Abstract
Introduction
Older age is a melanoma risk factor. Elderly individuals are likelier to have immunosenescence, which could help melanoma cells escape immune surveillance. Hence, it is believed that elderly people cannot mount a potent immune response to checkpoint inhibitors to eliminate melanoma.
Objectives
To investigate age-related differences in the time to progression, overall survival, and immunotherapy-related adverse events among patients with metastatic melanoma who received checkpoint inhibitors.
Methods
We retrospectively identified patients at our institution between January 2012 and December 2016 with stage IV melanoma who received at least 1 dose of ipilimumab, pembrolizumab, nivolumab, or combined ipilimumab and nivolumab. Demographic, pathologic, and clinical characteristics were obtained. Immune-related response criteria were used to define responses.
Results
Twenty-nine patients were younger than age 65 years and 31 were age 65 years or older. Time to progression was comparable between the age groups (hazard ratio = 0.79, 95% confidence interval = 0.37–1.70, p = 0.46). Overall survival was not significantly different after immunotherapy between groups (hazard ratio = 0.75, 95% confidence interval = 0.31–1.82, p = 0.491). Overall, immunotherapy-related adverse events were comparable between groups, with 62% in younger patients (18/29) and 45% in older patients (14/31 p = 0.19). Of 60 patients, 30 responded to immunotherapy. Nonresponders were more likely than responders to have BRAF-mutated melanomas (16 [53.3%] vs 8 [27.6%]; p = 0.04) and less likely to have immunotherapy-related adverse events (12 [40%] vs 20 [66.7%]; p = 0.04).
Conclusion
Aging does not seem to affect response to checkpoint inhibitors. Elderly patients with metastatic melanoma should be treated similarly to younger patients.
Keywords: checkpoint inhibitors, elderly, immune surveillance, immunosenescence, ipilimumab, melanoma, nivolumab, pembrolizumab
INTRODUCTION
The incidence of cutaneous melanoma is increasing faster than any other potentially preventable cancer in the US.1 An estimated 96,480 new cases of cutaneous melanoma were diagnosed in the US in 2019.2 High-dose interleukin-2 has been the agent of choice to treat metastatic melanoma since 1985.3 With high-dose interleukin-2, long-term survivals are observed in 5% to 10% of patients. However, because of the severe toxicity profile, its use is restricted to a minority of patients who are physically fit enough to withstand such therapy.3
The advent of immunotherapy with checkpoint inhibitors has revolutionized the management of metastatic melanoma. It is known now that the cytotoxic T-lymphocyte antigen-4 (CTLA-4) imposes a negative feedback on T cells, leading to inactivation of their cytotoxic function. Targeting CTLA-4 with ipilimumab helps restore T-cell activity against melanoma.4 The programmed death-1 protein (PD-1) is an immune checkpoint receptor expressed by activated T cells. The PD-1 binds to its ligands PDL1 and PDL2, on melanoma cells, which deactivate the T cell, allowing melanoma cells to escape immune surveillance. The CTLA-4 inhibitor, ipilimumab, and the PD-1 inhibitors, pembrolizumab and nivolumab, are approved by the US Food and Drug Administration to treat metastatic melanoma.5
Checkpoint inhibitors help activate T cells but also can give rise to immunotherapy-related adverse events (irAEs) such as immune-mediated colitis, rash, autoimmune pneumonitis, pruritus, nausea, anemia, arthralgia, vomiting, constipation, immune-mediated hepatitis, immune-mediated nephritis and renal dysfunction, autoimmune endocrine deficiencies (hypothyroidism, hypophysitis, and adrenal insufficiency), autoimmune encephalitis, and fatigue.6
Age is an important prognostic factor in cutaneous melanoma. Melanoma has an aggressive biology, and, with advancing age, carries a worse prognosis.7–9 Differences in the natural history of melanoma between younger and older patients are believed to be partially the result of immunosenescence that helps melanoma cells escape an effective immune surveillance.8 All immune cells originate from the hematopoietic stem cells in the bone marrow, and as we age, there is a 2-fold to 4-fold decline in the proliferative capacity of these stem cells compared with younger people.10 Although, production of pro-B cells decreases markedly with aging, T-cell precursors seem to be less affected.11 Aging results in decreased Toll-like receptor function. Toll-like receptors have been found to induce the protective adaptive immune responses in antitumor immunity,12 reduced cytokine production,13 and decreased production of nitric oxide and reactive oxygen species by macrophages.14 Likewise, the ability of NK (natural killer) cells to produce interferron-γ becomes modestly impaired in older individuals, thus impairing the ability to destroy melanoma cells.15 Moreover, aging results in a decline in the number and function of T cells and dendritic cells (the most potent antigen-presenting cells).10 It also reduces the costimulatory molecule CD28, which impairs the ability of T cells to proliferate and secrete interleukin-2.16
Our basic understanding of immunosenescence has broadened, giving rise to the myth among some physicians that checkpoint inhibitors may not be as effective in treating the elderly patients with metastatic melanoma as it is in treating the younger ones. This study was conducted to investigate age-related differences in outcomes among patients with metastatic melanoma who received immunotherapy with checkpoint inhibitors.
