Abstract
BACKGROUND:
Left ventricular assist device (LVAD) therapy improves the hemodynamics of advanced heart failure patients. However, it is unknown whether hemodynamic optimization improves clinical outcomes. The aim of this study was to investigate whether hemodynamic optimization reduces hospital readmission rate in LVAD patients.
METHODS AND RESULTS:
LVAD patients undergoing an invasive hemodynamic ramp test were prospectively enrolled and followed for 1 year. LVAD speed was optimized using a ramp test, targeting the following goals: central venous pressure <12 mm Hg, pulmonary capillary wedge pressure <18 mm Hg, and cardiac index >2.2 L/(min·m2). The frequency and cause of hospital readmissions were compared between patients who achieved (optimized group) or did not achieve (nonoptimized group) these goals. Eighty-eight outpatients (median 61 years old, 53 male) underwent ramp testing 236 days after LVAD implantation, and 54 (61%) had optimized hemodynamics after LVAD speed adjustment. One-year survival after the ramp study was comparable in both groups (89% versus 88%). The total hospital readmission rate was lower in the optimized group compared with the nonoptimized group (1.15 versus 2.86 events/y, P<0.001). This result was predominantly because of a reduction in the heart failure readmission rate in the optimized group (0.08 versus 0.71 events/y, P=0.016).
CONCLUSIONS:
LVAD patients, in whom hemodynamics were optimized, had a significantly lower rate of hospital readmissions, primarily because of fewer heart failure admissions. These findings highlight the importance of achieving hemodynamic optimization in LVAD patients.
Keywords: blood pressure, central venous pressure, heart failure, hemodynamics, patients
Continuous-flow left ventricular assist devices (LVADs) have improved survival rates of appropriately selected patients with Stage D heart failure (HF).1 However, LVAD patients experience high rehospitalization rates because of multiple diverse comorbidities. This fact has shifted the focus of research in the area of mechanical circulatory support toward the identification of strategies which to reduce adverse events, improve quality of life, and lower cost of care.2
One of the approaches to achieve these goals has been optimization of LVAD speed and medical therapies, which have the potential to enhance device performance.3 Although existing guidelines have few specific recommendations regarding optimization of LVAD speed,4 the utility of the hemodynamic ramp test for this purpose has recently been shown in carefully conducted studies.3
Although it is plausible that optimization of hemodynamics may reduce recurrent HF events and potentially other serious complications,5 to date, no prospective studies have confirmed the prognostic implication of hemodynamic optimization in LVAD patients.6 This study was undertaken to determine if optimization of hemodynamic values reduces the rate of adverse events in LVAD recipients.
METHODS
Patient Selection
The data, analytic methods, and study materials will be made available from corresponding author to any researchers for purposes of reproducing the results or replicating the procedure. Clinically stable LVAD outpatients (HVAD or HeartMate II) underwent the previously described hemodynamic ramp study3 as part of our routine institutional LVAD follow-up protocol between April 2014 and January 2017 and were prospectively followed for 1 year afterward until December 2017. On April 1, 2014, the senior author of this article (Dr Uriel) established a prospective hemodynamic ramp test database in which every patient sign an informed consent to participate in the study, and the data are collected at that time of ramp test with specified study coordinator (D. Rodgers). Patients with suspicion for device malfunction and those receiving continuous inotrope infusion or intravenous diuretics were excluded from this study. The study protocol was approved by the University of Chicago Institutional Review Board. The primary end point was all-cause readmission rate at 1 year after the ramp test.
Ramp Test Protocol
Patients underwent a hemodynamic and echocardiographic LVAD speed ramp study as detailed previously.3 Right heart catheterization was used to measure intracardiac pressures over a range of LVAD speeds after the confirmation of an international normalized ratio between 1.8 and 3.0. The recorded hemodynamic parameters included central venous pressure (CVP), pulmonary artery pressures (PAP), and pulmonary capillary wedge pressure (PCWP). Cardiac output and cardiac index (CI) were calculated by the indirect Fick method.7 Echocardiographic parameters including left ventricular size and valve insufficiencies were collected as detailed previously.8
After completion of data acquisition at baseline LVAD speed, pump speeds were lowered to 2300 rpm (HVAD) or 8000 rpm (HeartMate II). Then, device speeds were increased by 100 rpm increments (HVAD) or by 400 rpm increments (HeartMate II). After a 2-minute stabilization period, the same parameters were recorded. This procedure was repeated until a maximum speed of each device.
At the conclusion of each test, the attending cardiologist reviewed the data, and the device was set at the speed which yielded, whenever possible, an optimal hemodynamic profile, which required all 3 of the following: CVP <12 mm Hg, PCWP <18 mm Hg, and CI >2.2 L/(min·m2). Secondary goals of speed optimization included achievement of intermittent aortic valve opening, minimal aortic insufficiency, and minimal mitral regurgitation.4 Repeat hemodynamics data were obtained at the set LVAD speed irrespective of LVAD speed change. In addition to speed optimization, physicians also used the results to guide adjustment of medical therapies.
Follow-Up Protocol
All patients were followed at the set LVAD speed and received guideline-directed medical therapy, including aspirin and warfarin, with a goal of international normalized ratio appropriate for each device.4 During the 1-year follow-up period, all deaths and all hospital readmissions because of any causes were collected. Changes in medication doses over the month after the ramp test were also obtained.
Readmission rates were standardized by calculating re-admission number per patient-year. Readmission cause were adjudicated into one of the following categories: (1) HF, defined as hospitalizations to treat volume overloaded or pulmonary congestion with intravenous diuretics; (2) gastrointestinal bleeding, described by the Interagency Registry for Mechanically Assisted Circulatory Support as any clinically suspected or documented bleeding from the gastrointestinal tract9; (3) stroke, defined by Interagency Registry for Mechanically Assisted Circulatory Support as symptomatic ischemic cerebral infarction or intracranial hemorrhage, diagnosed by the attending neurologist; (4) Ventricular tachyarrhythmia requiring chemical or electrical cardioversion by either antitachycardia pacing or device shock; and (5) driveline infection according to the Interagency Registry for Mechanically Assisted Circulatory Support definition.
Statistical Analyses
Statistical analyses were performed with SPSS Statistics 22 (SPSS, Inc, Chicago, IL). Two-sided P-values <0.05 were considered statistically significant. Continuous variables were expressed as mean±SD and compared between groups using unpaired t test when normally distributed or expressed as median (25% interquartile, 75% interquartile) and compared using Mann-Whitney U test when nonnormally distributed. Categorical variables were compared between groups using Fisher exact test as necessary. Event rates were calculated from total event numbers divided by total observational period and compared between groups using negative binomial regression analyses.
