Abstract
Background
Heart failure (HF) patients show abnormal autonomic activities, which may stem from altered functional connectivity (FC) between different brain sites.
Methods and Results
we evaluate insular and cerebellar FC with other brain areas, before, during, and after the Valsalva challenge, with functional magnetic resonance imaging in 35 HF and 35 control subjects. Significant insular FC emerged with striatum, thalamus, and anterior cingulate. While left and right cerebellar cortices showed significant FC with each other constituting the cerebellum network. The insula and cerebellum networks showed significant negative FC with each other at baseline, challenge, and recovery phases. The challenge induced increased FC within the insula and the cerebellum networks in both HF and controls. However, HF subjects showed more increased insular network FC, but less enhanced cerebellar FC. During the recovery phase, the negative FC between the insular network and cerebellum enhanced significantly in controls, but not in HF. Lower left ventricle ejection fraction was correlated with lower insula network FC, and impaired negative FC between cerebellum and the insula network in HF.
Conclusions
Increased insular FC in HF subjects might contribute to exaggerated sympathetic tone. While impaired cerebellar FC and diminished negative interactions between cerebellum and insular systems may indicate impaired parasympathetic functions in HF.
Keywords: Functional magnetic resonance imaging, Cerebellum, Insula, Functional Connectivity
INTRODUCTION
Heart failure (HF) patients exhibit a range of autonomic issues that fail to regulate sympathetic and parasympathetic neural outputs in response to body position, motor, or respiratory challenges (1, 2). Such malfunctions are reflected as abnormal arterial pressure, heart rate, and sweating in the condition (2, 3). Although impaired autonomic regulatory clinical outcomes in HF are substantial, the underlying neural network mechanisms controlling timely- and sustained-responses to processes that require cardiovascular adjustment, such as the Valsalva maneuver, are unknown.
The Valsalva maneuver is a non-invasive procedure to examine both sympathetic and parasympathetic autonomic functions (4). The autonomic challenge consists of a voluntary forced expiratory effort against a closed upper airway, which raises intrathoracic pressure, and results in a sequence of hemodynamic changes and cardiovascular regulatory reflex. During the challenge period (i.e., sympathetic phase), healthy subjects show heart rate increase, which returns quickly to baseline following expiratory pressure release (i.e., parasympathetic phase) (4). However, HF patients usually fail to demonstrate the normal increase in heart rate during the sympathetic phase, and show a slower and gradual decline during the parasympathetic phase, rather a quick drop as seen in healthy controls (5, 6). In addition, HF patients also show abnormal blood pressure changes during and after the autonomic challenges (7). Since previous HF studies suggest that aberrant autonomic outflows from forebrain to medullary sites are underlying such deficits (5–7), disturbed functional communications between different autonomic regulatory areas, including the insular and cerebellar regions, may also contribute to these abnormal physiological responses to the Valsalva challenge.
Brain structural injury appears in both gray and white matter sites in HF subjects that provide a structural basis for disturbed brain network communications in the condition (8–10). Structural damage in two crucial autonomic regulatory networks are of major concern: the insular-limbic-thalamo-striatal network and the cerebellar network. The insular-limbic-thalamo-striatal network is a set of brain sites responsible for autonomic, somatic, and motoric functions, including insular, anterior cingulate, hypothalamus, thalamus, caudate, putamen, and globus pallidus areas (11). The insula, as the cortical hub of this network, serves autonomic regulatory and interoceptive awareness functions via projections to visceral, thalamic, brainstem, and limbic areas (12), and have been identified as the central command center to ensure heart rate and blood pressure increase at body movement (13). The cerebellar network, including both cerebellar cortices and deep nuclei, plays important roles in dampening extremes of blood pressure and modulates vestibulo-cardiovascular responses, with cerebellar injuries in animal studies leading to fatal outcomes with extreme blood pressure or attenuated heart rate increase (14). Such brain imaging studies emphasized widespread structural impairments in the insular-limbic-thalamo-striatal and cerebellar networks. However, no study has examined the functional communications or coordination of these two autonomic regulatory networks, as well as their responses to autonomic challenges in HF.
In this study, we used blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to evaluate the functional connectivity (FC), which quantifies the synchronization or coordination of neural activities of anatomically-distinct brain regions, within and between the insular and cerebellar networks. Brain FC is dynamic, which is distinct from structural connectivity, i.e. white matter fibers connecting different brain areas, and changes within minutes to seconds due to subject’s physiological, psychological, and pathological states (15–18). Since the Valsalva maneuver includes different phases, brain FC may change according to different physiological states before, during, and after the autonomic challenge. However, no study has examined how FC varies in different stages of the Valsalva maneuver, which are assessed here. We hypothesize that FC in the insular and cerebellar networks will be altered in HF at baseline, and during different phases of the Valsalva maneuver compared to healthy controls.
MATERIALS AND METHODS
Subjects
We recruited 35 hemodynamically-optimized HF subjects (i.e. drug dosages were titrated to reach targeted hemodynamic goals as per AHA/ACC HF guidelines (19) and 35 age- and gender-comparable healthy controls. Demographic, clinical, and physiologic data of HF and control subjects are summarized in Table 1. All HF patients were recruited from the Ahmanson- UCLA Cardiomyopathy Center. The diagnosis of HF was based on national diagnostic criteria (20), and all subjects included in this study were with New York Heart Association Functional Class II at the time of MRI. All HF patients were without any history of valvular congenital heart defects, pregnancy induced cardiomyopathy, drug abuse, or previous history of stroke, carotid vascular disease, head injury, or any diagnosed psychiatric condition. HF patients were treated with guideline-directed medical therapy, including angiotensin receptor blockers or angiotensin-converting enzyme inhibitors, beta blockers, and diuretics, and were stabilized for hemodynamics and body-weight for at least six months prior to the participation in MRI studies.