METHODS
Patients
All patients with metastatic melanoma (M1a, b, or c), regardless of pathologic type (cutaneous, mucosal, and ocular), who received immunotherapy with checkpoint inhibitors at our institute between January 2012 and December 2016, were included in this retrospective study. Evaluable patients received at least 1 dose of ipilimumab, pembrolizumab, nivolumab, or combined ipilimumab and nivolumab.
Baseline characteristics included age, sex, melanoma pathologic type, BRAF mutation status, prior melanoma-directed therapies, Eastern Cooperative Oncology Group (ECOG) performance status, baseline serum lactate dehydrogenase levels, and presence of brain metastases. The Charlson Comorbidity Index, which predicts the 1-year mortality for a patient who may have a range of comorbid conditions, such as heart disease, AIDS, or cancer (a total of 22 conditions), was calculated. Each condition was assigned a score of 1, 2, 3 or 6, depending on the risk of dying associated with each one the score was calculated and reported for every patient. IrAEs including fever, fatigue, diarrhea and biopsy-confirmed colitis, hypothyroidism, adrenal insufficiency, rash, itching, vitiligo, central nervous system adverse events, and other adverse events believed to be caused by immune therapy, were noted. The date of death and/or the date of melanoma recurrences were recorded.
End Points and Assessment
The primary objective of this study was to evaluate the baseline demographic, clinical, and pathologic characteristics between responders and nonresponders to immune checkpoint inhibitors among patients with metastatic melanoma, then to investigate the age-related differences (< 65 years vs ≥ 65 years) in the time to progression, overall survival, and irAEs. Responses to checkpoint inhibitors were defined as complete response, partial response, or stable disease observed on positron emission tomography or total-body computed tomography scans obtained 6 months after the initiation of immunotherapy. Any evidence of radiologic progression (an increase in tumor burden of at least 25% compared with baseline) at 6 months was considered progressive disease. Because of lack of documentation, irAEs were not graded but were recorded as all irAEs of any severity.
The study protocol was approved by the institutional review board at the University of Arkansas for Medical Sciences, Little Rock, AR.
Statistical Analysis
We used bivariate analyses to describe the distribution of response to immunotherapy by demographics and pathologic characteristics. Study participants enrolled in the study at the time of immunotherapy initiation. Participation in the study ended because of disease progress, termination of immunotherapy, death, or the end of our study (February 1, 2017). The Cox proportional hazards regression model was used to assess the response to immunotherapy and the overall survival by age group. Because our eligible participants were all non-Hispanic whites and had stage IV melanoma, we did not need to adjust for race and disease stage to control confounding in our Cox proportional hazards models. All analyses were conducted using Stata 14.0 software (StataCorp, College Station, TX).
RESULTS
Responders versus Nonresponders
Of 96 patients with metastatic melanoma diagnosed at the University of Arkansas for Medical Sciences, 36 patients were excluded from the study because they received treatment elsewhere. Thus, 60 patients were available for this retrospective review. As shown in Table 1, 36 (60%) of 60 patients were men. Cutaneous melanoma was the predominant type. Forty percent of patients (n = 24) harbored the BRAF mutation. Prior treatment before starting immunotherapy was documented in 22 (36.6%) of 60 patients. Adverse events to immunotherapy were found in 32 (53.3%) of 60 patients.
