Abstract
Leukocyte-associated Ig-like receptor 1 (LAIR1) is an ITIM-bearing collagen receptor expressed by leukocytes and is implicated in immune suppression. However, using a divalent soluble LAIR1/Fc recombinant protein to block interaction of cell surface LAIR1 with matrix collagen, we found that whereas Th1 responses were enhanced as predicted, Th17 responses were strongly inhibited. Indeed, LAIR1 on both T cells and monocytes was required for optimal Th17 responses to collagen type (Col)V. For pre-existing “natural” Th17 response to ColV, the LAIR1 requirement was absolute, whereas adaptive Th17 and Th1/17 immune responses in both mice and humans were profoundly reduced in the absence of LAIR1. Furthermore, the addition of C1q, a natural LAIR1 ligand, decreased Th1 responses in a dose-dependent manner, but it had no effect on Th17 responses. In IL-17-dependent murine organ transplant models of chronic rejection, LAIR1+/+ but not LAIR1−/− littermates mounted strong fibroproliferative responses. Surface LAIR1 expression was higher on human Th17 cells as compared with Th1 cells, ruling out a receptor deficiency that could account for the differences. We conclude that LAIR1 ligation by its natural ligands favors Th17 cell development, allowing for preferential activity of these cells in collagen-rich environments. The emergence of cryptic self-antigens such as the LAIR1 ligand ColV during ischemia/reperfusion injury and early acute rejection, as well as the tendency of macrophages/monocytes to accumulate in the allograft during chronic rejection, favors Th17 over Th1 development, posing a risk to long-term graft survival.
Lung transplantation is complicated by chronic rejection, which manifests itself as obliterative bronchiolitis (OB) or its clinical correlate bronchiolitis obliterans syndrome. Improvements in immunosuppression have decreased acute rejection, but chronic rejection remains a major obstacle for long-term survival (1). One reason for this is the development of CD4+ Th17 autoimmunity directed against collagen type V (ColV) (2) and K-α1-tubulin (3). Th17-driven autoimmune responses are different as compared with the Th1-driven response in several aspects. When the Th1 response to tetanus toxoid/diphtheria toxoid (TT/DT) and the Th17 response to ColV were compared in a trans-vivo delayed type hypersensitivity (Tv-DTH) bioassay, the Th1 response required IFN-γ (2) and IL-12 (V.V. Agashe, E. Jankowska-Gan, and W.J. Burlingham, unpublished observations), could use either dendritic cells (DCs) or monocytes as APCs (4), and was independent of purinergic P2X7R function (5). In contrast, the Th17 response required IL-17 and TNF-α, but not IFN-γ. It had an absolute requirement for IL-1β, as well as monocytes as APCs, and was strictly P2X7R-dependent (2, 5, 6). Besides the critical role of the Th17 response to ColV in bronchiolitis obliterans syndrome, a “mixed” Th1/17 ColV response was found in coronary artery disease patients and in models of atherosclerosis (5, 7, 8).
Recently, we have described pre-existing or natural Th17 responses to certain self-antigens, including ColV, vimentin, and K-α1-tubulin in normal healthy individuals and in naive CBA mice (9). These pre-existing responses could be detected after the removal of CD39+ or CD25+ T regulatory (Treg) cells or by the neutralization of TGF-β1 (9). Interestingly, these Th17 cells responded in an HLA-DR-specific fashion to 160-aa fragments of ColV α1, but not to 15mer ColV peptides (9). Thus, some tertiary structure was required for the natural Th17 response. Although a number of receptors might be involved, we chose the leukocyte-associated Ig-like receptor 1 (LAIR1) for in-depth study, because it is constitutively expressed and has been implicated in both inhibitory and activating functions (10, 11).
LAIR1 is a transmembrane receptor expressed by a variety of immune cells (10). It binds collagenous domains via its extracellular domain and contains two ITIMs in its cytoplasmic tail. LAIR1 ligands include fibrillar collagens and collagenous domains containing proteins, for example, C1q and mannose binding lectin (10, 12, 13). Traditionally, LAIR1 has been described as an inhibitory receptor, because LAIR1 ligation by anti-LAIR1 Ab recruited Src homology region 2 domain-containing phosphatase (SHP)1 and SHP2 and decreased NK cell cytotoxicity (14). LAIR1 also inhibited T and B cells (15, 16). However, LAIR1−/− mice were not hyperresponsive and were no more susceptible to experimental autoimmune encephalomyelitis and colitis than were their LAIR1+/+ littermates (17). Thus, the role of LAIR1 in vivo remains unresolved. Because most data about LAIR1 inhibition were developed from in vitro studies and with an anti-LAIR1 Ab, rather than the natural ligands, we sought to determine the role of LAIR1 in the context of Th17 responses, including chronic allograft rejection model (18–20), as well as a trans-vivo bioassay system (21, 22) using natural ligands for LAIR1.
Materials and Methods
Human samples
Blood was drawn from lung, lung/heart, or kidney transplant patients and coronary artery disease patients after written informed consent was obtained, in accordance with Institutional Review Board guidelines. PBMCs were isolated using Ficoll-Hypaque and frozen for later use.
Antigens
Collagen type I (Coll) and ColV were a gift from Dr. D.S. Wilkes or Dr. D.D. Brand (University of Tennessee Health Science Center, Memphis, TN), vimentin was from R&D Systems (Minneapolis, MN), K-α1-tubulin was a gift from Dr. T. Mohanakumar (Dignity Health St. Joseph’s Hospital and Medical Center, Phoenix, AZ), TT/DT vaccine was from Sanofi-Aventis (Bridgewater, NJ), C1q was from Complement Technology (Tyler, TX), and ColV peptides were from GenScript (Piscataway, NJ) or the University of Wisconsin-Madison peptide synthesis core.
The soluble LAIR1/Fc protein (sLAIR1/Fc) construct (human LAIR1 fused to Fc of human IgG1 expressed in the J558 cell line) was obtained from Dr. M. Colona and purified in-house. An ELSIA was used to determine the binding capabilities of sLAIR1/Fc. Plates were coated overnight with various proteins of interest (10 μg/ml) and blocked with 2% BSA. sLAIR1/Fc was serially diluted onto the plate and incubated overnight. The bound sLAIR1/Fc was detected with an HRP-labeled anti-human IgG1 Ab (Sigma-Aldrich, St. Louis, MO). For inhibition studies, sLAIR1/Fc (1 μg/ml) was preincubated overnight with the inhibitor of interest. This mixture was then transferred to the ColV-coated plates and incubated overnight. sLAIR1/Fc bound to the ColV-coated plate was detected as described. A section of mouse footpad was stained with sLAIR1/Fc and the bound protein was detected with an anti-human Fc HRP-conjugated secondary Ab.
Mice
B6 and B10 mice were purchased from Envigo (Indianapolis, IN) or Taconic Biosciences (Cambridge City, IN), LAIR1 knockout (LAIR−/−) mice were a gift from Dr. John E. Coligan (17), and CB17 SCID mice were from Biotron Laboratory. Male and female mice were used. Animals were housed in a specific pathogen-free facility and experiments conducted in accordance with the Institutional Animal Care and Use Committee guidelines. Mice were immunized s.c. or intradermally with 50–100 μg of bovine ColV or ColV α1 peptide p599 emulsified in CFA. In certain experiments animals received a boost in IFA and some mice also received 75 μl of TT/DT pediatric vaccine i.p. Animals were euthanized 2–3 wk after immunizations, and spleen and lymph nodes were collected and homogenized to get a single-cell suspension for experimental use. Bone marrow was flushed from the tibia and femur into monocyte media (PBS plus 2% FBS plus 1 mM EDTA). The marrow was filtered, RBCs were lysed, and cells were resuspended in monocyte media.