Table 1:
Demographic, biophysical, and clinical data of HF and control subjects.
| Variables | HF (n = 35) | Controls (n = 35) | p values |
|---|---|---|---|
| Age range (years) | 40–66 | 40–61 | – |
| Mean age (years) | 54.5±7.7 | 51.2±5.9 | 0.054 |
| Gender (Male: Female) | 24:11 | 22:13 | 0.621 |
| BMI (kg/m2) | 26.8±5.1 | 25.4±3.0 | 0.050 |
| Education (years) | 15.0±3.1 | 16.1±2.2 | 0.076 |
| Heart rate (beats/min) | 73.5±11.6 | 69.7±11.8 | 0.182 |
| Systolic BP (mm Hg) | 111.3±22.4 | 117.8±16.8 | 0.175 |
| Diastolic BP (mm Hg) | 67.0±10.5 | 76.4±12.8 | 0.001 |
| LVEF (%) | 28.4±9.4 | - | - |
| Etiology | Ischemic (n=21); Non-Ischemic (n=14) | ||
| Medications | Diuretics (n=31); Betablocker (n=32); Angiotensin receptor (n=12); ACE inhibitors (n=19) |
HF, Heart failure; BMI, Body mass index; BP, Blood pressure; LVEF, Left ventricle ejection fraction.
Control subjects were recruited through advertisements at the UCLA campus and West Los Angeles area. All control subjects were in good health, without any clinical history of cardiovascular, stroke, respiratory, neurological, or psychiatric disorders that may introduce brain changes. All participants gave written informed consent before MRI scanning or other data collection, and the study protocol was approved by the Institutional Review Board at the UCLA.
Valsalva Maneuver
The Valsalva maneuver was performed by all participants in a sequence of four 16-s vigorous and forceful exhalations into a mouthpiece, spaced 120 s apart, to a target expiratory pressure of 30 mmHg level, with 160 s baseline before the first challenge period. The mouthpiece was connected to a pressure gauge, which allowed continuous assessment of exhalation pressure. The pressure signal was calibrated before each experiment. The closed exhalation circuit allowed a slow air leak, guaranteeing an open glottis, which ensured raised intra-thoracic pressure. Following each 16-s challenge period, the closed exhalation circuit was removed and the subject breathed normally for 120 s. A light signal was used to indicate challenge onset for the Valsalva effort. Subjects were instructed to, upon seeing the light signal, take a breath and exhale against a resistance, maintaining a target pressure. A second light was illuminated when subjects achieved 30 mmHg target pressure. Subjects practiced the Valsalva maneuver prior to MRI data collection, and the research team observed each challenge to ensure the target pressure was achieved and maintained for each of the four expiratory efforts.
Magnetic Resonance Imaging
All HF and control subjects underwent for brain structural and functional imaging in a 3.0-Tesla MRI scanner (Siemens, Erlangen, Germany). We used foam pads on either side of the head to minimize head motion during scanning. High-resolution T1-weighted images were acquired using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence [repetition time (TR) = 2200 ms; echo-time (TE) =2.34, 2.41 ms; flip angle (FA) = 9°; field of view (FOV) = 230×230 mm2; matrix size = 320×320; voxel size = 0.72×0.72×0.9 mm3). BOLD-fMRI data were collected with an echo planar imaging based pulse sequence in the axial plane (TR = 2000 ms; TE = 30 ms; FA = 90°; FOV = 230×230 mm2; matrix size = 64×64; voxel size =3.6× 3.6× 4.2; slice thickness = 4.2 mm; volumes = 352). The Valsalva maneuver was performed by all the subjects during the fMRI scanning. We continuously recorded breathing rate, heart rate, and oxygen saturation levels from each subject during MRI using an abdominal pneumatic belt and a finger pulse oximeter for safety reasons.
Data Processing
Prior to FC analysis, fMRI data were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) software. The initial 10 volumes were discarded to avoid signal saturation issues, and remaining 342 volumes were realigned to eliminate potential head-motion and co-registered to T1-weighted images. Since the Valsalva maneuver requires the movement of mouth and forceful exhalations, head-motion is inevitable. To eliminate signal contamination from head motion, the effects of six rigid-body motion parameters and their first and second derivatives were regressed-out as covariates from the time series of each voxel. In addition, challenge-induced linear trend and global brain signal changes were also regressed out from the time series of each voxel. BOLD images were then spatially-normalized to Montreal Neurological Institute (MNI) space, using nonlinear transformation procedures, and smoothed with a 4-mm full-width at half-maximum Gaussian kernel.
For FC analysis, four regions of interest (ROI) from standard AAL template were selected as seed regions, including the left and right insula and cerebellum crus 2. FC maps were generated for each ROI of individual subject from the fMRI data of the baseline (140–0 s before the first challenge onset), the Valsalva challenge phase (0–16 s after the onset of each of the four challenges), and the recovery phase (16–40 s after the each challenge onset), respectively. Functional connectivities between regional mean time series for seed regions and whole-brain voxels were calculated with a canonical correlation approach. A positive FC value indicated positive correlation between the mean fMRI time course of the seed region and a specific brain area (i.e. co-activation of these areas), while a negative FC value showed negative correlation between the neural activities of the seed region and a specific brain area (i.e. the time series changes in the opposite direction between these regions, while one region is activated the other region is deactivated). Individual FC maps were converted into z-scored maps with Fisher’s z transformation and subjected to second-level, random-effects analysis to generate group-level statistical maps.
Statistical Analyses
Demographic and physiologic data were examined by the Chi-square (categorical values) and independent samples t-tests (numerical values). We performed one-sample t-tests on the baseline FC maps, Valsalva challenge phase, and recovery phase, respectively, to examine brain areas that were significantly associated with the activities of ROIs during different phases in HF and control subjects [covariates: age and sex; False discovery rate (FDR) corrections for multiple comparisons, P < 0.05]. We compared the FC maps between challenge phase and baseline, between recovery phase and baseline, as well as between recovery and challenge phase, using paired t-tests in healthy control (HC) and HF groups (FDR, P < 0.05). We compared the FC maps between HF and control subjects using analysis of covariance (ANCOVA; covariates: age and sex; FDR, P < 0.05) for the three phases, respectively. We further examined cross-subject partial correlations between the FC maps and left ventricle ejection fraction (LVEF) values in HF subjects by calculating partial correlations between FC value of each brain voxel and LVEF values (covariates: age and sex; AlphaSim corrections for multiple comparisons, corrected P < 0.05, with voxel-level p < 0.005, cluster size > 25 voxels).
RESULTS
Demographic and physiological variables
No significant differences in age, gender, or education appeared between groups (Table 1). In addition, HF patients did not differ in BMI, heart rate, or systolic blood pressure compared to controls. However, HF subjects had lower diastolic blood pressure over healthy controls (p = 0.001).
