Abstract
Frailty is a geriatric syndrome characterized by diminished muscle strength, endurance, and weakened physical function. Physical frailty is often unaddressed clinically as it tends to manifest among chronic illnesses and sarcopenia, and consensus criteria for frailty diagnosis remains elusive. Whole-body vibration training (WBVT) has been used to improve deficits in lower body muscular strength and functional performance in healthy and high functioning older adults; therefore, the purpose of this study was to determine the effects of WBVT on physical frailty in skilled nursing home residents. This study compared the effects of 12 wks (2x/wk) of WBVT (n = 10) to standard care, which served as the control (CON: n = 10), on isometric knee extension strength (KE), body composition, and functional performance in 20 (16 female) pre-frail and frail skilled nursing home residents (82 ± 5 yrs). Frailty was assessed using the FRAIL scale and function was measured using the short physical performance battery (SPPB). WBVT consisted of 4 lower body exercises (partial squat, narrow squat, wide squat, calf raise) during vertical vibration (25 – 40 Hz). Data were analyzed using two-way ANOVA (group × time) and post-hoc paired and independent t-tests. Significance was set at p ≤ 0.05. There were significant group-by-time interactions for KE and SPPB. Post-hoc paired t-tests revealed that WBVT improved KE (22.3 ± 4.0 to 29.0 ± 4.5 kg) and improvement in SPPB performance approached significance (4.5 ± 2.3 to 5.2 ± 2.1 units, p = 0.089). WBVT was well tolerated and occurred without adverse health complications. WBVT can be used to counteract losses in leg strength without adverse health complications in skilled nursing home residents.
Keywords: Muscle strength, body composition, physical function, activities of daily living, frailty, older adults
INTRODUCTION
Frailty is a multifaceted geriatric syndrome characterized by diminished skeletal muscle strength, endurance, and weakened function (20, 30). Consequently, frailty increases dependency, falls risk, hospitalization, and death when exposed to acute stressors (11, 37). In the United States (U.S.), 1.5 million older adults are institutionalized annually, and approximately 33% are admitted to long-term care institutions exclusively due to physical frailty and their dependence in performing activities of daily living (ADL) (39). According to the American Medical Association, approximately 40% of older adults 80 years and older suffer from frailty syndrome (2), as well as the majority of nursing home residents (22).
While frailty is a widely recognized medical syndrome, the criteria used to best identify frailty syndrome is debated (30). Several frailty instruments have been developed; however, these tools often require substantial time and/or detailed medical history to complete (20, 32, 35). The FRAIL scale is a validated frailty instrument and an attractive alternative to other assessments because of its feasibility in the clinical and research settings (3). The FRAIL scale consists of 5 domains that include components from the Cardiovascular Health Study (fatigue, muscle resistance, ambulation, weight loss) and Frailty Index (number of illnesses), which are used to identify older adults as robust, pre-frail, or frail.
Skilled nursing facilities serve the purpose of providing advanced nursing care and/or rehabilitation services; however, these facilities tend to foster an inactive environment, as much of the residents’ interactions occur by bedside or in common areas near their bedroom (23). Bates-Jensen et al. reported that the average time spent in bed across a sample population of 15 nursing homes was 17 hours per day with some residents spending as many as 22 hours (4). While physical frailty can manifest in the presence of sarcopenia and disease, it is reasonable that chronic leg disuse is a secondary contributor to muscle weakness and poor physical function in nursing home residents. Resistance training is a feasible intervention for counteracting losses of muscular strength and performance in frail older adults (17). Despite the widely documented benefits of resistance training, many older adults do not lift weights; therefore, there is value in exploring exercise alternatives that are tolerable and beneficial for institutionalized older adults.
Whole-body vibration exercise training (WBVT) has been explored as an alternative to conventional resistance training. Roelants, Delecluse, & Verschueren reported significant increases in isometric (12.4%) and dynamic (12.1%) leg strength after 12 wks of WBVT in postmenopausal (65 yrs) women that were similar to a resistance training only group (36). In a study with overweight and obese postmenopausal women (56 yrs), leg press strength increased by 8.3% (18) and 19.4% (19) after a 6- and 12-wk lower extremity WBVT, respectively. Community-dwelling older adults (72 yrs) improved timed up-and-go (TUG) performance, sit to stand time, and balance with 6 wks of WBVT (21). In nursing home residents (78 yrs), 6 wks of lower extremity WBVT improved TUG and balance more than a control group performing the same exercises without vibration (5). The TUG, sit-to-stand, and gait speed tests are all objective measures of functional performance used to predict future health outcomes in older adults.
Improvements in functional performance with WBVT may be partly explained by enhanced muscular activity evoked by the vibration stimulus delivered during exercise. It is hypothesized that the deformation of muscle tissue caused by vibration activates the muscle spindle resulting in reflex activation of the α-motor neuron and subsequent muscular contraction (12). When young (26 yrs) and older (64 yrs) adults were exposed to vertical vibration while standing, surface electromyography detected significantly greater muscle activity of the lower extremities in both groups compared to standing without vibration with muscle activity during WBVT greatest in the older group (26). Similar to the effects of resistance training and plyometric training, vibration stimulus increases the gravitational load on the neuromuscular system (40) thereby providing a stimulus that modifies the functional capacity of skeletal muscle.
Absence of consensus criteria for the clinical diagnosis of frailty syndrome and the complex health issues of long-term nursing care residents might partly explain the limited exploration of WBVT as a treatment for physical frailty. The purpose of this investigation was to compare the effects of 12 wks of unloaded, lower extremity WBVT to standard care control (CON) on measures of body composition, strength, and physical function, in pre-frail and frail older adults living in a skilled nursing facility. It was hypothesized that WBVT would reduce total fat and body mass, increase leg strength, and improve short physical performance battery (SPPB) and TUG performance compared to CON.