Predictors of failure to optimize the hemodynamic profile were investigated by univariate and multivariate logistic regression analyses among baseline characteristics. Predictors of any readmissions were investigated by univariate and multivariate Cox proportional hazard ratio analyses among baseline characteristics including optimized hemodynamics. Variables with P<0.05 in the univariate analyses were used into the multivariate models by using a forward stepwise method. Kaplan-Meier analysis and log-rank test were performed for the comparison in readmission-free rate.
RESULTS
Baseline Characteristics at Ramp Test
A total of 88 LVAD patients (median 61 years old and 53 male) were enrolled (Table 1). Most patients were implanted as destination therapy (76%), and 58% had a nonischemic cause for HF. Most of them had New York Heart Association functional class I or II (83/88 [95%]). Ramp tests were performed at 236 (91, 647) days after LVAD implantation.
Table 1.
Baseline Characteristics at Ramp Test
| N=88 | |
|---|---|
| Demographics | |
| Age, y | 61 (53, 67) |
| Sex, male | 53 (60%) |
| Race, white | 49 (56%) |
| Body mass index | 27.6 (24.0, 34.6) |
| Preramp LVAD duration, d | 236 (91, 647) |
| Nonischemic cause | 51 (58%) |
| Destination therapy | 67 (76%) |
| Post-LVAD exchange | 2 (2%) |
| Device | |
| HeartMate II | 57 (65%) |
| HVAD | 31 (35%) |
| Comorbidity | |
| Hypertension | 49 (56%) |
| Diabetes mellitus | 36 (41%) |
| History of stroke | 13 (15%) |
| Atrial fibrillation | 34 (39%) |
| History of ventricular tachyarrhythmias | 18 (21%) |
| New York Heart Association functional class | |
| Class I | 35 (40%) |
| Class II | 48 (55%) |
| Class III | 5 (5%) |
| Laboratory data | |
| Hemoglobin, g/dL | 11.8±1.7 |
| Hematocrit, % | 36.4 (33.7, 38.5) |
| eGFR, mL/(min·1.73 m2) | 54 (39, 71) |
| Serum total bilirubin, mg/dL | 0.6 (0.4, 0.8) |
| Preramp readmission rate | |
| Total readmission rate, events/y | 1.13 |
| HF readmission rate, events/y | 0.16 |
| Non-HF readmission rate, events/y | 0.97 |
Data are expressed as n (%), mean±SD, or median (25% quartile, 75% quartile). eGFR indicates estimated glomerular filtration rate; HF, heart failure; and LVAD, left ventricular assist device.
Hemodynamic Ramp Test Results
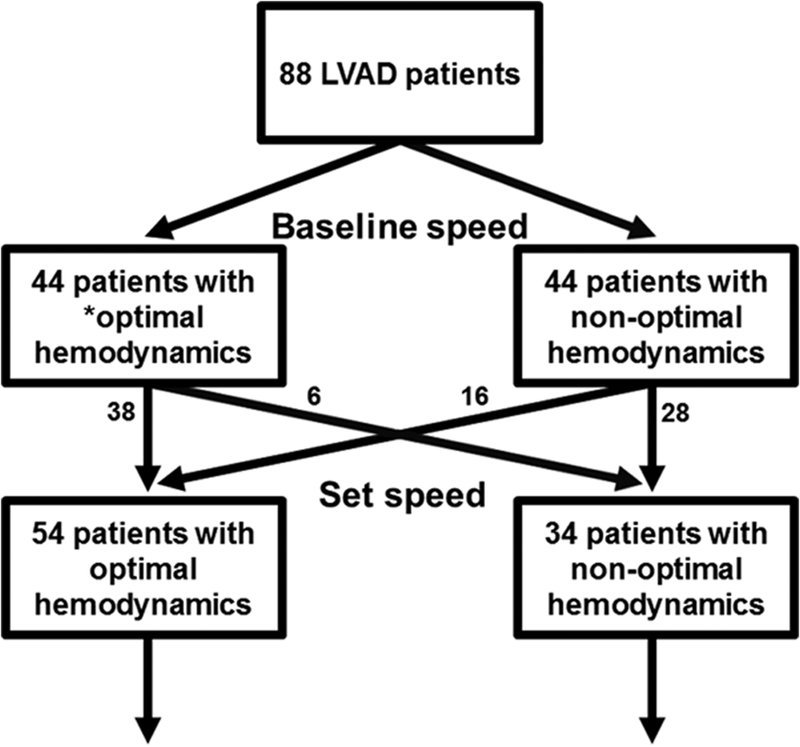
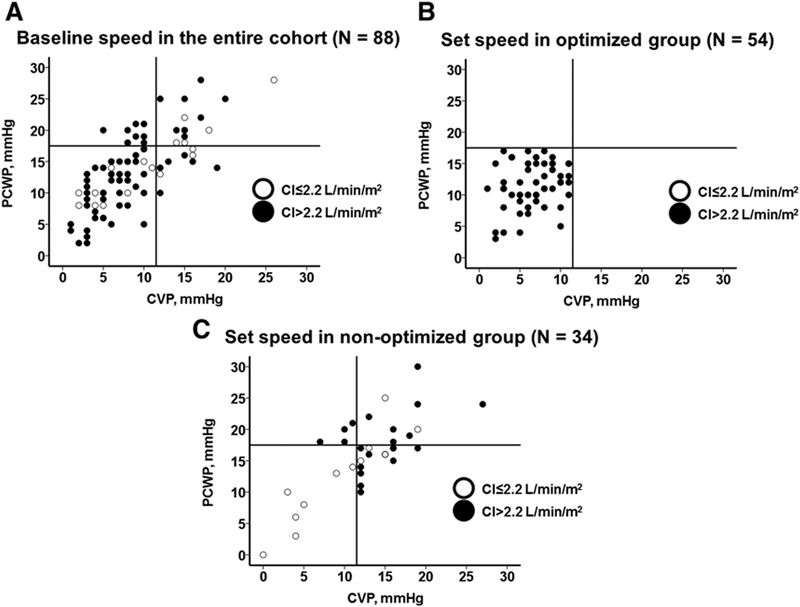
Baseline LVAD speeds were 9188±372 rpm for Heart-Mate II and 2677±162 rpm for HVAD. At baseline, 44 out of 88 patients (50%) had optimized hemodynamic profiles, whereas 44 (50%) had at least one of the 3 hemodynamic parameters outside the range (Figure 1). There was no difference in the distribution of New York Heart Association functional class between the optimized group (I, 21; II, 20; III, 3) and the nonoptimized group (I, 14; II, 28; III, 2) at baseline LVAD speed (P=0.23). Distributions of hemodynamics at baseline LVAD speed are shown in Figure 2A and in Table 2 with other clinical variables.
