Abstract
The combination of a calcineurin inhibitor (cyclosporine [CSA] or tacrolimus [Tac]) and methotrexate (MTX) or mycophenolate mofetil (MMF) are widely used approaches to graft-versus-host disease (GVHD) prevention. There is limited and conflicting data, however, on the comparative effectiveness of MMF compared to MTX
We analyzed data from the CIBMTR for adult patients undergoing first myeloablative hematopoietic cell transplant (HCT) from an HLA-identical matched related (MRD) (N=3979) or matched unrelated donor (URD) (N=4163) using CSA+MMF, CSA+MTX, Tac+MMF or Tac+MTX for GVHD prevention from 2000–2013.
Within the MRD cohort, 2252 received CSA+MTX, 1391 Tac+MTX, 114 CSA+MMF, and 222 Tac+MMF. Recipients of CSA+MMF had a higher incidence of acute grade 2–4 (HR 1.65, 95% CI 1.24–2.20, P<0.001) and grade 3–4 (HR 1.92, 95% CI 1.31–2.83, P<0.001) GVHD compared to Tac+MTX. The use of CSA+MMF was also associated with inferior overall survival (OS) (HR 2.31, 95% CI 1.73–3.09, P<0.001) due to higher transplant-related mortality (TRM) (HR 4.03, 95% CI 2.61–6.23, P<0.001) versus Tac+MTX. Within the URD cohort, 974 received CSA+MTX, 2697 Tac+MTX, 68 CSA+MMF, and 424 Tac+MMF. CSA+MMF was again significantly associated with a higher incidence of grade 3–4 acute GVHD (HR 2.31, 95% CI 1.57–3.42, P<0001), worse OS (HR 2.36, 95% CI 1.67–3.35, P<0.001), and higher TRM (HR 3.09, 95% CI 2.00–4.77, P<0.001), compared to Tac+MTX, and other regimens.
This large retrospective comparison of MMF versus MTX in combination with CSA or Tac thus demonstrates significantly worse GVHD and survival outcomes with CSA+MMF compared to Tac+MTX.
Keywords: GVHD, MMF, MTX, myeloablative, allogeneic HCT
INTRODUCTION:
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality after allogeneic hematopoietic cell transplant (HCT). While the combination of a calcineurin inhibitor (CNI), such as tacrolimus (Tac) or cyclosporine (CSA) with methotrexate (MTX) has been a standard practice over the past several decades for GVHD prevention, this treatment is associated with several unfavorable toxicities, including mucositis [1], delayed engraftment [2], and hepatic toxicities, primarily due to MTX [3]. Mycophenolate mofetil (MMF) was initially shown to have synergy with CSA in preventing GVHD and improving survival in experimental models, [4] and despite a limited number of prospective randomized trials comparing its efficacy to MTX, the combination of CNI and MMF is commonly used in both reduced-intensity conditioning (RIC) and myeloablative (MAC) transplants. [5–10]
While small prospective studies have suggested similar outcomes of MMF and MTX in MAC transplant with improved toxicity profiles, [8–11] others have demonstrated more severe acute GVHD, primarily in unrelated donor (URD) transplants [12, 13]. Previous retrospective studies evaluating MMF have also confirmed improved toxicity but with similar GVHD and survival outcomes compared to MTX; [14, 15] whereas a more recent retrospective study of 414 patients undergoing MAC and RIC HCT demonstrated a relatively high incidence of grade III-IV acute GVHD (22.3% in related and 36.5% in unrelated donors) and non-relapse mortality (NRM) (33.3% in related and 46.5% in unrelated donors).[16] In addition, a large Center for International Blood and Marrow Transplant Research (CIBMTR) analysis comparing bone marrow (BM) versus peripheral blood (PB) grafts in URD transplant demonstrated significantly worse outcomes in overall survival (OS), NRM, and acute (a) and chronic (c)GVHD with MMF compared to MTX, further raising the question of MMF’s efficacy compared to MTX in this setting.[17]
Given these conflicting results, we sought to determine the rates of GVHD and OS in patients undergoing first myeloablative transplant using MTX versus MMF in combination with CSA or Tac.
METHODS:
Data Source:
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 420 transplantation centers worldwide that contribute data on consecutive allogeneic and autologous HCTs to a centralized statistical center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in the capacity of the CIBMTR as a public health authority under HIPPAA regulations. Additional details regarding the source have been previously described.[18]
Patients:
Patients age 18 years or older who underwent a first HLA-identical sibling or 8/8 or 7/8 HLA-matched unrelated donor HCT for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), or myelodysplastic syndrome (MDS) and received a CNI (CSA or Tac) in combination with MTX or MMF for GVHD prophylaxis reported to the CIBMTR from 2000–2013 were included. Transplants using anti-thymocyte globulin (ATG) were included, however ex-vivo T-cell depleted grafts, use of alemtuzumab, and post-transplant cyclophosphamide were excluded. Haploidentical, syngeneic and cord blood transplants were also excluded.
Study Endpoints and definitions:
The primary endpoints of this study are incidence of grade II-IV and III-IV aGVHD, cGVHD, and OS. Secondary endpoints include relapse, transplant-related mortality (TRM), and disease-free survival (DFS). The composite endpoint of GVHD-relapse-free survival (GRFS), including survival without grade 3–4 aGVHD, cGVHD requiring systemic treatment, relapse or death was also evaluated. GVHD was graded according to historical consensus criteria.[19, 20] Disease status was categorized into early, intermediate, and advanced.[21] Relapse was defined by hematologic criteria by submitting centers with non-relapse mortality as a competing event. TRM was defined as death without evidence of disease recurrence, relapse was considered a competing event. DFS was defined as time to treatment failure (death or relapse). For relapse, TRM, and DFS, patients alive in continuous complete remission were censored at last follow-up. For GVHD, death without the event was considered a competing event. HLA matching was defined as described previously.[22]
Statistical Analysis:
Patient, disease, and transplant-related variables for donor types were compared using chi-square statistics for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities for relapse, TRM, and GVHD were calculated using the cumulative incidence method to account for competing risks. Kaplan-Meier estimates were used to calculate the probability of DFS and OS. Multivariate Cox regression models were constructed to evaluate hazard ratios (HR) for endpoints of aGVHD, cGVHD, relapse, TRM, DFS and OS of GVHD prophylaxis regimens (CSA+MTX, CSA+MMF, Tac+MMF) compared with Tac+MTX as the reference. Pair-wise comparisons were also made between each combination of Tac or CSA and MTX or MMF. Analyses were performed separately in MRD and URD recipients given the known differences in GVHD between these groups as well as patterns of use of GVHD prophylaxis. Other variables in the multivariable model included: age at transplant, race, gender, Karnofsky performance status, Sorror HCT comorbidity index, disease diagnosis (AML, ALL, MDS, CML), disease status (early, intermediate, advanced), donor age (for URD), donor HLA match (7/8 versus 8/8, for URD), donor-recipient CMV match, donor-recipient sex match, graft source (PB versus BM, use of ATG, conditioning regimen, use of total body irradiation (TBI), and year of transplantation. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. When the test indicated differential effects over time (non-proportional hazards), models were constructed breaking the post-transplant time course into 2 periods, using the maximized partial likelihood method to find the most appropriate breakpoint, which may have been different for different outcomes. Several outcomes, including cGVHD, TRM and OS, demonstrated differential effects over time and are thus reported for different time-points. A backward stepwise procedure was used to identify all significant risk factors and develop models for each outcome, using a P value threshold of 0.05. Interactions between the main variable (GVHD prophylaxis) and adjusted covariates were tested at the significance level of 0.01.
RESULTS:
Transplantation from an HLA-identical related donor
Patient, Disease, and Transplant Characteristics
Patient characteristics are summarized in Table 1. In the MRD cohort, CSA+MTX was the most common GVHD prophylaxis regimen (N=2252), followed by Tac+MTX (N=1391), Tac+MMF (N=222) and CSA+MMF (N=114). Most patients receiving CSA+MTX (65%) were transplanted prior to 2005, reflected by a larger proportion of transplants for CML (29%). ATG was used infrequently, but more commonly in the CSA+MMF (11%) and Tac+MMF (14%) group compared to CSA+MTX (5%) and Tac+MTX (4%). The use of BM was more common in the CSA+MMF (28%) and CSA+MTX (36%) groups compared to Tac+MTX (8%) and Tac+MMF (7%).
