tTreg TCRs derived from Yae62β Tg mice allowed for TCRα sequencing methods to associate clonotype frequencies with self-reactivity properties. High throughput sequencing of Vα2+ tTreg cells, isolated from neonatal and adult YAe62β mice, revealed that tTreg cells with specificity for age-dependent antigens are selectively enriched in the neonatal thymus as compared to the adult thymus (Fig. 1k). Collectively, these data are consistent with the requirement for specific DC subsets in the thymic selection and peripheral activation of certain tTreg clonotypes in vivo14, 22, and support the hypothesis that neonatal tTreg cells can recognize age- and inflammation-dependent changes in antigen presentation.
Identification of self-pMHC epitopes recognized by neonatal tTreg cells TCRs
To characterize potential self-peptides recognized by neonatal tTreg cells, we curated mass spectrometry approaches that assessed the IAb immunopeptidome and synthesized the unique species to create an IAb self-peptide library (see Methods). Eighty individual neonatal tTreg hybridomas were cultured with pools of self-peptides and IAb expressing fibroblasts as the APC, and IL-2 secretion was measured. T cell specificity for an individual peptide was de-convoluted from the reactivity patterns. Using this method, seventeen unique self-peptides were identified that stimulated at least one neonatal tTreg hybridoma with an EC50 value of 1μM or less (Fig. 2a, Supplementary Table 1).
Figure 2.
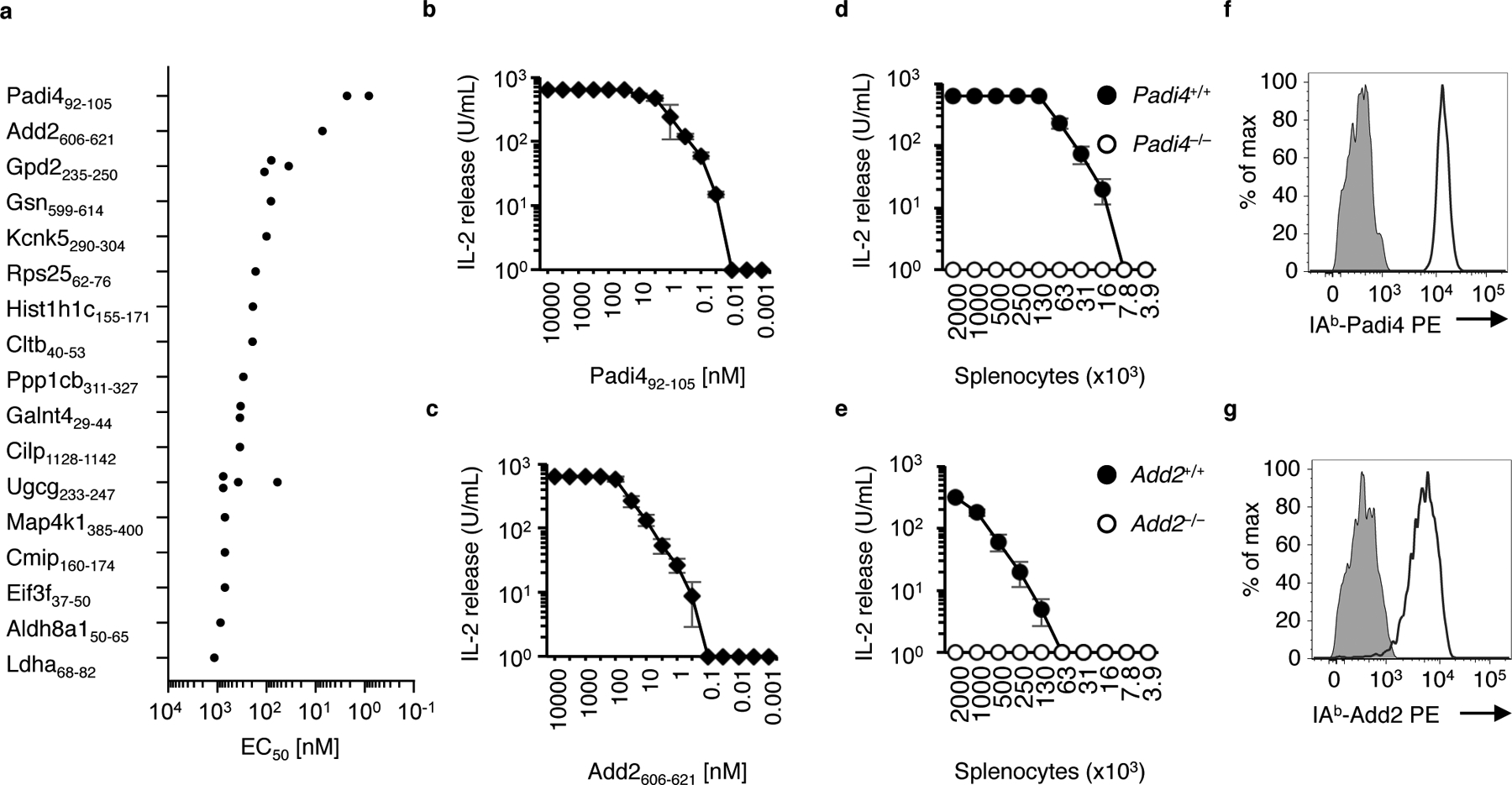
Identification of self-ligands recognized by neonatal tTreg TCR using an immunopeptidome library screen. (a) Chart of EC50 values of IL-2 release for tTreg hybridomas responding to titrating concentrations of library-identified self-peptides. (b-e) IL-2 release of the (b) 4699 tTreg hybridoma and (c) 6287 4699 tTreg hybridoma cultured with titrating concentrations of identified Padi492–105 or Add2606–621 presented by IAb expressing mouse fibroblasts, or (d) with titrating concentrations of C57BL/6 or Padi4−/− splenocytes, or (e) with titrating concentrations of C57BL/6 or Add2−/− splenocytes. Data are example of three independent experiments giving similar results. Error bars show standard deviation. (f) IAb-Padi4 tetramer staining of the 4699 tTreg hybridoma, and (g) IAb-Add2 tetramer staining of the 6287 tTreg hybridoma (black line). Gray negative control stains are the IAb-Add2 tetramer staining of the 4699 tTreg hybridoma and the IAb-Padi4 tetramer staining the 6287 tTreg hybridoma.
The 4699 tTreg hybridoma was found react to Peptidyl arginine deiminase, type IV, (Padi492–105), and the 6287 T cell hybridoma recognizes Adducin 2 (Add2606–621) (Fig. 2b,c). Activation assays using splenocytes from Padi4−/− mice and Add2−/− mice demonstrated that the in vitro self-reactivity of the 4699 and 6287 tTreg hybridomas required their identified self-ligand (Fig. 2d,e). IAb tetramers carrying Padi492–105 and Add2606–621 were created, and found to specifically stain the 4699 and 6287 tTreg hybridomas, respectively (Fig. 2f,g). The ability of these self-pMHC tetramers to stain tTreg hybridomas suggested that these reagents would allow for visualization of the Padi4-and Add2- specific tTreg repertoires in vivo.
Development of Padi4-specific tTreg cells is restricted to the neonatal period
Padi4- and Add2-specific Vα2+ CD4SP thymocytes are readily detected using self-pMHC tetramers in one to three week old YAe62β mice, with many further differentiating into tTreg cells. These Foxp3-GFP+ tTreg express the classical phenotypic markers including CD25, GITR, PD-1 and CD44 (Fig. 3a, top row, Supplementary Fig. 2a). Starting at 4wks of age, a severe depletion of both Padi4-reactive thymic CD4SP and tTreg cells was observed, (Fig. 3a, bottom row). Analyses of Padi4−/− mice revealed that the neonatal development of Padi4-specific tTreg cells, and the adult loss of Padi4-specific CD4SP are dependent upon the expression of the identified self-antigen (Fig. 3b). The age-dependent alteration of the Padi4-specific thymocyte repertoire occurs both as a percent of total cells, and as an absolute number of cells (Fig. 3c–f). Thus, development of Padi4-specific tTreg stops in the adult thymus, and is not simply diluted out by changes in thymic cellularity. A similar age-dependent tTreg selection occurs for Add2-reactive thymocytes (Supplementary Fig. 2b–d). Further, the high frequencies of IAb-Padi4 tetramer bright CD4SP thymocytes in 6 weeks old Padi4−/− YAe62β indicate thymocytes can rearrange Padi4-specific TCRs in adult mice (Fig. 3b). These data demonstrate that the development of Padi4-specific tTreg cells is unique to neonatal thymus.