Table 1.
Demographic and pathologic characteristics among patients with stage IV melanoma, by response to immunotherapya
| Characteristic | Responders (n = 30) | Nonresponders (n = 30) | p value |
|---|---|---|---|
| Age at initiation of immunotherapy, y median, (IQR) | 66.9 (54.3–73.3) | 62.7 (54.3–69.1) | 0.48 |
| Sex | 0.6 | ||
| Men | 19 (63.3) | 17 (56.7) | |
| Women | 11 (36.7) | 13 (43.3) | |
| Melanoma type | 0.06 | ||
| Cutaneous | 28 (93.3) | 26 (86.7) | |
| Mucosal | 0 (0.0) | 4 (13.3) | |
| Ocular | 2 (6.7) | 0 (0.0) | |
| BRAF mutation | 0.04 | ||
| No | 21 (70.0) | 14 (46.7) | |
| Yes | 8 (26.7) | 16 (53.3) | |
| Missing | 1 (3.3) | 0 (0.0) | |
| Previous treatment | 0.59 | ||
| None | 18 (60.0) | 20 (66.7) | |
| Yes | 12 (40.0) | 10 (33.3) | |
| Charlson Comorbidity Index, median, (IQR) | 6 (6,8) | 9 (6,10) | 0.003 |
| ECOG score, median, (IQR) | 0 (0,1) | 0 (0,1) | 0.51 |
| Brain metastasis | 0.78 | ||
| No | 21 (70.0) | 20 (66.7) | |
| Yes | 9 (30.0) | 10 (33.3) | |
| Elevated baseline serum LDH level | 0.28 | ||
| No | 27 (90.0) | 24 (80.0) | |
| Yes | 3 (10.0) | 6 (20.0) | |
Data are presented as number (percentage) unless indicated otherwise.
ECOG = Eastern Cooperative Oncology Group; IQR = interquartile range; LDH = lactate dehydrogenase.
Patients who did not respond to immunotherapy were more likely to have a BRAF mutation, a higher Charlson index, and a lower irAE profile. No difference between responders and nonresponders was noted regarding the type of immunotherapy used (Table 1). Overall irAEs were present in 20 (66.7%) of responders compared with 12 (40%) of nonresponders (p = 0.03). Endocrinopathies, hepatitis, pneumonitis, dermatitis, and central nervous system adverse effects were similar in both responders and nonresponders. Thirty percent of patients (n = 10) who responded to immunotherapy had colitis compared with 7% (n = 2) in nonresponders (p = 0.03). Similarly, rheumatologic adverse effects were more common in responders (p = 0.007). There was no statistical difference between responders and nonresponders regarding the presence or absence of brain metastasis (p = 0.78).
Time to Progression and Overall Survival by Age Group
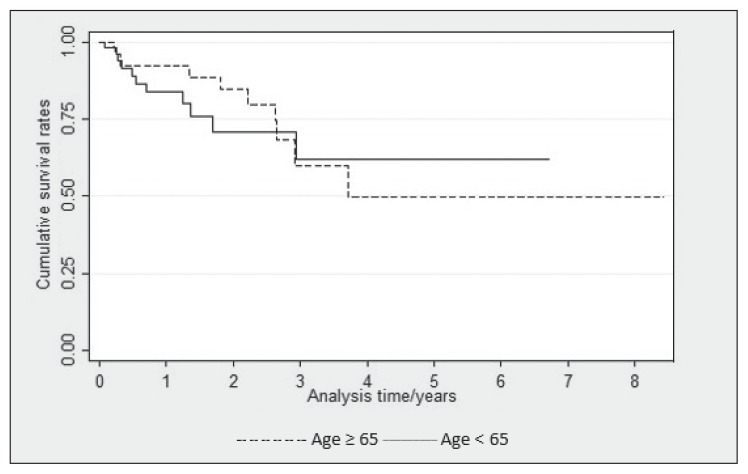
Twenty-nine patients were younger than age 65 years and 31 were age 65 years and older. After adjustment for sex, melanoma type, and presence of brain metastasis, there was no significant difference in survival after immunotherapy between the 2 age groups (hazard ratio [HR] = 0.75, 95% confidence interval [CI] = 0.31–1.82, p = 0.491; Table 2 and Figure 1). Similarly, the time to progression was compared between groups, and after adjustment for sex and melanoma type, the time to progression was found to be comparable with no statistically significant difference (HR = 0.79, 95% CI = 0.37–1.70, p = 0.46; Table 3).