Murine transplantation
All transplants were between B10 donors and B6 LAIR1−/− recipients or B6 LAIR+/+ recipients. Heterotopic cardiac and orthotopic lung transplants were performed as described previously (18–20). Heart grafts were monitored by abdominal palpitation (23). The hearts and the lung were collected 42 and 21 d posttransplant, respectively, and used for H&E and trichrome staining. A blinded pathologist scored the transplants for rejection and fibrosis. The cardiac grafts were scored according to the criteria in the Heart Rejection Study Group (24). Briefly, the scoring included no rejection (grade 0), focal (grade 1A) or diffuse (grade 1B) interstitial mononuclear infiltrates without myocyte necrosis, focal aggressive cellular rejection with myocyte damage (grade 2), multifocal aggressive infiltrates with myocyte damage (grade 3A), diffuse inflammatory process with necrosis (grade 3B), and diffuse aggressive polymorphous/mononuclear infiltrate with edema, hemorrhage, vasculitis, and necrosis (grade 4). Fibrosis was scored on a graded scale as follows: 0, no fibrotic areas; 1, increased numbers of interstitial collagen fibers; 2, 0–10% of area is fibrotic, 3, 10–50% of area is fibrotic; and 4, 50–100% of area is fibrotic. Acute vascular rejection score was calculated from the severity of involved epicardial and intramyocardial vessels (0, negative; +1, minimal intimal inflammation; +2, moderate intimal and medial inflammation; and +3, severe transmural inflammation).
The lung transplant recipients were euthanized, and the native and donor lungs were harvested, glutaraldehyde fixed, and paraffin embedded. A portion of the lower lobe of each lung was sectioned and used for H&E and trichrome staining. Lung transplants were scored following the International Society of Heart and Lung Transplant 2007 classification (25). Scores include the alveolar perivascular inflammation (American College of Rheumatology [ACR]-A score) as minimal (A1), mild (A2), moderate (A3), and severe (A4), and bronchial inflammation (ACR-B score) as low grade and high grade. Additionally, peribronchial fibrosis in this model was graded as negative (0), mild (1), moderate (2), and severe (3). When OB lesions were identified, they were labeled as present (yes) or absent (no). The hydroxyproline assay was used to determine the collagen content of the lung grafts as previously described (26, 27).
Tv-DTH assay
A Tv-DTH assay was performed by the cotransfer of 7–10 × 106 human PBMCs or mouse splenocytes and the appropriate Ag into the footpads of CB17-SCID or B6 mice (21, 28). Murine footpad thickness was measured with a gauge before and 18–20 h after the cotransfer of cells. Background swelling seen with the buffer alone was subtracted to determine the Ag-specific response. Ag concentrations per injection were: for human studies, ColV peptide (5 μg), ColV peptide (1 μg), TT/DT (15 μg), donor or donor-matched alloantigen lysate (8–12 μg), and EBV (8 μg); for murine studies, ColV and ColI peptides (20 μg), ColV peptide (5 μg), and TT/DT (25 μg). In certain experiments, sLAIR1/Fc (0.05–1 μg) or C1q (0.5–10 μg) was titrated in with the cells and the Ag of interest. To determine the cytokine dependency of certain immune responses, neutralizing Abs were added: purified anti-mouse IFN-γ (551216; BD Biosciences), anti-mouse IL-17A (555068; BD Biosciences), anti-mouse TNF-α (14-7423-85; eBioscience), anti-mouse IL-1β (14-7012-85; eBioscience), or anti-mouse TGF-β1 (555052; BD Biosciences) were added at 10 μg per injection.
Cell isolations
Untouched CD3+ T cells were isolated and depleted of CD25+ cells according to the manufacturer’s instructions (130-095-130, 130-091-072; Miltenyi Biotec). Untouched murine CD11b+Ly6G− monocytes were isolated from the bone marrow (19761 or 19861; Stemcell Technologies) according to the manufacturer’s instructions.
Flow cytometry
PBMCs (1 × 106) were Fc blocked (130-059-901; Miltenyi Biotec), stained, and acquired on a BD LSR II or a BD LSRFortessa (5). For intracellular cytokine staining, 1 × 106 PBMCs were cultured in the presence of the appropriate antiegn (ColI or ColV) in DMEM containing 5% FBS at 37°C. After overnight incubation, the cells were stimulated with PMA (10 ng/ml) and ionomycin (1 μg/ml) for 5 h, with the addition of brefeldin A (420601; BioLegend) for the last 3 h. The following Abs were used: CD3-FITC or CD3–Alexa Fluor 700 (317306/100216; BioLegend or 557943; BD Biosciences), CD11b-BV421 (562632; BD Biosciences), CD56-allophycocyanin (555518; BD Biosciences), CD14–Alexa Fluor 700 (325614; BioLegend), CD16-allophycocyanin-H7 (560195; BD Biosciences), LAIR1-PE (550811; BD Biosciences), HLA-DR–PE-Cy7 (25-9956-41; eBioscience), CD123-BV421 (306017; BioLegend), lineage-BV510 (348807; BioLegend), CD11c-allophycocyanin (559877; BD Biosciences), PE-IgG1 κ isotype control (55749; BD Biosciences), CD196/CCR6-FITC (11-1969-42; eBioscience), CD194/CCR4-PECy7 (561034; BD Biosciences), CD4-BUV395 (563552/563790; BD Biosciences), CD183/CXCR3-allophycocyanin (550967; BD Biosciences), IFN-γ-BV421 (563376; BD Biosciences), IL-17-PE (559502; BD Biosciences), γδ TCR-FITC (118105; BioLegend). Data were analyzed using FlowJo (Treestar).