Table 1:
Characteristics of patients receiving myeloablative transplant treated with Tac/CSA in combination with MTX or MMF
| Matched Related Donor | Matched Unrelated Donor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSA+MMF N=114 | CSA+MTX N=2252 | TAC+MMF N=222 | TAC+MTX N=1391 | P-Value | CSA+MMF N=68 | CSA+MTX N=974 | TAC+MMF N=424 | TAC+MTX N=2697 | P-Value | |
| Age at transplant, years, median (range) | 48 (19–69) | 48 (18–72) | 49 (18–70) | 48 (18–71) | <0.001 | 42 (18–72) | 40 (18–69) | 46 (18–73) | 45 (18–75) | <0.001 |
| Race | ||||||||||
| Caucasian | 95 (83) | 1746 (78) | 210 (95) | 1142 (82) | <0.001 | 59 (87) | 904 (93) | 386 (91) | 2475 (92) | 0.05 |
| Non-Caucasian | 16 (14) | 393 (17) | 11(5) | 166 (12) | 7 (10) | 39 (4) | 30 (7) | 160 (6) | ||
| Missing | 3 (3) | 113 (5) | 1(<1) | 83 (6) | 2 (3) | 31 (3) | 8 (2) | 62 (2) | ||
| Sorror co-morbidity index | ||||||||||
| Prior to 2007 | 57 (50) | 2033 (90) | 71 (32) | 555 (40) | 32 (47) | 820 (84) | 216 (51) | 1354 (50) | <0.001 | |
| 0–1 | 30 (26) | 155 (7) | 68 (31) | 412 (30) | 16 (24) | 84 (9) | 104 (25) | 669 (25) | ||
| 2+ | 25 (22) | 52 (2) | 82 (37) | 419 (30) | <0.001 | 13 (19) | 53 (5) | 103 (24) | 657 (24) | |
| Missing | 2 (2) | 12 (<1) | 1(<1) | 5 (<1) | 7 (10) | 17 (2) | 1 (<1) | 17 (<1) | ||
| KPS prior to transplant | ||||||||||
| <90 | 36 (32) | 441 (20) | 71 (32) | 455 (33) | <0.001 | 23 (38) | 232 (24) | 168 (40) | 838 (31) | <0.001 |
| ≥ 90 | 67 (59) | 1763 (78) | 68 (31) | 886 (64) | 42 (62) | 669 (69) | 245 (58) | 1666 (62) | ||
| Missing | 11 (10) | 48 (2) | 82 (37) | 50 (4) | 0 | 73 (7) | 11 (3) | 193 (7) | ||
| Disease | ||||||||||
| AML | 62 (54) | 879 (43) | 133 (60) | 741 (53) | <0.001 | 41 (60) | 451 (46) | 245 (58) | 1519 (56) | <0.001 |
| ALL | 12 (11) | 490 (22) | 42 (19) | 271 (19) | 12 (18) | 226 (23) | 70 (17) | 479 (18) | ||
| CML | 24 (21) | 648 (29) | 15 (7) | 166 (12) | 8 (12) | 199 (20) | 51 (12) | 291 (11) | ||
| MDS | 16 (14) | 136 (6) | 32 (14) | 213 (15) | 7 (10) | 98 (10) | 58 (14) | 408 (15) | ||
| Disease status at transplant * | 46 (40) | 977 (43) | 124 (56) | 741 (53) | <0.001 | 27 (40) | 470 (48) | 195 (46) | 1334 (49) | 0.002 |
| Early | 35 (31) | 881 (39) | 34 (15) | 323 (23) | 19 (28) | 278 (29) | 90 (21) | 635 (24) | ||
| Intermediate | 29 (25) | 377 (17) | 60 (27) | 309 (22) | 21 (31) | 216 (22) | 136 (32) | 691 (26) | ||
| Advanced | 4 (4) | 17 (<1) | 4 (2) | 18 (1) | 1 (1) | 10 (1) | 3 (<1) | 37 (1) | ||
| Missing | ||||||||||
| Donor type | ||||||||||
| HLA-identical sibling | 114 | 2252 | 222 | 1391 | 0 | 0 | 0 | 0 | <0.001 | |
| Unrelated, 8/8-matched | 0 | 0 | 0 | 0 | 46 (68) | 686 (70) | 316 (75) | 2130 (79) | ||
| Unrelated, 7/8-matched | 0 | 0 | 0 | 0 | 22 (32) | 288 (30) | 108 (25) | 567 (21) | ||
| Donor-recipient gender Match | 40 (35) | 750 (33) | 78 (35) | 429 (31) | 0.96 | 25 (37) | 341 (35) | 174 (41) | 1032 (38) | 0.34 |
| Male/Male | 28 (25) | 500 (22) | 48 (22) | 326 (23) | 17 (25) | 275 (28) | 125 (29) | 775 (29) | ||
| Male/Female | 24 (21) | 545 (24) | 51 (23) | 342 (25) | 11 (16) | 185 (19) | 54 (13) | 447 (17) | ||
| Female/Male | 22 (19) | 456 (20) | 45 (20) | 294 (21) | 15 (22) | 173 (18) | 71 (17) | 442 (16) | ||
| Female/Female | 0 | 1 (<1) | 0 | 0 | 0 | 0 | 0 | 1 (<1) | ||
| Missing | ||||||||||
| Donor-recipient CMV status | 44 (39) | 1247 (55) | 77 (35) | 558 (40) | <0.001 | 19 928) | 164 (17) | 108 (25) | 567 (21) | 0.003 |
| Neg/Neg | 16 (14) | 208 (9) | 25 (11) | 142 (10) | 9 (13) | 102 (10) | 44 (10) | 263 (10) | ||
| Neg/Pos | 19 (17) | 273 (12) | 63 (28) | 331 (24) | 24 (35) | 339 (35) | 138 (33) | 948 (35) | ||
| Pos/Neg | 32 (28) | 430 (19) | 54 (24) | 337 (24) | 15 (22) | 306 (31) | 122 (29) | 801 (30) | ||
| Pos/Pos | 3 (3) | 94 (4) | 3 (1) | 23 (2) | 1 (1) | 64 (7) | 12 (3) | 118 (4) | ||
| Missing | ||||||||||
| Graf t type | ||||||||||
| Bone marrow | 41 (36) | 633 (28) | 15 (7) | 114 (8) | <0.001 | 17 (25) | 479 (49) | 53 (13) | 813 (30) | <0.001 |
| Peripheral blood | 73 (54) | 1619 (72) | 207 (93) | 1277 (92) | 51 (75) | 500 (51) | 371 (88) | 1884 (70) | ||
| ATG use | ||||||||||
| Yes | 13 (11) | 118 (5) | 30 (14) | 55 (4) | <0.001 | 23 (34) | 146 (15) | 144 (34) | 718 (27) | <0.001 |
| No | 101 (89) | 2134 (95) | 192 (86) | 1336 (96) | 45 (66) | 828 (85) | 280 (66) | 1979 (73) | ||
| TBI | ||||||||||
| Yes | 30 (26) | 834 (37) | 99 (45) | 600 (43) | <0.001 | 24 (35) | 614 (63) | 141 (33) | 1128 (42) | <0.001 |
| No | 84 (74) | 1418 (63) | 123 (55) | 791 (57) | 44 (65) | 360 (37) | 283 (67) | 1569 (58) | ||
| Conditioning regimen | ||||||||||
| Bu + Cy ± Others | 54 (47) | 1215 (54) | 32 (14) | 461 (33) | <0.001 | 25 (37) | 284 (29) | 64 (15) | 688 (26) | <0.001 |
| ATG+ Bu ± Cy ± Flu ± | 11 (10) | 88 (4) | 8 (4) | 49 (4) | 15 (22) | 60 (6) | 101 (24) | 533 (20) | ||
| Others | 9 (8) | 47 (2) | 72 (32 ) | 248 (18) | 2 (3) | 11 (1) | 98 (23) | 288 (11) | ||
| Bu + Flu ± others | 25 (22) | 749 (33) | 72 (32) | 494 (36 ) | 16 (24) | 516 (53) | 88 (21) | 897 (33) | ||
| TBI ± Cy ± others | 1 (<1) | 29 (1) | 22 (10) | 5 (<1) | 6 (9) | 85 (9) | 41 (10) | 177 (7) | ||
| ATG + TBI ± Cy ± others | 4 (4) | 65 (3) | 5 (2) | 101 (7) | 2 (3) | 12 (3) | 12 (3) | 54 (2) | ||
| TBI + Etop ± others | 10 (9) | 68 (3) | 11 (5) | 33 (2) | 2 (3) | 5 (<1) | 20 (5) | 60 (2) | ||
