Abstract
Objective
To establish a simple screening method for diabetes based on myoinositol (MI) in urine samples collected at home.
Research design and methods
Initially, we evaluated the stability of urinary MI (UMI) at room temperature (RT; 25°C) and 37°C in 10 outpatients with type 2 diabetes. We then enrolled 115 volunteers without a current or history of diabetes. In all subjects, glucose intolerance was diagnosed by 75 g oral glucose tolerance test (75gOGTT). To assess the association between UMI or urine glucose (UG) and plasma glucose (PG), urine samples were also collected at 0 and 2 hours during 75gOGTT. All the subjects collected urine samples at home before and 2 hours after consuming the commercially available test meal. UMI levels at wake-up time (UMIwake-up), before (UMIpremeal) and 2 hours after the test meal (UMI2h-postprandial) were measured using an enzymatic method. ΔUMI was defined as UMI2h-postprandial minus UMIpremeal.
Results
Differing from UG, UMI was stable at RT and 37°C. UMI was increased linearly along with an increase in PG, and no threshold for UMI was observed. UMI was closely associated with blood glucose parameters obtained from a 75gOGTT and hemoglobin A1c (HbA1c) at hospital after adjustment for age, sex, body mass index and serum creatinine. UMIwake-up, UMIpremeal, UMI2h-postprandial and ΔUMI at home were higher in diabetic subjects than non-diabetic subjects even after the above adjustment. Receiver operating characteristics curve (ROC) analyses revealed that for the screening of diabetes, the area under the curve for ROC for UMI2h-postprandial and ΔUMI (0.83 and 0.82, respectively) were not inferior to that for HbA1c ≥48 mmol/mol, which is the American Diabetes Association (ADA) criteria for diabetes.
Conclusions
MI measurement in urine samples collected at home before and after the meal would be a simple, non-invasive and valuable screening method for diabetes.
Keywords: screening, myoinositol, urinary
Significance of this study.
What is already known about this subject?
Urinary myoinositol (UMI) is increased in subjects with diabetes. Therefore, measuring UMI during a 75 g oral glucose loading is a useful and non-invasive screening method for diabetes at hospital. However, it has not been widely accepted because it cannot be used as a diagnosis of diabetes, even if it is performed at hospital. We therefore conducted studies to establish a simple screening method for diabetes based on myoinositol levels in urine samples collected at home.
What are the new findings?
Different from urinary glucose (UG), UMI was stable at room temperature (RT) and 37°C. The estimated shelf life of UMI was sufficiently long to permit a urine sample collected at home to be mailed to the laboratory without preservative. Different from UG, no threshold for UMI was observed, and UMI was increased linearly along with an increase in plasma glucose. Therefore, the properties of UMI permit it to screen early stage of diabetes.
UMI levels from urine samples self-collected at home were closely associated with blood glucose parameters obtained from a 75 g oral glucose tolerance test (75gOGTT) and HbA1c at hospital. UMI was higher in subjects with diabetes than non-diabetes at wake-up time, premeal, 2 hours after ingestion of test meal and ΔUMI0–2h at home. For the screening of diabetes, the area under the curve for receiver operating characteristics for UMI at 2 hours after ingestion of test meal and ΔUMI0–2h at home were not inferior to that for HbA1c ≥48 mmol/mol (6.5%), which is the ADA criteria for diabetes at hospital (0.83, 0.82, and 0.90, respectively).
Introduction
According to the International Diabetes Federation, the population of subjects with diabetes is explosively increasing in the world.1 The number of patients with diabetes is estimated to be about 400 million,2 and a quarter of them are not aware that they have diabetes.3 Moreover, the lifestyle or pharmaceutical interventions for subjects with impaired glucose tolerance (IGT) but not impaired fasting glucose (IFG) could prevent the progression of diabetes and cardiovascular disease.4–7 Therefore, to prevent them, the early detection of glucose intolerance (GI), especially postprandial hyperglycemia, is highly desirable.8 9 However, in the incipient stage of diabetes, there are few subjective symptoms.10 11 Because of this, the majority of such patients rarely visit a hospital, leading to a delay in the initiation of treatment.12 13 Furthermore, 70% of subjects with diabetes live in developing countries, and access to medical care is frequently limited because of limited finances and a shortage of physicians.3 Therefore, there is an urgent need to develop a simple, non-invasive, inexpensive, and precise mass-screening method that is available at home and would enable them to take the first steps toward undergoing further advanced examinations such as measuring fasting plasma glucose (FPG), hemoglobin A1c (HbA1c) and a 75 g oral glucose tolerance test (75gOGTT) at hospital.
Significance of this study.
How might these results change the focus of research or clinical practice?
Screening for diabetes by mailing self-collected urine samples from home to the laboratory to measure UMI would enable us to select appropriate subjects who would need to have advanced examinations such as a 75gOGTT. The UMI test would be suitable for subjects with limited access to medical care because of financial problems and locality and would be the first step towards further medical examinations. It would lead to a more cost-effective screening for diabetes.
