Abstract
Asthma causes enormous suffering and cost for children in the US and around the world [[1], [2], [3]]. Co-morbid gastroesophageal reflux disease (GERD) makes asthma management more difficult due to increased symptoms. Proton pump inhibitor (PPI) drugs are effective at improving to GERD symptoms, however they have demonstrated only modest and variable effects on asthma control in the setting of co-morbid GERD. Importantly, PPI metabolism and efficacy depend on CYP2C19 genotype. The Genotype Tailored Treatment of Symptomatic Acid Reflux in Children with Uncontrolled Asthma (GenARA) study is a randomized, double-blind, placebo-controlled trial to determine if genotype-tailored PPI dosing improves asthma symptoms among children with inadequately controlled asthma and GERD symptoms. This study has an innovative design to both assess the efficacy of genotype-tailored PPI dosing and perform pharmacokinetic modeling of the oral PPI Lansoprazole. Children ages 6–17 years old with clinician-diagnosed asthma and mild GERD symptoms will submit a saliva sample for CYP2C19 genotyping. Participants will undergo a two-step randomization to: (1) genotype-tailored versus conventional dosing of open-label oral lansoprazole for pharmacokinetic modeling, and (2) genotype-tailored lansoprazole daily versus placebo for 24 weeks to determine the effect of genotype-tailored PPI dosing on asthma control. Measures of asthma control, spirometry, and nasal washes during acute illnesses will be collected at 8-week intervals throughout the study. GenARA will better define the effects of CYP2C19 genotype on the dose response of lansoprazole in children and adolescents and assess if a novel dosing regimen improves GERD and asthma control.
1. Introduction
Asthma remains difficult to control in many patients particularly children with co-morbid conditions such as obesity and gastroesophageal reflux disease. Year after year, asthma is a leading cause of pediatric urgent care visits, ED visits, hospitalizations, and ICU admissions [4]. Asthma also causes chronic symptoms that do not always lead to healthcare utilization but cause diminished quality of life with reduced sleep quality, missed school days, and reduced extracurricular participation. Personalized approaches tailored to at-risk, high morbidity groups hold promise for improving asthma care. Decades of data resulting from animal models [[5], [6], [7], [8]], epidemiologic studies [9,10], human esophageal acid instillation studies [[11], [12], [13], [14], [15], [16], [17]], pH probe asthma symptom correspondence studies [18], and surgical fundoplication follow-up studies [[19], [20], [21], [22]] all suggest that GERD contributes to poor asthma control. Past trials studying the effect of proton pump inhibitors on asthma symptoms in children have shown inconsistent results (Table 1 ). The current literature suggests that anti-GERD medications do not consistently improve asthma outcomes in a diverse cohort of patients with GERD symptoms. Partial or inconsistent response from a drug that is known to have variable clearance within the population (as is the case with PPIs) is consistent with a pharmacogenetic effect.
Table 1.
Pediatric asthma studies involving proton pump inhibitors (PPI).
| Type | Study | Patient Selection | n | PPI (dose in mg) | Duration | PPI effect | Comparison |
|---|---|---|---|---|---|---|---|
| Obs | Khooshoo et al. [23] | 5–10.5 years old asthma + GERD | 46 | Lansoprazole 30 mg/day (+prokinetic) | 46 wks | Yes | PPI and surgically tx GERD vs no GERD +PPI, no GERD untx. |
| RCT | Størdal et al. [24] | 7–16 years old asthma + GERD | 38 | Omeprazole 20 mg/day | 12 wks | No | PPI vs placebo |
| Obs | Yüksel et al. [25] | 1–16 years old non-atopic asthma + GERD | 50 | Lansoprazole 1 mg/kg/day | 12 wks | Yes | pre-PPI tx vs post-PPI tx |
| Obs | Khoshoo et al. [26] | 8–15 years old asthma + GERD after 1 year of PPI/prokinetic | 44 | Esomeprazole 40 mg/day | 52 wks | Yes | PPI vs ranitidine |
| Obs | Khoshoo et al. [27] | 6–13 years old non-atopic asthma + GERD | 30 | Esomeprazole 40 mg or lansoprazole 30 mg/day (+prokinetic) | 104 wks | Yes | PPI/GERD vs no PPI/no GERD |
| Obs | Bediwy et al. [28] | 5–11 years old asthma + GERD | 59 | Esomeprazole 20 mg/day | 12 wks | Yes | PPI/GERD vs placebo/no GERD |
| RCT | Holbrook et al. [29] | 6–17 years old asthma | 306 | Lansoprazole 15 mg/day (<30 kg) or 30 mg/day (≥30 kg) | 24 wks | No | PPI vs placebo |
Obs – observational study, RCT – randomized controlled trial, PPI – proton pump inhibitor, GERD – gastroesophageal reflux disease.
PPIs inhibit gastric H+/K+ ATPase (the final effector in the acid secretion pathway of gastric parietal cells) and are a first-line for therapy of GERD. Most PPIs are metabolized primarily by the CYP2C19 hepatic enzyme. The CYP2C19 gene is highly polymorphic so the metabolism and pharmacokinetics of PPIs is variable [[30], [31], [32], [33], [34], [35]]. Depending on the CYP2C19 diplotype, individuals can be classified as poor metabolizers (PM), normal metabolizers (NM), intermediate metabolizers (IM), extensive metabolizers (EM), or ultra-rapid metabolizers (UM) (Table 2 ) [36]. Little to no PK research among CYP2C19 metabolizer phenotypes has been conducted in children. Currently PPI dosing for children is largely extrapolated from adult findings which is a major health and safety concern.
Table 2.
