Abstract
Background
Cerebral arteriopathy is a risk factor for incident and recurrent childhood AIS. There are no standardized criteria to quantify arteriopathy severity.
Aims
To evaluate a method of scoring severity of steno-occlusive arteriopathy in childhood arterial ischemic stroke (AIS) and its association with recurrence.
Methods
In a single-center prospectively enrolled cohort of 49 children with first AIS and arteriopathy, a composite cerebrovascular stenosis score (CVSS) was measured by two independent raters as the sum of stenosis scores in each of 18 intracranial large and medium arteries, where 0 = none; 1 = low-grade, 1–50%; 2 = high-grade, >50–99%; 3 = occlusion, 100%. Cox proportional-hazards models were used to determine the association of CVSS with recurrence. The analysis was stratified by presence or absence of moyamoya arteriopathy (syndrome or disease).
Results
At a median follow-up period of 2.5 years (range: 0.8–9), 18/49 children (37%) experienced a recurrence. Median time to recurrence was 0.2 (range: 0.02–2.8) years. Interrater agreement was good, with an intraclass correlation coefficient of 0.77 [95% confidence interval (CI) 0.63–0.87, P < 0.001). Higher CVSS was associated with higher recurrence rate [hazard ratio (HR) per point 1.09, 95% CI 1.04-1–16, P = 0.001]. Among those with moyamoya arteriopathy, CVSS was associated with recurrence (HR per point of CVSS 1.11, 95% CI 1.03–1.19, P = 0.004), but there was no association in those without moyamoya arteriopathy (HR per point of CVSS 0.91, 95% CI 0.75–1.09, P = 0.32).
Conclusions
The CVSS is a reliable measure of severity of steno-occlusive arteriopathy in childhood stroke. This preliminary study suggests that higher CVSS is associated with stroke recurrence in children with moyamoya arteriopathy.
Keywords: cerebrovascular disease, childhood stroke, method, magnetic resonance imaging, risk factors, stenosis
Introduction
Steno-occlusive large-vessel intracranial disease is associated with a threefold higher rate of recurrent stroke compared with the overall rate in childhood arterial ischemic stroke (AIS) (1). Recurrence is predicted by progressive rather than nonprogressive arteriopathy, and predictors of progressive arteriopathy are arterial occlusion and moyamoya arteriopathy (2-4). There is no well-established and tested method for measuring and classifying vasculopathy severity or progression in children. In addition, the pathophysiologies of radiographically disparate arteriopathic diseases and risk factors for progression are incompletely under-stood (3-5).
In adults with large-vessel intracranial or internal carotid artery disease, the severity of stenosis predicts recurrent stroke (6,7). This literature on severity of stenosis, combined with grading scales for moyamoya arteriopathy that account for multiple vessel involvement, formed the conceptual basis for a composite cerebrovascular stenosis score (CVSS) (8). To our knowledge, the CVSS is the first assessment score designed for all childhood steno-occlusive arteriopathy. It is intended to guide risk stratification at the time of a primary acute stroke presentation.
In a large cohort study, the rate of recurrence was highest with moyamoya arteriopathy, compared with other forms of arteriopathy (9). The frequency of stroke recurrence with moyamoya arteriopathy, including idiopathic primary moyamoya disease and secondary moyamoya syndrome, ranges from 20% over 4 years to 38% over 10 years (10,11). The rate of disease progression may be high with moyamoya arteriopathy of any type. In a single-center case series of children with asymptomatic moyamoya due to sickle-cell anemia or neurofibromatosis type 1, new-onset transient ischemic attack (TIA), stroke, headache, seizure, or symptomatic hemorrhage occurred in 54% of patients. Radiographic progression in this study, defined either as progressive narrowing of the anterior cerebral, middle cerebral, or internal carotid arteries with collateral vessels or as development of sulcal hyperintensity on fluid-attenuated inversion recovery MRI (an indicator of slow cerebral blood flow) or radiographic infarction, occurred in 45% over 5.4 ± 3.8 years (12).
