Abstract
Homeostasis in healthy tissues strongly relies on cell-to-cell adhesion and cell-to-extracellular matrix interactions. For instance, normal epithelial cells maintain tissue structure by adhering to each other and to the extracellular matrix. The proteins that mediate these distinct interactions are collectively called cell adhesion molecules and are divided into four major groups: cadherins, integrins, selectins, and immunoglobulins. They not only physically anchor cells, but also critically integrate signaling between the extracellular microenvironment and cells. These signals include biochemical cues, as adhesion proteins can both act as ligand-activated receptors and activate mechanotransduction triggered by changes in the physical environment. Molecular mechanisms related to cell adhesion signaling have been extensively studied, especially because mutations and changes in expression of these proteins, particularly cadherins and integrins, are frequently associated with diseases ranging from developmental intellectual disability to cancer. In fact, two major hallmarks of cancer, loss of cell-to-cell adhesion and anchorage-independent growth, are both dependent on cell adhesion molecules. Despite many studies elucidating the relationships between malignant transformation and metastasis and cellular adhesion processes, several areas still await exploration. Here, we highlight recently discovered roles of adhesion molecules in collective cancer cell migration and discuss the utility of three-dimensional models in studying cell-cell adhesion. We also describe recent therapeutic approaches targeting adhesion molecules.
Keywords: integrin, cadherin, cell adhesion, cancer, cancer biology, cell adhesion molecules, cancer stem cells, collective migration, epithelial-mesenchymal transition, integrin, organoids, targeted treatment
Introduction
Cell adhesion molecules are mostly transmembrane receptor proteins widely expressed through the normal epithelium and endothelium and by distinct immune cells. These proteins promote cell-to-cell and cell-to-extracellular matrix adhesion and are composed of three domains: an intracellular domain, a transmembrane domain, and an extracellular domain (1, 2). The intracellular domain interacts with the cytoskeleton, directly or via scaffolding proteins, and is responsible for signaling, whereas the extracellular domain interacts with other cell adhesion molecules or the extracellular matrix. Thus, cell adhesion molecules often integrate the extracellular cues with cell intrinsic signaling, affecting intracellular responses, cytoskeletal organization, intracellular signaling, and gene expression (3, 4).
Based on their protein sequence and structures, cell adhesion molecules can be divided into four major groups: cadherins, integrins, selectins, and immunoglobulins (Igs)3. This division is strongly linked to the distinct types of cellular junctions built by these proteins expressed on the cell surface (summarized in Fig. 1). Even though the primary role of adhesion molecules is to maintain cell-to-cell contact and attachment to the extracellular matrix, they also function as signaling effector molecules involved in cellular functions, such as cell growth, survival, and transcriptional activity (5–7). In this review, we will focus on describing the distinct roles that the two major groups of adhesion molecules, cadherins and integrins, play in cancer biology.
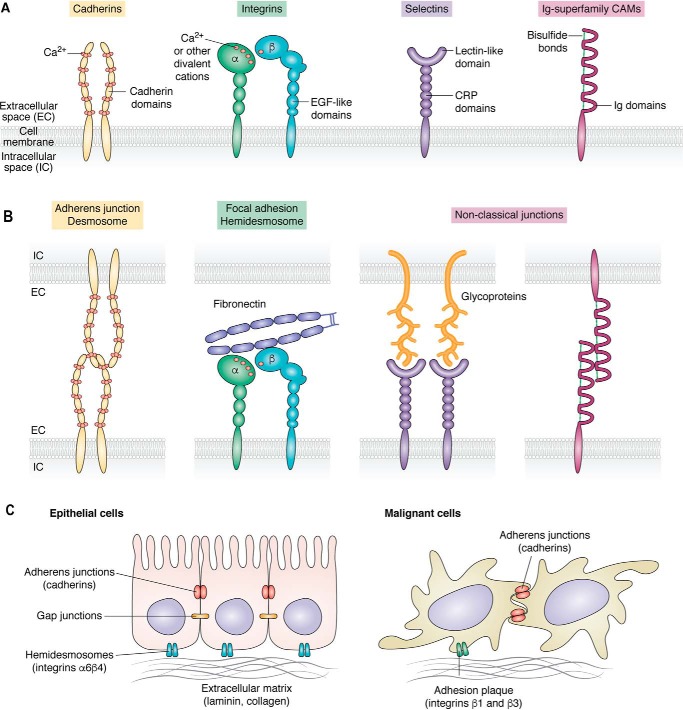
Figure 1.
Cell adhesion molecules in normal and cancer cells A, schematic representation of the structure of four major classes of cell adhesion molecules. B, interactions between cell adhesion molecules on the cell surface create different types of cellular adhesions, including adherens junctions, gap junctions, desmosomes, and hemidesmosomes. C, alterations in cell adhesion molecules in the transition between the normal epithelial cell state (left) and malignancy (right). The localization of the distinct cell adhesion complex types is shown. EGF, epidermal growth factor; CRP, complement regulatory protein domains.
Cell adhesion molecules structure and function in normal tissues
Cadherins and integrins are among the most studied classes of adhesion receptors. Homotypic cell-cell adhesions are mediated by cadherins, whereas adhesion between the cell and its extracellular matrix is mediated by integrins. Because cadherins and integrins comprise a wide variety of interactions at different levels, they are considered to be directly related elements that are part of a large adhesive network (3, 6, 8).
Cadherins are calcium-dependent transmembrane proteins that play a fundamental role in junctional adhesions, holding cells within tissues together, maintaining their intracellular cohesion, and preserving tissue architecture. These proteins are indispensable for morphogenesis, tissue remodeling, and maintenance of tissue barriers (4, 9). The structure of cadherins comprises several domains, typically presenting one signal sequence, a protein precursor, one single transmembrane domain, and five ectodomains. Four of the ectodomains correspond to the protein module of the immunoglobulin-like fold called an extracellular cadherin domain, which binds to calcium ions to facilitate its proper folding. The fifth domain contains four conserved cysteines and an N-terminal extracellular domain. Typically, any protein containing one or more extracellular cadherin domain is designated as a member of the cadherin family. The cadherin superfamily includes a wide variety of proteins, and there are many isoforms distributed in a tissue-specific manner (10, 11).
Some cadherins, termed classical cadherins, have been shown to mediate homotypic adhesive interactions. The first classical cadherins identified in vertebrates were E- and N-cadherin. Classical cadherins depend on a proteolytic removal of the pro-domain to exert their adhesive capacity (10, 12). In addition, these proteins form an adhesive dimer interface where a swapping of paired extracellular cadherin 1 N-terminal domains generates a 2-fold symmetric interaction. These strand-swapped interfaces are preferentially formed between cadherins from apposed cells, mostly in the presence of calcium. The assembly of cadherins between apposed adherent cells generates junctional structures that are densely packed (13, 14).
Integrins are the major class of receptors involved in homotypic and heterotypic adhesive events. These proteins transmit signals via inside-out and outside-in signaling and comprise a large and complex family of transmembrane glycoproteins. Structurally, these proteins are heterodimeric transmembrane receptors formed by noncovalent association of two such transmembrane glycoproteins. These two subunits are identified as α and β, whereby 18 integrin α subunits and eight β subunits are so far known to heterodimerize into 24 different integrins (15). The subunits α and β are composed by one transmembrane domain, one ectodomain, and a short cytoplasmic tail domain. Their extracellular domains form elongated stalks and a globular ligand-binding head region that mostly binds with other glycoproteins and connective tissue components, such as collagens, laminins, and fibronectin. Integrin β subunits harbor a metal ion-dependent adhesion site, which coordinates for example with magnesium ions. The metal ion–dependent adhesion site coordination with magnesium ions is altered due to integrin ligand binding, causing conformational changes that lead to an open and active integrin conformation (5, 16–18). The vast majority of integrins connect with the actin cytoskeleton in cell-matrix adhesions via cytoskeletal linker proteins (e.g. talin, paxillin, and vinculin). These connections between integrins and the actin cytoskeleton are necessary for activation of downstream pathways. Thus, integrins provide a link between the outside environment and cellular responses related to motility, such as immune cell trafficking, hemostasis, and migration of cancer cells (18–20). Many pathways related to growth factor response depend on integrin-mediated adhesion to the extracellular matrix or integrin-dependent intracellular signaling, linking integrin to cell proliferation and anchorage-dependent survival (21–23).
