Abstract
Background
The incidence of infective endocarditis, a serious heart infection that can result from injection drug use, has increased in step with the opioid epidemic. Harm reduction services aimed at decreasing infectious complications of injection drug use are limited in rural areas; however, it is unknown whether the burden of opioid use–associated infective endocarditis varies between rural and urban populations.
Methods
We used 2003–2016 National (Nationwide) Inpatient Sample data and joinpoint regression to compare trends in hospitalization for opioid use–associated infective endocarditis between rural and urban populations.
Results
Rates of US hospitalizations for opioid use–associated infective endocarditis increased from 0.28 to 3.86 per 100 000 rural residents, as compared with 1.26 to 3.49 for urban residents (overall difference in annual percent change P < .01). We observed 2 distinct trend periods, with a period of little change between 2003 and 2009/2010 (annual percent change, 0.0% rural vs –0.08% urban) followed by a large increase in hospitalization rates between 2009/2010 and 2016 (annual percent change, 0.35% rural vs 0.36% urban). Over the study period, opioid use–associated infective endocarditis hospitalizations shifted toward younger age groups for both rural and urban residents, and rural resident hospitalizations increasingly occurred at urban teaching hospitals. For both groups, Medicaid was the most common payer.
Conclusions
The increase in US hospitalizations for opioid use–associated infective endocarditis over the past decade supports the importance of public health efforts to reduce injection-related infections in both urban and rural areas. Future studies should examine factors affecting the higher increase in rate of these hospitalizations in rural areas.
Keywords: harm reduction, injection drug use, infective endocarditis, opioid use disorder, rural health
Hospitalizations for injection drug use–associated infective endocarditis have increased over the past decade in the United States [1, 2]. People who inject drugs are at increased risk of developing infective endocarditis and other blood-borne infections, particularly when using unsafe injection practices (eg, licking needles, injecting through unclean skin). Thus, in addition to decreasing opioid use through prevention and comprehensive treatment programs, harm reduction strategies such as education and provision of sterile supplies are critical for reducing infective endocarditis among persons who inject drugs.
In the United States, opioid use disorder treatment providers and syringe site programs offering harm reduction services are more often located in urban areas [3, 4], meaning rural persons who inject drugs must travel greater distances to access these services [5]. Given the relative inaccessibility of these resources in rural areas, we hypothesized that hospitalizations for opioid use–associated infective endocarditis have increased at a greater rate for persons living in rural vs urban areas during the recent opioid epidemic in the United States.
METHODS
Study Population
We used the National (Nationwide) Inpatient Sample, developed for the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality, to estimate hospitalization rates for opioid use–associated infective endocarditis by rural–urban residency status between 2003 and 2016 [6]. These data are a nationally representative sample of all hospital discharge records from US community hospitals (2012–2016) and, for data before 2012, a nationally representative sample of all US community hospitals from which discharge records were retained. We used annual census-based rural and urban population denominators [7] to calculate rates of hospitalizations per 100 000 residents. The Maine Medical Center Institutional Review Board determined that this study was not human subjects research.
Assessment of Opioid Use–Associated Infective Endocarditis
We used prior research studies to compile code lists of International Classification of Diseases, Clinical Modification (ICD-CM), diagnosis codes for opioid use (ie, abuse, dependence, adverse effects, poisoning from opioids, or unspecified use) [8] and infective endocarditis (Supplementary Table 1) [1]. We focused on opioid use because it is strongly associated with injection drug use, and no ICD-CM diagnosis codes identify drug use by mode of administration. We excluded opioid poisonings due to intentional injury because we considered them unlikely to result in infective endocarditis. We used the Centers for Medicare and Medicaid Services’ General Equivalence Mappings to verify that ICD-9-CM codes mapped to ICD-10-CM codes, as our analysis spanned the ICD-CM-9/10 transition (October 1, 2015) [9]. Only hospitalizations with at least 1 diagnosis code for both opioid use and infective endocarditis were classified as opioid use–associated infective endocarditis.