Fully developed moyamoya arteriopathy, with high-grade artery occlusive disease in the internal carotid and proximal circle of Willis and formation of collateral arteries, may not be evident at the time of first stroke. Magnetic resonance angiography (MRA) criteria to characterize moyamoya arteriopathy have been proposed. Houkin and colleagues described a grading system similar to the scale proposed here, but their system accounts for stenosis in only four paired arteries because it is intended exclusively for moyamoya arteriopathy (8). Sometimes the diagnosis of moyamoya is not fully evident at the time of the first stroke. We seek here to determine whether a comprehensive scale designed for any arteriopathy can also be used to predict outcome with moyamoya. In 1969 Suzuki and Takaku described six stages of moyamoya disease on conventional angiography. Early stages include narrowing of the internal carotid and mainstem intracerebral arteries, followed by occlusive arterial changes with reduction of blood flow, and finally disappearance of the moyamoya with virtual disappearance of the internal carotid (13). Early Suzuki stages may not be identified as moyamoya arteriopathy. A standardized measure of disease severity for any arteriopathy has utility in measuring the effects of an intervention designed either to reduce recurrence or modify the course of progression. The Suzuki staging describes progression on conventional angiogram. The classification system proposed in this study is MRA-based and has the advantage of avoidance of an invasive study.
Aims
We developed a novel scoring method that assigns a CVSS to characterize radiologic severity of cerebral arteriopathy and its change over time. We tested the hypothesis that a higher CVSS, indicative of a more severe arteriopathy at the time of initial stroke presentation, is associated with a higher risk of recurrent stroke.
Methods
Subjects
Participants were identified from a single-center prospective consecutive cohort enrolled in a pediatric stroke registry at the Children’s Hospital of Philadelphia (CHOP). Informed consent was obtained in accordance with a protocol approved by the local institutional review board. The registry was queried for all children with first-ever acute AIS from January 1, 2002, to June 1, 2011. Medical records and imaging were reviewed for eligibility for the study. Inclusion criteria were age of 29 days to 18 years at stroke onset, availability of initial vascular imaging study, finding of an intracranial vascular abnormality on initial vascular imaging, and at least one clinical or radiographic follow-up encounter. Cases were excluded for nonvascular causes of stroke. Among 176 children with first-onset AIS enrolled in the registry in this time interval, 49 were eligible and were included in the analysis.
Vascular imaging and classification
Source data included MRA studies performed at CHOP and at outside facilities that were uploaded to the CHOP radiology system. Images were acquired on both 1.5- and 3-Tesla scanners. Only one study involved postcontrast imaging. For two studies, only reformatted images without source imaging were available. The diagnosis of moyamoya or other arteriopathy subtype was not necessarily established with first stroke presentation. Here we classify participants as having moyamoya or other arteriopathy if the diagnosis was made at any time in the study interval during which they received care. Moyamoya arteriopathy was defined by characteristic unilateral or bilateral stenosis or occlusion of the internal carotid arteries and proximal circle of Willis vessels with collateral formation. Moyamoya arteriopathy includes idiopathic moyamoya disease and moyamoya syndrome. Determination of arteriopathy subtype was done centrally by one author (RI) who was blinded to CVSS scoring; it was based on review of clinical charts and imaging findings. Subtypes, as adapted from Sebire et al., included moyamoya arteriopathy, focal cerebral arteriopathy involving proximal segmental middle cerebral artery stenosis with or without involvement of the distal internal carotid artery, infectious arteritis, intracranial extension/embolism from cervical vessel disease, and ‘other’ focal or multifocal intracranial occlusion or stenosis (14). Cases classified as extension/embolism from cervical disease were considered ‘confirmed’, if there was definitive imaging evidence of cervical dissection, or ‘suspected’ if there was high clinical suspicion and imaging was consistent but not definitive for dissection. The ‘other’ category included unifocal or multifocal stenosis or occlusion not conforming to the other categories, such as isolated basilar artery stenosis.