FC patterns in HF and control subjects
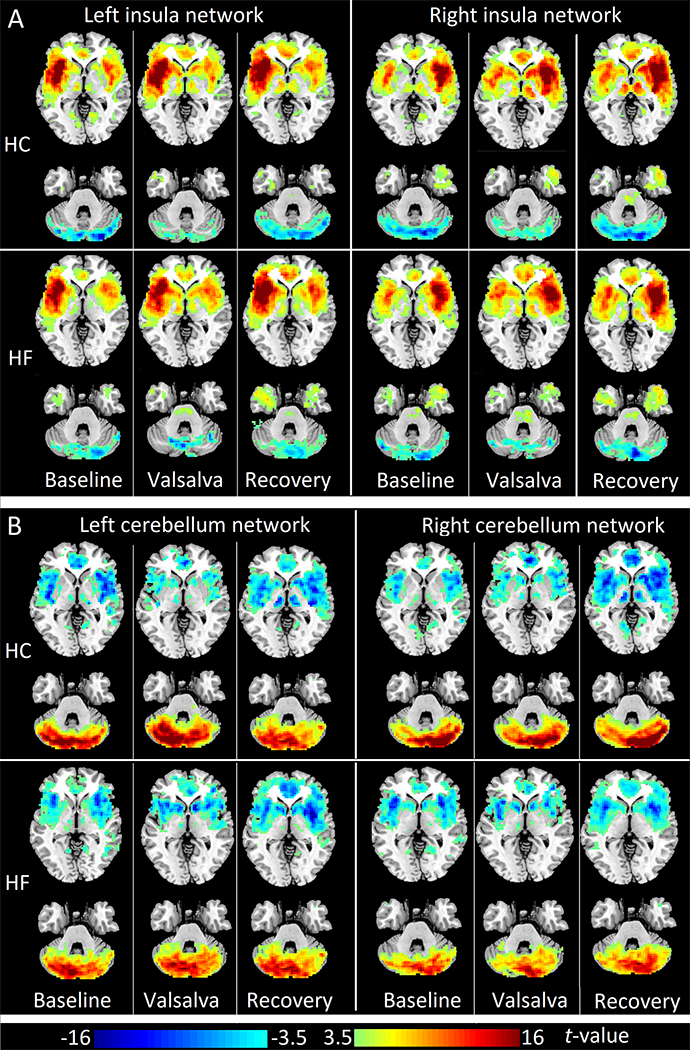
The insula and cerebellum networks showed similar patterns in both HC and HF groups throughout different phases of the Valsalva maneuver. Left and right insula showed significant positive FC with the contralateral insula, bilateral striatum, thalamus, and anterior cingulate cortex (ACC), at baseline, challenge, and recovery phases (FDR corrected P < 0.05). These sites constitute the insular-limbic-thalamo-striatal network (referred here as the insula network). Also, left and right insula showed significant negative FC with bilateral cerebellar cortices at different phases of the Valsalva maneuver (Fig. 1A, FDR corrected P < 0.05). Left and right cerebellar cortices exhibited significant positive FC with the contralateral cerebellar cortices, as well as significant negative FC with bilateral insula, striatum, ACC, and thalamus (Fig. 1B, FDR corrected P < 0.05).
Figure 1:
Insular and cerebellum networks during baseline, Valsalva challenge, and recovery in healthy controls and heart failure subjects. (A) One sample t-tests (FDR corrected p<0.05; covariates, age and gender) of left insular FC maps (left panel) and right insular FC maps (right panel) in HC (upper panel) and HF (lower panel). (B) One sample t-tests (FDR corrected p<0.05; covariates, age and gender) of left cerebellum FC maps (left panel) and right cerebellum FC maps (right panel) in HC (upper panel) and HF (lower panel). Three columns in each sub figure represent results in three phases, from left to right: baseline, Valsalva challenge, and recovery phase. Two representative slices in neurological convention (left side of the brain is shown on the left) are shown for each phase. Warm color indicates positive functional connectivity (FC), cold color shows negative FC. HC: healthy controls; HF: heart failure.
FC pattern changes between baseline, challenge, and recovery phases in HF and control subjects
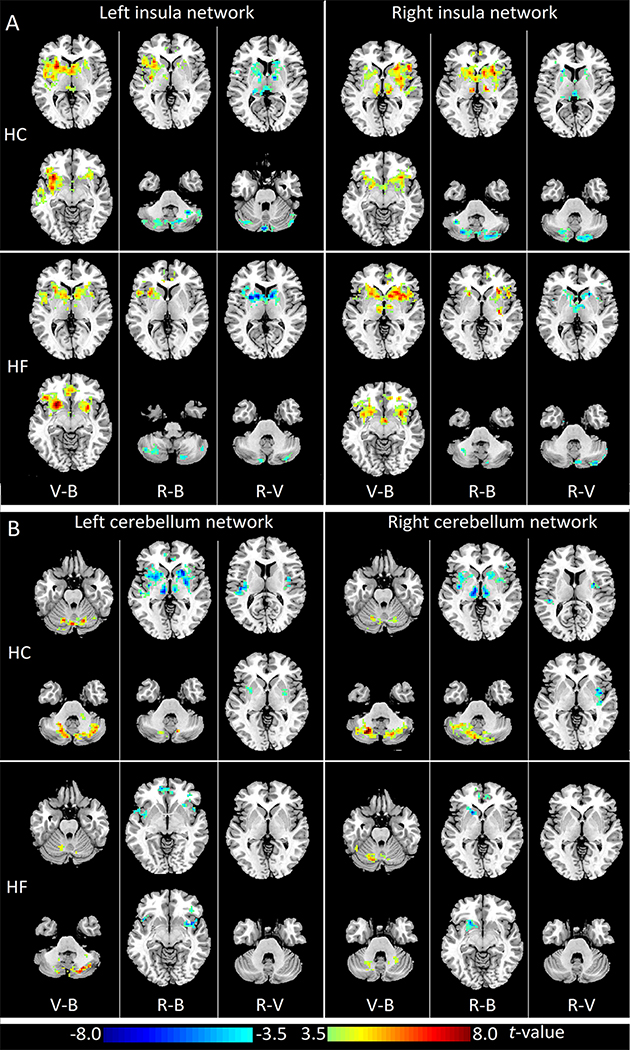
In HC subjects, left and right insula showed significantly increased FC with the contralateral insula, bilateral striatum, and thalamus during challenge over baseline period. During recovery phase, left and right insula showed increased FC within the insular-thalamo-striatal network, as well as enhanced negative FC with cerebellar cortices compared with baseline. However, these increased FC within the insula network was less extensive compared to the challenge phase. Similarly, HF subjects showed the highest FC within left and right insula networks during the challenge phase, and the recovery phase showed the strongest negative FC between insula and cerebellar networks. (Fig. 2A, supplementary table 1, FDR corrected P < 0.05).