ELISPOT
Plates with coated with the appropriate primary Ab, that is, purified anti-mouse IFN-γ (551216; BD Biosciences), IL-17A (16-7175-85; eBioscience), or IL-4 (554434; BD Biosciences), and incubated overnight at 4°C. Wells were blocked with 1% BSA at room temperature for 2 h and the washed with HL1 serum-free media (Lonza, Walkersville, MD) supplemented with penicillin/streptomycin and 2 mmol/l L-glutamine. Murine lymphocytes were added at 2.5 × 106 to 4 × 106 cells per well and stimulated with media, ColI (10 ×g per well), ColV (10 ×g per well), or TT. Plates were incubated at 37°C at 5% CO2 for 24–48 h. Following incubation, cells were lysed with H2O and wells washed four times with PBS plus Tween 20, followed by four washes in PBS. Secondary Abs anti-mouse IFN-γ (554410; BD Biosciences), IL-17A (13-7177-85; eBioscience), or IL-4 (554390; BD Biosciences) diluted in PBS containing 1% BSA were added to the wells. Plates were incubated at room temperature for 2 h. After washing, spots were developed with streptavidin-alkaline phosphatase and 5-bromo-4-chloro-3-indolyl phosphate substrate (SEL002; R&D Systems) according to the manufacturer’s instructions. Spots were counted using the AID ELSIPOT plate reader system (Autoimmun Diagnostika, Strasburg, Germany). Triplicate wells were run for each Ag condition, and results are expressed as an average number of spots per million cells producing cytokine (18).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
Results
Pre-existing Th17 responses to ColV are abolished by genetic deletion of LAIR1
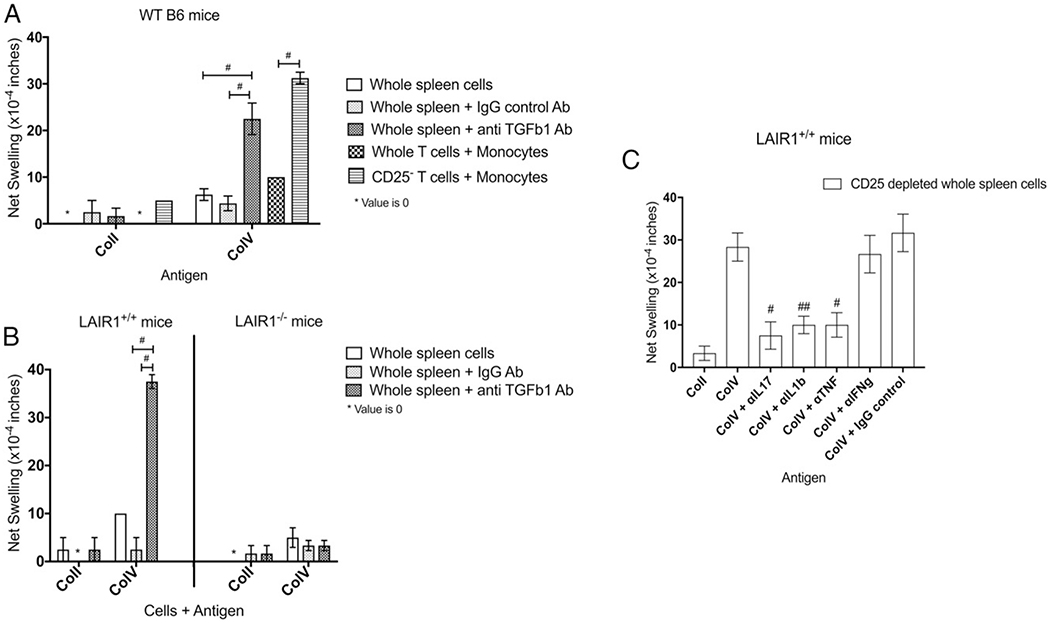
We first sought to determine whether B6 mice (background of the LAIR1−/− mice) had pre-existing responses to ColV, similar to those reported for the CBA mice (9). Using the trans-vivo bioassay system we demonstrated that whole spleen cells from naive wild-type (WT) B6 mice did not respond to ColV (Fig. 1A). The addition of an isotype control did not reveal any significant responses to ColV (Fig. 1A). However, the neutralization of TGF-β1 in the same system revealed a statistically significant increase in response to ColV (Fig. 1A). No responses to ColI were detected (Fig. 1A).
FIGURE 1.
Natural responses against ColV in C57BL/6 and LAIR1+/+ mice, but not in the LAIR1−/− littermate controls. Spleen cells from naive WT B6, LAIR1+/+, or LAIR1−/− littermate control mice were tested for their pre-existing natural immune responses to ColV. (A) Whole spleen cells from WT B6 mice (n = 8–12, pooled) were tested for their reactivity to ColI and ColV. The neutralization of TGF-β1 lead to the uncovering of a response against ColV but not ColI. Neither the whole spleen cells alone nor the addition of an IgG control isotype Ab revealed any responses to any Ag. Unseparated T cells and monocytes did not demonstrate a robust response to ColV. However, the CD25− T cells plus monocytes demonstrated a robust response to ColV, but not to ColI. #p < 0.0005 by unpaired t test. (B) Spleen cells from LAIR1+/+ and LAIR1−/− littermates (n = 7–9, pooled) were tested for their natural ability to respond to ColI and ColV. After neutralizing TGF-β1, the LAIR+/+ mice revealed strong responses to ColV, comparable to those seen with the WT B6 mice. No responses were detected in the LAIR1−/− mice. #p < 0.0001 by unpaired t test. (C) CD25-depleted spleen cells from LAIR1+/+ mice (n = 8, pooled) were tested for their reactivity to ColV in the presence of neutralizing Abs to various cytokines. Neutralization of IL-17, TNF-α, and IL-1β decreased the ColV response. Importantly, the neutralization of IFN-γ did not affect the natural ColV response. #p < 0.05, ##p < 0.005 by unpaired t test.
To determine whether this response could be recapitulated using isolated cells (9), we isolated CD25− T cells and CD11b+Ly6G− monocytes and used them in the same assay. Whereas the unseparated T cell population and monocytes gave a higher response to ColV than to ColI (Fig. 1A), the CD25− T cells and monocytes revealed a strong natural response to ColV, but not to ColI (Fig. 1A).
Cells from LAIR1+/+ littermates responded similarly to ColV upon TGF-β1 neutralizations as did WT B6 mice (Fig. 1B), but cells from naive LAIR1−/− mice failed to respond to ColV (Fig. 1B). The use of isolated CD25− T cells and monocytes from the LAIR1−/− mice also did not reveal any significant responses to ColV, ruling out changes in cytokine production by Treg cells (Supplemental Fig. 1A).
Additionally, to determine the cytokines responsible for the natural ColV response, we depleted CD25+ cells from the spleen of naive LAIR1+/+ mice. The response of the CD25-depleted fraction to ColV was analyzed in the presence of neutralizing Abs against IL-17, IL-1β, TNF-α, and IFN-γ. The neutralization of the former three, but not IFN-γ, decreased the natural response to ColV (Fig. 1C), indicating that the natural ColV response is a Th17 response. Further in vitro analysis of cells from LAIR1+/+ and LAIR1−/− mice revealed the source of IL-17 to be αβ TCR+CD4+ T cells (Supplemental Fig. 1B). These results together suggest that the Th17 ColV response is LAIR1-dependent, and that the lack of LAIR1 abolishes this response.
LAIR1 is also required for the induced ColV and ColV peptide response in mice
Having established that the natural Th17 response to ColV is absent in LAIR1−/− mice, we next aimed at determining the LAIR1 dependency of the induced ColV response (5). To test this, LAIR1+/+ and LAIR1−/− littermates were immunized with ColV. ELISPOT analysis of spleen cells from LAIR1+/+ mice revealed the production of IL-17 and IFN-γ in response to ColV stimulation. Conversely, the production of both cytokines was decreased in the LAIR1−/− mice (Fig. 2A). The IL-17 and IFN-γ spots were similar in magnitude in the LAIR1+/+ mice, indicating that the induced ColV response is a Th1/17 response, not a Th17 response.
FIGURE 2.
LAIR1 is required for optimal induced Th1/17 responses to ColV and ColV peptide in WT B6 and LAIR1+/+ mice. Cells from immunized B6 WT, LAIR1+/+, and LAIR1−/− mice were tested in the ELISPOT and the Tv-DTH assay. (A) Lymphocytes from ColV-immunized WT B6 LAIR1+/+ (n = 7) and B6 LAIR1−/− (n = 11) mice were cultured in vitro with ColV and analyzed for the expression of three cytokines, IL-17, IFN-γ, and IL-4. A statistically significant decrease in IL-17 spots. #p < 0.05, B6 LAIR1−/− mice as compared with the B6 LAIR1+/+ mice (by unpaired t test; values >1000 were excluded in the analysis). (B) Lymphocytes from ColV-immunized WT B6 mice (n = 38) were tested for their ability to respond to ColV in the presence of neutralizing Abs to various cytokines. Neutralization of IL-17, TNF-α, and IFN-γ and gave statistically significant decreases in ColV reactivity. #p < 0.05, ##p < 0.005, ###p < 0.0005 by paired t test. (C) Using isolated T cells and monocytes, robust Th1 responses to TT/DT were seen in the LAIR1+/+ and the LAIR1−/− mice. However, a statistically significant decrease in ColV response was seen in the LAIR1−/− group as compared with the LAIR1+/+ group. No significant differences in TT/DT and ColV responses were noted when only one of the cell type, either the T cell or the APC, was LAIR1−/− (n = 3 separate experiments, with cells pooled from four mice in each group). No responses to ColI were detected in either group. #p < 0.005, by unpaired t test. (D) Lymphocytes from p599-immunized WT B6 mice were tested for their ability to respond to ColI and ColV Ags and to ColV peptide p599 in the presence of neutralizing Abs to various cytokines. Left, Lymphocytes from p599-immunized WT B6 mice (n = 25) responded equally well to p599 and whole ColV, but not to ColI and p909 (28). #p < 0.05, ##p < 0.0001, unpaired t test. Right, Neutralizing IL-17, IL-1β, and TNF-α, but not IFN-γ, decreased the response to ColV peptide p599 (n = 4). #p < 0.05, ##p ≤ 0.005 by paired t test.