Figure 3.
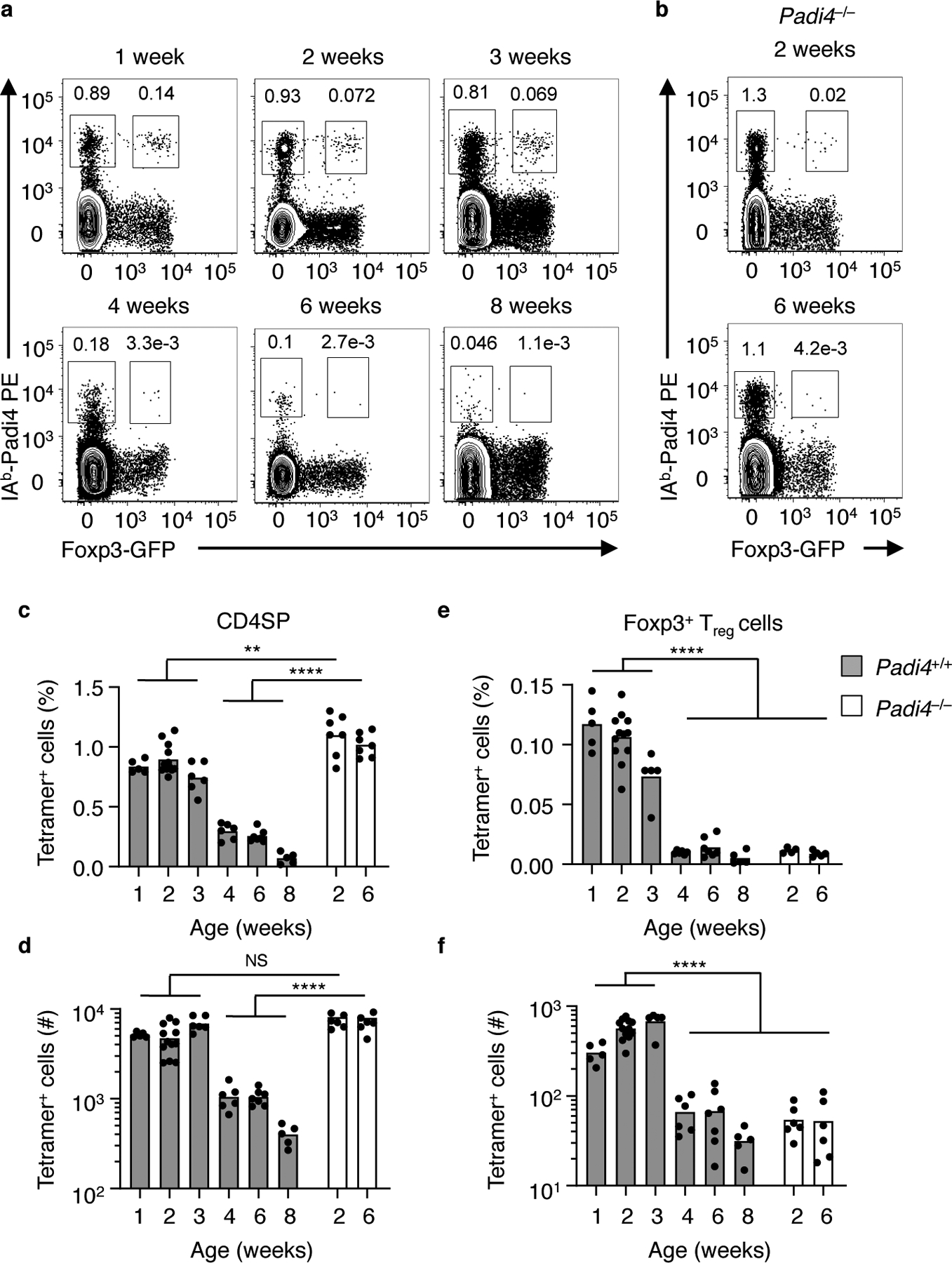
Development of Padi492–105-specific tTreg cells is restricted to the neonatal thymus. (a, b) Flow cytometry of Vα2+ CD4+CD8− thymocytes in (a) YAe62β.Foxp3-GFP and (b) Padi4−/−.YAe62β.Foxp3-GFP mice at different ages; numbers adjacent to outlined areas indicate percent IAb-Padi4 tetramer+ Foxp3-GFP− (left) and Foxp3-GFP+ (right). (c-f) Frequency and total cell numbers of IAb-Padi4+ (c, d) CD4+CD8− Foxp3− and (e, f) Foxp3-GFP+ tTreg cells in WT and Padi4−/− YAe62β.Foxp3-GFP mice at different ages. Bars represent the data mean, n = 6, 12, 6, 6, 6, 6 WT mice at 1, 2, 3, 4, and 8 weeks old, n = 7 Padi4−/− 2 and 6 weeks old. (c-f) NS P>0.05, **P<0.01, ***P<0.01, ****P<0.0001 one-way ANOVA Tukey multiple comparisons test.
Within the mature T cell repertoire accumulation of Padi4-reactive tTreg cells in the spleen begins at 1 week of age, and requires expression of endogenous Padi4 (Fig. 4a–f). As a percentage of the total, Padi4-reactive tTreg cells are diluted correlating with the age-dependent reduction in thymic output (Fig. 4c). The total number Padi4-reactive tTreg cells within the spleen peaks at four weeks, and slowly decrease through 8 weeks of age (Fig. 4d). An early accumulation of Padi4-specific CD4+ Tconv cells similarly develops (Fig. 4e, f). Splenic Padi4-reactive tTreg cells share phenotypic similarity with perinatal tagged tTreg subsets4, including high expression levels of PD-1, CD44, CD25, GITR, NRP1 and CTLA4 (Fig. 4g). The corresponding CD4+ Tconv cells in these mice express PD-1, NRP1, CD73 and FR4 (Supplementary Fig. 3), a phenotype similar to studies of antigen-experienced anergic CD4 T cells23. In contrast, Padi4-reactive T cells that developed in Padi4−/− mice are almost exclusively CD44loCD62Lhi naïve-phenotype CD4 Tconv cells (Supplementary Fig. 3).
Figure 4.