Immunoglobulin-like cell adhesion molecules (Ig-CAMs) have highly glycosylated extracellular domains consisting of variable number of immunoglobulin-like loops (24). The extracellular domain of Ig-CAM may be anchored in the membrane by glycophosphatidylinositol anchors or linked to a transmembrane domain. Homotypic interactions between Ig-CAMs can drive cell-to-cell adhesion, whereas the cytoplasmic tail of these proteins may interact with cytoskeletal proteins. The most well-known members of this superfamily are major histocompatibility complex class I and II molecules and T-cell receptor complex. Other members include ICAM, VCAM, MadCAM-1, and ALCAM, which are all important in leukocyte trafficking (25).
Selectins are another class of adhesion molecules related to immune function. Selectins mediate cell-cell adhesions by binding to carbohydrates in a calcium-dependent manner (26). These transmembrane proteins are responsible for the initial steps of leukocyte rolling, which initiates migration of the immune cell through the blood vessel wall into the surrounding tissue (27).
All of molecules described above play distinct roles in context-dependent cell-cell and cell-extracellular matrix adhesion. However, the ability to transduce the signals from the environment and trigger intracellular responses, as well as outside-in signaling, provides adhesion molecules with functional versatility.
Role of adhesion molecules in migration
Whereas integrins play a key role in single-cell migration, which requires complete loss of adherens junctions that is mediated by E-cadherin, integrins also sense the environment and forces that generate movement. Integrins perform these various functions by their conformational changes that are triggered by their binding either to the extracellular matrix or to intracellular proteins that alter the binding affinity of integrin, affect their clustering, and recruit cytoskeletal linker proteins (18). These changes remodel nascent or focal adhesions and generate tension, whereas coordinated assembly and disassembly of these adherent structures generate forces of cellular movement (28–30).
Single-cell migration and invasion are vital for many physiological processes, including immune cell trafficking. However, in morphogenesis and wound healing, an alternative process of collective cell migration has also evolved (reviewed in Ref. 31). In this process, assemblies of cells move together, as the cell-cell junctions remain intact, allowing neighboring cells to adhere to each other during the movement. Adherens junctions in collective migration are maintained by homotypic cadherin interactions between the cells in a group (32). Other members of the adhesion molecule family, including Igs L1CAM, NCAM, and ALCAM, can also support this function (33, 34). Integrins also play a role in collective adhesion, as they can bind intercellular deposits of extracellular matrix and in this way support cell cohesion (35). Variability of adhesion molecules and signaling contexts results in plasticity of cell-cell junctions and leads to distinct modes of collective migration, ranging from sheet migration to movement of cellular strands and clusters (36). Thus, adhesion molecules are key proteins regulating all modes of cellular movement in tissue plasticity and remodeling.
Loss of cell adhesion during malignant transformation
In the classic view of malignant transformation in the epithelium, cells lose their dependence on integrin-mediated interactions with the extracellular matrix and resulting signaling events (Fig. 1C). During this process, adherens junctions that are mediated by E-cadherin and that are crucial for cell-cell adhesion are lost. This can be a result of a direct genetic mutation, which occurs in 4% of all cancers (37, 38), or a decrease in E-cadherin expression due to its promoter methylation or transcriptional repression (39–44). Additional alterations are also observed in the specialized multiprotein complexes present in stratified epithelia named hemidesmosomes. Integrin α6β4 present in hemidesmosomes interacts with the keratin intermediate filament instead of actin filaments via binding to plectin isoform 1a (P1a), which is a unique characteristic when compared with other members of this family. In tumor cells, the lack of polarity is displayed by actin protrusions, which will disassemble the hemidesmosomes and mediate cell migration and invasion. After hemidesmosomes disassemble, α6β4 integrin becomes phosphorylated and relocates to an F-actin–rich protrusion, where integrin interacts with actin filaments (45). All of these changes are associated with epithelial-mesenchymal transition, which renders cancer cells more motile and invasive (46).
Further, epithelial-mesenchymal transition (EMT) is a reversible transcriptional program considered the primary element driving tumor progression to metastasis (47). Epithelial-mesenchymal transition allows epithelial cells to adopt a more mesenchymal state, which enhances cellular migration. This process in normal physiological conditions contributes to embryogenesis, organ development, and wound healing, whereas in cancer, it supports several malignant traits, including metastatic spread and drug resistance (48). In tumors, the EMT phenotype was also reported to be associated with a more inflammatory tumor microenvironment and was thus proposed as a potential biomarker for immune checkpoint blockade agents (49, 50). However, the impact of epithelial-mesenchymal transition in the efficiency of this blockade is still unknown.
One of the hallmarks of epithelial-mesenchymal transition is the “cadherin switching” characterized by loss of E-cadherin expression and increased expression of N-cadherin, as well as changes in the integrin repertoire (51). This promotes a shift from tight cell-to-cell and cell-basement membrane connections, mediated by E-cadherin and α6β4 integrins, to N-cadherin-dependent adhesions, mediated by β1 and β3 integrins. Thus, EMT allows cells to adhere readily to collagen, a component of the extracellular matrix, rather than the basement membrane. N-cadherin also activates the Rho-family GTPase signaling pathway, enhances fibroblast growth factor signaling, and modulates the Wnt signaling pathway (52). All of these pathways contribute to the aggressive tumor phenotype with the capacity to escape from the primary tumor location to secondary sites (52). Moreover, EMT induced by silencing of E-cadherin has also been shown to protect cells from anoikis, cell death triggered by disrupted anchorage to basement membrane (53). However, E-cadherin was also recently shown to act as a survival factor in metastatic invasion of breast cancer by limiting reactive oxygen species-mediated apoptosis (54). Thus, context-dependent adhesion molecule switching not only contributes to cancer cell motility, but also promotes their proliferation and survival ability in the bloodstream, thereby increasing the probability of distant metastases forming.
The role of adhesion molecule switching during the epithelial-mesenchymal transition and malignancy has been extensively studied by overexpression of the EMT-driving transcription factors in cell line models (55). Down-regulation of E-cadherin, α6β4 integrins, and epithelial cell adhesion molecules during the transition is orchestrated by twist-related protein 1, zinc finger E-box–binding homeobox proteins, and the transcriptional repressors SNAIL and SLUG (44). The same factors promote overexpression of N-cadherin as well as β1 and β3 integrins. However, recent studies have shown that complete state transition is rarely observed, and in more physiological conditions, cancer cells express a combination of epithelial and mesenchymal markers, thereby creating a heterogeneous continuum rather than two binary states (47, 56). In breast cancer models, among these hybrid epithelial-mesenchymal cells, intermediate levels of integrin β4 expression mark a population of cells with higher tumor-initiating capacity than integrin 4high and integrin β4low populations (57). Integrin β4 mRNA was also associated with relapse-free survival in triple-negative breast cancer (57). It is not clear which molecular mechanisms mediated through integrin β4 are responsible for the tumor-initiating properties and maintenance of the intermediate epithelial-mesenchymal state.
These recent findings point to the seemingly opposing traits related to malignancy-cellular adhesion loss that could lead to increased motility and pro-tumorigenic downstream signaling mediated by adhesion molecules. Thus, altering the expression of certain adhesion molecules may allow cancer cells to increase their malignant potential in different ways.
