Abstract
Embryonic large molecule derived from yolk sac (ELYS) is a constituent protein of nuclear pores. It initiates assembly of nuclear pore complexes into functional nuclear pores toward the end of mitosis. Using cellular, molecular, and genetic tools, including fluorescence and Electron microscopy, quantitative PCR, and RNAi-mediated depletion, we report here that the ELYS ortholog (dElys) plays critical roles during Drosophila development. dElys localized to the nuclear rim in interphase cells, but during mitosis it was absent from kinetochores and enveloped chromatin. We observed that RNAi-mediated dElys depletion leads to aberrant development and, at the cellular level, to defects in the nuclear pore and nuclear lamina assembly. Further genetic analyses indicated that dElys depletion re-activates the Dorsal (NF-κB) pathway during late larval stages. Re-activated Dorsal caused untimely expression of the Dorsal target genes in the post-embryonic stages. We also demonstrate that activated Dorsal triggers apoptosis during later developmental stages by up-regulating the pro-apoptotic genes reaper and hid. The apoptosis induced by Reaper and Hid was probably the underlying cause for developmental abnormalities observed upon dElys depletion. Moreover, we noted that dElys has conserved structural features, but contains a noncanonical AT-hook–like motif through which it strongly binds to DNA. Together, our results uncover a novel epistatic interaction that regulates Dorsal dynamics by dElys during development.
Keywords: NF-κB transcription factor, nuclear envelope, nuclear pore, nuclear organization, nuclear translocation, nuclear structure, development, Drosophila, Drosophila genetics
Introduction
The embryonic large molecule derived from yolk sac (ELYS)3 was characterized in the mouse as a putative transcription factor important for hematopoiesis (1). ELYS was characterized to possess nuclear localization signal (NLS), nuclear export signal, N-terminal β-propeller region, central helical region, and C-terminally disordered region (1, 2). AT-hook motifs present in ELYS allow DNA binding, and the transactivation domains (acidic region) can induce transcription (1). The β-propeller–like domain present in ELYS mediates interaction with other nucleoporins and facilitates the nuclear pore complex (NPC) assembly (3). NPC is a multiprotein assembly of nucleoporins (Nups) with their sizes varying from ∼60 MDa in yeast to ∼125 MDa in metazoans. Nucleoporins assemble into subcomplexes and are present on the cytoplasmic, nuclear membrane, and nucleoplasmic faces of nuclear pores (4, 5). In metazoans, at the onset of mitosis, subcomplexes of NPCs dissociate from each other and get redistributed inside the cell. ELYS was reported to be an integral member of the Nup107 complex and essential for post-mitotic NPC assembly (6–10). In interphase, ELYS is present at the nuclear envelope and nucleoplasm, but in mitosis, it associates with chromatin, kinetochores, and spindles (8). Importantly, the conserved ELYS domain is required for its NPC and kinetochore localization (11), where ELYS helps in microtubule polymerization (12). ELYS tethers Nup107 complex to kinetochores and initiates their inclusion into post-mitotic nuclear pores (9).
ELYS is critically required for embryonic development as ELYS null mice die during embryonic stage E3.5 to E5.5, well before the onset of embryonic hematopoiesis (1, 13). However, conditional inactivation of the ELYS locus in adult mice showed reduced effects, and mice behaved normally (14). ELYS, also known as Mel-28, is required for the maintenance of the nuclear morphology and embryonic development in Caenorhabditis elegans (7, 10). The ELYS ortholog in zebrafish, flotte lotte (flo), is critically required for early embryonic and pharyngeal skeleton development (15). Additionally, flo deletion disrupted NPC formation, and defective nuclear import induced replication stress in intestinal progenitor cells (16).
In addition to the NPC assembly, the depletion of ELYS leads to phosphorylation-dependent mislocalization of lamin B receptor (LBR) from the nuclear periphery (17). ELYS recruits protein phosphatase-1 (PP1) to the kinetochores, and a reduction in phosphorylation levels of various molecules drives nuclear envelope assembly (18). Thus, ELYS being a nucleoporin and putative transcription factor is a critical molecule that may have a significant role to play in the early developmental events of an organism. However, no directed study has been performed on ELYS to obtain mechanistic insight of important roles played by ELYS in cellular homeostasis and early developmental events.
We chose Drosophila melanogaster (fruit flies) to address the importance of ELYS in early developmental processes. CG14215, an ELYS-like molecule in Drosophila (hereafter as dElys), is known to be present at the nuclear periphery and in association with the promoter region of genes undergoing active transcription (19, 20). Here, we report the genetic, molecular, and cellular characterization of dElys. dElys binds with DNA through its noncanonical AT-hook–like motif. RNAi-mediated depletion of dElys induces loss of nucleoporins and lamins, thus affecting the nuclear pore and nuclear lamina assembly. Importantly, upon dElys depletion, Dorsal (NF-κB) accumulates inside the nucleus in later larval stages, without any immune challenge, and induces apoptosis. Through our results, we strongly imply that dElys is a key developmental molecule required in cellular processes integral to normal cellular homeostasis. In addition to maintaining the nuclear architecture, dElys contributes to the development by regulation of the Dorsal dynamics into post-embryonic tissues.
Results
ELYS is highly conserved in Drosophila
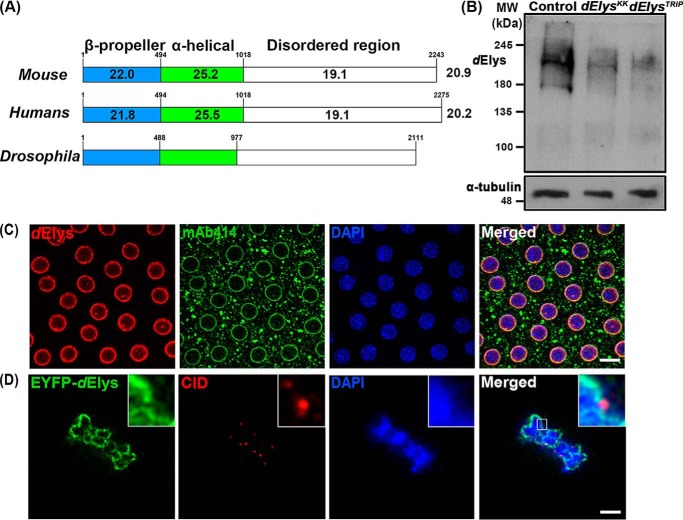
ELYS is a DNA-binding nucleoporin that coordinates NPC assembly at the end of mitosis. Drosophila ELYS (dElys, CG14215) is known to localize at the nuclear periphery and is found to be present at the promoter region of genes undergoing active transcription (19, 20). However, any mechanistic details on the dElys function are not available. For molecular and cellular characterization of dElys, we started with primary sequence alignment and report that an overall ∼20–21% identity exists among dElys and mouse and human ELYS (Fig. 1A). Using bioinformatics tools, we have predicted the presence and conserved arrangement of structural features like the N-terminal β-propeller domain (aa 1–488), central helical domain (aa 489–977), and a C-terminal unstructured region (Fig. 1A). Homology-based 3D structure predictions of the N-terminal β-propeller domain of dElys identified a highly-conserved seven-bladed β-propeller structure (Fig. S1A) appreciably similar to the reported structure of the N-terminal domain of mouse ELYS (3, 21). Evolutionary divergence analysis indicates that dElys is present in a clade quite distinct and distant from vertebrate ELYS (Fig. S1B).
Figure 1.
ELYS is conserved in Drosophila. A, graphical representation of sequence identity of dElys with mouse and human ELYS. dElys is 20.9 and 20.2% identical with mouse and human ELYS, respectively. Domain-wise identity is mentioned in each domain. Conserved secondary structures between Drosophila, mouse, and human ELYS are represented. β-Propeller N-terminal domain, α-helical central domain, and disordered C-terminal regions are conserved in dElys as in higher orthologs. B, antibody generated against dElys identified a band of ∼215 kDa in lysates obtained from third instar larval head complex from WT control and ubiquitous dElys knockdown lines as indicated. α-Tubulin was used as a loading control. C, syncytial Drosophila embryos stained with dElys antibody (red), mAb414 (green) marking FG-nucleoporins, and DNA stained with DAPI. Scale bar, 5 μm. D, high-resolution image of metaphase-arrested Drosophila S2-expressing EYFP–tagged dElys (green) stained for kinetochores with an anti-CID antibody (red). Scale bar, 2 μm.
We raised polyclonal antibodies against the C-terminal antigenic fragment (aa 1796–2111) of dElys. Immunoblotting with anti-dElys antibody detected a band of ∼215 kDa in control head complex lysates and a band of decreased intensity in lysates prepared from tissues where dElys was ubiquitously knocked down (ubiquitous knockdown was achieved by driving dElys RNAi with the Act5C–GAL4 driver, to be denoted as ubiquitous here on) (Fig. 1B). dElys antibodies detected a conserved and strong nuclear rim staining pattern in syncytial embryos overlapping with mAb414 antibodies recognizing FG-nucleoporins in nuclear pores (Fig. 1C). dElys antibodies also detected a similar overlapping pattern at the nuclear rim of salivary gland nuclei from GFP–Nup107-expressing organisms (Fig. S1C). Moreover, the EYFP–dElys-expressing salivary glands also had a nuclear rim localization pattern identical to endogenous dElys (Fig. S1D). In salivary gland nuclei, dElys antibodies detect a pattern co-localizing with the nuclear lamina–associated lamin-B receptor at the nuclear periphery (Fig. S1E). We probed whether dElys also exhibits cell-cycle–dependent subcellular localization changes as seen in other organisms. Metaphase-arrested EYFP–dElys-expressing Drosophila S2 cells were co-stained with the centromere identifier (CID, Drosophila CENP-A homolog) (22) to mark the kinetochores. dElys localized to mitotic chromosomes but not on the kinetochores. Rather, dElys was observed in the close vicinity of CID puncta. It is important to note that ELYS recruits the Nup107 complex to the kinetochores in cell culture, Xenopus egg extracts, and C. elegans embryos (6–8, 12). However, the Nup107 complex was reported to be absent from kinetochores in Drosophila (23).
ELYS is also known as an AT-hook–containing transcription factor 1 (AHCTF1). Using a canonical mouse ELYS AT-hook motif (Mm_Elys_AT-hook), we have predicted three AT-hook–like motifs in dElys. We noticed a lack of glycine and proline amino acids in the conserved –RGRP– core (overscored) present in several characterized AT-hook proteins (Fig. S2A) (24). We thus asked whether such a divergent noncanonical AT-hook can bind DNA. A C-terminal fragment (aa 1858–1961) of dElys bearing all three predicted AT-hook–like motifs, dElys_AT-1, -2, and -3, was purified and tested in a DNA-binding assay. Together with this, we mutated the arginines in each predicted AT-hook–like motif individually (mAT-1, mAT-2, and mAT-3, where “m” denotes arginines of the AT-hook mutated to alanines) or combined the mutations of all three AT-hooks (mAT-1 + 2 + 3) (Fig. S2B) and tested the ability of purified proteins (Fig. S2C) to bind with AT-rich or non-AT–rich DNA. It is evident from the EMSA experiments and their quantitation that mutations in AT-hook 1 and 3 (mAT-1 and mAT-3) show a significant reduction in their ability to bind with both AT-rich and non-AT–rich DNA (Fig. S2, D–G). Our data further established that the presence of glycine and proline is not as important in the –RGRP– core as the positively charged arginine. The alternately placed positively charged amino acids are known to play more important role in AT-hook functions (25). Through our initial in silico, biochemical, and cellular analysis, we suggest that dElys is a canonical ELYS molecule despite low sequence identity.
dElys is essential for normal development in Drosophila
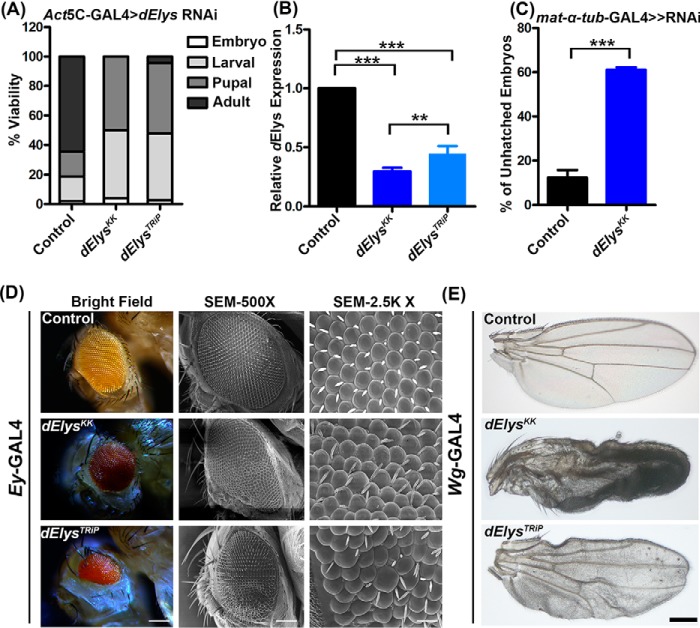
After assigning features of a nucleoporin and determining the localization dynamics, we asked whether dElys is essential for development in Drosophila. We performed in vivo depletion of dElys using RNAi line (v103547) from the Vienna Drosophila Resource Centre (VDRC) (dElysKK). To validate phenotypes observed with the dElysKK RNAi line, we also generated a TRiP-based RNAi line (dElysTRiP). The ubiquitous knockdown of dElys by the two RNAi lines led to severe consequences on viability and induced lethality with a varying extent at each developmental stage. Although no adults emerged from the dElysKK RNAi line, a few viable flies (∼4%) emerged out in dElysTRiP. dElys-depleted embryos were largely unaffected (∼4% lethality), but a significantly increased lethality occurred at the larval stage (∼46%), and ∼50% of pupae died in the late pupal stage of development (Fig. 2A). The quantitative PCR-based assessment of knockdown in dElys transcript levels suggests ∼70 and ∼55% knockdown in dElysKK and dElysTRiP RNAi lines, respectively (Fig. 2B). Although dElysKK has two predicted off-targets, CG15643 and CG7051, in addition to dElys, the expression level of CG15643 remained unperturbed, while the CG7051 levels decreased by ∼20% during dElys knockdown (p < 0.05) (Fig. S3, A and B). CG7051 is reported to have an exclusive expression in male germ cells (26) and hence would not have contributed to the phenotypes observed. We have used the dElysKK line in all subsequent studies because it showed a comparatively significant knockdown, and the pVALIUM10-based TRiP line is relatively ineffective in knockdown during oogenesis as well as a lack of uniform knockdown (27).