Additionally, the neutralization of IL-17, TNF-α, and IFN-γ significantly decreased the response to ColV (Fig. 2B) in WT B6 mice. Neutralization of IL-1β was trending toward significance. Interestingly, note that IFN-γ was found to be significant, but the results varied between individual mice (Fig. 2B). This supported the idea that the induced ColV response was more Th1/17.
To confirm the lack of Th17 responses in LAIR1−/− mice, we isolated CD3+ T cells and monocytes from TT/DT- and ColV- immunized LAIR1+/+ and LAIR1−/− mice and tested them in various combinations. The absence of LAIR1 on either the monocyte or the T cell alone did not abrogate the ColV response (Fig. 2C). Only when LAIR1 was absent on both cell types did we find a statistically significant drop in ColV responses (Fig. 2C). No responses to ColI were detected, and the TT/DT response remained consistent in all groups (Fig. 2C). These results suggested that the absence of LAIR1 on both the T cell and the monocyte is required for the decrease in Th17 ColV responses.
We also determined the response to the I-Ab-specific ColV α1 peptide p599 in WT B6 mice (28). Lymphocytes from these p599 immunized mice responded to p599 as well as ColV, but not to Coll and p909 (Fig. 2D). Unlike the induced ColV response, which was Th1/17, the induced ColV peptide p599 response was dependent only on IL-17, IL-1β, and TNF-α, but not on IFN-γ (Fig. 2D). These results indicated that the LAIR1, although absolutely required for the pre-existing Th17 response to ColV, was also partially required for the induced Th17 and induced Th1/17 responses to ColV.
sLAIRl/Fc binds ColV, the larger 160-aa fragments, but not the 15mer peptides of ColV α1
To further understand the role of LAIR1 on Th1 and Th17 responses, we employed an antagonist of LAIR1, sLAIR1/Fc. The protein was purified and characterized in-house (Supplemental Fig. 2A). Consistent with the published data on LAIR1, sLAIR1/Fc bound all collagens tested, including the larger 160-aa-long fragment of ColV α1, along with C1q, but did not bind fibrinogen, fibronectin, or the 15mer peptides of ColV (Fig. 3A).
FIGURE 3.
sLAIR1/Fc binds to collagenous domain–containing proteins, the 160-aa fragments, but not the 15mer peptides of ColV α1. The sLAIR1/Fc construct was tested for its ability to bind various proteins and peptides of interest in an ELISA assay. (A) sLAIR1/Fc bound all the proteins of interest (collagens and C1q) but failed to bind fibrinogen and fibronectin. sLAIR1/Fc also bound the larger 160-aa fragment of ColV α1, F5, but not the 15mer ColV peptides p629 and p1049 (n = 2–3 individual experiments; a representative plot is shown). (B) Inhibition of sLAIR1/Fc binding to a plate coated with ColV after preincubation of sLAIR1/Fc with various proteins and peptides of interest is shown. Preincubation with certain proteins (CollV, ColXI, and C1q) but not others (tetanus Ag, ColV peptides) inhibited sLAIR1/Fc from binding to the plate-bound ColV (n = 2–3 experiments; representative plots from one experiment are shown).
The inability of sLAIR1/Fc to bind the peptides was further confirmed with inhibition studies. Preincubation of a fixed concentration of sLAIR1/Fc with increasing concentrations of ColV or ColXI completely inhibited its binding to the plate-bound ColV (Fig. 3B). The preincubation with C1q only partially inhibited its binding to the plate-bound ColV (Fig. 3B). However, the preincubation with peptides did not inhibit its binding to the plate coated with ColV (Fig. 3B). Similar results were obtained when increasing concentrations of sLAIR1/Fc were titrated into a fixed inhibitor concentration (Supplemental Fig. 2B).
Staining of a mouse footpad with sLAIR1/Fc also demonstrated its binding to collagens present in the tissue as evidenced by the trichrome stain (Supplemental Fig. 2C). Collectively, these results indicate that the tertiary structure of its ligands is important for LAIR1 binding and that LAIR1 binds collagens and C1q differently.
Antagonism of collagen–LAIR1 interaction increases Th1 responses but decreases Th17 responses
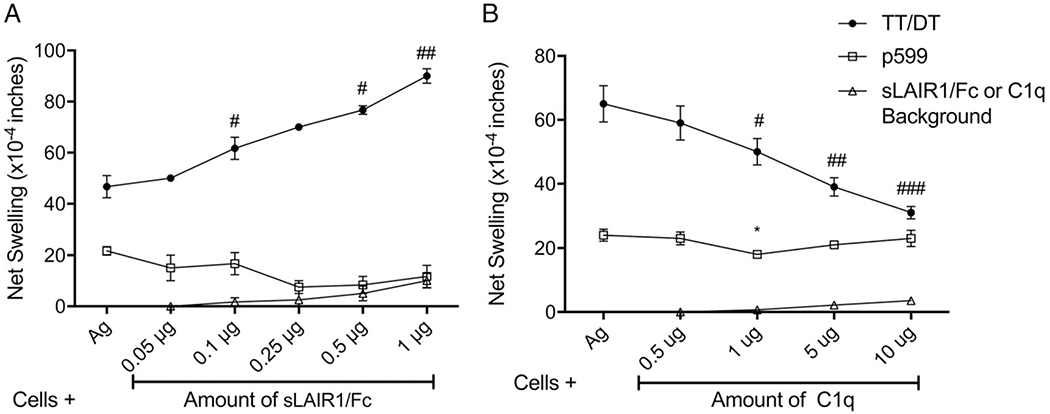
To determine the in vivo role of LAIR1 in the effector end of the immune response in a collagen-rich environment, we employed the Tv-DTH assay. Lymphocytes from TT/DT- and ColV peptide–immunized mice or PBMCs from ColV-reactive patients were injected into the footpads of B6 or SCID mice along with the Ag of interest, with increasing concentrations of sLAIR1/Fc. This blocked the access of the injected cells to matrix collagen through their surface LAIR1 receptors, and the use of ColV α1 peptides in the assay enabled us to rule out any effect ColV might have had as an LAIR1 ligand.
Because in the case of Th1 cells we were inhibiting an inhibitory response, increasing sLAIR1/Fc concentrations gave a statistically significant increase in the Th1 TT/DT-induced swelling response (Fig. 4A) in cells from immunized WT B6 mice. Conversely, increasing amounts of sLAIR1/Fc decreased the Th17 ColV peptide response (Fig. 4A). Utilizing C1q, an LAIR1 agonist due to its collagen “tail” domain (29), decreased the Th1 TT/DT response but failed to affect the Th17 ColV peptide response (Fig. 4B) in cells from immunized WT B6 mice.
FIGURE 4.