Definition of CYP2C19 metabolizer phenotype.
| Allele1 | Genotype | Allele activity | Metabolizer phenotype | Frequency (pan-ethnic cohort) [33] |
|---|---|---|---|---|
| *1/*1 | Wild type (WT) | 2 active alleles | Normal | 41% |
| Metabolizer (NM) | ||||
| *1/*n | Heterozygous WT | 1 active allele | Intermediate | 21% |
| 1 inactive allele | Metabolizer (IM) | |||
| *2/*2 or *n/*n | Homozygous mutant | 2 inactive alleles | Poor | 3% |
| Metabolizer (PM) | ||||
| *1/*17 | Heterozygous WT | 1 active allele | Extensive | 24% |
| 1 increased activity allele | Metabolizer (EM) | |||
| *17/*17 | Homozygous mutant | 2 increased activity alleles | Ultrarapid | 4% |
| Metabolizer (UM) | ||||
| *2/*17 | Heterozygous mutant | 1 inactive allele | Normal metabolizer (NM) | 6% |
| 1 increased activity allele |
1 - *2, *3, *8, or *9 refer to loss-of-function (inactive) alleles; n – refers to any of the loss-of-function alleles.
Therefore, the GenARA study was designed as a randomized, double-blind, placebo-controlled 24 week intervention study to determine if genotype-tailored PPI dosing improves asthma symptoms among children with inadequately controlled asthma and recent evidence of mild GERD symptoms. GenARA will measure change in the ‘Juniper’ Asthma Control Questionnaire (ACQ) as the primary outcome, while evaluating asthma and GERD control and lansoprazole safety and pharmacokinetics [37].
1.1. Study design
The GenARA study is a multi-center controlled, 24-week parallel group interventional trial involving 64 children with asthma and GERD symptoms randomized to either genotype-tailored lansoprazole or placebo (Table 3 ). The study was reviewed by the Thrasher Research Fund, the Food and Drug Administration (IND 130170), and by the Institutional Review Boards at all participating sites.
Table 3.
Schedule for collection of response data.
| Visit | V1 | V2 | V3 | V4 | V5 |
|---|---|---|---|---|---|
| Time (weeks) | 0 | 2 | 10 | 18 | 26 |
| Consent/Assent | X | ||||
| Screening | X | ||||
| Baseline hx | X | ||||
| Saliva for genotyping | X | ||||
| Asthma and GERD counseling | X | X | |||
| PK | X | ||||
| Diary Card | X | X | X | X | X |
| Health history | X | X | X | X | |
| Med adherence | X | X | X | ||
| Spirometry | X | X | X | X | X |
| Asthma questionnaires | X | X | X | X | X |
| GSAS | X | X | X | X | |
| Adverse event screening | X | X | X | X | X |
| Pregnancy testing | X | X | X | X | X |
| Nasal samples | X | X | X | X |
PK – pharmacokinetic testing, GSAS – GERD Symptom Assessment Score.
1.2. Study population
GenARA selection criteria were established to study children with inadequately controlled asthma and mild GERD-related symptoms.
Criteria for enrollment – inclusion:
-
•
Age: 6–17 year olds with documented clinician-diagnosed asthma.
-
•
Evidence of recent uncontrolled asthma (must meet at least one of the following): (1) ACQ > 1.2; (2) Use of short-acting beta-agonist for asthma symptoms twice/week or more on average over the past month; (3) Nocturnal awakenings with asthma symptoms more than once per week on average over the last month; or (4) Two or more emergency department visits, unscheduled provider visits, prednisone courses or hospitalizations for asthma in the past 12 months.
-
•
Currently on stable dose of daily inhaled corticosteroid medication for asthma control equivalent to 88 μg of fluticasone or greater for at least 6 weeks from the time of enrollment. Participant must be on National Asthma Education and Prevention Program (NAEPP) controller step 2, 3, 4 or 5 [38].
-
•
Currently with mild GERD symptoms reported at visit 1 defined by a score on the Pediatric GERD Symptom Assessment Score >15 and <80 [39]. GSAS ranges from 0 to 490 with a higher score representing worse GERD symptoms.
Criteria for enrollment – exclusion: Participants could not be taking or have any of the following: daily CYP2C19 substrates, inducers, or inhibitors medication; past or current history of severe GERD or related disorders (erosive esophagitis, peptic ulcer disease, eosinophilic esophagitis) which in the opinion of the pediatric gastroenterology safety specialist/study physician requires treatment with acid-blocking agents (since participant may receive placebo); daily use of a PPI for >4 consecutive weeks in the past 6 months; previous intubation for asthma; admission to intensive care unit for >24 h for asthma in the past year; previous surgery involving the esophagus or stomach (anti-reflux surgery, peptic ulcer surgery, trache-esophageal fistula repair); forced expiratory volume in 1 s (FEV1) <60% of predicted at enrollment; any major chronic illness that would interfere with participation in the intervention or completion of the study procedures; history of phenylketonuria; medication use: treatment of GERD symptoms with over-the-counter antacids 4 days/week or more on average over past month; theophylline preparations, azoles, anti-coagulants, insulin for Type I diabetes, digitalis, oral iron supplements when administered for iron deficiency within 1 month; any investigational drugs within the past 2 months; drug allergies: previous allergic reaction from lansoprazole or other proton pump inhibitor medication or adverse reaction to aspartame; inability to complete baseline measurements in a satisfactory manner according to the judgment of the research coordinator or site PI; <75% completion of daily diary for asthma symptoms, SABA use and ICS medication adherence during the run-in period; plan for family to move from study location within the next 6 months.
1.3. Study medication and dosing
Participants eligible for randomization will be administered an open label dose of either conventional or genotype-tailored lansoprazole at visit 2 for pharmacokinetic analysis. Participants will then be subsequently randomized to either genotype-tailored lansoprazole (genotype-tailored dosing as in Table 4 ) or placebo. Placebo includes an equal volume of the liquid suspension sweetener that has a similar taste and consistency. Both active commercially-available lansoprazole and placebo liquid is manufactured and supplied by Cutis Pharma, Inc. (Wilmington, MA).