Cell adhesion in invasiveness by collective migration
As described above, loss of cell adhesion has been classically viewed as a pro-tumorigenic feature. However, in many epithelial tumor types, normal cell adhesion through cell junctions is retained despite the more mesenchymal phenotype of cells within a tumor. This enables cancer cells to adopt a collective migration mode (58). Collective migration is defined as two or more cells moving together across a two-dimensional layer of extracellular matrix while retaining their cell-cell junctions or when they move into a three-dimensional interstitial tissue scaffold. This process is key to normal morphogenesis and epithelial homeostasis as an important strategy for local tissue infiltration (59).
The invasion by collective migrating carcinomas is characterized by a fine balance between cell-cell and cell-extracellular matrix adhesions (60). Three hallmarks define collective cell migration: cells remain physically and functionally connected; cell-cell junctions are maintained; and, in most cases, the movement of cell groups modifies the tissue along the migration path (31). Cell-cell junctions play a crucial role in this process by controlling the collective movement of proteins, in particular cadherins, the immunoglobulin superfamily members, and integrin. This process critically depends on the coupling of the actin cytoskeleton to multiple cells, transmitting forces, and guiding the signaling that drives the migration of the group of cells (reviewed in Refs. 42 and 43).
Integrin can control collective migration in several ways, one of them involving focal adhesion kinase and the tyrosine-protein kinase Src capacity of up-regulating integrin expression, which contributes to the processes of integrin-mediated cell spreading and migration. Their inhibition effects integrin-mediated signaling, thereby generating a suppression of E-cadherin–dependent collective cell movement. Further, it is known that focal adhesion kinase in complex with the Src presents a central role in cancer through its ability to promote proliferation and resistance in tumor cells, thereby expanding the influence of integrin signaling when involved in the inhibition of this complex (62, 63).
Evidence of collective migration has also been identified in vivo, as clusters of circulating tumor cells have been found in the bloodstream of cancer patients (64–67). In vivo experiments have shown that clusters of circulating tumor cells are derived from oligoclonal tumors and are not just a mere aggregation of cells in circulation (67). Clusters of circulating tumor cells are migrating out of the primary tumor as a collective and are thus more efficient in establishing metastases than individual cancer cells. Abundance of circulating tumor cell clusters is clinically relevant, as it correlates with worse patient outcomes in different tumor types (68–70). Characterization of circulating tumor cell clusters revealed that the cell adherens junction protein plakoglobin is crucial for cluster formation, and its knockdown resulted in reduced metastatic spread (67). Whereas circulating tumor cell clusters express mesenchymal markers, the presence of cell junctions points again to an intermediate epithelial-mesenchymal state, where aspects of epithelial cell adhesion are maintained (71).
Collective migration requires remodeling of the extracellular matrix, to facilitate the movement of a group of cells. This remodeling may also be driven by cancer cells at the leading front of the invasive group. Recent studies have shown that cancer-associated fibroblasts (CAFs) can remodel the matrix and create tracks, which can then be used by migrating cancer cells (72). Moreover, by generating heterotypic junctions between N-cadherin on the CAF membranes and E-cadherin on the cancer cell membrane, CAFs can generate intercellular physical force and drive collective invasion of cancer cells (73). A better understanding of the mechanisms behind the collective migration will be of significant clinical relevance, as this process is often associated with increased resistance to chemotherapy, radiation, and targeted approaches.
Cell-cell interactions and collective migration in three-dimensional cultures
In vitro studies have shown that mesenchymal cells in primary tumors adopt collective invasion traits as a result of confinement by the increase of the extracellular matrix density (74). Thus, it is possible that clusters of circulating tumor cells are generated in an increasingly dense extracellular matrix as a result of “cell jamming.” This hypothesis could help explain why breast tissue density is associated with an increased risk of invasive and metastatic cancer (75). It also underlines the tight connection between the microenvironment and the control of cell adhesion. To address the clinically significant switch between single-cell and collective migration, more studies focusing on the role of the tumor microenvironment and its physical properties are needed. However, collective migration occurs when two or more cells move together across a two-dimensional layer of extracellular matrix while retaining their cell-cell junctions or when they move into a three-dimensional interstitial tissue scaffold (59). Modeling of the collective migration process in standard two-dimensional cultures has proven to be challenging, and novel approaches that recapitulate the physical environment of cancer cells are now advancing this field. Three-dimensional cell culture conditions that promote formation of multicellular spheroids, spherical aggregates of cancer cells that produce their own extracellular matrix but do not adhere to the culture dish surface, have been developed for many tumor types. Cancer cells grown as spheroids can switch between single-cell epithelial-mesenchymal transition–driven migration and collective migration in response to extracellular matrix porosity and confinement (74). Switching toward the collective migration mode depends on pericellular proteolysis, whereby secretion of matrix metalloproteinases enables proteolysis and remodeling of the extracellular matrix and generates space for multicellular assembly movement. However, the exact mechanisms of remodeling by cellular clusters and their adhesion to remodel extracellular matrix, which is required for motility, remain largely unknown (31, 76).
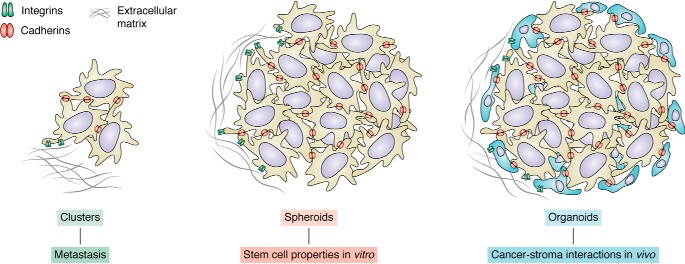
Recent advances in organotypic cultures allow for three-dimensional reconstruction of spheroids and organoids from patient samples (Fig. 2). Spheroid cultures allow for in vitro maintenance of intratumor heterogeneity that exists in patient samples but is mainly lost in standard two-dimensional cultures. Organoids are multicellular assemblies that contain not only tumor cells, but also distinct populations of tumor-associated stroma cells, which contribute to the generation of the extracellular matrix and remodeling (reviewed in Ref. 77). Interestingly, both of these in vitro three-dimensional tumor models are significantly better at reflecting drug responses of the patients from which they were derived. This would point to the significance of cell-to-cell contact, adhesion, and extracellular matrix interactions in therapy responses. Further studies of the properties of organoid cultures are needed to dissect the molecular mechanisms of cell clustering and interactions. Whereas most of the current drug discovery efforts focus on cytotoxic effects, we expect that as the three-dimensional models become more widely adopted in high-throughput screening platforms, new compounds that could affect cellular adhesion will also emerge.
Figure 2.
Cancer cell clusters, spheres, and organoids. Shown are aspects of cancer cell biology where cell adhesion is crucial yet not well-understood.
Integrins, three-dimensional cultures, and cancer stemlike cells
Organoids and tumor spheroids provide a more faithful model of human tumors, as they retain a higher frequency of cancer stemlike cells compared with traditional cultures. Specifically, the first protocols for cancer stemlike cell culture were derived from adult stem cell cultures. These relatively rare populations of cancer cells can give rise to diversely differentiated subpopulations and are more resistant to standard therapies.
The first protocols for cancer stemlike cell culture were derived from adult stem cell cultures. Just like normal stem cells, cancer stemlike cells rely on asymmetric cell division to retain the pool of undifferentiated cancer stemlike cells, a property often controlled by contact of cancer stemlike cells with the stem cell niche. These interactions, both in normal and cancer tissues, are driven by integrins. Depending on the tumor type and genetic background, integrins α6β1, α6β4, and αVβ3 have all been implicated in stem cell self-renewal, by supporting the interactions between cancer stem cells and their niches (78, 79). Integrins were also shown to provide the anchorage-dependent context that allows for asymmetric cell division of different progenitor and stem cells (80–82) and could play similar functions in cancer stem cells.
By activation of focal adhesion kinase, integrin complexes affect the Wnt and hedgehog signaling pathways, which are key to self-renewal and expansion of the stem cell pool and are considered essential regulators of oncogenesis (83). It is thus plausible that adhesion molecules expressed in cancer cells in three-dimensional organoid cultures provide signaling that mimics the supportive environment of the stem cell niches. This is another unexplored area where studies of cellular adhesion could lead to the identification of novel therapeutic approaches.