Figure 2:
Changes of insular and cerebellar functional connectivity (FC) during the different phases of Valsalva maneuver in healthy controls and heart failure subjects. (A) Paired t-tests (FDR corrected p<0.05) of left insular FC maps (left panel) and right insular FC maps (right panel) in HC (upper panel) and HF (lower panel). (B) Paired t-tests (FDR corrected p<0.05) of left cerebellum FC maps (left panel) and right cerebellum FC maps (right panel) in HC (upper panel) and HF (lower panel). Three columns in each sub figure represent results of comparisons between different phases, from left to right: Valsalva challenge phase vs. baseline (V-B), recovery phase vs. baseline (R-B), and recovery phase vs. Valsalva challenge (R-V). Two representative slices in neurological convention are shown for each comparison. Warm color indicates increased FC values, cold color shows decreased FC values. HC: healthy controls; HF: heart failure.
In both groups, left and right cerebellar cortices showed increased FC within the cerebellum network during the challenge phase compared to baseline. During the recovery phase, bilateral cerebellar cortices showed significantly enhanced negative FC with the insula network, including the bilateral insular cortices, putamen, thalamus, and ACC, compared to baseline in HC. The negative FC between bilateral cerebellar cortices and bilateral insula and putamen during the recovery phase was also stronger than that in the challenge phase in control subjects. However, HF subjects showed significantly enhanced negative left cerebellar cortex FC with only bilateral insula and ACC, but not bilateral putamen during the recovery phase. Also, right cerebellar cortex showed increased negative FC with only left putamen and ACC, but not other areas in the insula network in HF subjects (Fig. 2B, supplementary table 1, FDR corrected P < 0.05).
FC pattern differences between HF and control subjects
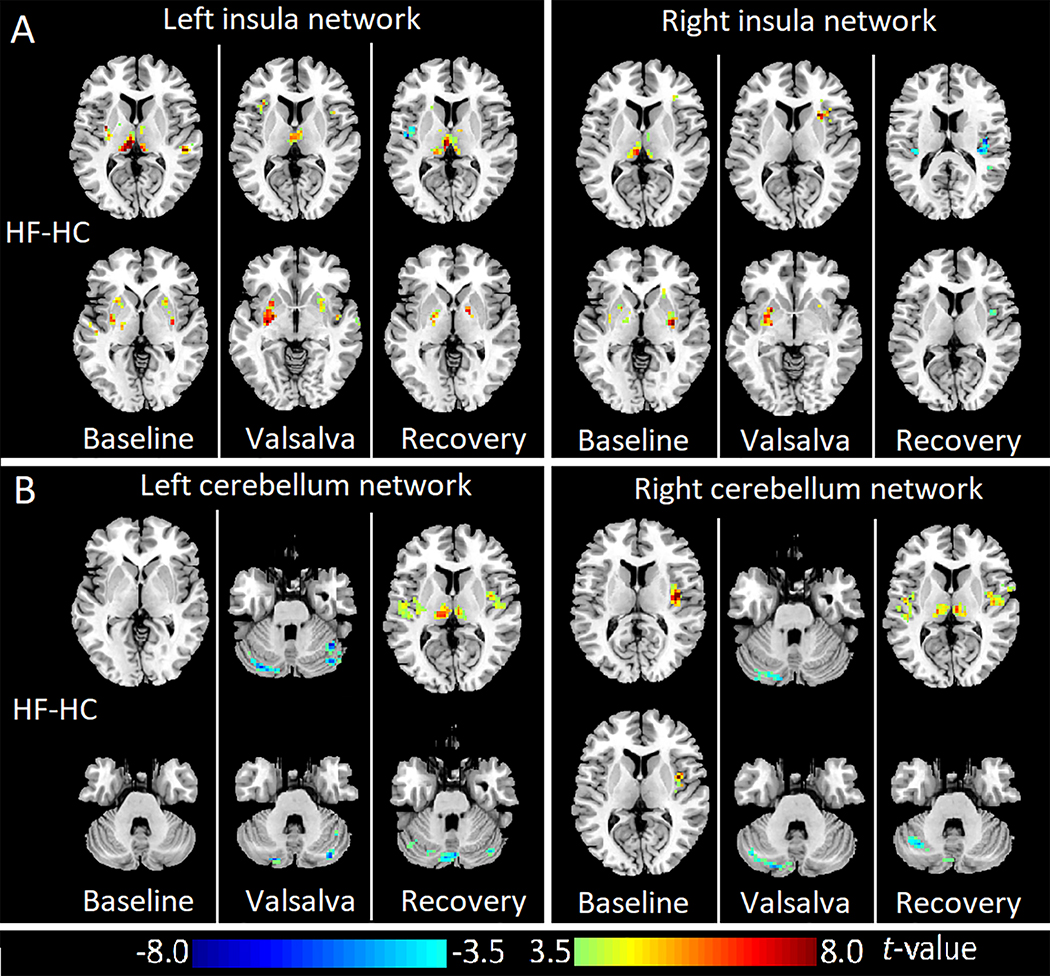
HF showed increased FC between bilateral insula and putamen, as well as thalamus at both baseline and challenge periods over control subjects. In the recovery phase, left insula showed higher FC with bilateral thalamus and globus pallidus, but lower FC with left posterior insula in HF compared to controls. In addition, right insula also showed lower FC with bilateral posterior insula in HF subjects (Fig. 3A, FDR corrected P < 0.05).