Myoinositol (MI; molecular weight 180.16) is structurally similar to D-glucose, and it is widely distributed in multiple organs.14 15 The reabsorption of MI in renal tubules competes with urinary glucose (UG) in cases of hyperglycemia, resulting in high concentrations of MI being excreted into the urine.16 It has been reported that urinary myoinositol (UMI) levels are increased in subjects with diabetes compared with controls.16 17 In healthy subjects, approximately 16~30 mg/day of MI is excreted in the urine, whereas, in subjects with diabetes, this level is increased to about 150~220 mg/day.17 18 It has been reported that ΔUMI, which is defined by a 2-hour post-75 g oral glucose loading UMI minus preload UMI, is a useful and non-invasive method for screening for GI.19 20 However, different from a regular 75gOGTT, measuring UMI during a 75 g oral glucose loading cannot be used as a diagnosis of GI, even if it is performed at hospital. Therefore, measuring UMI to screen for GI in hospital has not been widely accepted. We therefore conducted studies to establish a simple screening method for undiagnosed diabetes based on MI levels in urine samples collected at home.
Materials and methods
Measurement of UMI
UMI was measured using an enzyme cycling method with MI dehydrogenase (Lucica MI, Asahi KASEI Pharma Co).20 21 The sensitivity of detection was 10 µmol/L, and the coefficient of variation (CV) was 0.5%~1.1%. Interassay and intra-assay CV were 0.5%~1.1% and 0.4%~1.3%, respectively.21 To reduce the influence of renal function, UMI was corrected by urinary creatinine (UCr), except for stability test.
Study design and population
Study 1: testing the stability of UMI, UG and UCr at room temperature (RT) and 37°C
Regarding mailing urine samples collected at home to a laboratory, we first evaluated the stability of UMI, UG, and UCr at RT (25°C) and under relatively severe conditions such as 37°C. Urine samples from 10 arbitrary outpatients with type 2 diabetes mellitus (T2DM) at Ehime University Hospital were stored without preservative in an incubator at 25°C or 37°C for 0, 1, 2, 3, 5, and 7 days. UMI, UG and UCr concentrations were measured at each of the above time points to assess the stability.
Study 2: the clinical usefulness of MI in urine samples collected at home before and after the ingestion of the test meal
We consecutively recruited Japanese volunteers who were attending medical check-up at Kitaishikai Hospital, Saijyo Central Hospital and Ehime University Hospital. Subjects with a current or history of diabetes and chronic renal dysfunction based on an estimated glomerular filtration rate (eGFR) of <30 ml/min/1.73m2 were excluded. To diagnose GI (IGT or diabetes), we performed a 75gOGTT at hospital, and their HbA1c levels were measured. A diagnosis of IGT and diabetes were defined according to either FPG or 2-hour plasma glucose level after a 75gOGTT or the HbA1c based on American Diabetes Association (ADA) criteria.22
To assess the association between UMI or UG and plasma glucose (PG), we also collected urine samples at 0 and 2 hours after glucose ingestion from a sequence of 46 subjects at Ehime University Hospital. These urine samples, without preservative, were immediately shipped to a single laboratory (Bio Medical Laboratories, Inc) at ambient temperature (5°C–26°C in Ehime prefecture), and UMI and UG levels were measured within 24 hours of the urine collection.
Within a week after the 75gOGTT at hospital, the participants ingested a test meal (commercially available energy bar: Calorie Mate 500 kcal: carbohydrate 51 g, fat 28 g, protein 11 g, Otsuka Pharmaceutical Co, Ltd) at home.23 24 There was a close correlation between plasma glucose levels at 2-hour post-75gOGTT and at 1 hour postingestion of this test meal (R2=0.67, p<0.001).
The participants collected urine samples (15 mL) at home at three points: (1) wake-up time (fasting first urine), (2) premeal (0 hour, 08:00), and (3) 2 hours after ingestion of the test meal (2-hour postprandial, 10:00). These urine samples without preservative were shipped to a single laboratory at ambient temperature, and the UMI and UCr levels were then measured within 2 days of their collection (online supplementary figure 1). The ΔUMI was defined as the 2-hour postprandial UMI minus the premeal UMI.
bmjdrc-2019-000984supp001.pdf (164KB, pdf)
Written informed consent was obtained from all participants prior to their enrollment in this study.
Statistical analysis
To assess the stability of UMI, UG, and UCr, we calculated the CV from 0 to 7 days. We considered samples to be stable if CV was within 5%. We also estimated the shelf life as previously described.25–27 Briefly, the shelf life data were calculated from the regression line (95% confidence limits line) of 6 data points (0, 1, 2, 3, 5, and 7 days) and the lower acceptance criteria of 90% of the reference (day 0).
To evaluate the association between glucose parameters and UMI, we performed multivariate regression analyses adjusted for age, sex, body mass index (BMI), and serum creatinine. The Mann-Whitney test was used to compare the subjects with or without diabetes. Differences in longitudinal data in the two groups were assessed by repeated-measure analysis of variance (ANOVA). To compare the normal glucose tolerance (NGT), IGT, and T2DM, one-way ANOVA or Kruskal-Wallis test were used. The values were expressed as the mean±SD or SE. To determine the utility of UMI for screening of diabetes or GI, we performed receiver operating characteristics (ROC) curve analyses based on STARD 2015 guidelines.28 Based on a previous report,19 we calculated the required sample size. The data indicated that a sample size of 44 would be needed to detect a minimum difference between the groups for a 5% change (α=0.05, 90% power). Statistical analyses were carried out using JMP V.13. Difference yielding p<0.05 was considered to be statistically significant.