Image analysis and scoring
Non-contrast MRA 3D time-of-flight sequences with maximum-intensity projection reformats were scored by two independent raters (DMM and SMS). The raters visually inspected 18 large intracranial vessels: bilateral intracranial internal carotid arteries, A1 segments, A2 segments, middle cerebral arteries, posterior cerebral arteries, superior cerebellar arteries, intracranial vertebral arteries, posterior inferior cerebellar arteries and the unpaired anterior communicating artery, and the basilar artery. The anterior and middle cerebral arteries were scored through their second segment. The posterior cerebral artery was scored through the level of the dorsal brainstem. The 18 vessels were inspected from the base of the brain cranially. The vessels were followed as they converged and branched in the direction of blood flow. When there was a focal irregularity in the contour of the vessel or an interruption was seen in the linear borders of a vessel, the vessel was noted as abnormal on the scoring sheet. Good visualization of similar-caliber vessels and comparison with a paired vessel segment were used to strengthen a rater’s certainty about abnormality. An abnormality in the vessel was categorized by the amount of visually estimated narrowing compared with the pre- and poststenosis lumen. A vessel was assigned a score of 1 if the percentage change in the caliber of the lumen was ≤50% (low-grade stenosis) and a score of 2 if the percent change in the caliber of the lumen was 50–99% (high-grade stenosis). If there was a complete interruption of flow or 100% change in caliber, the vessel was noted to have an occlusion. Five small-caliber vessels – the anterior communicating artery, bilateral superior cerebellar arteries, and posterior inferior cerebellar arteries – were felt to be too small to estimate the degree of stenosis on MRA. These five vessels were not scored for stenosis; they were scored only for the presence of an occlusion.
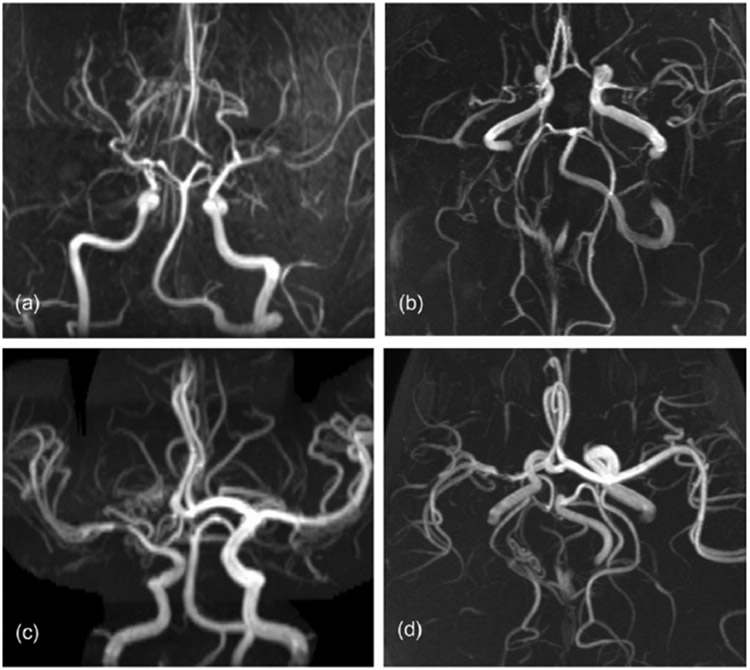
To calculate the CVSS, the data were combined, and occlusion was assigned a score of 3. An abnormal vessel was scored 1 for low-grade stenosis, 2 for high-grade stenosis, or 3 for occlusion. The individual scores across the 18 vessels were added (Appendix S1). Only patients with arteriopathy as a cause of stroke were included, so the anticipated range of scores was 1–54. Time to score a study was approximately 15 min. Illustrative images are shown for cases with mild and severe steno-occlusive arteriopathy with their respective CVSS (Fig. 1).