Table 4.
Genotype-tailored dosing adjustments.
| Phenotype | Daily dosing by weight |
||
|---|---|---|---|
| % change from conventional | <30 kg | ≥30 kg | |
| UM | 100% ↑ | 30 mg (10 ml) | 60 mg (20 ml) |
| EM | 50% ↑ | 22.5 mg (7.5 ml) | 45 mg (15 ml) |
| NM | 0% | 15 mg (5 ml) | 30 mg (10 ml) |
| IM | 30% ↓ | 10.5 mg (3.5 ml) | 21 mg (7 ml) |
| PM | 60% ↓ | 6 mg (2 ml) | 12 mg (4 ml) |
UM – ultra rapid metabolizer, EM – extensive metabolizer, NM – normal metabolizer, IM – intermediate metabolizer, PM – poor metabolizer.
Table adapted from Lima [35].
1.4. Randomization procedure
Eligible participants will be monitored for 10–17 days after screening and prior to randomization (run-in period) to assess asthma and GERD severity and diary card adherence. Participants will undergo a two-step randomization process. First, participants will be given a single oral dose of lansoprazole which will be randomly assigned as either conventionally-dosed lansoprazole or dosing according to Table 4 (genotype-tailored dosing). Second, they will be randomized for the 24-week intervention period to either genotype-tailored PPI dosing or matched placebo. Randomization for the 24-week intervention will be in a 1:1 ratio and stratified by site. Neither participants nor the research staff will be informed of the treatment group assignment to maintain the double-mask.
1.5. Safety monitoring
Lansoprazole is commercially available and is generally well-tolerated. Side effects will be assessed at 4 clinic visits and 3 telephone visits during the trial (Fig. 1 ). For patients with mild GERD and some persisting degree of asthma symptoms, there exists reasonable equipoise regarding the role of long-term PPI use, and thus use of a placebo-control with safety monitoring in place is justifiable. All participants regardless of randomization will receive education about diet and lifestyle modifications to reduce GERD as outlined in the patient handout: GERD in Children and Adolescents, published jointly by two expert organizations [40]. All subjects will continue on their previously prescribed asthma control regimen. During the study, participants can seek care from their pre-specified asthma physician. The study PI is also available for asthma problems and other clinical care questions. Pre-specified threshold criteria for starting oral steroids for an asthma exacerbation are also in place consistent with past NIH-funded pediatric asthma trials [41].
Fig. 1.
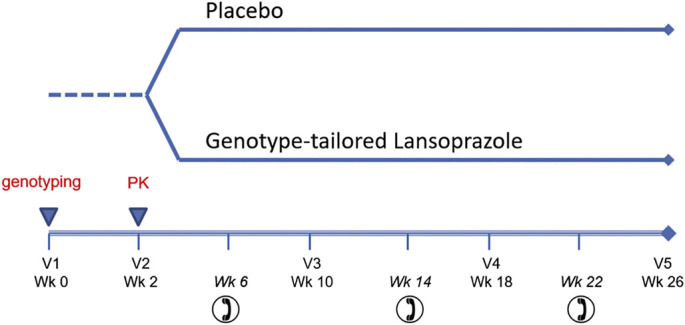
Study schema. PK – pharmacokinetic samples drawn at 2 and 5 h after a single open label oral dose, V – visit, Wk – week.
1.6. Data collection and study visits
Trained research staff will determine eligibility using a baseline medical history, anthropometrics, pulmonary function/lung responsiveness testing, asthma symptom questionnaires, and pregnancy testing (females) at visit 1. Guardians will sign informed consent documents. Participants will start on a monitored 2-week run-in period. If participants are adherent to diary cards, they meet eligibility for randomization at visit 2. <75% completion of daily diary for asthma symptoms, SABA use, and ICS medication adherence during the run-in period prompts an extension of the run-in for an additional 1–2 weeks at the discretion of the investigator. The timing and details of data collection are in Table 3. The screening visit (V1) includes informed consent, instructions regarding study format and testing per Table 3. Saliva collection for genotyping will be performed to identify metabolizer phenotype for each participant. Visit 1 also signifies the start of the run-in period. At visit 1, staff will review the participant's asthma action plan. Participants will be given daily diary cards to document study drug adherence, asthma symptoms, asthma medication use, and asthma-related healthcare contact. At the randomization visit (V2), participants will return diary cards, have nasal blow training and collection, and undergo eligibility for randomization. They will be given an open label dose of lansoprazole for pharmacokinetic analysis. After 24 weeks of intervention, participants return their daily diary cards at termination visit (V5) and had testing performed per Table 3. They will receive an exit letter detailing their genotype and counseling information regarding asthma and GERD.
1.7. Outcome measures
1.7.1. Asthma symptom scoring
Change in the ‘Juniper’ Asthma Control Questionnaire (ACQ) is the primary outcome. This measure has been used in many well-designed multi-center asthma trials with children [29,42]. The ACQ ranges from 0 to 6 (higher values indicate worse asthma control) with a change of 0.5 reflecting a clinically meaningful difference in asthma control and considers a broad set of control indicators including use of bronchodilators, cough, nocturnal symptoms, level of activity, and pulmonary function. At all visits we will also perform the Asthma Symptom Utility Index (ASUI) [43]. We will compute rate and prevalence of asthma symptom exacerbations for both treatment groups.
1.7.2. Lung function
We will use an approved spirometric system appropriately calibrated and procedures conducted per American Thoracic Society standards [44]. Outcomes include forced vital capacity (FVC), forced expiratory volume 1 s (FEV1), and FEV1/FVC. Raw data and percent predicted values will be recorded and based on accepted normative data.