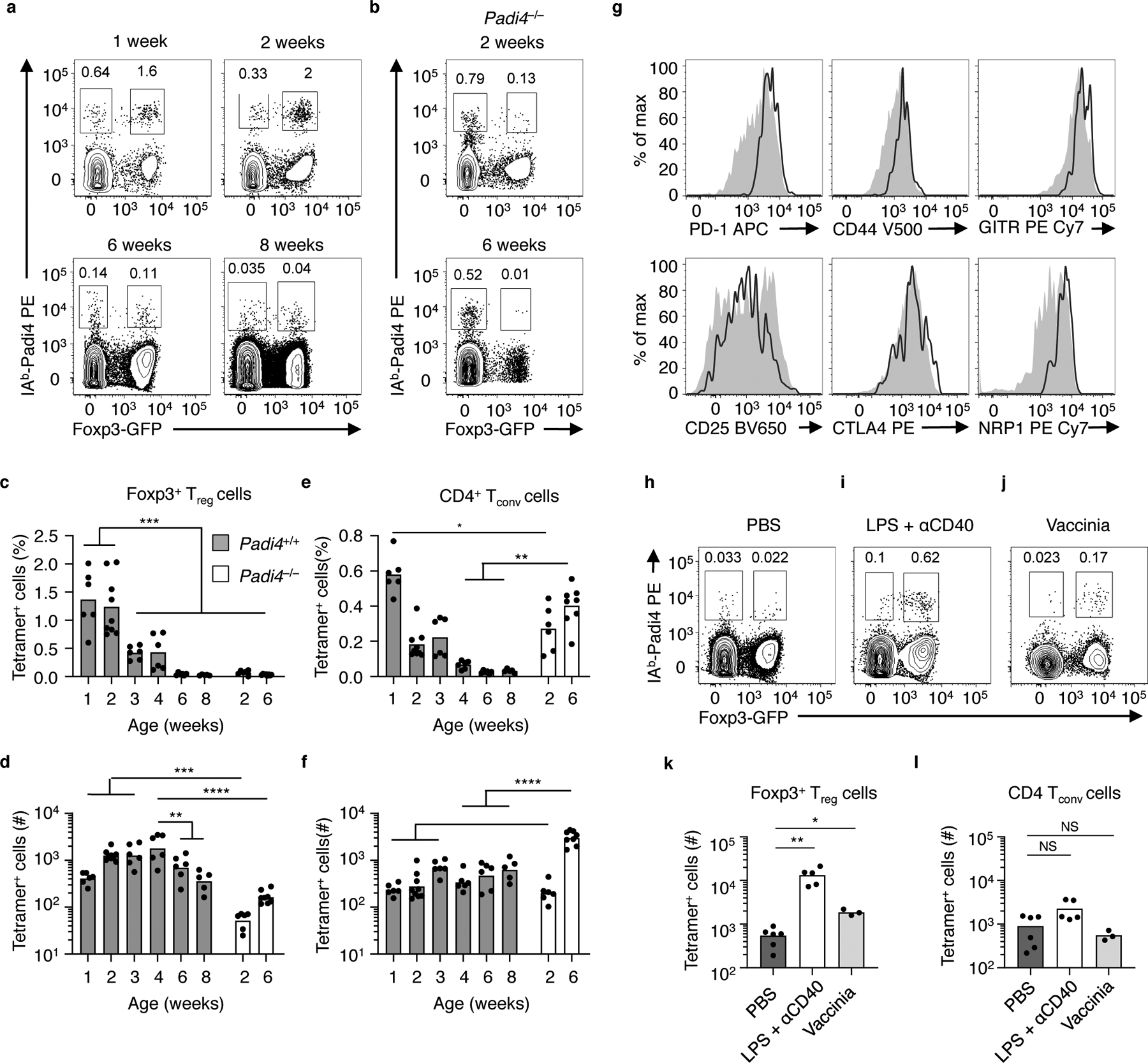
Padi4-specific tTreg cells seed the peripheral repertoire during the neonatal window and respond to inflammation. (a, b) Flow cytometry of splenic Vα2+ CD4+ T cells in (a) YAe62β.Foxp3-GFP mice and (b) Padi4−/−.YAe62β.Foxp3-GFP mice at different ages (weeks). (c-f) Frequency and (d) total numbers of IAb-Padi4 tetramerpos Vα2+ CD4+ Foxp3-GFPpos tTreg cells and (e, f) Vα2+ CD4+ Foxp3-GFPneg CD4 Tconv cells in YAe62β.Foxp3-GFP and Padi4−/−.YAe62β.Foxp3-GFP mice at different ages. (g) Flow cytometry analyzing the expression of PD-1, CD44, GITR, CD25, CTLA4 and Nrp1 on IAb-Padi4 tetramerpos Vα2+CD4+Foxp3-GFPpos tTreg cells isolated from 2 week old YAe62β.Foxp3-GFP mice (black line). Gray histograms are IAb-Padi4 tetramerneg Vα2+CD4+Foxp3-GFPpos tTreg cells in the same mice. (h-l) Flow cytometry of Vα2+ CD4+ T cells isolated from 8 week old YAe62β.Foxp3-GFP mice following i.p. injection of (h) PBS, (i) LPS + αCD40 and (j) rVaccina virus 5 days prior. Quantification of IAb-Padi4 tetramerpos (k) CD4+Foxp3-GFPpos tTreg cells and (l) Foxp3-GFPneg CD4 Tconv cells present in YAe62β.Foxp3-GFP mice following i.p. injection of PBS, LPS+αCD40 or rVac virus. (a g) Data are derived from 3 independent experiments giving similar results, bars represent the data mean, n = 6, 9, 6, 6, 6, 6 WT mice at 1, 2, 3, 4, and 8 weeks old, n = 6 and 8 Padi4−/− 2 and 6 weeks old. (h-l) Data pooled from 3 independent experiments with similar results, with 9, 5 and 6 mice per group, bars represent the data mean. Significance identified using a one-way ANOVA Tukey multiple comparisons test, and (k,l) non-parametric Dunn’s multiple comparison test. ns P>0.05, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
The increased reactivity of the 4699 tTreg hybridoma to LPS+αCD40-activated APCs (Fig. 1f) suggested that Padi4-specific tTreg cells might sense inflammation in vivo. To test this idea, inflammation was induced in YAe62β mice by i.p. injection with LPS+αCD40 or vaccinia virus infection. Padi4-specific tTreg cells expanded within the spleen in both models of inflammation whereas no significant changes in the frequency of Padi4- specific CD4+ Tconv cells were observed (Fig. 4h–l). These data demonstrate that Padi4-specific tTreg cells are exported during the neonatal window, are maintained in the peripheral repertoire and can accumulate during inflammation.
Negative selection eliminates Padi4-specific thymocytes from the adult thymus
The restricted development of Padi4-specific tTreg cells to the neonatal thymus suggested mechanisms actively limit their generation in adult mice. To probe this question, we first quantified the frequency at which Padi4-specific thymocyte are present at different thymic developmental stages, in neonate and adult mice, which do or do not express endogenous Padi4. Pre-selection CD4+CD8+ (DP) TCRβint CCR7lo thymocytes that undergo positive selection on MHC-II molecules upregulate TCRβ and CCR7 expression, and down regulate CD8 expression to become a CD4SP. Within the CD4SP compartment, thymocytes can be further delineated based on maturation status: semi-mature (SM), mature 1 (M1) and mature 2 (M2)9, 10, 24. Within the neonatal and adult thymus, a very small fraction of DP TCRβhi CCR7pos thymocytes express Foxp325, with larger frequencies occurring at the CD4SP M1 and M2 stages (Fig. 5a,b Supplementary Fig. 4).
Figure 5.