Assessment of Rurality
Rurality of patient residence was determined at the county level in the National (Nationwide) Inpatient Sample using 2 classification schemes. For 2003–2006, the 4-level Urban Influence Codes were used, and starting in 2007, the 6-level National Center for Health Statistics Classification Scheme was used (versions released in 2005 and 2014). We defined urban counties as central or fringe large metro areas with a population ≥1 million people and rural counties as micropolitan (centered around a city of 10 000 to 50 000 people) or noncore counties; these urban and rural levels were identified equivalently in each classification scheme. We excluded residents living in suburban counties (27.5% of total hospital discharges for opioid use–associated infective endocarditis) from our analysis; however, we conducted a standalone post hoc analysis of this group for exploratory purposes.
Data Analysis
We estimated trends in opioid use–associated infective endocarditis hospitalizations by rural–urban status between 2003 and 2016. We also compared demographics, hospital characteristics, and hospital outcomes between rural and urban opioid use–associated infective endocarditis hospitalizations. Total hospital charges were adjusted for inflation [10, 11].
We used survey procedures to account for the complex survey design and trend weights to correct for changes in the National (Nationwide) Inpatient Sample sampling design [12]. We compared proportions using Rao Scott chi-square tests and mean values using linear regression. We used Joinpoint regression software with Monte Carlo permutation models to determine when there was a significant change in the slope of the trend line and to calculate the annual percent change estimates for each segment. A test for parallelism compared the overall slope of the trend lines between rural and urban residents. We used SAS EG, version 7.15 (SAS Institute, Cary, NC, USA), and Joinpoint Regression Software (4.7.0, National Cancer Institute).
RESULTS
Between 2003 and 2016, there were 42 801 hospitalizations of rural (unweighted n = 1541) and urban (unweighted n = 7191) patients for opioid use–associated infective endocarditis in the United States. For both rural and urban residents, opioid use–associated infective endocarditis hospitalizations shifted toward younger age groups over the study interval, with the mean age of rural hospitalizations decreasing from 43.5 years to 36.6 years (P < .0001) and the mean age of urban hospitalizations decreasing from 41.3 years to 38.8 years (P < .0001) (Table 1). The percentage of female hospitalizations did not change over the study period for rural (P = .31) or urban residents (P = .90). Over the course of the study, the distribution of race/ethnicity changed for urban residents, with persons of non-Hispanic white race/ethnicity making up a greater percentage of urban hospitalizations (52.6% in 2003–2007 vs 73.7% in 2012–2016; P < .0001). The distribution of race/ethnicity did not change over time for rural hospitalizations (P = .20). Over the course of the study period, the distribution of income quartile (defined using the median household income of residents in the patient’s ZIP code) changed for urban residents hospitalized for opioid use–associated infective endocarditis (P < .0001), with 43% of urban resident hospitalizations in the lowest income quartile in 2003–2007 vs 33.1% in 2012–2016. In contrast, rural resident hospitalizations were increasingly likely to be in the lowest income quartile over the study period (53.0% in 2003–2007 vs 58.3% in 2012–2016), although this trend did not reach statistical significance (P = .17). Overall, Medicaid was the most common payer for both urban and rural resident hospitalizations.
Table 1.