Table 2.
Cox regression for overall survival among patients with stage IV melanoma
| Age group, y | Survival status after immunotherapy initiation | Hazard ratio (95% confidence interval) | Median survival, y | ||
|---|---|---|---|---|---|
| Alive, no. (%) | Dead, no. (%) | Crude | Adjusteda | ||
| < 65 | 18(46.2) | 11 (52.4) | 1.0 | 1.0 | 4.14 |
| ≥ 65 | 21 (53.8) | 10 (47.6) | 0.81 (0.34–1.92) | 0.75 (0.31–1.82) | 5.00 |
Adjusted for sex, melanoma type, and brain metastatis.
Figure 1.
Kaplan-Meier survival plot for overall survival among patients with stage IV melanomaa
a Adjusted for sex, melanoma type, and brain metastasis (p = 0.491) in patients younger than age 65 years or age 65 years and older.
Table 3.
Cox regression for disease progression in patient with stage IV melanoma
| Age group, y | Disease progression | Hazard ratio (95% confidence interval) | Median time to progression, y | ||
|---|---|---|---|---|---|
| No, no. (%) | Yes, no. (%) | Crude | Adjusteda | ||
| < 65 | 13 (43.3) | 16 (53.3) | 1.0 | 1.0 | 0.33 |
| ≥ 65 | 17 (56.7) | 14 (46.7) | 0.78 (0.38–1.61) | 0.79 (0.37–1.70) | 0.99 |
Adjusted for sex and melanoma type.
Age-Related Differences in Immunotherapy-Related Adverse Events
Overall irAEs in the 2 age groups were comparable, with 62% in the younger patients (18/29) and 45% in the older patients (14/31; p = 0.19). The irAEs, including endocrinopathies, colitis, hepatitis, pneumonitis, dermatitis, and central nervous system adverse effects, were similar in both age groups. Interestingly, rheumatologic adverse effects were more common in younger patients (p = 0.035; Table 4).
Table 4.
Immune-related adverse events (AEs; number of patients)
| Adverse event | Age < 65 years (n = 29) | Age ≥ 65 years (n = 31) | p value | ||
|---|---|---|---|---|---|
| AEs | No AEs | AEs | No AEs | ||
| Overall adverse events | 18 | 11 | 14 | 17 | 0.190 |
| Endocrinopathies | 8 | 21 | 4 | 27 | 0.155 |
| Colitis | 8 | 21 | 4 | 27 | 0.833 |
| Hepatitis | 1 | 28 | 1 | 30 | 0.962 |
| Pneumonitis | 3 | 26 | 0 | 31 | 0.066 |
| Dermatitis | 4 | 25 | 6 | 25 | 0.563 |
| Rheumatologic disease | 6 | 23 | 1 | 30 | 0.035a |
| CNS adverse effects | 1 | 28 | 1 | 30 | 0.962 |
Boldface indicates significant.
CNS = central nervous system.