| Others | ||||||||||
| Year of transplant | ||||||||||
| 2000–2004 | 41 (36) | 1460 (65) | 33 (15) | 248 (18) | <0.001 | 11 (16) | 560 (57) | 49 (12) | 506 (19) | <0. |
| 2005–2008 | 29 (25) | 635 (28) | 82 (37) | 464 (33) | 34 (50) | 321 (33) | 219 (52) | 1148 (43) | 001 | |
| 2009–2013 | 44 (39) | 157 (7) | 107 (48) | 679 (49) | 23 (34) | 93 (10) | 156 (37) | 1043 (39) | ||
| Follow-up of survivors, months, median (range) | 70 (3–168) | 61 (1–194) | 65 (3–150) | 66 (3–174) | <0.001 | 76 (37–169) | 97 (6–193) | 72 (25-191) | 72 (5–172) | <0.001 |
Tac- tacrolimus; CSA-Cyclosporine; MTX- methotrexate; MMF- mycophenolate mofetil; KPS- Karnofsky performance status; AML- acute myeloid leukemia; ALL- acute lymphoblastic leukemia; CML- chronic myeloid leukemia; MDS- myelodysplastic syndrome; CMV-cytomegalovirus; ATG- antithymocyte globulin; TBI- total body irradiation; Bu- busulfan; Cy- cyclophosphamide; Flu-fludarabine; Etop- etoposide
Engraftment and GVHD
Median time to neutrophil engraftment was 16 days for CSA+MTX (range, 1–72); followed by CSA+MMF 14 days (7–29); Tac+MTX 13 days (1–111); and Tac+MMF 12 days (98–25). Platelet recovery by day 28 was also low with CSA+MTX (75%) and CSA+MMF (76%) compared to Tac+MTX (82%) and Tac+MMF (98%) (Table 2)
Table 2:
Univariate Outcomes and Cause of Death in Myeloablative Related and Unrelated Donor Transplant
| Matched Related Donor (MRD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N eval | CSA+MMF Prob (95% CI) | N eval | CSA+MTX Prob (95% CI) | N eval | Tac+MMF Prob (95% CI) | N eval | Tac+MTX Prob (95% CI) | P-Value | |
| Acute GVHD, II-IV | |||||||||
| Day 100 | 114 | 44 (35–53%) | 2218 | 34 (32–36%) | 220 | 35 (28–41%) | 1369 | 32 (20–35%) | 0.07 |
| Acute GVHD, III-IV | |||||||||
| Day 100 | 114 | 26 (18–34%) | 2203 | 18 (16–19%) | 220 | 18 (13–23%) | 1369 | 14 (12–16%) | 0.005 |
| Chronic GVHD | |||||||||
| 6 months | 113 | 20 (13–28%) | 2194 | 27 (25–29%) | 219 | 28 (23–35%) | 1372 | 26 (24–28%) | 0.35 |
| 1 year | 30 (21–39%) | 41 (39–43%) | 45 (39–52%) | 46 (44–99%) | <0.001 | ||||
| Relapse | |||||||||
| 1 year | 112 | 37 (28–46%) | 2212 | 21 (19–23%) | 219 | 28 (23–35%) | 1368 | 30 (28–33%) | <0.001 |
| 2 years | 40 (31–49%) | 27 (25–29%) | 45 (39–52%) | 36 (34–39%) | <0.001 | ||||
| Transplant-related mortality | |||||||||
| 1 year | 112 | 30 (22–39%) | 2212 | 18 (16–19%) | 219 | 14 (10–19%) | 1368 | 13 (11–15%) | <0.001 |
| 2 years | 35 (26–44%) | 20 (18–21%) | 17 (12–22%) | 17 (15–19%) | <0.001 | ||||
| Disease-free survival | |||||||||
| 1 y ear | 112 | 33 (25–42%) | 2212 | 61 (59–63%) | 219 | 54 (47–60%) | 1368 | 57 (54–60%) | <0.001 |
| 2 years | 25 (18–34%) | 54 (52–56%) | 45 (39–52%) | 47 (44–49%) | <0.001 | ||||
| Overall Survival | |||||||||
| 1 year | 114 | 46 (37–55%) | 2252 | 69 (67–71%) | 222 | 66 (59–72%) | 1391 | 68 (66–71%) | <0.001 |
| 2 years | 35 (26–44%) | 61 (59–64%) | 52 (45–59%) | 55 (53–58%) | <0.001 | ||||
| GVHD-Relapse-free | |||||||||
| Survival (GRFS) | 114 | 2214 | 221 | 1377 | |||||
| 1 year | 16 (10–23%) | 27 (25–29%) | 20 (15–25%) | 20 (18–22%) | <0.001 | ||||
| ANC recovery | |||||||||
| 14 days | 113 | 46 (37–55%) | 2228 | 30 (28–32%) | 220 | 66 (60–72%) | 1379 | 50 (48–53%) | <0.001 |
| Platelet recovery | |||||||||
| 28 days | 105 | 76 (68–84%) | 2159 | 75 (74–77%) | 201 | 89 (84–93%) | 1349 | 82 (80–84%) | <0.001 |
| Cause of Death | 80 | 992 | 132 | 780 | |||||
| Primary disease | 33 (41) | 384 (39) | 71 (54) | 404 (52) | |||||
| Graft failure | 2 (3) | 8 (<1) | 1 (<1) | 1 (<1) | |||||
| GVHD | 24 (30) | 222 (22) | 28 (21) | 158 (20) | |||||
| IPN | 5 (6) | 102 (10) | 10 (8) | 70 (9) | |||||
| Infection | 3 (4) | 57 (6) | 4 (3) | 20 (3) | |||||
| Organ failure | 8 (10) | 91 (9) | 7 (5) | 59 (8) | |||||
| Secondary | 0 | 14 (1) | 2 (2) | 10 (1) | |||||
| malignancy | 4 (5) | 86 (9) | 7 (5) | 40 (5) | |||||
| Other | 1 (1) | 28 (3) | 2 (2) | 18 (2) | |||||
| Missing | |||||||||
| Unrelated donor (URD) | |||||||||
| N eval | CSA+MMF Prob (95% CI) | N eval | CSA+MTX Prob (95% CI) | N eval | Tac+MMF Prob (95% CI) | N eval | Tac+MTX Prob (95% CI) | ||
| Acute GVHD, II-IV | |||||||||
| Day 100 | 68 | 57 (46–69%) | 964 | 50 (47–53%) | 419 | 55 (50–59%) | 2678 | 49 (47–51%) | 0.11 |
| Acute GVHD, III-IV | |||||||||
| Day 100 | 68 | 40 (28–52%) | 965 | 23 (20–25%) | 420 | 25 (21–30%) | 2673 | 20 (19–22%) | 0.001 |
| Chronic GVHD | |||||||||
| 6 months | 66 | 21 (12–32%) | 962 | 35 (32–38%) | 417 | 35 (30–40%) | 2673 | 28 (27–30%) | <0.001 |
| 1 year | NE | 48 (44–51%) | 51 (46–56%) | 45 (43–47%) | 0.01 | ||||
| Relapse | |||||||||
| 1 year | 64 | 23 (14–25%) | 954 | 23 (20–25%) | 415 | 28 (24–32%) | 2652 | 27 (25–29%) | 0.03 |
| 2 years | 25 (15–36%) | 27 (24–30%) | 32 (28–37%) | 31 (30–33%) | 0.03 | ||||
| Transplant-related mortality | |||||||||
| 1 year | 64 | 41 (29–53%) | 954 | 26 (24–29%) | 415 | 24 (20–28%) | 2652 | 20 (19–22%) | <0.001 |
| 2 years | 44 (32–56%) | 30 (27–33%) | 29 (25–34%) | 24 (22–26%) | <0.001 | ||||
| Disease-free survival | |||||||||
| 1 y ear | 64 | 36 (25–48%) | 954 | 51 (48–54%) | 415 | 48 (43–53%) | 2652 | 53 (51–54%) | 0.02 |
| 2 years | 31 (21–43%) | 43 (40–47%) | 39 (34–43%) | 0.02 | |||||
| Overall Survival | |||||||||