Characteristics of 8732 Hospitalizations for Opioid Use–Associated Infective Endocarditis, 2003–2016 National Inpatient Sample, Weighted %
| 2003–2007 | 2008–2011 | 2012–2016 | P a | |||||
|---|---|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | Urban | Rural | Urban | Rural | |
| Unweighted discharges | 1999 | 187 | 1333 | 245 | 3859 | 1109 | - | - |
| Weighted discharges | 9494 | 902 | 6397 | 1168 | 19 295 | 5545 | - | - |
| Mean age, y | 41.3 | 43.5 | 42.9 | 43.3 | 38.8 | 36.6 | <.0001 | <.0001 |
| Age categories | <.0001 | <.0001 | ||||||
| ≤25 y | 9.9 | 12.1 | 12.8 | 15.4 | 16.5 | 18.4 | ||
| 26–35 y | 23.6 | 28.0 | 26.9 | 28.8 | 35.3 | 39.1 | ||
| 36–45 y | 30.5 | 28.2 | 22.2 | 18.5 | 18.6 | 20.6 | ||
| 46–55 y | 26.5 | 12.8 | 22.2 | 21.9 | 16.1 | 11.5 | ||
| 56–65 y | 6.3 | 5.2 | 11.1 | 8.5 | 8.7 | 6.3 | ||
| ≥66 y | 3.2 | 13.6 | 4.8 | 6.9 | 4.8 | 4.1 | ||
| Sexb | .90 | .31 | ||||||
| Male | 52.2 | 50.6 | 55.2 | 48.8 | 52.4 | 46.3 | ||
| Female | 47.8 | 49.4 | 44.7 | 51.2 | 47.6 | 53.7 | ||
| Race/ethnicityb | <.0001 | .20 | ||||||
| White | 52.6 | 87.6 | 62.8 | 89.2 | 73.7 | 91.3 | ||
| Black | 30.2 | 6.1 | 21.6 | 4.1 | 13.2 | 2.1 | ||
| Hispanic | 12.6 | 3.0 | 11.7 | 3.9 | 9.6 | 2.7 | ||
| Asian/Pacific Islander | 0.4 | 0.8 | 0.4 | 0.9 | 0.6 | 0.6 | ||
| Native American | 0.3 | 1.7 | 0.2 | 0 | 0.5 | 2.3 | ||
| Other | 3.9 | 0.8 | 3.3 | 1.9 | 2.6 | 1.0 | ||
| Payerb | <.0001 | .0001 | ||||||
| Medicare | 11.3 | 22.8 | 15.0 | 22.1 | 14.3 | 15.8 | ||
| Medicaid | 43.4 | 38.9 | 43.4 | 36.4 | 52.7 | 52.9 | ||
| Private insurance | 14.4 | 12.7 | 13.0 | 11.8 | 14.1 | 10.8 | ||
| Self-pay | 21.5 | 15.7 | 20.4 | 20.0 | 13.8 | 16.4 | ||
| No charge | 2.9 | 0.5 | 2.3 | 4.0 | 1.8 | 1.2 | ||
| Other | 6.5 | 9.4 | 5.8 | 5.8 | 3.4 | 3.0 | ||
| Income quartile | <.0001 | .17 | ||||||
| 1 (lowest income) | 43.0 | 53.0 | 36.6 | 54.9 | 33.1 | 58.3 | ||
| 2 | 22.0 | 32.2 | 19.5 | 34.2 | 21.0 | 31.7 | ||
| 3 | 18.8 | 11.5 | 25.1 | 9.5 | 23.4 | 8.7 | ||
| 4 (highest income) | 16.2 | 3.3 | 18.9 | 1.4 | 22.5 | 1.3 | ||
| Type of hospital | .49 | .0001 | ||||||
| Rural | 0.3 | 55.5 | 0.2 | 54.0 | 0.1 | 37.6 | ||
| Urban nonteaching | 24.8 | 13.4 | 32.8 | 12.6 | 23.7 | 11.9 | ||
| Urban teaching | 74.9 | 31.1 | 67.0 | 33.4 | 76.2 | 50.5 | ||
| Hospital region | .45 | .22 | ||||||
| Northeast | 32.8 | 17.9 | 27.8 | 7.6 | 28.5 | 12.2 | ||
| Midwest | 15.7 | 20.8 | 17.2 | 19.9 | 16.9 | 22.1 | ||
| South | 30.3 | 41.6 | 31.6 | 51.3 | 30.3 | 49.4 | ||
| West | 21.2 | 19.8 | 23.4 | 21.3 | 24.3 | 16.3 | ||
| Transferred in from another hospital/facilityc | - | - | 10.8 | 22.3 | 15.5 | 38.2 | - | - |
aFor comparison of 2003–2007 vs 2012–2016 data.
bMay not sum to 100 due to rounding.
cBetween 2008, when data on this variable were first obtained, and 2016.