Figure 3:
Abnormal insular and cerebellum networks in heart failure during baseline, Valsalva challenge, and recovery over control subjects. (A) ANCOVA (FDR corrected p<0.05; covariates, age and gender) of left insular FC maps (left panel) and right insular FC maps (right panel) comparing HF with healthy subjects. (B) ANCOVA (FDR corrected p<0.05; covariates, age and gender) of left cerebellum FC maps (left panel) and right cerebellum FC maps (right panel) comparing HF with healthy subjects. Three columns in each sub figure represent results of comparison results in three phases, from left to right: baseline, Valsalva challenge, and recovery. Two representative slices in neurological convention are shown for each phase. Warm color indicates increased FC values, cold color shows decreased FC values. HC: healthy controls; HF: heart failure.
Left cerebellar network did not show significant difference between groups at the baseline; however, decreased FC appeared between left and right cerebellar cortices in challenge and recovery phases in HF compared to healthy controls. Left cerebellum also showed impaired negative FC with bilateral insula and thalamus during the recovery period in HF over control subjects. Right cerebellum showed impaired negative FC with right insula at baseline, and decreased FC with the contralateral cerebellar cortices in challenge and recovery phases in HF compared to healthy controls. Right cerebellum showed decreased negative FC with bilateral insula and thalamus during the recovery phase in HF over controls (Fig. 3B, FDR corrected P < 0.05).
Correlations between FC and LVEF values in HF
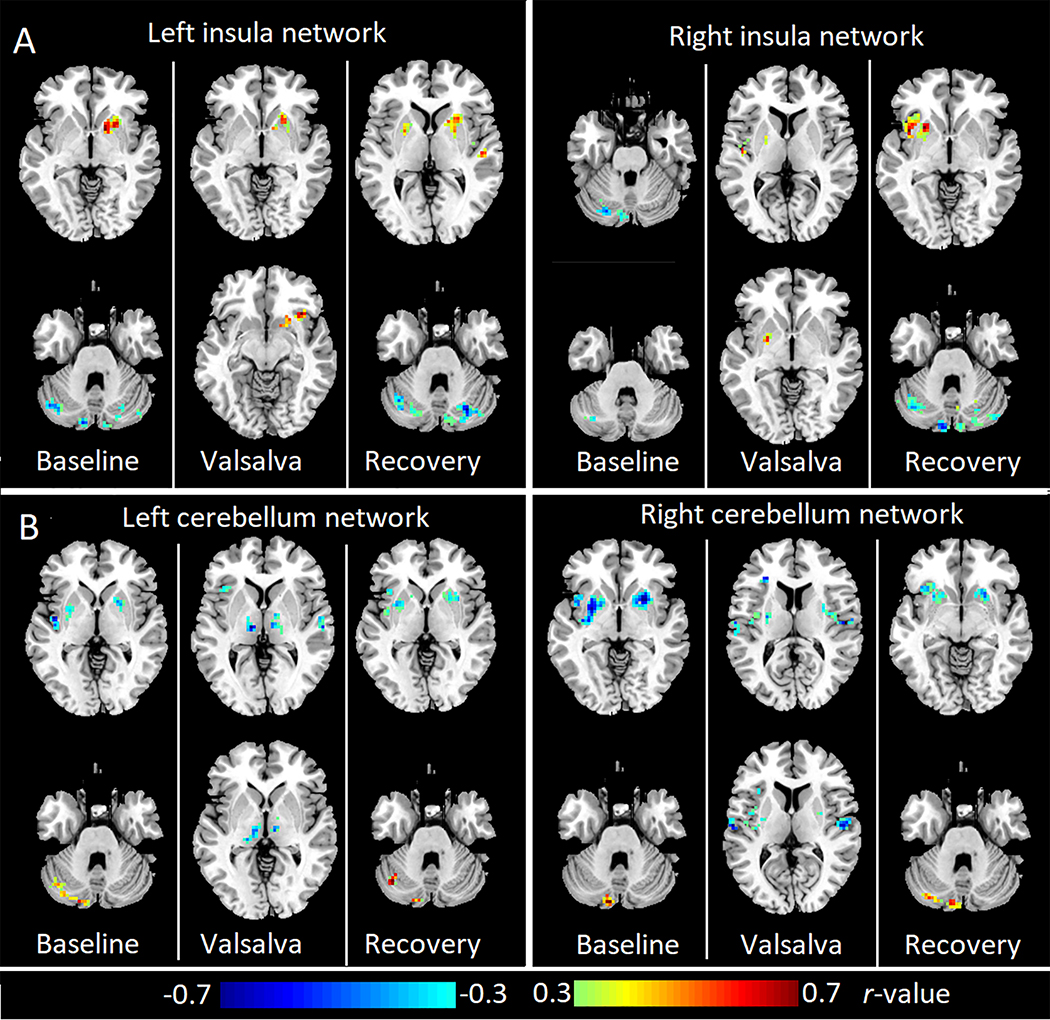
Partial correlation analysis showed that the left insula FC with striatum was positively correlated with LVEF during all of the three phases. The left insula FC with cerebellum was negatively correlated with LVEF at baseline and recovery phases. The right insula FC with left insula and left putamen was positively correlated with the LVEF during challenge and recovery phases, while the right insula FC with cerebellar cortices was negatively correlated with LVEF at baseline and recovery periods (Fig 4. A, AlphaSim corrected P < 0.05)
Figure 4:
Brain areas with significant correlation between LVEF and insular or cerebellar FC in HF subjects during baseline, Valsalva challenge, and recovery phases. (A) Partial correlation (Alphasim corrected p<0.05) between LVEF and left insula FC maps (left panel) or right insula FC maps (right panel). (B) Partial correlation (Alphasim corrected p<0.05) between LVEF and left cerebellum FC maps (left panel) or right cerebellum FC maps (right panel). Three columns in each sub figure represent results in three phases, from left to right: baseline, Valsalva challenge, and recovery phase. Two representative slices in neurological convention are shown for each phase. Warm color indicates positive correlation and cold color shows negative correlation. HC: healthy controls; HF: heart failure.
The FC within the left cerebellum network showed significant positive correlation with LVEF at baseline and recovery. While the left cerebellum FC with bilateral insula, putamen, and thalamus was negatively correlated with LVEF. Similar correlation patterns appeared in the right cerebellum network as well. The right cerebellum FC with the contralateral cerebellar cortices was positively correlated with LVEF at baseline and recovery, while right cerebellum FC with bilateral insula and putamen was negatively correlated with LVEF (Fig 4. B, AlphaSim corrected P < 0.05).