Figure 2.
dElys is essential for the normal development of Drosophila. A, quantification representing the lethality stages of the dElys knockdown organism. RNAi lines were driven with ubiquitous Act5C–GAL4 driver. Control represents WT flies crossed with the Act5C–GAL4 driver. B, quantitative PCR for dElys knockdown using two different RNAi lines. Data are represented from at least three independent experiments. Statistical significance was derived from one-way ANOVA followed by Tukey's post hoc test. Error bars represent S.E. *** represents p < 0.0001, and ** represents p < 0.001. C, hatching rate analysis of maternal depletion of control and dElys in embryos using mat-α-tub-GAL4 ≫ RNAi driver. D, eye-specific knockdown of dElys using the Ey-GAL4 drivers. The 1st column shows the stereomicroscopic image; the 2nd column represents the SEM image (×500), and the 3rd column shows an SEM image (×2.5K). Observations were made from at least three independent experiments. Scale bar, 200 μm in the stereomicroscopic image; 20 μm in SEM column 1, and 2 μm in SEM column 3). Control represents WT flies crossed with Ey-GAL4 driver. E, dElys depletion in the wing using the Wg-GAL4 driver. Representative images are shown for wing phenotypes. Observations were made from at least three independent experiments. Scale bar, 200 μm. Control is WT flies crossed with Wg-GAL4driver.
Maternally contributed dElys will hinder the analysis for developing an understanding of dElys functions in early development. We thus used the mat–α-tub–GAL4 driver to deplete maternally contributed dElys before the induction of zygotic transcription to overcome this issue. Maternally depleted dElys (dElysKK RNAi line) embryos have an abnormal shape, and they show a very slow rate of development compared with control embryos (data not shown). Hatching rate analysis showed that maternal dElys-depleted embryos showed ∼62% lethality as compared with ∼12% lethality observed with control embryos obtained by crossing control flies with the driver line (Fig. 2C).
To avail more insights on the importance of dElys in development, we silenced dElys in a tissue-specific manner in eyes and wings using eyeless (Ey) and wingless (Wg) drivers, respectively. dElys-depleted flies have a shrunken eye with a significant reduction in the area occupied by ommatidia. Also, the ommatidia shape was irregular, and their arrangement was in disarray, leaving behind an eye cavity (Fig. 2D, 1st and 2nd vertical panels). Moreover, a significant number of dElys-depleted ommatidia show loss of eye bristles, duplication of bristles, and an irregular orientation (Fig. 2D, 3rd vertical panel, SEM ×2.5K). Similarly, dElys-silenced wings look crumpled with signs of vein atrophy and significant cell death in the wing blade tissues (Fig. 2E). The severity of these phenotypes varied between the two different dElys RNAi lines used in this study, yet the observations made regarding eye and wing phenotypes were consistent. Our data thus suggest an essential requirement of dElys for the normal development of Drosophila.
Loss of dElys results in the nuclear pore and nuclear lamina assembly defects
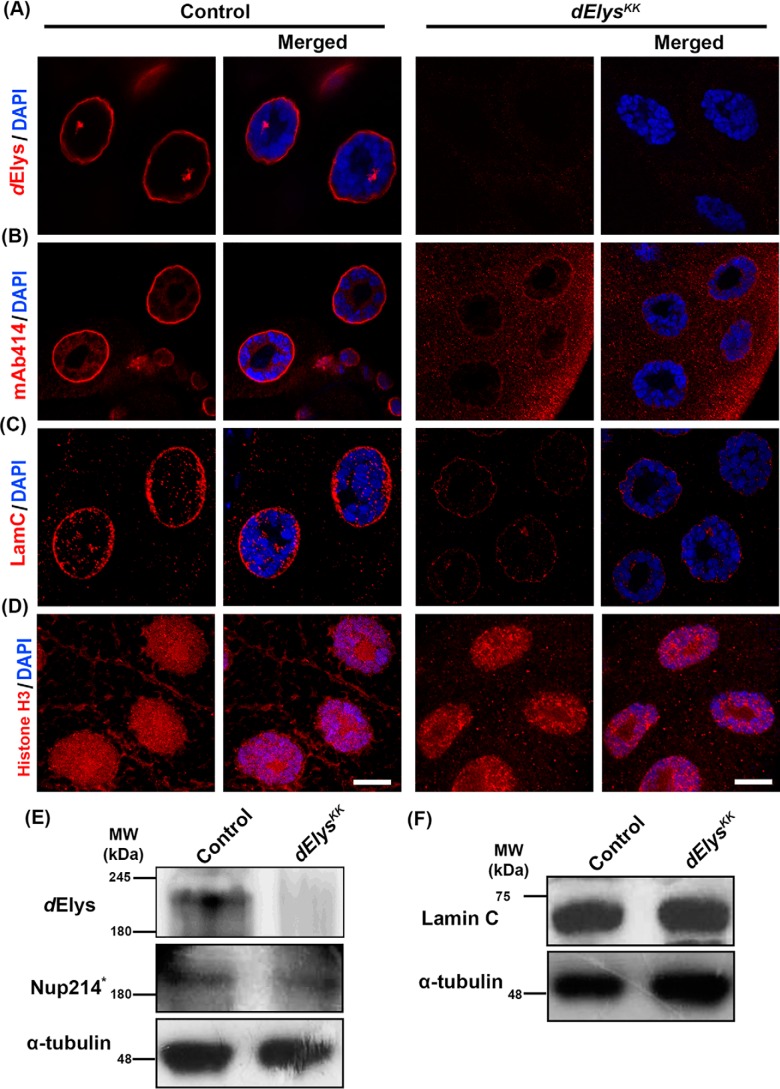
We next asked whether the dElys is functionally conserved in Drosophila. We assessed the localization of various nucleoporins representing different subcomplexes of NPC when dElys is ubiquitously knocked down. When salivary gland nuclei from dElys knockdown flies were stained for Nup43, representing the Nup107 complex, Nup98, a mobile nucleoporin (Fig. S4, A and B), and mAb414 antibody recognizing FG-repeats, they were found to be largely absent from the nuclear membrane (Fig. 3, A and B, right panels). Concomitantly, a marked increase in the cytoplasmic staining for these nucleoporins was observed in the dElys-depleted salivary gland tissues indicating a redistribution of nucleoporins. Quantitation of nuclear membrane intensities for different Nups also corroborates the fact that dElys affects efficient assembly of nucleoporins into NPC (dElys: control, 1.00 ± 0.04, and dElysKK, 0.15 ± 0.01; Nup43: control, 1.00 ± 0.08, and dElysKK, 0.29 ± 0.02; Nup98: control, 1.07 ± 0.12, and dElysKK, 0.31 ± 0.02; FG-repeat nucleoporins: control, 1.00 ± 0.07, and dElysKK, 0.36 ± 0.01) (Fig. S4, G–J). We have further confirmed with histone H3 staining that dElys depletion does not alter chromatin structure significantly (histone H3: control, 1.00 ± 0.06, and dElysKK, 0.85 ± 0.03) (Fig. 3D) and hence could be used for normalization of intensities of every molecule tested. Detection of the protein levels of these nucleoporins in the larval head–complex lysate suggests no significant decrease in their levels except for Nup98 (Fig. 3E and Fig. S4P). Our data thus establish a conserved role for dElys in post-mitotic NPC assembly in Drosophila.
Figure 3.
dElys is essential for the nuclear pore complex and nuclear lamina assembly in Drosophila. A and B, detection of dElys (A) and FG-repeat nucleoporins (detected by mAb414, B) on the nuclear rim assessed in third instar larval control, (1st and 2nd vertical panels) and dElys-depleted (ubiquitous Act5C–GAL4 driven, 3rd and 4th vertical panels) salivary gland nuclei. DNA is stained with DAPI. Scale bar, 5 μm. Control is WT flies crossed with the Act5C–GAL4 driver. C, assessment of lamin C localization in third instar larval control, and dElys-depleted (ubiquitous Act5C–GAL4 driven) salivary gland nuclei as mentioned in A and B. Scale bar, 5 μm. D, representative images of histone H3 (red) staining in control and dElys-depleted (ubiquitous Act5C–GAL4-driven) third instar larval salivary gland nuclei. DNA is stained with DAPI. Scale bar, 5 μm. Control is WT flies crossed with the Act5C–GAL4 driver. E, Western blot analysis of indicated molecules tested in control and dElys knockdown (ubiquitous Act5C–GAL4–driven) third instar larval head lysate. * indicates detection with mAb414 antibodies. α-Tubulin was used as a loading control. Control is WT flies crossed with the Act5C–GAL4 driver. Each Western blotting was performed at least three times. F, Western blot analysis for nuclear lamina molecule lamin C as done in E. α-Tubulin was used as a loading control. Stripes separated from the same experiment were probed with additional antibodies as mentioned in Fig. S4P.
ELYS regulates the incorporation of LBR in the nuclear envelope in a phosphorylation-dependent manner (17, 28). So, we asked whether the nuclear lamina assembly is also affected by dElys. Salivary gland tissues, ubiquitously depleted for dElys, when stained for lamin B, LBR, and lamin C showed reduced localization in the nuclear lamina (Fig. S4, C and D, and Fig. 3C, respectively). Quantitation of nuclear lamina intensities for lamina-associated molecules suggests a clear decrease under dElys-depleted conditions, which emphasizes the importance of dElys in the recruitment of these molecules to the nuclear lamina (LBR: control, 1.02 ± 0.07, and dElysKK, 0.28 ± 0.01; lamin B: control, 0.98 ± 0.04, and dElysKK, 0.50 ± 0.04; lamin C: control, 0.99 ± 0.04, and dElysKK, 0.43 ± 0.01). Our data constitute the first report highlighting the in vivo importance of dElys in the localization of lamin B and lamin C to the nuclear lamina. It is also important to note that during the dElys depletion experiments, protein levels of lamins and LBR remained unaffected (Fig. 3F and Fig. S4Q). These observations suggested that dElys is indispensable for the maintenance of the nuclear lamina and ultimately the nuclear architecture.