METHODS
Participants
Thirty-seven older adults (men and women) ≥ 65 years of age were recruited from the long-term care units of 3 skilled nursing facilities in Tallahassee, Florida using organized informational meetings and referrals from each facility’s medical and physical therapy staff. Only participants with a SPPB total score greater than 1 and less than 9 were included. There were no restrictions to socioeconomic status or race. All participants received a physician’s consent to verify there were no underlying conditions that would contraindicate WBVT and/or functional performance testing. Informed consent was signed on behalf of the participant by a legal guardian, court appointed guardian, power of attorney, or surrogate if the participant was unable to sign on his or her own behalf. In addition, each participant signed an assent form, which established his or her willingness to participate in the study.
Several participants suffered from cognitive impairment, but only those who were capable of following instructions were included in the study. Participants were excluded if they had unstable cardiovascular disease, uncontrolled hypertension (≥ 160/100 mmHg), uncontrolled diabetes (blood glucose > 250 mg/dl), musculoskeletal deformity, symptomatic rheumatoid or osteoarthritis, neuromuscular disease, hernias, symptomatic known unrepaired aortic aneurysm, hip or knee replacement, recent hospitalization with in the past 6 months due to myocardial infarction, stroke or fractures, or terminally ill with cancer. Participants that were exercising outside of prescribed physical therapy were excluded. This study was approved by the University’s Institutional Review Board. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (31).
Protocol
This was a 12-wk non-randomized controlled trial. Participants were assigned to one of two groups after baseline testing: WBVT or CON. WBVT participants were recruited from only one skilled nursing facility. Recruitment and training were restricted to one facility due to the use of only one WBV plate. CON participants were recruited from all three skilled nursing facilities. Each of the skilled nursing facilities provided similar levels of healthcare and housed residents of similar health and functional status. In Tallahassee, admission to a skilled nursing facility is random; however, factors such as rehabilitation potential, willingness to be institutionalized, payment source or insurance type, and doctor’s referral can influence admittance to the facility. All of the skilled nursing facilities sampled in this study offered long-term care and rehabilitative services.
All visits and data collection occurred on site at the skilled nursing facilities. On the first visit, participants received the informed consent and assent, and a physician’s consent form. Once consent and assent were obtained, the participant’s demographic information was collected. Information about the presence of underlying illnesses and other medical information were reported by the participant and confirmed with the on-site medical records. Once the physician’s consent was received, the participants were scheduled for their second visit.
During visit two, frailty status, depression, functional performance, and grip strength were assessed in this order. The FRAIL scale uses 5 domains to assess frailty: Fatigue, Resistance (ability to climb a flight of stairs), Ambulation (ability to walk one block), Illness (number of illnesses counted), and Loss of weight (weight change within 6 months) (27, 29, 41). Fatigue, resistance, and ambulation categories were assessed using responses to questions selected from the SF-36 Health Survey. Illnesses and weight loss were documented from medical records. One to two deficits indicated pre-frailty and ≥ 3 deficits indicated frailty syndrome. Depression was measured using the Geriatric Depression Scale-15 (GDS-15); it is a self-report assessment used to identify depression in older adults (15, 16). The scale is a 15-item short form of the original 30-item Geriatric Depression Scale that consists of yes or no questions. Since most participants had vision impairments, questions were read aloud to each participant and responses were recorded.
Functional performance was measured using the SPPB (24) and the TUG test (34), respectively. Participants were familiarized with each protocol prior to testing. Participants performed each test once unless they failed to follow the protocol. Measurements obtained when a participant failed to follow protocol were considered invalid, and the test was repeated until performed correctly. Participants were instructed to wear the shoes they normally wore while ambulating in the facility or they wore treaded socks if they did not own shoes.
The SPPB is comprised of 3 separate tasks: repeated chair stands, balance in 3 standing positions (semi-tandem stand, side-by-side stand, tandem stand), and the 8-foot walk. A summary ordinal score of 0 to 12 (higher score indicates greater function) is reported based on the performance of the 3 tests. The repeated chair stands test required a participant to stand up straight from a chair as quickly as possible 5 times without rest in between stands and with his or her arms folded across the chest. Using a stopwatch, the investigator measured the time required to complete this task. Balance testing assessed a participant’s ability to stand in 3 different standing positions: semi-tandem, side-by-side, and tandem for 10 seconds. Gait speed was assessed using an 8-foot (2.44 meters) walk course. Participants could use a walking aid to walk at usual pace while timed by a stopwatch. The TUG was performed with participants beginning the test seated in a standard armchair with an easily identifiable line 3 meters (10 ft.) away on the floor. All participants were permitted to use an assistive walking aid to complete the test. On the word “go”, the participant stood up from the chair, walked as quickly as possible to the line marked on the floor, turned around, walked back to the chair, and then sat down. A stopwatch was used to time the participant. Researchers stopped timing when the participant sat back down.
Isometric handgrip strength was measured as maximal voluntary contraction with a calibrated Lafayette handgrip dynamometer (Lafayette, IN). Each participant held the hand dynamometer at his or her side while seated. The participant was asked to inhale and upon exhalation squeeze the hand dynamometer with maximum effort. Three trials were used for each hand and trials alternated between hands. Isometric handgrip strength was recorded as the sum of the highest measurement from each hand. The single highest score was recorded to identify strength deficits to diagnose sarcopenia.