DISCUSSION
Overview
Insula showed significant FC with bilateral striatum, thalamus, and ACC, areas that constitute the insular network in HF and control subjects. While left and right cerebellar cortices showed significant FC with each other constituting the cerebellum network; the insula and cerebellum networks showed significant negative FC with each other at baseline, challenge, and recovery phases in HF and controls. At baseline, HF patients showed increased insular FC with bilateral striatum and thalamus over control subjects. The Valsalva challenge induced increased FC within the insula network, and within the cerebellum network in both HC and HF subjects. However, compared to healthy subjects, HF showed more increased FC within the insula network, but less enhanced FC between bilateral cerebellar cortices. During the recovery phase, the negative FC between the insula and the cerebellum networks enhanced significantly in healthy controls, but not in HF. Further analysis showed significant positive correlations between LVEF and FC within the insula network, as well as stronger negative interactions between cerebellar cortices and the insula network.
The insular and cerebellar FC can be the central neurobiological parameters of autonomic functions. The Valsalva maneuver elicited increased insular FC (Figure 2A) accompanied by increased heart rate in healthy controls (5), indicating enhanced insular FC is associated with sympathetic activity in normal condition. Hyper sympathetic activity during rest is one of the major phenomena in HF patients; we observed higher insular network FC during baseline in HF than controls (Figure 3A), again indicating increased insular FC is associated with enhanced baseline sympathetic tone in HF condition. On the other hand, the cerebellum showed negative FC with the insula network (Figure 1B), and this negative FC between cerebellum and insula was enhanced during the Valsalva challenge and recovery phase (Figure 2B), suggesting an inhibitory role of cerebellum to limit extreme blood pressure and heart rate changes in normal condition. HF subjects showed decreased cerebellar FC during the challenge and impaired negative FC between the cerebellum and insula during the recovery phase (Figure 3B) accompanied by slower drop of heart rate, as shown before (5), again suggesting that cerebellar FC might be associated with parasympathetic activation. These evidences support each other and demonstrate the strong associations between brain FC and autonomic function in both normal and HF condition
Exaggerated insular network FC at baseline
Both sympathetic and parasympathetic branches of the autonomic nervous system are affected in HF patients. At rest, the sympathetic outflow in HF subjects is often exaggerated, the parasympathetic activity is lessened, and the interaction between these two systems is altered (21). The increased baseline FC within the insular network may be associated with exaggerated resting sympathetic tone in HF. With fiber connections with multiple brain areas, insula plays important roles in cardiovascular, vestibular, somatosensory, and motor modulation (11). Insular cortical and subcortical FC, especially with the ACC, thalamus, and basal-ganglia, underscore the site’s roles in autonomic control, along with somatosensory integration and pain-regulations (12). Abnormal insular activities may encourage a pro-arrhythmic state (22). Previous studies found insular structural impairment would result in malfunction of autonomic regulation and lead to exaggerated sympathetic tone, with an accompanying higher incidence in myocardial infarction and heart failure (23). Increased insular FC with other autonomic control areas, such as striatum and thalamus, could shift cardiovascular balance towards increased basal sympathetic tone, a pro-arrhythmic condition, and may contribute to the higher risk of cardiac mortality (23).
Increased insular and impaired cerebellar FC networks during challenge
The Valsalva maneuver triggers enhanced sympathetic activities, including heart rate increase and blood pressure changes, during the challenge (4, 6). Previous fMRI studies have reported increased neural activities in the insula accompanying increased heart rate (5, 6, 10). Positron emission tomography study also showed that activation in insula, putamen, ACC, and thalamus were associated with increased heart rate during autonomic challenge (24). We found here enhanced insular FC with basal ganglia, thalamus, and ACC in healthy controls during challenge, suggesting that increased insular network FC contributes to sympathetic activation in normal condition. However, HF subjects showed more intensified insular network FC at both baseline and challenge periods over the controls, indicating that heightened insular network contributes to exaggerated baseline sympathetic tone and activates sympathetic outflow during autonomic challenge. Lower LVEF was associated with lower insula FC with putamen in HF during different phases of the Valsalva maneuver, suggesting that increased insular FC in HF compared to controls might be a compensatory mechanism in the condition. The over-activated insular network in HF during the challenge may reflect autonomic nervous system effort to trigger sympathetic outflow and increase heart rate to compensate low heart output during autonomic challenge (6); increased heart rate may help the brain to develop stronger compensatory mechanism in HF patients.
In addition to increased insular network FC, the challenge also induced increased FC within the cerebellum network in both control and HF subjects. Since the Valsalva maneuver involves an interaction between motoric (active forced expiration), as well as autonomic regulatory components, increased FC within insula and cerebellum networks may have implications for both aspects. As important parts of the motor circuit, the thalamus, putamen, globus pallidus, and cerebellum play significant roles in motor planning (16). The disruption of this circuitry will result in ataxia, difficulties in smooth pursuit of action, and other movement disorders (17, 25). In our study, HF showed higher insular FC with thalamus, putamen, and globus pallidus during challenge over controls, suggesting a higher demand for central recruitment to maintain the expiratory strain and motor coordination pattern. Also, HF showed less cerebellar FC increase than controls during the challenge, indicating impaired cerebellar functions in motor and autonomic regulations in HF.
For autonomic regulation, cerebellar cortices are key for timely coordination of sympathetic and parasympathetic attributes, and for dampening extremes of hypotension/hypertension induced by physiological challenges or pathological conditions (6, 26). Impaired cerebellar FC in HF subjects would not only lead to deficits in respiratory motor coordination, but also to sympathetic deficits, such as heart rate and blood pressure dysregulations. Moreover, lower LVEF was correlated with lower FC between cerebellar cortices during baseline and recovery periods. These data demonstrated that impaired cerebellum network FC was directly associated with deficits in heart functions.
Impaired negative interactions between cerebellar and insular networks during recovery
During the recovery phase, cerebellum showed significantly enhanced negative FC with the insula, thalamus, and striatum in HC. However, negative FC between the cerebellum and insular networks was less significant in HF. Following the expiratory pressure release, parasympathetic system is activated and heart rate returns quickly to baseline in HC, but HF patients show a slower heart rate decline, as well as abnormal blood pressure during the recovery (5, 6). The absence of negative interactions between the cerebellar and insular networks in HF may underlie the impaired parasympathetic functions, and may result in tardy decrease of heart rate and abnormal blood pressure changes.