Effect of sLAIR1/Fc, an LAIR1 antagonist, and C1q, an LAIR1 agonist, on the Th1 tetanus and the Th17 ColV peptide response in immunized WT B6 mice. sLAIR1/Fc and C1q were used to determine the effect of LAIR1 on Th1 and Th17 responses in cells obtained from TT/DT and ColV-immunized B6 mice. (A) Addition of sLAIR1/Fc increased the Th1 TT/DT responses (black line) with increasing sLAIR1/Fc concentrations. Conversely, the Th17 ColV p599 peptide response (blue line) showed a decreasing trend with increasing sLAIR1/Fc concentrations (n = 3 experiments with pooled splenocytes from two to four mice). Using a paired t test between Ag and each subsequent concentration of sLAIR1/Fc, for p599, p = 0.0942 at 0.5 μg of sLAIR1/Fc. For TT/DT, #p < 0.05, ##p < 0.005. (B) The introduction of C1q, an LAIR1 ligand, decreased the Th1 TT/DT response (black line) but failed to cause any significant difference in the Th17 ColV p599 peptide response (blue line) (n = 4–6 experiments, with cells pooled from two to seven immunized mice). Using a paired t test between Ag and each subsequent concentration of C1q, for p599, *p < 0.005. For TT/DT, #p < 0.05, ##p < 0.01, ###p < 0.005.
Similar results were seen with PBMCs from ColV-reactive patients; that is, increasing sLAIR1/Fc concentrations significantly increased the Th1 TT/DT swelling response but decreased the Th17 ColV peptide response (Fig. 5A). The addition of C1q showed a trend toward a decrease in the Th1 TT/DT responses (Fig. 5B), but it did not affect the Th17 ColV peptide response (Fig. 5B). Owing to the lack of ColV peptide-reactive patient samples, PBMCs from a single patient were used in Fig. 5B. These results support the idea that LAIR1 differentially regulates Th1 and Th17 responses, inhibiting the former but not the latter.
FIGURE 5.
Effect of sLAIR1/Fc, an LAIR1 antagonist, and C1q, an LAIR1 agonist, on Th1 and Th17 responses in ColV-reactive heart or lung transplant patient PBMCs. PBMCs from ColV-reactive heart or lung transplant patients were tested for their ability to respond to various ColV peptides or donor responses and TT/DT or EBV in the presence of increasing concentrations of sLAIR1/Fc or C1q. (A) Addition of sLAIR1/Fc increased the Th1 TT/DT responses (black line) with increasing sLAIR1/Fc concentrations. Conversely, the Th17 ColV peptide response (blue line) decreased to baseline with increasing sLAIR1/Fc concentrations (n = 4 experiments with three patients). Using a paired t test between Ag and each subsequent concentration of sLAIR1/Fc, for ColV peptides, #p < 0.05, ##p < 0.005. For TT/DT, #p < 0.05. (B) Increasing concentrations of C1q decreased the Th1 tetanus response in a dose-dependent manner (black line) but did not affect the Th17 ColV peptide response (blue line) (n = 2 experiments with one patient). Using a paired t test between Ag and each subsequent concentration of C1q, for TT/DT, p = 0.09 at 5 μg, and p = 0.07 at 10 μg. (C). The antagonism of the surface LAIR1 receptor increased the Th1 EBV response but decreased the Th17 alloantigen response (n = 5 experiments with three patients. Using a paired t test between Ag and each subsequent concentration of sLAIR1/Fc, #p < 0.05, ##p < 0.005.
To determine whether this pattern holds true for other induced Th17 versus Th1 responses, we used the Th1 EBV and the Th1/17 anti-donor Ag response in chronic kidney graft rejection (5). Increasing sLAIR1/Fc concentrations significantly increased the EBV response (Fig. 5C) but significantly decreased the anti-donor response (Fig. 5C) in chronically rejecting kidney transplant patients. These results strongly suggest that LAIR1 is differentially regulating Th1 and Th1/17 responses.
However, artificial LAIR1 ligands inhibited both Th1 and Th17 responses. For example, the anti-LAIR1 Ab completely inhibited both the Th17 ColV peptide response (Supplemental Fig. 3) as well as the Th1 response to tetanus toxoid (data not shown). Notably, the addition of natural ligands of LAIR1, for example, C1q or ColI, did not inhibit the Th17 responses (Supplemental Fig. 3).
The differential effects of LAIR1 on Th1 and Th17 cells cannot be attributed to the lower expression of LAIR1 on human Th17 cells or their APCs
We hypothesized that the differential regulation of Th1 and Th17 responses by LAIR1 could be explained by a lack of, or by a differential expression of, LAIR1 expression on these cells. Therefore, we analyzed the LAIR1 expression on PBMCs from ColV-reactive patients. CXCR3+CD4+ Th1 cells were found to have lower LAIR1 expression as compared with the CCR4+ CCR6+ CD4 Th17 cells (Fig. 6A), both in terms of the percentage of LAIR1+ cells and the median fluorescence intensity of LAIR1 (Fig. 6A). Similar to the published data, both the DCs and monocytes expressed LAIR1 (Fig. 6B), and plasmacytoid DCs (pDCs) also expressed the highest levels of LAIR1 (30). Surprisingly, both the CD16+ and CD16− monocytes expressed LAIR1 at levels comparable to the pDCs (Fig. 6B). Because most of the ColV-specific T cells expressed the αβ TCR (Supplemental Fig. 1B), the expression of LAIR1 on γδ T cells was excluded from this publication. These results indicate that the differential regulation of Th1 and Th17 cells seen in vivo is not due to a lack of LAIR1 expression on Th17 cells.
FIGURE 6.
LAIR1 expression on chemokine receptor gated Th1 cells, Th17 cells, DCs, and monocytes. PBMCs from ColV-reactive patients (n = 6) were stained to distinguish between Th1 cells, Th17 cells, DCs, and monocytes. T cells were gated on CCR4 and then on CCR6 and CXCR3 for differentiating Th1(CCR4+CXCR3+CCR6−) and Th17 (CCR4+CCR6+CXCR3+/−) cells. DCs were defined as lineage− (CD3, CD14, CD16, CD19, CD20, CD56), HLA-DR+ and then differentiated as mDCs (CD11c+CD123−) and pDCs (CD11c−CD123+). Monocytes were defined as CD3−CD14+CD11b+CD56− and then gated as CD16+ or CD16− monocytes. (A) T cells with a Th17 phenotype (CCR4+, CCR6+) had a higher percentage of cells that stained positive for LAIR1, as compared with the Th1 cells (CXCR3+). The Th17 cells also had a higher MFI of LAIR1 as compared with the Th1 cells. #p < 0.05 by paired t test. (B) Of all the APCs analyzed, mDCs, pDCs, and CD16+ and CD16− monocytes expressed LAIR1. The MFI, however, was higher on pDCs and CD16+ and CD16− monocytes as compared with mDCs. #p < 0.05 by paired t test. mDC, myeloid DC; MFI, median fluorescence intensity.
LAIR1−/− mice mount deficient chronic rejection responses to heart and lung allografts
Given that the LAIR1−/− mice were completely unable to make a natural Th17 response to ColV, and they were defective in their induced Th17 responses, we determined whether they would develop chronic rejection in a Th17-dependent murine transplant models (18) (Supplemental Fig. 4). B10 hearts or lungs were transplanted into LAIR1+/+ or LAIR1−/− recipients.
A significant decrease was seen in cellular infiltration in the heart grafts from LAIR1−/− mice as compared with the LAIR1+/+ mice, along with a trend toward a lower amount of collagen deposition and vascular rejection (Fig. 7A, 7B, Table I). Only one of the B6 LAIR1−/− mice demonstrated collagen deposition and vascular rejection and thus a statistical significance could not be achieved for vascular rejection between the LAIR1+/+ and LAIR1−/− groups (Fig. 7B, Table I).