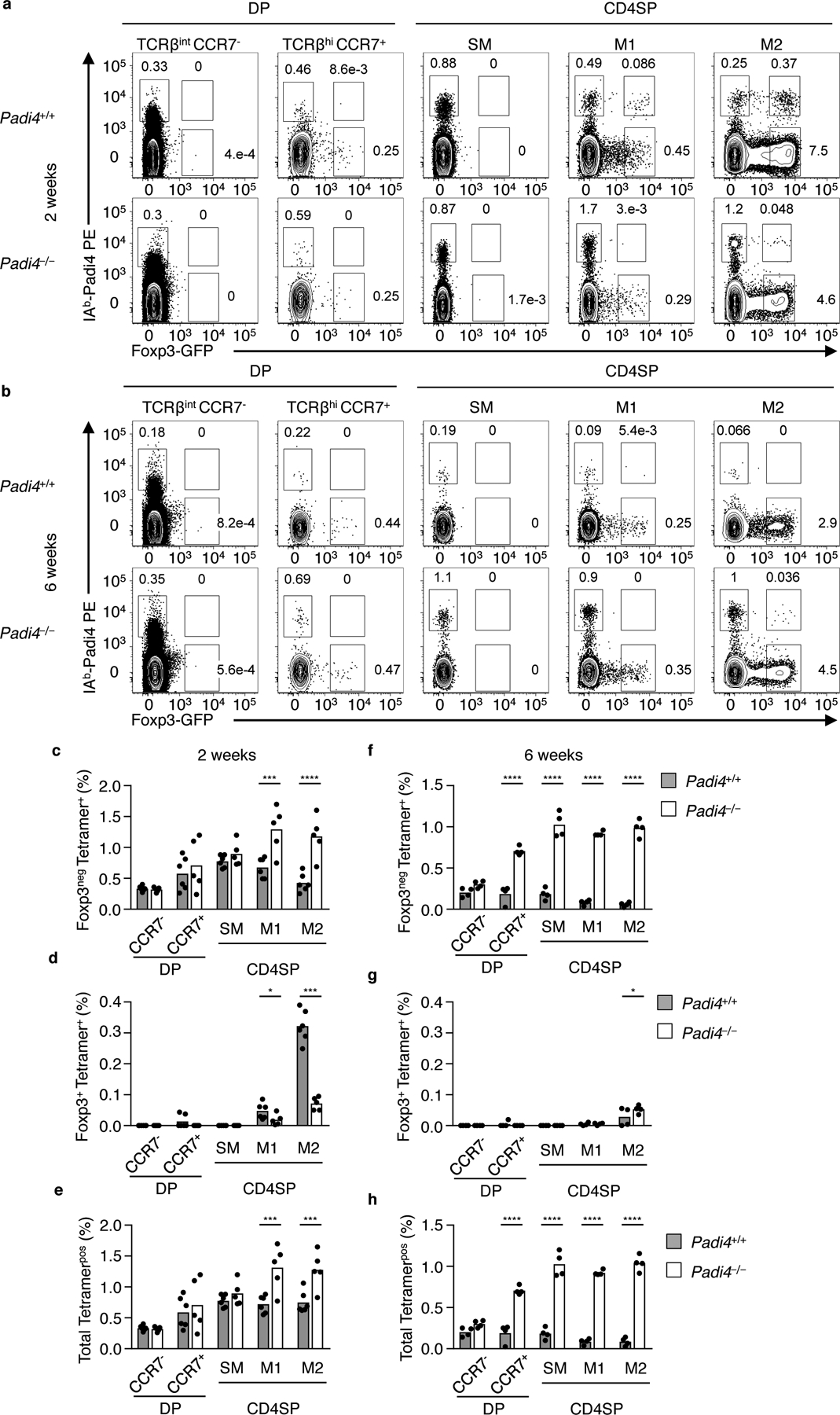
Padi4-specific thymocytes are subject to temporally regulated, stage-specific changes in negative selection. Flow cytometry analyses of (a) neonatal and (b) adult Vα2+ DP and CD4SP thymocytes isolated from Padi4+/+.YAe62β.Foxp3-GFP (top row) and Padi4−/−.YAe62β.Foxp3-GFP (bottom row) mice for Foxp3 expression and IAb-Padi4 tetramer binding. Subsets shown are pre-selection DP (CD4+ CD8+ TCRβint CCR7neg), post-selection DP (CD4+CD8+ TCRβhi CCR7pos), and TCRβhi CCR7pos CD4SP thymocytes pre-gated on CD69+ MHC-Ilo (SM), CD69+ MHC-Ihi (M1) and CD69lo MHC-Ihi (M2). (c-g) Quantification of the frequency of IAb-Padi4 tetramerpos (c,f) Foxp3neg, (d,g) Foxp3pos and (e,h) total (Foxp3neg + Foxp3+) thymocytes within each thymic subset. (c-h) Data are derived from 3 independent experiments giving similar results, bars represent the data mean, n = 6 WT and Padi4−/− mice at 2 and 6 weeks old. Significance identified using a one-way ANOVA Sidak’s multiple comparison test. ns P>0.05, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Within the neonatal thymus, a small fraction of Padi4-specific thymocytes begin to express Foxp3 at the CD4SP M1 stage (Fig. 5a, top row). At the CD4SP M1 stage, a significant decrease in Foxp3neg Padi4-specific thymocytes is also observed, in comparison to Padi4−/− mice (Fig. 5c–e). The increase in Padi4-specific tTreg cells and decrease in Foxp3neg CD4SP is magnified at the CD4SP M2 stage. In adult mice, negative selection depletes Padi4-specific thymocytes beginning at the DP stage (Fig. 5b,f-h). This largely eliminates Padi4-specific thymocytes prior to upregulating TCRβ and CCR7 expression, or downregulating CD8 in Padi4+/+ but not Padi4−/− mice (Fig. 5b–h, Supplementary Fig. 4). The development of Add2-specific CD4SP is similarly abrogated in adult thymus (Supplementary Fig. 4k–o). These data argue that the thymocyte stage at which Padi4-specific thymocytes recognize cognate self-antigen changes between the neonate and adult thymus.
Limiting Padi4 expression to radioresistant cells allows for adult tTreg development
Mixed Padi4+/+ and Padi4−/− bone marrow (BM) chimeric mice were generated to reveal the cellular sources of Padi4 that control negative selection in adult mice. Donor Padi4−/− BM into Padi4−/− mice (KO -> KO) and Padi4+/+ BM into Padi4+/+ mice (WT -> WT) phenocopy their parental strains; Padi4 deficiency in both the BM and recipient radioresistant compartments resulted in the generation of a high frequency of Padi4-specific CD4SP thymocytes, whereas mice sufficient in Padi4 in both compartments demonstrated profound deletion at the TCRβint CCR7neg DP to TCRbhi CCR7pos CD4SP transition (Fig. 6a–h). Further, neither KO -> KO or WT -> WT BM chimeric mice generated significant populations of Padi4-specific tTreg cells (Fig. 6i,j). The exclusive expression of Padi4 in BM cells (WT -> KO) showed a significant elimination of Padi4-specific thymocytes at the DP to CD4SP transition, and minimal development of tTreg cells (Fig. 6a–j). Consistent with these data, flow sorted thymic CD11c+ Xcr1neg cDC (migratory Sirpa cDC26) presents IAb-Padi4 to T cell hybridomas (Fig. 6k,l).
Figure 6.
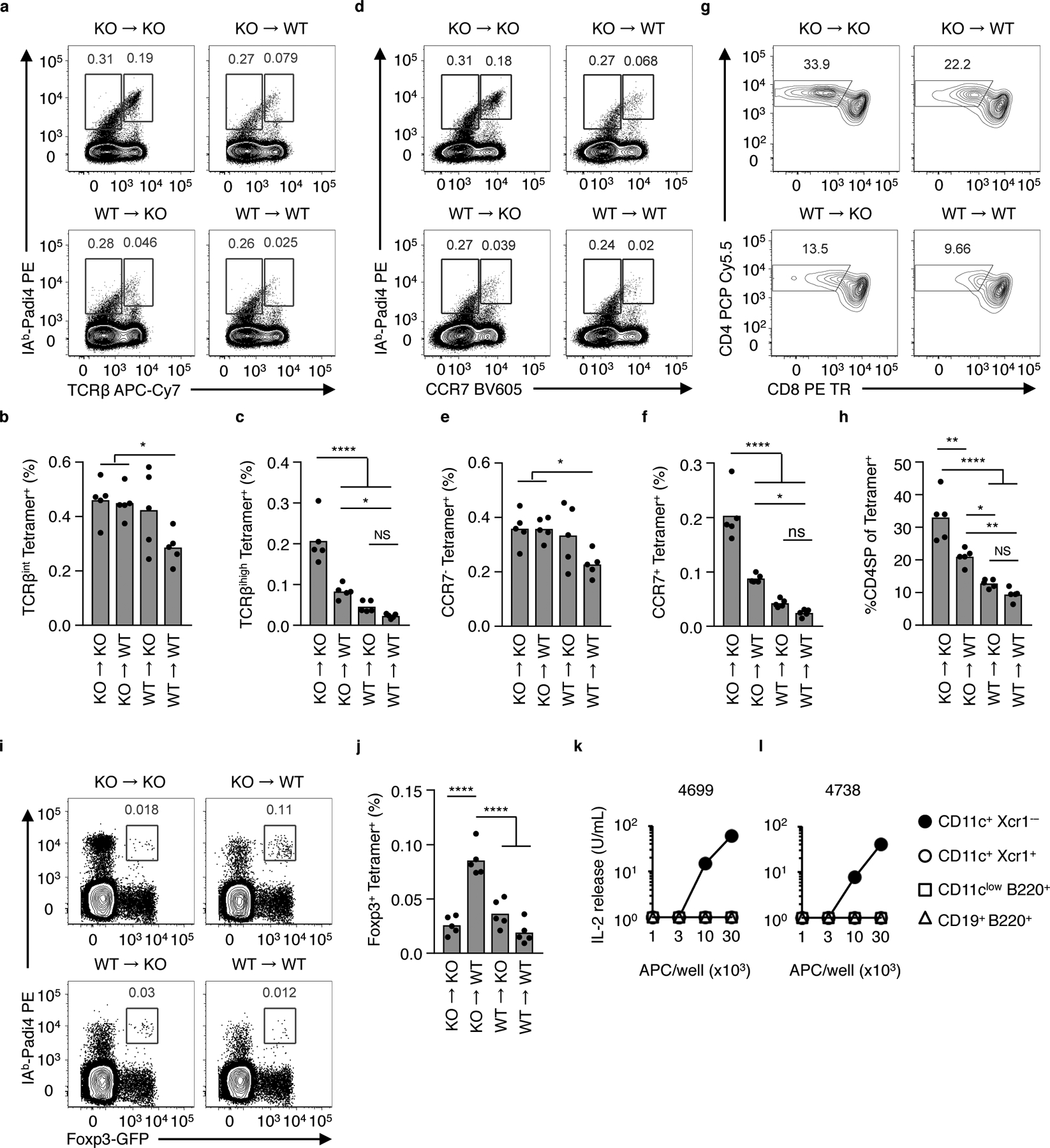
Limiting Negative Selection restores development of Padi4-specific tTreg cells in the adult thymus. (a-j) Eliminating Padi4 expression in BM derived cells increases the frequency of Padi4-specific TCRβhi CCR7+ CD4SP thymocytes and tTreg cells. Flow cytometry analyses of thymocytes isolated from mixed WT and Padi4−/− (KO) YAe62β bone marrow chimeric mice. (a, b) Total Vα2+ thymocytes analyzed for IAb-Padi4 tetramer binding and (a) TCRβ and (b) CCR7 expression. (c) Flow cytometry analyses of total Vα2+ IAb-Padi4 tetramerpos thymocytes for CD4 and CD8 expression. (d-h) Quantification of Vα2+ IAb-Padi4 tetramerpos (d) TCRβint (e) TCRβhi (f) CCR7neg (g) CCR7pos and (h) CD4SP thymocytes. (i) Vα2+ CD4SP thymocytes analyzed for IAb-Padi4 tetramer binding and Foxp3 expression, and (j) quantification of IAb-Padi4 tetramerpos Foxp3-GFP+ CD4SP thymocytes. Data is from two