Blood pressure regulation is impaired both during the challenge and recovery periods of the Valsalva maneuver in HF (27), and abnormal cerebellar FC may directly result in this dysregulation. Cerebellar cortices interact with other autonomic control areas that play important roles in limiting blood pressure extremes, as well as breathe-to-breathe blood pressure changes (28, 29). In our study, as the respiratory straining goes on, the cerebellar FC increase significantly in control, but not in HF subjects, indicating a less sufficient control of hypertension. During the recovery phase, HF patients typically show little or no rise in blood pressure (27), and lower FC between cerebellar cortices over controls. The impaired cerebellar network FC in HF may contribute to dysregulation of blood pressure after the challenge.
Since increased insular network FC could shift autonomic balance towards increased sympathetic tone, the negative interaction between cerebellum and insula networks may indicate a suppressive effect of cerebellum system on the insula system, which brings the heart rate and blood pressure back to baseline during recovery (23). Various studies showed that the cerebellum can exert a tonic suppressor or inhibitory influence on subcortical structures within the striatal-limbic circuit (30), and electrical cerebellar stimulation inhibited sympathetic tone and increased parasympathetic activity to the cerebrovascular bed, resulting in vasodilation and an increase in mean carotid blood flow (31). Our results showed that the enhanced negative FC between cerebellar and insular systems as seen in HC was absent in HF, implying impaired cerebellar functions to dampen excessive sympathetic tones. In addition, lower LVEF was also correlated with decreased negative interaction between cerebellum and insula networks, indicating that proper coordination between cerebellar cortices and insular networks is essential for maintaining sympathetic and parasympathetic balance, as well as normal heart functions. The lack of suppressive effects from cerebellum may result in prolonged activation in the insular network during the recovery phase, likely leading to a prolonged sympathetic activation and delayed return to baseline heart rate and blood pressure levels in HF.
Pathological processes contributing to abnormal functional connectivity
Although the precise pathological processes underlying the altered FC in HF are unclear, but may include several possible mechanisms. HF patients show compromised cerebral perfusion, resulting from low cardiac output, and insular and cerebellar functional changes may have developed from ischemia or inadequate perfusion accompanying the syndrome (32). Cerebellar, insular, and subcortical sites are especially vulnerable to hypoxia/ischemia, and even short-term exposure to hypoxia can cause severe neural injury (33). Previous brain structural studies showed brain tissue changes in various autonomic control areas in HF subjects (10). We believe that a portion of abnormal FC responses to the Valsalva maneuver may derive from impaired neurons within the structures and axons affecting the neural pathways connecting the insula, striatum, and cerebellum to other brain sites. In addition, initial injury to the autonomic control sites in the brain in HF subjects, including the insular and cerebellar areas, may result in abnormal central neural outflow and compromise vascular activity and heart rate regulation, which in turn can lead to altered brain perfusion, introducing secondary brain injury in limbic and other brain areas (34).
Limitations
One major limitation of this study is that we could not directly link brain FC changes to sympathetic and parasympathetic activities, although demonstrated the dynamic FC changes in different stages of the Valsalva maneuver. For decades, heart rate responses to the Valsalva maneuver have been used as a robust test of circulatory integrity (4). Our previous studies have also repeatedly showed abnormal physiological response to Valsalva challenge in HF patients (5, 6). This study further revealed aberrant brain FC dynamics accompanied with these physiological changes in HF. However, dynamic heart rate changes are often result from both sympathetic and parasympathetic nerve activities, and such co-existence of both activities may confound the finding, and this may be the case why both insular and cerebellar network activations were observed during different phases of the Valsalva maneuver. Although peripheral markers of autonomic activities can be obtained indirectly, e.g., by measuring heart rate, blood pressure, sweat release, or skin blood flow, these responses are confounded by both sympathetic and parasympathetic actions, and are slower compared to directly-recorded autonomic nerve activity using electrodes. Microneurography, in which a tungsten microelectrode is inserted percutaneously into a peripheral nerve in awake human subjects, allows to record autonomic nerve activities to either muscle or skin (35), and further studies combining fMRI and microneurography may help to separate effects of sympathetic and parasympathetic activities on brain FC patterns in HF patients.
Conclusions
HF patients show both persistent increased sympathetic and impaired parasympathetic tones, and abnormal insular and cerebellar FC found here would have greatly contributed to those altered autonomic functions. Our findings indicate that increased insular network FC at baseline and during challenge in HF might be a compensatory mechanism for low heart output, which contributes to the exaggerated sympathetic tone in HF. While impaired cerebellum FC network and the diminished negative interactions between cerebellar and insular systems may indicate a loss of the normal suppressive action from the cerebellum on the insula network, which may contribute to impaired sympathetic-vagal balance in the condition.
The FC changes during the Valsalva maneuver might be useful as clinical marker of autonomic health state in HF patients. Increased FC within the insular-limbic-thalamo-striatal circuitry may indicate exaggerated sympathetic outflow, and diminished negative FC between the insular and cerebellar sites might reflect impaired parasympathetic regulation in HF subjects. Moreover, the significant correlations between these FC changes and LVEF suggest that brain functional changes are associated with disease severity in HF, and could be used as a non-invasive way to evaluate the central nervous system autonomic functions and overall brain health in HF condition
Supplementary Material
Highlights.
Hyper connectivity emerged in the insular-limbic-thalamo-striatal network in HF
HF showed reduced cerebellar connectivity during the challenge and recovery phases
Altered connectivity appeared between the cerebellum and insula in HF during the recovery phase
Lower LVEF was correlated with lower insular network functional connectivity in HF
ACKOWLEDGEMENTS
We thank Mrs. Karen Harada, Ms. Cristina Cabrera-Mino, Mr. Luke Ehlert, and Dr. Sadhana Singh for assistance with data collection.
FUNDING
This research work was supported by National Institutes of Health R01 NR-013625, R01 NR-014669, and American Heart Association 17POST33440099 (BR).