1.7.3. GERD symptom scoring
Change in the Pediatric GERD Symptom Assessment Questionnaire (GSAS) will be measured. This is a 10-item tool that has been validated in children in the assessment of gastroesophageal reflux disease related symptoms such as chest, abdominal pain, pain, choking with eating, swallowing dysfunction, regurgitation and nausea [27]. The GSAS ranges from 0 to 490 and a score ≥ 15 reflects significant GERD symptoms. A value of 80 will be used as a maximum cut-off for mild symptoms as this was 2 SD from the mean GSAS for children without baseline GERD [29,45].
1.7.4. Pharmacokinetics
1.7.4.1. Quantification of lansoprazole and its main metabolites in plasma
Concentrations for lansoprazole and metabolites will be quantified using liquid chromatography/mass spectroscopy as described [46,47]. This method is more sensitive than high performance liquid chromatography for metabolites requiring less plasma or blood.
1.7.4.2. PK modeling
To characterize the impact of genetic polymorphisms on the dose-concentration response relationship of lansoprazole, we developed a physiologically-based pharmacokinetic (PBPK) model that integrates information on the drug's physiochemical properties (e.g. molecular weight, logP, pKa, and pH-deependent solubility), human anatomy and physiology. This PBPK model was developed based on available literature data and used prospectively in sensitivity analyses which showed that peak plasma concentrations is approximately 2 h and shifts later at higher gastric pH. Based on this preliminary analysis, blood will be drawn at 2 and 5 h post open-label genotype-tailored dosing. Using population PK-PD techniques, we will compute the area under the curve of all participants. We will perform an efficacy analysis to develop a pediatric population PK/PD model, which will establish a relationship between dosing conventions and plasma concentrations of lansoprazole by metabolizer phenotype. Plasma concentrations will also be compared to change in GERD symptoms. This model will be developed and qualified in three steps as outlined below.
-
A.
Exploratory analysis of PK and GERD symptom score data
An exploratory analysis will be performed to determine the relationship between lansoprazole PK and GERD scores, to identify potential outliers, and to explore the impact of genetic polymorphisms in CYP2C19 and changes in GERD symptoms.
-
B.
Development of a population pharmacokinetic (pop-PK) model
A non-linear mixed effects modeling approach will be performed in NONMEM (v7.2 or higher) to determine the structural model that adequately describes the PK of lansoprazole and allows for the estimation of respective model parameters. Once an appropriate structural model has been selected, the variance structure of the model will be informed using pooled data from the intensive and sparse sampling periods of this study. The significance of covariates, particularly of genetic polymorphisms in CYP2C19, will be assessed using an automated stepwise covariate model building method. This method involves a forward selection and backward elimination of covariates in which one model for each relevant parameter-covariate relationship is prepared and tested in a univariate manner. Statistical criterion such as a p-value <.01 (objective function value (OFV); ΔOFV of 6.64 for 1 degree of freedom assuming a chi-squared distribution) for the forward selection and a p-value <.001 (ΔOFV of 10.83 for 1 degree of freedom assuming a chi-squared distribution) for the backward elimination will be used to indicate relations that are of interest for inclusion. The drop in OFV will be used to rank the covariate with the most important parameter-covariate relationship. The model with the largest drop in OFV will be used to include the covariate with the largest parameter-covariate relationship into the model. Goodness-of-fit and appropriateness of the final model from the SCM will be assessed by diagnostic plots, physiological meaningfulness of the obtained parameter estimates, visual predictive checks, and non-parametric bootstraps.
-
C.
Development of a population PK/PD model: Various empirical population PK/PD models have been reported for other PPIs in adults but none in children [48,49]. A population PK/PD model for the efficacy of lansoprazole in children will be established by linking the pop-PK model developed in (b) to an appropriate PD model. In order to identify an appropriate PD model, Emax, sigmoidal Emax and indirect response will be explored and the best model selected based on standard goodness-of-fit plots, reduction in OFV and visual predictive checks [50]. In addition, age-dependent changes in synthesis rates of proton pumps as well as circadian rhythms related to gastric acid secretions and food effects will be considered during model development [14].
1.7.5. Respiratory tract sampling
The ‘nasal blow’ collection technique has been previously validated within the AsthmaNet consortium [51]. Parents will be trained at visit 2 to identify early respiratory infection symptoms and to collect and store nasal wash samples. Multiplex PCR analysis will be performed for 22 relevant respiratory viral and bacterial pathogens (see Table 5 ) using TaqMan assays validated by the CDC [52]. Inflammatory mechanisms will be assessed through type I interferon (IFN-α, IFN-β), IL-8, and leptin levels in nasal wash samples.
Table 5.
Pathogens to be interrogated in respiratory samples.
| M. catarrhalis | Adenovirus |
|---|---|
| S. aureus | Enterovirus |
| K. pneumoniae | Rhinovirus |
| C. pneumoniae | Parainfluenza virus 4 |
| M. pneumoniae | Parainfluenza virus 3 |
| S. pneumoniae | Parainfluenza virus 2 |
| H. influenza | Parainfluenza virus 1 |
| P. aeruginosa | Respiratory syncytial virus |
| Bocavirus | Human metapnuemovirus |
| Human coronavirus 1/2 | Influenza B |
| Human coronavirus 3/4 | Influenza A |
1.8. Oversight of the study
The GenARA study was initiated at Duke Children's Hospital and was expanded to the Nemours Children's Health System, Jacksonville, Florida. A data and safety monitoring board was created to monitor for safety and data integrity and met yearly. An investigational new drug application was submitted under the section 505 (i) of the Federal Food, Drug, and Cosmetic Act for the genotype-tailored lansoprazole intervention and granted 4/29/16 (IND130170).