| 1 year | 68 | 40 (28–52%) | 974 | 57 (54–60%) | 424 | 54 (49–59%) | 2697 | 60 (58–62%) | 0.001 |
| 2 years | 34 (23–45%) | 49 (46–52%) | 44 (39–49%) | 50 (48–52%) | 0.005 | ||||
| GVHD-Relapse-free | |||||||||
| Survival (GRFS) | 67 | 968 | 422 | 2691 | |||||
| 1 year | 10 (4–19%) | 16 (13–18%) | 10 (7–13%) | 17 (15–18%) | <0.001 | ||||
| ANC recovery | |||||||||
| 14 days | 67 | 43 (32–55%) | 970 | 26 (23–28%) | 424 | 70 (65–74%) | 2686 | 41 (39–43%) | <0.001 |
| Platelet recovery | |||||||||
| 28 days | 67 | 64 (52–75%) | 960 | 59 (56–62%) | 402 | 83 (79–86%) | 2646 | 68 (67–70%) | <0.001 |
| Cause of Death | 48 | 628 | 293 | 1659 | |||||
| Primary disease | 13 (27) | 209 (33) | 111 (38) | 686 (41) | |||||
| Graft failure | 0 | 9 (1) | 1(<1) | 12 (<1) | |||||
| GVHD | 16 (33) | 167 (27) | 90 (31) | 393 (24) | |||||
| IPS | 7 (15) | 73 (12) | 30 (10) | 170 (10) | |||||
| Infection | 4 (8) | 35 (6) | 11(4) | 66 (4) | |||||
| Organ failure | 5 (10) | 69 (11) | 22 (8) | 169 (10) | |||||
| Secondary | 0 | 2 (<1) | 3 (1) | 15 (<1) | |||||
| malignancy | 3 (6) | 58 (9) | 17 (6) | 120 (7) | |||||
| Other | 0 | 6 (<1) | 8 (3) | 29 (2) | |||||
| Missing | |||||||||
CSA- cyclosporine; MMF- mycophenoalte mofetil; MTX- methotrexate; Tac- tacrolimus; GVHD- graft-versus-host diseae; ANC- absolute neutrophil count; IPN- idiopathic pneumonia syndrome
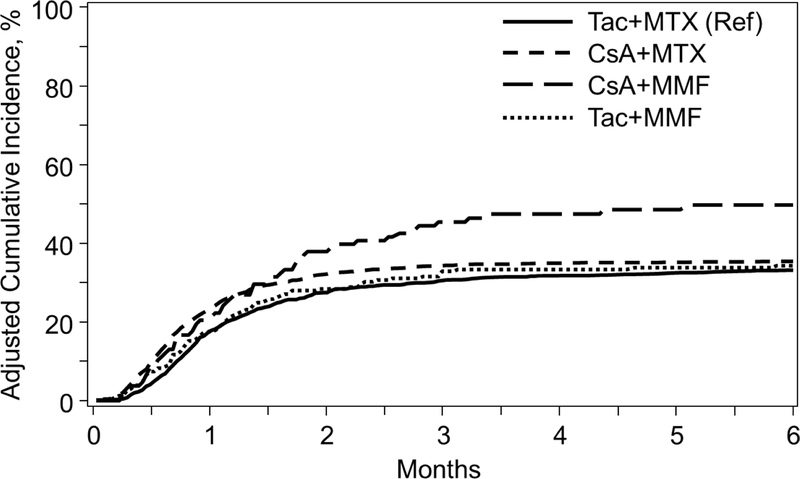
The cumulative incidence of grade II-IV and III-IV aGVHD at day 100 is shown in Table 2. In multivariable analysis (MVA), CSA+MMF (HR 1.65, P<0.001) and CSA+MTX (HR 1.17, P=0.010) were associated with worse grade II-IV aGVHD relative to reference Tac+MTX (Table 3, Figure 1A). CSA+MMF was also associated with more grade III-IV aGVHD (HR 1.92, P<0.001) versus Tac+MTX. While the primary analysis focused on comparisons of GVHD prophylaxis regimens relative to Tac+MTX as the reference, pairwise comparisons between each regimen was also evaluated and demonstrated a higher incidence of grade III-IV aGVHD with CSA+MMF relative to CSA+MTX (HR 1.71, 95% confidence interval [CI] 1.17–2.50, P=0.006). CSA+MMF also fared worse relative to CSA+MTX (HR1.41, 95% CI 1.06–1.86, P=0.017) and Tac+MMF (HR 1.57, 95% CI 1.10–2.22, P=0.012) for grade II-IV aGVHD, but did not reach our pre-defined level of significance (P=0.01). Complete MVA tables detailing additional patient, disease, and transplant-factors are in the Supplementary Tables.Given differential effects over time specific for this outcome, MVA for cGVHD were divided into those experiencing cGVHD <5 months and ≥ 5 months (median time to cGVHD onset: 5.6 months). Relative to Tac+MTX, CSA+MTX was associated with higher rates of cGVHD <5 months of transplant (HR 1.41, P<0.001), however ≥5 months of transplant, this association reversed (HR 0.74, P<0.001) (Table 3). Pairwise comparisons of each GVHD prophylaxis regimens relative to each other did not reveal any further significant associations (Supplementary Table)
Table 3:
Multivariate Outcomes in Myeloablative Related and Unrelated Donor Transplant
| Tac+MTX | CSA+MMF | CSA+MTX | Tac+MMF | |
|---|---|---|---|---|
| Matched Related donor (MRD) HR (95% CI), P-value | ||||
| aGVHD grade 2–4 | Ref (1.00) | 1.65 (1.24–2.20) P<0.001 | 1.17 (1.04–1.33) P=0.010 | 1.05 (0.83–1.34) P=0.661 |
| aGVHD grade 3–4 | 1.92 (1.31–2.83) P<0.001 | 1.13 (0.92–1.37) P=0.283 | 1.19 (0.85–1.66) P=0.319 | |
| cGVHD* <5 months | 1.34 (0.83–2.17) P=0.233 | 1.41 (1.20–1.67) P<0.001 | 1.28 (0.94-.74) P=0.119 | |
| cGVHD* ≥5 months | 0.56 (0.34–0.95) P=0.030 | 0.74 (0.65–0.85) P<0.001 | 0.92 (0.71–1.19) P=0.512 | |
| TRM* <5 months | 4.03 (2.61–6.23) P<0.001 | 2.29 (1.78–2.95) P<0.001 | 1.17 (0.71–1.94) P=0.537 | |
| TRM* ≥5 months | 1.38 (0.78–2.44) P=0.275 | 0.83 (0.67–1.03) P=0.095 | 0.90 (0.59–1.36) P=0.609 | |
| Relapse | 1.43 (1.05–1.93) P=0.022 | 0.86 (0.74–0.98) P=0.029 | 0.92 (0.73–1.14) P=0.444 | |
| OS* <5 months | 2.31 (1.73–3.09) P<0.001 | 1.27 (1.10–1.48) P=0.002 | 1.05 (0.81–1.37) P=0.704 | |
| OS* ≥5 months | 0.94 (0.62–1.43) P=0.767 | 0.74 (0.64–0.87) P<0.001 | 0.92 (0.70–1.20) P=0.530 | |
| Unrelated donor (URD) HR (95% CI), P-value | ||||
| aGVHD, grade 2–4 | Ref (1.00) | 1.49 (1.08–2.07) P=0.016 | 1.00 (0.90–1.12) P=0.961 | 1.14 (0.99–1.32) P=0.066 |
| aGVHD, grade 3–4 | 2.31 (1.57–3.42) P<0.001 | 1.02 (0.87–1.20) P=0.806 | 1.26 (1.02–1.56) P=0.030 | |
| cGVHD* <4 months | 1.54 (0.79–2.98) P=0.203 | 1.62 (1.35–1.93) P<0.001 | 1.34 (1.04–1.73) P=0.022 | |
| cGVHD* ≥4 months | 0.92 (0.52–1.63) P=0.779 | 0.85 (0.74–0.98) P=0.025 | 1.47 (1.24–1.75) P<0.001 | |
| TRM* <4 months | 3.09 (2.00–4.77) P<0.001 | 1.24 (1.02–1.51) P=0.030 | 1.02 (0.77–1.36) P=0.896 | |
| TRM* ≥4 months | 0.89 (0.42–1.90) P=0.763 | 1.00 (0.84–1.20) P=0.979 | 1.45 (1.16–1.81) P=0.001 | |
| Relapse | 0.81 (0.50–1.32) P=0.398 | 0.97 (0.85–1.12) P=0.699 | 0.93 (0.78–1.12) P=0.449 | |
| OS* <4 months | 2.36 (1.67–3.35) P<0.001 | 1.23 (1.08–1.41) P=0.002 | 1.20 (1.01–1.44) P=0.044 | |
| OS* ≥4 months | 1.10 (0.64–1.89) P=0.730 | 0.91 (0.79–1.05) P=0.221 | 1.34 (1.12–1.61) P=0.001 | |
Given differential effects over time (non-proportional hazards), models were constructed breaking the post-transplant time course into 2 periods, using the maximized partial likelihood method to find the most appropriate breakpoint.