While analyzing the nuclear periphery localization of nucleoporins and lamins, we noticed that the Ran-GTPase, required for the coordination of the nucleo-cytoplasmic transport of protein cargo, is mislocalized upon dElys depletion. In dElys-depleted salivary gland tissues, Ran reactivity was lacking from the nuclear periphery and appeared redistributed throughout the cell (Ran: control, 1.03 ± 0.09, and dElysKK, 0.29 ± 0.05) (Fig. S4, E and N). Upon dElys knockdown, the absence of Ran-GTPase, Nups, and lamins prompted us to ask whether everything associated with the nuclear periphery is re-distributed. TBP-associated factors that form TFIID show nuclear rim and strong cytoplasmic staining in the Drosophila embryo (29). Accordingly, TATA-box binding protein-1 (TBP1, dTBP in Drosophila) when stained with polyclonal anti-yeast TBP antibodies (as used in Ref. 30) presented the characteristic nuclear periphery staining pattern even in the dElys-depleted salivary gland tissues (dTBP: control, 1.00 ± 0.11, and dElysKK, 0.76 ± 0.12) (Fig. S4, F and O). Unaltered protein levels of these molecules indicate that dElys is required for their recruitment to the nuclear envelope, possibly during post-mitotic nuclear buildup where dElys is the first molecule associating with DNA. A similar loss of FG-nucleoporins from the nuclear rim was noticed in salivary glands when dElys was knocked down using a TRiP-based RNAi line (Fig. S5A, bottom-most panels). To further confirm the dElys function in NPC assembly, we attempted to rescue dElys depletion phenotype by multiple gene copy approach, where we provided an extra copy of dElys (UAS–dElys) through a transgene to exhaust the dsRNA generated by RNAi (dElysKK), leaving behind a significant amount of dElys to perform its function. The FG-repeat nucleoporins analyzed in this combination (dElysKK; UAS-dElys) showed partial rescue of its level on the nuclear periphery (FG-repeat nucleoporins: control, 1.00 ± 0.13; dElysKK, 0.43 ± 0.01; dElysKK; UAS-dElys, 0.71 ± 0.03) (Fig. S5A, 3rd horizontal panel, and B), suggesting that the phenotypes observed were indeed a result of dElys depletion.
dElys knockdown shows activated Dorsal pathway and induced apoptosis
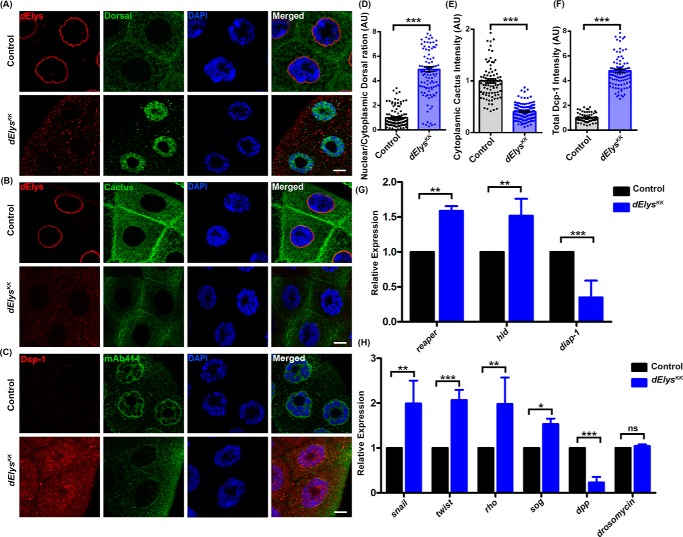
To deduce the underlying molecular mechanism that drives dElys depletion phenotypes, we sought to check for perturbed nucleo-cytoplasmic traffic of various signaling molecules. Dorsal (NF-κB) is an important transcription factor, which stably associates with Cactus and remains inactive in the cytoplasm under normal conditions. The rapid shuttling of Dorsal into the nucleus requires a stimulus (31, 32) and is accompanied by ubiquitination-dependent degradation of the Cactus, NF-κB inhibitor (IκB) in the cytoplasm (33, 34). In salivary glands isolated from ubiquitous knockdown of control, the Dorsal signal was predominantly diffused in the cytoplasm (Fig. 4A, upper panels). In contrast, dElys-depleted salivary gland tissues showed an intense Dorsal signal inside the nucleus (Fig. 4A, lower panels). Ratiometric quantification of the Dorsal intensities in the cytoplasm and nucleus further highlighted that Dorsal signals significantly increased inside the nucleus upon dElys depletion (nuclear/cytoplasmic Dorsal: control, 1.00 ± 0.07, and dElysKK, 4.93 ± 0.20) (Fig. 4D). Increased nuclear localization of Dorsal was also seen in salivary glands obtained from dElys knockdown using the TRiP-based RNAi line (Fig. S5C, 4th horizontal panel). Upon dElys depletion, the increased retention of Dorsal inside the nucleus is not accompanied by an increase in dorsal transcript levels (Fig. S6A). Thus, dElys regulates the redistribution of Dorsal, but not its expression. The accumulation of Dorsal inside the nucleus prompted us to ask whether the cytoplasmic level of Cactus has been reduced. The cytoplasmic Cactus signal is significantly diminished in dElys-depleted salivary gland tissues as compared with control tissues (Fig. 4B). The quantification of Cactus staining further corroborated the observation that dElys depletion may induce Cactus degradation and Dorsal accumulation inside the nucleus (Cactus: control, 1.00 ± 0.03, and dElysKK, 0.40 ± 0.01) (Fig. 4E). Our attempts to rescue Dorsal nuclear localization by providing an extra copy of dElys using transgene (UAS–dElys) led to Dorsal receding from the nuclei of the third instar larval salivary glands, suggesting a specific role of dElys in nuclear Dorsal localization (nuclear/cytoplasmic Dorsal: control, 1.00 ± 0.07, and dElysKK, 7.03 ± 0.47; dElysKK; UAS-dElys, 1.33 ± 0.05) (Fig. S5, C, 3rd horizontal panel, and D). Although Dorsal is known to be a pro-growth molecule, studies suggest that activated Dorsal can also induce apoptosis in cells (35–37). The phenotypes observed upon dElys depletion may be a consequence of the unfavorable accumulation of Dorsal inside the nucleus and thus could induce cell death.
Figure 4.
Dorsal is activated in dElys depletion and shows induced apoptotic response. A, detection of Dorsal with anti-Dorsal (green) and dElys antibodies (red) in control (upper panels) and dElys RNAi (ubiquitous Act5C–GAL4 driven, lower panels) third instar larval salivary gland nuclei. DNA is stained with DAPI. Scale bar, 5 μm. Control is WT flies crossed with the Act5C–GAL4 driver. B, detection of Cactus in third instar larval salivary gland cells with anti-Cactus (green) as mentioned in A. Scale bar, 5 μm. C, detection of apoptosis in third instar larval salivary gland cells with anti-Drosophila caspase-1 antibody (red) and FG-nucleoporins by mAb414 antibodies in control (upper panels) and dElys depletion (ubiquitous Act5C–GAL4 driven, lower panels). DNA is stained with DAPI. Scale bar, 5 μm. Control is WT flies crossed with the Act5C–GAL4 driver. D–F, quantification of intensities of indicated molecules in control and dElys RNAi condition. The intensity of each molecule was normalized to the intensity of DAPI. Data are represented from at least three independent experiments. At least 45 nuclei were analyzed from 7 to 8 pairs of salivary glands. Statistical significance was derived from the Student's t test. Error bars represent S.E. *** represents p < 0.0001. G, expression analysis of apoptosis regulatory genes, reaper, hid, and diap-1 by quantitative PCR in cDNA from third instar larval head complex of control and dElys RNAi (ubiquitous Act5C–GAL4 driven). Data are represented from at least three independent experiments. Statistical significance was derived from the Student's t test. The error bars represent the standard deviation. *** represents p < 0.0001, and ** represents p < 0.001. H, expressions of Dorsal target genes snail, twist, rho, dpp, short gastrulation (sog), and drosomycin analyzed by quantitative PCR in control and dElys RNAi (ubiquitous Act5C–GAL4 driven) third instar larval head complex lysate. Data are represented from at least three independent experiments. Statistical significance was derived from the Student's t test. The error bars represent the standard deviation. *** represents p < 0.0001, and ** represents p < 0.001, and * represents p < 0.05; ns is nonsignificant.
We next probed whether the abnormal development of the eye and wing tissues, as well as lethality observed upon dElys depletion, is due to apoptosis. We employed acridine orange staining as a primary assay to infer the induction of apoptosis in salivary glands. dElys-depleted tissues accumulated significantly more acridine orange than normal tissues suggesting activation of cell death (data not shown). To further assert whether dElys depletion can induce cell death (apoptotic response), we probed for cleaved Death caspase-1 (Dcp-1). Dcp-1 is critical for normal embryonic development, and an elevation in Dcp-1 levels is a hallmark for apoptosis (38–40). Dcp-1 staining of salivary gland tissues from ubiquitous dElys knockdown showed increased immunoreactivity and increased Dcp-1 positive puncta (cleaved Dcp-1: control, 1.00 ± 0.03, and dElysKK, 4.79 ± 0.13) (Fig. 4, C and F). We next asked whether nuclearly-accumulated Dorsal causes any alterations in the expression of apoptotic genes. We probed for the transcript levels of pro-apoptotic genes reaper and hid and anti-apoptotic diap-1 (Drosophila inhibitor of apoptosis 1). cDNA was prepared from the third instar larval head complex isolated from the ubiquitous knockdown of control and dElys, and the transcript levels were assessed by quantitative real-time PCR. We observed a significant increase in the level of the pro-apoptotic reaper and hid expression, although there was a complementary decrease in the level of anti-apoptotic diap-1 upon dElys depletion (Fig. 4G). The relative levels of diap-1, reaper, and hid are important determinants of cell survival or cell death. Together, the quantitative PCR and Dcp-1 immunostaining data indicate an indisputable correlation toward apoptotic induction upon dElys depletion. Accumulation of Dorsal upon dElys knockdown should bring a change in the levels of its target genes. The quantitative PCR-based detection of snail, twist, rho, and sog levels revealed an increase in their expression. However, the levels of dpp (decapentaplegic), a gene suppressed by Dorsal, decreased in dElys-depleted samples as compared with control (Fig. 4H). To rule out the possibility of an activated Dorsal pathway under immune response, we checked for the expression of the anti-microbial peptide drosomycin in dElys-depleted cDNA. The unaltered drosomycin levels suggest that dElys depletion-dependent activation of the Dorsal pathway does not have a microbial infection and immunity component to it (Fig. 4H).
Dorsal-dependent expression of snail, twist, and sog during early development is important for cellularization (41). We used the third instar larval head complex for cDNA preparation, which is quite late in the development. Expression of snail, twist, and sog could be detected in the late larval stages and that may be the cause of apoptosis in dElys-depleted organisms inducing developmental defects. Studies indicate that Drosophila apoptotic response activators include snail, twist, dpp, and rho (42–45). It is thus not surprising to find resurrected expression of Dorsal targets during late developmental stages that could induce apoptosis and developmental defects observed under dElys-depleted conditions.
Accumulation and re-activation of Dorsal in post-embryonic tissues is dElys specific
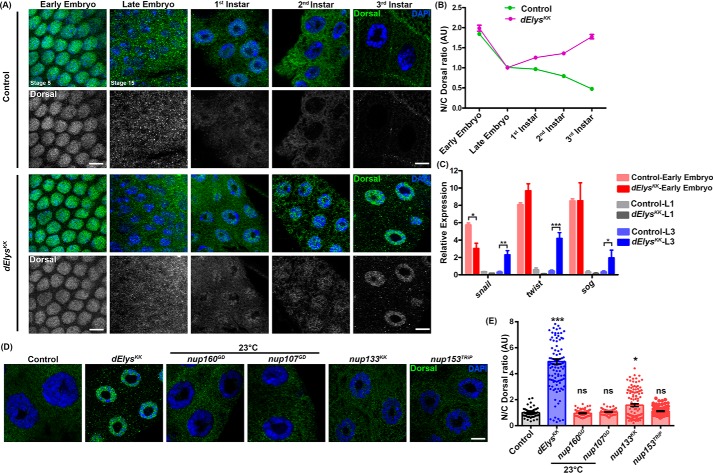
We set out to investigate whether dElys depletion-dependent nuclear retention of Dorsal is a temporal event of later developmental stages or whether Dorsal is entrapped in the nucleus even after the embryonic stage. First, we analyzed the Dorsal levels in early embryonic stages (stage 5) in control and dElys knockdown were driven by mat–α-tub–GAL4. The nuclear levels of Dorsal remain high and unperturbed in both dElys and control-depleted embryos (Fig. 5A, 1st vertical panel). Significant nuclear Dorsal levels along with increased cytoplasmic localization of Dorsal was observed in presumptive embryonic salivary glands from late embryonic stages (stage 15) of control and dElysKK organisms (Fig. 5A, 2nd vertical panel). We then looked for Dorsal signals in the nuclei of salivary glands isolated from each successive larval stage. A gradual but steady decrease in the nuclear Dorsal signal was observed in the first instar to third instar stage nuclei of the ubiquitous control knockdown organisms. The salivary gland nuclei from the first instar larva of ubiquitous dElys depletion have the Dorsal signal inside the nucleus that is comparable with control depletion (Fig. 5A, 3rd vertical panel). However, we observed an increase in nuclear levels of Dorsal in the second instar larval stage of dElys-depleted organisms, whereas control nuclei have only residual or no Dorsal signals (Fig. 5A, 4th vertical panel). Importantly, the salivary glands from the third instar larval stage of ubiquitous dElys depletion show a prominent nuclear accumulation and a complementary decrease in the Dorsal signal inside the cytoplasm. But the control third instar salivary gland nuclei had no Dorsal inside the nucleus, and the Dorsal levels remained restricted to cytoplasm only (Fig. 5A, 5th vertical panel). Quantification of nuclear Dorsal signal intensities from control and dElys-depleted salivary gland nuclei further established that Dorsal starts to re-distribute in the nucleus during post-embryonic developmental stages upon dElys depletion (Fig. 5B). To further support this observation, we followed the expression of three Dorsal targets, namely snail, twist, and sog, which are key determinants of dorsoventral polarity in successive developmental stages. Under dElys-depleted conditions, the expression of snail, twist, and sog is high during the early embryonic stage, which diminished during the first instar larval stage after specifying dorsoventral polarity. But a resurrected expression of these key molecules was observed during the third instar stage when dElys was depleted. Under the control depletion condition, there is no significant expression observed during the late larval stages (Fig. 5C). Our results thus strongly suggest that dElys plays an important role in regulating the Dorsal signaling during post-embryonic stages and its temporal sequestration in the cytoplasm.