1.9. Data management
Study data were collected and managed using the Research Electronic Data Capture (REDCap) electronic data capture system and tools, hosted by collaborators within the Duke Clinical Research Institute. REDCap was chosen because it is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
1.10. Analysis
1.10.1. Sample size calculation
We determined that 64 participants will need to be randomized according to a 1:1 allocation ratio, with a goal of 32 receiving genotype-tailored lansoprazole and 32 receiving placebo. Power estimation for the effect of lansoprazole on ACQ change from baseline was performed using a two-sided t-test with an error rate of 0.05. Based on preliminary data from this patient population, we anticipated the mean ACQ at entry will be 1.2 with a standard deviation of 0.7 and aimed to detect an ACQ change of 0.5 [45]. We assumed 20% attrition with a final goal of 50 participants completing intervention to achieve a power of 93.4%. We also had >90% power to detect a 50% difference between groups in respiratory infections and 66% difference to detect a 5% difference in FEV1. Due to the low assumed background rate of 1 exacerbation/year, we were not powered at 80% to detect a difference in asthma exacerbations.
1.10.2. Data analysis
We assumed an intention-to-treat approach. When the trial ends, we will use a two sample t-test and ANCOVA to determine whether the change in ACQ from the randomization to termination visit differs between treatment groups, (α = 0.05). Other secondary outcomes that are continuous numeric variables will be analyzed similarly. For count data (asthma exacerbations, episode of poor and respiratory tract infections), we will employ Poisson regression analysis. The statistical package SAS 9.4 (SAS Institute Inc., Cary, NC) will be used. All tests were two-tailed at a level of significance of 0.05.
2. Discussion
The GenARA study aims to determine if genotype-tailored dosing of lansoprazole in children with inadequately controlled asthma leads to improved asthma control. Considering the frequent prescription of PPIs in patients with asthma, understanding the relationship between genotype-tailored dosing and asthma control is crucial to determine its efficacy and safety.
Prior studies from both adults and children demonstrate that the efficacy of PPIs to treat GERD and related conditions are closely linked to plasma concentrations. Numerous studies in adults have shown that CYP2C19 variants markedly influence the PK and PD of PPI [30,32,34,[53], [54], [55], [56]]. Patients needing acid-suppression who are EM or UM have higher treatment failure rates compared to NM when given conventional dosing [32,55,57]. H. pylori cure rates are significantly higher among PM compared to EM [31,58] and higher doses of PPI are required to eradicate H. pylori in EM [58,59]. Recent studies in children have also demonstrated the importance of CYP2C19 variants [[60], [61], [62]]. The clearance of pantoprazole was estimated in pediatric patients from birth to 16 years and was 60% lower in PMs compared to EMs [63]. Our group compared drug levels following a single dose of conventionally dosed lansoprazole with CYP2C19 genotype/metabolizer phenotype in 56 children with GERD and found a direct relationship between CYP2C19 diplotype and lansoprazole concentration [64]. We also recently found that EM children on conventional PPI dosing and undergoing esophageal pH probe testing had inadequate acid blockade compared to NM + PM. In addition, our group recently assessed all children who failed PPI therapy and subsequently underwent surgical gastric fundoplication who also had available endoscopic tissue samples for DNA genotyping. The children who failed PPI and required fundoplication were significantly more likely to have the EM phenotype compared to controls [4]. Significantly fewer children failing PPI and needing fundoplication had the PM phenotype compared to controls. Collectively, these data support our hypothesis that EM and UM children are being underdosed on conventional PPI dosing and will benefit from a higher (genotype-tailored) dose of PPI to achieve optimal anti-secretory response.
In addition, treatment with PPI have consistently been associated with a number of adverse side-effects including C. dificile colitis [[65], [66], [67]] and respiratory infections [[67], [68], [69], [70]]. Our group showed that PPI use at conventional doses for 6 months in asthmatic children without significant GERD at baseline was associated with significantly greater sinusitis, strep throat and URI [29]. In a subsequent genetic sub-study, we showed that participants with IM or PM had significantly higher lansoprazole levels compared to NM and were significantly more likely to suffer respiratory adverse events related to URI and strep/sore throat [64]. These same PM participants developed worse asthma controlled (triggered by URI) compared to NM after 4 months on conventional PPI dosing [71]. Conventional dosing of PPI in these IM and PM patients is likely to improve GERD symptoms at the cost of greater risk of URIs. Since URIs are the most common trigger for loss of asthma control in children, conventional dosing may not ultimately not help asthma control due to greater URIs. Instead, we propose that these IM or PM patients could be given a genotype-tailored dose providing normal serum concentrations and improved GERD symptoms with the risk of excess URI. We will collect nasal wash samples from participants with URIs to microbiologically confirm URI.
These results will have broad applicability because patients possessing significant CYP2C19 variants are a sizeable segment of the population. In fact, only about 40% of the population has the NM phenotype while IM/PM, EM and UM phenotypes make up 24%, 24% and 4% of the population, respectively [36].
Loss of function alleles (*n = loss of function allele, e.g. CYP2C19 SNPs: *2 rs4244285; *3 rs4986893; *8 rs41291556l *9 rs17884712) reduce drug clearance and increase area under the curve (AUC). CYP2C19*17 is a gain of function allele that increases drug clearance. Our prior PK data in children support the use of five distinct metabolizer phenotypes which warrant distinct dosing adjustments from the conventional two-tiered dosing. We have developed an evidenced-based dosing algorithm tailored to Extensive (EM), Ultra-rapid (UM), Intermediate (IM) and Poor metabolizers (PM) that is based on pediatric PK principles after analyzing the limited extant pediatric data. These estimates are also in agreement with that proposed by Furuta [57], but are as yet untested in children.