Fig. 1.
(a, b) Severe bilateral moyamoya disease, composite cerebrovascular stenosis score (CVSS) = 10. (c, d) Unilateral variant moyamoya, CVSS = 5.
Clinical data
Medical records were abstracted for data on clinical presentation, past medical history, associated medical conditions, stroke risk factor diagnostic studies, hospital course, and clinical status at clinic follow-up including symptoms of recurrent stroke or TIA. It is standard practice in our center for all patients to undergo standardized diagnostic testing for stroke risk factors according to an institutional clinical stroke care pathway that includes vascular imaging, cardiac evaluation, and thrombophilia testing. Stroke subtype is classified in the stroke registry into one of the following etiologic categories based on combined clinical and radiologic data: cardioembolic, intracranial arteriopathy, aortocervical arteriopathy, infectious or autoimmune arteritis, hemoglobinopathy, thrombophilia, and tumor-related stroke. Determination of date of onset of first clinical stroke was confirmed by chart review. Acute AIS was defined as an acute clinical deficit consistent with ischemia in an arterial distribution and confirmed by the finding of an acute infarct on neuroimaging corresponding to the localization of the clinical deficit, consistent with the recommendations by the National Institute of Neurological Disorders and Stroke’s Stroke Common Data Elements project (15,16). Data on demographics and on chronic health conditions associated with arteriopathy and thrombophilia were abstracted from the medical record by a single investigator (SMS). This investigator reviewed all subsequent follow-up stroke clinic visit charts and neuroimaging for recurrent events. Chart review was performed before scoring.
Radiographic follow-up
All follow-up neuroimaging and radiographic reports were reviewed for new ischemic lesions, defined as any of the following: hypodensity on CT, a new MRI lesion with restricted diffusion, or an MRI T2-hyperintense lesion not present on the prior neuroimaging.
Recurrent stroke or TIA
Electronic and paper medical records from pediatric stroke clinic visits, pediatric neurology clinic visits, and other physician visits were reviewed. Recurrent events were defined as (i) new clinically evident acute AIS with neuroimaging evidence of new infarct; (ii) clinically silent stroke, defined as neuroimaging evidence of new infarct without clinical symptoms; or (iii) TIA, defined clinically as a sudden focal neurological deficit of presumed vascular origin lasting <24 h without evidence of corresponding infarction on imaging (16). A TIA was adjudicated when this was clearly documented in a physician note. In three patients in whom the diagnosis was unclear from the record, the treating physician was contacted to determine his or her impressions of the clinical event. When there were multiple recurrent events, the first event was used for the analysis.
Statistical analysis
All analyses were performed using statistical software (SPSS, IBM, Armonk, NY, USA; Stata, StataCorp, College Station, TX, USA). Age, CVSS score, total follow-up time, number of follow-up visits, and number of follow-up imaging studies were not normally distributed and are reported as medians with ranges. Median estimate and proportion of participants were compared among participants with and without recurrence using the Wilcoxon rank-sum test and Fisher’s exact test as appropriate. Cox proportional-hazards models were used to compare the relative rate of recurrence for continuous CVSS, clustered by participant as the two raters’ scores are not independent (scores based on same participant). The selection of threshold scores with maximized potential to predict recurrence was based on visualization of the trend in hazard ratio by score. These were tested using nonparametric receiver operating characteristic (ROC) curves. Due to the small number of study participants, univariate analyses for recurrence were performed for moyamoya arteriopathy, chronic condition without moyamoya arteriopathy, and thrombophilia. Only one variable acted as an effect modifier, so multiple covariate models were not tested. To test for interaction with moyamoya arteriopathy, an interaction term was added to the Cox proportional-hazards model with CVSS score and moyamoya as independent variables. Interrater reliability of the CVSS scores was compared between the two raters with an intraclass correlation coefficient (ICC) using one-way analysis of variance. The ICC was considered to indicate moderate agreement if 0.41 to 0.60, substantial agreement if 0.61 to 0.80, and almost perfect (excellent) agreement if 0.81 to 1.00 (17). A two sided P value of ≤0.05 was considered statistically significant.