Figure 5.
dElys depletion re-distributes Dorsal in the nucleus during the larval stage and specific to dElys. A, nuclear localization of Dorsal (green) assessed in control and dElys depletion in each stage of development. The 1st two vertical panels show early and late embryonic stages, and the next three vertical panels show each successive larval stage salivary glands. DNA is stained with DAPI. Scale bar, 5 μm. Early embryonic depletion was driven with mat–α-tub–GAL4. Control is WT flies crossed with the mat–α-tub–GAL4 (for early embryos) and ubiquitous Act5C–GAL4 (for late embryo and later developmental stages) driver, respectively. Stages of embryonic development are mentioned. B, quantitation of nuclear/cytoplasmic intensity ratio of Dorsal measured through developmental stages of Drosophila and plotted for each stage showing the distribution of Dorsal in embryos and salivary gland cells. Data represent at least three independent experiments. Data are represented from at least three independent experiments. Error bar represents S.E. C, Dorsal target genes snail, twist, and sog expression was analyzed in successive developmental stages by quantitative-PCR. The red bar represents analysis in whole early embryos; the gray bar represents analysis in head complex tissues of the first instar larva, and the blue bar represents analysis in head complex tissues of third instar larva. Control and dElys depletion graphs are mentioned with different shades in the image. Data are represented from at least three independent experiments. The error bar represents the standard deviation. *** represents p < 0.0001, ** represents p < 0.001, and * represents p < 0.05. D, Dorsal nuclear localization assessed in control and nucleoporin knockdown third instar larval salivary glands (ubiquitous Act5C–GAL4 driven). DNA is stained with DAPI. Scale bar, 5 μm. Control is WT flies crossed with the Act5C–GAL4 driver. RNAi-mediated knockdown of Nup160GD and Nup107GD was carried out at 23 °C to obtain third instar larva. E, quantitation of nuclear/cytoplasmic intensity ratio of Dorsal in control and nucleoporins knockdown salivary gland nuclei (ubiquitous Act5C–GAL4 driven). Data are represented from at least three independent experiments. At least 45 nuclei were analyzed from 7 to 8 pairs of salivary glands. Statistical significance derived from one-way ANOVA followed by Tukey's post hoc test. The error bar is S.E. *** represents p < 0.0001, and * represents p < 0.05; ns is nonsignificant.
We next asked whether this nuclear re-localization of Dorsal is specific to dElys depletion or a general consequence of a lack of properly-assembled nuclear pores. To check this, we investigated Dorsal localization in salivary gland nuclei from the knockdown of critical nucleoporins nup160, nup133, nup107, and nup153. Members of the Nup107 complex play a key regulatory role in post-mitotic and interphase NPCs assembly, although Nup153 is a critical molecule for interphase NPCs assembly only (46–48). RNAi-mediated depletion of nup160 and nup107 caused lethality at the first instar larval stage; however, when reared at 23 °C, organisms from these crosses could survive until third instar larva. The salivary gland nuclei from nup160 and nup107 depletion do not show significant accumulation of Dorsal inside the nucleus (nuclear/cytoplasmic Dorsal: control, 1.00 ± 0.05, and nup160GD, 0.95 ± 0.02; nup107KK: 1.05 ± 0.02) (Fig. 5D). When compared with control, nup153 depletion shows no significant difference in Dorsal signals inside the nuclei. Only nup133 depletion showed little accumulation of nuclear Dorsal, which is still significantly lower than dElys (nuclear/cytoplasmic Dorsal: nup153TRiP, 1.11 ± 0.03, and nup133KK, 1.57 ± 0.10) (Fig. 5D). The knockdown of these nucleoporins suggests a drastic loss in their expression (Fig. S6, B–E). mAb414 staining of salivary gland nuclei indicated that removal of these other nucleoporins had induced NPC assembly defects (Fig. S7). Accordingly, the mRNA export was also perturbed that led to accumulation of mRNA inside the nucleus (nuclear mRNA: control, 1.00 ± 0.11; dElysKK, 1.87 ± 0.23; nup160GD, 3.68 ± 0.28; nup107KK, 7.29 ± 0.59; and nup153TRiP, 3.84 ± 0.28) (Fig. S8, A and B). Moreover, GFP–NLS import assay showed increased GFP–NLS signals in the cytoplasm suggesting that the import process is also affected (cytoplasmic/nuclear GFP–NLS: control, 1.00 ± 0.08; dElysKK, 1.85 ± 0.07; nup160GD, 2.32 ± 0.19; nup107KK, 2.13 ± 0.15; and nup133KK, 2.03 ± 0.08) (Fig. S8, C and D). Quantitation of nuclear intensities of the Dorsal signal demonstrates that the Dorsal signal inside the nucleus increased by ∼5-fold upon dElys depletion, but the same remains largely unchanged upon depletion of other tested nucleoporins involved in NPC assembly (Fig. 5E). We suggest that the resurrected residence of the Dorsal inside the nucleus is dElys-specific and not the outcome of the absence of functional NPCs in the nuclear envelope.
dElys depletion-induced apoptosis is Dorsal-mediated
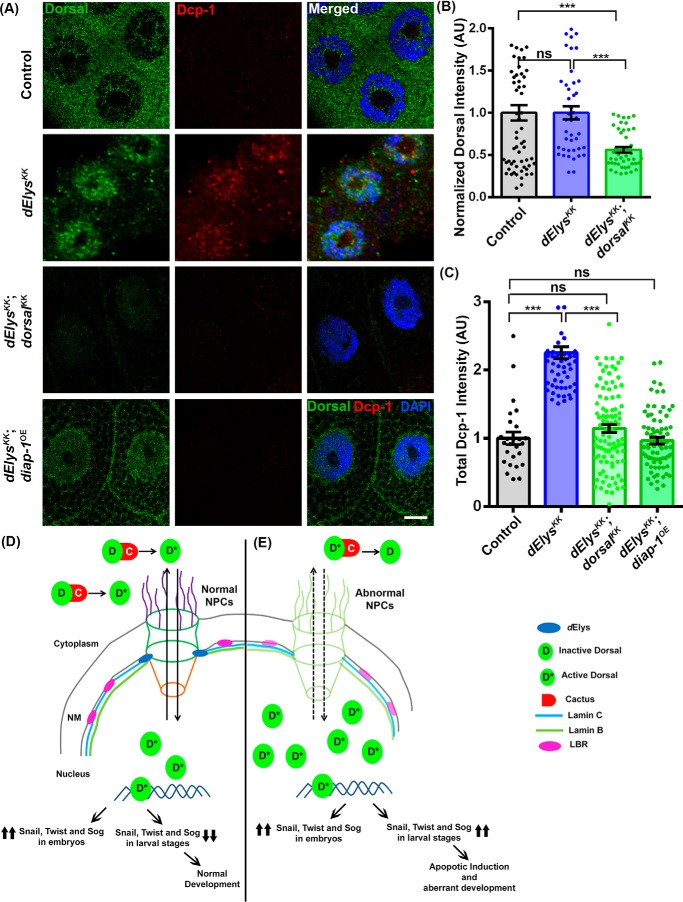
We next examined whether the apoptosis and the nuclear localization of Dorsal are independent consequences of dElys depletion. We have created a genetic combination of dElys depletion in the dorsal null background, but the embryos showed lethality at the early embryonic stage (∼stage 2) and hence could not be used in any relevant study (Fig. S9A). Analysis of dElys-depleted or dorsal null organisms revealed increased apoptosis (Dcp-1 staining) by embryonic stage 11 as compared with control (Fig. S9B). Hence, we employed the combined knockdown of dElys and dorsal and analyzed the apoptotic response from co-depleted salivary glands. In control depletion, Dorsal remained cytoplasmic, and no Dcp-1 staining was observed (Fig. 6, A, 1st horizontal panels, B, and C). However, dElys depletion induced accumulation of Dorsal, and the enhanced punctate staining of Dcp-1 was observed (Fig. 6, A, 2nd horizontal panels, B, and C). When dElys and dorsal were co-depleted, nuclear levels of Dorsal and punctate Dcp-1 staining both reduced significantly in salivary gland tissues (total Dorsal intensity: control, 1.00 ± 0.09; dElysKK, 1.00 ± 0.07; dElysKK: dorsalKK, 0.56 ± 0.03; and total Dcp-1 intensity: control, 1.00 ± 0.09; dElysKK, 2.25 ± 0.08; dElysKK: dorsalKK, 1.14 ± 0.05) (Fig. 6, A, 3rd horizontal panels, B, and C).
Figure 6.
Dorsal depletion and diap-1 overexpression can rescue dElys depletion apoptotic effects. A, apoptotic induction assessed in control (1st horizontal panels), dElys RNAi (2nd horizontal panels), dElys, dorsal co-depletion (ubiquitous Act5C–GAL4-driven, 3rd horizontal panels), and dElys RNAi; diap-1 overexpressed (salivary gland–specific expression, 4th horizontal panels). Third instar larval salivary gland tissues were isolated from above genetic combination of organisms and stained with Dorsal (green) and Dcp-1 (red) antibodies. DNA is stained with DAPI. Scale bar, 5 μm. Control is WT flies crossed with the Act5C–GAL4 driver as well as salivary gland–specific drivers. B and C, quantitation of signal intensity of Dorsal (B) and Dcp-1 (C) in the indicated samples. Data are derived from at least three independent experiments. At least 45 nuclei were analyzed from 7 to 8 pairs of salivary glands. Statistical significance was derived from one-way ANOVA followed by Tukey's post hoc test. Error bars represent S.E. *** represents p < 0.0001, and ns is nonsignificant. A theoretical model integrating the observations of the study is shown. D, under normal conditions, dElys initiates postmitotic NPC assembly leading to functional nuclear pore in the nuclear membrane and normal organization of the nuclear lamina. Nuclear-cytoplasmic shuttling of Dorsal occurs conventionally leading to normal development. Dorsal is transported back to the cytoplasm through functional nuclear pores after completing the transcriptional regulation of key target genes in the nucleus. Inside cytoplasm, Dorsal is kept inactive by binding with Cactus, by certain post-translational modifications, or may be degraded. E, under dElys-depleted condition, nucleoporins and nuclear lamina components are mislocalized. The NPC assembly and functional nuclear pore formation are perturbed, and the nuclear lamina organization also is compromised. Dorsal translocates into the nucleus during early developmental stages, exits as normal, but during larval stage re-enters the nucleus due to the defective nucleo-cytoplasmic shuttling of certain post-translational regulators of Dorsal in the nucleus or loss of its independent interaction with dElys. Resurrected Dorsal inside nucleus activates transcription of Dorsal target genes which rather remain shut during the post-embryonic stage. The mistimed transcription of developmentally regulated proteins may induce apoptosis resulting in lethality and developmental defects. Thus, the presence of dElys is critical for Dorsal localization, growth versus apoptosis fate choice and to the conventional developmental program of the organism.
We next examined whether apoptosis can be rescued by overexpressing diap-1 (diap-1OE) in the dElys knockdown. As ubiquitous overexpression of diap-1 is lethal, we used localized expression using salivary gland-specific drivers, Fkh-GAL4; C-147-GAL4. Salivary gland-specific dElys depletion elicited the Dcp-1 signal, but the diap-1 overexpression in the dElys depletion background brings the Dcp-1 levels back to normal, subsiding the apoptotic response (total Dcp-1 intensity: dElysKK, diap-1OE, 0.96 ± 0.04) (Fig. 6, A, 4th horizontal panel, and C). We further intended to rescue this phenotype in adult wings by using nubbin-GAL4; Ubx-GAL4 for expressing diap-1 in dElys depletion background. We could partially rescue the apoptosis and wing phenotypes when diap-1 is expressed in wing imaginal discs (Fig. S10A) of dElys knockdown (dElysKK; diap-1OE) organisms. The disc shape appears normal, and the Dcp-1 staining is reduced in the wing pouch region of the wing imaginal disc from dElysKK; diap-1OE organisms as compared with dElysKK organisms (total Dcp-1 intensity in Wing imaginal discs: control, 1.00 ± 0.30, and dElysKK, 6.42 ± 1.42; dElysKK: diap-1OE, 3.41 ± 0.42) (Fig. S10, B and C). The wings in adults arising from this genetic combination show improved morphology compared with dElys depletion (Fig. S10D). We attributed this partial rescue to the weak expression of Ubx-Gal4 in the wing-pouch area, which forms the major surface of wings. Together, our data strongly suggest that the induction of apoptosis upon dElys depletion is Dorsal-mediated, and the abundance of DIAP-1 counters the apoptotic phenotypes of dElys depletion.