independent experiments giving similar results, n = 5 mice per group, bars represent the data mean. (k,l) IL-2 response of the (k) 4699 and (l) 4738 tTreg hybridomas cultured with titrating numbers of CD11c+ Xcr1neg cDC (filled circle), CD11c+ Xcr1pos cDC (open circle), CD11clo B220+ pDC (open squares) and CD19+ B220+ B cells (open triangle) isolated from thymus of adult C57BL/6 mice. Data is from two independent experiments giving similar results. Significance identified using a one-way ANOVA Tukey multiple comparisons test, ns P>0.05, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Exclusive expression of Padi4 in radioresistant cells result in a partial rescue of Padi4-specific T cell development. In Padi4−/− BM into Padi4+/+ mice (KO -> WT), significantly more Padi4-specific CCR7pos TCRβhi CD4SP thymocytes are generate, as compared to mice that express Padi4 in both compartments (Fig. 6a–h). Further, the elimination of Padi4 expression in BM-derived cells resulted in the differentiation of Padi4-specific CD4SP cells into tTreg cells (Fig. 6i,j). These data argue within the adult thymus, Padi4-expression in adult thymic stromal cells is sufficient to generate Padi4-specific tTreg cells, however, BM derived cells present IAb-Padi4 to developing thymocytes in a manner sufficient to induce negative selection at the DP to CD4SP transition.
Neonatal Padi4-specific tTreg cells express TCRs with modest dwell-times
The neonatal development of Padi4-reactive tTreg cells and CD4+ Tconv cells, as well as the overall depletion of Foxp3neg M1 and M2 CD4SP cells, suggests thymocytes expressing Padi4-reactive TCRs are under selection pressures. To identify and characterize TCRs expressed on Padi4-specific thymocytes that audition for selection into the neonatal tTreg repertoire, IAb-Padi4 tetramerpos Foxp3-GFPneg CD4SP thymocytes were FACS sorted from 2-week old YAe62β mice, and eight novel TCRs were cloned (Fig. 7a,b). In vitro activation assays of T cell hybridomas expressing these TCRs showed a ~105-fold range of EC50 values to the Padi492–105 epitope (Fig. 7c, Supplementary Table 2).
Figure 7.
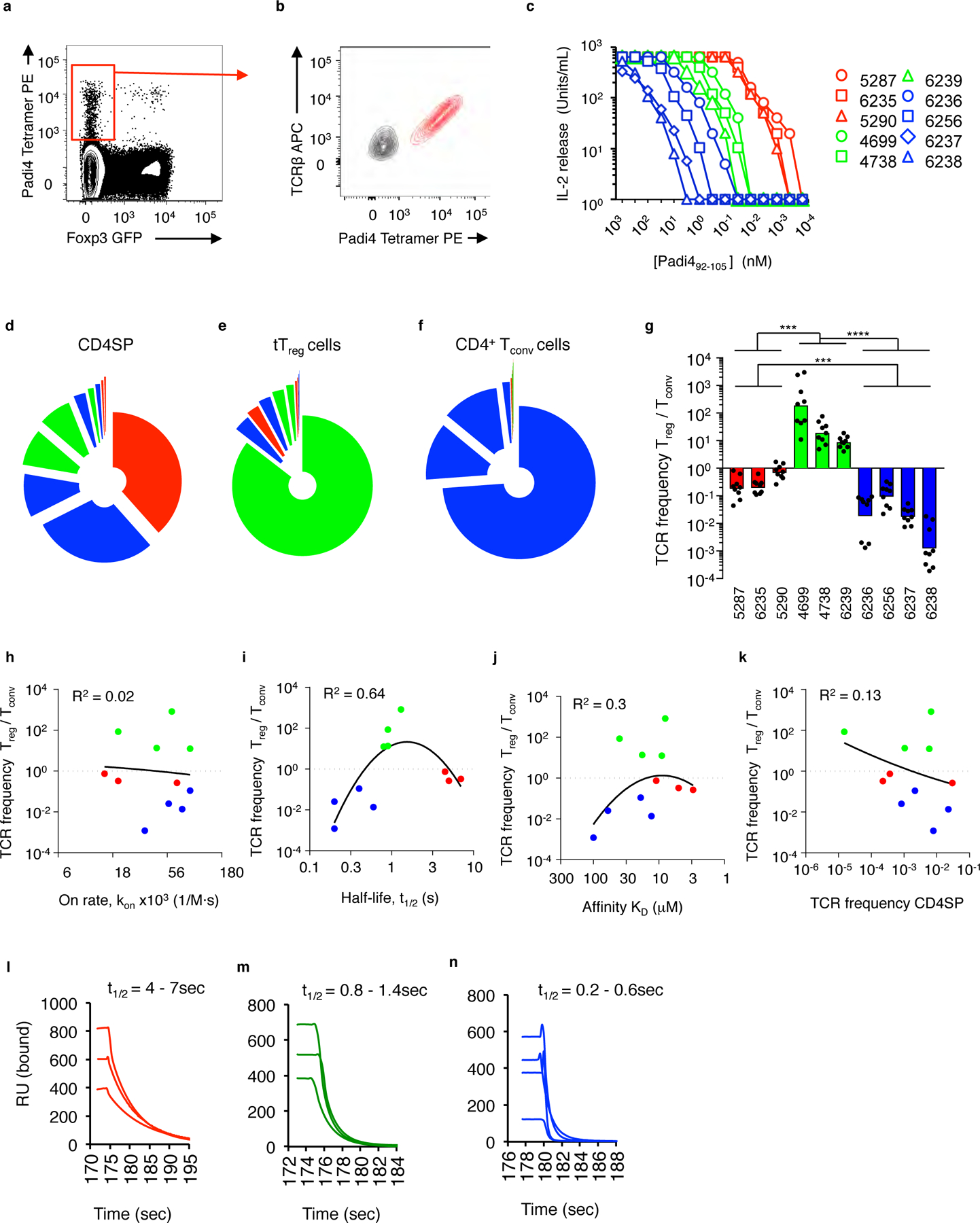
Padi4-specific neonatal tTreg cells express TCRs with modest dwell times. (a) IAb-Padi4 tetramerpos Vα2+ Foxp3-GFPneg CD4SP thymocytes isolated from YAe62β.Foxp3-GFP were sorted, and (b) TCRs cloned and re-expressed in T cell hybridomas. (c) IL-2 response of Padi4-reactive T cell hybridomas cultured with IAb-expressing fibroblasts and titrating concentration of soluble Padi492–105 peptide; see key for name of individual TCRs. (d-f) Relative distribution of Padi4-reactive TCR clonotypes carried in the (d) thymic Foxp3-GFPneg CD4SP, (e) splenic CD4+ Foxp3-GFPpos tTreg and (f) splenic CD4+ Foxp3-GFPneg Tconv cell repertoires of 2-week old YAe62β.Foxp3-GFP mice. Colored pie slices represent individual TCRs described in (c). (g) Frequency distribution of individual Padi4-reactive T cell clonotypes carried in splenic CD4+ tTreg versus CD4+ Tconv cell repertoires of 2-week old YAe62β.Foxp3-GFP mice. Data are derived from 4 independent experiments, (d-f) represented as mean fraction of repertoire, (g) bars represent the data geometric mean. (h-k) Centered second order non-linear regression analysis of 11 Padi4- and Add2-specific TCRs comparing the tTreg/Tconv cell frequency bias versus the TCR:self-pMHC (h) on-rate, kon (i) half-life, t1/2 and (j) equilibrium affinity, KD, and (k) the clonal frequency in Foxp3neg CD4SP thymocytes. Correlation plots are based on probable cure fit using Akaike’s Information Criteria, Prism 7.04. R2 values represent goodness of fit analysis. (l-n) Time scale of soluble (l) 5287, 6235, 5290, (m) 4699, 4783, 6239, and (n) 6236, 6256, 6237, 6238 TCRs disassociating from immobilized IAb-Padi4 measured by surface plasmon resonance. Sensograms are background subtracted from each TCR interacting with a non-cognate ligand. Data are derived from 4 biological replicates with similar results. (g) Significance identified using a Kruskal-Wallis test and Dunn’s multiple comparisons test, ***P<0.001, ****P<0.0001.
