Abstract
Objective
To retrospectively evaluate the safety and efficacy of percutaneous radiofrequency ablation (RFA) in patients with metachronous hepatic metastases arising from pancreatic adenocarcinoma who had previously received curative surgery.
Materials and Methods
Between 2002 and 2017, percutaneous RFA was performed on 94 metachronous hepatic metastases (median diameter, 1.5 cm) arising from pancreatic cancer in 60 patients (mean age, 60.5 years). Patients were included if they had fewer than five metastases, a maximum tumor diameter of ≤ 5 cm, and disease confined to the liver or stable extrahepatic disease. For comparisons during the same period, we included 66 patients who received chemotherapy only and met the same eligibility criteria described.
Results
Technical success was achieved in all hepatic metastasis without any procedure-related mortality. During follow-up, local tumor progression of treated lesions was observed in 38.3% of the tumors. Overall median survival and 3-year survival rates were 12 months and 0%, respectively from initial RFA, and 14.7 months and 2.1%, respectively from the first diagnosis of liver metastasis. Multivariate analysis showed that a large tumor diameter of > 1.5 cm, a late TNM stage (≥ IIB) before curative surgery, a time from surgery to recurrence of < 1 year, and the presence of extrahepatic metastasis, were all prognostic of reduced overall survival after RFA. Median overall (12 months vs. 9.1 months, p = 0.094) and progression-free survival (5 months vs. 3.3 months, p = 0.068) were higher in the RFA group than in the chemotherapy group with borderline statistical difference.
Conclusion
RFA is safe and may offer successful local tumor control in patients with metachronous hepatic metastases arising from pancreatic adenocarcinoma. Patients with a small diameter tumor, early TNM stage before curative surgery, late hepatic recurrence, and liver-only metastasis benefit most from RFA treatment. RFA provided better survival outcomes than chemotherapy for this specific group with borderline statistical difference.
Keywords: Pancreatic adenocarcinoma, Metachronous hepatic metastasis, Radiofrequency ablation
INTRODUCTION
Patients with pancreatic adenocarcinoma have a poor prognosis, with overall 5-year survival rates ranging from 0.4–8% (1,2). Surgical resection is the only form of curative treatment; however, only about 10% of patients are candidates for surgery because of the presence of advanced disease at the time of diagnosis (3,4,5). Even after curative surgery, cancer recurrence develops within 1–2 years of pancreatic surgery in over 60% of patients, with extrapancreatic recurrence being most frequently located in the liver (6,7,8). Most patients thereby qualify for palliative therapy, including systemic chemotherapy and/or radiation therapy (9). However, such options are of limited benefit as pancreatic carcinomas respond poorly to these treatment modalities (10,11).
Percutaneous radiofrequency ablation (RFA) has been recognized as a safe and effective local therapy for primary or metastatic liver malignancies (12,13,14,15,16,17,18,19). As most hepatic metastases recurring after surgery in patients with pancreatic adenocarcinoma are small (≤ 3 cm), percutaneous RFA is usually feasible. A few reports have described the promising outcomes of RFA for treatment of hepatic metastasis from pancreatic adenocarcinoma (20,21,22). In this study, we evaluated the safety and efficacy of percutaneous RFA in 60 patients with 94 metachronous hepatic metastasis, arising after curative resection of pancreatic adenocarcinoma. Further, we compared the survival outcomes of RFA treatment with those of chemotherapy for this specific group.
MATERIALS AND METHODS
Patient Population
Our institutional review board approved this study (AMC 2018-1139), and waived the requirement for patient consent due to the retrospective nature of the study. Patients were deemed eligible for RFA if they had less than five recurrent hepatic tumors from pancreatic adenocarcinoma, tumors ≤ 5 cm in maximum diameter, no evidence of vascular invasion, stable extrahepatic metastases (neither decreasing nor increasing in extent or severity by chemotherapy control) or no extrahepatic disease, and tumors that were detectable by ultrasonography (US) or CT with an acceptable and safe path (22). Patients were excluded if they had a recurrent hepatic tumor > 5 cm in maximum diameter, more than five tumors, an Eastern Cooperative Oncology Group performance status of 2–4, vascular invasion, progressive extrahepatic metastases, or coagulopathy (platelet count < 50 × 103/µL; international normalized ratio > 1.5).