Discussion
ELYS was found in stable association with the Nup107 complex of nuclear pores (6–8), and studies revealed that ELYS recruits Nup107 complex to the kinetochores and helps in regulation of Nup107 complex-mediated microtubule formation at the kinetochores (12, 49, 50). The biochemical characterization of AT-hook–like motif in the Drosophila ELYS ortholog (dElys) encoded by CG14215 has echoed the view that positively-charged amino acid residues play an important role in the DNA binding (Fig. 1), similar to what has been observed with noncanonical AT-hook of Drosophila TAF-1 (25). The DNA-binding ability enables ELYS to initiates NPC assembly at a precise location and not at ectopic sites (11, 49, 51). The conserved localization of dElys to the nuclear rim in interphase also suggests a conserved function of dElys in Drosophila. However, unlike other characterized ELYS molecules (8, 12, 49, 50), dElys is absent from kinetochores and appears to envelop chromatin in mitosis (Fig. 1). The absence of dElys from the kinetochores provides a suitable explanation for the lack of Drosophila Nup107 complex from the kinetochore during mitosis (23). Although dElys and Sec13 (a dNup107 complex member) localize to the active chromatin regions (52), it remains to be elucidated whether they both interact physically.
The ubiquitous knockdown of dElys, induced developmental defects and lethality in Drosophila (Fig. 2) mirroring the defects observed in other organisms following the conserved function of ELYS molecules (6, 11, 53, 54). The dElys phenocopies nucleoporin functions of the ELYS molecule characterized in other organisms and is involved in nucleo-cytoplasmic transport regulation and NPC assembly and interacts with nuclear lamina proteins (Fig. 3) as reported in mammalian cell lines, C. elegans and Xenopus egg extract studies (6, 7, 9, 16, 28, 53), suggesting a conserved functional role in Drosophila. Nucleo-cytoplasmic transport defect observed in dElys depletion highlights its conserved role as a nucleoporin (7, 10, 55–57). We observed a conserved role for dElys in LBR recruitment to help organize the nuclear lamina, similar to what has been reported previously (17, 28). Importantly, we report the mislocalization of both lamin B and C from the nuclear lamina upon dElys depletion (Fig. 3). We attribute this mislocalization of lamin B and C to the loss of Ran from the nuclear periphery due to the probable reduction of Nup358 upon dElys loss. RanGTP helps in the dissociation of lamin from the lamin–importin α/β complex for incorporation into the nuclear lamina (58). Loss of Ran from the nucleus in dElys depletion might have hampered the dissociation of the lamin–importin α/β complex, in turn affecting nuclear lamina assembly in these nondividing salivary gland cells. This aspect of nuclear lamina assembly regulation by dElys requires detailed investigation.
ELYS is known to initiate post-mitotic NPC assembly across different model organisms (6–11, 15, 49). Assembly of the NPCs, in turn, regulates the proper distribution of inner nuclear membrane proteins leading to correct nuclear architecture regulating proper nuclear transport (28, 59). So, NPC assembly, LBR localization, lamina assembly, and nuclear transport can be linked to nuclear pore–associated function of ELYS in Drosophila.
Dorsal (NF-κB) is a developmentally-regulated key transcription factor, and its nuclear localization and regulated activation of its downstream pathways are critical for normal development (34). The induction of the Dorsal signaling pathway plays an important role in the growth and development of the organism (60, 61). The ectopic presence of Dorsal inside the nucleus of dElys-depleted salivary gland tissues (Fig. 4) is in direct contrast to the presence of Dorsal inside the nucleus only during early embryonic stages or a microbial infection. It is rather uncommon for Dorsal to be present inside the nucleus in later stages of Drosophila development and could suggest an uncharacteristic Dorsal pathway activation. The Dorsal present inside the dElys-depleted third instar salivary gland nuclei is active and transcribes growth-promoting target genes snail, twist, and sog. Expression of these genes is generally restricted to embryonic stages of development. Besides, the expression of pro-apoptotic genes reaper and hid is also induced (Fig. 4). The snail, twist, and sog genes are known to induce apoptosis when expressed in an unregulated manner (43, 44). In accordance, an increase in apoptosis is evident in dElys-depleted salivary gland tissues (Fig. 4). The two contrasting paradigms of gene expression can generate a developmental imbalance resulting in apoptosis by a hitherto unknown mechanism. Dorsal can induce apoptotic responses, and emerging ideas suggest that Dorsal, depending on cues, helps in growth versus apoptotic fate choices (62). Thus, dElys depletion can regulate effective nucleo-cytoplasmic transport of important transcriptional regulators to induce apoptosis. Importantly, no other nucleoporin tested in our study, when depleted, phenocopied the Dorsal accumulation inside the nucleus (Fig. 5). These results emphasize a strong pore-independent function for dElys in Dorsal localization. Detailed analysis exploring the interactions between dElys, Dorsal, and upstream players of the Dorsal pathway will shed more light on nuclear pore independent functions of dElys. The apoptotic consequence of an aberrant Dorsal pathway activation seems to be a signature of dElys nucleoporin in Drosophila. Nucleoporin depletion and apoptosis are not uncommon, and our observation aligns with the apoptosis induction in astrocytoma cells when nup107 is depleted (63).
Our observation regarding the Dorsal pathway activation is in direct contrast to what is known for another nucleoporin mbo (Nup88) in Drosophila. The deletion of the mbo did not affect the general nuclear import/export and nuclear lamina assembly. Nup88 interacted feebly with Dorsal, but more importantly, nuclear levels of activated Dorsal were reduced in response to infection when Nup88 was deleted (64). Our observation can imply that in addition to genetic interaction, dElys may possess a physical interaction with Dorsal or Cactus. Nup88, along with other nucleoporins like Nup62, Nup153, and Nup214, plays important roles in developmental pathways (65–67), but a detailed mechanistic understanding of selective nuclear translocation of activated signals are lacking. ELYS targets protein phosphatases to the nucleus, which can dephosphorylate and export molecules back to the cytoplasm (18). Structural and functional conservation of dElys may suggest that dElys perhaps regulates the localization of an orthologous phosphatase to control the nucleo-cytoplasmic shuttling of Dorsal in Drosophila. It is also very likely that through phosphatases, dElys may regulate the phosphorylation-mediated degradation of Cactus, resulting in the nuclear translocation of Dorsal. It will be of great interest to identify whether such a phosphatase is involved in the shuttling process of Dorsal in Drosophila. Our results highlight the key difference between dElys and other nucleoporins and affirm a role for dElys in developmental signaling in addition to NPC and nuclear lamina organization.
NF-κB family transcription factors play a critical role in normal growth and development. Their dysregulation leads to cancer, chronic inflammatory disease, and developmental defects (68, 69). WntD, the product of Dorsal/Twist/Snail Signaling, is a feedback inhibitor of Dorsal signaling. WntD inhibits the nuclear translocation of Dorsal, thus inactivating expression of downstream targets (70, 71). Our report highlights that the novel role of the nucleoporin, dElys, in Dorsal regulation during the later stages of development is an addition to the growing pool of reports for molecules involved in the regulation of Dorsal signaling. Unlike WntD, dElys is a novel intracellular regulator of Dorsal signaling.
We suggest that dElys levels help maintain a tight balance between pro-growth and pro-apoptotic responses to achieve normal growth and development in Drosophila. dElys does regulate development and could contribute to normal growth in post-mitotic, nondividing cells by keeping the NF-κB trigger under control in a spatiotemporal manner (Fig. 6).
Our study thus provides an initial hint to a novel but yet-to-be-identified pathway that involves noncanonical AT-hook–like motifs containing dElys to regulate the spatiotemporal distribution of Dorsal to ensures proper growth and development in Drosophila. Suppression of Dorsal signaling after the embryonic stage by dElys is an added layer of regulation to ensure proper growth and development. Moreover, there may be additional signaling cascades that are perturbed by changes in dElys levels, which require further elucidation.
Materials and methods
In silico analysis
An ortholog of ELYS in Drosophila was identified by using mouse ELYS as the reference sequence. PSI-BLAST with three iterations using mouse ELYS and its AT-hook motif sequence was used against the nonredundant protein database at NCBI with default parameters. ELYS orthologs in other organisms were identified by similar searches in the NCBI database. T-coffee, a multiple sequence alignment server, was used for alignment (72). The aligned sequence was then used for visualization in Jalview and highlighted with a Clustal X color scheme. The phylogenetic tree was inferred from this analysis using Jalview (73). Protein sequence motifs were identified using the SMART database for the representative sequence from each organism (74). IBS was used to draw a scaled protein illustration for each protein (75). Percentage identity was calculated against mouse ELYS as a reference for each ELYS-like molecule. Secondary structure for CG14215 was predicted using PSIPRED version 3.3, DisoPRED, and DomPRED (76). Predicted secondary structure for N-terminal and the central helical domain was compared with the experimentally-proven secondary structure of human and mouse ELYS (3). 3D structure of NTD of CG1425 was modeled using a Phyre2 algorithm and mouse ELYS NTD structure as a template (21).
Fly strains and genetics
All flies were reared at 25 °C on standard corn meal–yeast–agar medium. RNAi crosses were grown at 28 °C for better expression of GAL4. nup160GD and nup107GD crosses were grown at 23 °C to obtain third instar larva. The RNAi line (KK 103547) for CG14215 was obtained from the VDRC. Other fly lines used in this study were obtained from the Bloomington Drosophila Stock Centre (BDSC) at Indiana University or VDRC. Controls used in this study are F1 progeny from Driver line crossed with W1118 flies. Fly lines used in this study are mentioned in Table S1. All the combinations and recombinations are made with standard fly genetics. Tissue-specific knockdown of dElys was achieved by Act5C–GAL4, Ey-GAL4, Wg-GAL4, Fkh-GAL4, mat–α-tub–GAL4 drivers obtained from BDSC. For maternal knockdown F1, virgin females from the cross of RNAi and mat–α-tub–GAL4 was crossed with RNAi males, and early embryos were collected from this cross.
Cloning and transgenic fly generation
We obtained the clone LD14710 containing the full-length CG14215-coding sequence in the pBluescript SK(−) vector from Drosophila Genomics Resource Centre (DGRC). This clone was used as a template for cloning full-length CG14215 into pTVW (Vector Laboratory, No. 1091), a fly transformation vector with gateway cassette, UASt promoter, and N-terminal EYFP tag as well as in pTW (Vector No. 1129) vector containing gateway cassette and UASt promoter (obtained from DGRC gateway-1 collection). Cloning was done using the Gateway cloning kit as per the manufacturer's instructions (Thermo Fisher Scientific). RNAi lines against CG14215 were generated by cloning 503 base pairs from exon 7 of CG14215 predicted using SnapDragon algorithm at the Drosophila RNAi screening center website (FlyRNAi.org), which do not have any predicted off-targets (77), and cloned into the gateway-based pVALIUM10 RNAi vector obtained from Drosophila RNAi screening center (TRiP). Transgenic flies were generated at the Fly Facility of the Centre for Cellular and Molecular Platforms at the National Center for Biological Sciences (C-CAMP-NCBS), Bangalore, India. Primers used in this study are mentioned in Table S2.
Drosophila S2 cell culture and transfections
Drosophila S2 cells were grown in Schneider's Drosophila medium (Gibco, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific) and 50 units/ml penicillin, 50 units/ml streptomycin, and 25 μg/ml amphotericin B (Gibco, Thermo Fisher Scientific) at 25 °C in a CO2-free incubator. Full-length CG14215 was cloned in pAVW (Vector No. 1087) from DGRC. S2 cells were transfected at 50% confluency with Effectene transfection reagent (Qiagen). Cells were grown for 3 days post-transfection. S2 cells were arrested in metaphase of mitosis by the addition of 20 μm MG-132 (RTU solution, Sigma) for 2 h at 25 °C.