To reveal the fate of thymocytes expressing Padi4-reactive TCR clonotypes, TCRα deep sequencing of thymic and splenic T cell subsets isolated from YAe62β mice was performed. These experiments were used to determined the frequency at which each Padi4-reactive TCR clonotype is carried in different T cell repertoires of neonatal mice: thymic Foxp3-GFPneg CD4SP, and splenic CD25+ Foxp3-GFP+ tTreg cells and CD4+ Tconv cells (Fig. 7 d–f). Three mature T cell lineage biases were observed. Lymphocytes expressing the most potent TCRs (colored red) were largely absent from the neonatal tTreg and CD4+ Tconv cell repertoires (Fig. 7e, f). The Padi4-reactive tTreg repertoire is predominately comprised of T cell clonotypes that have moderate potency (colored green), whereas the Padi4-reactive CD4+ Tconv cell repertoire is primarily lymphocytes expressing TCRs with weak potency (colored blue) (Fig. 7e,f). Frequency bias into the tTreg or Tconv cell repertoires ranged from 10–1,000-fold (Fig. 7g).
We next evaluated whether the observed T cell lineage biases were associated with a particular parameter of the TCR:IAb-Padi4 binding reaction. Surface plasmon resonance (SPR) analyses determined that the equilibrium affinity (KD) of this collection of Padi4-specific TCRs ranged from 3–90 μM, and the half-life (t1/2) or dwell time of the interactions ranged from ~0.2–7 sec (Supplementary Table 2, Supplementary Fig. 5). Centered second order non-linear regression analysis was carried out to test whether tTreg/CD4 Tconv cell selection bias best correlated with the TCR:self-MHC (a) half-life, t1/2 (b) equilibrium affinity, KD (c) on-rate, kon or (d) the clonotype frequency in thymic Foxp3neg CD4SP repertoire. Best probable curve fits show that the t1/2 is the best correlate for predicting tTreg development (Fig. 7h–k).
Padi4-specific TCRs that promoted neonatal tTreg development ranged from ~0.8–1.4 seconds. TCRs that induce neonatal negative selection have t1/2 of 4–7sec, whereas the t1/2 of TCRs that The CD4+ Tconv cell repertoire express TCRs with very short t1/2 (Fig. 7l–n). A similar ordering occurs when kinetic values are used to estimate the confinement time (ta) of the TCR:pMHC interaction (Supplementary Table 2)27, 28. In contrast, Padi4-specific TCRs with KD values of 8–20μM promoted negative selection (TCR 5290), biased towards the tTreg repertoire (TCRs 4699, 4738, 5292 and 6239) or were enriched in the CD4 Tconv cell lineage (6236 and 6256). Consistent with these data, the Add2606–621-reactive 6287 tTreg TCR has a KD of ~40μM and t1/2 ~0.9sec. These data argue that TCR:self-pMHC dwell time forms a kinetic window that promotes the generation of neonatal Padi4-specific tTreg cells.
Padi4-specific tTreg TCRs use conventional TCR:pMHC docking orientations.
To identify in molecular detail how TCRs that regulate neonatal thymocyte fate choice bind their self-ligands, we determined the crystal structures of six TCRs bound to IAb-Padi492–105. The set includes two TCRs that induce neonatal negative selection (TCRs 6235 and 5287), or promoted the development of neonatal tTreg cells (TCRs 4699 and 4738) or CD4+ Tconv cells (TCRs 6256 and 6236) (Fig. 8a–c, Supplementary Fig. 6). The resolutions of the TCR:pMHC complexes range from 2.7 – 3.4 Å (Supplementary Table 3). The overall buried surface area (BSA) of the complexes range from 1440–1500 Å2, with ~60% of the BSA derived from TCR:IAbα chain interactions (Fig. 8d–i, Supplementary Fig. 6). Each of the TCRs binds IAb-Padi4 using a conventional TCR:pMHC orientation, with crossing angles of 41–46° and incident angles of 13–15° (Fig. 8j,k).
Figure 8.
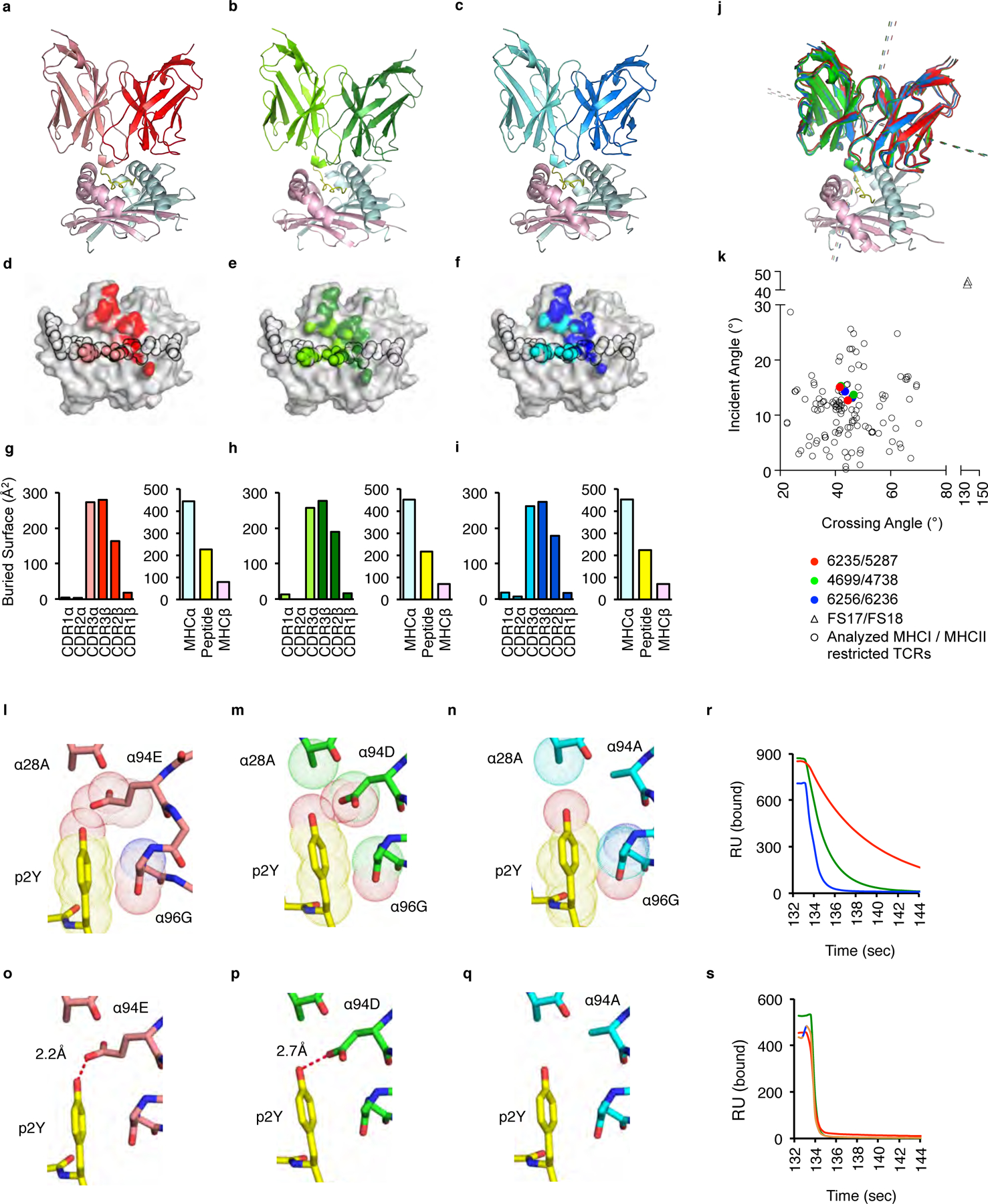
TCRs that promote neonatal negative selection, tTreg differentiation or CD4 Tconv cell development use conventional docking orientations on IAb-Padi4. (a-c) Ribbon diagrams of (a) 6235 (pdb: 6MNO), (b) 4699 (pdb:6MKD) and (c) 6256 (pdb: 6MNM) TCRs binding IAb-Padi4. The 6235 TCR is colored red (TCRβ) and pink (TCRα); the 4699 TCR is colored dark green (TCRβ) and light green (TCRα); The 6256 TCR is colored dark blue (TCRβ) and light blue (TCRα). IAb-Padi4 is colored cyan (IAbα chain), yellow (peptide), and magenta (IAbβ chain). (d-f) Projections of the (d) 6235, (e) 4699 and (f) 6256 TCRs bound to IAb-Padi4. The peptide residues are outlined in black. (g-i) The amount of buried surface area (BSA) of the (g) 6235:IAb-Padi4, (h) 4699:IAb-Padi4 and (i) 6256:IAb-Padi4 complexes contributed by TCRα and TCRβ loops, and the peptide or MHC chains. Figures were made with PyMol. (j) Overlay of 6235, 5287, 4699, 4378, 6256 and 6236 TCRs binding IAb-Padi4. Dashed lines indicate the crossing and incident angles. (k) Plot of the 6235, 5287, 4699, 4378, 6256 and 6236 TCR:IAb-Padi4 crossing and incident angles, compared to 129 human and mouse MHC-I and MHC-II TCR:pMHC complexes, including two iTreg reverse orientation (triangles)44. (l-q) Van der Waals interactions between the (l) 6235, (m) 4699 and (n) 6256 TCRα chains with the Padi4 P2Y residue, and (o-q) the presence, absence and distance of a hydrogen bond (red dash line) between the CDR3α 94 residue and the Padi4 P2Y hydroxyl moiety. (r, s) Time scale of soluble 6235 (red), 4699 (green) and 6256 (blue) TCRs disassociating from immobilized (r) IAb-Padi4 and (s) IAb-Padi4 p2F measured by surface plasmon resonance. Sensograms are background subtracted from each TCR interacting with a non-cognate IAb-Add2 ligand. SPR data are examples of 4 independent experiments, giving similar results.