Results
Study 1
UMI was stable at RT and 37°C
We first evaluated the stability of UMI and UG from 10 outpatients with T2DM. Their clinical characteristics are summarized in online supplementary table 1. Stability tests revealed that, at RT, the CV of UMI from 0 to 7 days were less than 5%. Even under severe conditions such as 37°C, UMI was stable and CVs were less than 5% except for one sample. In contrast, UG was unstable at RT. In most of the samples, the CV of UG from 0 to 7 days were more than 5% at RT. This phenomenon was even more obvious at 37°C (online supplementary table 1). The estimated shelf life of UMI was sufficiently long to permit a urine sample collected at home to be mailed to the laboratory when stored at RT and 37°C (46 days and 18 days, respectively). However, the estimated shelf life of UG was very short at RT and 37°C (1 day and 0 day, respectively). Furthermore, the estimated shelf life of UCr was 7 days at RT and 2 days at 37°C.
Therefore, we focused on the usefulness of UMI for screening for undiagnosed diabetes in urine samples collected at home.
Study 2
Characteristics of the participants in the study 2
We enrolled 115 Japanese volunteers without a current or past history of diabetes. Their clinical characteristics are summarized in table 1. A 75gOGTT revealed that 63 subjects had NGT, 29 had IGT, and 23 had T2DM. FPG and HbA1c in the T2DM group were 6.6±1.1 mmol/L (118±21.2 mg/dL) and 40.6±4.6 mmol/mol (5.9%±0.4 %), respectively. The average age was higher and renal function was lower in the IGT or T2DM group than the NGT group.
Table 1.
Characteristics of participants in the study 2
| NGT | IGT | T2DM | P value | |
| Age, years | 47.4±12.0 | 65.4±9.4 | 63.9±9.9 | <0.0001 |
| Gender, n (male/female) | 63 (15/48) | 29 (9/20) | 23 (12/11) | 0.0427 |
| BMI, kg/m2 | 23.4±4.0 | 24.2±3.5 | 24.5±3.0 | 0.3837 |
| FPG, mmol/L (mg/dL) | 5.0±0.4 (90±6.7) | 5.6±0.7 (101±11.9)*** | 6.6±1.1 (118±21.2)*** | <0.0001 |
| 2-hour PG, mmol/L (mg/dL) | 5.5±1.0 (99±18.0) | 8.9±1.0 (161±17.6)*** | 12.9±2.9 (233±51.4)*** | <0.0001 |
| FIRI, pmol/L (µIU/mL) | 37.5±17.4 (5.4±2.5) | 46.5±32.0 (6.7±4.6) | 46.5±29.1 (6.7±4.2) | 0.1412 |
| HbA1c, mmol/mol (%) | 31.2±3.7 (5.0±0.3) | 36.0±3.9 (5.4±0.4)*** | 40.6±4.6 (5.9±0.4)*** | <0.0001 |
| Total cholesterol, mmol/L (mg/dL) | 5.3±1.1 (203±40.9) | 5.2±0.9 (200±34.8) | 5.3±1.4 (203±53.3) | 0.92 |
| HDL cholesterol, mmol/L (mg/dL) | 1.7±0.5 (66±21.0) | 1.4±0.4 (54±14.8)* | 1.4±0.4 (52±14.8)** | 0.0018 |
| eGFR, ml/min/1.73m2 | 83.0±20.1 | 74.0±16.9* | 68.7±15.6** | 0.0005 |
The values were presented as mean±SD or n.
*P<0.05; versus NGT, **p<0.01; versus NGT, and ***p<0.001; versus NGT.
BMI, body mass index; eGFR, estimated glomerular filtration rate; FIRI, fasting immunoreactive insulin; FPG, fasting plasma glucose; HDL, high-density lipoprotein; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; PG, plasma glucose; T2DM, type 2 diabetes mellitus.