Role of growth factors in adhesion molecule signaling
Cell-to-cell contact maintenance is not the only biological function of adherent molecules related to cancer progression or inhibition. Ample evidence shows that these molecules can also activate signaling pathways independently of adhesion (84). One such function, relevant to cancer progression, is the ability of cell adhesion molecules to modulate growth factor receptor signaling, which is of great relevance because growth factor stimulation is one of the most important hallmarks of cancer (recently reviewed in Ref. 60). For example, the fibroblast growth factor receptor (FGFR) tyrosine kinase can be bound by several adhesion molecules, including N-cadherin, NCAM, and L1CAM (85). N-cadherin can trigger cis-dimerization of FGFR, which leads to autophosphorylation of the receptor and recruitment and phosphorylation of downstream factors (86). This growth-promoting signaling is independent of the ligand. The extracellular domain of N-cadherin can also interact with FGFR and inhibit its internalization, which leads to increased signaling in a ligand-independent fashion (87). Additionally, N-cadherin was also shown to prevent FGFR ubiqitination and degradation (88). Both FGFR and N-cadherin are often overexpressed in metastatic cells, which results in up-regulation of the mitogen-activated protein kinase pathway and increased motility and invasion of cancer cells (86). These interactions occur independently of the adhesive properties of cadherin and were shown to drive stemlike properties and epithelial-mesenchymal transition (89). Because the fibroblast growth factor receptor is frequently amplified in genomes of distinct cancer subtypes, targeting this mechanism could be of high clinical relevance.
Another receptor tyrosine kinase also frequently amplified in cancer is the epidermal growth factor receptor, which is a crucial factor of breast cancer metastasis (90, 91). Interestingly, downstream pathways of the epidermal growth factor receptor can also be affected by adhesion-independent interactions with the adhesion molecule desmoglein 1 (92). Because desmoglein 1 is a desomosome protein, which is usually down-regulated in malignant progression, restoration of its expression levels leads to attenuation of epidermal growth factor receptor signaling. This is due to the sequestration of the receptor within the membrane, preventing receptor internalization and activation of Erk1/2, ultimately leading to a decrease in the formation of cellular protrusions, such as invapodia, that are needed for invasion. Interactions between oncogenic receptor tyrosine kinases could thus be attractive targets for therapeutics.
Role of cell adhesion molecules in the tumor microenvironment
Expression of distinct adhesion molecules in malignancy is not limited to cancer cells, and it can be significantly altered in noncancerous cells of the tumor microenvironment. Cancer cell secretion of pro-angiogenic factors results in increased blood vessel generation (93). However, the endothelial cells that line these new vessels have lower than normal expression of endothelial adhesion molecules, such as intercellular adhesion molecules 1 and 2, vascular cell adhesion molecule 1, E-selectin, and CD34 (94). This endothelial anergy, characterized as unresponsiveness to inflammatory signaling, results in decreased adhesion of immune cells to the wall of the vessel, leading to reduced effector T-cell infiltration into the tumor, which is a key mechanism of immune evasion of solid malignancies (95).
Adhesion molecules are also key in leukocyte-cancer cell interactions. For example, dendritic cells responsible for phagocytosis and cancer-derived antigen presentation interact with dying cancer cells via integrins, such as integrin αVβ4 (96, 97). On the other hand, dendritic cells can also express β2 integrin, which promotes anti-inflammatory interactions. Integrin αE, which is expressed by dendritic cells, but also by myeloid-derived suppressor cells and regulatory T cells, promotes pro- or anti-tumorigenic action of immune cells, depending on its cellular context (94). The exact role of distinct classes of adhesion molecules expressed by dendritic cells in the anti-tumor immune response remains to be determined.
Cadherins and integrins as targets for cancer therapy
Cadherin as well as integrin expression and activation directly influence human malignancies. Due to their broad impact in malignant transformations, they are considered potential targets for cancer therapy (94). Cadherin-mediated cell-cell adhesion disruption is frequently observed in solid and hematological tumors. By analyzing CD34+ cell populations from patients with myelodysplastic syndrome, it was shown that the promoter of E-cadherin often hypermethylated, which results in decreased protein expression (98). Further, comparison of bone marrow and blood revealed that in normal control samples, the E-cadherin promoter was essentially unmethylated, and 78% of the leukemia samples presented abnormal hypermethylation of this region, thus reinforcing the E-cadherin gene as a common target for hypermethylation in hematologic malignancies (99).
Leukemic stem cell self-renewal is largely dependent on N-cadherin, which causes future evasion of chemotherapy effects. The overexpression of N-cadherin in hematopoietic stem and progenitor cells was shown to promote and preserve hematopoietic stem and progenitor cells during serial bone marrow transplantations. Further, inhibition of N-cadherin resulted in a significant reduction in long-term engraftment. These findings suggest that N-cadherin–mediated cell adhesion is functionally essential for the regulation of hematopoietic stem and progenitor cell activity in the bone marrow and thus an interesting target for the treatment of leukemia (100).
Integrins are considered prime promising anti-cancer drug targets, and their antagonists have been shown to achieve success by impeding integrin trafficking that involves invasion and migration of cells in tumors, which are crucial in promoting cancer metastasis (4). Some αV integrin antibodies have been evaluated in late-stage clinical trials, such as abituzumab (Merck KGaA, Darmstadt, Germany) and intetumumab (Centocor, Malvern, PA). Both bind the integrin αV subunit and inhibit all five αV integrins. They were both shown to inhibit ligand binding and tumor growth in xenograft models. Abituzumab showed a favorable safety profile for progressive castration-resistant prostate cancer patients with bone metastases after chemotherapy. Intetumumab showed the potential to be efficacious as a single agent for the treatment of metastatic melanoma and displayed a manageable safety profile. The results presented support further larger clinical studies (101–104).
Integrin β2 inhibitors function by inhibiting cell adhesion and trapping of white blood cells. Antibodies and small molecules have been generated, but in most cases, issues were encountered during the trials (105). Here, the shortage of animal models, including knockout mouse models, is one of the big difficulties because proper in vivo models are required to validate the target. Further roadblocks are encountered due to the low therapeutic effect, the lack of selectivity, and the presence of side effects, in particular hepatotoxicity. In addition to the compounds that have already reached clinical trials, there are a wide variety of recent studies on integrin inhibition as a target for cancer therapy strategy (106).
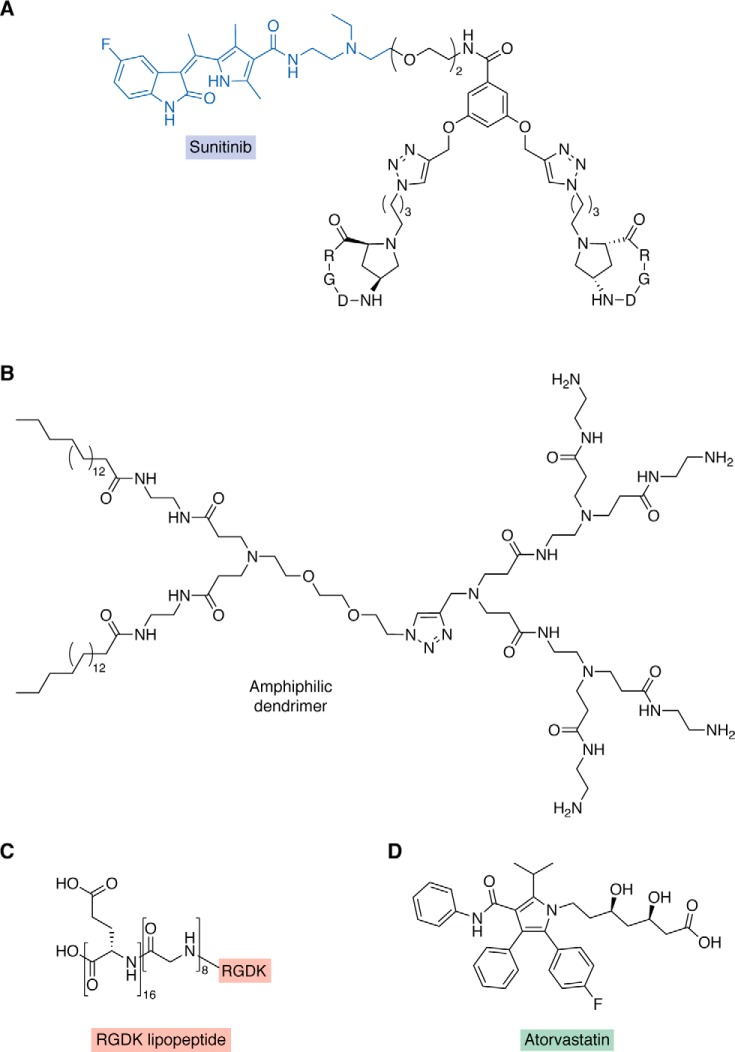
Another study using the integrin-targeted approach was undertaken for drug-resistant ovarian carcinoma. Conjugates presenting a selective binder for the extracellular portion of integrin αVβ3 covalently linked to sunitinib, a tyrosine kinase inhibitor, were screened for their anti-tumor potential. This strategy resulted in one active compound (Fig. 3A) that inhibits the growth of drug-sensitive and -resistant cells in the micromolar range, reduces cell migration and invasive abilities, and presents an increased potency compared with sunitinib (107).