The type of hospitals where opioid use–associated infective endocarditis hospitalizations occurred also changed over the study period. The distribution of hospital type changed for rural (P = .0001) but not urban residents (P = .49), with the percentage of rural residents hospitalized at urban teaching hospitals increasing by 62% over the study period (from 31.1% to 50.5%). Nearly all urban residents were hospitalized at urban hospitals over the study period. An increasing percentage of rural hospitalizations for opioid use–associated infective endocarditis occurred in the South (41.6% in 2003–2007 vs 49.4% in 2012–2016); however, these changes in regional distribution did not reach statistical significance (P = .22). The regional distribution of hospitalizations of urban residents did not change over the study period (P = .45). Additionally, rural resident hospitalizations were more likely than urban ones to be the result of a transfer from another facility (eg, 2012–2016 transfers: rural 38.2%, urban 15.5%; P < .0001).
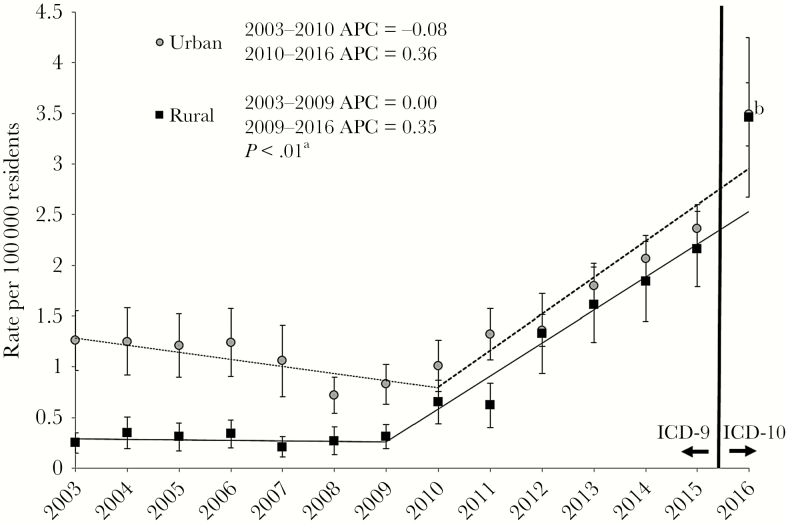
Between 2003 and 2016, rates of hospitalization for opioid use–associated infective endocarditis increased from 0.28 to 3.86 per 100 000 among rural residents compared with 1.26 to 3.49 per 100 000 for urban residents. Joinpoint regression identified the years 2009 and 2010 as the start of a significant change in slope for rural and urban residents, respectively. For the 2003–2009/2010 time segment, the annual percent change for rural residents was 0.0%, whereas the annual percent change for urban residents slightly decreased (–0.08%); the annual percent change estimates for rural and urban residents were similar for the 2009/2010–2016 time segment slope (0.35% vs 0.36%) (Figure 1). Overall, the increase in annual percent change was higher for rural vs urban residents (P < .01).
Figure 1.
Rates of hospitalization for injection drug use–associated infective endocarditis for patients by rural–urban status of location of residence, 2003–2016. Error bars represent 95% confidence intervals for observed annual rates. Trend lines (rural, solid line; urban, dashed line) represent regression estimates for trend periods, as determined using joinpoint regression. aUrban vs rural (overall), P < .01. bRate estimates for 2016 overlap. Abbreviation: APC, annual percent change; ICD, International Classification of Diseases.