Antibody generation and Western blotting
The C-terminal 343 amino acids of CG14215, which is a unique and most antigenic and hydrophilic part, were amplified from the LD14710 clone and subcloned into pET28a(+) vector. Protein was expressed in Escherichia coli BL21 (DE3) cells, induced using 200 μm IPTG (Sigma), and incubated at 18 °C overnight. Cells were pelleted down, lysed in 100 mm NaH2PO4, 10 mm Tris-HCl buffer, pH 8.0, containing 8 m urea and 1% Triton X-100, 50 μg/ml lysozyme, and 1× protease inhibitor mixture (Roche Applied Science). The recombinant protein was purified over Ni-NTA beads and eluted with low pH at 4.5 in 100 mm NaH2PO4, 10 mm Tris-HCl buffer, pH 8.0, containing 8 m urea. Protein was dialyzed against 100 mm NaH2PO4, 10 mm Tris-HCl buffer, pH 8.0, containing a decreasing amount of urea up to 2 m. Protein was concentrated using a centrifugal concentrator of 30 kDa (Amersham Biosciences, GE Healthcare). Protein was flash-frozen in liquid nitrogen and stored at −80 °C. Polyclonal antibody against CG14215 was generated in the rabbit at Abgenex, Bhubaneswar, Odisha, India. Antibodies were affinity-purified over purified antigens chemically cross-linked to N-hydroxysuccinimidyl-Sepharose beads (Sigma) and eluted with low pH, neutralized, and dialyzed against PBS overnight at 4 °C. Antibody against full-length dNup43 was also generated using an identical protocol.
Larval head complexes were dissected in cold PBS from third Instar larva and lysed in Laemmli buffer. A total of two equivalent head complexes were loaded in each well of the gel. Drosophila S2 cells were pelleted down and lysed in 50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 1 mm MgCl2,1 mm EDTA, 10% glycerol, 0.4% sodium deoxycholate, 1% Triton X-100, 0.5% SDS, 1% Nonidet P-40, 2× Protease inhibitor cocktail (PIC) (modified from Ref. 78). 5 and 10 μg of total protein was resolved onto 8% SDS-PAGE and transferred to methanol-activated polyvinylidene difluoride membrane (Merck-Millipore). Polyclonal anti-dElys antibody was used at 1:1000, and anti-Nup43 antibody (1:500), anti-Nup98 (1:2500), anti-lamin B (1:2500), anti-lamin C (1:2500), anti-mAb414 (1:5000), anti-Ran (1:5000), and anti-α-tubulin (1:5000) dilutions with overnight incubation at 4 °C were used. The horseradish peroxidase–coupled secondary antibody was used at 1:15,000 dilutions for the detection of proteins in Western blotting developed with SuperSignal West Pico chemiluminescent substrate (Pierce, Thermo Fisher Scientific).
Immunostaining
In vivo localization of dElys was revealed by immunostaining of Drosophila embryos. mat–α-tub–GAL4-driven W1118 and dElysKK-staged syncytial blastoderm embryos were collected on apple juice agar plates for 1 h and aged to 2.5–3 h at 25 °C. Embryos were processed, as mentioned elsewhere (79, 80). Briefly, embryos were dechorionated in a 1:1 solution of sodium hypochlorite (∼4% w/v available chlorine) and water until appendages from 80% of embryos disappear. Embryos were washed thoroughly in embryo wash buffer containing 0.2% NaCl and 0.05% Triton X-100 and fixed with 4% formaldehyde in heptane for 45 min at room temperature. Embryos were then devitellinized in 100% methanol for 30 min at room temperature. Embryos were blocked in 5% neutralized goat serum (The Jackson Laboratory). Processed embryos were then used for immunostaining with the anti-dElys antibody (1:1000), mAb414 (1:500, Bio-legend), and anti-Dorsal (1:20, 7A4, DSHB). Embryos were mounted in Vectashield mounting medium (Vector Laboratories).
For immunostaining of Drosophila salivary glands, ubiquitous Act5C–GAL4-driven first, second, or third instar larvae were dissected in cold PBS for isolation of salivary glands. Glands were fixed in freshly prepared 4% formaldehyde for 30 min at room temperature and washed thoroughly with 0.2% PBST (PBS + 0.2% Triton X-100). Salivary glands were blocked with 5% neutralized goat serum (The Jackson Laboratory) and stained with the following: anti-dElys (1:1000), anti-dNup43 (1:250), and mAb414 (1:500, Bio-legend); anti-Ran (1:5000, BD Biosciences); anti-lamin Dm0 (1:1000, ADL67.10, DSHB, deposited by Prof. Paul A. Fisher, Stony Brook University (81)); anti-lamin C (1:2000, gift from Prof. Paul A. Fisher, Stony Brook University (81)); anti-LBR (1:500, gift from Prof. George Krohne, University of Wurzburg, Germany (82)); anti-Nup98 (1:1000, gift from Dr. Cordula Schulz, University of Georgia (83)); anti-TBP (1:200 (30)) for overnight at 4 °C and also probed with anti-Dorsal (1:20, 7A4, DSHB (34)), 1:50 for Dorsal antibody gift from Dr. Zeitlinger (Stowers Institute); and anti-Cactus (1:50, 3H12, DSHB (34)). For apoptotic induction, salivary glands were probed with an anti-cleaved-Dcp-1 antibody (1:100, Cell signaling technology, #9578 (38)). Eye and wing imaginal discs were dissected from third instar larva and processed as described above for probing of apoptotic induction with the Dcp-1 antibody. Secondary antibodies used were anti-rabbit Alexa Fluor 568 (1:800, Thermo Fisher Scientific), anti-mouse Alexa Fluor 488 (1:800, Thermo Fisher Scientific), anti-guinea pig FITC (1:400, Jackson laboratories), anti-mouse Cy5 (1:500, Jackson Laboratories). DNA was stained with DAPI (1:5000, Thermo Fisher Scientific). Salivary glands were mounted in Vectashield mounting medium (Vector Laboratories).
Drosophila S2 cells were immunostained by immobilizing on 0.25 mg/ml concanavalin A–coated coverslips for 2 h at 25 °C. MG-132–arrested cells were fixed by 100% methanol at −20 °C, blocked with 5% neutralized goat serum (The Jackson Laboratory), and immunostained anti-dElys (1:1000), anti-α-tubulin (1:1000, DSHB), and anti-CID (1:1000, a gift from Prof. Steve Henikoff, Fred Hutchinson Cancer Research Center, Seattle, WA (22)). All the steps of immunostaining of S2 cells were followed as per Ref. 84.
All the samples were imaged on Carl Zeiss LSM 780 up-right confocal microscope equipped with a ×63/1.4 N.A. oil immersion lens. Super-resolution microscopy of anti-CID antibody-labeled EYFP–dElys transfected cells was done with a Carl Zeiss LSM 800 Airyscan inverted microscope. Images were processed for super-resolution in the in-built mathematical algorithm of Zen.2 software of LSM 800 Airyscan. Images were processed with ImageJ (National Institutes of Health) or Fiji software and Adobe Photoshop CS6 (Adobe Corp.).
Intensity quantification
Average signal intensities for each molecule were measured by using Zen-2012 (Carl Zeiss, Image Analysis software) or Fiji software, and graphs were plotted with GraphPad software (Prism). For the quantification of Dorsal and GFP–NLS nuclear localization, five different regions of interest (ROI) were defined per nucleus and the cytoplasm around it. The average intensity from each ROI was measured using Zen-2012 (Carl-Zeiss, Image Analysis software), and the mean of those intensities was taken per nucleus and cytoplasm around it. The ratio of the nucleus and cytoplasmic mean intensity was calculated for the final graph. Dcp-1 signal intensities were measured using five ROIs per cell, and the mean of that is used for the final graph. Nuclear rim localization of different molecules was calculated as average intensity per nucleus, including the nuclear rim. All the experiments were performed as at least three independent replicates.
Bright-field microscopy
For imaging of eye and wing phenotype of dElys knockdown flies, 3 days old flies were anesthetized with diethyl ether (Merck) and immobilized on sticky gum for proper orientation of Drosophila eye. At least 10 flies from each knockdown experiment were imaged from three independent experiments for each genotype. Drosophila eyes were imaged using Leica fluorescent stereomicroscope M205 FA using a ×123 magnification of particular equipment. For wing imaging in case of wing phenotypes with a dElys knockdown and dElysKK; diap-1OE, 3-day-old fly wings from each genotype were dissected and placed on a glass slide under a coverslip and imaged directly under Leica upright light microscope DM2500 with a ×10 magnification (85). At least 15 pairs of wings from each genotype were examined from three independent crosses.
Scanning EM (SEM)
Drosophila eyes from eye-specific knockdown of dElys were imaged using a scanning electron microscope for detailed analysis of eye structure perturbations. Three-day-old flies from each RNAi cross was processed as per Ref. 86. Briefly, flies were anesthetized and fixed with 2.5% glutaraldehyde in PBS for 2 h at 4 °C. Flies were washed thoroughly twice with PBS+ 4% sucrose. Flies were dehydrated through a graded ethanol series and were subjected to critical point drying. Samples were mounted on aluminum stubs with carbon-conductive tape. Flies were coated with gold particles in a sputter coating apparatus. Samples were imaged using a Carl Zeiss Gemini II FESEM microscope. At least 10 flies from each genotype were imaged from three independent experiments.
In vitro DNA binding experiment
To analyze the DNA-binding activity of AT-hook–like motifs of dElys, we purified a fragment spanning all three AT-hook–like DNA-binding motifs by cloning the C-terminal 104 amino acids (1858–1961) from clone LD14710 into pET28a (+) vector. The His6-tagged AT-hook motif fragment was purified by expressing in E. coli BL21 (DE3) cells. Protein was induced by 200 μm IPTG (Sigma) overnight at 18 °C. Cells were lysed in lysis buffer containing 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 20 mm imidazole, 1% Triton X-100, and 50 μg/ml lysozyme on ice. Protein was purified over Ni-NTA beads by eluting in 300 mm imidazole in 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm MgCl2, and 0.5 mm EDTA. Protein was dialyzed overnight against dialysis buffer, 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm MgCl2, and 0.5 mm EDTA.
For site-directed mutagenesis of arginine residues, we used Q5 site-directed mutagenesis kit (New England Biolabs) with primers coding mutated nucleotides. Primers used for mutagenesis are mentioned in Table S2. The clone described above was used as a template for site-directed mutagenesis where conserved arginines were mutated to alanine as shown in Fig. S2. Mutations were confirmed using DNA sequencing (IISER-Bhopal sequencing facility). Clones were transformed into E. coli BL21 (DE3) cells, and proteins were purified as mentioned earlier.
In vitro DNA-binding experiment was performed by incubating the template that contains 50% AT richness and 90% AT richness with purified WT and mutant proteins. A known cytosolic protein that does not bind to DNA was used as a negative control. DNA binding experiment was performed in binding buffer containing 100 mm Tris, 500 mm KCl, 10 mm DTT, pH 7.5, for 30 min at room temperature and analyzed onto a 0.8% agarose gel and detected by a UV transilluminator (UVP). Purified DNA used for the binding experiment is 5 nm, and the total purified protein was used at 0.5 μm concentrations. EMSA was performed by using the Lightshift Chemiluminescent EMSA kit (Pierce, Thermo Fisher Scientific) by using Sdic DNA oligonucleotides as described previously (25).
Total mRNA fluorescence in situ hybridization (mRNA-FISH)
Total mRNA-FISH was performed as per Ref. 87, with some modifications. Salivary glands from third instar larvae were dissected and fixed in 4% paraformaldehyde for 20 min at room temperature followed by the second fixation in 100% methanol for 10 min at 4 °C. Tissues were dehydrated in graded ethanol series for 5 min each. Cy3-labeled poly(dT) oligonucleotides (IDT) were denatured in hybridization buffer containing 50% formamide, 2× SSC (saline sodium citrate), 10% dextran sulfate, and 0.05% BSA at 70 °C for 5 min. Denatured poly(dT)–Cy3 was added at 0.1 pmol/μl concentration to the fixed tissue and incubated overnight at 37 °C for hybridization. Tissues were washed in 2× SSC twice. DAPI was added for staining DNA and mounted in Vectashield. Tissues were imaged using the LSM 780 laser-scanning confocal microscope.
Quantitative-PCR
Total RNA was isolated from control and dElys knockdown with either mat–α-tub–GAL4-driven early embryos or ubiquitous Act5C–GAL4-driven first and third instar larval head complex using total tissue RNA isolation kit (Favorgen Biotech). One μg of total RNA was used to synthesize cDNA using iScript cDNA synthesis (Bio-Rad). cDNA was diluted five times, and 1 μl of cDNA from each genotype was used as a template. Semi-quantitative PCR was done using gene-specific primers and RpL49 as control. Real-time PCR on the same cDNA was done in Lightcycler 480 (Roche Applied Science) at standard cycling conditions and probed with SYBR Green (Bio-Rad) in real-time using dElys and actin RT primers. Quantification of each reaction was done by calculating ΔCT values. ΔCT was normalized against CT values of actin. The graph was plotted as fold change in dElys expression using GraphPad software (Prism). Primers used in quantitative PCR are mentioned in Table S2.
