Abstract
Objective
Compared with hemoglobin A1c (HbA1c), continuous glucose monitoring (CGM) may better capture risk of diabetes complications in patients with chronic kidney disease (CKD), including diabetic peripheral neuropathy (DPN). We hypothesized that glucose time in range (TIR), measured by CGM, is associated with DPN symptoms among participants with type 2 diabetes mellitus (type 2 DM) and moderate-to-severe CKD.
Research design and methods
We enrolled 105 people with type 2 DM treated with insulin or sulfonylurea, 81 participants with CKD (estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2) and 24 matched control participants with eGFR ≥60 mL/min/1.73 m2. Each participant wore a CGM for two 6-day periods. Calculated glycemic measures included TIR (glucose 70–180 mg/dL) and glucose management indicator (GMI). DPN symptoms were assessed using the Michigan Neuropathy Screening Instrument (MNSI) questionnaire, with a positive MNSI score defined as ≥2 symptoms.
Results
Participants with CKD had a mean age of 68 years, diabetes duration 20 years, eGFR 38 mL/min/1.73 m2 and HbA1c 7.8%, 61 mmol/mol. Sixty-two participants reported ≥2 DPN symptoms, 51 (63%) with CKD and 11 (46%) controls. Less TIR and higher GMI were associated with higher risk of MNSI questionnaire score ≥2 (OR 1.25 (95% CI 1.02 to 1.52) per 10% lower TIR, and OR 1.79 (95% CI 1.05 to 3.04) per 1% higher GMI, adjusting for age, gender and race). Similar results were observed when analyses were restricted to participants with CKD. In contrast, there was no significant association of HbA1c with DPN symptoms.
Conclusions
Symptoms of DPN were common among participants with long-standing type 2 DM and CKD. Lower TIR and higher GMI were associated with DPN symptoms.
Keywords: diabetes, diabetic foot, nephropathy, neuropathy, glucose monitoring
Significance of this study.
What is already known about this subject?
For type 2 diabetes mellitus (type 2 DM), the role of glycemia in the development of diabetic peripheral neuropathy is not entirely understood.
Hemoglobin A1c (HbA1c) is a fairly crude marker of glycemia, particularly in patients with chronic kidney disease, and continuous glucose monitor is a powerful tool in the therapeutic management of diabetes.
What are the new findings?
We demonstrate that less time in range and higher glucose management indicator were significantly associated with the prevalence of diabetic peripheral neuropathy.
Laboratory value HbA1c was not found to be associated with peripheral neuropathy.
How might these results change the focus of research or clinical practice?
Our data suggest that CGM metrics may provide clinicians and researchers especially valuable information for evaluating risk of diabetes complications.
Introduction
Diabetic peripheral neuropathy (DPN) is the most common complication of diabetes and leads to physical disability, with profound effect on quality of life, morbidity and mortality.1–4 In particular, DPN contributes to diabetic foot ulcers, lower extremity amputations and infection.
Diabetes complications tend to coexist, but surprisingly little has been published regarding the relationship between chronic kidney disease (CKD) and DPN, and existing data do not demonstrate a clear association. This is in contrast to diabetic retinopathy, for which there is a clear relationship with CKD, particularly type 1 diabetes mellitus (type 1 DM) and in type 2 diabetes mellitus (type 2 DM).5 6 Patients with CKD are at high risk of diabetic foot disease, and lower extremity amputation is two to six times greater among those with CKD and diabetes than diabetes alone.7 8 These risks may plausibly be explained in part by coexisting DPN.
Hyperglycemia is a known risk factor for the development and progression of both DPN and CKD.9–12 Improved glycemic control has been shown to dramatically reduce the risk of DPN in people with type 1 DM but only modestly reduce the incidence of DPN in type 2 DM.13–15 This discrepancy highlights that for type 2 DM, the role of glycemia in the development of DPN is not entirely understood.
Compared with hemoglobin A1c (HbA1c), continuous glucose monitoring (CGM) may better capture risk of complications, including DPN, through precisely measured time in range (TIR), glucose management indicator (GMI) and measures of glucose variability. TIR has been found to be strongly associated with the development or progression of diabetic retinopathy and microalbuminuria in type 1 and type 2 DM.16 17 However, more data are needed to evaluate the relationship of TIR with microvascular diabetes outcomes, and no prior studies have identified an association of TIR or GMI with DPN.18
We aimed to 1) describe the prevalence of DPN symptoms among participants with type 2 DM and moderate-to-severe CKD and 2) examine the association of glycemia (as measured by CGM) with DPN symptoms among our target population.