On the third visit, participants met with researchers to determine their body mass, waist circumference, body composition, and leg strength. Body mass was measured using a dual ramp wheelchair scale (Rice Lake Weighing Systems, Rice Lake, Wisconsin) and height was measured using a wall-mounted stadiometer (Seca, Chino, California). Waist circumference was measured using a spring-loaded tape measure (Northcoast Medical, Gilroy, California) at eye level and at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest at the smallest area of the torso (42). Body composition was measured using a hand-foot bioelectrical impedance (BIA) machine (Biodynamics Model 310e, Seattle, Washington) at a single frequency of 50 KHz. Measurements included fat weight (kg), body fat %, and lean weight (kg).
Isometric leg strength was measured using a mechanical push-pull dynamometer (Baseline Instruments, White Plains, NY). The dynamometer was factory calibrated and properly “zeroed” before each measurement according to manufacturer’s instructions. At the time of measurement, participants sat in the upright position at the end of their bed with knees flexed at 90°. The dynamometer was placed on the distal tibia approximately 10 cm above the lateral malleolus. The researcher then stood opposite of the leg being tested and held the dynamometer perpendicular to the limb. Participants were instructed to exert maximal effort against the dynamometer, while avoiding explosive movements. Participants maintained a fixed position during the entire procedure by grasping the edge of their bed with both hands for stabilization.
Knee extension force was measured by a break-test, which used the measured peak force that the tester had to apply to overcome the participant’s force, indicated by a slight movement of the participant’s leg in the opposite direction of the voluntary contraction (9, 10). All participants were familiarized with the strength test and performed the measurement at least twice to demonstrate the protocol prior to testing. Measurements were repeated 3 times on each leg in an alternating fashion. The average of the 3 measurements from each leg were then added together and recorded as the average isometric leg strength. Average strength was reported instead of peak strength as a conservative approach to minimizing tester bias with this measurement tool. Measurements obtained when the participant failed to follow protocol were considered invalid, and the test was repeated until performed correctly.
Sarcopenia was determined by the presence of low muscle mass plus poor muscular strength and/or poor physical performance (14). The total lean mass measured by BIA was converted to total skeletal muscle (SM) mass using the following equation (13).
SM mass was then converted to a skeletal muscle mass index (SMI) by dividing the calculated SM by the participant’s height in meters squared (kg/m2). The estimated BIA-cutoff points for low muscle mass used were 8.87 kg/m2 and 6.42 kg/m2 for men and women, respectively (13). Poor muscular strength was determined by a grip strength < 30 kg for men and < 20 kg for women using the single highest measurement recorded at baseline (14). A baseline SPPB total score of ≤ 8 indicated poor functional performance (14). Sarcopenic obesity was identified in participants who were sarcopenic with a waist circumference measurement > 88 cm for women and > 102 cm for men.
Prior to the intervention, participants completed 2 wks of familiarization training. During familiarization, the participants were introduced to the equipment and shown how to perform each of the assigned exercises. Participants met with the investigator twice a week for approximately 1 hour to learn how to execute 4 lower body exercises on the WBVT platform without vibration. Participants completed 2 sets of 10 repetitions of partial squats performed at 30°, narrow squats and wide squats performed at 60°, and calf raises with body weight. Participants performed all 4 exercises standing with knees slightly bent (10–15°) at the starting position of each exercise to prevent transmission of vibration to the trunk and head. Dynamic exercises were performed at a rate of 2 seconds during ascension (concentric contraction) and 3 seconds during descension (eccentric contraction), using a metronome. One to three-minute rest intervals were taken between sets and exercises. Rest entailed the use of a chair to sit down on a wooden platform that was constructed to the height of the WBV plate. The inclusion of the platform was believed to improve safety during exercise routine, as it allowed participants to take several small steps to transfer from the ground to the vibration plate and rest between sets and exercises without stepping down from the plate when fatigued.
After familiarization participants in the WBVT group trained twice a week for 12 consecutive wks. WBVT was progressive and contraction speeds were consistent with slow resistance training. Participants completed 3 sets of 10 repetitions of partial squats, narrow squats, wide squats, and calf raises. If the participant could not complete all the repetitions of the third set, the participant was allowed to rest and then complete the remaining repetitions. For participants who demonstrated a poor tolerance for the dynamic exercises during familiarization (n = 2), an amended exercise program was implemented. These participants completed one dynamic repetition during the first set of each exercise and then performed static partial squat at 30° for the remainder of the set. Volume was increased by adding one dynamic repetition to the first set of each exercise except during wks 4, 7 and 10, when vibration intensity was increased. All participants were exposed to 50 seconds of vibration stimulus per exercise set.
The Pro5 Airdaptive Model by PowerPlate (Performance Health Systems, Northbrook, IL) was used to deliver vertical vibration at an initial frequency of 25 Hz. Frequency was increased by 5 Hz every third week so that all participants were training at 40 Hz by week 10. Amplitude remained at 1 mm for the program’s entirety. Each WBV session lasted approximately 45 minutes. During WBVT sessions, rate of perceived exertion (RPE) was measured using the Borg scale. Rest time between sets and exercises were recorded using a stopwatch.
In addition to WBVT, participants were instructed to continue with their day-to-day activities and follow their doctors’ orders. Doctor’s orders were different for all participants and primarily consisted of prescribed medicines, dietary supplements, occupational therapy, and physical therapy. WBVT was not used in place of physical therapy. Physical therapy activities included gait, balance and transfer training for approximately 1 hour ranging from 3–5 times per wk. For 12 wks participants in the CON group were instructed to continue with their day-to-day activities and follow their doctors’ orders. Participants were instructed to refrain from beginning or participating in exercise programs outside of any prescribed physical therapy.