Differing from UG, UMI was increased linearly along with an increase in plasma glucose, and no threshold for UMI was observed
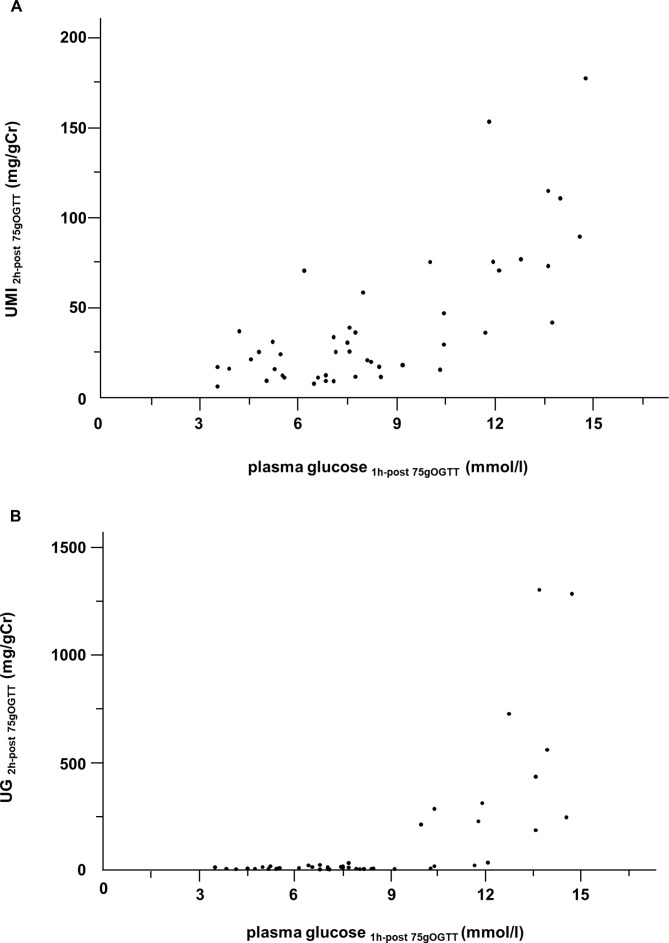
To examine the influence of plasma glucose levels on the excretion of UMI or UG, we collected urine samples at 2 hours after a 75gOGTT from a series of 46 subjects at Ehime University Hospital and measured the UMI and UG levels (figure 1). It is well known that UG has a renal threshold for glucose. Consistent with previous reports,29 30 UG at 2-hour post-75gOGTT (UG2h-post-75gOGTT) was detected via an increase in plasma glucose at 1-hour post-75gOGTT (plasma glucose1h-post-75gOGTT), and the threshold concentration for detection was approximately 8.9–10.5 mmol/L (160–190 mg/dL). In contrast, no such threshold for UMI at 2-hour post-75gOGTT (UMI2h-post-75gOGTT) was observed. UMI2h-post-75gOGTT was increased linearly along with an increase in plasma glucose1h-post-75gOGTT (R2=0.67, p<0.001).
Figure 1.
The relation between plasma glucose concentration at 1-hour post-75gOGTT and UMI (A) or UG (B) excretion at 2-hour post-75gOGTT (n=46). UMI2h-post-75gOGTT, UMI/Cr at 2-hour post-75gOGTT; UG2h-post-75gOGTT, UG/Cr at 2-hour post-75gOGTT; and plasma glucose1h-post-75gOGTT, plasma glucose level at 1-hour post-75gOGTT. 75gOGTT, 75 g oral glucose tolerance test; UG, urinary glucose; UMI, urinary myoinositol.
Relation between glucose parameters from blood samples collected at hospital and MI levels from urine samples collected at home
We next assessed the relation between glucose and HbA1c levels from blood samples collected at hospital and UMI levels in urine samples collected at home (n=115, table 2). Multivariate regression analyses revealed that after adjustment for age, sex, BMI and serum creatinine, UMI for all of the three time frames, including wake-up time (UMIwake-up), premeal (UMIpremeal), 2-hour postprandial (UMI2h-postprandial), and ΔUMI were associated with glucose parameters obtained from a 75gOGTT and HbA1c at hospital. UMI2h-postprandial at home was most closely associated with HbA1c and plasma glucose level fasting and at 2 hours after a 75gOGTT at ADA criteria for diabetes.
Table 2.
Relation between glucose parameters from blood samples collected at hospital and UMI levels from self-collected urine samples at home
| Dependent variables | Independent variables | Non-standardized β
(95% CI) |
Standardized β | P value |
| 75gOGTT Fasting plasma glucose |
ln UMIwake-up | 18.5 (9.5 to 27.4) | 0.36 | <0.0001* |
| ln UMIpremeal | 16.3 (7.2 to 25.4) | 0.30 | 0.0006* | |
| ln UMI2h-postprandial | 21.9 (14.0 to 29.8) | 0.44 | <0.0001* | |
| ln Δ UMI | 4.9 (1.2 to 8.6) | 0.24 | 0.0097* | |
| 75gOGTT 1-hour plasma glucose |
ln UMIwake-up | 54.0 (24.9 to 83.1) | 0.29 | 0.0004* |
| ln UMIpremeal | 48.5 (18.1 to 78.8) | 0.25 | 0.0020* | |
| ln UMI2h-postprandial | 73.8 (47.9 to 99.7) | 0.41 | <0.0001* | |
| ln Δ UMI | 12.5 (0.9 to 24.0) | 0.18 | 0.0347 | |
| 75gOGTT 2-hour plasma glucose |
ln UMIwake-up | 37.4 (3.7 to 71.0) | 0.19 | 0.0298 |
| ln UMIpremeal | 33.3 (0.2 to 66.3) | 0.17 | 0.0488 | |
| ln UMI2h-postprandial | 72.5 (44.5 to 100.6) | 0.40 | <0.0001* | |
| ln Δ UMI | 17.8 (5.3 to 30.2) | 0.25 | 0.0058* | |
| 75gOGTT AUC0 – 2h glucose |
ln UMIwake-up | 81.9 (37.2 to 126.7) | 0.29 | 0.0005* |
| ln UMIpremeal | 73.3 (27.4 to 119.2) | 0.24 | 0.0020* | |
| ln UMI2h-postprandial | 121.0 (82.9 to 159.1) | 0.44 | <0.0001* | |
| ln Δ UMI | 23.8 (6.1 to 41.5) | 0.22 | 0.0089* | |
| HbA1c | ln UMIwake-up | 0.33 (0.06 to 0.59) | 0.20 | 0.0152 |
| ln UMIpremeal | 0.32 (0.06 to 0.59) | 0.19 | 0.0165 | |
| ln UMI2h-postprandial | 0.55 (0.33 to 0.78) | 0.36 | <0.0001* | |
| ln Δ UMI | 0.13 (0.03 to 0.23) | 0.21 | 0.0133 |
Multivariable regression analyses adjusted for age, sex, BMI, and serum creatinine. Dependent variables: fasting plasma glucose, 1-hour post-75gOGTT plasma glucose, 2-hour post-75gOGTT plasma glucose, AUC0–2h glucose of 75gOGTT, and HbA1c. Independent variables: the logarithm of UMIwake-up, UMI at wake-up time (fasting first urine); UMIpremeal, UMI at premeal (fasting second urine); and UMI2h-postprandial, UMI at 2-hour postprandial. ΔUMI was defined by 2-hour postprandial UMI minus premeal UMI.