DISCUSSION
Aging is accompanied by functional decline in both innate and adaptive immunity.17 We found no significant differences, when adjusted for sex, type of melanoma, and presence of brain metastasis, in the time to progression and the overall survival between the younger than age 65 and age 65 years and older groups who received checkpoint inhibitors for treatment of metastatic melanoma. Our results were similar to those of other studies.18 In one study, 855 patients with unresectable stage III or stage IV melanoma received ipilimumab after failure to respond or intolerance to at least 1 prior systemic treatment. There were no statistically significant differences in the median progression-free survival and overall survival between the older (> 70 years) and the younger (≤ age 70 years) group.19 In the US Expanded Access Program, the 1-year survival rate in patients with metastatic melanoma treated with ipilimumab was not different among age 65 years and younger compared with age 65 years and older, which was 38% and 37%, respectively.20 Another study of 95 patients, treated with immunotherapy for metastatic melanoma, showed that the survival and response rates, to checkpoint inhibitors, in patients older than age 80 years were very similar to those for younger patients.21
Immunotoxicity is an indirect marker of the efficacy of immunotherapy. Our results revealed that responders, regardless of age, had a higher rate of irAEs (66.7%) than did nonresponders (40%; p = 0.04). Immune-mediated colitis, in particular, was higher in responders compared with nonresponders (approximately 30% [n = 10] vs 7% [n = 2], p = 0.03). One prior study showed significantly improved response rates in patients in whom immune-mediated enterocolitis developed because of ipilimumab.22 Other studies showed a strong correlation between the treatment response rate and irAEs,23–25 but these studies pertain to patients who received anti-CTLA-4; the data are conflicting regarding anti-PD-1 immunotherapy. The results of recently concluded multicenter randomized controlled trials, including CheckMate 03726 and KEYNOTE-006,27 showed significantly improved progression-free survival and decreased rates of adverse effects in patients receiving anti-PD-1 immunotherapy compared with those receiving anti-CTLA-4. Our study findings lend support to the correlation between response rate and irAEs irrespective of the type of immunotherapy administered.
There is also a strong correlation between the development of vitiligo and the tumor response in patients receiving immunotherapy.28–30 In our study, vitiligo developed in only 2 patients (3.3%) and both had complete response to immunotherapy. A systematic review was conducted of 137 studies comprising 139 treatment arms (11 general immune stimulation, 84 vaccine trials, 28 antibody-based trials, and 16 adoptive T-cell transfer studies) and including a total of 5737 patients.30 The overall cumulative incidence of vitiligo was 3.4% (95% CI = 2.5%–4.5%). Vitiligo development was significantly associated with better progression-free survival (HR = 0.51; 95% CI = 0.32–0.82; p < 0.005) and overall survival (HR = 0.25; 95% CI = 0.10–0.61; p < 0.003), indicating that these patients have 2 to 4 times less risk of disease progression and death, respectively, compared with patients without vitiligo development.30
In our study, 7 (11.7%) of the patients treated with checkpoint inhibitors had immune-related rheumatologic adverse effects. Interestingly, this was particularly common in younger patients (p = 0.03). Another study showed that 1.3% of the total patients treated with nivolumab and ipilimumab experienced rheumatologic adverse events.31
A BRAF mutation has been associated with earlier age of onset, more aggressive clinical course, and decreased survival in patients who did not receive BRAF inhibitor therapy.32 Our study showed increased rates of the BRAF mutation in patients who failed to respond compared with the ones who responded to the immunotherapy (53% vs 27%, p = 0.04). However, BRAF inhibitors have improved survival in these patients with gene mutations.33 Sequential treatment with BRAF inhibitors and checkpoint inhibitors has emerged as a new strategy in the treatment of BRAF-mutated melanoma, but data remain conflicting regarding the preferred sequence.34–36 Nonetheless our study touches on the interaction between the BRAF mutation and the response to immunotherapy, and the data regarding this interaction per se are still lacking. A BRAF mutation contributes to immune escape. Boni et al37 showed that BRAF inhibition increases the expression of melanocyte differentiation antigens, which is associated with increased antigen-specific T-cell recognition; MEK inhibition, on the other hand, impairs T-lymphocyte function. It is not fully understood whether patients with a BRAF mutation should be treated with BRAF inhibitors first or immunotherapy first. Clinical trials are being conducted to clarify the appropriate sequence. In a retrospective study, progression-free survival and response rates were found to be similar irrespective of the timing of BRAF inhibitor therapy (before or after immunotherapy).35 In another study, a longer overall survival was found if ipilimumab was given before a BRAF inhibitor compared with a BRAF inhibitor followed by ipilimumab, or with either agent alone.38 The results of that study support the use of immunotherapy as first line in patients with BRAF mutations.38
Other studies have shown that immunotherapy in elderly patients may respond better in melanoma because of fewer regulatory T cells relative to CD8+ T cells in tumor deposits.39 Another study by Li et al40 showed that immune checkpoint inhibitors significantly prolonged the survival in both younger and older groups with melanoma. Anti-PD-1 agents were more efficient in older compared with younger patients with melanoma.