Hatching rate analysis
Embryos from control and dElys RNAi-driven with mat–α-tub–GAL4 were collected for 5 h and aged at 28 °C for 48 h after counting the total number of embryos. After 48 h, the number of intact, unhatched embryos were counted manually under the stereoscope. The percentage of unhatched embryos was determined by the ratio of unhatched embryos to the total number of embryos collected. Each experiment was performed with at least three independent replicates.
Author contributions
S. J. K. M. and R. K. M. conceptualization; S. J. K. M., V. K., and R. K. M. formal analysis; S. J. K. M. investigation; S. J. K. M. visualization; S. J. K. M., V. K., and R. K. M. methodology; S. J. K. M. and R. K. M. writing-original draft; S. J. K. M., V. K., and R. K. M. writing-review and editing; V. K. and R. K. M. resources; V. K. and R. K. M. supervision; R. K. M. funding acquisition; R. K. M. validation; R. K. M. project administration.
Supplementary Material
Acknowledgments
We thank the Bloomington Drosophila Stock Centre (BDSC) and Vienna Drosophila Resource Centre (VDRC) for fly lines used in this study; the DGRC for a genomic clone of CG14215; and the Developmental Studies Hybridoma Bank (DSHB) and the University of Iowa for monoclonal antibodies. We thank Prof. Paul A. Fisher (Stony Brook University) for anti-lamin C; Prof. George Krohne (University of Wurzburg) for anti-LBR; Dr. Cordula Schulz (University of Georgia) for the anti-Nup98; Prof. Steve Henikoff (Fred Hutchinson Cancer Research Center, Seattle, WA) for anti-CID; Dr. Julia Zeitlinger (Stowers Institute, Kansas City, MO) for anti-Dorsal, and Dr. R. S. Tomar (Indian Institute of Science Education and Research-Bhopal) for the anti-TBP antibody. S. J. M. thanks Neha Upadhyay for help with real-time PCR and analysis. We thank the Indian Institute of Science Education and Research-Bhopal Central Instrumentation Facility for DNA sequencing, SEM, and confocal microscopes.
This work was supported by an extramural grant from the Science and Engineering Research Board (SERB), Ministry of Science and Technology, Government of India, Grant EMR/2016/001819 (to R. K. M.) and Intramural Funding from the Indian Institute of Science Education and Research-Bhopal. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S10 and Tables S1 and S2.
- ELYS
- embryonic large molecule derived from yolk sac
- CID
- centromere identifier
- DAPI
- 4′,6-diamidino-2-phenylindole
- ANOVA
- analysis of variance
- NPC
- nuclear pore complex
- NLS
- nuclear localization signal
- BDSC
- Bloomington Drosophila Stock Centre
- EYFP
- enhanced yellow fluorescent protein
- aa
- amino acid
- Ni-NTA
- nickel-nitrilotriacetic acid
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- LBR
- lamin B-receptor
- VDRC
- Vienna Drosophila Resource Centre
- ROI
- regions of interest
- oligo
- oligonucleotide
- FISH
- fluorescence in situ hybridization
- SEM
- scanning electron microscopy
- DGRC
- Drosophila Genomics Resource Centre.
References
- 1. Kimura N., Takizawa M., Okita K., Natori O., Igarashi K., Ueno M., Nakashima K., Nobuhisa I., and Taga T. (2002) Identification of a novel transcription factor, ELYS, expressed predominantly in mouse foetal haematopoietic tissues. Genes Cells 7, 435–446 10.1046/j.1365-2443.2002.00529.x [DOI] [PubMed] [Google Scholar]
- 2. Okita K., Nobuhisa I., Takizawa M., Ueno M., Kimura N., and Taga T. (2003) Genomic organization and characterization of the mouse ELYS gene. Biochem. Biophys. Res. Commun. 305, 327–332 10.1016/S0006-291X(03)00772-1 [DOI] [PubMed] [Google Scholar]
- 3. Bilokapic S., and Schwartz T. U. (2013) Structural and functional studies of the 252-kDa nucleoporin ELYS reveal distinct roles for its three tethered domains. Structure 21, 572–580 10.1016/j.str.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hetzer M. W. (2010) The nuclear envelope. Cold Spring Harb. Perspect. Biol. 2, a000539 10.1101/cshperspect.a000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoelz A., Debler E. W., and Blobel G. (2011) The structure of the nuclear pore complex. Annu. Rev. Biochem. 80, 613–643 10.1146/annurev-biochem-060109-151030 [DOI] [PubMed] [Google Scholar]
- 6. Franz C., Walczak R., Yavuz S., Santarella R., Gentzel M., Askjaer P., Galy V., Hetzer M., Mattaj I. W., and Antonin W. (2007) MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 8, 165–172 10.1038/sj.embor.7400889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galy V., Askjaer P., Franz C., López-Iglesias C., and Mattaj I. W. (2006) MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr. Biol. 16, 1748–1756 10.1016/j.cub.2006.06.067 [DOI] [PubMed] [Google Scholar]
- 8. Rasala B. A., Orjalo A. V., Shen Z., Briggs S., and Forbes D. J. (2006) ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc. Natl. Acad. Sci. U.S.A. 103, 17801–17806 10.1073/pnas.0608484103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasala B. A., Ramos C., Harel A., and Forbes D. J. (2008) Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol. Biol. Cell 19, 3982–3996 10.1091/mbc.e08-01-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandez A. G., and Piano F. (2006) MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr. Biol. 16, 1757–1763 10.1016/j.cub.2006.07.071 [DOI] [PubMed] [Google Scholar]
- 11. Gómez-Saldivar G., Fernandez A., Hirano Y., Mauro M., Lai A., Ayuso C., Haraguchi T., Hiraoka Y., Piano F., and Askjaer P. (2016) Identification of conserved MEL-28/ELYS domains with essential roles in nuclear assembly and chromosome segregation. PLoS Genet. 12, e1006131 10.1371/journal.pgen.1006131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yokoyama H., Koch B., Walczak R., Ciray-Duygu F., González-Sanchez J. C., Devos D. P., Mattaj I. W., and Gruss O. J. (2014) The nucleoporin MEL-28 promotes RanGTP-dependent γ-tubulin recruitment and microtubule nucleation in mitotic spindle formation. Nat. Commun. 5, 3270 10.1038/ncomms4270 [DOI] [PubMed] [Google Scholar]
- 13. Okita K., Kiyonari H., Nobuhisa I., Kimura N., Aizawa S., and Taga T. (2004) Targeted disruption of the mouse ELYS gene results in embryonic death at peri-implantation development. Genes Cells 9, 1083–1091 10.1111/j.1365-2443.2004.00791.x [DOI] [PubMed] [Google Scholar]
- 14. Gao N., Davuluri G., Gong W., Seiler C., Lorent K., Furth E. E., Kaestner K. H., and Pack M. (2011) The nuclear pore complex protein Elys is required for genome stability in mouse intestinal epithelial progenitor cells. Gastroenterology 140, 1547–1555.e10 10.1053/j.gastro.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davuluri G., Gong W., Yusuff S., Lorent K., Muthumani M., Dolan A. C., and Pack M. (2008) Mutation of the zebrafish nucleoporin elys sensitizes tissue progenitors to replication stress. PLoS Genet. 4, e1000240 10.1371/journal.pgen.1000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Jong-Curtain T. A., Parslow A. C., Trotter A. J., Hall N. E., Verkade H., Tabone T., Christie E. L., Crowhurst M. O., Layton J. E., Shepherd I. T., Nixon S. J., Parton R. G., Zon L. I., Stainier D. Y., Lieschke G. J., and Heath J. K. (2009) Abnormal nuclear pore formation triggers apoptosis in the intestinal epithelium of elys-deficient zebrafish. Gastroenterology 136, 902–911 10.1053/j.gastro.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mimura Y., Takagi M., Clever M., and Imamoto N. (2016) ELYS regulates the localization of LBR by modulating its phosphorylation state. J. Cell Sci. 129, 4200–4212 10.1242/jcs.190678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hattersley N., Cheerambathur D., Moyle M., Stefanutti M., Richardson A., Lee K. Y., Dumont J., Oegema K., and Desai A. (2016) A nucleoporin docks protein phosphatase 1 to direct meiotic chromosome segregation and nuclear assembly. Dev. Cell 38, 463–477 10.1016/j.devcel.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ilyin A. A., Ryazansky S. S., Doronin S. A., Olenkina O. M., Mikhaleva E. A., Yakushev E. Y., Abramov Y. A., Belyakin S. N., Ivankin A. V., Pindyurin A. V., Gvozdev V. A., Klenov M. S., and Shevelyov Y. Y. (2017) Piwi interacts with chromatin at nuclear pores and promiscuously binds nuclear transcripts in Drosophila ovarian somatic cells. Nucleic Acids Res. 45, 7666–7680 10.1093/nar/gkx355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pascual-Garcia P., Debo B., Aleman J. R., Talamas J. A., Lan Y., Nguyen N. H., Won K. J., and Capelson M. (2017) Metazoan nuclear pores provide a scaffold for poised genes and mediate induced enhancer-promoter contacts. Mol. Cell 66, 63–76.e6 10.1016/j.molcel.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelley L. A., Mezulis S., Yates C. M., Wass M. N., and Sternberg M. J. (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vermaak D., Hayden H. S., and Henikoff S. (2002) Centromere targeting element within the histone fold domain of Cid. Mol. Cell. Biol. 22, 7553–7561 10.1128/MCB.22.21.7553-7561.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katsani K. R., Karess R. E., Dostatni N., and Doye V. (2008) In vivo dynamics of Drosophila nuclear envelope components. Mol. Biol. Cell 19, 3652–3666 10.1091/mbc.e07-11-1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aravind L., and Landsman D. (1998) AT-hook motifs identified in a wide variety of DNA-binding proteins. Nucleic Acids Res. 26, 4413–4421 10.1093/nar/26.19.4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metcalf C. E., and Wassarman D. A. (2006) DNA binding properties of TAF1 isoforms with two AT-hooks. J. Biol. Chem. 281, 30015–30023 10.1074/jbc.M606289200 [DOI] [PubMed] [Google Scholar]
- 26. Fatima R. (2011) Drosophila Dynein intermediate chain gene, Dic61B, is required for spermatogenesis. PLoS One 6, e27822 10.1371/journal.pone.0027822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ni J. Q., Zhou R., Czech B., Liu L. P., Holderbaum L., Yang-Zhou D., Shim H. S., Tao R., Handler D., Karpowicz P., Binari R., Booker M., Brennecke J., Perkins L. A., Hannon G. J., and Perrimon N. (2011) A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8, 405–407 10.1038/nmeth.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clever M., Funakoshi T., Mimura Y., Takagi M., and Imamoto N. (2012) The nucleoporin ELYS/Mel28 regulates nuclear envelope subdomain formation in HeLa cells. Nucleus 3, 187–199 10.4161/nucl.19595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Georgieva S., Kirschner D. B., Jagla T., Nabirochkina E., Hanke S., Schenkel H., de Lorenzo C., Sinha P., Jagla K., Mechler B., and Tora L. (2000) Two novel Drosophila TAF(II)s have homology with human TAF(II)30 and are differentially regulated during development. Mol. Cell. Biol. 20, 1639–1648 10.1128/MCB.20.5.1639-1648.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choudhury S. D., Vs A., Mushtaq Z., and Kumar V. (2017) Altered translational repression of an RNA-binding protein, Elav, by AOA2-causative Senataxin mutation. Synapse 71, 10.1002/syn.21969 [DOI] [PubMed] [Google Scholar]
- 31. Rushlow C. A., Han K., Manley J. L., and Levine M. (1989) The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell 59, 1165–1177 10.1016/0092-8674(89)90772-1 [DOI] [PubMed] [Google Scholar]
- 32. DeLotto R., DeLotto Y., Steward R., and Lippincott-Schwartz J. (2007) Nucleo-cytoplasmic shuttling mediates the dynamic maintenance of nuclear Dorsal levels during Drosophila embryogenesis. Development 134, 4233–4241 10.1242/dev.010934 [DOI] [PubMed] [Google Scholar]
- 33. Belvin M. P., Jin Y., and Anderson K. V. (1995) Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 9, 783–793 10.1101/gad.9.7.783 [DOI] [PubMed] [Google Scholar]
- 34. Whalen A. M., and Steward R. (1993) Dissociation of the dorsal–cactus complex and phosphorylation of the dorsal protein correlate with the nuclear localization of dorsal. J. Cell Biol. 123, 523–534 10.1083/jcb.123.3.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaltschmidt B., Kaltschmidt C., Hofmann T. G., Hehner S. P., Dröge W., and Schmitz M. L. (2000) The pro- or anti-apoptotic function of NF-κB is determined by the nature of the apoptotic stimulus. Eur. J. Biochem. 267, 3828–3835 10.1046/j.1432-1327.2000.01421.x [DOI] [PubMed] [Google Scholar]