Results
Baseline clinical and vascular characteristics
The median age of the participants at the time of first-ever stroke was 6.4 (range: 0.4–17.8) years. The proportion male was 73%. The arteriopathy subtype, associated chronic medical conditions, and findings on thrombophilia testing are summarized in Table 1. Anatomic localization of arteriopathy was predominantly in the anterior circulation in 28/49 (57%), predominantly in the posterior circulation in 16/49 (33%), and in both anterior and posterior circulations in 5/49 (10%). There were 14 participants with moyamoya arteriopathy, and seven participants had a chronic condition associated with a nonmoyamoya arteriopathy. Thrombophilia testing was available for 47 participants (96%), of whom 30 (64%) had at least one abnormality.
Table 1.
Arteriopathy subtype, associated medical diagnoses and thrombophilia
| Arteriopathy subtype | N |
|---|---|
| Moyamoya arteriopathy | 14 |
| Idiopathic (9) | |
| Hemoglobinopathy (2) | |
| Fibromuscular dysplasia, systemic (1) | |
| NF1 with cranial radiation (2) | |
| Focal cerebral arteriopathy (MCA, ICA) | 9 |
| Infectious arteritis | 4 |
| Bacterial meningitis (3) | |
| HIV (1) | |
| Focal or multifocal vessel occlusion/stenosis from intracranial extension or embolism from cervical vessel disease | 12 |
| Cervical carotid artery dissection, confirmed (1) | |
| Cervical vertebral artery dissection, confirmed (7) | |
| Cervical vertebral artery dissection, suspected (4) | |
| Other focal or multifocal vessel occlusion/stenosis: | 10 |
| Isolated vessel hypoplasia or stenosis in posterior circulation (4) | |
| Other COW vessel occlusion, suspected embolic or thrombotic (6) | |
| Associated medical diagnoses* | N |
| NF1 | 2 |
| Hemoglobinopathy | 3 |
| Trisomy 21 | 3 |
| Hypertension | 4 |
| Immunologic disorder† | 3 |
| Thrombophilia | N |
| Factor V Leiden mutation heterozygote | 3 |
| Prothrombin gene mutation heterozygote | 1 |
| Elevated lipoprotein a (>30 mg/dL) | 14 |
| Lupus anticoagulant (elevated dRVVT) | 10 |
| Elevated anticardiolipin Ab | 4 |
| Elevated beta-2 glycoprotein Ab | 4 |
| Protein S deficiency | 5 |
| Antithrombin III deficiency | 1 |
| Elevated factor VIII | 1 |
Associated medical conditions and thrombophilias are not mutually exclusive and may be multiple in a given patient.
Immunologic disorders include HIV, autoimmune lymphoproliferative syndrome, and SAMHD1-mutation-related systemic autoimmune disease.
MCA, middle cerebral artery; ICA, internal carotid artery; COW, circle of Willis; HTN, hypertension; NF1, neurofibromatosis type I; dRVVT, dilute Russell’s viper venom time; Ab, antibody.
Image analysis and CVSS
All 49 patients included in the final analysis had vascular imaging of adequate quality to allow for scoring in all 18 vessels. The scores ranged from 1 to 25 for rater 1 and from 0 to 26 for rater 2. The median score for rater 1 was 6 [interquartile range (IQR) 3.5–9], and that for rater 2 was 4 (IQR 3–6.5) (P = 0.037, Wilcoxon rank-sum). There was substantial agreement in CVSS between the two raters. ICC was 0.77 [95% confidence interval (CI) 0.63–0.87, P < 0.001]. ICC for the number of vessels involved was 0.76 (95% CI 0.61–0.86), P < 0.001, also indicating substantial agreement.