Figure 1. Flow sheet of the present study.
*Optimal hemodynamic profile requires all 3 of the following: central venous pressure <12 mm Hg, pulmonary capillary wedge pressure <18 mm Hg, and cardiac index >2.2 L/(min·m2). LVAD indicates left ventricular assist device.
Figure 2. Relationships among central venous pressure (CVP), pulmonary capillary wedge pressure (PCWP), and cardiac index (CI).
All cohort at baseline speed (A), optimized group at set speed (B), and nonoptimized group at set speed (C) are shown.
Table 2.
Baseline Characteristics, Hemodynamic and Echocardiographic Variables at Baseline LVAD Speed and at Set LVAD Speed and the Direction of LVAD Speed Change
| Optimized Group (N=54) | Nonoptimized Group (N=34) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 60 (53, 67) | 61 (52, 72) | 0.97 |
| Sex, male | 34 (63%) | 19 (56%) | 0.33 |
| Race, white | 30 (56%) | 19 (56%) | 0.35 |
| Body mass index | 28.4 (23.5, 35.7) | 29.2 (24.8, 33.0) | 0.54 |
| Preramp duration, d | 194 (73, 497) | 364 (110, 871) | 0.35 |
| Nonischemic cause | 26 (48%) | 25 (74%) | 0.016* |
| Destination therapy | 37 (69%) | 30 (88%) | 0.029* |
| Post-LVAD exchange | 0 (0%) | 2 (6%) | 0.15 |
| Device | |||
| HeartMate II | 31 (57%) | 25 (74%) | 0.10 |
| HVAD | 23 (43%) | 9 (26%) | 0.10 |
| Comorbidity | |||
| Hypertension | 32 (59%) | 17 (50%) | 0.26 |
| Diabetes mellitus | 21 (39%) | 15 (44%) | 0.40 |
| History of stroke | 7 (13%) | 6 (18%) | 0.38 |
| Atrial fibrillation | 16 (30%) | 18 (53%) | 0.025* |
| History of ventricular tachyarrhythmias | 9 (17%) | 9 (26%) | 0.20 |
| New York Heart Association functional class | 0.53 | ||
| Class I | 23 (43%) | 12 (35%) | … |
| Class II | 29 (54%) | 19 (56%) | … |
| Class III | 2 (4%) | 3 (9%) | … |
| Laboratory data | |||
| Hemoglobin, g/dL | 11.7±1.5 | 11.9±2.0 | 0.46 |
| Hematocrit, % | 36.0 (34.2, 38.4) | 37.0 (32.9, 40.6) | 0.38 |
| eGFR, mL/(min·1.73m2) | 55 (41, 76) | 50 (38, 70) | 0.38 |
| Serum total bilirubin, mg/dL | 0.5 (0.4, 0.8) | 0.7 (0.5, 0.8) | 0.55 |
| Preramp readmission rate | |||
| Total readmission rate, events/y | 1.08 | 1.21 | … |
| HF readmission rate, events/y | 0.18 | 0.12 | … |
| Non-HF readmission rate, events/y | 0.90 | 1.10 | … |
| Baseline LVAD speed | |||
| CVP, mm Hg | 6 (4, 9) | 12 (8, 16) | <0.001† |
| PCWP, mm Hg | 12.2±5.0 | 16.1±6.3 | 0.003‡ |
| CI, L/(min·m2) | 2.76 (2.48, 3.10) | 2.44 (2.04, 2.91) | 0.30 |
| CVP <12 mm Hg | 49 (91%) | 16 (47%) | <0.001* |
| PCWP <18 mm Hg | 45 (83%) | 20 (59%) | 0.011* |
| CI >2.2 L/(min·m2) | 48 (89%) | 20 (59%) | 0.001* |
| dPAP–PCWP >5 mm Hg | 38 (70%) | 23 (68%) | 0.48 |
| PAPi | 2.7 (1.9, 4.7) | 1.5 (1.0, 2.4) | 0.015† |
| MAP, mm Hg | 88.6±11.5 | 89.9±11.5 | 0.63 |
| SVR, dynes/(s·cm−5) | 1183 (952, 1555) | 1333 (985, 1581) | 0.54 |
| LVDd, cm | 5.98±1.24 | 5.83±1.21 | 0.63 |
| Intermittent AV opening | 23 (43%) | 9 (26%) | 0.095 |
| Aortic insufficiency | 1 (0, 2) | 1 (0, 2) | 0.56 |
| MR grade | 0 (0, 1) | 0 (0, 1) | 0.93 |
| Set LVAD speed | |||
| CVP, mm Hg | 7 (4, 9) | 13 (10, 16) | <0.001† |
| PCWP, mm Hg | 11.4±3.6 | 16.0±6.2 | <0.001‡ |
| CI, L/(min·m2) | 2.76 (2.46, 3.13) | 2.38 (2.04, 2.84) | 0.041† |
| CVP <12 mm Hg | 54 (100%) | 11 (32%) | <0.001* |
| PCWP <18 mm Hg | 54 (100%) | 21 (62%) | <0.001* |
| CI >2.2 L/(min·m2) | 54 (100%) | 21 (62%) | <0.001* |
| dPAP–PCWP >5 mm Hg | 42 (78%) | 21 (62%) | 0.09 |
| PAPi | 2.7 (2.0, 5.4) | 1.3 (1.0, 2.0) | <0.001† |
| MAP, mm Hg | 91.6±11.9 | 92.5±13.8 | 0.76 |
| SVR, dynes/(s·cm−5) | 1259 (950, 1536) | 1320 (934, 1611) | 0.32 |
| LVDd, cm | 5.93±1.17 | 5.79±1.16 | 0.59 |
| Intermittent AV opening | 26 (48%) | 13 (38%) | 0.25 |
| Aortic insufficiency | 1 (0, 2) | 1 (0, 2) | 0.63 |
| MR grade | 0 (0, 1) | 0 (0, 1) | 0.79 |
| Changes in LVAD speed | |||
| Decreased | 11 (20%) | 6 (18%) | 0.76 |
| Unchanged | 17 (31%) | 11 (32%) | 0.82 |
| Increased | 26 (49%) | 17 (50%) | 0.85 |
Aortic insufficiency and MR were graded as follows: 0, none; 1, trace; 2, mild; 3, mild to moderate; 4, moderate; 5, severe. AV indicates aortic valve; CI, cardiac index; CVP, central venous pressure; dPAP, diastolic pulmonary artery pressure; eGFR, estimated glomerular filtration ratio; HF, heart failure; LVAD, left ventricular assist device; LVDd, left ventricular diastolic diameter; MAP, mean arterial pressure; MR, mitral valve regurgitation; PAPi, pulmonary artery pressure index; PCWP, pulmonary capillary wedge pressure; and SVR, systemic vascular resistance.