Total charges, length of stay, and number of procedures for opioid use–associated infective endocarditis hospitalizations are shown in Table 2. Total charges increased for hospitalizations of both urban and rural residents between 2003 and 2016, and they were 23% higher for urban vs rural hospitalizations in 2012–2016 (P < .0001). The mean number of procedures increased slightly over the study period, from 3.1 to 3.6 for urban residents (P = .0005) and 3.0 to 3.3 for rural residents (P = .26). The mean length of hospitalization increased by 4% (15.1 to 15.7 days; P = .27) for urban patients and 18% for rural patients (13.3 to 15.7 days; P = .09), although neither change reached statistical significance. In 2012–2016, most discharges were to home or another facility. In 2012–2016, the distribution of discharge dispositions varied between rural and urban hospitalizations (P = .0001), with urban hospitalizations more likely to result in a patient-directed discharge (ie, against medical advice; 17.1% vs 11.7%) and less likely to be discharged home (37.4% vs 43.7%) than rural hospitalizations. Overall, the percentages of in-hospital deaths were high (range, 3.8–8.0%) but appeared to decrease over time for both urban (P < .0001) and rural residents (P = .21).
Table 2.
Outcomes of 8732 Hospitalizations for Opioid Use–Associated Infective Endocarditis, 2003–2016 National Inpatient Sample
| 2003–2007 | 2008–2011 | 2012–2016 | P a | |||||
|---|---|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | Urban | Rural | Urban | Rural | |
| Unweighted discharges | 1999 | 187 | 1333 | 245 | 3859 | 1109 | - | - |
| Weighted discharges | 9494 | 902 | 6397 | 1168 | 19 295 | 5545 | - | - |
| Inflation-adjusted total charges, mean | $93 038 | $72 121 | $132 179 | $86 819 | $154 179 | $125 444 | <.0001 | <.0001 |
| Length of stay, mean, d | 15.1 | 13.3 | 16.5 | 12.0 | 15.7 | 15.7 | .27 | .09 |
| No. of procedures, mean | 3.1 | 3.0 | 3.6 | 3.4 | 3.6 | 3.3 | .0005 | .26 |
| Discharge disposition, weighted %b | <.0001 | .21 | ||||||
| In-hospital death | 7.4 | 6.6 | 8.0 | 3.8 | 5.6 | 5.9 | ||
| Transferred to another facility | 33.3 | 38.3 | 33.8 | 42.0 | 39.8 | 38.7 | ||
| Home | 42.5 | 48.8 | 43.2 | 46.4 | 37.4 | 43.7 | ||
| Patient-directed dischargec | 16.8 | 6.3 | 14.9 | 7.9 | 17.1 | 11.7 |
aFor comparison of 2003–2007 vs 2012–2016 data.
bMay not sum to 100 due to rounding.
cDefined as patient leaving early (ie, “against medical advice”).
The rate of hospitalization for opioid use–associated endocarditis for suburban residents, analyzed in an exploratory post hoc analysis, was 0.52 per 100 000 residents in 2003 and 3.88 per 100 000 residents in 2016 (Supplementary Figure 1). The annual percent change was 0.0% between 2003 and 2011, at which point a change in slope was detected; the annual percent change was 0.51% from 2011 to 2016. Among suburban residents, the mean age of those hospitalized for opioid use–associated endocarditis decreased slightly during the study period, from 40.0 years in 2003–2007 to 37.2 years in 2012–2016 (P = .002) (Supplemental Table 2). The percentage of hospitalizations among suburban residents who were female (P = .03), white (P < .0001), had their hospitalization paid for by Medicaid (P < .0001), and received care in the South (P < .0001) increased between 2003 and 2016. The distribution of discharge dispositions varied between 2003 and 2016 (P = .01); the percentage of in-hospital deaths appeared to decrease slightly, from 7.2% in 2003–2007 to 6.3% in 2012–2016 (Supplementary Table 3).
DISCUSSION
Using nationally representative data on US hospital discharges between 2003 and 2016, we observed an annual percent increase in opioid use–associated infective endocarditis hospitalizations for both rural and urban residents, with a slightly greater overall annual percent increase observed for rural vs urban residents. Between 2003 and 2009/2010, urban residents had a lower annual percent increase relative to rural residents; the annual percent increases between 2009/2010 and 2016 were nearly identical. Previous investigations of opioid use–associated infective endocarditis and rurality were limited by the use of small convenience samples and aggregated data. Among infective endocarditis patients receiving care at a tertiary care hospital in North Carolina between 2009 and 2014, patients with documented injection drug use were 2.4 times as likely to be from a rural county as patients without injection drug use [13]; however, no association between residing in a rural county and injection drug use–associated infective endocarditis was observed at hospitals in Virginia (2000–2016) [14] or Portland, Maine (2013–2016) [15]. Our study is the first to characterize rural–urban differences in opioid use–associated infective endocarditis hospitalization rate trends nationally. The annual percent increase for suburban residents also increased.