Follow-up and recurrence
All patients had either clinical or radiographic follow-up. Almost all of the patients n = 47, 96%) had follow-up with a pediatric stroke neurologist. There was a median of 4 (range 1–12) follow-up visits, with 82% having >1 follow-up visit. Radiographic follow-up was available for 43 (88%), all of whom had at least one follow-up MRI. Per patient, there was a median of three follow-up MRI studies (IQR 3–5 studies). The median interval from the first to the last imaging study was 2.2 (range 0.04– 8.3) years. Among 49 participants followed for a median of 2.5 (range 0.1–9) years, there were 18 children with recurrent events (37%). Median time to recurrence was 0.2 (range: 0.02–2.8) years. There was no difference in median age, proportion male, duration of follow-up, number of follow-up visits, or number of follow-up imaging studies for participants with and without recurrence (Table 2). Among all participants with recurrence, the first recurrent event was a clinical stroke in five participants, a TIA in eight participants, and a silent infarct in five participants. Of 14 participants with moyamoya arteriopathy, 10 had a recurrent stroke or TIA. Among participants with moyamoya arteriopathy, the first recurrent event was a clinical stroke in four participants, a TIA in five participants, and a silent infarct in one participant. Among participants without moyamoya arteriopathy, the first recurrent event was a clinical stroke in three participants, a TIA in two participants, and a silent infarct in three participants. Ten of 18 participants (56%) with recurrence had subsequent or stuttering TIAs and strokes. Among 10 participants with recurrent clinical or silent strokes, there were 46 vessels affected by some degree of stenosis, and the recurrence occurred in the distal territory of 24 vessels. There were no cases of recurrence in a previously unaffected vessel. Revascularization surgery was performed in eight participants with moyamoya arteriopathy, of whom seven had recurrence: two preoperative, two postoperative, and three during a perioperative period at an outside hospital.
Table 2.
Comparison of groups
| Participants without recurrence (n = 31) |
Participants with recurrence (n = 18) |
P value | |
|---|---|---|---|
| Age (years), median (range) | 6.42 (0.37–17.85) | 8.96 (1.28–16.96) | 0.32* |
| Duration of total follow-up (years), median (range) | 2.53 (0.08–9.04) | 2.50 (0.09–7.41) | 0.83* |
| Number of follow-up imaging studies, median (range) | 3 (0–14) | 4 (0–11) | 0.11* |
| Number of follow-up visits, median (range) | 4 (1–12) | 6 (1–12) | 0.54* |
| Male, n (%) | 23 (74) | 13 (72) | 1.00† |
Wilcoxon rank-sum test.
Fisher’s exact test.
Age [hazard ratio (HR) 1.04, 95% CI 0.96–1.14, P = 0.33), chronic condition without moyamoya arteriopathy (0.29, 95% CI 0.07–1.22, P = 0.09), and thrombophilia (HR 1.14, 95% CI 0.44– 2.96, P = 0.78) were not associated with recurrence rate. Higher CVSS was associated with higher rate of recurrence [HR per point 1.09, 95% CI 1.04–1.16, P = 0.001]. An interaction term for CVSS and moyamoya was significant, P = 0.017. Therefore, CVSS was tested separately in those with and without moyamoya arteriopathy. In those with moyamoya arteriopathy, the CVSS was associated with time to recurrence, with a HR per CVSS point of 1.11 (95% CI 1.03–1.19, P = 0.004). In those without moyamoya arteriopathy, the CVSS was not associated with time to recurrence, with a HR of 0.91 (95% CI 0.75–1.09, P = 0.32) (Table 3).
Table 3.
Hazard ratios for outcome of recurrence
| Variable | Univariate HR* (95% CI) | P value |
|---|---|---|
| CVSS | 1.09 (1.4–1.16) | 0.001 |
| Age | 1.04 (0.96–1.14) | 0.33 |
| Chronic condition without moyamoya | 0.29 (0.7–1.22) | 0.09 |
| Thrombophilia | 1.14 (0.44–2.96) | 0.78 |
| Variable | Multivariable HR* (95% CI) | P value |
| CVSS | 0.91 (0.75–1.09) | 0.32 |
| Moyamoya | 1.11 (1.3–1.19) | 0.004 |
Per one-point increase in score.