Methods
Study population and design
The Continuous Glucose Monitoring to Assess Glycemia in Chronic Kidney Disease (CANDY) study was a prospective observational cohort study designed to examine hyperglycemia, glycemic variability and biomarkers of glycemic control among people with type 2 DM and CKD.19 Participants with type 2 DM were enrolled between 7 August 2015 and 12 July 2017 from Nephrology clinics, the Diabetes Care Center and referring clinics associated with the University of Washington, Seattle, Washington, USA. We recruited 81 participants with moderate-to-severe CKD (estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2). We then recruited 24 control participants (with eGFR ≥60 mL/min/1.73 m2) from the same source population, matching on the characteristics of age, duration of diabetes, HbA1c and glucose-lowering medication use of participants with CKD. Because a primary objective of the CANDY study was to evaluate incidence of hypoglycemia in this population, eligible participants were treated with a sulfonylurea and/or insulin as their diabetes medication regimen. Exclusion criteria were age <18 years, history of kidney transplant, dialysis treatment, treatment with erythropoietin, current use of clinical CGM, pregnancy or current therapy for cancer and inability to speak English. Of the 149 consented participants, 105 completed the study. Nine participants were found to meet exclusion criteria after consent obtained, 13 declined to participate and 17 were lost to follow-up. One participant completed the study, but the data from the CGM were insufficient for analysis.
Continuous glucose monitoring
The Medtronic Enlite sensor (Medtronic, Northridge, California, USA) was used to monitor glycemia for two 6-day periods, separated by 2 weeks. With this sensor, interstitial glucose levels were recorded every 5 min, with a detection range of 40–400 mg/dL. Each participant was also provided with a Freestyle Lite glucose meter (Abbott, Alameda, California, USA) for self-monitored blood glucose at least twice a day to calibrate the CGM. Study physicians evaluated each CGM report, excluding periods of time with evidence of CGM malfunction or marked dyssynchrony (>30%) between CGM and fingerstick glucose values.
Glycemia metrics evaluated included measurements of overall glycemia and glucose variability over the 12-day period. TIR, glucose 70–180 mg/dL, was computed by calculating the percentage of CGM glucose readings in target range.20 Time above range (TAR, glucose >180 mg/dL) was calculated in a similar manner. GMI, which is the estimation of HbA1c based on CGM data, was calculated based on mean CGM glucose over the 12 days using the previously published equation.20 21 Per cent coefficient of variation was calculated as the SD divided by mean multiplied by 100 of all CGM glucose concentrations measured every 5 min over 12 days.
Assessment of peripheral neuropathy symptoms
At the first study visit, participants were given the Michigan Neuropathy Screening Instrument (MNSI) questionnaire to assess symptoms of peripheral neuropathy. The MNSI questionnaire has been validated to assess distal DPN compared with gold standard diagnostic testing and has been widely used in large clinical trials, including Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC), Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D).12 14 15 22 A MNSI total score was calculated using the published scoring algorithm, assigning one point for 12 of the 14 questions in the questionnaire. The algorithm excludes questions 4 (cramping) and 10 (weakness) from the total score, as question 4 is in part a measure of impaired circulation, and question 10 is in part a measure of general asthenia.22
MNSI questionnaire score ≥2 was our primary outcome definition for DPN because it was also used in the ACCORD and BARI-2D studies.14 15
Secondary outcomes included MNSI questionnaire ≥4, which represents more advanced disease and was used in DCCT/EDIC,13 and a composite outcome of MNSI questionnaire score ≥2 or reported use of neuropathy medications, to include participants with DPN who are no longer symptomatic due to treatment.23
Clinical data
Race, ethnicity, education, general health, smoking and medical history were defined by self-report. Medications were inventoried with assistance from electronic health records. Serum creatinine was measured at two separate study visits (2 weeks apart) using methods traceable to isotope dilution mass spectrometry, and eGFR was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The mean of the two eGFR values was used for analyses. HbA1c was measured by HPLC at the University of Missouri, conforming to National Glycohemoglobin Standardization Program (NGSP) standards, from whole blood collected at the end of each CGM period; the mean of the two values was used for analyses.
Statistical analysis
Linear regression with robust Huber-White SEs was used to test differences in MNSI score by CKD status, adjusting for age, sex and race. Logistic regression was used to test differences in the prevalence of DPN by CKD status, TIR, GMI and other glycemia metrics and clinical characteristics. In analyses that were considered secondary because they may overly adjust for causal intermediates, differences in DPN were additionally adjusted for HbA1c or neuropathy meds. All analyses were performed using SPSS statistical software (IBM, Released 2017, IBM SPSS Statistics for Windows, V.25.0. Armonk, New York, USA). A two-tailed p value <0.05 was taken as evidence of statistical significance in all analyses.