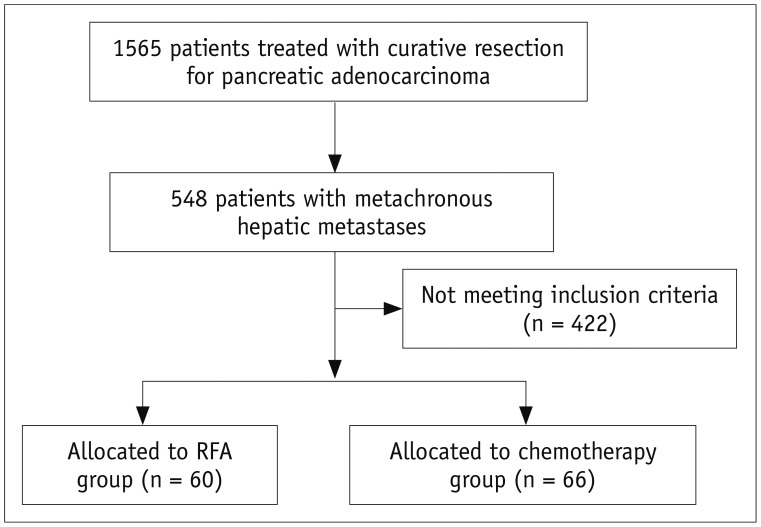
From December 2002 to August 2017, 60 patients (35 men, 25 women; age range, 38–78 years; mean age ± standard deviation [SD], 60 ± 9.1 years) with 94 metachronous hepatic metastases arising from pancreatic adenocarcinoma underwent percutaneous RFA and were included in the present study (Fig. 1). Twenty-eight of the 60 patients have been previously reported (21). Four (6.6%) of the 60 patients with hepatic recurrence and distant metastases received systemic chemotherapy for recurrence prior to RFA. All patients underwent curative resection of their adenocarcinomas, consisting of Whipple surgery in 23 patients, distal pancreatectomy in 20, pylorus-preserving pancreaticoduodenectomy in 15, and total pancreatectomy in two. The stage of the pancreatic ductal adenocarcinoma was classified according to the 8th TNM staging system by American Joint Committee on Cancer. The stages were IA (n = 3), IB (n = 22), IIA (n = 2), IIB (n = 28), and III (n = 5) before the time of curative surgery. The pancreatic adenocarcinomas were well-differentiated (n = 1), moderately differentiated (n = 50), or poorly differentiated (n = 9). For comparisons during the same period, we included 66 patients who received chemotherapy only and met the same eligibility criteria described (Fig. 1). The baseline characteristics of the patients and tumors are summarized in Table 1. The median time between the diagnosis of hepatic metastases and RFA treatment was 0.6 months (range, 1 day–5.6 months). Of the 60 included patients who underwent RFA, 49 had hepatic recurrences only, seven had both hepatic and local recurrences (in the resection areas), and four had hepatic recurrence and distant metastases. Adjuvant chemotherapy was indicated for patients with tumor stages IB or greater taking into account the patient performance status, patient willingness, age, and cost (21). Of the sixty patients, 48 (80%) received adjuvant chemotherapy after curative surgery for pancreatic adenocarcinoma. Tumor sizes ranged from 0.6–5 cm in maximum dimension (median, 1.5 cm). For 17 (28.3%) of the 60 patients, recurrent hepatic tumors were diagnosed histologically according to the results of image-guided percutaneous needle biopsy, while contrast-enhanced CT and/or whole-body positron emission tomography [F-18 Fludeoxyglucose] scans after surgery were used for 43 patients (71.7%).
Fig. 1. Patient selection flow chart.
RFA = radiofrequency ablation
Table 1. Baseline Characteristics of Patients.