P<0.05 by Fisher exact test.
P<0.05 by Mann-Whitney U test.
P<0.05 by unpaired t test.
After adjustment of LVAD speed (9385±432 rpm for HeartMate II and 2709±154 rpm for HVAD), hemodynamic profile was improved in 16 out of 44 (36%) of the patients with abnormal hemodynamics at baseline, whereas hemodynamics worsened in 6 out of 44 (14%) patients with normal hemodynamics at baseline (Appendix Table I in the Data Supplement). These resulted in a total of 54 out of 88 patient (61%) with optimal hemodynamics (Figure 1). Distribution of each hemodynamic variable at the set LVAD speed in the optimized group (Figure 2B) and the nonoptimized group (Figure 2C) are shown in Figure 2 and in Table 2 with other clinical variables. Notably, only 11 out of 34 of the nonoptimized patients (32%) achieved CVP <12 mm Hg, whereas 21 out of 34 (62%) achieved PCWP <18 mm Hg, and 21 out of 34 (62%) achieved CI >2.2 L/(min·m2).
There were no statistically significant differences in the rate of hemodynamic optimization between HeartMate II and HVAD (55% [31/56] versus 72% [23/32], P=0.10). Comparison of baseline characteristics stratified by the optimization of hemodynamics is shown in Table 2.
Medication changes in the month after the ramp test are shown in Appendix Table II in the Data Supplement. Of note, 35% of the nonoptimized patients experienced increases in diuretic dose versus 19% of the optimized group.
Predictors of Failure to Hemodynamic Optimization
Among nonischemic cardiomyopathy, atrial fibrillation, destination therapy, and pulmonary artery pulsatility index (PAPi) at baseline speed, which were significant in the univariate analyses (P<0.05 for all), only lower PAPi was an independent predictor of failure to optimize hemodynamics at set LVAD speed (odds ratio, 0.68; 95% CI, 0.50–0.92; P=0.014; Table 3). Among those with abnormal hemodynamics at baseline speed (n=44), destination therapy and elevated CVP at baseline were associated with persistently nonoptimized hemodynamics (P<0.05 for both; Appendix Table III in the Data Supplement).
Table 3.
Predictors of Failure of Hemodynamic Optimization at Set LVAD Speed Among Baseline Characteristics
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y | 1.00 (0.96–1.04) | 0.97 | ||
| Sex, male | 1.34 (0.56–3.22) | 0.51 | ||
| Race, white | 1.21 (0.70–2.08) | 0.50 | ||
| Body mass index | 0.98 (0.93–1.04) | 0.53 | ||
| Preramp LVAD duration, d | 1.00 (1.00–1.00) | 0.35 | ||
| Nonischemic cardiomyopathy | 2.99 (1.18–7.58) | 0.02* | ||
| Destination therapy | 3.45 (1.05–11.3) | 0.04* | ||
| HeartMate II device | 2.06 (0.81–5.24) | 0.13 | ||
| Hypertension | 0.69 (0.29–1.63) | 0.40 | ||
| Diabetes mellitus | 1.24 (0.52–2.96) | 0.63 | ||
| History of stroke | 1.44 (0.44–4.71) | 0.55 | ||
| Atrial fibrillation | 2.67 (1.10–6.52) | 0.031* | ||
| History of ventricular tachyarrhythmias | 1.80 (0.63–5.12) | 0.27 | ||
| CVP at baseline speed, mm Hg | 1.02 (0.96–1.09) | 0.48 | ||
| PCWP at baseline speed, mm Hg | 1.00 (0.96–1.05) | 0.90 | ||
| Cardiac index at baseline speed, L/(min·m2) | 1.00 (0.51–1.97) | 0.99 | ||
| PAPi at baseline speed | 0.68 (0.50–0.92) | 0.014* | 0.68 (0.50–0.92) | 0.014* |
| Hemoglobin, g/dL | 1.11 (0.86–1.43) | 0.43 | ||
| Hematocrit, % | 1.05 (0.95–1.15) | 0.37 | ||
| eGFR, mL/(min·1.73 m2) | 0.99 (0.97–1.01) | 0.99 | ||
| Serum total bilirubin, mg/dL | 0.81 (0.40–1.63) | 0.56 | ||
| Preramp total admission, events/y | 1.04 (0.82–1.33) | 0.74 | ||
| Preramp HF admission, events/y | 0.77 (0.30–1.99) | 0.59 | ||
| Preramp non-HF admission, events/y | 1.07 (0.83–1.40) | 0.60 | ||
CVP indicates central venous pressure; eGFR, estimated glomerular filtration ratio; HF, heart failure; LVAD, left ventricular assist device; OR, odds ratio; PAPi, pulmonary artery pressure index; and PCWP, pulmonary capillary wedge pressure.
P<0.05 by logistic regression analyses.
Event-Free Rate Comparison
In the year after the ramp test, the patients with optimal hemodynamics experienced 6 deaths (11%) and 56 readmissions; the patients with abnormal hemodynamics experienced 4 deaths (12%) and 82 readmissions.