Statistical Analysis
Power analysis was determined using a study by Zhang et al. in 2014 where significant differences in mean TUG performance scores were observed between a control group (30.39 ± 9.24 sec) and WBVT group (21.34 ± 4.42 sec) (43). Based on these data the effect size was calculated to be 0.979. Based on an α level of 0.05 and 80% power the minimum number of participants per group was 9. Accounting for rate of attrition (≈ 20%), it was determined that at least 24 participants would be recruited for this study.
Descriptive statistics were calculated for all variables and included means and standard deviations. One-way analysis of variance (ANOVA) was used to analyze baseline data between the WBVT and CON groups. Homogeneity of variances was assessed by Levene’s test. Dependent variables were analyzed using a 2 × 2 factorial ANOVA (group × time) with repeated measures on the last factor. If sphericity was violated, the Greenhouse-Geisser test was used. When a significant group-by-time interaction and/or time effect was identified, between-groups and within-group comparisons were performed using independent and paired t-tests. Independent t-tests were used to compare changes in strength between high (n = 8) and low (n = 2) dynamic volume participants and to compare the magnitude of change in SPPB performance between WBVT and CON groups. Pearson product moment correlations were performed to evaluate relationships on selected dependent measures. Significance was accepted at p < 0.05. The Statistical Package for the Social Sciences (v21) software packages (SPSS, Chicago, IL) was used to analyze the data.
RESULTS
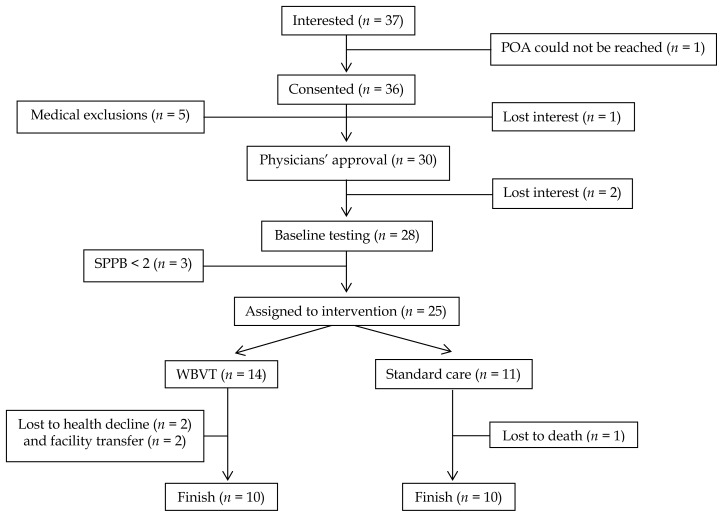
A total of 37 older adults were interested in participating in this non-randomized controlled clinical trial; however, only 30 older adults consented and received a physician’s approval to participate. Due to loss of interest (n = 2) and physical function below the inclusion criteria (n = 3), only 25 of the 30 older adults were assigned to either a WBVT group or CON group. Twenty participants completed the 12-wk study. Figure 1 presents a flow diagram of participants through the study. All WBVT participants (n = 10) completed the 24 training sessions.
Figure 1.
Participant Flow Through Study. POA: power of attorney; WBVT: whole body vibration training. Medical exclusions included epilepsy (n = 1), dialysis (n = 1), advanced dementia (n = 2), and underlying lung cancer (n = 1).
Tables 1–3 present the participants’ characteristics at baseline for the groups. The mean age of participants who completed the intervention was 82.4 ± 5.2 years (73 – 94 yrs). Body mass index (BMI) ranged from underweight to stage II obesity (15.3–38.2 kg/m2). Hypertension, depression, cognitive impairment, and diabetes mellitus were the most frequent medical conditions reported. Nineteen participants were treated with more than 5 pharmaceuticals. All participants required an assistive device for ambulation. Primary assistive ambulatory devices included wheelchair (n = 9), rollator (n = 10), and basic folding walker (n = 1). In the WBVT group, three participants received occupational therapy during the 12 wks and two participants received physical therapy for 2 wks during training. Length of stay at the skilled nursing facility was not different between groups. There were no differences in baseline clinical characteristics between the two groups (Tables 1–3). Females made up 80% (n = 16) of the sample population with 25% of participants being African American (n = 4).
Table 1.
Demographics of WBVT and Control Groups (n = 20).
| Age (years) | Height (cm) | Weight (kg) | |
|---|---|---|---|
| Control (n = 10) | 83.5 ± 5.9 | 165.4 ± 9.6 | 71.9 ± 17.6 |
| WBVT (n = 10) | 81.3 ± 4.4 | 166.6 ± 7.4 | 68.6 ± 13.7 |
Note. Values are means ± SD. WBVT: whole-body vibration training.
Table 2.
Clinical Characteristics of WBVT and Control Groups (n = 20).
| Pre-frail | Frail | Sarcopenia | Sarcopenic Obesity | Falls | Medications | |
|---|---|---|---|---|---|---|
| Control (n = 10) | 1 | 9 | 3 | 1 | 1(0–5) | 13.2 ± 4.4 |
| WBVT (n = 10) | 4 | 6 | 6 | 3 | 2 (0–5) | 16.2 ± 7.3 |
Note. Values are means ± SD. Values in parentheses are ranges. Medication number includes any medicine or supplement prescribed by the primary physician including daily medications and those taken as needed. WBVT: whole-body vibration training.
Table 3.
Prevalence of Chronic Disease in Participants (n = 20).
| Cognitive Impairment | MI | Depression | Arthritis | DM | HTN | OST | Stroke | KD | |
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 10) | 40% | 30% | 70% | 60% | 50% | 80% | 50% | 30% | 30% |
| WBVT (n = 10) | 60% | 20% | 80% | 40% | 60% | 90% | 40% | 30% | 20% |
Note. Cognitive impairment includes presence of Alzheimer’s disease or dementia. WBVT: whole-body vibration training; MI: myocardial infarction/angina pectoris; DM: diabetes mellitus; HTN: hypertension; OST: osteoporosis; KD: kidney disease.