Figure 1A.
Incidence of Acute Grade II-IV GVHD among GVHD Prophylaxis Regimens in Myeloablative Matched Related Donors
TRM
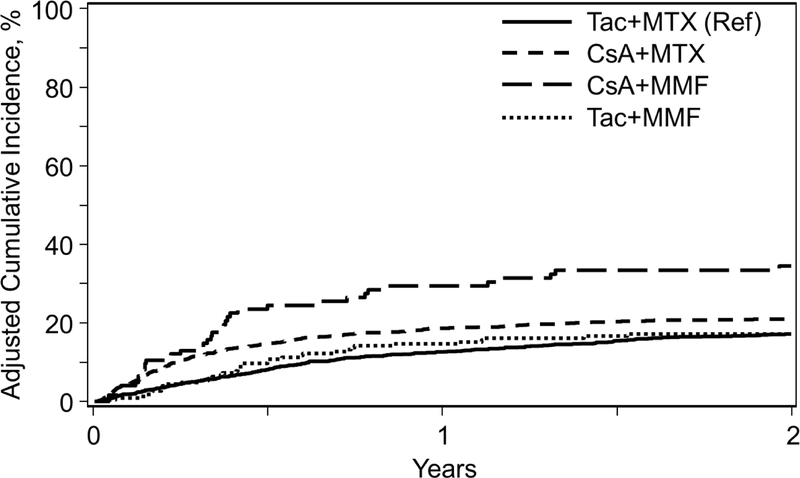
The incidence of TRM at 1-year was significantly higher for CSA+MMF (30%) compared to other GVHD regimens (Table 2). In MVA, both CSA+MMF (HR 4.03, P<0.001) and CSA+MTX (HR 2.29, P<0.001) were significantly associated with higher TRM compared to Tac+MTX <5 months of transplant, but not after 5 months given time varying effects (Table 3, Figure 2A). Pairwise comparisons between all GVHD prophylaxis regimens also demonstrate worse TRM<5 months of transplant with CSA+MMF compared to CSA+MTX (HR 1.76, 95% CI 1.17–2.65, P=0.007) and Tac+MMF (HR 3.43, 95% CI 0.77–3.03, P<0.001); and subsequently CSA+MTX relative to Tac+MMF (HR 1.96, 95% CI 1.19–3.22, P=0.0074) (Supplementary Table).
Figure 2A.
Incidence of Treatment-Related Mortality among GVHD Prophylaxis Regimens in Myeloablative Matched Related Donors
Relapse
The 1-year cumulative incidence of relapse for each regimen is shown in Table 2. In MVA, there was no significant difference in relapse between any GVHD prophylaxis regimen relative to Tac+MTX. Pairwise comparisons between all groups, however, demonstrate a higher association of relapse with CSA+MMF relative to CSA+ MTX (HR 1.67, 95% CI 1.23–2.27, P=0.001) (Supplementary Table).
DFS and OS
CSA+MMF recipients had poor DFS and OS compared to all other GVHD regimens (Table 2). One-year probabilities of GRFS confirmed significantly worse outcomes with CSA+MMF (10%) compared to CSA+MTX (27%), Tac+MMF (20%), and Tac+MTX (20%).
In MVA, CSA+MMF was associated with poor DFS (HR 1.64, P<0.001) relative to Tac+MTX. Pair-wise comparisons between each regimen also demonstrate inferior DFS with CSA+MMF versus CSA+MTX (HR 1.63, 95% CI 1.29–2.05, P<0.001) and Tac+MMF (HR 1.74, 95% CI 1.32–2.30, P<0.001) (Supplementary index).
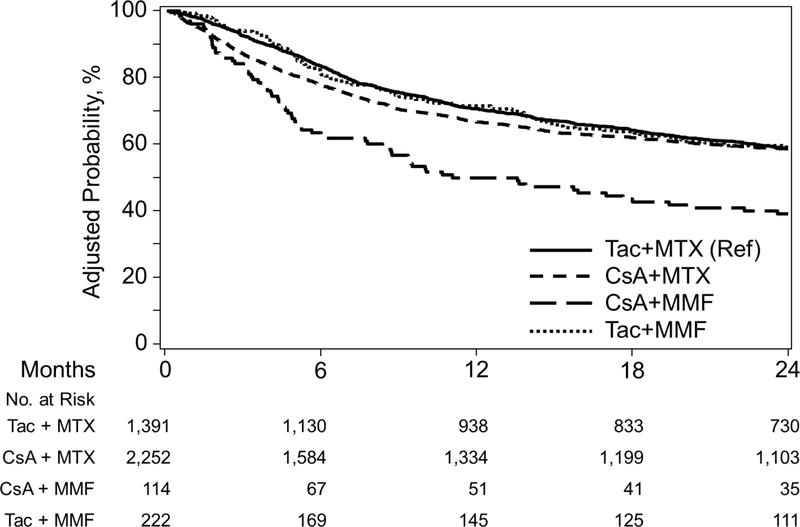
Two-year OS within the CSA+MMF cohort was 48%, compared to 67% with CSA+MTX, 69% with Tac+MMF, and 71% for Tac+MTX. Adjusted OS curves are shown in Figure 3A. In MVA, CSA+MMF was significantly associated with worse outcome (HR 2.31, P<0.001) compared to Tac +MTX < 5 months of transplant. CSA+MTX was also associated with worse survival (HR 1.27, P=0.002) compared to Tac+MTX within the first 5 months, with a reversal of this association ≥5 months (HR 0.74, P=0.003) given non-proportional hazards over time (Table 3). Pairwise comparisons also demonstrated inferior survival of CSA+MMF relative to CSA+MTX (HR 1.82, 95% CI 1.36–2.42, P<0.001) and Tac+MMF (HR 2.17, 95% CI 1.53–3.12, P<0.0001) <5 months of transplant (Supplementary index)
Figure 3A.
Adjusted Overall Survival among GVHD Prophylaxis Regimens in Myeloablative Matched Related Donors
Transplantation from an unrelated donor
Patient, Disease, and Transplant Characteristics
In the URD cohort, Tac+MTX was the predominant GVHD prophylaxis regimen (N=2697), followed by CSA+MTX (N=974), Tac+MMF (N=424), and CSA+MMF (N=68) (Table 1). Similar to MRD, there were more patients in the CSA+MTX (57%) cohort transplanted before 2005. The use of BM as a cell source was also more common in CSA+MTX group (49%) compared to other regimens—CSA+MMF (25%), Tac+MMF (13%), Tac+MTX (30%); while ATG was used less commonly with CSA+MTX (15%) relative to CSA+MMF (34%), Tac+MMF (34%), and Tac+MTX (27%).