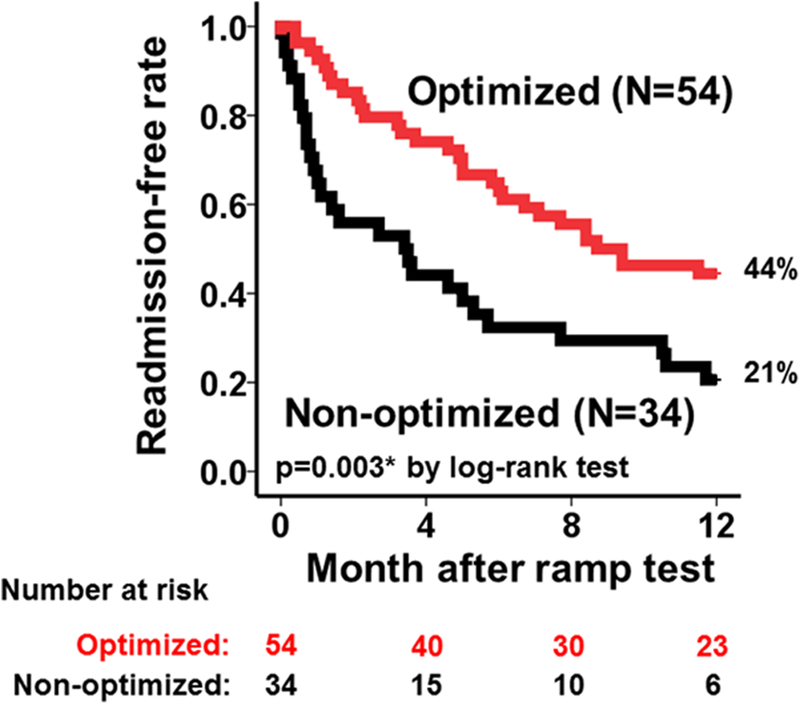
There was no difference in survival rates between the 2 groups (P=not significant). The optimized group had significantly higher hospital admission-free survival rate compared with the nonoptimized group (Figure 3; 44% versus 21%; P=0.003) with a Cox hazard ratio of 0.47 (95% CI, 0.28–0.79, P=0.005).
Figure 3. Readmission-free survival rates between optimized and non-optimized group during 1-year observational period.
*P<0.05 by the log-rank test.
The hospital admission-free survival rates were comparable when patients were stratified into 3 groups by the preramp LVAD duration (<3 months, 38%; 3 months to 1 year, 31%; >1 year, 38%; P=0.67; Appendix Figure I in the Data Supplement).
Among baseline characteristics, optimized hemodynamics (hazard ratio, 0.40; 95% CI, 0.23–0.67) and hemoglobin level (hazard ratio, 0.77; 95% CI, 0.65–0.91) were independent predictors of any readmissions after ramp tests (P<0.05 for both; Table 4).
Table 4.
Predictors of Any Readmissions After Ramp Test Among Baseline Characteristics
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.01 (0.99–1.03) | 0.46 | ||
| Sex, male | 1.28 (0.76–2.18) | 0.36 | ||
| Race, white | 1.24 (0.90–1.70) | 0.20 | ||
| Body mass index | 0.98 (0.94–1.01) | 0.23 | ||
| Preramp LVAD duration, d | 1.00 (1.00–1.00) | 0.34 | ||
| Nonischemic cardiomyopathy | 0.88 (0.52–1.48) | 0.62 | ||
| Destination therapy | 1.21 (0.65–2.24) | 0.56 | ||
| HeartMate II device | 1.21 (0.70–2.10) | 0.49 | ||
| Hypertension | 0.78 (0.46–1.31) | 0.35 | ||
| Diabetes mellitus | 1.32 (0.78–2.23) | 0.30 | ||
| History of stroke | 0.80 (0.38–1.70) | 0.57 | ||
| Atrial fibrillation | 0.96 (0.56–1.64) | 0.87 | ||
| History of ventricular tachyarrhythmias | 1.08 (0.57–2.04) | 0.81 | ||
| Optimized hemodynamics | 0.46 (0.28–0.78) | 0.004* | 0.40 (0.23–0.67) | 0.001* |
| Hemoglobin, g/dL | 0.80 (0.67–0.95) | 0.010* | 0.77 (0.65–0.91) | 0.002* |
| Hematocrit, % | 0.93 (0.87–0.99) | 0.018* | ||
| eGFR, mL/(min·1.73m2) | 1.0 (0.99–1.01) | 0.85 | ||
| Serum total bilirubin, mg/dL | 1.18 (0.88–1.59) | 0.27 | ||
| Preramp total admission, events/y | 1.10 (0.95–1.27) | 0.21 | ||
| Preramp HF admission, events/y | 0.86 (0.48–1.54) | 0.61 | ||
| Preramp non-HF admission, events/y | 1.13 (0.97–1.31) | 0.12 | ||
eGFR indicates estimated glomerular filtration ratio; HF, heart failure; HR, hazard ratio; LVAD, left ventricular assist device.
P<0.05 by Cox proportional hazard ratio regression analyses.
Readmission Rate Comparison
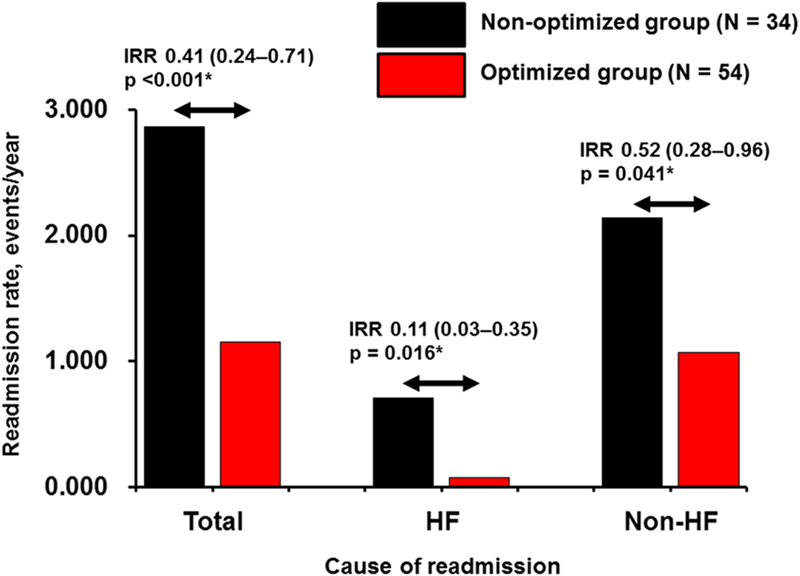
The all-cause readmission rate after ramp test in the entire cohort was 1.81 events/y. They were significantly less frequent in the optimized group compared with the nonoptimized group (1.15 versus 2.86 events/y, incidence rate ratio, 0.41; 95% CI 0.24–0.71; P<0.001; Figure 4). Approximately half of the between-group difference in all-cause readmission rate (0.63/1.71) was attributable to the lower HF readmission rate in the optimized group (0.08 versus 0.71 events/y; incidence rate ratio, 0.11, 95% CI, 0.03–0.35; P=0.016). Consistently, in the overall cohort, the most common cause of readmission was HF (20%), followed by gastrointestinal bleeding (17%). Differences in the readmission rates for all other major causes were statistically nonsignificant between the 2 groups (P >0.05 for all, data not shown).