*P values remained significant after Bonferroni’s correction.
AUC, area under the curve; BMI, body mass index; 75gOGTT, 75 g oral glucose tolerance test; UMI, urinary myoinositol.
UMI levels before and after ingestion of test meal at home were higher in subjects with diabetes
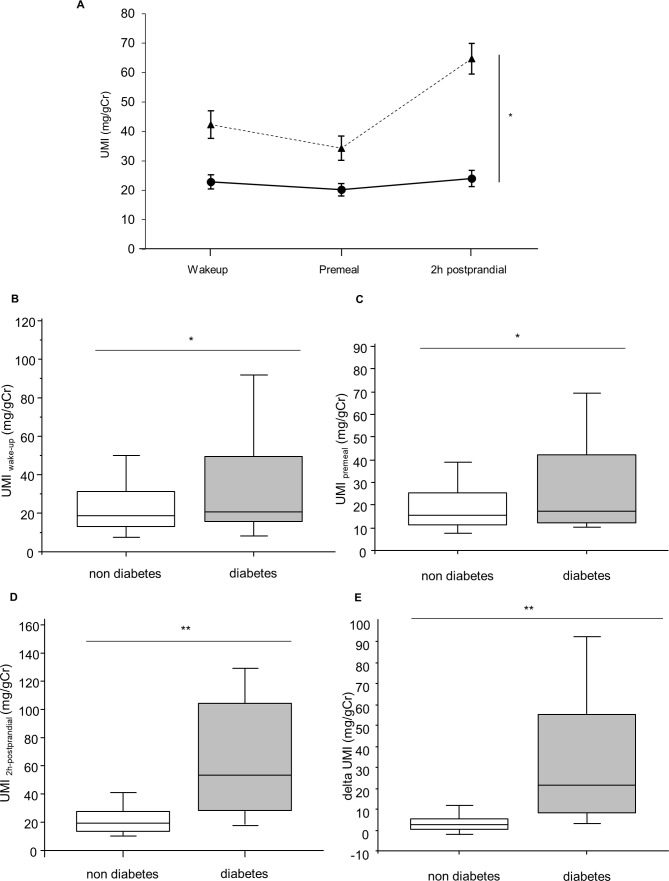
Repeated measures ANOVA (figure 2A) and Mann-Whitney test (figure 2B–E) showed significant differences between diabetes and non-diabetes in UMI before and after ingestion of test meal at home. UMIwake-up, UMIpremeal, UMI2h-postprandial, and ΔUMI were higher in subjects with diabetes than non-diabetes, even after adjusted for age, sex, BMI and serum creatinine (43.5±5.2 vs 23.2±2.5, p<0.01, 36.2±4.6 vs 20.3±2.3, p<0.01, 67.8±5.8 vs 24.3±2.9, p<0.001, and 33.7±4.2 vs 4.3±2.1, p<0.001, respectively).
Figure 2.
Comparison of UMI between subjects with or without diabetes during ingestion of test meal. (A) Solid line: subjects without diabetes (n=92); dotted line: subjects with diabetes (n=23). Error bars represent SE *p<0.001 computed by repeated-measures ANOVA. (B–E) Box plots indicating the 5th and 95th percentiles (vertical lines), 25th and 75th (boxes), and 50th percentiles (horizontal lines). *P<0.05 and **p<0.0001 computed by Mann-Whitney test. UMIwake-up, UMI at wake-up time; UMIpremeal, UMI at premeal; UMI2h-postprandial, UMI at 2-hour postprandial; ΔUMI, UMI2h-postprandial minus UMIpremeal. ANOVA, analysis of variance; UMI, urinary myoinositol.