Engraftment and GVHD
Median time to neutrophil engraftment with CSA+MTX was 17 days (range 6–42), followed by 14 days for both CSA+MMF, (9–27) and Tac+MTX, (2–205), and 12 days (5–48) Tac+MMF. Platelet recovery by day 28 was also low in CSA+MTX recipients (59%) compared to CSA+MMF (64%), Tac+MTX (68%), and Tac+MMF (83%) (Table 2).
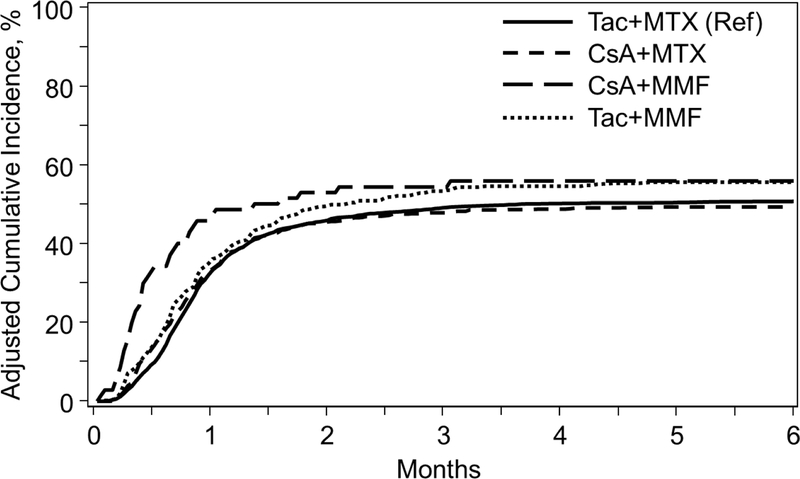
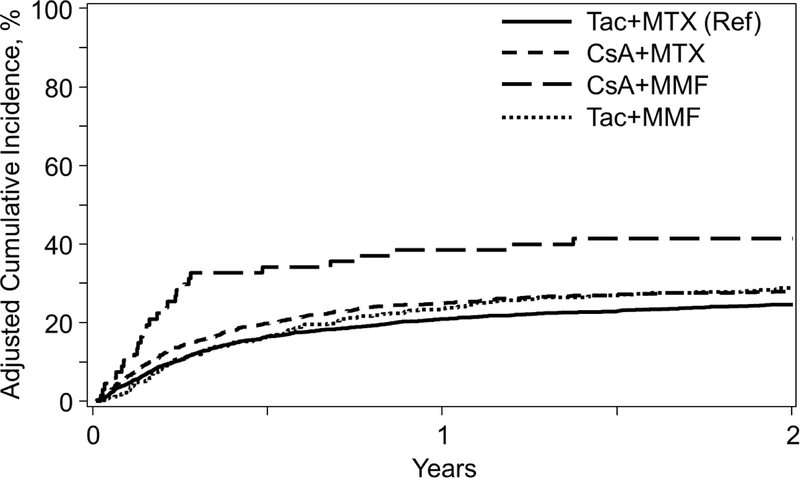
The cumulative incidence of grade II-IV aGVHD at day 100 was highest in the CSA+MMF group (57%) compared to other regimens (Table 2). Day100 grade III-IV aGVHD was also highest for CSA+MMF (40%) compared to CSA+MTX (23%); Tac+MMF (25%); and Tac+MTX (20%). In MVA, CSA+MMF was significantly associated with grade III-IV aGVHD (HR 2.31, P<0.001), as well as worse grade II-IV aGVHD (HR 1.49, P=0.016) compared to Tac+MTX, although this did not reach the pre-defined significance level (Table 3, Figure 1B). Pairwise comparisons also demonstrate a similar higher risk of grade III-IV aGVHD with CSA+MMF compared to CSA+MTX (HR 2.27, 95% CI 1.51–3.40, P<0.001) and Tac+MMF (HR 1.83, 95% CI 1.19–2.81, P=0.006); and grade II-IV (HR 1.49, 95% CI 1.07–2.08, P=0.019) with CSA+MMF compared to CSA+MTX (not reaching pre-defined level of significance). (Supplementary index). Similar to the MRD cohort, MVA results for cGVHD were divided into those experiencing cGVHD <4 months and ≥4 months given differential effects over time specific for this outcome. Relative to Tac+MTX, CSA+MTX was associated with higher rates of cGVHD <4 months of transplant (HR 1.62, P<0.001). After 4 months of transplant, Tac+MMF was associated with higher cGVHD (HR 1.47, P<0.001) relative to Tac+MTX. Pairwise comparisons also demonstrate higher rates of cGVHD with Tac+MMF compared to CSA+MTX (HR 1.73, 95% CI 1.41–2.12, P<0.001) ≥4 months (Supplementary index).
Figure 1B.
Incidence of Acute Grade II-IV GVHD among GVHD Prophylaxis Regimens in Myeloablative Unrelated Donors
TRM
The incidence of TRM at 1-year was highest for CSA+MMF (41%) compared to other regimens (Table 2). In MVA, CSA+MMF (HR 3.09, P<0.001) was significantly associated with higher TRM versus Tac+MTX <4 months of transplant. After 4 months, Tac+MMF (HR 1.45, P=0.001) was associated with increased TRM relative to Tac+MTX (Table 3, Figure 2B). Pairwise comparisons also demonstrate worse TRM with CSA+MMF relative to CSA+MTX (HR 2.49, 95%CI 1.58–3.91, P<0.001) and Tac+MMF (HR 3.03, 95% CI 1.85–5.00, P<0.001) <4 months of transplant. After 4 months, Tac+MMF was also associated with worse TRM relative to CSA+MTX (HR 1.45, 95% CI 1.12–1.87, P=0.0053) (Supplementary index)
Figure 2B.
Incidence of Treatment-Related Mortality among GVHD Prophylaxis Regimens in Myeloablative Unrelated Donors
Relapse
There were no significant associations between any GVHD prophylaxis regimens with respect to relapse relative to Tac+MTX or any other GVHD regimen in the URD cohort.
DFS and OS
CSA+MMF recipients had poor 1-year DFS (36%) and OS (34%) compared to other GVHD prophylaxis regimens (Table 2). One-year GRFS was low at 10% for both CSA+MMF and Tac+MMF, compared to CSA+MTX (16%) and Tac+MTX (17%) (Table 2).
In MVA, no prophylaxis regimen was associated with worse DFS relative to Tac+MTX. Older age, mismatched donor, donor age, poor performance status, disease, disease status, and conditioning regimen were associated with DFS (Supplementary index).
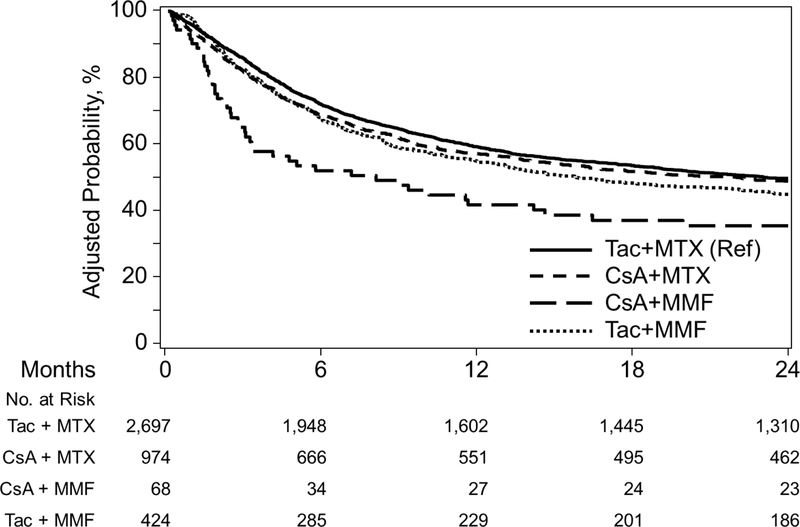
CSA+MMF (HR 2.36, P<0.001), and CSA+MTX (HR1.23, P=0.002) were associated with worse OS compared to Tac+MTX <4 months of transplant. After 4 months, Tac+MMF demonstrated worse OS (HR 1.34, P=0.001) versus Tac+MTX (Figure 3B). Pairwise comparisons also demonstrate worse survival with CSA+MMF compared to CSA+MTX (HR 1.92, 95% CI 1.342.75, P=0.0004) and Tac+MMF (HR 1.96, 95% CI 1.35–2.85, P=0.0005). After 4 months, Tac+MMF was associated with worse survival versus CSA+MTX (HR 1.47, 95% CI 1.18–1.81, P=0.0004) (Supplementary index).