Figure 3.
Different drug discovery approaches to targeting integrin for cancer therapy. A, conjugate presenting a selective binder for the extracellular region of integrin aVb3 (black) covalently linked to sunitinib (blue) (79). B, amphiphilic dendrimer composed with a dual-targeting peptide bearing an Arg-Gly-Asp-Lys (RGDK) lipopeptide (red) (C) (81). D, atorvastatin identified as a novel candidate for drug repurposing for undruggable integrins (82).
From a genome-wide study of taxol resistance, the miR-29c miRNA was identified, which is directly related to the resistance observed in therapy against nasopharyngeal carcinoma. Regulation of taxol resistance by miR-29c was demonstrated to occur due the inhibition of integrin β1. When silencing miR-29c, a marked increase in growth of taxol-resistant nasopharyngeal carcinoma tumor occurred, whereas the knockdown of integrin β1 reversed the tumor growth. These results indicate that miR-29c has the potential to be used in taxol-resistant nasopharyngeal carcinoma therapy by inhibiting integrin β1 (108) and support the interest in designing compounds targeting integrin for the treatment of drug-resistant tumors.
Further, siRNA is a novel emerging therapy approach for treating various diseases, such as cancer. In this approach, the specific delivery to the desired target cells is necessary to achieve high therapeutic efficacy. Drug delivery systems such as dendrimers have been used as promising strategies to siRNA delivery. One example of this approach was the design of an amphiphilic dendrimer (Fig. 3B) composed with a dual-targeting peptide bearing an RGDK lipopeptide (Fig. 3C) warhead designed to interact with integrin. This targeted system enhanced siRNA delivery, increasing gene silencing, and anticancer activity, reducing proliferation of cells (106).
Integrin has also been studied as a cancer target by employing a drug-repurposing approach. It is known that integrin β3 could potentially overcome chemoresistance due to its abundant expression in drug-resistant cells that have a mesenchymal phenotype. Thus, an in silico screening approach was used to search for drugs that mimic the changes of the transcriptome level caused by the knockdown of integrin β3. By this approach, atorvastatin (Fig. 3D) was identified as a novel candidate for drug repurposing, which represents an alternative path to drug discovery for undruggable integrins. It was shown that atorvastatin sensitized cancer cells to conventional chemotherapeutic drugs, such as doxorubicin (109).
The examples discussed above represent a minor fraction of the broad diversity of approaches that have been studied in past years involving cadherins and integrins as drug targets for cancer. They clearly show the promising nature of these strategies and the plurality of paths that can be applied to achieve their goals.
Outlook
Cell adhesion molecules could constitute an attractive therapeutic target group, as their extracellular domains could be easily accessed by antibodies or small-molecule inhibitors. Multiple clinical trials have been initiated based on successful reduction of tumor growth by integrin targeting, especially αV and β1 (101–105). Thus far, most of the clinical trials have not significantly affected the disease-free or overall survival in patients (67). This may be due to the redundancy in signaling mediated by integrins, but also due to adverse effects caused by disruption of integrin-dependent functions in normal tissues. New studies targeting cell adhesion in combination with other drugs are under way. A bispecific antibody targeting β2 integrin LFA-1 and Id was recently developed (61). In preclinical trials for B cell lymphoma, this antibody targeted cancer cells exclusively, despite expression of β2 integrin on several normal cell types. Thus, this or similar approaches that use cell adhesion proteins as targeted therapy delivery systems are of great interest. Future studies of the mechanisms of cellular adhesion and interactions between tumor cells and the cells of the microenvironment are also warranted to open new avenues for more effective cancer treatment.
Acknowledgment
We thank Dr. Erumbi Rangarajan (The Scripps Research Institute) for insightful discussions.
This is publication number 29874 from The Scripps Research Institute. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Ig
- immunoglobulins
- Ig-CAM
- immunoglobulin-like cell adhesion molecule
- EMT
- epithelial-mesenchymal transition
- CAF
- cancer-associated fibroblast
- FGFR
- fibroblast growth factor receptor.
References
- 1. Makrilia N., Kollias A., Manolopoulos L., and Syrigos K. (2009) Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 27, 1023–1037 10.3109/07357900902769749 [DOI] [PubMed] [Google Scholar]
- 2. Moh M. C., and Shen S. (2009) The roles of cell adhesion molecules in tumor suppression and cell migration: a new paradox. Cell Adh. Migr. 3, 334–336 10.4161/cam.3.4.9246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mui K. L., Chen C. S., and Assoian R. K. (2016) The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci. 129, 1093–1100 10.1242/jcs.183699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko K. S., Arora P. D., Bhide V., Chen A., and McCulloch C. A. (2001) Cell-cell adhesion in human fibroblasts requires calcium signaling. J. Cell Sci. 114, 1155–1167 [DOI] [PubMed] [Google Scholar]
- 5. Freemont A. J., and Hoyland J. A. (1996) Cell adhesion molecules. Clin. Mol. Pathol. 49, M321–M330 10.1136/mp.49.6.M321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canel M., Serrels A., Frame M. C., and Brunton V. G. (2013) E-cadherin-integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 126, 393–401 10.1242/jcs.100115 [DOI] [PubMed] [Google Scholar]
- 7. Windisch R., Pirschtat N., Kellner C., Chen-Wichmann L., Lausen J., Humpe A., Krause D. S., and Wichmann C. (2019) Oncogenic deregulation of cell adhesion molecules in leukemia. Cancers (Basel) 11, 311 10.3390/cancers11030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber G. F., Bjerke M. A., and DeSimone D. W. (2011) Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 124, 1183–1193 10.1242/jcs.064618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shapiro L., and Weis W. I. (2009) Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 1, a003053 10.1101/cshperspect.a003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boggon T. J., Murray J., Chappuis-Flament S., Wong E., Gumbiner B. M., and Shapiro L. (2002) C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296, 1308–1313 10.1126/science.1071559 [DOI] [PubMed] [Google Scholar]
- 11. Shashikanth N., Petrova Y. I., Park S., Chekan J., Maiden S., Spano M., Ha T., Gumbiner B. M., and Leckband D. E. (2015) Allosteric regulation of E-cadherin adhesion. J. Biol. Chem. 290, 21749–21761 10.1074/jbc.M115.657098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Häussinger D., Ahrens T., Aberle T., Engel J., Stetefeld J., and Grzesiek S. (2004) Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography. EMBO J. 23, 1699–1708 10.1038/sj.emboj.7600192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel S. D., Ciatto C., Chen C. P., Bahna F., Rajebhosale M., Arkus N., Schieren I., Jessell T. M., Honig B., Price S. R., and Shapiro L. (2006) Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124, 1255–1268 10.1016/j.cell.2005.12.046 [DOI] [PubMed] [Google Scholar]