Results
Participant characteristics
The 81 participants with CKD had a mean age of 68 years, diabetes duration 20 years, body mass index 33.8 kg/m2, eGFR 38 mL/min/1.73 m2 and HbA1c 7.8%; 74% were white, 89% used insulin (table 1). In comparison, the 24 control participants had overlapping but slightly lower distributions of age and duration of diabetes and a similar distribution of glucose-lowering medication use (by design). History of myocardial infarction (MI), congestive heart failure (CHF) and stroke was reported in 16%, 22% and 15% of participants with CKD, respectively, and 4% of participants without CKD for each, MI, CHF and stroke. Statin use was reported in 93% of participants with CKD and 83% of control participants. Twenty-four per cent of individuals reported use of neuropathic medications. Gabapentin was the most commonly reported neuropathic medication, with 22% of CKD and 17% of controls reporting use. Duloxetine or venlafaxine were reported in 5% of participants with CKD and 4% of controls.
Table 1.
Baseline demographic and clinical characteristics of participants
| CKD | Controls | |
| (n=81) | (n=24) | |
| Demographics | ||
| Age (years) | 68 (10) | 64 (10) |
| Male | 52 (64) | 15 (63) |
| Race/ethnicity | ||
| White | 60 (74) | 20 (83) |
| Black | 12 (15) | 2 (8) |
| Other | 9 (11) | 2 (8) |
| Hispanic ethnicity | 8 (10) | 3 (13) |
| Highest level of education | ||
| High school | 22 (27) | 7 (29) |
| Trade school | 17 (21) | 7 (29) |
| College | 23 (28) | 7 (29) |
| Graduate school | 19 (24) | 3 (13) |
| Health history | ||
| Current smoking | 2 (3) | 3 (13) |
| History of MI | 13 (16) | 1 (4) |
| History of CHF | 18 (22) | 1 (4) |
| History of stroke | 12 (15) | 1 (4) |
| Duration of diabetes (years) | 20 (10.55) | 16 (8.35) |
| Medication use | ||
| Insulin | 72 (89) | 21 (88) |
| Insulin dose (units/kg/day) | 0.56 (0.43) | 0.65 (0.49) |
| Insulin secretagogues | 18 (22) | 5 (21) |
| Sulfonylureas | 17 (21) | 5 (21) |
| Meglitinides | 1 (1) | 0 (0) |
| Other glucose-lowering agents | 29 (36) | 18 (75) |
| DPP4i | 3 (4) | 0 (0) |
| GLP1 agonists | 11 (14) | 5 (21) |
| Biguanides | 18 (22) | 14 (58) |
| SGLT2i | 1 (1) | 5 (21) |
| TZDs | 0 (0) | 0 (0) |
| Alpha-glucosidase inhibitor | 0 (0) | 0 (0) |
| Antihypertensive medications | ||
| ACEi/ARBs | 60 (74) | 21 (88) |
| Beta-blockers | 38 (47) | 5 (21) |
| Lipid-lowering medications | ||
| Statins | 75 (93) | 20 (83) |
| Neuropathy medications | ||
| Gabapentin | 18 (22) | 4 (17) |
| Duloxetine/Venlafaxine | 4 (5) | 1 (4) |
| Lidocaine patch | 3 (4) | 0 |
| Tricyclic antidepressants | 0 (0) | 0 (0) |
| Pregabalin | 0 (0) | 0 (0) |
| Physical characteristics | ||
| Height (cm) | 171 (10) | 172 (9) |
| BMI | 33.8 (5.7) | 32.4 (6.2) |
| Systolic blood pressure (mm Hg) | 132 (21) | 136 (17) |
| Diastolic blood pressure (mm Hg) | 72 (13) | 78 (12) |
| Laboratory values | ||
| eGFR (mL/min/1.73 m2) | 38 (14) | 83 (11) |
| Urine ACR (mg/g) | 150 (28 to 637) | 14 (8 to 57) |
| HbA1c (%), mean (SD) | 7.8 (1.6) | 8.0 (1.5) |
| HbA1c (mmol/mol), mean (SD) | 62 (17) | 64 (16) |
| CGM variables | ||
| GMI (%), mean (SD) | 7.37 (0.96) | 7.09 (0.73) |
| % CV of CGM sensor glucose readings, mean (SD) | 31 (6) | 29 (7) |
| % Time below range (<70 mg/dL) | 2 (3) | 2 (3) |
| % Time in range (70–180 mg/dL) | 62 (23) | 69 (22) |
| % Time above range (>180 mg/dL) | 37 (23) | 29 (22) |
Cell contents are N (%) or mean (SD), except for urine ACR which is median (25th percentile, 75th percentile).