Although our study discussed all the checkpoint inhibitors as 1 group, further research will be necessary to identify differences, if any, between these agents. In addition, because our study is a retrospective study with a small sample size, it is likely to have practitioner bias because of the lack of randomization. It is a single-institution study in the Southern US. Further randomized multicenter studies with larger sample sizes will be useful to better evaluate the differences between these 2 age groups in terms of response to treatment, survival, and adverse effect profile.
CONCLUSION
Aging does not seem to affect the response to checkpoint inhibitors. Time to progression, overall survival, and immune-mediated adverse events were similar in younger and older patients with metastatic melanoma receiving checkpoint inhibitors. Autoimmunity owing to checkpoint inhibitors, especially immune-mediated colitis and vitiligo, are markers of better response. Elderly patients with metastatic melanoma should be treated similarly to younger patients, even with combination therapy such as ipilimumab and nivolumab. Future studies should investigate better biomarkers, such as PDL1, to predict response to checkpoint inhibitors.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Editor’s note
An abstract of this article was previously published in Joshi K, Atwal D, Ravilla R, et al. Outcomes of immunotherapy in advance melanoma in relation to age. J Clin Onc 2018 Feb 10;36(5 Suppl):187. DOI: https://doi.org/10.1200/JCO.2018.36.5_suppl.187
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Authors’ Contributions
Dinesh Atwal, MD; Fade Mahmoud, MD; Krishna Joshi, MD; and Rahul Ravilla, MD, did protocol writing, data collection, and data analysis. Issam Makhoul, MD; Laura Hutchins, MD; Naveen Yarlagadda, MD; Sunil Kakadia, MD; and Yadav Pandey, MD, helped with the literature review, discussion, and writing the manuscript.
All authors vouch for the accuracy and completeness of the data and analyses, and all have given final approval to the manuscript.
References
- 1.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015 Mar 30;107(6):djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Statistics Center. 2019 estimates [Internet] Atlanta, GA: American Cancer Society; 2019. [cited 2019 December 22]. Available from: https://cancerstatisticscenter.cancer.org. [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999 Jul;17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996 Mar 22;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 5.Atrash S, Makhoul I, Mizell JS, Hutchins L, Mahmoud F. Response of metastatic mucosal melanoma to immunotherapy: It can get worse before it gets better. J Oncol Pharm Pract. 2017 Apr;23(3):215–9. doi: 10.1177/1078155215627503. [DOI] [PubMed] [Google Scholar]
- 6.Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract. 2017 Dec;23(8):620–4. doi: 10.1177/1078155216667635. [DOI] [PubMed] [Google Scholar]
- 7.Austin PF, Cruse CW, Lyman G, Schroer K, Glass F, Reintgen DS. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol. 1994 Nov;1(6):487–94. doi: 10.1007/BF02303614. [DOI] [PubMed] [Google Scholar]
- 8.Weiss SA, Han J, Darvishian F, et al. Impact of aging on host immune response and survival in melanoma: An analysis of 3 patient cohorts. J Transl Med. 2016 Oct 19;14(1):299. doi: 10.1186/s12967-016-1026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balch CM, Soong SJ, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013 Nov;20(12):3961–8. doi: 10.1245/s10434-013-3100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zavala WD, Cavicchia JC. Deterioration of the Langerhans cell network of the human gingival epithelium with aging. Arch Oral Biol. 2006 Dec;51(12):1150–5. doi: 10.1016/j.archoralbio.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Effros RB, Walford RL. The immune response of aged mice to influenza: Diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983 Oct 15;81(2):298–305. doi: 10.1016/0008-8749(83)90237-X. [DOI] [PubMed] [Google Scholar]
- 12.Kawarada Y, Ganss R, Garbi N, Sacher T, Arnold B, Hämmerling GJ. NK− and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol. 2001 Nov 1;167(9):5247–53. doi: 10.4049/jimmunol.167.9.5247. [DOI] [PubMed] [Google Scholar]