Table 4 presents the average rest times and RPEs throughout the intervention. There was a decrease in RPE from the first 3 wks of WBVT (WK 1–3: 12.6 ± 2.2 units) to the last 3 wks of WBVT (WK 10–12: 11.6 ± 2.6 units) that approached significance (p = 0.069). Rest time between sets and exercises significantly decreased (p = 0.046) from the first 3 wks of WBVT (117 ± 16 s) to the last 3 wks of WBVT (109 ± 16 s). Volume is reported as the product of repetitions and sets. Each WBVT participant (n = 8) completed 2880 dynamic repetitions of lower body exercise. Participants who completed the amended WBVT program (n = 2) completed 87% less dynamic volume than the other participants by completing 384 dynamic repetitions. When WBVT participants were separated into a high (n = 8) and low (n = 2) dynamic volume groups, average RPE during the 12 wks was not different (p = 0.5) between the high (RPE = 12.0 ± 7.4) and low (RPE = 12.4 ± 1.3) groups. All WBVT participants (n = 10) were exposed to 50 seconds of vibration per set with a 12-wk total of 240 minutes of vertical vibration stimulus.
Table 4.
Rest Time, Rate of Perceived Exertion, and Volume During WBVT (n = 10).
| Rest (s) | RPE | Volume (reps) | ||
|---|---|---|---|---|
| n = 8 | n = 2 | |||
| Weeks 1–3 | 117 ± 16 | 12.6 ± 2.2 | 720 | 24 |
| Weeks 4–6 | 112 ± 15 | 12.1 ± 2.8 | 720 | 72 |
| Weeks 7–9 | 107 ± 19 | 11.8 ± 2.6 | 720 | 120 |
| Weeks 10–12 | 109 ± 16* | 11.6 ± 2.6† | 720 | 168 |
Note. Values are means ± SD. WBVT: whole-body vibration training; RPE: rate of perceived exertion; s: seconds; reps: repetitions.
Significantly different from weeks 1–3 (p < 0.05).
p = 0.069.
Table 5 presents the pre- and post-measurements of body composition. There were no differences in baseline characteristics between the two groups in body composition measured by anthropometry and BIA. Baseline measurements of SMI determined that 9 participants (8 Females) were below the BIA threshold for low lean mass. Waist circumference measurements identified central obesity in 10 females and 3 males.
Table 5.
Body Composition Measurements (n = 20).
| WBVT (n = 10) | CON (n = 10) | |||
|---|---|---|---|---|
| PRE | POST | PRE | POST | |
| Weight (kg)a | 68.6 ± 13.6 | 69.4 ± 13.6 | 71.9 ± 17.6 | 73.9 ± 17.7* |
| BMI (kg/m2)a | 24.7 ± 4.6 | 25.0 ± 4.5 | 26.4 ± 6.6 | 27.1 ± 6.4* |
| Waist circumference (cm) | 93.3 ± 13.7 | 92.8 ± 13.1 | 93.3 ± 15.8 | 94.4 ± 16.0 |
| Total lean mass (kg) | 44.2 ± 8.8 | 44.8 ± 8.3 | 47.5 ± 12.5 | 47.7 ± 13.1 |
| Total fat mass (kg) | 24.3 ± 6.7 | 24.8 ± 6.7 | 24.8 ± 10.9 | 26.2 ± 11.5 |
| Total body fat (%) | 35.3 ± 5.0 | 35.4 ± 4.7 | 34.1 ± 9.5 | 35.2 ± 10.5 |
| SMI (kg/m2) | 6.71 ± 1.61 | 6.73 ± 1.45 | 7.34 ± 2.03 | 7.29 ± 2.21 |
Note. Values are means ± SD. BMI: body mass index; SMI: skeletal muscle index. WBVT: whole-body vibration training.
significant time effect (p < 0.05).
p < 0.05, significantly different from baseline.
After the 12-wk intervention there were no significant group-by-time interactions for any of the body composition measures. There was a statistically significant effect of time on body mass (F1,18 = 8.869, p = 0.008, η2 = 0.330) and BMI (F1,18, = 7.470, p = 0.014, η2 = 0.293) in the WBVT group. There was a significant increase in body mass (p = 0.022) and BMI (p = 0.028) in CON; however, body weight and BMI were not different between groups post-intervention (p > 0.05).
Table 6 presents the data for strength and functional performance. There were no differences in strength between WBVT and CON at baseline. There was a significant group-by-time interaction (F1,18 = 5.434, p = 0.032, η2 = 0.232) and time effect (F1,18 = 4.778, p = 0.042, η2 = 0.210) for isometric knee extension strength. WBVT significantly improved isometric knee extension strength (p = 0.003) that ranged from < +1% to +79%. CON recorded declines in leg strength that ranged from < −2% to −44% (n = 5) and increased leg strength that ranged from < +2% to +51% (n = 5).
Table 6.