Figure 4. Readmission rate of each cause in the optimized group and nonoptimized group.
HF indicates heart failure; and IRR, incidence rate ratio. *P<0.05 by negative binomial regression analysis.
All-cause readmission rates stratified by the change in hemodynamics during ramp test are shown in Appendix Table IV in the Data Supplement. Among those with initially nonoptimized hemodynamics (n=44), 16 became optimized and had numerically lower readmission rate compared with 28 patients whose hemodynamics remained still nonoptimized (incidence rate ratio, 0.64; 95% CI, 0.30–1.37; P=0.26).
As for the device comparison, there were no statistical differences in readmission rates between HeartMate II and HVAD (1.98 versus 1.65 events/y, P=0.15).
At 3 months after ramp test, hemoglobin and estimated glomerular filtration ratio were higher, and serum total bilirubin was lower in the optimized group compared with the nonoptimized group (Appendix Table V in the Data Supplement).
Comparison in Readmission Rates Between Preramp and Postramp Period
In the entire cohort, the all-cause readmission rate numerically increased from the preramp to postramp period (1.02–1.81 events/y; Appendix Table VI in the Data Supplement). Numerically, readmission rate increased in the nonoptimized group but remained unchanged in the optimized group.
DISCUSSION
We analyzed in this study the prognostic implications of optimized hemodynamics achieved during LVAD speed optimization. Our main findings are as follows: (1) the hemodynamic ramp test identified that 50% of patients had nonoptimized hemodynamics at their baseline LVAD speed, despite being stable outpatients; (2) ramp study–guided LVAD speed adjustment improved hemodynamic optimization from 50% to 61%; (3) the target CVP (<12 mm Hg) was the most difficult hemodynamic goal to achieve; (4) patients with lower PAPi at baseline speed were less likely to achieve optimal hemodynamics; (5) patients who remained persistently hemodynamically nonoptimized had a higher CVP and were more likely to have been implanted as destination therapy; (6) patients with optimized hemodynamics experienced significantly lower rates of hospital readmissions, primarily because of reduced rates of HF admissions; and (7) optimized hemodynamics was an independent predictor of any readmissions after ramp test.
Hemodynamic Stabilization Through Ramp Tests
Although all participants were clinically stable outpatients, 50% had abnormal hemodynamics at their baseline LVAD speed. Furthermore, we could not distinguish between the optimized group and the nonoptimized group by using New York Heart Association functional classification. This finding highlights the potential role of invasive hemodynamic tests to augment the current standard of care for assessing the hemodynamic status of a patient.3
Another important lesson learned from the ramp study is that hemodynamics cannot be optimized in 39% of patients. The hemodynamic parameter that most commonly led to failure of hemodynamic optimization was CVP (in 11 patients). This finding implies that 13% of our entire cohort has some degree of late right ventricular (RV) failure. The Evolving Mechanical Support Research Group previously reported an 8% rate of clinically diagnosed late RV failure.10 The higher rate in our cohort demonstrates the value of invasive assessment to detect hemodynamic disturbances that have not yet presented clinically. Many of these patients may have had early stages of RV failure that only presented later, as shown by the HF admission rate in the nonoptimized group. LVAD speed adjustment directly affects loading conditions for the left ventricle but has a neutral or sometimes deleterious effect on RV function.11 Thus, it is not surprising that elevated CVP was often a cause of nonoptimized hemodynamics. The role that the RV plays in post-LVAD hemodynamic optimization is further emphasized by the differences in PAPi between the 2 groups. At both the baseline speed and the set speed, PAPi was lower in the nonoptimized group, and PAPi was an only independent predictor of failure to optimize hemodynamics in the multivariate model. Furthermore, the group of 44 patients that presented nonoptimized and remained persistently nonoptimized had a significantly higher CVP at baseline LVAD speed. Nonischemic cardiomyopathy was also identified as a predictor of nonoptimization in the univariate analysis, and it is possible that this association may also be related to RV dysfunction, which is typically more present in nonischemic cardiomyopathies than ischemic cardiomyopathies. Taken as a whole, it is clear that RV failure is a major factor behind difficulty in optimizing post-LVAD hemodynamics. The hemodynamic ramp study can play a valuable role in the long-term management of LVAD patient by providing early identification of these patients who are at increased risk for HF admissions.
Atrial fibrillation was the other significant predictor of nonoptimization in the univariate analysis. A possible explanation for this finding is that atrial fibrillation may impair RV filling because of loss of atrial contraction. In the context of preexisting RV dysfunction, the loss of the atrial kick may be significant enough to promote the development of RV failure, and consequently, a failure of hemodynamic optimization. The prognostic impact of atrial fibrillation in the LVAD population is outside the scope of this study, but future research should investigate the impact of different atrial fibrillation treatment strategies on outcomes, including hemodynamics and quality of life.
Although the ramp test is not always sufficient in optimizing hemodynamics, particularly among those with underlying RV dysfunction, it may also alert clinicians to patients who are at higher risk of adverse outcomes and indicate the need for more intensive management including adjustment of HF medications.3
Survival and Readmission During LVAD Support
The 1-year survival in our cohort was acceptable and was comparable irrespective of hemodynamic optimization (87% and 88%). We should mention that this 1-year survival represents survival from the time of the ramp test and not from device implantation.
Our findings highlight the challenge of repeated read-missions after LVAD implantation. The readmission rate in our cohort was 1.81 events/y, similar to the rates of other reports (≈2.0 events/y)12–14 and serves as a reminder that adverse events remain a significant barrier to expanded use of LVADs. Baras Shreibati et al2 have demonstrated that the cost-effectiveness of LVAD therapy continues to be adversely affected by frequent readmissions. Similarly, the high readmission rates in the ROADMAP study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients) challenges the ability of LVAD to be a therapy for less sick patient populations.15,16
The most frequent causes of readmission in our cohort were HF, in line with previous reports.12,17–19 LVAD therapy aims to overcome HF by decreasing left ventricular end-diastolic pressure and increasing cardiac output.20 Thus, the persistence of HF symptoms can have 2 sources: inadequate device optimization and persistent RV failure. As shown in our study, RV failure cannot always be addressed by speed optimization alone. In contrast, a significant percentage of cases with persistent HF may be because of inadequate LVAD speed setting, and for these patients, LVAD speed optimization may prevent subsequent HF readmissions. Follow-up of echocardiography including RV function or measurements of intracardiac pressures would improve better understanding of subtype of each HF (right-side, left-side, or both).