Innovative clinical trial design has been important to combat the lack of pediatric studies and the challenges therein. Our study was designed with a two-step randomization to study both pharmacokinetics and efficacy. Participants will be randomized to: (1) open label conventional or genotype-tailored lansoprazole for PK analysis then (2) genotype-tailored lansoprazole or placebo intervention. The part 2 randomization will not be stratified based on results of the part 1 randomization because we felt the impact on change in ACQ at 6 months would be minimal, though it is theoretically possible. To characterize the effect of PPI on asthma, we also made specific choices to safely and specifically measure efficacy. Lansoprazole has been approved by the FDA down to age 12 months for the indications GERD, duodenal ulcers, H. pylori eradication, benign gastric ulcer, NSAID-associated gastric ulcer, eosinophilic esophagitis, and pathological hypersecretory conditions. It has been used in >10,000 patients in phase II and III clinical trials involving varying doses and durations and have been found to be well-tolerated in both short-term and long-term trials. Rarely (~1% of cases) patients have reported diarrhea, constipation, abdominal pain, and nausea with use of lansoprazole. The current FDA label for lansoprazole states that PPI therapy may be associated with increased risk for C. difficile associated diarrhea. Long term and multiple daily dose PPI therapy may be associated with an increase risk of osteoporosis-related fractures of the hip, wrist, or spine, and hypomagnesemia rarely. To include only ‘mild’ GERD, we chose to exclude children at enrollment with a GSAS >80. Previous studies in our patient population have shown that children without diagnosed GERD had a GSAS score ranging from 0 to 276 with a mean (SD) = 18.6 (29.5) [45]. This threshold was chosen as it was 2 SD above the mean GSAS for a group of children without reported GERD at baseline [29]. We selected the ACQ as our primary outcome because: (1) it is a sensitive measure of asthma control, (2) it is validated for children and adults in Spanish and English, and (3) its simplicity.
In summary, we have developed an innovative clinical trial to assess the efficacy of genotype-tailored PPI dosing for the treatment of asthma. This study will benefit children with both GERD and asthma in the future through better understanding of dose response and characterization of the safety profile of lansoprazole. This trial design may be applicable to other clinical trials as the discovery of pharmacogenomics for other therapies and the need for pediatric pharmacokinetic studies continues to grow. If results from the GenARA study can show that genotype-tailored dosing is more efficacious and safer for children with asthma, the future treatment of children with asthma and GERD will be greatly improved.
Funding source
This study was funded by the Thrasher Research Fund (ID#12917)
Financial disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Akinbami L.J. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 2.Swallen K.C. Overweight, obesity, and health-related quality of life among adolescents: the National Longitudinal Study of Adolescent Health. Pediatrics. 2005;115(2):340–347. doi: 10.1542/peds.2004-0678. [DOI] [PubMed] [Google Scholar]
- 3.Lang J.E., Hossain M.J., Lima J.J. Overweight children report qualitatively distinct asthma symptoms: Analysis of validated symptom measures. J. Allergy Clin. Immunol. 2015 Apr;135(4):886–893. doi: 10.1016/j.jaci.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franciosi J.P. Association between CYP2C19 extensive metabolizer phenotype and childhood anti-reflux surgery following failed proton pump inhibitor medication treatment. Eur. J. Pediatr. 2018;177(1):69–77. doi: 10.1007/s00431-017-3051-4. [DOI] [PubMed] [Google Scholar]
- 5.Allen G.B. Acid aspiration-induced airways hyperresponsiveness in mice. J. Appl. Physiol. 2009;107(6):1763–1770. doi: 10.1152/japplphysiol.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang I.M. Airway responses to esophageal acidification. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(1):R211–R219. doi: 10.1152/ajpregu.00394.2007. [DOI] [PubMed] [Google Scholar]
- 7.Hamamoto J. Esophageal stimulation by hydrochloric acid causes neurogenic inflammation in the airways in guinea pigs. J. Appl. Physiol. 1997;82(3):738–745. doi: 10.1152/jappl.1997.82.3.738. [DOI] [PubMed] [Google Scholar]
- 8.Lopes F.D. PubMed - NCBI; 2018. Pulmonary Responses to Tracheal or Esophageal Acidification in Guinea Pigs With Airway Inflammation. [DOI] [PubMed] [Google Scholar]
- 9.Havemann B.D., Henderson C.A., El-Serag H.B. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56(12):1654–1664. doi: 10.1136/gut.2007.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakkar K. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125(4):e925–e930. doi: 10.1542/peds.2009-2382. [DOI] [PubMed] [Google Scholar]
- 11.Wright R.A., Miller S.A., Corsello B.F. Acid-induced esophagobronchial-cardiac reflexes in humans. Gastroenterology. 1990;99(1):71–73. doi: 10.1016/0016-5085(90)91231-t. [DOI] [PubMed] [Google Scholar]
- 12.Wu D.N. Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest. 2000;118(6):1553–1556. doi: 10.1378/chest.118.6.1553. [DOI] [PubMed] [Google Scholar]
- 13.Zhu G.C. Experimental study for the mechanism of gastroesophageal-reflux-associated asthma. Dis. Esophagus. 2014;27(4):318–324. doi: 10.1111/dote.12108. [DOI] [PubMed] [Google Scholar]
- 14.Schan C.A. Gastroesophageal reflux-induced bronchoconstriction. An intraesophageal acid infusion study using state-of-the-art technology. Chest. 1994;106(3):731–737. doi: 10.1378/chest.106.3.731. [DOI] [PubMed] [Google Scholar]