Strength and Functional Performance Measurements (n = 20).
| WBVT (n = 10) | CON (n = 10) | |||
|---|---|---|---|---|
| PRE | POST | PRE | POST | |
| Isometric HG (kg) | 39.9 ± 9.2 | 38.8 ± 9.5 | 33.9 ± 10.4 | 35.2 ± 11.4 |
| Isometric KE (kg) a,b | 22.3 ± 4.0 | 29.0 ± 4.5* | 23.8 ± 6.3 | 23.6 ± 9.6 |
| SPPB total (units) b | 4.5 ± 2.3 | 5.2 ± 2.1¥ | 4.1 ± 1.9 | 3.7 ± 2.3 |
| Gait speed (m/s) | 0.44 ± 0.18 | 0.48 ± 0.20 | 0.35 ± 0.11 | 0.36 ± 0.13 |
| TUG (sec) | 27.41 ± 9.42 | 26.06 ± 9.76 | 32.7 ± 16.04 | 33.79 ± 15.16 |
| 30-second arm curl | 13 ± 3 | 14 ± 3† | 10.0 ± 5 | 9 ± 4 |
Note. Values are means ± SD. Isometric HG was the sum of the highest measurement from each hand. Isometric KE was the sum of mean measurements from each leg. HG: handgrip; KE: knee extension; SPPB: short physical performance battery; TUG: timed up-and-go; WBVT: whole-body vibration training. TUG (n = 9 per group).
significant time effect (p < 0.05).
significant group-by-time interaction (p < 0.05)
p < 0.050, significantly different from baseline.
p = 0.070, different from baseline.
p = 0.089, different from baseline.
There were no differences in functional performance between WBVT and CON at baseline. There was a significant group-by-time interaction for SPPB performance (F1,18 = 4.84, p = 0.041, η2 = 0.212). Total SPPB score improved non-significantly by 15.6% (p = 0.089) following the 12 wks of WBVT. The magnitude of change in SPPB performance was significantly greater (p = 0.041) in the WBVT group (Δ SPPB = +0.7 units) compared to CON (Δ SPPB = −0.4 units). WBVT participants either maintained (n = 4) or improved (n = 5) SPPB performance, while one participant declined. Most CON participants either maintained (n = 4) or experienced functional decline (n = 4), while two participants improved.
WBVT participants were separated into low and high exercise volume groups to assess differences in exercise adaptations. Independent t-tests revealed no differences between groups for changes in isometric knee extension strength or SPPB (p > 0.05). Dynamic exercise volume did not correlate with changes in isometric knee extension strength or SPPB (p > 0.05).
According to the number of depressive responses of the GDS-15, depression ranged from normal (0–4) to mild (5–8) in the WBVT group and from normal to moderate (9–11) in the CON group. The mean number of depressive responses for WBVT and CON were 2.6 ± 2.9 and 4.1 ± 3.0, respectively. Group averages reflected minimal to no depressive symptoms and groups were not different from each other at baseline indicating that medicines prescribed for depression were effective and/or participants were not depressed. There were no changes (p > 0.05) in depressive responses for WBVT (2.9 ± 3.1) or CON (3.8 ± 3.6) by the study’s completion.
DISCUSSION
The purpose of the study was to compare the effects of 12 wks of WBVT to CON on measures of body composition, leg strength, and lower extremity performance in older adults living in a skilled nursing facility. Baseline age and BMI were similar to other WBVT studies that sampled institutionalized frail older adults (38) and frail outpatients (41). All participants recorded deficits in both gait speed (< 0.8 m/s) and functional performance (SPPB: ≤ 8 units). Approximately 55% of participants recorded deficits in gait speed, functional performance, and handgrip strength (Males: < 30 kg; Females: < 20 kg).
In the present study, 12 wks of WBVT did not elicit changes in body composition measures; therefore, we rejected our hypothesis that WBVT would reduce total body fat and body mass compared to the CON group. While day-to-day physical activity was not measured, it is likely that limited physical activity from prolonged sitting or lying in bed contributed to the increase in body weight in the CON group. In line with our findings, body composition did not change in nursing home residents (80–95 years) that completed 6 or 8 wks of lower limb static (30–50 Hz, 2–5 mm) (5) or dynamic WBVT (30–35 Hz, 4 mm) (1). Studies that reported changes in muscle tissue using WBVT used a training frequency of 3x/wk, higher exercise volume, and imaging methods of body composition (7, 28). Increases in muscle cross sectional area (CSA) (Computed tomography) of the vastus medialis (+8.7%) and biceps femoris (+15.5%) were reported in community-dwelling females (79.3 years) following 10 wks of static and dynamic WBVT (20–40 Hz, 2–4 mm) (28). Lean mass CSA of the right leg increased 3.4% (p < 0.001) after 12 months of WBVT in community-dwelling older men (67 years) (7). Differences in training frequency/volume and measurement technique could partly explain why no changes in body composition were detected in the WBVT group. While BIA prediction equations of SM have been shown to correlate with magnetic resonance imaging predictions, BIA measurements in diseased older adults with altered hydration status (e.g. electrolyte imbalances, dehydration from inadequate fluid intake or use of diuretics, peripheral edema) are less accurate (13) .
Compared to baseline measurements, isometric knee extension strength significantly improved by ~33.7% following 12 wks of WBVT, thereby supporting our alternative hypothesis. This is comparable to the 38.8% improvement in maximal isometric leg press strength reported in 26 older women (79 yrs) following 10 wks of unloaded static and dynamic lower body WBVT (28). Conversely, interventions of longer duration have reported smaller magnitudes of change compared to the present study. Six months of WBVT improved maximal isometric leg strength in institutionalized older women (80.3 yrs) by ~15% (6), while 12 months of WBVT only improved isometric leg strength by 9.8% in older men (67.3 yrs) (7) and 9.4% in community-dwelling older adults (> 60 yrs) (8). It could be inferred that the initial training status of the residents reflects their poor health status and physical function; therefore, participants in the present study had more room for improvement.