Within the set, the 6235, 4699 and 6256 TCRs differ only at a single CDR3α residue (TCRα 94 E, D and A, respectively). This high degree of sequence homology allowed for detailed assessment of structural features that regulate TCR:self-pMHC binding kinetics, and ultimately neonatal thymocytes fate choice. Overall, the TCR and pMHC residues that form the binding interface and BSA are nearly identical. The most noteworthy alterations in the binding interface occur between the CDR3α 94 residue and the hydroxyl moiety of the Padi4 peptide P2 tyrosine (p2Y) (Fig. 8l–n). The 6235 CDR3α 94E residue contacts include a hydrogen bond with a length of 2.2Å, whereas the electrostatic interactions between the 4699 CDR3α 94D residue is at a less ideal distance of 2.7Å (Fig. 8o,p). The 6256 CDR3α 94A residue does not interact with the P2Y residue, with only the CDR1α 28 residue forming a van der Waals contact with the hydroxyl of the p2Y (Fig. 8n,q).
To test whether the Padi4 p2Y hydroxyl is the lynchpin controlling the differences in dwell times, SPR analyses of the three TCRs binding IAb-Padi4 carrying a phenylalanine at p2 (p2F) was performed. The 6235, 4699 and 6256 TCRs bind IAb-Padi4 with t1/2 of 5, 1.4 and 0.4 sec, respectively (Fig. 8r, Supplementary Table 2). In contrast, the three TCRs bound IAb-Padi4 p2F with near identical binding kinetics, including a more rapid t1/2 of ~0.2sec (Fig. 8s). Thus, unique interactions created between the 6235, 4699 and 6256 TCRα chains with the IAb-Padi4 p2Y hydroxyl manifest differences in the TCR:self-pMHC dwell times, which allows the 4699 TCR to bias thymocytes into the neonatal tTreg repertoire.
Discussion
Despite the critical nature of neonate-derived tTreg cells in establishing immune homeostasis, the ligand specificities of this T cell repertoire are largely unexplored. Here we identified self-ligands recognized by neonatal tTreg cells, and elucidated ligand recognition parameters that promote tTreg differentiation. Within the neonatal thymus, Padi4-specific thymocytes have a fate choice at the CD4SP stage: develop into tTreg or CD4 Tconv cells, or undergo deletion. Our results argue that the outcome of this selection branch point is clonotype specific, and predicated on the dwell time of the TCR:self-pMHC interaction. In the adult thymus, however, Padi4-reactive thymocytes undergo negative selection at the DP to CD4SP transition, precluding their ability to differentiate into CD4 Tconv or tTreg cells. Our results reveal roles for temporally regulated changes in negative selection and TCR:self-pMHC kinetics in framing the neonatal tTreg selection window.
TCR-centric hypotheses have been proposed to account for the ability of self-reactive thymocytes to escape negative selection and be exported as tTreg cells or autoimmune Tconv cells. One model, named the “hit and run” hypothesis, argues that the Foxp3 program results from altered or attenuated agonist TCR signals, which are insufficient to induce clonal deletion5. Attenuated TCR signals may arise from thymocytes recognizing low abundant thymic self-peptides or rare APCs that present them5, 12. This model arose, in part, to explain several aspects of tTreg development. Reporter models of TCR signaling, TCRβ chain usage and certain TCR-cognate self-ligand models argue that thymocytes interactions with agonist self-pMHC promote tTreg development, and that the tTreg repertoire carries increased self-reactivity, relative to the CD4 Tconv cell repertoire5, 17, 20, 29–35. However, agonist selection of tTreg cells has not been uniformly observed36, 37. Further, the TCR repertoires of CD4+ Tconv cells and Foxp3+ Treg cells display some clonotype overlap38, which may result from a broad range of TCR affinities being able to induce tTreg cell development39. Our observation that TCR:self-pMHC dwell time is a key variable in determining the outcome of neonatal T cell development is consistent with the ‘hit and run’ model, as TCR signal quality can be dependent upon TCR:pMHC binding kinetics40.
Non-canonical TCR docking on pMHC can result in the generation of abnormal TCR signal transduction events,41, 42, which may arise from altered TCR synapse formation and recruitment of co-stimulatory molecules43. How frequently altered binding modes are used by self-reactive thymocytes to escape negative selection is unclear, though the lone report of human induced Treg TCRs bound to pMHC revealed a reversed binding polarity44. In the examples presented here, the TCR:pMHC docking orientation was found to be within published examples of TCRs expressed by conventional foreign-reactive MHC-I and MHC-II restricted T cells. Nevertheless, the ~1–2sec dwell times used by Padi4-reactive tTreg cells are shorter than most T cells responding to pathogen challenge45, which may limit or alter certain aspects of TCR signaling and subsequent gene transcription during neonatal T cell development.