ACEi, angiotensin-converting-enzyme inhibitors; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; CGM, continuous glucose monitor; CHF, congestive heart failure; CKD, chronic kidney disease; CV, coefficient of variation; DPP4i, dipeptidyl peptidase 4 inhibitor; eGFR, estimated glomerular filtration rate; GLP1, glucagon-like peptide 1; GMI, glucose management indicator; HbA1c, hemoglobin A1c; MI, myocardial infarction; SGLT2i, sodium-glucose cotransporter 2 inhibitors; TZD, thiazolidinedione.
CKD and peripheral neuropathy
The mean MNSI questionnaire score for participants with CKD was 3.36 (2.97) and 2.33 (2.41) for controls (p=0.046), with an adjusted difference of 1.35 (95% CI 0.03 to 2.67), p=0.025. For most questions in the MNSI questionnaire, participants with CKD had a higher prevalence of symptoms compared with controls (figure 1). The most frequently reported symptom in both groups was cramping in the lower extremities (67% CKD, 50% control), which was not included in the algorithm used to calculate the total MNSI score. Eight participants (seven CKD, one control) reported a prior amputation. Sixty-two participants had DPN, as established by an MNSI questionnaire score ≥2, including 51 (63%) with CKD and 11 (46%) controls; adjusted OR 2.29 (95% CI 0.88 to 6.00). Estimates of association of CKD with secondary definitions of DPN (MNSI score ≥4, MNSI score ≥2 or use of neuropathic medications) were similar (table 2).
Figure 1.
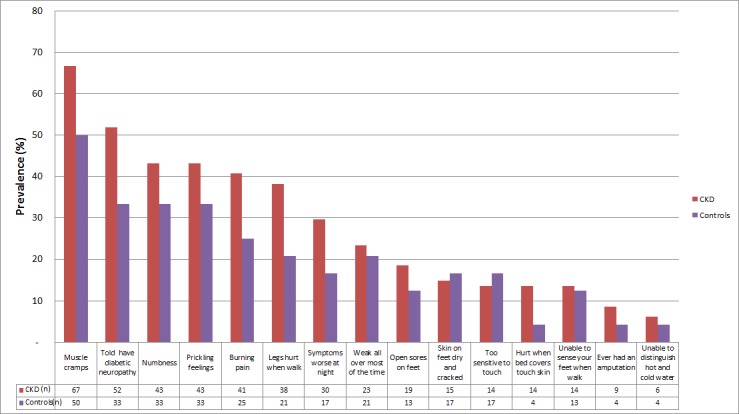
Prevalence of neuropathy symptoms based on Michigan Neuropathy Screening Instrument questionnaire response by chronic kidney disease (CKD) status.
Table 2.
Associations of CKD with distal peripheral neuropathy
| CKD (n=81) |
Controls (n=24) |
P value* | Adjusted difference (95% CI)† |
Adjusted OR (95% CI)† |
|
| Mean MNSI questionnaire score | 3.36 (2.97) | 2.33 (2.41) | 0.046 | 1.35 (0.03 to 2.67) | |
| MNSI score ≥2‡ | 51 (63%) | 11 (46%) | 0.160 | 2.29 (0.88 to 6.00) | |
| MNSI score ≥2 or use of one or more neuropathy medications | 56 (69%) | 13 (54%) | 0.222 | 2.31 (0.86 to 6.20) | |
| MNSI score ≥4 | 36 (44%) | 8 (33%) | 0.358 | 2.16 (0.78 to 5.94) |
*Unadjusted t-test or χ2 test.
†Adjusted for age, gender, race
‡ Primary outcome of interest.
CKD, chronic kidney disease; MNSI, Michigan Neuropathy Screening Instrument.
TIR, glycemia and peripheral neuropathy
Among all participants, mean TIR was 63%±23%. For those participants who were within the target range >70% of the time, DPN prevalence was 43%, and those who were within target range <70% of the time, DPN prevalence was 74%. The prevalence of DPN was inversely correlated with TIR (figure 2) and significantly associated, adjusting for age, sex and race (OR 1.25 (95% CI 1.02 to 1.52) per 10% (144 min) lower TIR). TAR was also associated with prevalence of DPN (table 3). GMI was 7.48% for participants with DPN and 7.06% for participants without DPN. GMI was significantly associated with the prevalence of DPN, adjusting for age, sex and race (OR 1.79 (95% CI 1.05 to 3.04) per 1% higher GMI). Conversely, there was no significant relationship of HbA1c, glucose variability as measured by %CV or diabetes duration with DPN. With further adjustment for body mass index, eGFR, use of insulin and use of sulfonylureas, estimates of association were similar, with wider CIs as expected for multiple covariates applied to a relatively small group of participants. Similar results were observed when secondary definitions of DPN were evaluated as outcomes (online supplementary table 1). In analyses stratified by CKD status, estimates for associations of TIR, GMI and other glycemia metrics with DPN were similar to those in the whole population or further from the null (table 4).