Twelve wks of WBVT did not significantly improve SPPB, TUG, or gait speed in pre-frail and frail older adults; therefore, we rejected our hypothesis that WBVT would improve measures of functional performance compared to the CON group. Our results agree with Sievänen et al. who reported that 10 wks of twice weekly WBVT failed to significantly improve SPPB score and TUG performance in a group of frail institutionalized older adults (84 yrs) with low functional performance (mean SPPB total = 3.7); however, meaningful improvements in SPPB performance were reported in some participants (38). A one unit change in SPPB score is recognized as a substantial change in functional performance in older adults (33). In our study, improvements in SPPB performance approached significance (p = 0.089). Post-intervention measurements indicated that five participants improved their SPPB score by at least one unit, while four participants maintained their functional performance, and a single participant declined. In the CON group, SPPB performance declined in four participants, remained unchanged in four participants, and increased in two participants. A change in SPPB score of 0.5 units is clinically meaningful (33); however, the SPPB is a functional performance test that utilizes integers to score performance. Thus, a meaningful change less than one unit cannot be detected in an individual, but could be used to assess average change in groups (25). Using this estimate of change, we found a clinically meaningful change in SPPB performance in the WBVT group (ΔSPPB = +0.7) and no change in the CON (ΔSPPB = −0.4).
There were no adverse events associated with WBVT. Unwanted side effects such as an itchy nose, headache, and lower limb soreness were common during the beginning of WBVT. WBVT was well tolerated by this group of pre-frail and frail older adults, even at higher frequencies of 35–40 Hz; intolerance to vibration frequencies as low as 18 Hz has been previously reported in frail institutionalized older adults using a side-alternating vibration device (38). Although WBVT was tolerable, the heterogeneity of medical conditions, diseases, disabilities, and treatments in the skilled nursing facility residents made the WBVT challenging to implement. Skilled nursing facilities house large target populations for strength and physical function therapies, but the high prevalence of contraindications to WBVT such as hip or knee implants, cardiac pacemakers, and deep vein thrombosis dramatically reduces the population size for this treatment.
There are several limitations to this study that should be discussed. The sample size of our study was small and produced a number of statistically non-significant results that may have been clinically significant. It is possible that with a larger sample size that we may have detected more notable changes in our dependent variables. While participant characteristics were not different at baseline, group assignments were not randomized; therefore, the influence of confounding variables were not minimized. Researchers were not blinded to group assignments during testing to eliminate tester bias. It is not known how many CON participants took part in physical therapy because the therapy staff of that facility declined to share and verify this information; therefore, we cannot explain why some participants improved in this group. Although, the majority of CON participants maintained or decreased on their measures suggesting that either more physical therapy or an exercise intervention is needed.
While evidence suggests that vibration stimulus with exercise improves functional performance more than exercise alone in nursing home residents (5), the addition of a non-vibration exercise control group would have strengthened our study design. Detectable changes in isometric leg strength may have been limited by use of the mechanical push-pull dynamometer in this sample population. We chose the mechanical push-pull dynamometer because of its portability, cost, and convenience; however, several participants reported discomfort in their legs during strength testing, which may have interfered with capturing true changes in strength from WBVT. Increased leg sensitivity from leg edema, neuropathy, peripheral artery disease, varicose veins, or a previous leg injury also may have impaired effort during strength testing.
The mechanisms underlying WBVT on the musculoskeletal system are not entirely understood, and this study did not include EMG or molecular analysis, so it is not possible to directly discuss the mechanisms of WBVT on muscular strength and functional performance in frailty syndrome. Future studies on frail older adults should include larger sample sizes, utilize a higher training frequency, individualize training programs to optimize WBV-induced changes in strength and performance, and include measurements that can better explain the mechanisms of WBVT in older frail adults.
WBVT was well tolerated and occurred without adverse health complications. This study demonstrated that WBVT could be used to counteract losses in leg strength in pre-frail and frail older skilled nursing home residents. Interventions with greater frequency and duration may be more beneficial for this population and may help them to improve their functional performance.
ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to Seven Hills Health and Rehabilitation Center, Heritage Healthcare Center, Jaqui Griffith, and the participants of the long-term facilities, without whom this study would not have been possible.
REFERENCES
- 1.Álvarez-Barbosa F, del Pozo-Cruz J, del Pozo-Cruz B, Alfonso-Rosa RM, Rogers ME, Zhang Y. Effects of supervised whole body vibration exercise on fall risk factors, functional dependence and health-related quality of life in nursing home residents aged 80+ Maturitas. 2014;79(4):456–463. doi: 10.1016/j.maturitas.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 2.American Medical Association. White Paper on Elderly Health Report of the Council on Scientific Affairs. Arch Intern Med. 1990;150(12):2459–2472. [PubMed] [Google Scholar]