Thymocyte extrinsic factors contribute to the ability of tTreg cells and autoimmune CD4 T cells to develop. Cytokine availability and antigenic niches limit the total number of tTreg cells and the frequency of individual clonotypes2, 46. As mice age, peripheral mature Foxp3+ tTreg cells recirculate to the thymus, and limit the production of adult-selected tTreg cells by limiting IL-2 but not necessarily TCR signaling47, 48. Our observation that the TCR:self-pMHC dwell time correlates with thymic selection outcome supports the hypothesis that competitive forces manifest on individual clonotypes, and not necessarily between different clonotypes that recognize the same self-antigen.
Maturation of the thymus from the perinate and neonate ages to adult includes changes to thymic APC populations. The maturation process includes the seeding of the thymus with migratory DC populations, and changes in MHC-II antigen processing machinery within medullary epithelial cells4, 26. These processes can influence tTreg selection, and provide a window in which pathogenic Tconv cells can more efficiently develop49. Closing the temporal window of Padi4-specific tTreg development involves a thymocyte stage-specific switch in antigen recognition, which results in negative selection of adult Padi4-specific thymocytes at the DP to CD4SP transition. Deletion in the adult thymus occurs prior to upregulating CCR7, a chemokine receptor that promotes thymocyte migration from the cortex to medulla8, suggesting cortical residency. The dichotomy of neonate versus adult selection of Padi4-specific T cell development outcomes occurs despite studies showing that negative selection to ubiquitously expressed antigens is fully functional during the neonatal period4, 50. Our data argue that age-dependent changes in negative selection constrict the development of certain tTreg specificities to the neonatal thymus.
Supplementary Material
Acknowledgments
Supported by the US National Institutes of Health (DK095077, AR071269 and AI109858) to E.S.H. X-ray diffraction data were collected at the LRL-CAT (31-ID) beamline at APS at Argonne National Laboratory for PDB IDs 6MKD and 6MKR and the FMX (17-ID-2) beamline at NSLS II at Brookhaven National Laboratory for PDB IDs 6MNM, 6MNO, 6MNN, 6MNG.
Footnotes
Data Availability
Accession codes.
NCBI Sequence Read Archive: TCR sequence data, PRJNA534321. Coordinates and structure factors for the 6235 TCR:IAb-Padi4, 4699 TCR:IAb-Padi4, 6256 TCR:IAb-Padi4, 5287 TCR:IAb-Padi4, 4378 TCR:IAb-Padi4 and 6236 TCR:IAb-complexes are available from the Protein Data Bank under accession numbers 6MNO, 6MKD, 6MNM, 6MKR, 6MNG, 6MNN. All additional data that support the findings of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests
References
- 1.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol 2014, 15(9): 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein L, Robey EA, Hsieh CS. Central CD4(+) T cell tolerance: deletion versus regulatory T cell differentiation. Nat Rev Immunol 2019, 19(1): 7–18. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008, 133(5): 775–787. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 2015, 348(6234): 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol 2016, 16(4): 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathis D, Benoist C. Aire. Annu Rev Immunol 2009, 27: 287–312. [DOI] [PubMed] [Google Scholar]
- 7.Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, et al. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell 2015, 163(4): 975–987. [DOI] [PubMed] [Google Scholar]
- 8.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 2006, 6(2): 127–135. [DOI] [PubMed] [Google Scholar]
- 9.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med 2013, 210(2): 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med 2013, 210(4): 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med 1997, 185(2): 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissler KA, Caton AJ. The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of Foxp3(+) regulatory T cells. Immunol Rev 2014, 259(1): 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013, 339(6124): 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry JSA, Lio CJ, Kau AL, Nutsch K, Yang Z, Gordon JI, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 2014, 41(3): 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med 2009, 206(6): 1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gratz IK, Campbell DJ. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front Immunol 2014, 5: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard JD, Gilmore DC, Dileepan T, Nawrocka WI, Chao JL, Schoenbach MH, et al. Identification of Natural Regulatory T Cell Epitopes Reveals Convergence on a Dominant Autoantigen. Immunity 2017, 47(1): 107–117 e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spence A, Purtha W, Tam J, Dong S, Kim Y, Ju CH, et al. Revealing the specificity of regulatory T cells in murine autoimmune diabetes. Proc Natl Acad Sci U S A 2018, 115(20): 5265–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Nguyen P, Liu W, Cheng C, Steeves M, Obenauer JC, et al. T cell receptor CDR3 sequence but not recognition characteristics distinguish autoreactive effector and Foxp3(+) regulatory T cells. Immunity 2009, 31(6): 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieback E, Hilgenberg E, Stervbo U, Lampropoulou V, Shen P, Bunse M, et al. Thymus-Derived Regulatory T Cells Are Positively Selected on Natural Self-Antigen through Cognate Interactions of High Functional Avidity. Immunity 2016, 44(5): 1114–1126. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, et al. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol 2016, 17(2): 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leventhal DS, Gilmore DC, Berger JM, Nishi S, Lee V, Malchow S, et al. Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells. Immunity 2016, 44(4): 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalekar LA, Schmiel SE, Nandiwada SL, Lam WY, Barsness LO, Zhang N, et al. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat Immunol 2016, 17(3): 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Y, Wang X, Jameson SC, Hogquist KA. Late stages of T cell maturation in the thymus involve NF-kappaB and tonic type I interferon signaling. Nat Immunol 2016, 17(5): 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med 2005, 202(7): 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med 2009, 206(3): 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govern CC, Paczosa MK, Chakraborty AK, Huseby ES. Fast on-rates allow short dwell time ligands to activate T cells. Proc Natl Acad Sci U S A 2010, 107(19): 8724–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aleksic M, Dushek O, Zhang H, Shenderov E, Chen JL, Cerundolo V, et al. Dependence of T cell antigen recognition on T cell receptor-peptide MHC confinement time. Immunity 2010, 32(2): 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001, 2(4): 301–306. [DOI] [PubMed] [Google Scholar]
- 30.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol 2002, 3(8): 756–763. [DOI] [PubMed] [Google Scholar]
- 31.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 2007, 8(4): 351–358. [DOI] [PubMed] [Google Scholar]
- 32.Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, et al. CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity 2015, 43(5): 896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med 2003, 197(4): 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011, 208(6): 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stadinski BD, Shekhar K, Gomez-Tourino I, Jung J, Sasaki K, Sewell AK, et al. Hydrophobic CDR3 residues promote the development of self-reactive T cells. Nat Immunol 2016, 17(8): 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med 2004, 200(10): 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity 2007, 27(3): 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol 2006, 7(4): 401–410. [DOI] [PubMed] [Google Scholar]
- 39.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity 2012, 37(3): 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreotti AH, Joseph RE, Conley JM, Iwasa J, Berg LJ. Multidomain Control Over TEC Kinase Activation State Tunes the T Cell Response. Annu Rev Immunol 2018, 36: 549–578. [DOI] [PubMed] [Google Scholar]
- 41.Hahn M, Nicholson MJ, Pyrdol J, Wucherpfennig KW. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat Immunol 2005, 6(5): 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams JJ, Narayanan S, Liu B, Birnbaum ME, Kruse AC, Bowerman NA, et al. T cell receptor signaling is limited by docking geometry to peptide-major histocompatibility complex. Immunity 2011, 35(5): 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubert DA, Gordo S, Sabatino JJ Jr., Vardhana S, Gagnon E, Sethi DK, et al. Self-reactive human CD4 T cell clones form unusual immunological synapses. J Exp Med 2012, 209(2): 335–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beringer DX, Kleijwegt FS, Wiede F, van der Slik AR, Loh KL, Petersen J, et al. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat Immunol 2015. [DOI] [PubMed] [Google Scholar]
- 45.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol 2005, 3(11): e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol 2009, 10(6): 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C, et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol 2015, 16(6): 628–634. [DOI] [PubMed] [Google Scholar]
- 48.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol 2015, 16(6): 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huseby ES, Sather B, Huseby PG, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity 2001, 14(4): 471–481. [DOI] [PubMed] [Google Scholar]
- 50.Dong M, Artusa P, Kelly SA, Fournier M, Baldwin TA, Mandl JN, et al. Alterations in the Thymic Selection Threshold Skew the Self-Reactivity of the TCR Repertoire in Neonates. J Immunol 2017, 199(3): 965–973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