FIGURE 7.
Murine organ transplantation from a B10 donor into B6 LAIR1+/+ or B6 LAIR1−/− recipients. Murine hearts were transplanted from a B10 donor to an LAIR1+/+ (n = 2) or LAIR1−/− (n = 4) recipient as described. (A) Representative H&E (top) and trichrome (bottom) staining. Original magnification ×20. LAIR1−/− recipients consistently demonstrated a lower infiltration of cells into the graft, as well as lower collagen deposition around the blood vessels, as compared with the LAIR1+/+ littermates. (B) Murine left lungs were transplanted from a B10 donor to an LAIR1+/+ (n = 2) or LAIR1−/− (n = 5) recipient as described. Left, A decrease in acute cellular and vascular rejection was seen between the LAIR1+/+ and LAIR1−/− mice. #p < 0.01 by unpaired t test. Right, Statistical significance for vascular rejection could not be achieved due to a single outlier. *p = 0.4466, by unpaired t test. (C) Native and transplanted lung at day 21 posttransplant, along with the corresponding H&E and trichrome staining (original magnification ×40). OB lesions are indicated by arrows (18). The first and second columns shows representative histology from LAIR1+/+ mice, one that developed OB and one that did not develop OB. The LAIR1−/− (third column) grafts did show cellular infiltration, but they had markedly reduced collagen deposition around the airways as compared with the LAIR1+/+ recipients. (D) A decrease in both perivascular inflammation and peribronchial fibrosis was noted between the LAIR1−/− mice. A statistically significant difference in peribronchial fibrosis was noted between the LAIR1+/+ and LAIR1−/− mice, ##p < 0.05 by unpaired t test. Statistical significance was trending toward a decrease for perivascular inflammation, *p = 0.0522 by unpaired t test. (E) Quantity of collagen deposition in the grafts, using the hydroxyproline assay (26, 27). Native lung (n = 4) was used as a baseline control (PBS). The LAIR1−/− (n = 4) mice had lower collagen content than the LAIR1+/+ mice that did not develop OB (n = 4) and was comparable to the baseline control.
Table I.
Histological analysis of murine cardiac allografts
| Transplant No.a | Donor | Recipient | POD | Acute Cellular Rejection | Myocardial Fibrosis Score | Acute Vascular Rejection |
|---|---|---|---|---|---|---|
| 1 | B10 | B6 LAIR1−/− | 42 | 2 | 1 | 2 |
| 2 | B10 | B6 LAIR1−/− | 41 | 1B | 0 | 0 |
| 3 | B10 | B6 LAIR1−/− | 42 | 1A | 0 | 0 |
| 4 | B10 | B6 LAIR1−/− | 41 | 1B | 0 | 0 |
| 5 | B10 | B6 LAIR1+/+ | 41 | 3A | 0 | 3 |
| 6 | B10 | B6 LAIR1+/+ | 40 | 3A | 1 | 0 |
Histological analysis of B10 cardiac allografts transplanted into a B6 LAIR1+/+ or B6 LAIR1−/− recipient. The cardiac grafts were scored according to the criteria in the Heart Rejection Study Group (24). POD, postoperative day.
Similar results were seen in the lung transplant model (18). The LAIR1+/+ mice with chronic rejection but no OB showed a low level of cellular infiltration and collagen deposition around the airways (Fig. 7C). The LAIR1+/+ mice that developed OB demonstrated high levels of cellular infiltration and collagen deposition (Fig. 7C). Additionally, one can observe the OB lesions within the airways (arrows). However, the LAIR1−/− mice did show cellular infiltration into the graft, but little collagen deposition (Fig. 7C) and no OB lesions. Perivascular inflammation (ACR-A) scores were decreased in the LAIR1−/− mice as compared with the LAIR1+/+ mice, but due to a single outlier the values were trending toward statistical significance (Fig. 7D, Table II). However, a significant difference was visible in fibrosis between the LAIR1+/+ and LAIR1−/− groups (Fig. 7D, Table II). The transplanted lungs were analyzed for their collagen content, with the native lung used as a baseline control (PBS). Lung allografts from non-OB LAIR1+/+ mice but not the LAIR1−/− mice showed a significant increase in the amount of collagen (Fig. 7E).
Table II.
Histological analysis of murine lung allografts
| Transplant No.a | Donor | Recipient | POD | ACR-A | ACR-B | Peribronchial Fibrosis | OB |
|---|---|---|---|---|---|---|---|
| 1 | B10 | B6 LAIR1−/− | 21 | 1 | Low | 0 | No |
| 2 | B10 | B6 LAIR1−/− | 21 | 2 | Low | 1 | No |
| 3 | B10 | B6 LAIR1−/− | 21 | 1 | Negative | 0 | No |
| 4 | B10 | B6 LAIR1−/− | 21 | 1 | Low | 1 | No |
| 5 | B10 | B6 LAIR1−/− | 21 | 0 | Negative | 0 | No |
| 6 | B10 | B6 LAIR1+/+ | 21 | 3 | Low | 3 | Yes |
| 7 | B10 | B6 LAIR1+/+ | 21 | 2 | Low | 2 | No |
Histological analysis of B10 lung allografts transplanted into a B6 LAIR1+/+ or B6 LAIR1−/− recipient. Lung transplants were scored following the International Society of Heart and Lung Transplant 2007 classification (25). POD, postoperative day.
Discussion
In this study we analyzed the role of LAIR1 in Th1 versus Th17 responses. We set out to test the hypothesis that the ITIM-bearing LAIR1 receptor will inhibit both responses (10). Recently, LAIR1 has been found to have diverse roles in the immune system; for example, LAIR1 signaling induced stem cell-like proliferative capabilities to acute myeloid leukemia cells, resulting in worse disease outcome (11). Additionally, LAIR1 was found to aid in the pathogenesis of the myeloid-based chronic myeloid leukemia (31). These results clearly indicate that LAIR1 can have both activating and inhibitory functions. Because we were analyzing the role of LAIR1 in the context of in vivo studies, we used the Tv-DTH assay, which enabled us to analyze the role of LAIR1 in the presence of its ligands (21). Additionally, the bioassay was more sensitive to the influence of IL-17/Th17 cells, as compared with an in vitro assay (Figs. 1, 2).
Similar to the previously published data on CBA mice (9), we found that WT B6 mice also had pre-existing responses to ColV regulated via a TGF-β1 mechanism (Fig. 1A). Although the B6 LAIR1+/+ littermates had robust pre-existing responses to ColV similar to the WT B6 mice, the B6 LAIR1−/− littermates completely lacked these responses. (Fig. 1A, 1B). Neither the neutralization of TGF-β1 (Fig. 1B) nor the depletion of CD25+ Treg cells (Supplemental Fig. 1A) revealed any pre-existing ColV responses in the LAIR1−/− mice, which were dependent on αβ TCR+ CD4+ T cells (Supplemental Fig. 1B), suggesting that LAIR1 is indispensable for the natural Th17 response to ColV in naive mice. Whether the lack of ColV response was due to a functional unresponsiveness of the cells or the complete absence of a responder population remains to be determined. Tetramer analysis of the T cell population in LAIR1−/− mice will answer these questions.
We have shown that the natural Th17 response to ColV can be induced by the 160-aa-long ColV α1 fragments but not the 15mer peptides of ColV (9). Additionally, in this study, we have demonstrated that sLAIR1/Fc binds the ColV fragment but not the peptide (Fig. 3, Supplemental Fig. 2). Therefore, our data strongly suggests that a secondary signal, generated by LAIR1 ligation, by its natural GPO ligands, is required for the of Th17 responses to ColV. Interestingly, also note that the secondary signal, in case of ColV responses, can be triggered by the Ag itself. Additionally, C1q did not completely inhibit sLAIR1/Fc from binding the ColV coated on the plate, indicating differences in LAIR1 ligand binding. Such differences have also been noted between the LAIR1-collagen and the LAIR1-anti-LAIR1 Ab interaction (32).