Figure 3B.
Adjusted Overall Survival among GVHD Prophylaxis Regimens in Myeloablative Unrelated Donors
DISCUSSION
In this current large CIBMTR study, we demonstrate significantly worse aGVHD, TRM, GFRS, and OS with CSA+MMF compared to Tac+MTX in both MRD and URD myeloablative transplant settings. Patients receiving CSA+MMF also had inferior TRM and survival outcomes compared to both CSA+MTX and Tac+MMF. Overall, these findings indicate a significant inferiority of CSA+MMF compared to all other combinations of Tac or CSA and MMF or MTX in both MRD and URD myeloablative transplantation.
The combination of MMF and Tac or CSA has previously been shown in retrospective and small prospective studies to be associated with significantly faster engraftment, shorter hospital stay, and less mucositis compared to MTX [8, 23, 24]. While this current analysis confirms generally faster engraftment rates with MMF compared to MTX, it also indicates superior engraftment with Tac over CSA. Previous studies, including three large prospective randomized trials, have also demonstrated a significantly lower incidence of aGVHD for patients receiving Tac compared to CSA, although these findings never translated into a survival benefit.[25–27] Similar to these studies, we evaluated MRD and MUD cohorts separately given the known differences in GVHD outcomes and differences in the patterns of use of GVHD prophylaxis. Our current study also suggested a more potent immunosuppressant effect of Tac, as we demonstrate significantly worse grade II-IV aGVHD, early (<4 months) cGVHD, and TRM with CSA+MTX compared to Tac+MTX in the MRD cohort; and worse early cGVHD and OS in the URD setting. Likewise, CSA+MMF was also associated with worse TRM, DFS, and OS compared to Tac+MMF, with a trend toward worse GVHD in MRD recipients; while in the URD setting CSA+MMF was associated with worse grade III-IV aGVHD, TRM, and OS compared to Tac+MMF. In fact, the immunosuppressive effect of Tac may be powerful enough to compensate for the inferiority of MMF, as we found no statistically significant differences between Tac+MMF and Tac+MTX in the MRD setting for any GVHD and survival outcomes. Tac+MMF may thus be a reasonable substitute in recipients of a MRD transplant when there is a concern for engraftment or severe mucosal toxicity.
In both the MRD and URD cohorts, CSA+MMF was associated with worse TRM and OS compared to CSA+MTX, secondary to significantly worse severe aGVHD, further suggesting a superiority of MTX over MMF. We did not detect any differences in cGVHD in the CSA+MMF group, and this may be due to the relatively higher proportion of patients receiving bone marrow grafts. While GVHD and survival was similar between Tac+MMF and Tac+MTX in the MRD setting, Tac+MMF was associated with higher cGVHD, TRM and worse survival greater than 4 months after transplant compared to Tac+MTX in the URD group with a trend toward worse aGVHD outcomes. The composite endpoint of GRFS also demonstrates significantly worse outcome with CSA+MMF compared to other regimens in the MRD setting, while both CSA+MMF and Tac+MMF had poor GRFS compared to MTX-containing regimens in URD transplantation, again indicating an advantage of MTX over MMF for GVHD prophylaxis.
There are several important considerations to take into account in this analysis, and given the retrospective nature of this evaluation, we also acknowledge several limitations. The reason for choosing MMF versus MTX (or Tac versus CSA) as GVHD prophylaxis is unknown in this study. We recognize this may reflect biases of specific institutional protocols, or a preference to choose a GVHD regimen with less mucositis or faster engraftment due to patient co-morbidities. This potential preferential bias may be reflected by the larger number of patients within the MTX cohorts, particularly among unrelated donors, compared to MMF-based regimens. These preferential differences are also highlighted by imbalances in other baseline characteristics (e.g. year of transplant, use of ATG, graft source) between patient groups which could potentially impact outcomes. Although these factors were all evaluated in multivariable analyses, it may be difficult to fully account for all these differences and thus must be taken into consideration in interpretation of these results. The dosing and duration of MMF and MTX used was also unknown in this dataset, and reflects a major limitation in this study. Although it is not standard to measure levels of the active metabolite of MMF, mycophenolic acid (MPA), pharmacokinetic studies in GVHD treatment demonstrate that the concentration of MPA is significantly greater in responders compared to non-responders.[28, 29] Prior studies have also demonstrated that patients with lower MPA steady state concentrations have increased severe GVHD and non-relapse mortality compared to those with higher levels, especially in unrelated donors.[30, 31] While it has been suggested that a dose of 45mg/kg/day is the optimal dose for the prevention of acute GVHD [10], other studies have demonstrated a superiority of higher MMF doses, administered three times a day (3 grams) rather than the more common twice daily (2 grams) [32, 33]. Interestingly, pharmacokinetic analyses have also demonstrated differences in MPA clearance between CSA and Tac, with CSA increasing clearance of MPA by 33.8% compared to Tac, requiring higher doses of MMF when used in combination with CSA [34]. This is a potentially important consideration to take into account when in interpreting the differences in outcome between CSA/MMF and Tac/MMF. Unfortunately specific dosing of MMF and levels of MPA in this study remain unknown and thus the potential effect of increased dosing, interaction with calcineurin inhibitor, and concentrations of MMF on GVHD outcomes limit our conclusions. Additionally, the duration of MMF is also likely to play an important role in which there may have been differences in practice in this study. Although the optimal duration of MMF after transplant has not been well-established, it is suggested that a prolonged course of MMF is associated with low incidences of reported GVHD.[7, 35] While longer durations of MMF may potentially account for the generally lower incidences of cGVHD within the first few months of transplant, there is likely to have been significant variation between dosing and duration of MMF which we were unable to take into account in this analysis
Furthermore, standard dosing of MTX is 15mg/m2 day 1, followed by 10mg/m2 day 3, 6, and 11, and it is likely that there were at least some modifications to this dosing schedule which would have been included in this analysis. Standard doses of MTX are often held or reduced due to severe mucositis or other toxicities, and the subsequent effect of this on GVHD outcomes is unclear [36]. In addition, a reduced dose schema of 5mg/m2 days 1, 3, 6, and 11, is frequently used and was previously developed to decrease the risk of mucosal and hepatic complications. This regimen has been widely used and shown to be effective in combination with CSA or Tac as GVHD prophylaxis with historically similar outcomes as standard dosing.[37–40] These reduced doses of MTX, however, have never been directly compared to standard dosing of MTX, and thus never been proven to be equivalent; but would have been included in this analysis. Finally, we do not have any data on the dosing or duration of Tac or CSA. There is likely to have been variation in practice regarding goal trough levels and duration of prophylactic therapy which may have affected incidence of GVHD.
We also recognize there are several important secondary outcomes in evaluating MMF versus MTX which we were not able to analyze in this retrospective study. MTX is part of a regimen that is known to be associated with significant morbidity including severe mucositis, prolonged hospitalization, use of total parenteral nutrition (TPN), delayed count recovery, and liver and renal toxicities. While there is insufficient data captured in this analysis to adequately address these questions, despite many attempts to substitute MTX in GVHD prophylaxis, there remains a failure to demonstrate a superior regimen. [8, 10, 41, 42]
In conclusion, this is the largest study to date evaluating MMF-based versus MTX-based GVHD prophylaxis and despite limitations of this retrospective analysis, demonstrates significantly inferior outcomes with CSA+MMF compared to all other GVHD regimens in both the related and unrelated donor settings. While no combination of calcineurin inhibitor and MMF or MTX was found to be superior to Tac+MTX, this study also confirms the potent immunosuppressive effect of Tac; and Tac+MMF may be a reasonable substitute in the related donor setting.