Hemodynamic Optimization and Reduced Readmission
There has been a renewed interest in the importance of invasive hemodynamics measurement during the past decade. Denardo et al21 reported in a subanalysis of the ESCAPE trial (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) that patients with lower PCWP and higher CI had improved survival. Shah et al6 recently demonstrated that functional capacity, which was assessed by 6-minute walk distance, improved when patients’ hemodynamics were normalized by invasive hemodynamic ramp tests. The dramatic reduction in HF readmission in patients implanted with an implantable PAP monitor emphasizes the importance of hemodynamic management in HF patients in general and LVAD patients in particular.22–24
The current study is the first to report the association of optimal hemodynamics with reduced readmissions in LVAD recipients and confirms previously published hypothesis that the results of the ramp test have important prognostic implications.3 The goals of speed optimization are to reduce left ventricular end-diastolic pressure and improve cardiac output. These changes will then permit easier control of patients’ volume status, improve end-organ dysfunction, and increase the ability to manage the patients in the ambulatory setting.
The first randomized trial testing the impact of hemodynamic optimization in LVAD patients is ongoing. The Ramp-it-Up study (https://www.clinicaltrials.gov. Unique identifier: ) is a multicenter pilot study randomizing patients to speed optimization using either echocardiographic or invasive hemodynamic assistance. Patients are followed for 6 months after the ramp test with endpoints that include event-free survival (defined as survival free of hospital readmissions for HF, arrhythmias, or stroke), 6-minute walk test distance, New York Heart Association classification, and quality of life assessed by Kansas City Cardiomyopathy Questionnaire. The results of the present study provide additional support for the hypothesis being tested in this Ramp-it-Up study.
It is important to emphasize that optimal hemodynamics, although reducing HF-related admissions, do not significantly reduce the rates of other adverse events which burden LVAD recipients. Furthermore, RV failure is sometimes refractory to LVAD speed adjustment. Further investigations are needed to identify strategies that reduce all LVAD-related adverse events, including RV failure.
Study Limitations
There are several limitations of the current study. First, the study was performed at a single center for only one year. Second, we enrolled only clinically stable outpatients, and these results should not be extrapolated to patients with decompensated HF. Third, the duration of LVAD support before the ramp test spanned a wide range. This wide range may have set up a time bias, and the implication of ramp tests performed at a specific timing is next concern.25 However, hospital read-mission-free survival rates were statistically comparable irrespective of the timing of ramp test. Furthermore, LVAD duration before ramp test days was not a predictor for worse outcome (P=0.35), and the times on support before the ramp test were statistically comparable between the groups (P=0.34). Fourth, we only included HeartMate II and HVAD patients, and our results may not be extrapolated to other devices. Fifth, we demonstrated that RV failure was a key to consider hemodynamic optimization, whereas we did not show RV echo parameters, which would strengthen the mechanism of the implication of hemodynamic optimization. Sixth, the goal of performing that ramp tests was to achieve a specific hemodynamic profile; however, we did not explicitly optimize other hemodynamic parameters, such as decoupling between the diastolic PAP and PCWP.26 Seventh, we focused on the implication of optimized hemodynamics but not the implication of LVAD speed adjustment itself, which will be evaluated in the ongoing Ramp-it-Up study. Eighth, management protocols for each type of adverse event and clinical thresholds for hospital admission may vary among institutions.
Despite these limitations, we think that the current findings provide significant insight into the importance of LVAD speed adjustment and hemodynamic optimization. The findings add to the growing amount of data that support the routine evaluation of hemodynamics as well as efforts to achieve hemodynamic optimization in the LVAD population.
Conclusions
Use of ramp tests to optimize hemodynamics in LVAD patients lead to lower hospital readmissions, primarily driven by fewer HF admissions. The results suggest that routine use of these tests may improve outcomes and also alert clinicians to patients at higher risk of adverse events in whom more intensive medical therapy and monitoring may be warranted.
Supplementary Material
WHAT IS NEW?
In this large prospective study, we demonstrated for the first time the clinical implication of hemodynamic optimization through hemodynamic and echocardiographic ramp test in patients with a left ventricular assist device: optimized hemodynamics was associated with lower readmission rate.
WHAT ARE THE CLINICAL IMPLICATIONS?
We found that the optimized hemodynamics was associated with lower readmission rate, largely because of reduced heart failure readmission in patients with a left ventricular assist device.
The findings suggest to clinicians a routine hemodynamic and echocardiographic ramp test after left ventricular assist device implantation to assess hemodynamic status and adjust device speed setting.
Disclosures
Dr Imamura receives financial support from a postdoctoral fellowship for Research Abroad of Japan Society for the Promotion of Science. Dr Uriel receives grant support from Abbott and Medtronic. Dr Sayer is a consultant for Medtronic. Dr Jeevanandam is a consultant for Abbott. Dr Burkhoff is a consultant for Medtronic. The other authors report no conflicts.
Footnotes
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.118.005094.