The induced Th17 response, which was completely dependent on IL-17, TNF-α, and IL-1β, was also diminished in the LAIR1−/− mice (Fig. 2A, 2C). The induced ColV response in patients following lung transplantation was shown to be completely dependent on monocytes as APCs (2). Monocytes expressed high levels of LAIR1 (Fig. 6B), and the addition of ColV to purified monocytes induced the production of IL-1β and TNF-α (5). The need for monocytes as APCs, the indispensable requirement of IL-1β, and the expression of PLZF T cells (9) indicate the ColV response to be a true innate Th17 response. It is highly possible that the deposition of ColV in the transplanted organ provides a damage-associated molecular pattern signal to the monocytes inducing the production of IL-1β and TNF-α. These cytokines further support the development of the Th17 response to ColV posttransplant. Such a possibility is supported by the fact that LAIR1−/− mice had lower fibrous (i.e., collagen) deposition and chronic rejection, despite having cellular infiltration into the transplanted organ.
The addition of sLAIR1/Fc and C1q into the assay system further supported the notion that LAIR1 inhibits Th1 but is required for Th17 responses (Figs. 4, 5). Similar results were seen with Th1/17 anti-donor responses in kidney transplant patients with chronic rejection (Fig. 5C). This suggests that if we could modulate the immune response with LAIR1 antagonists, it might be possible to induce suppression of Th17 but maintain Th1 immunity, similar to the P2X7R (5). One such approach is the use of LAIR2, a naturally occurring soluble form of LAIR1. Use of LAIR2 inhibited the complement-mediated cytotoxicity in an ex vivo model of Ab mediated rejection (33).
In the B10-to-B6 model, the grafts downregulated complement regulatory proteins, resulting in complement activation and enhanced C3a deposition. Increased C3a upregulated IL-17 production that, in a feedback loop, further decreased the complement regulatory proteins (34). Thus, the intervention with a complement inhibitor, for example, C1 inhibitor, will be helpful in acute rejection and prevent complement activation during chronic rejection (35). However, our data suggest that free C1q in the system during chronic rejection, although not detrimental, might not inhibit Th17 cells. We are currently investigating whether the pre-existing responses to ColV contribute to the development of fibrosis and chronic rejection following organ transplantation (2), in atherosclerosis (8) or in chronic pulmonary hypertension caused by hypoxia (36, 37) (L.V. Gonzalez-Bosc, E. Jankowska-Gan, and W.J. Burlingham, unpublished observations).
Despite an increased LAIR1 expression (Fig. 6) on Th17 cells, they were not inhibited by LAIR1, supporting the concept that LAIR1 is actually required for Th17 responses. The differential regulation of Th1 and Th17 by LAIR1-collagen engagement could be due to the differential recruitment of secondary messengers in Th1 and Th17 cells, similar to the recruitment of CAMK1 and CREB in acute myeloid leukemia cells (11). Thus, the recruitment of secondary messengers, either inhibitory or activating, likely depends on the type of LAIR1-ligand engagement on the cell surface (receptor oligomerization due to GPO repeats in collagens versus dimerization using an Ab). Recently, it was published that CREB was essential for Th17 cells in vivo and that its absence in CD4 T cells lead impaired Th17 differentiation and the development of autoimmunity (38). We hypothesize that the CREB required for Th17 development could be recruited by LAIR1 binding via SHP1.
Another possibility is that LAIR1 is in fact required for the inhibition of the corresponding ColV-specific Treg cells that inhibit ColV-specific Th17 cells. It is possible that LAIR1 in fact inhibits the Treg cells, thus augmenting the Th17 responses. However, such a phenomenon would not fully explain the lack of responses seen in LAIR1−/− mice. Another possibility is that LAIR1 might inhibit Treg cells but provides a positive signal to Th17 cells. If the level of LAIR1 expression is inversely related to its inhibitory function (Fig. 6), then a low level of LAIR1 on Treg cells as compared with the Th17 cells (V.V. Agashe and W.J. Burlingham, unpublished observations) predicts that exposure of LAIR1 ligands might upset Treg/Th17 equilibrium in the LAIR1+/+ mice, leading to chronic rejection, but that in LAIR1−/− mice the Treg and Th17 cells would remain in equilibrium, thereby decreasing chronic rejection. It is interesting to speculate that lower LAIR1 expression on cells inhibits them, but increased LAIR1 induces a positive signal. Lastly and importantly, note that LAIR1 ligands bind differently to the receptor. Preincubation of sLAIR1/Fc with C1q did not completely inhibit sLAIR1/Fc binding to collagens coated on the plate, where incubation with matrix collagen V or XI did (Fig. 3B). These possibilities, however, do not answer all the questions regarding LAIR1 but provide exciting new avenues for LAIR1 research.
Thus, the analysis of TT/DT and EBV responses confirmed that LAIR1 can be a powerful inhibitory receptor in the immune system through the phosphatase activity of SHP1, as described previously (10). However, it is essential for the development of Th17 responses and our results argue for a unique nonredundant role of LAIR1 in Th17 responses.
Supplementary Material
Acknowledgments
We thank Dr. J.E. Coligan (National Institute of Allergy and Infectious Diseases, Bethesda, MD) for providing the LAIR1−/− mice.
This work was supported by National Institutes of Health Grants 1P01AI084853 (to V.V.A., E.J.-G., M.K., J.A.S., L.D.H., J.F.K., J.R.T., D.R., D.S.W., and W.J.B.), 1R01AI119140 (to V.V.A., E.J.-G., J.A.S., L.D.H., and W.J.B.), and R01AI110617 (to E.J.-G. and J.A.S.).
V.V.A. conceived, designed, and performed the experiments; analyzed the data; and wrote the paper. E.J.-G., M.K., J.A.S., L.D.H., J.F.K., J.R.T., and D.R. performed experiments and histology analysis. M.C. provided technical (reagents) support and reviewed the paper. M.D. initiated the project. D.S.W. reviewed the paper. W.J.B. conceived and designed the experiments and helped write the paper.
Abbreviations used in this article:
- ACR
American College of Rheumatology
- ColI
collagen type I
- ColV
collagen type V
- DC
dendritic cell
- LAIR1
leukocyte-associated Ig-like receptor 1
- OB
obliterative bronchiolitis
- pDC
plasmacytoid DC
- SHP
Src homology region 2 domain–containing phosphatase
- sLAIR1/Fc
soluble LAIR1/Fc protein
- Treg
T regulatory
- TT/DT
tetanus toxoid/diphtheria toxoid
- Tv-DTH
trans-vivo delayed type hypersensitivity
- WT
wild-type
Footnotes
The online version of this article contains supplemental material.
Disclosures
D.S.W. is a cofounder of Immune-Works, Inc., a biotechnology company involved in designing therapeutics for various forms of lung diseases. The other authors have no financial conflicts of interest.