- 3.Aprahamian I, de Castro Cezar NO, Izbicki R, Lin SM, Paulo DLV, Fattori A, Yassuda MS. Screening for frailty with the FRAIL scale: A comparison with the phenotype criteria. J Am Med Dir Assoc. 2017;18(7):592–596. doi: 10.1016/j.jamda.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Bates-Jensen BM, Alessi CA, Cadogan M, Levy-Storms L, Jorge J, Yoshii J, Schnelle JF. The minimum data set bedfast quality indicator: differences among nursing homes. Nurs Res. 2004;53(4):260–272. doi: 10.1097/00006199-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bautmans I, Van Hees E, Lemper JC, Mets T. The feasibility of whole body vibration in institutionalised elderly persons and its influence on muscle performance, balance and mobility: a randomised controlled trial. BMC Geriatr. 2005;5(1):17. doi: 10.1186/1471-2318-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaerts A, Delecluse C, Boonen S, Claessens AL, Milisen K, Verschueren SM. Changes in balance, functional performance and fall risk following whole body vibration training and vitamin D supplementation in institutionalized elderly women. A 6 month randomized controlled trial. Gait Posture. 2011;33(3):466–472. doi: 10.1016/j.gaitpost.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Bogaerts A, Delecluse C, Claessens AL, Coudyzer W, Boonen S, Verschueren SM. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(6):630–635. doi: 10.1093/gerona/62.6.630. [DOI] [PubMed] [Google Scholar]
- 8.Bogaerts AC, Delecluse C, Claessens AL, Troosters T, Boonen S, Verschueren SM. Effects of whole body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial) Age Ageing. 2009;38(4):448–454. doi: 10.1093/ageing/afp067. [DOI] [PubMed] [Google Scholar]
- 9.Bohannon RW. Hand-held dynamometer measurements obtained in a home environment are reliable but not correlated strongly with function. Int J Rehabil Res. 1996;19(4):345–348. doi: 10.1097/00004356-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Bohannon RW. Hand-held dynamometry: a practicable alternative for obtaining objective measures of muscle strength. Isokinet and Exerc Sci. 2012;20(4):301–315. [Google Scholar]
- 11.Campitelli MA, Bronskill SE, Hogan DB, Diong C, Amuah JE, Gill S, Maxwell CJ. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16(1):133. doi: 10.1186/s12877-016-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56(9):1710–1715. doi: 10.1111/j.1532-5415.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Zamboni M. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: The acceptability and performance of the 15 item geriatric depression scale (GDS15) and the development of short versions. Family Practice. 1994;11(3):260–266. doi: 10.1093/fampra/11.3.260. [DOI] [PubMed] [Google Scholar]
- 16.De Craen AJ, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry. 2003;18(1):63–66. doi: 10.1002/gps.773. [DOI] [PubMed] [Google Scholar]
- 17.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. JAMA. 1990;263(22):3029–3034. [PubMed] [Google Scholar]
- 18.Figueroa A, Kalfon R, Madzima TA, Wong A. Effects of whole-body vibration exercise training on aortic wave reflection and muscle strength in postmenopausal women with prehypertension and hypertension. Journal Hum Hypertens. 2013;28(2):118–122. doi: 10.1038/jhh.2013.59. [DOI] [PubMed] [Google Scholar]
- 19.Figueroa A, Kalfon R, Madzima TA, Wong A. Whole-body vibration exercise training reduces arterial stiffness in postmenopausal women with prehypertension and hypertension. Menopause. 2014;21(2):131–136. doi: 10.1097/GME.0b013e318294528c. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 21.Furness TP, Maschette WE. Influence of whole body vibration platform frequency on neuromuscular performance of community-dwelling older adults. J Strength Cond Res. 2009;23(5):1508–1513. doi: 10.1519/JSC.0b013e3181a4e8f9. [DOI] [PubMed] [Google Scholar]
- 22.Gabrel CS. Characteristics of elderly nursing home current residents and discharges: data from the 1997 National Nursing Home Survey. Advance Data. 2000;(312):1–15. [PubMed] [Google Scholar]
- 23.Grant PM, Granat MH, Thow MK, Maclaren WM. Analyzing free-living physical activity of older adults in different environments using body-worn activity monitors. J Aging Phys Act. 2010;18(2):171–184. doi: 10.1123/japa.18.2.171. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13(6):538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lienhard K, Vienneau J, Nigg S, Friesenbichler B, Nigg BM. Older adults show higher increases in lower-limb muscle activity during whole-body vibration exercise. J Biomech. 2017;52:55–60. doi: 10.1016/j.jbiomech.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Lopez D, Flicker L, Dobson A. Validation of the frail scale in a cohort of older Australian women. J Am Geriatr Soc. 2012;60(1):171–173. doi: 10.1111/j.1532-5415.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 28.Machado A, García-López D, González-Gallego J, Garatachea N. Whole-body vibration training increases muscle strength and mass in older women: A randomized-controlled trial. Scand J Med Sci Sports. 2010;20(2):200–207. doi: 10.1111/j.1600-0838.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 29.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley JE, Vellas B, Van Kan GA, Anker SD, Bauer JM, Bernabei R, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navalta JW, Stone WJ, Lyons TS. Ethical Issues Relating to Scientific Discovery in Exercise Science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng TP, Feng L, Nyunt MSZ, Larbi A, Yap KB. Frailty in older persons: multisystem risk factors and the Frailty Risk Index (FRI) J Am Med Dir Assoc. 2014;15(9):635–642. doi: 10.1016/j.jamda.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 34.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 36.Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52(6):901–908. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- 37.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Sievänen H, Karinkanta S, Moisio-Vilenius P, Ripsaluoma J. Feasibility of whole-body vibration training in nursing home residents with low physical function: a pilot study. Aging Clin Exp Res. 2014;26(5):511–517. doi: 10.1007/s40520-014-0206-2. [DOI] [PubMed] [Google Scholar]
- 39.Thompson DD. Aging and sarcopenia. J Musculoskelet Neuronal Interact. 2007;7(4):344–345. [PubMed] [Google Scholar]
- 40.Torvinen S, Kannus P, Sievaénen H, Jaérvinen TA, Pasanen M, Kontulainen S, Jaérvinen TL, Jaérvinen M, Oja P, Vuori I. Effect of a vibration exposure on muscular performance and body balance. Randomized cross-over study. Clin Physiol Funct Imaging. 2002;22(2):145–152. doi: 10.1046/j.1365-2281.2002.00410.x. [DOI] [PubMed] [Google Scholar]
- 41.Van Kan GA, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Medical Dir Assoc. 2008;9(2):71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva: 2011. Dec 8–11, 2008. [Google Scholar]
- 43.Zhang L, Weng C, Liu M, Wang Q, Liu L, He Y. Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: a pilot randomized controlled trial. Clin Rehabil. 2014;28(1):59–68. doi: 10.1177/0269215513492162. [DOI] [PubMed] [Google Scholar]