Figure 2.
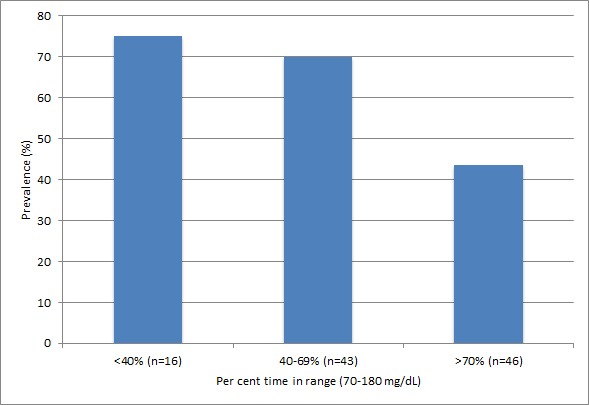
Prevalence of distal peripheral neuropathy according to time in range asceratined by continuous glucose monitoring.
Table 3.
Association of glycemia with distal peripheral neuropathy
| Exposure | Unadjusted | Model 1 | Model 2 | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Time in range (70–180 mg/dL) (per 10% lower)* | 1.26 (1.04 to 1.53) | 0.021 | 1.25 (1.02 to 1.52) | 0.031 | 1.23 (0.99 to 1.52) | 0.060 |
| Time above range (>180 mg/dL) (per 10% higher)* | 1.24 (1.03 to 1.50) | 0.024 | 1.23 (1.02 to 1.50) | 0.035 | 1.22 (0.99 to 1.50) | 0.061 |
| % coefficient of variations (per 6% higher (1 SD)) | 1.25 (0.83 to 1.86) | 0.283 | 1.24 (0.82 to 1.88) | 0.312 | 1.19 (0.75 to 1.90) | 0.457 |
| GMI (per 1% higher) | 1.82 (1.08 to 3.07) | 0.024 | 1.79 (1.05 to 3.04) | 0.033 | 1.81 (1.02 to 3.20) | 0.042 |
| HbA1c (per 1% or 11 mmol/mol higher) | 1.29 (0.97 to 1.70) | 0.08 | 1.25 (0.93 to 1.69) | 0.139 | 1.29 (0.93 to 1.79) | 0.129 |
| Duration of diabetes (per 10 years longer) | 1.15 (0.77 to 1.71) | 0.51 | 1.19 (0.77 to 1.82) | 0.433 | 1.13 (0.73 to 1.75) | 0.575 |
Sixty-two of 105 participants with a total MNSI questionnaire score ≥2 were defined as having distal peripheral neuropathy in this analysis.
Model 1 is adjusted for age, gender, race. Model 2 additionally adjusts for BMI, eGFR, use of insulin and use of sulfonylureas. In model 2, all outcomes except duration of diabetes additionally adjust for duration of diabetes.
*10%=144 minutes/day.
BMI, body mass index; eGFR, estimated glomerular filtration rate; GMI, glucose management indicator; HbA1c, hemoglobin A1c; MNSI, Michigan Neuropathy Screening Instrument.
Table 4.
Associations of glycemia with diabetic peripheral neuropathy stratified by CKD status
| CKD | Controls | P value for interaction | |
| Adjusted OR* (95% CI) | Adjusted OR* (95% CI) | ||
| Time in range (70–180 mg/dL) (per 10% lower)† | 1.27 (1.01 to 1.61) | 0.97 (0.61 to 1.53) | 0.69 |
| Time above range (>180 mg/dL) (per 10% higher)† | 1.26 (1.01 to 1.58) | 0.92 (0.58 to 1.47) | 0.63 |
| Per cent coefficient of variations (per 6% higher (1 SD)) | 1.04 (0.63 to 1.71) | 3.10 (0.87 to 11.01) | 0.37 |
| GMI (per 1% higher) | 1.93 (1.05 to 3.55) | 0.68 (0.16 to 2.84) | 0.58 |
| HbA1c (per 1% or 11 mmol/mol higher) | 1.33 (0.94 to 1.88) | 0.48 (0.14 to 1.65) | 0.83 |
| Duration of diabetes (per 10 years longer) | 1.16 (0.73 to 1.87) | 0.91 (0.23 to 3.65) | 0.58 |
Sixty-two of 105 participants with a total MNSI questionnaire score ≥2 were defined as having distal peripheral neuropathy in this analysis.