REFERENCES
- 1.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. [DOI] [PubMed] [Google Scholar]
- 2.Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-effectiveness of left ventricular assist devices in ambulatory patients with advanced heart failure. JACC Heart Fail. 2017;5:110–119. doi: 10.1016/j.jchf.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 3.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, Jorde UP, Juricek C, Ota T, Jeevanandam V, Burkhoff D, Lang RM. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016;4:208–217. doi: 10.1016/j.jchf.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 4.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J; International Society for Heart and Lung Transplantation. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 5.Saeed O, Jermyn R, Kargoli F, Madan S, Mannem S, Gunda S, Nucci C, Farooqui S, Hassan S, Mclarty A, Bloom M, Zolty R, Shin J, D’Alessandro D, Goldstein DJ, Patel SR. Blood pressure and adverse events during continuous flow left ventricular assist device support. Circ Heart Fail. 2015;8:551–556. doi: 10.1161/CIRCHEARTFAILURE.114.002000 [DOI] [PubMed] [Google Scholar]
- 6.Shah P, Badoe N, Phillips S, Abdullah K, May CW, Nabut JL, Singh R, de-Filippi C, Desai SS. Unrecognized left heart failure in LVAD recipients: the role of routine invasive hemodynamic testing. ASAIO J. 2018;64:183–190. doi: 10.1097/MAT.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 7.Tehrani DM, Grinstein J, Kalantari S, Kim G, Sarswat N, Adatya S, Sayer G, Uriel N. Cardiac output assessment in patients supported with left ventricular assist device: discordance between thermodilution and indirect fick cardiac output measurements. ASAIO J. 2017;63:433–437. doi: 10.1097/MAT.0000000000000528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, Restaino SW, Mancini DM, Flannery M, Takayama H, John R, Colombo PC, Naka Y, Jorde UP. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60:1764–1775. doi: 10.1016/j.jacc.2012.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–3047. doi: 10.1161/CIRCULATIONAHA.111.040246 [DOI] [PubMed] [Google Scholar]
- 10.Rich JD, Gosev I, Patel CB, Joseph S, Katz JN, Eckman PM, Lee S, Sundareswaran K, Kilic A, Bethea B, Soleimani B, Lima B, Uriel N, Kiernan M; Evolving Mechanical Support Research Group (EMERG) Investigators. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J Heart Lung Transplant. 2017;36:50–58. doi: 10.1016/j.healun.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Addetia K, Uriel N, Maffessanti F, Sayer G, Adatya S, Kim GH, Sarswat N, Fedson S, Medvedofsky D, Kruse E, Collins K, Rodgers D, Ota T, Jeevanandam V, Mor-Avi V, Burkhoff D, Lang RM. 3D morphological changes in LV and RV during LVAD ramp studies. JACC Cardiovasc Imaging. 2018;11(2 pt 1):159–169. doi: 10.1016/j.jcmg.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 12.Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, D’Alessandro D, Stevens G, Goldstein DJ. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95:1276–1281. doi: 10.1016/j.athoracsur.2012.12.039 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez RE, Singh SK, Hoang DT, Ali SW, Elayda MA, Mallidi HR, Frazier OH, Meyers DE. Present-day hospital readmissions after left ventricular assist device implantation: a large single-center study. Tex Heart Inst J. 2015;42:419–429. doi: 10.14503/THIJ-14-4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smedira NG, Hoercher KJ, Lima B, Mountis MM, Starling RC, Thuita L, Schmuhl DM, Blackstone EH. Unplanned hospital readmissions after Heart-Mate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail. 2013;1:31–39. doi: 10.1016/j.jchf.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Starling RC, Estep JD, Horstmanshof DA, Milano CA, Stehlik J, Shah KB, Bruckner BA, Lee S, Long JW, Selzman CH, Kasirajan V, Haas DC, Boyle AJ, Chuang J, Farrar DJ, Rogers JG; ROADMAP Study Investigators. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: the ROADMAP Study 2-year results. JACC Heart Fail. 2017;5:518–527. doi: 10.1016/j.jchf.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 16.Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, Loebe M, Moazami N, Long JW, Stehlik J, Kasirajan V, Haas DC, O’Connell JB, Boyle AJ, Farrar DJ, Rogers JG; ROADMAP Study Investigators. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP Study. J Am Coll Cardiol. 2015;66:1747–1761. doi: 10.1016/j.jacc.2015.07.075 [DOI] [PubMed] [Google Scholar]
- 17.Akhter SA, Badami A, Murray M, Kohmoto T, Lozonschi L, Osaki S, Lushaj EB. Hospital readmissions after continuous-flow left ventricular assist device implantation: incidence, causes, and cost analysis. Ann Thorac Surg. 2015;100:884–889. doi: 10.1016/j.athoracsur.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 18.Haglund NA, Davis ME, Tricarico NM, Keebler ME, Maltais S. Readmissions after continuous flow left ventricular assist device implantation: differences observed between two contemporary device types. ASAIO J. 2015;61:410–416. doi: 10.1097/MAT.0000000000000218 [DOI] [PubMed] [Google Scholar]
- 19.Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Stulak JM, Joyce L, Daly R, Park SJ, Kushwaha SS. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163. doi: 10.1016/j.jacc.2012.09.041 [DOI] [PubMed] [Google Scholar]
- 20.Giridharan GA, Koenig SC, Soucy KG, Choi Y, Pirbodaghi T, Bartoli CR, Monreal G, Sobieski MA, Schumer E, Cheng A, Slaughter MS. Left ventricular volume unloading with axial and centrifugal rotary blood pumps. ASAIO J. 2015;61:292–300. doi: 10.1097/MAT.0000000000000201 [DOI] [PubMed] [Google Scholar]
- 21.Denardo SJ, Vock DM, Schmalfuss CM, Young GD, Tcheng JE, O’Connor CM. Baseline hemodynamics and response to contrast media during diagnostic cardiac catheterization predict adverse events in heart failure patients. Circ Heart Fail. 2016;9:e002529. doi: 10.1161/CIRCHEARTFAILURE.115.002529. [DOI] [PubMed] [Google Scholar]
- 22.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 23.Givertz MM, Stevenson LW, Costanzo MR, Bourge RC, Bauman JG, Ginn G, Abraham WT; CHAMPION Trial Investigators. Pulmonary artery pressure-guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017;70:1875–1886. doi: 10.1016/j.jacc.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 24.Feldman DS, Moazami N, Adamson PB, Vierecke J, Raval N, Shreenivas S, Cabuay BM, Jimenez J, Abraham WT, O’Connell JB, Naka Y. The utility of a wireless implantable hemodynamic monitoring system in patients requiring mechanical circulatory support. ASAIO J. 2018;64:301–308. doi: 10.1097/MAT.0000000000000670 [DOI] [PubMed] [Google Scholar]
- 25.Maltais S, Aaronson KD, Teuteberg JJ, Slaughter MS, Najjar SS, Jeevanandam V, Pham DT, McGee EC Jr, Leadley K, Kormos RL. Adverse event rates change favorably over time for patients bridged with the heartware left ventricular assist device. ASAIO J. 2017;63:745–751. doi: 10.1097/MAT.0000000000000585 [DOI] [PubMed] [Google Scholar]
- 26.Imamura T, Chung B, Nguyen A, Rodgers D, Sayer G, Adatya S, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, Juricek C, Kagan V, Jeevanandam V, Mehra M, Burkhoff D, Uriel N. Decoupling between diastolic pulmonary artery pressure and pulmonary capillary wedge pressure as a prognostic factor after continuous flow ventricular assist device implantation. Circ Heart Fail. 2017;10:e003882. doi: 10.1161/CIRCHEARTFAILURE.117.003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.