Our study also highlights several recent trends in the demographic characteristics of patients hospitalized with opioid use–associated infective endocarditis. Between 2003 and 2016, rural residents hospitalized with opioid use–associated infective endocarditis were increasingly more likely to be white, to be low income, and to receive care at urban hospitals, consistent with national trends for overall heroin overdose–associated hospitalizations [16]. We observed an increase, albeit non–statistically significant, in the proportion of rural hospitalizations for opioid use–associated infective endocarditis occurring in the South, similar to what has been reported for incident HIV [17], for which unsafe injection practice is also a major risk factor. A high proportion of opioid use–associated infective endocarditis occurring in this region may be related to the relative lack of harm reduction services, increased stigma, and poor regional uptake of Medicaid expansion through the Affordable Care Act [18], which likely further limits health care accessibility and affordability of substance use treatment programs.
Hospitalizations for opioid use–associated infective endocarditis doubled in cost between 2003 and 2016, a relatively modest increase compared with the near quadrupling of inpatient charges for overall opioid abuse/dependence hospitalizations with and without serious infections observed between 2002 and 2012 [19]. The observed increase in inpatient charges likely results from increasing costs for identical care and greater use of resources [19], rather than a change in typical length of stay or number of procedures, which increased only slightly over the study period. The higher cost of hospitalizations of urban vs rural residents could also be attributed to differences in charges between rural and urban hospitals.
Our study has several limitations. The National (Nationwide) Inpatient Sample does not allow for identification of unique patients; however, the high percentage of rural patients transferred from another hospital or emergency department (eg, 2012–2016, 38.2%) suggests that rural residents might be more likely to have multiple hospitalizations for opioid use–associated infective endocarditis represented in the data. However, we did not observe a difference in the percentage of rural vs urban patients transferred to another facility, which would not support this hypothesis. We used opioid use ICD codes to identify hospitalizations with opioid use–associated infective endocarditis, and it is possible that some hospitalizations were misclassified with respect to opioid use, but the direction of this misclassification and differences by rural–urban status are unknown. Further, by only examining opioid use–associated infective endocarditis, we missed infective endocarditis hospitalizations associated with other nonopioid drug use (eg, cocaine, methamphetamines). Finally, although we cross-walked ICD-9-CM and ICD-10-CM codes using the Centers for Medicare and Medicaid Services’ General Equivalence Mappings, the ICD-9-CM /ICD-10-CM transition may have created a spurious jump in opioid use–associated infective endocarditis hospitalizations, similar to what has been observed for all overall opioid-related hospitalizations [8]; however, we do not expect that this would vary between rural and urban populations.
Increasing rates of hospitalizations for opioid use–associated endocarditis, a costly disease with high mortality, call for dedicated public heath efforts to support harm reduction and recovery, including expansion of syringe site programs and access to treatment providers, which have been shown to reduce infections related to injection drug use [20, 21]. Programs suited for rural communities, which may be particularly vulnerable, include mobile sites, distribution networks, community outreach, and telemedicine [22]. Future studies should investigate whether factors affecting opioid use–associated infective endocarditis hospitalizations in rural populations, such as access to harm reduction services and treatment providers, are independent of those driving overall opioid use–associated hospitalizations.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by a grant from the National Institutes of Health (U54 GM115516 to the Northern New England Clinical and Translational Research Network) and the Maine Economic Improvement Fund (to K.A.A.).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. K.T., J.C., K.A., and E.K.N. developed the study scope, objectives, and methods, with contributions from B.A. J.C. performed data analysis with substantial contributions from K.A. J.C. and E.K.N. drafted the manuscript with critical review and feedback from all authors.