*adjusted for age, gender, and race
†10%=144 minutes/day
CKD, chronic kidney disease; GMI, glucose management indicator; HbA1c, hemoglobin A1c; MNSI, Michigan Neuropathy Screening Instrument.
bmjdrc-2019-000991supp001.pdf (44.4KB, pdf)
Discussion
DPN symptoms were common among people with long-standing type 2 DM and CKD in this observational cohort study. On average, participants with CKD reported one more neuropathy symptom than control subjects. Hyperglycemia was also common, with glucose >180 mg/dL during approximately one-third of time monitored by CGM. Less TIR and higher GMI were significantly associated with the prevalence of DPN. In contrast, HbA1c in this population with CKD was not associated with DPN, suggesting that CGM-derived measures of glycemia for these individuals more closely reflect the risk of DPN due to hyperglycemia than even well-measured biomarkers of mean glycemia which are known to underestimate in CKD.24 25
CKD and diabetic neuropathy have been widely studied, but the focus has been primarily on cardiac autonomic neuropathies, with few studies evaluating CKD and peripheral neuropathy.26–29 About 60%–70% of patients with CKD have some type of uremic polyneuropathy.30 However, uremia appears to have little effect specifically on the peripheral nervous system, as measured by nerve conduction studies, when eGFR is >12 mL/min/1.73 m2. In our study, 67% of participants with CKD reported an MNSI score ≥2, and the mean eGFR was 38 mL/min/1.73 m2 well above the threshold of 12 mL/min/1.73 m2 at which peripheral neuropathy due to uremia was previously reported. This suggests that the DPN symptoms we observed were likely due to diabetes, which is further supported by the significant associations of TIR and GMI with DPN in our study population.
There are several possible explanations for our observation that participants with CKD had more neuropathy symptoms than those without CKD, despite similar current diabetes treatments and glycemic control. First, participants with CKD may have had more risk factors for microvascular complications, such as cardiovascular disease and poor glycemic control earlier in the course of diabetes, and these risk factors might have led to both CKD and DPN, confounding the observed association. Second, CKD may act synergistically with type 2 DM to cause DPN by increasing inflammation, oxidative stress or other nerve damage pathways.31 Third, we cannot rule out that differences in symptoms were due to perceptions of neuropathy that are augmented by CKD, rather than physical nerve damage. Finally, there may be yet to be identified genetic risk factors that predispose individuals to microvascular complications of diabetes.
In demonstrating associations of TIR and GMI with DPN, our study is consistent with clinical trials reporting that glycemic control prevents or delays the development and progression of DPN. Our data parallel the DCCT/EDIC findings that intensive diabetes therapy reduced the risk of microvascular outcomes. In the DCCT/EDIC trial of type 1 DM, intensive diabetes therapy led to a dramatic reduction in DPN and its progression (78% relative risk reduction).13 In type 2 DM, enhanced glycemic control has been demonstrated to more modestly reduce the risk of developing, by DPN 5%–9%.14 15 32 For example, in the ACCORD trial, intensive glycemic therapy arm was associated with a lower prevalence of MNSI score ≥2 at the end of the study (HR 0.92 (95% CI 0.86 to 0.99)).15 The apparent blunted effects of intensive glycemic control on DPN comparing type 2 DM with type 1 DM may be due to multiple comorbidities also affecting peripheral nerves in type 2 DM, and this may be even more pronounced in our population of participants with CKD.27 33 Nonetheless, we observed significant correlations of both TIR and GMI with DPN symptoms.
HbA1c is a fairly crude marker of glycemia, particularly in patients with CKD, and CGM is a powerful tool in the therapeutic management of diabetes. Core glucose metrics determined by CGM, including TIR and GMI, provide more precise real-time and retrospective information about glycemic control.34 For example, for any given mean glucose level, HbA1c tends to be lower in end-stage kidney disease, while GMI would remain unbiased.24 Our data suggest that CGM measures may be more strongly associated with risk of developing DPN in this population. These measures are being considered as new therapeutic targets and more studies are needed to determine how CGM metrics correlate with diabetes complications.16 21
Glucose variability has been demonstrated to be an independent risk factor for free radical damage and diabetes complications, yet we did not find that glucose variability was associated with DPN.35 36 There is inconsistency in the relationship between glucose variability and DPN in the literature, and some research indicates that for type 2 DM, glucose variability may be regarded as a marker of beta-cell function rather than direct cause of DPN.37–39
To our knowledge, this is the first study to demonstrate associations of TIR and GMI with DPN. TIR findings parallel the DCCT/EDIC evaluation of TIR and the development and progression of other microvascular outcomes.16 Our study also contributes to the literature of DPN in participants with CKD, which to date is scant. Other strengths of the study include the use of a validated questionnaire to define our outcome of interest and a thorough inventory of neuropathy medications. In addition, we used CGM for sufficient periods of time to characterize glycemic patterns, standardized across participants. We evaluated contemporary glycemic metrics that are being considered as new therapeutic target in diabetes management. This study was limited by the relatively small sample size and the cross-sectional observational study design; therefore, we were not able to assess the temporal relationship between CKD or glycemia and DPN. Our relatively small amount of CGM data may not accurately reflect the long-term glycemic patterns throughout 20 years of living with diabetes. We did not use a physical examination-based definition for DPN, which means that our primary outcome was based on subjective report of symptoms. However, other studies have demonstrated the MNSI questionnaire closely correlates with physical examination findings, with MNSI score ≥4 threshold most consistent with the physical examiation.22 Additionally, subjective data are most relevant to patient experience and quality of life.