- 36. Radhakrishnan S. K., and Kamalakaran S. (2006) Pro-apoptotic role of NF-κB: implications for cancer therapy. Biochim. Biophys. Acta 1766, 53–62 10.1016/j.bbcan.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 37. Ryan K. M., Ernst M. K., Rice N. R., and Vousden K. H. (2000) Role of NF-κB in p53-mediated programmed cell death. Nature 404, 892–897 10.1038/35009130 [DOI] [PubMed] [Google Scholar]
- 38. DeVorkin L., Go N. E., Hou Y. C., Moradian A., Morin G. B., and Gorski S. M. (2014) The Drosophila effector caspase Dcp-1 regulates mitochondrial dynamics and autophagic flux via SesB. J. Cell Biol. 205, 477–492 10.1083/jcb.201303144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCall K., and Steller H. (1998) Requirement for DCP-1 caspase during Drosophila oogenesis. Science 279, 230–234 10.1126/science.279.5348.230 [DOI] [PubMed] [Google Scholar]
- 40. Song Z., McCall K., and Steller H. (1997) DCP-1, a Drosophila cell death protease essential for development. Science 275, 536–540 10.1126/science.275.5299.536 [DOI] [PubMed] [Google Scholar]
- 41. Reeves G. T., and Stathopoulos A. (2009) Graded dorsal and differential gene regulation in the Drosophila embryo. Cold Spring Harb. Perspect. Biol. 1, a000836 10.1101/cshperspect.a000836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell K., Lebreton G., Franch-Marro X., and Casanova J. (2018) Differential roles of the Drosophila EMT-inducing transcription factors Snail and Serpent in driving primary tumour growth. PLoS Genet. 14, e1007167 10.1371/journal.pgen.1007167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gullaud M., Delanoue R., and Silber J. (2003) A Drosophila model to study the functions of TWIST orthologs in apoptosis and proliferation. Cell Death Differ. 10, 641–651 10.1038/sj.cdd.4401222 [DOI] [PubMed] [Google Scholar]
- 44. Lim H. Y., and Tomlinson A. (2006) Organization of the peripheral fly eye: the roles of Snail family transcription factors in peripheral retinal apoptosis. Development 133, 3529–3537 10.1242/dev.02524 [DOI] [PubMed] [Google Scholar]
- 45. Neisch A. L., Speck O., Stronach B., and Fehon R. G. (2010) Rho1 regulates apoptosis via activation of the JNK signaling pathway at the plasma membrane. J. Cell Biol. 189, 311–323 10.1083/jcb.200912010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Orjalo A. V., Arnaoutov A., Shen Z., Boyarchuk Y., Zeitlin S. G., Fontoura B., Briggs S., Dasso M., and Forbes D. J. (2006) The Nup107-160 nucleoporin complex is required for correct bipolar spindle assembly. Mol. Biol. Cell 17, 3806–3818 10.1091/mbc.e05-11-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vollmer B., Lorenz M., Moreno-Andrés D., Bodenhöfer M., De Magistris P., Astrinidis S. A., Schooley A., Flötenmeyer M., Leptihn S., and Antonin W. (2015) Nup153 recruits the Nup107-160 complex to the inner nuclear membrane for interphasic nuclear pore complex assembly. Dev. Cell 33, 717–728 10.1016/j.devcel.2015.04.027 [DOI] [PubMed] [Google Scholar]
- 48. Zuccolo M., Alves A., Galy V., Bolhy S., Formstecher E., Racine V., Sibarita J. B., Fukagawa T., Shiekhattar R., Yen T., and Doye V. (2007) The human Nup107-160 nuclear pore subcomplex contributes to proper kinetochore functions. EMBO J. 26, 1853–1864 10.1038/sj.emboj.7601642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gillespie P. J., Khoudoli G. A., Stewart G., Swedlow J. R., and Blow J. J. (2007) ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing. Curr. Biol. 17, 1657–1662 10.1016/j.cub.2007.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mishra R. K., Chakraborty P., Arnaoutov A., Fontoura B. M., and Dasso M. (2010) The Nup107-160 complex and γ-TuRC regulate microtubule polymerization at kinetochores. Nat. Cell Biol. 12, 164–169 10.1038/ncb2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sonneville R., Craig G., Labib K., Gartner A., and Blow J. J. (2015) Both chromosome decondensation and condensation are dependent on DNA replication in C. elegans embryos. Cell Rep. 12, 405–417 10.1016/j.celrep.2015.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuhn T. M., Pascual-Garcia P., Gozalo A., Little S. C., and Capelson M. (2019) Chromatin targeting of nuclear pore proteins induces chromatin decondensation. J. Cell Biol. 218, 2945–2961 10.1083/jcb.201807139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cerveny K. L., Cavodeassi F., Turner K. J., de Jong-Curtain T. A., Heath J. K., and Wilson S. W. (2010) The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. Development 137, 2107–2115 10.1242/dev.047753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lupu F., Alves A., Anderson K., Doye V., and Lacy E. (2008) Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev. Cell 14, 831–842 10.1016/j.devcel.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aitchison J. D., Blobel G., and Rout M. P. (1995) Nup120p: a yeast nucleoporin required for NPC distribution and mRNA transport. J. Cell Biol. 131, 1659–1675 10.1083/jcb.131.6.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Emtage J. L., Bucci M., Watkins J. L., and Wente S. R. (1997) Defining the essential functional regions of the nucleoporin Nup145p. J. Cell Sci. 110, 911–925 [DOI] [PubMed] [Google Scholar]
- 57. Fabre E., and Hurt E. (1997) Yeast genetics to dissect the nuclear pore complex and nucleo-cytoplasmic trafficking. Annu. Rev. Genet. 31, 277–313 10.1146/annurev.genet.31.1.277 [DOI] [PubMed] [Google Scholar]
- 58. Adam S. A., Sengupta K., and Goldman R. D. (2008) Regulation of nuclear lamin polymerization by importin α. J. Biol. Chem. 283, 8462–8468 10.1074/jbc.M709572200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hawryluk-Gara L. A., Shibuya E. K., and Wozniak R. W. (2005) Vertebrate Nup53 interacts with the nuclear lamina and is required for the assembly of a Nup93-containing complex. Mol. Biol. Cell 16, 2382–2394 10.1091/mbc.e04-10-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Espín-Palazón R., and Traver D. (2016) The NF-κB family: key players during embryonic development and HSC emergence. Exp. Hematol. 44, 519–527 10.1016/j.exphem.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 61. Govind S. (1999) Control of development and immunity by rel transcription factors in Drosophila. Oncogene 18, 6875–6887 10.1038/sj.onc.1203223 [DOI] [PubMed] [Google Scholar]
- 62. Barkett M., and Gilmore T. D. (1999) Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18, 6910–6924 10.1038/sj.onc.1203238 [DOI] [PubMed] [Google Scholar]
- 63. Banerjee H. N., Gibbs J., Jordan T., and Blackshear M. (2010) Depletion of a single nucleoporin, Nup107, induces apoptosis in eukaryotic cells. Mol. Cell. Biochem. 343, 21–25 10.1007/s11010-010-0494-6 [DOI] [PubMed] [Google Scholar]
- 64. Uv A. E., Roth P., Xylourgidis N., Wickberg A., Cantera R., and Samakovlis C. (2000) members only encodes a Drosophila nucleoporin required for rel protein import and immune response activation. Genes Dev. 14, 1945–1957 [PMC free article] [PubMed] [Google Scholar]
- 65. Genenncher B., Wirthmueller L., Roth C., Klenke M., Ma L., Sharon A., and Wiermer M. (2016) Nucleoporin-regulated MAP kinase signaling in immunity to a necrotrophic fungal pathogen. Plant Physiol. 172, 1293–1305 10.1104/pp.16.00832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu L., Kang Y., Cöl S., and Massagué J. (2002) Smad2 nucleo-cytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFβ signaling complexes in the cytoplasm and nucleus. Mol. Cell 10, 271–282 10.1016/S1097-2765(02)00586-5 [DOI] [PubMed] [Google Scholar]
- 67. Yang X., Gu Q., Lin L., Li S., Zhong S., Li Q., and Cui Z. (2015) Nucleoporin 62-like protein activates canonical Wnt signaling through facilitating the nuclear import of β-catenin in zebrafish. Mol. Cell. Biol. 35, 1110–1124 10.1128/MCB.01181-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Park M. H., and Hong J. T. (2016) Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 5, E15 10.3390/cells5020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xia Y., Shen S., and Verma I. M. (2014) NF-κB, an active player in human cancers. Cancer Immunol. Res. 2, 823–830 10.1158/2326-6066.CIR-14-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ganguly A., Jiang J., and Ip Y. T. (2005) Drosophila WntD is a target and an inhibitor of the Dorsal/Twist/Snail network in the gastrulating embryo. Development 132, 3419–3429 10.1242/dev.01903 [DOI] [PubMed] [Google Scholar]
- 71. Gordon M. D., Dionne M. S., Schneider D. S., and Nusse R. (2005) WntD is a feedback inhibitor of Dorsal/NF-κB in Drosophila development and immunity. Nature 437, 746–749 10.1038/nature04073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Notredame C., Higgins D. G., and Heringa J. (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 10.1006/jmbi.2000.4042 [DOI] [PubMed] [Google Scholar]
- 73. Waterhouse A. M., Procter J. B., Martin D. M., Clamp M., and Barton G. J. (2009) Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schultz J., Milpetz F., Bork P., and Ponting C. P. (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U.S.A. 95, 5857–5864 10.1073/pnas.95.11.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu W., Xie Y., Ma J., Luo X., Nie P., Zuo Z., Lahrmann U., Zhao Q., Zheng Y., Zhao Y., Xue Y., and Ren J. (2015) IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31, 3359–3361 10.1093/bioinformatics/btv362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jones D. T. (1999) Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 10.1006/jmbi.1999.3091 [DOI] [PubMed] [Google Scholar]
- 77. Flockhart I. T., Booker M., Hu Y., McElvany B., Gilly Q., Mathey-Prevot B., Perrimon N., and Mohr S. E. (2012) FlyRNAi.org–the database of the Drosophila RNAi screening center: 2012 update. Nucleic Acids res. 40, D715–D719 10.1093/nar/gkr953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Andlauer T. F., Scholz-Kornehl S., Tian R., Kirchner M., Babikir H. A., Depner H., Loll B., Quentin C., Gupta V. K., Holt M. G., Dipt S., Cressy M., Wahl M. C., Fiala A., Selbach M., Schwarzel M., and Sigrist S. J. (2014) Drep-2 is a novel synaptic protein important for learning and memory. Elife 3, 10.7554/eLife.03895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Harel A., Zlotkin E., Nainudel-Epszteyn S., Feinstein N., Fisher P. A., and Gruenbaum Y. (1989) Persistence of major nuclear envelope antigens in an envelope-like structure during mitosis in Drosophila melanogaster embryos. J. Cell Sci. 94, 463–470 [DOI] [PubMed] [Google Scholar]
- 80. Johansen K. M., and Johansen J. (2004) Studying nuclear organization in embryos using antibody tools. Methods Mol. Biol. 247, 215–234 10.1385/1-59259-665-7:215 [DOI] [PubMed] [Google Scholar]
- 81. Riemer D., Stuurman N., Berrios M., Hunter C., Fisher P. A., and Weber K. (1995) Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. J. Cell Sci. 108, 3189–3198 [DOI] [PubMed] [Google Scholar]
- 82. Wagner N., Weber D., Seitz S., and Krohne G. (2004) The lamin B receptor of Drosophila melanogaster. J. Cell Sci. 117, 2015–2028 10.1242/jcs.01052 [DOI] [PubMed] [Google Scholar]
- 83. Parrott B. B., Chiang Y., Hudson A., Sarkar A., Guichet A., and Schulz C. (2011) Nucleoporin98–96 function is required for transit amplification divisions in the germ line of Drosophila melanogaster. PLoS One 6, e25087 10.1371/journal.pone.0025087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Buster D. W., Nye J., Klebba J. E., and Rogers G. C. (2010) Preparation of Drosophila S2 cells for light microscopy. J. Vis. Exp. 1982 10.3791/1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Velentzas P. D., Velentzas A. D., Pantazi A. D., Mpakou V. E., Zervas C. G., Papassideri I. S., and Stravopodis D. J. (2013) Proteasome, but not autophagy, disruption results in severe eye and wing dysmorphia: a subunit- and regulator-dependent process in Drosophila. PloS one 8, e80530 10.1371/journal.pone.0080530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wolff T. (2011) Preparation of Drosophila eye specimens for scanning electron microscopy. Cold Spring Harb. Protoc. 2011, 1383–1385 10.1101/pdb.prot066506 [DOI] [PubMed] [Google Scholar]
- 87. Capelson M., Liang Y., Schulte R., Mair W., Wagner U., and Hetzer M. W. (2010) Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell 140, 372–383 10.1016/j.cell.2009.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.