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Yusen RD, and Stehlik J; International Society for Heart and Lung Transplantation. 2013. The registry of the international society for heart and lung transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J. Heart Lung Transplant. 32: 951–964. [DOI] [PubMed] [Google Scholar]
- 2.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. 2007. IL-17–dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J. Clin. Invest. 117: 3498–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, and Mohanakumar T. 2008. De novo production of K-α1 tubulin-specific antibodies: role in chronic lung allograft rejection. J. Immunol. 180: 4487–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derks RA, Jankowska-Gan E, Xu Q, and Burlingham WJ. 2007. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J. Immunol. 179: 3443–3451. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan JA, Jankowska-Gan E, Shi L, Roenneburg D, Hegde S, Greenspan DS, Wilkes DS, Denlinger LC, and Burlingham WJ. 2014. Differential requirement for P2X7R function in IL-17 dependent vs. IL-17 independent cellular immune responses. Am. J. Transplant. 14: 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, and Sallusto F. 2007. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells. Nat. Immunol. 8: 942–949. [DOI] [PubMed] [Google Scholar]
- 7.Park AC, Huang G, Jankowska-Gan E, Massoudi D, Kernien JF, Vignali DA, Sullivan JA, Wilkes DS, Burlingham WJ, and Greenspan DS. 2016. Mucosal administration of collagen V ameliorates the atherosclerotic plaque burden by inducing interleukin 35-dependent tolerance. J. Biol. Chem 291: 3359–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dart ML, Jankowska-Gan E, Huang G, Roenneburg DA, Keller MR, Torrealba JR, Rhoads A, Kim B, Bobadilla JL, Haynes LD, et al. 2010. Interleukin-17–dependent autoimmunity to collagen type V in atherosclerosis. Circ. Res. 107: 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan JA, Jankowska-Gan E, Hegde S, Pestrak MA, Agashe VV, Park AC, Brown ME, Kernien JF, Wilkes DS, Kaufman DB, et al. 2017. Th17 responses to collagen type V, kα1-tubulin, and vimentin are present early in human development and persist throughout life. Am. J. Transplant. 17: 944–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyaard L 2008. The inhibitory collagen receptor LAIR-1 (CD305). J. Leukoc. Biol. 83: 799–803. [DOI] [PubMed] [Google Scholar]
- 11.Kang X, Lu Z, Cui C, Deng M, Fan Y, Dong B, Han X, Xie F, Tyner JW, Coligan JE, et al. 2015. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat. Cell Biol. 17: 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, and Meyaard L. 2006. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 203: 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olde Nordkamp MJ, van Eijk M, Urbanus RT, Bont L, Haagsman HP, and Meyaard L. 2014. Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J. Leukoc. Biol. 96: 105–111. [DOI] [PubMed] [Google Scholar]
- 14.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, and Phillips JH. 1997. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 7: 283–290. [DOI] [PubMed] [Google Scholar]
- 15.Meyaard L, Hurenkamp J, Clevers H, Lanier LL, and Phillips JH. 1999. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J. Immunol. 162: 5800–5804. [PubMed] [Google Scholar]
- 16.van der Vuurst de Vries AR, Clevers H, Logtenberg T, and Meyaard L. 1999. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptormediated signaling. Eur. J. Immunol. 29: 3160–3167. [DOI] [PubMed] [Google Scholar]
- 17.Tang X, Tian L, Esteso G, Choi SC, Barrow AD, Colonna M, Borrego F, and Coligan JE. 2012. Leukocyte-associated Ig-like receptor-1–deficient mice have an altered immune cell phenotype. J. Immunol. 188: 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, and Wilkes DS. 2011. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am. J. Transplant. 11: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corry RJ, Winn HJ, and Russell PS. 1973. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16: 343–350. [DOI] [PubMed] [Google Scholar]
- 20.Niimi M 2001. The technique for heterotopic cardiac transplantation in mice: experience of 3000 operations by one surgeon. J. Heart Lung Transplant. 20: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 21.Jankowska-Gan E, Hegde S, and Burlingham WJ. 2013. Trans-vivo delayed type hypersensitivity assay for antigen specific regulation. J. Vis. Exp. (75):e4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Pocock GM, Sharma A, Peery SL, Fites JS, Felley L, Zarnowski R, Stewart D, Berthier E, Klein BS, et al. 2016. Human iNKT cells promote protective inflammation by inducing oscillating purinergic signaling in monocyte-derived DCs. Cell Rep. 16: 3273–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ, and Burlingham WJ. 2007. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J. Immunol. 179: 6749–6761. [DOI] [PubMed] [Google Scholar]
- 24.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, and Zerbe A, The International Society for Heart Transplantation. 1990. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: heart rejection study group. J. Heart Transplant. 9: 587–593. [PubMed] [Google Scholar]
- 25.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. 2007. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 26: 1229–1242. [DOI] [PubMed] [Google Scholar]
- 26.Mares DC, Heidler KM, Smith GN, Cummings OW, Harris ER, Foresman B, and Wilkes DS. 2000. Type V collagen modulates alloantigeninduced pathology and immunology in the lung. Am. J. Respir. Cell Mol. Biol. 23: 62–70. [DOI] [PubMed] [Google Scholar]
- 27.Woessner JF Jr. 1961. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 93: 440–447. [DOI] [PubMed] [Google Scholar]
- 28.Keller MR, Haynes LD, Jankowska-Gan E, Sullivan JA, Agashe VV, Burlingham SR, and Burlingham WJ. 2013. Epitope analysis of the collagen type V-specific T cell response in lung transplantation reveals an HLA-DRB1*15 bias in both recipient and donor. PLoS One 8: e79601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son M, Santiago-Schwarz F, Al-Abed Y, and Diamond B. 2012. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl. Acad. Sci. USA 109: E3160–E3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonaccorsi I, Cantoni C, Carrega P, Oliveri D, Lui G, Conte R, Navarra M, Cavaliere R, Traggiai E, Gattorno M, et al. 2010. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNa production. PLoS One 5: e15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Shojaee S, Buchner M, Geng H, Lee JW, Klemm L, Titz B, Graeber TG, Park E, Tan YX, et al. 2015. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. [Published erratum appears in 2016 Nature 534: 138.] Nature 521: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang X, Narayanan S, Peruzzi G, Apara A, Natarajan K, Margulies DH, Coligan JE, and Borrego F. 2009. A single residue, arginine 65, is critical for the functional interaction of leukocyte-associated inhibitory receptor-1 with collagens. J. Immunol. 182: 5446–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olde Nordkamp MJ, Boross P, Yildiz C, Jansen JH, Leusen JH, Wouters D, Urbanus RT, Hack CE, and Meyaard L. 2014. Inhibition of the classical and lectin pathway of the complement system by recombinant LAIR-2. J. Innate Immun. 6: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, Benson HL, Shilling R, Wu Q, Weber DJ, Wagner SR, et al. 2013. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J. Immunol. 191: 4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danobeitia JS, Ziemelis M, Ma X, Zitur LJ, Zens T, Chlebeck PJ, Van Amersfoort ES, and Fernandez LA. 2017. Complement inhibition attenuates acute kidney injury after ischemia-reperfusion and limits progression to renal fibrosis in mice. PLoS One 12: e0183701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maston LD, Jones DT, Giermakowska W, Howard TA, Cannon JL, Wang W, Wei Y, Xuan W, Resta TC, and Gonzalez Bosc LV. 2017. Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 312: L609–L624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandya PH, Fisher AJ, Mickler EA, Temm CJ, Lipking KP, Gracon A, Rothhaar K, Sandusky GE, Murray M, Pollok K, et al. 2016. Hypoxiainducible factor-1α regulates CD55 in airway epithelium. Am. J. Respir. Cell Mol. Biol. 55: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Ni L, Chang D, Lu H, Jiang Y, Kim BS, Wang A, Liu X, Zhong B, Yang X, and Dong C. 2017. Cyclic AMP-responsive element-binding protein (CREB) is critical in autoimmunity by promoting Th17 but inhibiting Treg cell differentiation. EBioMedicine 25: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.