Furthermore, UMIwake-up, UMI2h-postprandial and ΔUMI were higher in subjects with GI than NGT, even after adjusted for age, sex, BMI and serum creatinine (36.1±4.2 vs 20.7±3.6, p<0.01, 47.7±5.0 vs 21.8±4.4, p<0.01, and 19.3±3.6 vs 3.4±3.2, p<0.05, respectively).
ROC curve analyses to determine the optimum cut-off values of UMI associated with diabetes
To further assess the usefulness of UMI levels in urine samples collected at home for the screening of diabetes, we performed ROC curve analyses (table 3). For the screening of diabetes, the area under the curve (AUC) for ROC (AUCROC) for UMI2h-postprandial was 0.83, with a sensitivity of 76% and a specificity of 81% at a cut-off value of 32 mg/gCr (p<0.0001). The AUCROC for ΔUMI was 0.82 with a sensitivity of 80% and a specificity of 80% at a cut-off value of 7.4 mg/gCr (p<0.0001). The AUCROC for UMI2h-postprandial and ΔUMI were not statistically inferior to that for HbA1c ≥48 mmol/mol (6.5%) using the ADA criteria for diabetes. Furthermore, for the screening of GI, the AUCROC for UMI2h-postprandial was 0.74 and ΔUMI was 0.69 (p<0.0001, online supplementary table 2).
Table 3.
Receiver operating characteristics (ROC) curve for potential predictors of diabetes
| Parameter | AUCROC (95% CI) | Cut-off | P value of AUCROC compared with HbA1c | Sensitivity | Specificity | PPV | NPV |
| HbA1c, mmol/mol (%) | 0.90 (0.83 to 0.98) | 48 (6.5)* | – | 0.08 | 1.00 | 1.00 | 0.80 |
| UMIwake-up, mg/gCr | 0.64 (0.49 to 0.78) | 18 | <0.001 | 0.73 | 0.49 | 0.27 | 0.87 |
| UMIpremeal, mg/gCr | 0.62 (0.48 to 0.76) | 14 | <0.0001 | 0.64 | 0.40 | 0.23 | 0.80 |
| UMI2h-postprandial, mg/gCr | 0.83 (0.73 to 0.93) | 32 | 0.13 | 0.76 | 0.81 | 0.53 | 0.92 |
| ΔUMI, mg/gCr | 0.82 (0.71 to 0.93) | 7.4 | 0.18 | 0.80 | 0.80 | 0.53 | 0.94 |
ΔUMI was defined as 2-hour postprandial UMI minus premeal UMI.
*American Diabetes Association criteria for diabetes as HbA1c ≥48 mmol/mol (6.5%) was used as reference standard.
AUCROC, area under the ROC curve; NPV, negative predictive value; PPV, positive predictive value; UMI, urinary myoinositol; UMI2h-postprandial, UMI at 2-hour postprandial; UMIpremeal, UMI at premeal (fasting second urine); UMIwake-up, UMI at wake-up time (fasting first urine).
These results suggest that measuring MI in urine samples collected at home before and after the ingestion of the commercially available test meal would be a simple and non-invasive screening method for diabetes.
Discussion
In the present study, we found that: (1) MI level in urine samples collected at home was associated with blood glucose parameters obtained from a 75gOGTT and HbA1c at hospital; (2) UMI2h-postprandial was closely associated with plasma glucose level at before and 2 hours after a 75gOGTT at criteria for testing for diabetes in ADA; (3) UMI was higher in diabetic subjects than non-diabetic subjects and in subjects with GI than NGT even after adjusted for age, sex, BMI and serum creatinine. We obtained similar results when we included five subjects with isolated IFG in GI; and (4) no adverse events were observed in our UMI test.
We also found that, different from UG, no threshold for UMI was detected. UMI increased linearly with increasing plasma glucose (figure 1). MI is transported from extracellular fluid via three inositol transporters: sodium-dependent MI transporters 1 and 2, and H+-myoinositol transporter, which cotransports myoinositol with H+.14 31 These transporters are competed by D-glucose when hyperglycemia is present before UG is detected.14 Actually, UMI levels were increased in subjects with diabetes even when their UG was not detected by a urine dipstick test (no.1, 108 mg/gCr and no.10, 97 mg/gCr in online supplementary table 1). Therefore, the properties of UMI permit it to screen early stage of T2DM. Indeed, in the present study, subjects had no current or history of diabetes, the majority of them were diagnosed by 2-hour plasma glucose level after a 75gOGTT, and HbA1c in the T2DM group was 40.6±4.6 mmol/mol (5.9%±0.4 %).
Shelf life testing demonstrated that different from UG, UMI was stable at RT and under more severe conditions as 37°C without preservative. However, compared with UMI, UCr was unstable at 37°C. The estimated shelf life of UCr was 7 days at RT and 2 days at 37°C. Therefore, correction for UCr is not acceptable under conditions of high temperature over 2 days. Concerning mailing a sample under severe conditions of high temperature, we further analyzed the usefulness of UCr-uncorrected UMI on the screening of diabetes. Even though the AUCROC of UMI2h-postprandial and ΔUMI were slightly decreased (0.83 to 0.80 and 0.82 to 0.77, respectively), using UCr-uncorrected UMI may be reasonable under the condition of high temperature over 37°C (online supplementary table 3).