| Variable | RFA Group | Chemotherapy Group | P |
|---|---|---|---|
| Patients | 60 | 66 | |
| Age (years old), mean ± SD | 59.9 ± 9.3 | 60.4 ± 8.7 | 0.736 |
| Sex, n (%) | 0.931 | ||
| Male | 35 (58.3) | 38 (57.6) | |
| Female | 25 (41.7) | 28 (42.4) | |
| TNM stage before curative surgery, n (%) | 0.654 | ||
| ≤ IIA | 26 (43.3) | 26 (39.4) | |
| ≥ IIB | 34 (56.7) | 40 (60.6) | |
| Differentiations, n (%) | 0.880 | ||
| Well differentiated | 1 (1.7) | 2 (3.0) | |
| Moderately differentiated | 50 (83.3) | 54 (81.8) | |
| Poorly differentiated | 9 (15.0) | 10 (15.2) | |
| Period to recurrence, n (%) | 0.632 | ||
| < 12 months | 45 (75.0) | 47 (71.2) | |
| ≥ 12 months | 15 (25.0) | 19 (28.8) | |
| Maximal tumor size, n (%) | 0.269 | ||
| > 1.5 cm | 35 (58.3) | 32 (48.5) | |
| ≤ 1.5 cm | 25 (41.7) | 34 (51.5) | |
| Tumor number, n (%) | 0.132 | ||
| Single | 38 (63.3) | 33 (50.0) | |
| Multiple | 22 (36.7) | 33 (50.0) | |
| Presence of extrahepatic metastasis, n (%) | 0.686 | ||
| Yes | 11 (18.3) | 14 (21.2) | |
| No | 49 (81.7) | 52 (78.8) |
n = number, RFA = radiofrequency ablation, SD = standard deviation
Radiofrequency Ablation Technique
RFA was performed percutaneously under US, with the patient under conscious sedation (dexmedetomidine 1 µg/kg and remifentanil 1 µg/kg) and local anesthesia with 5–10 mL of 1% lidocaine. Prophylactic antibiotics were not used before the RFA procedure. CT-guided RFA (n = 3) was performed in patients with a poor US window. Artificial ascites using 5% dextrose in water solution was injected in the peritoneal space for 8 patients. Fusion imaging of real time US with CT (n = 3) or MRI (n = 1) was used. A single electrode (ValleyLab, Burlington, MA, USA) (n = 38) or an electrode cluster (ValleyLab) (n = 22) was used, with radiofrequency current being emitted for 12 or 15 minutes using a 200 W generator set to deliver maximum power and employing the automatic impedance control method. Each tumor received 1–4 ablations (mean, 1.5 ablations) per session, according to tumor size and shape. For all tumors ≤ 2 cm in diameter, a single electrode with a 3 cm exposed tip was used (19). For tumors ≥ 2 cm in diameter, a cluster electrode or multiple overlapping insertions of a single electrode were used (19). The endpoint of the RFA was an ablative margin of at least 1 cm (19,23,24). At the end of the RFA procedure, the electrode path was cauterized to prevent bleeding, and tumor seeding during retraction of the electrode.
Follow-Up and Evaluation of Data
All patients were transferred to the CT suite immediately after the RFA procedure, and an immediate post-RFA examination was performed with contrast-enhanced CT, to evaluate success of the ablation, and possible complications. Major complications were defined as any event requiring additional treatment, including an increased level of care, hospital stay beyond observation status (including re-admission after initial discharge), permanent adverse sequelae including substantial morbidity or disability, or death (25,26). All other complications were classified as minor. Ablations were considered to be complete, and technical success achieved if the ablation zone completely covered the tumor, and there was no irregular enhancement of the ablated area (26). If residual tumor was present in the ablated area, an additional session of RFA was performed.
Follow-up contrast-enhanced CT was performed one month after ablation and every two-three months thereafter. Repeat RFA was employed to treat local tumor progression, and new intrahepatic focal lesions in patients with less than five tumors with a largest diameter ≤ 5 cm, no vascular invasion, and stable extrahepatic disease.
The terminology and reporting criteria of the Society of Interventional Radiology were used for reporting the follow-up findings (26). Local tumor progression was defined as the appearance of new tumor foci at the edge of the ablation zone, during follow-up contrast-enhanced imaging. Local tumor progression, progression-free survival, and overall survival rates were calculated using the Kaplan-Meier method. Progression-free survival was defined as the time elapsed between treatment initiation, and tumor progression or death from any cause (27). The overall survival periods were measured in months, from the date of diagnosis of hepatic metastasis, and from the time of initial RFA, to a patient's death. The local tumor progression was calculated from initial ablation to the first imaging evidence of local tumor progression. Tumor size was dichotomized relative to the median diameter (1.5 cm), and the local tumor progression curves of study patients were compared, according to the tumor size using log-rank test.