- 13.van Duin D, Mohanty S, Thomas V, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007 Jan 15;178(2):970–5. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 14.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: Impact on macrophage function. Aging Cell. 2004 Aug;3(4):161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 15.Le Garff-Tavernier M, Béziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010 Aug;9(4):527–35. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaltoft K. Cytokine-driven immortalization of in vitro activated human T lymphocytes. CD28 expression correlates inversely with cell population doublings. Exp Clin Immunogenet. 1998;15(2):84–9. doi: 10.1159/000019058. [DOI] [PubMed] [Google Scholar]
- 17.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: Is it ever too old to become young again? Nat Rev Immunol. 2009 Jan;9(1):57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 18.Lebbé C, McDermott DF, Robert C, et al. Ipilimumab improves survival in previously treated advanced melanoma patients with poor prognostic factors: subgroup analysis from a phase III trial. Proceedings of the European Society of Medical Oncology 2010 Congress; 2010 Oct 8–12; Milan, Italy. Abstract 3505. [Google Scholar]
- 19.Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res. 2014 Apr 4;33(1):30. doi: 10.1186/1756-9966-33-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence D, McDermott D, Hamid O, et al. Treatment of patients (pts) with stage III or IV melanoma on an ipilimumab (Ipi) Expanded Access Program (EAP): Results for 3 mg/kg cohort. Paper presented at Society for Melanoma Research Congress; 2012 Nov 8–11; Hollywood, CA. [Google Scholar]
- 21.Friedman CF, Horvat TZ, Minehart J, et al. Efficacy and safety of checkpoint blockade for treatment of advanced melanoma (mel) in patients (pts) age 80 and older (80+) J Clin Oncol. 2016 May;34(15 Suppl):10009. DOI: https://10.1200/JCO.2016.34.15_suppl.10009. [Google Scholar]
- 22.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006 May 20;24(15):2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005 Sep 1;23(25):6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007 Nov 15;13(22 Pt 1):6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13(1):211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015 Jul 2;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert C, Schachter J, Long GV, et al. KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015 Jun 25;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol. 2017 Feb;44(2):117–22. doi: 10.1111/1346-8138.13520. [DOI] [PubMed] [Google Scholar]
- 29.Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016 Jan;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 30.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J Clin Oncol. 2015 Mar 1;33(7):773–81. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 31.Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2016;76:1–3. doi: 10.1136/annrheumdis-2016-209595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011 Apr 1;29(10):1239–46. doi: 10.1200/JCO.2010.32.4327. DOI: https://10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 33.Paulson KG, Thompson JA. A second chance for success with BRAF and MEK inhibitors in melanoma. Lancet Oncol. 2017 Apr;18(4):418–9. doi: 10.1016/S1470-2045(17)30151-1. [DOI] [PubMed] [Google Scholar]
- 34.Aya F, Fernandez-Martinez A, Gaba L, et al. Sequential treatment with immunotherapy and BRAF inhibitors in BRAF-mutant advanced melanoma. Clin Transl Oncol. 2017 Jan;19(1):119–24. doi: 10.1007/s12094-016-1514-0. [DOI] [PubMed] [Google Scholar]
- 35.Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014 Jun 1;120(11):1695–701. doi: 10.1002/cncr.28620. [DOI] [PubMed] [Google Scholar]
- 36.Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: Data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest. 2014 May;32(4):144–9. doi: 10.3109/07357907.2014.885984. [DOI] [PubMed] [Google Scholar]
- 37.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010 Jul 1;70(13):5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 38.Ascierto PA, Margolin K. Ipilimumab before BRAF inhibitor treatment may be more beneficial than vice versa for the majority of patients with advanced melanoma. Cancer. 2014 Jun 1;120(11):1617–9. doi: 10.1002/cncr.28622. [DOI] [PubMed] [Google Scholar]
- 39.Pawelec G. Unexpected benefits of aging for favorable responses to PD-1 blockade in melanoma? Clin Cancer Res. 2018 Nov 1;24(21):5193–4. doi: 10.1158/1078-0432.CCR-18-1475. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Yang X, Feng Y, et al. The impact of immunosenescence on the efficacy of immune checkpoint inhibitors in melanoma patients: A meta-analysis. OncoTargets Ther. 2018 Oct 26;11:7521–7. doi: 10.2147/OTT.S165368. [DOI] [PMC free article] [PubMed] [Google Scholar]