These results suggest that the UMI test would be suitable for subjects with limited access to medical care because of financial problems and locality and would be the first step towards further medical examinations. For example, subjects could mail urine samples collected at home before and after the ingestion of prescribed test meal like commercially available energy bar to a laboratory for analysis at ambient temperature, and when UMI was high, they could be advised to visit a medical institution for further advanced examinations of diabetes.
A 75gOGTT at a hospital is widely used as the gold standard for the screening and diagnosis of GI. However, (1) a 75gOGTT usually requires multiple (2–5 times) blood collections, (2) there is a risk of hyperglycemia and problems associated with blood collection, (3) it is a relatively costly test,32 and (4) the test can be a burden for the staff if many subjects are scheduled at one time. However, the UMI test is (1) available at home, (2) non-invasive, (3) inexpensive (compared with a 75gOGTT at hospital, at one-tenth the price in Japan), and (4) the UMI test has a few limitations in terms of the number of samples. These results suggest that the UMI test, as discussed here, would be more suitable for mass screening for diabetes or GI than a 75gOGTT.
Previous studies have reported that UMI was increased in cases of renal failure.33 34 Therefore, to reduce the impact of renal function on UMI, (1) we excluded subjects with chronic renal dysfunction (eGFR of <30 ml/min/1.73m2), (2) UMI were corrected by UCr, and (3) we performed multivariate regression analyses adjusted for serum creatinine.
There are some limitations in this study. First, the period from the last meal to wake-up time when subjects first collected fasting urine was dependent on the individual. Therefore, the influence of the supper on UMIwake-up may be different. Second, in the present study, we excluded subjects with eGFR <30 ml/min/1.73m2. Therefore, to further clarify the influence of renal function on UMI for screening for diabetes, study of larger general population including subjects with several renal dysfunction will be necessary. Third, the urine samples were shipped to a single laboratory at ambient temperature at our region (5°C–26°C), and the UMI and UCr levels were then measured within 2 days of the urine collection. Therefore, further investigation of samples under various conditions, periods and temperature of storage will be necessary.
In conclusion, measuring MI levels in urine samples collected at home before and after the ingestion of the test meal would be a simple, non-invasive, and valuable screening method for diabetes in subjects without chronic renal dysfunction. UMI test at home would be more suitable for mass screening for diabetes as the first step toward further investigation such as a 75gOGTT for a more definitive diagnosis at hospital.
Acknowledgments
We wish to thank Akiko Otaki and Risa Kagawa at Ehime University for technical assistance.
Footnotes
Contributors: All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship. Specifically, MT, YT, FY, SK and HO designed the experiments. MT, YT, FY, MY, GH, SS, JF and SK collected the data. MT, YT, FY and SK analyzed data. MT, YT and HO wrote the manuscript. YT is the guarantors of this work. All of the authors give full consent for publication of the present manuscript. All authors are responsible for the integrity of the work as a whole.
Funding: This study was supported by Japan Diabetes Foundation, OMRON Corporation, ASAHI KASEI Pharma Corporation, and Sysmex Corporation.
Disclaimer: The funders were not involved in the design of the study, the collection, analysis, and interpretation of data, writing the report, or the decision to submit the report for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Study protocols were approved by the research ethics committee of each hospitals.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50. 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 2. NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Center for Chronic Disease Prevention and Health Promotion National diabetes statitics report, 2017. Available: www.cdc.gov [Accessed 16 Sep 2019].
- 4. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 5. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DECODE Study Group, the European Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161:397–405. 10.1001/archinte.161.3.397 [DOI] [PubMed] [Google Scholar]
- 7. Takao T, Suka M, Yanagisawa H, et al. Impact of postprandial hyperglycemia at clinic visits on the incidence of cardiovascular events and all-cause mortality in patients with type 2 diabetes. J Diabetes Investig 2017;8:600–8. 10.1111/jdi.12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selph S, Dana T, Blazina I, et al. Screening for type 2 diabetes mellitus: a systematic review for the U.S. preventive services Task force. Ann Intern Med 2015;162:765–76. 10.7326/M14-2221 [DOI] [PubMed] [Google Scholar]
- 9. Barry E, Roberts S, Oke J, et al. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ 2017;356:i6538 10.1136/bmj.i6538 [DOI] [PubMed] [Google Scholar]