- 14. Geiger B., Volk T., Volberg T., and Bendori R. (1987) Molecular interactions in adherens-type contacts. J. Cell Sci. Suppl. 8, 251–272 10.1242/jcs.1987.supplement_8.14 [DOI] [PubMed] [Google Scholar]
- 15. Bachmann M., Kukkurainen S., Hytönen V. P., and Wehrle-Haller B. (2019) Cell adhesion by integrins. Physiol. Rev. 99, 1655–1699 10.1152/physrev.00036.2018 [DOI] [PubMed] [Google Scholar]
- 16. Hynes R. O., and Zhao Q. (2000) The evolution of cell adhesion. J. Cell Biol. 150, F89–F96 10.1083/jcb.150.2.F89 [DOI] [PubMed] [Google Scholar]
- 17. Ginsberg M. H. (2014) Integrin activation. BMB Rep. 47, 655–659 10.5483/BMBRep.2014.47.12.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- 19. Burridge K., and Chrzanowska-Wodnicka M. (1996) Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463–518 10.1146/annurev.cellbio.12.1.463 [DOI] [PubMed] [Google Scholar]
- 20. Aksorn N., and Chanvorachote P. (2019) Integrin as a molecular target for anti-cancer approaches in lung cancer. Anticancer Res. 39, 541–548 10.21873/anticanres.13146 [DOI] [PubMed] [Google Scholar]
- 21. Mainiero F., Murgia C., Wary K. K., Curatola A. M., Pepe A., Blumemberg M., Westwick J. K., Der C. J., and Giancotti F. G. (1997) The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 16, 2365–2375 10.1093/emboj/16.9.2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ieda M., Tsuchihashi T., Ivey K. N., Ross R. S., Hong T.-T., Shaw R. M., and Srivastava D. (2009) Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev. Cell 16, 233–244 10.1016/j.devcel.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz M. A., and Assoian R. K. (2001) Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553–2560 [DOI] [PubMed] [Google Scholar]
- 24. Wai Wong C., Dye D. E., and Coombe D. R. (2012) The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int. J. Cell Biol. 2012, 340296 10.1155/2012/340296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cayrol R., Wosik K., Berard J. L., Dodelet-Devillers A., Ifergan I., Kebir H., Haqqani A. S., Kreymborg K., Krug S., Moumdjian R., Bouthillier A., Becher B., Arbour N., David S., Stanimirovic D., and Prat A. (2008) Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat. Immunol. 9, 137–145 10.1038/ni1551 [DOI] [PubMed] [Google Scholar]
- 26. Springer T. A. (2009) Structural basis for selectin mechanochemistry. Proc. Natl. Acad. Sci. U.S.A. 106, 91–96 10.1073/pnas.0810784105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Springer T. A. (1990) Adhesion receptors of the immune system. Nature 346, 425–434 10.1038/346425a0 [DOI] [PubMed] [Google Scholar]
- 28. Sun Z., Tseng H.-Y., Tan S., Senger F., Kurzawa L., Dedden D., Mizuno N., Wasik A. A., Thery M., Dunn A. R., and Fässler R. (2016) Kank2 activates talin, reduces force transduction across integrins and induces central adhesion formation. Nat. Cell Biol. 18, 941–953 10.1038/ncb3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parsons J. T., Horwitz A. R., and Schwartz M. A. (2010) Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 10.1038/nrm2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Pascalis C., and Etienne-Manneville S. (2017) Single and collective cell migration: the mechanics of adhesions. Mol. Biol. Cell 28, 1833–1846 10.1091/mbc.e17-03-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedl P., and Gilmour D. (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- 32. Peglion F., Llense F., and Etienne-Manneville S. (2014) Adherens junction treadmilling during collective migration. Nat. Cell Biol. 16, 639–651 10.1038/ncb2985 [DOI] [PubMed] [Google Scholar]
- 33. Gavert N., Ben-Shmuel A., Raveh S., and Ben-Ze'ev A. (2008) L1-CAM in cancerous tissues. Expert Opin. Biol. Ther. 8, 1749–1757 10.1517/14712598.8.11.1749 [DOI] [PubMed] [Google Scholar]
- 34. Lehembre F., Yilmaz M., Wicki A., Schomber T., Strittmatter K., Ziegler D., Kren A., Went P., Derksen P. W. B., Berns A., Jonkers J., and Christofori G. (2008) NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J. 27, 2603–2615 10.1038/emboj.2008.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belvindrah R., Hankel S., Walker J., Patton B. L., and Müller U. (2007) β1 integrins control the formation of cell chains in the adult rostral migratory stream. J. Neurosci. 27, 2704–2717 10.1523/JNEUROSCI.2991-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friedl P., and Mayor R. (2017) Tuning collective cell migration by cell-cell junction regulation. Cold Spring Harb. Perspect. Biol. 9, a029199 10.1101/cshperspect.a029199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cerami E., Gao J., Dogrusoz U., Gross B. E., Sumer S. O., Aksoy B. A., Jacobsen A., Byrne C. J., Heuer M. L., Larsson E., Antipin Y., Reva B., Goldberg A. P., Sander C., and Schultz N. (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., and Schultz N. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1–pl1 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vleminckx K., Vakaet L. Jr., Mareel M., Fiers W., and van Roy F. (1991) Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66, 107–119 10.1016/0092-8674(91)90143-M [DOI] [PubMed] [Google Scholar]
- 40. Perl A. K., Wilgenbus P., Dahl U., Semb H., and Christofori G. (1998) A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 10.1038/32433 [DOI] [PubMed] [Google Scholar]
- 41. Hennig G., Löwrick O., Birchmeier W., and Behrens J. (1996) Mechanisms identified in the transcriptional control of epithelial gene expression. J. Biol. Chem. 271, 595–602 10.1074/jbc.271.1.595 [DOI] [PubMed] [Google Scholar]
- 42. Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J., and García de Herreros A. (2000) The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84–89 10.1038/35000034 [DOI] [PubMed] [Google Scholar]
- 43. Cano A., Pérez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., and Nieto M. A. (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 10.1038/35000025 [DOI] [PubMed] [Google Scholar]
- 44. De Craene B., and Berx G. (2013) Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13, 97–110 10.1038/nrc3447 [DOI] [PubMed] [Google Scholar]
- 45. Borradori L., and Sonnenberg A. (1999) Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Invest. Dermatol. 112, 411–418 10.1046/j.1523-1747.1999.00546.x [DOI] [PubMed] [Google Scholar]
- 46. Kalluri R., and Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pastushenko I., Brisebarre A., Sifrim A., Fioramonti M., Revenco T., Boumahdi S., Van Keymeulen A., Brown D., Moers V., Lemaire S., De Clercq S., Minguijón E., Balsat C., Sokolow Y., Dubois C., et al. (2018) Identification of the tumour transition states occurring during EMT. Nature 556, 463–468 10.1038/s41586-018-0040-3 [DOI] [PubMed] [Google Scholar]
- 48. Thiery J. P., Acloque H., Huang R. Y. J., and Nieto M. A. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 49. Lou Y., Diao L., Cuentas E. R. P., Denning W. L., Chen L., Fan Y. H., Byers L. A., Wang J., Papadimitrakopoulou V. A., Behrens C., Rodriguez J. C., Hwu P., Wistuba I. I., Heymach J. V., and Gibbons D. L. (2016) Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin. Cancer Res. 22, 3630–3642 10.1158/1078-0432.CCR-15-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peng H., Zhang Y., Zhou Z., Guo Y., Huang X., Westover K. D., Zhang Z., Chen B., Hua Y., Li S., Xu R., Lin N., Peng B., and Shen S. (2019) Integrated analysis of ELMO1, serves as a link between tumour mutation burden and epithelial-mesenchymal transition in hepatocellular carcinoma. EBioMedicine 46, 105–118 10.1016/j.ebiom.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wheelock M. J., Shintani Y., Maeda M., Fukumoto Y., and Johnson K. R. (2008) Cadherin switching. J. Cell Sci. 121, 727–735 10.1242/jcs.000455 [DOI] [PubMed] [Google Scholar]