Conclusions
In conclusion and as hypothesized, lower TIR was associated with DPN symptoms, suggesting the importance of improved glycemic control in the prevention of DPN. We found that for every 10% lower TIR there is a 25% increased risk of DPN. Yet more studies are needed to understand why participants with CKD reported more symptoms of DPN despite similar glycemia. Laboratory value HbA1c in our CKD population was not found to be associated with DPN, whereas TIR and GMI were, suggesting CGM metrics may provide clinicians and researchers especially valuable information for evaluating risk of diabetes complications. Further research is needed to better understand the role that glycemic control plays in the development and progression of DPN in this population.
Acknowledgments
The authors would like to thank Medtronic for donating CGM equipment. The authors would also like to thank Abbott for donating self-monitored blood glucose equipment and supplies.
Footnotes
Contributors: LM, RK, NB, LZ, IBH and IHdB contributed to the conception and design of the study, analysis and interpretation of data, edited and reviewed the manuscript. NR carried out data collection. IA, ZB and DLT edited and reviewed the manuscript, and contributed to the discussion. All authors reviewed and approved the final version of the manuscript.
Funding: The CANDY Study was primarily supported by American Diabetes Association grant #4-15-CKD-20. Additional funding came from grants R01DK088762, R01DK087726 and T32DK007247 from the National Institute of Diabetes and Digestive and Kidney Diseases; a grant from Puget Sound Veterans Affairs Health Care System and an unrestricted grant from Northwest Kidney Centers.
Competing interests: IBH reports receiving research support from Medtronic Diabetes and consulting for Abbott Diabetes Care, Roche, Bigfoot and Becton Dickinson. IHdB reports consulting for Ironwood and Boehringer-Ingelheim.
Patient consent for publication: Not required.
Ethics approval: The study was reviewed and approved by Institutional Review Boards of the University of Washington and the Puget Sound Veterans Affairs Health Care System, Seattle, Washington, USA.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Protocols, consents, data and statistical analyses available on request.
References
- 1. Tentolouris N, Al-Sabbagh S, Walker MG, et al. . Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care 2004;27:1598–604. 10.2337/diacare.27.7.1598 [DOI] [PubMed] [Google Scholar]
- 2. Tesfaye S, Boulton AJM, Dyck PJ, et al. . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM 1998;91:733–7. 10.1093/qjmed/91.11.733 [DOI] [PubMed] [Google Scholar]
- 4. Argoff CE, Cole BE, Fishbain DA, et al. . Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006;81:S3–11. 10.1016/S0025-6196(11)61474-2 [DOI] [PubMed] [Google Scholar]
- 5. Grunwald JE, Alexander J, Maguire M, et al. . Prevalence of ocular fundus pathology in patients with chronic kidney disease. Clin J Am Soc Nephrol 2010;5:867–73. 10.2215/CJN.08271109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grunwald JE, Pistilli M, Ying G-S, et al. . Retinopathy and progression of CKD: the CRIC study. Clin J Am Soc Nephrol 2014;9:1217–24. 10.2215/CJN.11761113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care 2008;31:1331–6. 10.2337/dc07-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffstad O, Mitra N, Walsh J, et al. . Diabetes, lower-extremity amputation, and death. Diabetes Care 2015;38:1852–7. 10.2337/dc15-0536 [DOI] [PubMed] [Google Scholar]
- 9. Vincent AM, Russell JW, Low P, et al. . Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 2004;25:612–28. 10.1210/er.2003-0019 [DOI] [PubMed] [Google Scholar]
- 10. Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev 2006;22:257–73. 10.1002/dmrr.625 [DOI] [PubMed] [Google Scholar]
- 11. de Boer IH, Group DER, DCCT/EDIC Research Group . Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:24–30. 10.2337/dc13-2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nathan DM, Genuth S, Lachin J, et al. . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 13. The effect of intensive diabetes therapy on the development and progression of neuropathy. The diabetes control and complications trial Research Group. Ann Intern Med 1995;122:561–8. 10.7326/0003-4819-122-8-199504150-00001 [DOI] [PubMed] [Google Scholar]
- 14. Pop-Busui R, Lu J, Lopes N, et al. . Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the Bari 2D cohort. J Peripher Nerv Syst 2009;14:1–13. 10.1111/j.1529-8027.2009.00200.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ismail-Beigi F, Craven T, Banerji MA, et al. . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–30. 10.1016/S0140-6736(10)60576-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beck RW, Bergenstal RM, Riddlesworth TD, et al. . Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–5. 10.2337/dc18-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu J, Ma X, Zhou J, et al. . Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–6. 10.2337/dc18-1131 [DOI] [PubMed] [Google Scholar]