- 10. Alam U, Asghar O, Azmi S, et al. General aspects of diabetes mellitus. Handb Clin Neurol 2014;126:211–22. 10.1016/B978-0-444-53480-4.00015-1 [DOI] [PubMed] [Google Scholar]
- 11. Takahashi Y, Inoue Y, Hirata Y. The prevalence of neuropathic symptoms in diabetic patients newly referred to our Hospital. Tohoku J Exp Med 1983;141 Suppl:439–45. 10.1620/tjem.141.Suppl_439 [DOI] [PubMed] [Google Scholar]
- 12. Polonsky WH. Poor medication adherence in diabetes: what's the problem? J Diabetes 2015;7:777–8. 10.1111/1753-0407.12306 [DOI] [PubMed] [Google Scholar]
- 13. González L, Elgart JF, Gagliardino JJ. [Education of people with type 2 diabetes through peers with diabetes: is it cost effective?]. Medwave 2015;15:e6348 10.5867/medwave.2015.11.6348 [DOI] [PubMed] [Google Scholar]
- 14. Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013;95:1811–27. 10.1016/j.biochi.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 15. Thomas MP, Mills SJ, Potter BVL. The "other" inositols and their phosphates: Synthesis, biology, and medicine (with recent advances in myo-inositol chemistry). Angew Chem Int Ed Engl 2016;55:1614–50. 10.1002/anie.201502227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennington AS, Hill CR, Craig J, et al. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N Engl J Med 1990;323:373–8. 10.1056/NEJM199008093230603 [DOI] [PubMed] [Google Scholar]
- 17. Ostlund RE, McGill JB, Herskowitz I, et al. D-Chiro-Inositol metabolism in diabetes mellitus. Proc Natl Acad Sci U S A 1993;90:9988–92. 10.1073/pnas.90.21.9988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshii H, Uchino H, Ohmura C, et al. Clinical usefulness of measuring urinary polyol excretion by gas-chromatography/mass-spectrometry in type 2 diabetes to assess polyol pathway activity. Diabetes Res Clin Pract 2001;51:115–23. 10.1016/S0168-8227(00)00221-7 [DOI] [PubMed] [Google Scholar]
- 19. Ikezaki H, Furusyo N, Okada K, et al. The utility of urinary myo-inositol as a marker of glucose intolerance. Diabetes Res Clin Pract 2014;103:88–96. 10.1016/j.diabres.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 20. Sarashina G, Yamakoshi M, Noritake M, et al. A study of urinary myo-inositol as a sensitive marker of glucose intolerance. Clin Chim Acta 2004;344:181–8. 10.1016/j.cccn.2004.02.026 [DOI] [PubMed] [Google Scholar]
- 21. Yamakoshi M, Kawazu S. Lucica MI urinary myoinositol kit: a new diagnostic test for diabetes mellitus and glucose intolerance. Mol Diagn Ther 2008;12:189–91. 10.1007/BF03256283 [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 2019;42:S13–28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 23. Morioka N, Funada J-ichi, Takata Y, et al. Influence of meal intake on pulse wave indices in type 2 diabetes. Hypertens Res 2010;33:743–7. 10.1038/hr.2010.66 [DOI] [PubMed] [Google Scholar]
- 24. Funada J-ichi, Takata Y, Hashida H, et al. Dysfunctional central hemodynamic regulation after daily meal intake in metabolic syndrome. Atherosclerosis 2010;210:268–73. 10.1016/j.atherosclerosis.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 25. Capen R, Christopher D, Forenzo P, et al. Evaluating current practices in shelf life estimation. AAPS PharmSciTech 2018;19:668–80. 10.1208/s12249-017-0880-4 [DOI] [PubMed] [Google Scholar]
- 26. Bajaj S, Singla D, Skhuja N. Stability testing of pharmaceutical products. J App Pharm Sci 2012;2:129–38. [Google Scholar]
- 27. European medicines agancy International cooperation on harmonisation of technical requirements for registration of veterinary medicinal products (VICH). Statistical evaluation of stability data, 2013. Available: www.ema.europa.eu/en/documents/scientific-guideline/international-cooperation-harmonisation-technical-requirements-registration-veterinary-medicinal_en-0.pdf [Accessed 16 Sep 2019].
- 28. Bossuyt PM, Reitsma JB, Bruns DE, et al. Stard 2015: an updated list of essential items for reporting diagnostic accuracy studies. Clin Chem 2015;61:1446–52. 10.1373/clinchem.2015.246280 [DOI] [PubMed] [Google Scholar]
- 29. Lawrence RD. Renal threshold for glucose: normal and in diabetics. BMJ 1940;1:766–8. 10.1136/bmj.1.4140.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–94. 10.1152/physrev.00055.2009 [DOI] [PubMed] [Google Scholar]
- 31. Fu H, Li B, Hertz L, et al. Contributions in astrocytes of SMIT1/2 and HMIT to myo-inositol uptake at different concentrations and pH. Neurochem Int 2012;61:187–94. 10.1016/j.neuint.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 32. Zhang P, Engelgau MM, Valdez R, et al. Costs of screening for pre-diabetes among US adults: a comparison of different screening strategies. Diabetes Care 2003;26:2536–42. 10.2337/diacare.26.9.2536 [DOI] [PubMed] [Google Scholar]
- 33. Niwa T, Yamamoto N, Maeda K, et al. Gas chromatographic--mass spectrometric analysis of polyols in urine and serum of uremic patients. Identification of new deoxyalditols and inositol isomers. J Chromatogr 1983;277:25–39. [PubMed] [Google Scholar]
- 34. Pitkänen E. Changes in serum and urinary myo-inositol levels in chronic glomerulonephritis. Clin Chim Acta 1976;71:461–8. 10.1016/0009-8981(76)90097-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000984supp001.pdf (164KB, pdf)