Footnotes
CONFLICT OF INTEREST
All authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. American journal of physiology Heart and circulatory physiology. 2001;280(3):H969–76. [DOI] [PubMed] [Google Scholar]
- 2.Floras JS, Butler GC, Ando SI, Brooks SC, Pollard MJ, Picton P. Differential sympathetic nerve and heart rate spectral effects of nonhypotensive lower body negative pressure. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281(2):R468–75. [DOI] [PubMed] [Google Scholar]
- 3.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32(11):2530–3. [DOI] [PubMed] [Google Scholar]
- 4.Elisberg EI. HEART RATE RESPONSE TO THE VALSALVA MANEUVER AS A TEST OF CIRCULATORY INTEGRITY. Jama. 1963;186:200–5. [DOI] [PubMed] [Google Scholar]
- 5.Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, et al. Aberrant central nervous system responses to the Valsalva maneuver in heart failure. Congestive heart failure (Greenwich, Conn). 2007;13(1):29–35. [DOI] [PubMed] [Google Scholar]
- 6.Ogren JA, Macey PM, Kumar R, Fonarow GC, Hamilton MA, Harper RM, et al. Impaired cerebellar and limbic responses to the valsalva maneuver in heart failure. Cerebellum (London, England). 2012;11(4):931–8. [DOI] [PubMed] [Google Scholar]
- 7.Paton JF, Spyer KM. Brain stem regions mediating the cardiovascular responses elicited from the posterior cerebellar cortex in the rabbit. The Journal of physiology. 1990;427:533–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R, Yadav SK, Palomares JA, Park B, Joshi SH, Ogren JA, et al. Reduced regional brain cortical thickness in patients with heart failure. PloS one. 2015;10(5):e0126595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar R, Nguyen HD, Ogren JA, Macey PM, Thompson PM, Fonarow GC, et al. Global and regional putamen volume loss in patients with heart failure. European journal of heart failure. 2011;13(6):651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song X, Roy B, Fonarow GC, Woo MA, Kumar R. Brain structural changes associated with aberrant functional responses to the Valsalva maneuver in heart failure. 2018;96(9):1610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn FG. Anatomy of the insula functional and clinical correlates. Aphasiology. 1999;13(1):55–78. [Google Scholar]
- 12.Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, et al. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Human brain mapping. 2012;33(9):2005–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak M, Holm S, Biering-Sorensen F, Secher NH, Friberg L. “Central command” and insular activation during attempted foot lifting in paraplegic humans. Human brain mapping. 2005;25(2):259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes MJ, Cotter LA, Arendt HE, Cass SP, Yates BJ. Effects of lesions of the caudal cerebellar vermis on cardiovascular regulation in awake cats. Brain research. 2002;938(1–2):62–72. [DOI] [PubMed] [Google Scholar]
- 15.Song X, Zhou S, Zhang Y, Liu Y, Zhu H, Gao JH. Frequency-Dependent Modulation of Regional Synchrony in the Human Brain by Eyes Open and Eyes Closed Resting-States. PloS one. 2015;10(11):e0141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, Song X, Yuan Y, Li E, Liu J, Liu W, et al. Abnormal functional connectivity of the amygdala is associated with depression in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2015;30(2):238–44. [DOI] [PubMed] [Google Scholar]
- 17.Hu X, Song X, Li E, Liu J, Yuan Y, Liu W, et al. Altered Resting-State Brain Activity and Connectivity in Depressed Parkinson’s Disease. PloS one. 2015;10(7):e0131133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. European journal of heart failure. 1999;1(3):251–7. [DOI] [PubMed] [Google Scholar]
- 20.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. [DOI] [PubMed] [Google Scholar]
- 21.Frenneaux MP. Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart (British Cardiac Society). 2004;90(11):1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oppenheimer S The anatomy and physiology of cortical mechanisms of cardiac control. Stroke. 1993;24(12 Suppl):I3–5. [PubMed] [Google Scholar]
- 23.Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 1996;6(3):131–40. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Watanabe S, Hamaguchi T, Mine H, Terui T, Kanazawa M, et al. Brain activation associated with changes in heart rate, heart rate variability, and plasma catecholamines during rectal distention. Psychosomatic medicine. 2009;71(6):619–26. [DOI] [PubMed] [Google Scholar]
- 25.Song X, Hu X, Zhou S, Xu Y, Zhang Y, Yuan Y, et al. Association of specific frequency bands of functional MRI signal oscillations with motor symptoms and depression in Parkinson’s disease. Scientific reports. 2015;5:16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutherer LO, Lutherer BC, Dormer KJ, Janssen HF, Barnes CD. Bilateral lesions of the fastigial nucleus prevent the recovery of blood pressure following hypotension induced by hemorrhage or administration of endotoxin. Brain research. 1983;269(2):251–7. [DOI] [PubMed] [Google Scholar]
- 27.Shamsham F, Mitchell J. Essentials of the diagnosis of heart failure. American family physician. 2000;61(5):1319–28. [PubMed] [Google Scholar]
- 28.Rector DM, Richard CA, Harper RM. Cerebellar fastigial nuclei activity during blood pressure challenges. Journal of applied physiology (Bethesda, Md : 1985). 2006;101(2):549–55. [DOI] [PubMed] [Google Scholar]
- 29.Harper RM, Gozal D, Bandler R, Spriggs D, Lee J, Alger J. Regional brain activation in humans during respiratory and blood pressure challenges. Clinical and experimental pharmacology & physiology. 1998;25(6):483–6. [DOI] [PubMed] [Google Scholar]
- 30.Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. Journal of neuroscience research. 1976;2(2):133–46. [DOI] [PubMed] [Google Scholar]
- 31.McKee JC, Denn MJ, Stone HL. Neurogenic cerebral vasodilation from electrical stimulation of the cerebellum in the monkey. Stroke. 1976;7(2):179–86. [DOI] [PubMed] [Google Scholar]
- 32.Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev. 2009;14(2):89–99. [DOI] [PubMed] [Google Scholar]
- 33.Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy B, Woo MA, Wang DJJ, Fonarow GC, Harper RM, Kumar R. Reduced regional cerebral blood flow in patients with heart failure. European journal of heart failure. 2017;19(10):1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macefield VG, James C, Henderson LA. Identification of sites of sympathetic outflow at rest and during emotional arousal: Concurrent recordings of sympathetic nerve activity and fMRI of the brain. International Journal of Psychophysiology. 2013;89(3):451–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.