- 18. Hirsch IB, Sherr JL, Hood KK. Connecting the dots: validation of time in range metrics with microvascular outcomes. Diabetes Care 2019;42:345–8. 10.2337/dci18-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmad I, Zelnick LR, Batacchi Z, et al. . Hypoglycemia in people with type 2 diabetes and CKD. Clin J Am Soc Nephrol 2019;14:844–53. 10.2215/CJN.11650918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Battelino T, Danne T, Bergenstal RM, et al. . Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International consensus on time in range. Diabetes Care 2019;42:1593–603. 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bergenstal RM, Beck RW, Close KL, et al. . Glucose management indicator (GMI): a new term for estimating A1c from continuous glucose monitoring. Diabetes Care 2018;41:2275–80. 10.2337/dc18-1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herman WH, Pop-Busui R, Braffett BH, et al. . Use of the Michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications Trial/Epidemiology of diabetes interventions and complications. Diabet Med 2012;29:937–44. 10.1111/j.1464-5491.2012.03644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Association AD 11. microvascular complications and foot care. Diabetes Care 2019;42:S124–38. [DOI] [PubMed] [Google Scholar]
- 24. Hoshino J, Larkina M, Karaboyas A, et al. . Unique hemoglobin A1c level distribution and its relationship with mortality in diabetic hemodialysis patients. Kidney Int 2017;92:497–503. 10.1016/j.kint.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 25. Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. . A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007;30:1049–55. 10.2337/dc06-2127 [DOI] [PubMed] [Google Scholar]
- 26. Li J, Cao Y, Liu W, et al. . Correlations among diabetic microvascular complications: a systematic review and meta-analysis. Sci Rep 2019;9:3137 10.1038/s41598-019-40049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pop-Busui R, Boulton AJM, Feldman EL, et al. . Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care 2017;40:136–54. 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edwards JL, Vincent AM, Cheng HT, et al. . Diabetic neuropathy: mechanisms to management. Pharmacol Ther 2008;120:1–34. 10.1016/j.pharmthera.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pop-Busui R, Roberts L, Pennathur S, et al. . The management of diabetic neuropathy in CKD. Am J Kidney Dis 2010;55:365–85. 10.1053/j.ajkd.2009.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aggarwal HK, Sood S, Jain D, et al. . Evaluation of spectrum of peripheral neuropathy in predialysis patients with chronic kidney disease. Ren Fail 2013;35:1323–9. 10.3109/0886022X.2013.828261 [DOI] [PubMed] [Google Scholar]
- 31. Yilmaz MI, Saglam M, Caglar K, et al. . The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 2006;47:42–50. 10.1053/j.ajkd.2005.09.029 [DOI] [PubMed] [Google Scholar]
- 32. Ohkubo Y, Kishikawa H, Araki E, et al. . Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–17. 10.1016/0168-8227(95)01064-K [DOI] [PubMed] [Google Scholar]
- 33. Williams ME, Garg R. Glycemic management in ESRD and earlier stages of CKD. Am J Kidney Dis 2014;63:S22–38. 10.1053/j.ajkd.2013.10.049 [DOI] [PubMed] [Google Scholar]
- 34. Danne T, Nimri R, Battelino T, et al. . International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–40. 10.2337/dc17-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 2006;295:1707–8. 10.1001/jama.295.14.1707 [DOI] [PubMed] [Google Scholar]
- 36. Monnier L, Mas E, Ginet C, et al. . Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–7. 10.1001/jama.295.14.1681 [DOI] [PubMed] [Google Scholar]
- 37. Xu F, Zhao L-H, Su J-B, et al. . The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr 2014;6:139 10.1186/1758-5996-6-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu Y-M, Zhao L-H, Zhang X-L, et al. . Association of glycaemic variability evaluated by continuous glucose monitoring with diabetic peripheral neuropathy in type 2 diabetic patients. Endocrine 2018;60:292–300. 10.1007/s12020-018-1546-z [DOI] [PubMed] [Google Scholar]
- 39. Jin HY, Lee KA, Park TS. The impact of glycemic variability on diabetic peripheral neuropathy. Endocrine 2016;53:643–8. 10.1007/s12020-016-1005-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000991supp001.pdf (44.4KB, pdf)