Supplementary Material
Highlights:
CSA and MMF is associated with increased severe acute GVHD in myeloablative HCT.
CSA and MMF is associated with worse TRM and inferior survival in myeloablative HCT.
Tac and MMF may be a reasonable substitute to Tac and MTX, but only in MSD myeloablative HCT.
MMF-based regimens is associated with worse GRFS compared to MTX regimens in myeloablative HCT.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Sonis ST, et al. , Oral mucositis and the clinical and economic outcomes of hematopoietic stemcell transplantation. J Clin Oncol, 2001. 19(8): p. 2201–5. [DOI] [PubMed] [Google Scholar]
- 2.Storb R, et al. , Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood, 1989. 73(6): p. 1729–34. [PubMed] [Google Scholar]
- 3.Storb R, et al. , Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med, 1986. 314(12): p. 729–35. [DOI] [PubMed] [Google Scholar]
- 4.Yu C, et al. , Synergism between mycophenolate mofetil and cyclosporine in preventing graftversus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood, 1998. 91(7): p. 2581–7 [PubMed] [Google Scholar]
- 5.Maris MB, et al. , HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood, 2003. 102(6): p. 2021–30. [DOI] [PubMed] [Google Scholar]
- 6.McSweeney PA, et al. , Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood, 2001. 97(11): p. 3390–400. [DOI] [PubMed] [Google Scholar]
- 7.Sabry W, et al. , Graft-versus-host disease prophylaxis with tacrolimus and mycophenolate mofetil in HLA-matched nonmyeloablative transplant recipients is associated with very low incidence of GVHD and nonrelapse mortality. Biol Blood Marrow Transplant, 2009. 15(8): p. 919–29. [DOI] [PubMed] [Google Scholar]
- 8.Bolwell B, et al. , A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant, 2004. 34(7): p. 621–5. [DOI] [PubMed] [Google Scholar]
- 9.Bornhauser M, et al. , Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation, 1999. 67(4): p. 499–504. [DOI] [PubMed] [Google Scholar]
- 10.Nash RA, et al. , A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant, 2005. 11(7): p. 495–505. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton BK, et al. Long-term follow-up of a prospective randomized trial comparing CYA and MTX with CYA and mycophenolate mofetil for GVHD prophylaxis in myeloablative sibling donor hematopoietic cell transplantation. Bone Marrow Transplant, 2013. DOI: 10.1038/bmt.2013.89. [DOI] [PubMed] [Google Scholar]
- 12.Perkins J, et al. , A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant, 2010. 16(7): p. 937–47. [DOI] [PubMed] [Google Scholar]
- 13.Perkins J, et al. , A Randomized, Controlled Trial of Graft-Versus-Host Disease (GVHD) Prophylaxis Comparing Tacrolimus and Mycophenolate Mofetil to Tacrolimus and Methotrexate: Analysis of GVHD, Relapse and Survival. Blood, 2008. 112(11): p. 779–779. [Google Scholar]
- 14.Neumann F, et al. , Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant, 2005. 35(11): p. 1089–93. [DOI] [PubMed] [Google Scholar]
- 15.Pohlreich D, et al. , Decreased risk of acute gastrointestinal toxicity when substituting methotrexate with mycophenolate mofetil in the prevention of graft-versus-host disease in stem cell transplantation following myeloablative conditioning regimens. Bone Marrow Transplant, 2006. 37(2): p. 235–6; author reply 236–7. [DOI] [PubMed] [Google Scholar]
- 16.Al-Kadhimi Z, et al. , High incidence of severe acute graft-versus-host disease with tacrolimus and mycophenolate mofetil in a large cohort of related and unrelated allogeneic transplantation patients. Biol Blood Marrow Transplant, 2014. 20(7): p. 979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eapen M, et al. , Bone marrow or peripheral blood for reduced-intensity conditioning unrelated donor transplantation. J Clin Oncol, 2015. 33(4): p. 364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horowitz M, The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant, 2008. 42 Suppl 1: p. S1–S2. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, et al. , 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant, 1995. 15(6): p. 825–8. [PubMed] [Google Scholar]
- 20.Shulman HM, et al. , Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med, 1980. 69(2): p. 204–17. [DOI] [PubMed] [Google Scholar]
- 21.Armand P, et al. , A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood, 2012. 120(4): p. 905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisdorf D, et al. , Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant, 2008. 14(7): p. 748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton BK, et al. , Long-term follow-up of a prospective randomized trial comparing CYA and MTX with CYA and mycophenolate mofetil for GVHD prophylaxis in myeloablative sibling donor hematopoietic cell transplantation. Bone Marrow Transplant, 2013. 48(12): p. 1578–80. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton BK, et al. , Cyclosporine in combination with mycophenolate mofetil versus methotrexate for graft versus host disease prevention in myeloablative HLA-identical sibling donor allogeneic hematopoietic cell transplantation. Am J Hematol, 2015. 90(2): p. 144–8. [DOI] [PubMed] [Google Scholar]
- 25.Ratanatharathorn V, et al. , Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood, 1998. 92(7): p. 2303–14. [PubMed] [Google Scholar]
- 26.Nash RA, et al. , Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood, 2000. 96(6): p. 2062–8. [PubMed] [Google Scholar]
- 27.Hiraoka A, et al. , Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant, 2001. 28(2): p. 181–5. \ [DOI] [PubMed] [Google Scholar]
- 28.Kiehl MG, et al. , Mycophenolate mofetil in stem cell transplant patients in relation to plasma level of active metabolite. Clin Biochem, 2000. 33(3): p. 203–8. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson PA, et al. , Mycophenolate pharmacokinetics and association with response to acute graft-versus-host disease treatment from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant, 2010. 16(3): p. 421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott CL, et al. , Nonrelapse mortality and mycophenolic acid exposure in nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant, 2013. 19(8): p. 1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakahashi K, et al. , Pharmacokinetics-based optimal dose prediction of donor source-dependent response to mycophenolate mofetil in unrelated hematopoietic cell transplantation. Int J Hematol, 2011. 94(2): p. 193–202. [DOI] [PubMed] [Google Scholar]
- 32.Bejanyan N, et al. , Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant, 2015. 21(5): p. 926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson P, et al. , Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther, 2005. 78(5): p. 486–500. [DOI] [PubMed] [Google Scholar]
- 34.Li H, et al. , Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. J Clin Pharmacol, 2013. 53(4): p. 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa S, et al. , Extended mycophenolate mofetil administration beyond day 30 in allogeneic hematopoietic stem cell transplantation as preemptive therapy for severe graft-versus-host disease. Transplant Proc, 2009. 41(9): p. 3873–6. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton BK, et al. , Does day 11 omission of methotrexate due to toxicity influence the outcome in myeloablative hematopoietic cell transplant? Results from a single-center retrospective cohort study. Blood Cancer J, 2015. 5: p. e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yau JC, et al. , An effective acute graft-vs.-host disease prophylaxis with minidose methotrexate, cyclosporine, and single-dose methylprednisolone. Am J Hematol, 1991. 38(4): p. 288–92. [DOI] [PubMed] [Google Scholar]
- 38.Giralt SA, et al. , Etoposide, cyclophosphamide, total-body irradiation, and allogeneic bone marrow transplantation for hematologic malignancies. J Clin Oncol, 1994. 12(9): p. 1923–30. [DOI] [PubMed] [Google Scholar]
- 39.Przepiorka D, et al. , Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after matched unrelated donor marrow transplantation. Blood, 1996. 88(11): p. 4383–9. [PubMed] [Google Scholar]
- 40.Uberti JP, et al. , Tacrolimus as monotherapy or combined with minidose methotrexate for graft-versus-host disease prophylaxis after allogeneic peripheral blood stem cell transplantation: long-term outcomes. Bone Marrow Transplant, 2004. 34(5): p. 425–31. [DOI] [PubMed] [Google Scholar]
- 41.Cutler C, et al. , Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood, 2014. 124(8): p. 1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torlen J, et al. , A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica, 2016. 101(11): p. 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.