HR, hazard ratio; CI, confidence interval; VSS, composite cerebrovascular stenosis score.
The area under the ROC curve for predicting recurrent stroke in those with moyamoya arteriopathy was 0.69 (95% CI 0.49– 0.90). A CVSS score of 7 maximized the ability to predict recurrence in those with moyamoya arteriopathy with a sensitivity of 75% (95% CI 51–91%) and a specificity of 50% (95% CI 16–84%). The rate of a recurrent event was more than three times higher for a score of ≥7 compared with lower scores (HR 3.04, 95% CI 1.07–8.62, P = 0.04).
Discussion
We developed a novel semiquantitative scoring method to rate severity of cerebral arteriopathy in children with AIS. Our analysis shows this MRA-based method has substantial interrater reliability. Unlike the staging system by Suzuki and Takaku, our score is based on noninvasive MRA studies. It can be readily used by radiologists and nonradiologists. Previously described scoring methods for moyamoya disease have been shown to be sensitive and specific for detecting disease. Our method can help to stratify risk of a recurrent cerebrovascular event in children with moyamoya arteriopathy. With further validation, this score can be used to measure progression and response to treatment. For every one-point increase in stenosis score in those with moyamoya arteriopathy, the rate of recurrence increased by 11%, and a score of ≥7 conferred a threefold increase in recurrence risk compared with lower scores. All recurrent events occurred in the arterial distribution of a vessel with stenosis at first stroke. In our sample the CVSS did not predict recurrence among those with other types of arteriopathy. The confidence interval for CVSS as a predictor of recurrence in nonmoyamoya cases was narrow. This suggests limited power and resolution to test the value of the CVSS in this group. The above conclusion that the score is not useful in nonmoyamoya may be a type II error.
An important strength of this study is the use of a prospectively identified cohort of children with AIS with complete laboratory and neuroimaging evaluations in the majority of participants. Clinical notes included discussion about pertinent cerebrovascular symptoms. In the majority of those with a recurrent event, the event was documented in the physician’s clinical note.
There are a number of limitations to this study. First, the study sample was small and from a single referral center so it may not be representative of childhood AIS. For example, the frequency of chronic conditions here (37%) was higher than has been reported in larger cohorts of childhood AIS (19%) (18). Second, in childhood AIS, MRA may miss arterial and embolic disease that would be detected by catheter angiography (19). Third, MRA may overestimate stenosis or may be more error-prone due to turbulence and technical limitations as compared with catheter angiography (20). Fourth, MRAs from 1.5-tesla (T) and 3-T scanners were scored in the same fashion, even though a 3-T magnet may better distinguish high-grade stenosis from occlusion. Fifth, three forms of recurrence were grouped as a composite outcome, even though the three outcomes (clinically evident stroke, silent stroke, and TIA) may not carry the same clinical importance. Sixth, this was an observational study, so follow-up was not uniformly available or carried out systematically. More follow-up imaging studies were performed in participants with recurrence, and silent infarctions may have been missed in subjects with fewer follow-up images, but we believe this should have been rare. Our cohort was followed closely, with a median of three follow-up imaging studies over a median interval of 2.2 years. Clinical outcomes were less likely to be missed because early censoring was rare in participants without recurrence. Among these participants, only one had a follow-up interval (0.15 years) that was less than the median time to a recurrent event, and only five (17%) were followed for less than one year. Seventh, the median scores and IQRs for the two raters (6 vs. 4, and 3.5–9 vs. 3–6.5) were more divergent than we would have desired and may limit the precision of our paired score to predict outcome events. Finally, our small sample size with few recurrent events limited our power to identify a relationship of the CVSS with recurrence in children with arteriopathy types other than moyamoya. Future therapies designed to stabilize or improve the course of arteriopathy could benefit from a quantitative MRA-based grading scale as a biomarker of disease. Our CVSS should be replicated with more CVSS raters and validated in another cohort, ideally with more children with nonmoyamoya arteriopathies.