Prior presentation. A previous version of this work was presented at the American College of Preventive Medicine in May 2019.
References
- 1. Schranz AJ, Fleischauer A, Chu VH, et al. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med 2019;170(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Des Jarlais DC, Nugent A, Solberg A, et al. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:1337–41. [DOI] [PubMed] [Google Scholar]
- 4. Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5-year update. J Rural Health 2019; 35:108–12. [DOI] [PubMed] [Google Scholar]
- 5. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health 2011; 2011:948789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Healthcare Cost and Utilization Project (HCUP). HCUP National Inpatient Sample (NIS). Rockville, MD: Agency for Healthcare Research and Quality; 2012 Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 15 September 2019. [Google Scholar]
- 7. Barrett M, Coffey R, Levit K. Population Denominator Data Sources and Data for Use With the HCUP Databases. HCUP Methods Series Report #2018-03. Rockville, MD: Agency for Healthcare Research and Quality; 2018. Available at: www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Accessed 21 November 2019. [Google Scholar]
- 8. Heslin KC, Owens PL, Karaca Z, et al. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care 2017; 55:918–23. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Medicare & Medicaid Services. ICD-10 CM and GEMs 2018 Updated 11 August 2017. Available at: https://www.cms.gov/Medicare/Coding/ICD10/2018-ICD-10-CM-and-GEMs.html. Accessed 21 November 2019.
- 10. US Bureau of Economic Analysis. Personal consumption expenditures excluding food and energy (chain-type price index) [DPCCRG3A086NBEA], retrieved from FRED. Federal Reserve Bank of St. Louis: Available at: https://fred.stlouisfed.org/series/DPCCRG3A086NBEA. Accessed 21 January 2020. [Google Scholar]
- 11. Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res 2018; 53:175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healthcare Cost and Utlization Project (HCUP). Using the HCUP National Inpatient Sample to Estimate Trends. Rockville, MD: Agency for Healthcare Research and Quality; 2015 Available at: https://www.hcup-us.ahrq.gov/reports/methods/2006_05_NISTrendsReport_1988-2004.pdf. Accessed 21 November 2019. [Google Scholar]
- 13. Hartman L, Barnes E, Bachmann L, et al. Opiate Injection-associated infective endocarditis in the Southeastern United States. Am J Med Sci 2016; 352:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray ME, Rogawski McQuade ET, Scheld WM, Dillingham RA. Rising rates of injection drug use associated infective endocarditis in Virginia with missed opportunities for addiction treatment referral: a retrospective cohort study. BMC Infect Dis 2018; 18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thakarar K, Rokas KE, Lucas FL, et al. Mortality, morbidity, and cardiac surgery in injection drug use (IDU)-associated versus non-IDU infective endocarditis: the need to expand substance use disorder treatment and harm reduction services. PLoS One 2019; 14:e0225460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu DJ, McCarthy EP, Stevens JP, Mukamal KJ. Hospitalizations, costs and outcomes associated with heroin and prescription opioid overdoses in the United States 2001-12. Addiction 2017; 112:1558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatits, STD, and TB Prevention and Division of HIV/AIDS Prevention. HIV in the Southern United States.2019 Available at: https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf. Accessed 21 November 2019.
- 18. Kaiser Family Foundation. Status of state Medicaid expansion decisions: Interactive Map. Available at: https://www.kff.org/medicaid/issue-brief/status-of-state-medicaid-expansion-decisions-interactive-map/. Accessed 15 January 2020.
- 19. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff 2016; 35:832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe programmes in people who inject drugs - an overview of systematic reviews. BMC Public Health 2017; 17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane review and meta-analysis. Addiction 2018; 113:545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schranz AJ, Barrett J, Hurt CB, et al. Challenges facing a rural opioid epidemic: treatment and prevention of HIV and hepatitis C. Curr HIV/AIDS Rep 2018; 15:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.