- 15.Harding S.M. Gastroesophageal reflux-induced bronchoconstriction. Is microaspiration a factor? Chest. 1995;108(5):1220–1227. doi: 10.1378/chest.108.5.1220. [DOI] [PubMed] [Google Scholar]
- 16.Andersen L.I., Schmidt A., Bundgaard A. Pulmonary function and acid application in the esophagus. Chest. 1986;90(3):358–363. doi: 10.1378/chest.90.3.358. [DOI] [PubMed] [Google Scholar]
- 17.Mansfield L.E. The role of the vague nerve in airway narrowing caused by intraesophageal hydrochloric acid provocation and esophageal distention. Ann. Allergy. 1981;47(6):431–434. [PubMed] [Google Scholar]
- 18.Harding S.M., Guzzo M.R., Richter J.E. 24-h esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest. 1999;115(3):654–659. doi: 10.1378/chest.115.3.654. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman J.A. Long-term outcomes of laparoscopic antireflux surgery for gastroesophageal reflux disease (GERD)-related airway disorder. Surg. Endosc. 2006;20(12):1824–1830. doi: 10.1007/s00464-005-0329-9. [DOI] [PubMed] [Google Scholar]
- 20.Kiljander T.O. Gastroesophageal reflux and bronchial responsiveness: correlation and the effect of fundoplication. Respiration. 2002;69(5):434–439. doi: 10.1159/000064021. [DOI] [PubMed] [Google Scholar]
- 21.Larrain A. Medical and surgical treatment of nonallergic asthma associated with gastroesophageal reflux. Chest. 1991;99(6):1330–1335. doi: 10.1378/chest.99.6.1330. [DOI] [PubMed] [Google Scholar]
- 22.Sontag S.J. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am. J. Gastroenterol. 2003;98(5):987–999. doi: 10.1111/j.1572-0241.2003.07503.x. [DOI] [PubMed] [Google Scholar]
- 23.Khoshoo V. Role of gastroesophageal reflux in older children with persistent asthma. Chest. 2003;123(4):1008–1013. doi: 10.1378/chest.123.4.1008. [DOI] [PubMed] [Google Scholar]
- 24.Stordal K. Acid suppression does not change respiratory symptoms in children with asthma and gastro-oesophageal reflux disease. Arch. Dis. Child. 2005;90(9):956–960. doi: 10.1136/adc.2004.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuksel H. Frequency of gastroesophageal reflux disease in nonatopic children with asthma-like airway disease. Respir. Med. 2006;100(3):393–398. doi: 10.1016/j.rmed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Khoshoo V., Haydel R., Jr. Effect of antireflux treatment on asthma exacerbations in nonatopic children. J. Pediatr. Gastroenterol. Nutr. 2007;44(3):331–335. doi: 10.1097/MPG.0b013e31802fe89c. [DOI] [PubMed] [Google Scholar]
- 27.Khoshoo V. Bronchial hyperreactivity in non-atopic children with asthma and reflux: effect of anti-reflux treatment. Pediatr. Pulmonol. 2009;44(11):1070–1074. doi: 10.1002/ppul.21094. [DOI] [PubMed] [Google Scholar]
- 28.Bediwy A.S. Induced sputum substance p in children with difficult-to-treat bronchial asthma and gastroesophageal reflux: effect of esomeprazole therapy. Int. J. Pediatr. 2011;2011:967460. doi: 10.1155/2011/967460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Writing Committee for the American Lung Association Asthma Clinical Research, C Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307(4):373–381. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desta Z. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 2002;41(12):913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 31.Furuta T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab. Pharmacokinet. 2005;20(3):153–167. doi: 10.2133/dmpk.20.153. [DOI] [PubMed] [Google Scholar]
- 32.Hagymasi K. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2011;12(6):873–888. doi: 10.2217/pgs.11.4. [DOI] [PubMed] [Google Scholar]
- 33.Strom C.M. Testing for variants in CYP2C19: population frequencies and testing experience in a clinical laboratory. Genet. Med. 2012;14(1):95–100. doi: 10.1038/gim.0b013e3182329870. [DOI] [PubMed] [Google Scholar]
- 34.Wedlund P.J. The CYP2C19 enzyme polymorphism. Pharmacology. 2000;61(3):174–183. doi: 10.1159/000028398. [DOI] [PubMed] [Google Scholar]
- 35.Lima J.J., Franciosi J.P. Pharmacogenomic testing: the case for CYP2C19 proton pump inhibitor gene-drug pairs. Pharmacogenomics. 2014;15(11):1405–1416. doi: 10.2217/pgs.14.103. [DOI] [PubMed] [Google Scholar]
- 36.Strom C.M. Testing for variants in CYP2C19: population frequencies and testing experience in a clinical laboratory. Genet. Med. 2011;14(1):95. doi: 10.1038/gim.0b013e3182329870. [DOI] [PubMed] [Google Scholar]
- 37.Juniper E.F. Measuring quality of life in asthma. Am. Rev. Respir. Dis. 1993;147(4):832–838. doi: 10.1164/ajrccm/147.4.832. [DOI] [PubMed] [Google Scholar]
- 38.NAEPP . NHLBI/NIH, US Department of Health and Human Services; 2007. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (Full Report 2007) pp. 1–417. [Google Scholar]
- 39.Deal L. Age-specific questionnaires distinguish GERD symptom frequency and severity in infants and young children: development and initial validation. J. Pediatr. Gastroenterol. Nutr. 2005;41(2):178–185. doi: 10.1097/01.mpg.0000172885.77795.0f. [DOI] [PubMed] [Google Scholar]
- 40.GERD in Children and Adolescents . 2018. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. [Google Scholar]
- 41.Jackson D.J. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N. Engl. J. Med. 2018;378(10):891–901. doi: 10.1056/NEJMoa1710988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Lung Association Asthma Clinical Research, C Randomized comparison of strategies for reducing treatment in mild persistent asthma. N. Engl. J. Med. 2007;356(20):2027–2039. doi: 10.1056/NEJMoa070013. [DOI] [PubMed] [Google Scholar]