Supplementary Material
Appendix S1. Scoring sheet.
Footnotes
Conflict of interest: None declared.
This study was presented as a poster at the 65th Annual Meeting of the American Academy of Neurology in San Diego, March 16–23, 2013.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- 1.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics 2007; 119:495–501. [DOI] [PubMed] [Google Scholar]
- 2.Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol 2006; 59:620–6. [DOI] [PubMed] [Google Scholar]
- 3.Braun KP, Bulder MM, Chabrier S et al. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain 2009; 132:544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabrier S, Husson B, Lasjaunias P, Landrieu P, Tardieu M. Stroke in childhood: outcome and recurrence risk by mechanism in 59 patients. J Child Neurol 2000; 15:290–4. [DOI] [PubMed] [Google Scholar]
- 5.Dlamini N, Freeman JL, Mackay MT et al. Intracranial dissection mimicking transient cerebral arteriopathy in childhood arterial ischemic stroke. J Child Neurol 2011; 26:1203–6. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Eliasziw M, Gutnikov SA et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003; 361:107–16. [DOI] [PubMed] [Google Scholar]
- 7.Chimowitz MI, Kokkinos J, Strong J et al. The warfarin-aspirin symptomatic intracranial disease study. Neurology 1995; 45:1488–93. [DOI] [PubMed] [Google Scholar]
- 8.Houkin K, Nakayama N, Kuroda S, Nonaka T, Shonai T, Yoshimoto T. Novel magnetic resonance angiography stage grading for moyamoya disease. Cerebrovasc Dis 2005; 20:347–54. [DOI] [PubMed] [Google Scholar]
- 9.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation 2006; 114:2170–7. [DOI] [PubMed] [Google Scholar]
- 10.Emam AT, Ali AM, Babikr MA. Childhood stroke in Eastern Province, KSA: pattern, risk factors, diagnosis and outcome. Acta Paediatr 2009; 98:1613–9. [DOI] [PubMed] [Google Scholar]
- 11.Lee YY, Lin KL, Wang HS et al. Risk factors and outcomes of childhood ischemic stroke in Taiwan. Brain Dev 2008; 30:14–9. [DOI] [PubMed] [Google Scholar]
- 12.Lin N, Baird L, Koss M et al. Discovery of asymptomatic moyamoya arteriopathy in pediatric syndromic populations: radiographic and clinical progression. Neurosurg Focus 2011; 31:E6. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki J, Takaku A. Cerebrovascular ‘moyamoya’ disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969; 20:288–99. [DOI] [PubMed] [Google Scholar]
- 14.Sebire G, Fullerton H, Riou E, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr 2004; 16:617–22. [DOI] [PubMed] [Google Scholar]
- 15.Saver JL, Warach S, Janis S et al. Standardizing the structure of stroke clinical and epidemiologic research data: the National Institute of Neurological Disorders and Stroke (NINDS) Stroke Common Data Element (CDE) project. Stroke 2012; 43:967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroke CDE Working Group. Data standards. 2010. Available at: http://www.commondataelements.ninds.nih.gov/Stroke.aspx#tab=Data_Standards.
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 18.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, Deveber GA, Ganesan V. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol 2011; 69:130–40. [DOI] [PubMed] [Google Scholar]
- 19.Rollins N, Dowling M, Booth T, Purdy P. Idiopathic ischemic cerebral infarction in childhood: depiction of arterial abnormalities by MR angiography and catheter angiography. AJNR Am J Neuroradiol 2000; 21:549–56. [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmann E, Wilterdink JL, Kosinski A et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology 2007; 68:2099–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Scoring sheet.