- 52. Mrozik K. M., Blaschuk O. W., Cheong C. M., Zannettino A. C. W., and Vandyke K. (2018) N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 18, 939 10.1186/s12885-018-4845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Onder T. T., Gupta P. B., Mani S. A., Yang J., Lander E. S., and Weinberg R. A. (2008) Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 68, 3645–3654 10.1158/0008-5472.CAN-07-2938 [DOI] [PubMed] [Google Scholar]
- 54. Padmanaban V., Krol I., Suhail Y., Szczerba B. M., Aceto N., Bader J. S., and Ewald A. J. (2019) E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573, 439–444 10.1038/s41586-019-1526-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Banyard J., and Bielenberg D. R. (2015) The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 56, 403–413 10.3109/03008207.2015.1060970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kröger C., Afeyan A., Mraz J., Eaton E. N., Reinhardt F., Khodor Y. L., Thiru P., Bierie B., Ye X., Burge C. B., and Weinberg R. A. (2019) Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 116, 7353–7362 10.1073/pnas.1812876116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bierie B., Pierce S. E., Kroeger C., Stover D. G., Pattabiraman D. R., Thiru P., Liu Donaher J., Reinhardt F., Chaffer C. L., Keckesova Z., and Weinberg R. A. (2017) Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 114, E2337–E2346 10.1073/pnas.1618298114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., and Friedl P. (2007) Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9, 893–904 10.1038/ncb1616 [DOI] [PubMed] [Google Scholar]
- 59. Ilina O., and Friedl P. (2009) Mechanisms of collective cell migration at a glance. J. Cell Sci. 122, 3203–3208 10.1242/jcs.036525 [DOI] [PubMed] [Google Scholar]
- 60. Hamidi H., and Ivaska J. (2018) Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer 18, 533–548 10.1038/s41568-018-0038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cohen S., Haimovich J., and Hollander N. (2003) Anti-idiotype x anti-LFA-1 bispecific antibodies inhibit metastasis of B cell lymphoma. J. Immunol. 170, 2695–2701 10.4049/jimmunol.170.5.2695 [DOI] [PubMed] [Google Scholar]
- 62. Canel M., Serrels A., Miller D., Timpson P., Serrels B., Frame M. C., and Brunton V. G. (2010) Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res. 70, 9413–9422 10.1158/0008-5472.CAN-10-1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bolós V., Gasent J. M., López-Tarruella S., and Grande E. (2010) The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco. Targets Ther. 3, 83–97 10.2147/ott.s6909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stott S. L., Hsu C.-H., Tsukrov D. I., Yu M., Miyamoto D. T., Waltman B. A., Rothenberg S. M., Shah A. M., Smas M. E., Korir G. K., Floyd F. P. Jr., Gilman A. J., Lord J. B., Winokur D., Springer S., et al. (2010) Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. U.S.A. 107, 18392–18397 10.1073/pnas.1012539107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Molnar B., Ladanyi A., Tanko L., Sréter L., and Tulassay Z. (2001) Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin. Cancer Res. 7, 4080–4085 [PubMed] [Google Scholar]
- 66. Yu M., Bardia A., Wittner B. S., Stott S. L., Smas M. E., Ting D. T., Isakoff S. J., Ciciliano J. C., Wells M. N., Shah A. M., Concannon K. F., Donaldson M. C., Sequist L. V., Brachtel E., Sgroi D., et al. (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aceto N., Bardia A., Miyamoto D. T., Donaldson M. C., Wittner B. S., Spencer J. A., Yu M., Pely A., Engstrom A., Zhu H., Brannigan B. W., Kapur R., Stott S. L., Shioda T., Ramaswamy S., et al. (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 10.1016/j.cell.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Larsson A.-M., Jansson S., Bendahl P.-O., Levin Tykjaer Jörgensen C., Loman N., Graffman C., Lundgren L., Aaltonen K., and Rydén L. (2018) Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 20, 48 10.1186/s13058-018-0976-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Murlidhar V., Reddy R. M., Fouladdel S., Zhao L., Ishikawa M. K., Grabauskiene S., Zhang Z., Lin J., Chang A. C., Carrott P., Lynch W. R., Orringer M. B., Kumar-Sinha C., Palanisamy N., Beer D. G., Wicha M. S., Ramnath N., Azizi E., and Nagrath S. (2017) Poor prognosis indicated by venous circulating tumor cell clusters in early-stage lung cancers. Cancer Res. 77, 5194–5206 10.1158/0008-5472.CAN-16-2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hou J.-M., Krebs M. G., Lancashire L., Sloane R., Backen A., Swain R. K., Priest L. J. C., Greystoke A., Zhou C., Morris K., Ward T., Blackhall F. H., and Dive C. (2012) Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532 10.1200/JCO.2010.33.3716 [DOI] [PubMed] [Google Scholar]
- 71. Jia D., Li X., Bocci F., Tripathi S., Deng Y., Jolly M. K., Onuchic J. N., and Levine H. (2019) Quantifying cancer epithelial-mesenchymal plasticity and its association with stemness and immune response. J. Clin. Med. 8, E725 10.3390/jcm8050725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gaggioli C., Hooper S., Hidalgo-Carcedo C., Grosse R., Marshall J. F., Harrington K., and Sahai E. (2007) Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400 10.1038/ncb1658 [DOI] [PubMed] [Google Scholar]
- 73. Labernadie A., Kato T., Brugués A., Serra-Picamal X., Derzsi S., Arwert E., Weston A., González-Tarragó V., Elosegui-Artola A., Albertazzi L., Alcaraz J., Roca-Cusachs P., Sahai E., and Trepat X. (2017) A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237 10.1038/ncb3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Haeger A., Krause M., Wolf K., and Friedl P. (2014) Cell jamming: collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochim. Biophys. Acta 1840, 2386–2395 10.1016/j.bbagen.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 75. Elsamany S., Alzahrani A., Elkhalik S. A., Elemam O., Rawah E., Farooq M. U. H., Almatrafi M., Olayan K. F. (2014) Prognostic value of mammographic breast density in patients with metastatic breast cancer. Med. Oncol. 31, 96 10.1007/s12032-014-0096-3 [DOI] [PubMed] [Google Scholar]
- 76. Nguyen-Ngoc K.-V., Cheung K. J., Brenot A., Shamir E. R., Gray R. S., Hines W. C., Yaswen P., Werb Z., and Ewald A. J. (2012) ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl. Acad. Sci. U.S.A. 109, E2595–E2604 10.1073/pnas.1212834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Drost J., and Clevers H. (2018) Organoids in cancer research. Nat. Rev. Cancer 18, 407–418 10.1038/s41568-018-0007-6 [DOI] [PubMed] [Google Scholar]
- 78. Farahani E., Patra H. K., Jangamreddy J. R., Rashedi I., Kawalec M., Rao Pariti R. K., Batakis P., and Wiechec E. (2014) Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 35, 747–759 10.1093/carcin/bgu045 [DOI] [PubMed] [Google Scholar]
- 79. Cooper J., and Giancotti F. G. (2019) Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 35, 347–367 10.1016/j.ccell.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Taddei I., Deugnier M.-A., Faraldo M. M., Petit V., Bouvard D., Medina D., Fässler R., Thiery J. P., and Glukhova M. A. (2008) β1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 10, 716–722 10.1038/ncb1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Turaga S. M., and Lathia J. D. (2016) Adhering towards tumorigenicity: altered adhesion mechanisms in glioblastoma cancer stem cells. CNS Oncol. 5, 251–259 10.2217/cns-2016-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. López-Rovira T., Silva-Vargas V., and Watt F. M. (2005) Different consequences of β1 integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation, and intercellular communication. J. Invest. Dermatol. 125, 1215–1227 10.1111/j.0022-202X.2005.23956.x [DOI] [PubMed] [Google Scholar]