- 43.Revicki D.A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114(4):998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 44.Standardization of Spirometry, 1995 Update. American Thoracic Society. Am. J. Respir. Crit. Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 45.Lang J.E. Gastro-oesophageal reflux and worse asthma control in obese children: a case of symptom misattribution? Thorax. 2016;71(3):238–246. doi: 10.1136/thoraxjnl-2015-207662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song M. Simultaneous determination of lansoprazole and its metabolites 5′-hydroxy lansoprazole and lansoprazole sulphone in human plasma by LC-MS/MS: application to a pharmacokinetic study in healthy volunteers. J. Pharm. Biomed. Anal. 2008;48(4):1181–1186. doi: 10.1016/j.jpba.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 47.Zhang D. Pharmacokinetics of lansoprazole and its main metabolites after single intravenous doses in healthy Chinese subjects. Xenobiotica. 2012;42(11):1156–1162. doi: 10.3109/00498254.2012.687119. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z.Y. Pharmacokinetic and pharmacodynamic population modeling of orally administered rabeprazole in healthy Chinese volunteers by the NONMEM method. Eur. J. Drug Metab. Pharmacokinet. 2006;31(1):27–33. doi: 10.1007/BF03190639. [DOI] [PubMed] [Google Scholar]
- 49.Sheng Y.C. Effect of CYP2C19 genotypes on the pharmacokinetic/pharmacodynamic relationship of rabeprazole after a single oral dose in healthy Chinese volunteers. Eur. J. Clin. Pharmacol. 2010;66(11):1165–1169. doi: 10.1007/s00228-010-0892-4. [DOI] [PubMed] [Google Scholar]
- 50.Puchalski T.A. Pharmacodynamic modeling of lansoprazole using an indirect irreversible response model. J. Clin. Pharmacol. 2001;41(3):251–258. doi: 10.1177/00912700122010069. [DOI] [PubMed] [Google Scholar]
- 51.Bacharier L.B. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey J.J. Comparative analytical evaluation of the respiratory TaqMan Array Card with real-time PCR and commercial multi-pathogen assays. J. Virol. Methods. 2016;228:151–157. doi: 10.1016/j.jviromet.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukushima-Uesaka H. Genetic variations and haplotypes of CYP2C19 in a Japanese population. Drug Metab. Pharmacokinet. 2005;20(4):300–307. doi: 10.2133/dmpk.20.300. [DOI] [PubMed] [Google Scholar]
- 54.Serrano D. The influence of CYP2C19 genetic polymorphism on the pharmacokinetics/− pharmacodynamics of proton pump inhibitor-containing Helicobacter pylori treatments. Curr. Drug Metab. 2012;13(9):1303–1312. doi: 10.2174/138920012803341393. [DOI] [PubMed] [Google Scholar]
- 55.Shi S., Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur. J. Clin. Pharmacol. 2008;64(10):935–951. doi: 10.1007/s00228-008-0538-y. [DOI] [PubMed] [Google Scholar]
- 56.Stedman C.A., Barclay M.L. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment. Pharmacol. Ther. 2000;14(8):963–978. doi: 10.1046/j.1365-2036.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- 57.Furuta T., Sugimoto M., Shirai N. Individualized therapy for gastroesophageal reflux disease: potential impact of pharmacogenetic testing based on CYP2C19. Mol. Diagn. Ther. 2012;16(4):223–234. doi: 10.1007/BF03262211. [DOI] [PubMed] [Google Scholar]
- 58.Furuta T. Pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2004;5(2):181–202. doi: 10.1517/phgs.5.2.181.27483. [DOI] [PubMed] [Google Scholar]
- 59.Furuta T. Effect of high-dose lansoprazole on intragastic pH in subjects who are homozygous extensive metabolizers of cytochrome P4502C19. Clin. Pharmacol. Ther. 2001;70(5):484–492. doi: 10.1067/mcp.2001.119721. [DOI] [PubMed] [Google Scholar]
- 60.Gumus E. Evaluation of lansoprazole as a probe for assessing cytochrome P450 2C19 activity and genotype-phenotype correlation in childhood. Eur. J. Clin. Pharmacol. 2012;68(5):629–636. doi: 10.1007/s00228-011-1151-z. [DOI] [PubMed] [Google Scholar]
- 61.Kearns G.L., Leeder J.S., Gaedigk A. Impact of the CYP2C19*17 allele on the pharmacokinetics of omeprazole and pantoprazole in children: evidence for a differential effect. Drug Metab. Dispos. 2010;38(6):894–897. doi: 10.1124/dmd.109.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ward R.M. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD) Eur. J. Clin. Pharmacol. 2010;66(6):555–561. doi: 10.1007/s00228-010-0811-8. [DOI] [PubMed] [Google Scholar]
- 63.Knebel W. Population pharmacokinetic modeling of pantoprazole in pediatric patients from birth to 16 years. J. Clin. Pharmacol. 2011;51(3):333–345. doi: 10.1177/0091270010366146. [DOI] [PubMed] [Google Scholar]
- 64.Lima J.J. Association of CYP2C19 polymorphisms and lansoprazole-associated respiratory adverse effects in children. J. Pediatr. 2013;163(3):686–691. doi: 10.1016/j.jpeds.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janarthanan S. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am. J. Gastroenterol. 2012;107(7):1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 66.Kwok C.S. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am. J. Gastroenterol. 2012;107(7):1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 67.Wilhelm S.M., Rjater R.G., Kale-Pradhan P.B. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert. Rev. Clin. Pharmacol. 2013;6(4):443–451. doi: 10.1586/17512433.2013.811206. [DOI] [PubMed] [Google Scholar]
- 68.Eom C.S. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183(3):310–319. doi: 10.1503/cmaj.092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herzig S.J. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301(20):2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 70.Lambert A.A. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang J.E. Lansoprazole is associated with worsening asthma control in children with the CYP2C19 poor metabolizer phenotype. Ann. Am. Thorac. Soc. 2015;12(6):878–885. doi: 10.1513/AnnalsATS.201408-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