- 83. Ding M., and Wang X. (2017) Antagonism between Hedgehog and Wnt signaling pathways regulates tumorigenicity. Oncol. Lett. 14, 6327–6333 10.3892/ol.2017.7030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cavallaro U., and Dejana E. (2011) Adhesion molecule signalling: not always a sticky business. Nat. Rev. Mol. Cell Biol. 12, 189–197 10.1038/nrm3068 [DOI] [PubMed] [Google Scholar]
- 85. Williams E. J., Furness J., Walsh F. S., and Doherty P. (1994) Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron 13, 583–594 10.1016/0896-6273(94)90027-2 [DOI] [PubMed] [Google Scholar]
- 86. Suyama K., Shapiro I., Guttman M., and Hazan R. B. (2002) A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2, 301–314 10.1016/S1535-6108(02)00150-2 [DOI] [PubMed] [Google Scholar]
- 87. Utton M. A., Eickholt B., Howell F. V., Wallis J., and Doherty P. (2001) Soluble N-cadherin stimulates fibroblast growth factor receptor dependent neurite outgrowth and N-cadherin and the fibroblast growth factor receptor co-cluster in cells. J. Neurochem. 76, 1421–1430 10.1046/j.1471-4159.2001.00140.x [DOI] [PubMed] [Google Scholar]
- 88. Kon E., Calvo-Jiménez E., Cossard A., Na Y., Cooper J. A., and Jossin Y. (2019) N-cadherin-regulated FGFR ubiquitination and degradation control mammalian neocortical projection neuron migration. eLife 8, e47673 10.7554/eLife.47673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qian X., Anzovino A., Kim S., Suyama K., Yao J., Hulit J., Agiostratidou G., Chandiramani N., McDaid H. M., Nagi C., Cohen H. W., Phillips G. R., Norton L., and Hazan R. B. (2014) N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene 33, 3411–3421 10.1038/onc.2013.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sigismund S., Avanzato D., and Lanzetti L. (2018) Emerging functions of the EGFR in cancer. Mol. Oncol. 12, 3–20 10.1002/1878-0261.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bhargava R., Gerald W. L., Li A. R., Pan Q., Lal P., Ladanyi M., and Chen B. (2005) EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod. Pathol. 18, 1027–1033 10.1038/modpathol.3800438 [DOI] [PubMed] [Google Scholar]
- 92. Valenzuela-Iglesias A., Burks H. E., Arnette C. R., Yalamanchili A., Nekrasova O., Godsel L. M., and Green K. J. (2019) Desmoglein 1 regulates invadopodia by suppressing EGFR/Erk signaling in an Erbin-dependent manner. Mol. Cancer Res. 17, 1195–1206 10.1158/1541-7786.MCR-18-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. De Palma M., Biziato D., and Petrova T. V. (2017) Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 10.1038/nrc.2017.51 [DOI] [PubMed] [Google Scholar]
- 94. Harjunpää H., Llort Asens M., Guenther C., and Fagerholm S. C. (2019) Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 10, 1078 10.3389/fimmu.2019.01078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Klein D. (2018) The tumor vascular endothelium as decision maker in cancer therapy. Front. Oncol. 8, 367 10.3389/fonc.2018.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Albert M. L., Pearce S. F., Francisco L. M., Sauter B., Roy P., Silverstein R. L., and Bhardwaj N. (1998) Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 10.1084/jem.188.7.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Akakura S., Singh S., Spataro M., Akakura R., Kim J.-I., Albert M. L., and Birge R. B. (2004) The opsonin MFG-E8 is a ligand for the αvβ5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp. Cell Res. 292, 403–416 10.1016/j.yexcr.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 98. Corn P. G., Smith B. D., Ruckdeschel E. S., Douglas D., Baylin S. B., and Herman J. G. (2000) E-cadherin expression is silenced by 5′ CpG island methylation in acute leukemia. Clin. Cancer Res. 6, 4243–4248 [PubMed] [Google Scholar]
- 99. Melki J. R., Vincent P. C., Brown R. D., and Clark S. J. (2000) Hypermethylation of E-cadherin in leukemia. Blood 95, 3208–3213 10.1182/blood.V95.10.3208 [DOI] [PubMed] [Google Scholar]
- 100. Hosokawa K., Arai F., Yoshihara H., Iwasaki H., Hembree M., Yin T., Nakamura Y., Gomei Y., Takubo K., Shiama H., Matsuoka S., Li L., and Suda T. (2010) Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell 6, 194–198 10.1016/j.stem.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 101. O'Day S., Pavlick A., Loquai C., Lawson D., Gutzmer R., Richards J., Schadendorf D., Thompson J. A., Gonzalez R., Trefzer U., Mohr P., Ottensmeier C., Chao D., Zhong B., de Boer C. J., et al. (2011) A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br. J. Cancer 105, 346–352 10.1038/bjc.2011.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mitjans F., Sander D., Adán J., Sutter A., Martinez J. M., Jäggle C. S., Moyano J. M., Kreysch H. G., Piulats J., and Goodman S. L. (1995) An anti-αv-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J. Cell Sci. 108, 2825–2838 [DOI] [PubMed] [Google Scholar]
- 103. Mahalingam B., Van Agthoven J. F., Xiong J.-P., Alonso J. L., Adair B. D., Rui X., Anand S., Mehrbod M., Mofrad M. R. K., Burger C., Goodman S. L., and Arnaout M. A. (2014) Atomic basis for the species-specific inhibition of αV integrins by monoclonal antibody 17E6 is revealed by the crystal structure of αVβ3 ectodomain-17E6 Fab complex. J. Biol. Chem. 289, 13801–13809 10.1074/jbc.M113.546929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mitjans F., Meyer T., Fittschen C., Goodman S., Jonczyk A., Marshall J. F., Reyes G., and Piulats J. (2000) In vivo therapy of malignant melanoma by means of antagonists of αv integrins. Int. J. Cancer 87, 716–723 [DOI] [PubMed] [Google Scholar]
- 105. Raab-Westphal S., Marshall J. F., and Goodman S. L. (2017) Integrins as therapeutic targets: successes and cancers. Cancers (Basel) 9, 110 10.3390/cancers9090110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dong Y., Yu T., Ding L., Laurini E., Huang Y., Zhang M., Weng Y., Lin S., Chen P., Marson D., Jiang Y., Giorgio S., Pricl S., Liu X., Rocchi P., and Peng L. (2018) A dual targeting dendrimer-mediated siRNA delivery system for effective gene silencing in cancer therapy. J. Am. Chem. Soc. 140, 16264–16274 10.1021/jacs.8b10021 [DOI] [PubMed] [Google Scholar]
- 107. Sartori A., Corno C., De Cesare M., Scanziani E., Minoli L., Battistini L., Zanardi F., and Perego P. (2019) Efficacy of a selective binder of αVβ3 integrin linked to the tyrosine kinase inhibitor sunitinib in ovarian carcinoma preclinical models. Cancers (Basel) 11, 531 10.3390/cancers11040531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang L., Hu C., Chao H., Wang R., Lu H., Li H., and Chen H. (2019) miR-29c regulates resistance to paclitaxel in nasopharyngeal cancer by targeting ITGB1. Exp. Cell Res. 378, 1–10 10.1016/j.yexcr.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 109. Hong S.-K., Lee H., Kwon O.-S., Song N.-Y., Lee H.-J., Kang S., Kim J.-H., Kim M., Kim W., and Cha H.-J. (2018) Large-scale pharmacogenomics based drug discovery for ITGB3 dependent chemoresistance in mesenchymal lung cancer. Mol. Cancer 17, 175 10.1186/s12943-018-0924-8 [DOI] [PMC free article] [PubMed] [Google Scholar]