The groups were compared using Student's t test for continuous data and the chi-square (χ2) test for categorical data. Multivariate Cox regression analysis was used to explore possible independent factors (age, sex, TNM stage before curative surgery, pathological grade, tumor number, diameter of the largest tumor, time between surgery and development of recurrence, presence of other metastases outside of the liver) associated with overall survival after RFA. Age, tumor number, and diameter of the largest tumor, were each dichotomized into two groups according to the median values. The time between surgery and development of recurrence was dichotomized into two groups, 1 year or longer and shorter than 1 year (24,28), and TNM stage before curative surgery was dichotomized into two groups, ≥ IIB and ≤ IIA. Pathological grade was dichotomized into two groups, well or moderately differentiated, and poorly differentiated. Only variables associated with a p value < 0.1 in the univariable Cox analysis were entered into the multivariable model. Fisher's exact test was used to evaluate the relationship between occurrence of liver abscess and the presence of bilioenteric anastomosis. All statistical analyses were performed using SPSS (version 21; IBM Corp., Armonk, NY, USA). A two-sided p value < 0.05 was considered statistically significant.
RESULTS
Technical Success
Technical success was achieved in 89 of the 94 hepatic tumors (94.6%) after a single session of US (n = 87) or CT (n = 2) guided RFA. In four patients, a residual tumor was detected on immediate follow-up CT, with an additional US guided RFA session then being performed for residual tumors. The one remaining hepatic tumor was not well-delineated on US at the time of RFA, although a residual unablated area was observed on immediate follow-up CT. Secondary RFA under CT guidance was performed on the following day to treat this residual unablated area, resulting in the complete ablation of the residual tumor. The technical success rate of RFA, including additional ablation procedures performed on the same day or a day later, was 100%.
Major Complications
Eight major complications occurred after RFA for 94 tumors (8.5%, 8/94) in 8 of the 60 patients (13.3%, 8/60), although there was no procedure-related mortality. Liver abscesses developed at six ablated areas 3–83 days (median time, 30 days) after RFA. Liver abscesses occurred in 5 of 38 (13.2%) patients with a bilioenteric anastomosis (patients who underwent Whipple surgery or pylorus-preserving pancreaticoduodenectomy), and in 1 of 22 (4.5%) patients without a bilioenteric anastomosis; however, the liver abscesses were not significantly related to the presence of bilioenteric anastomoses (p = 0.399). All six liver abscesses were successfully managed with percutaneous drainage and antibiotic treatment. The remaining two major complications were intraperitoneal hemorrhages that occurred 1 day after RFA of superficially located tumors and required blood transfusion and percutaneous drainage. The major complications in the chemotherapy group were neutropenic fever requiring admission and medical treatment (n = 3), significant cytopenia requiring hold or switch of chemotherapy (n = 7), and severe nausea and vomiting requiring admission and medical treatment (n = 3). The major complication rate between the RFA (13.3%, 8/60) and chemotherapy (19.7%, 13/66) groups was statistically non-significant (p = 0.338).
Local Tumor Progression
During follow-up, local tumor progression of treated lesions was observed in 36 (38.3%) of the 94 tumors, with 5 of these 36 tumors being treated by repeat RFA. The cumulative local tumor progression rates at 6 months, 1 year, and 2 years were 23.3%, 41.2%, and 47.3%, respectively.
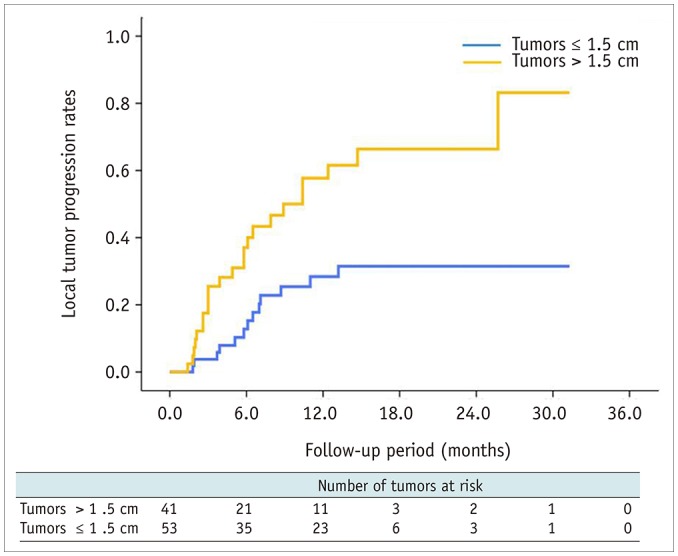
The local tumor progression rates for tumors ≤ 1.5 cm in diameter at 6 months, 1 year, and 2 years after RFA were 12.8%, 28.4%, and 31.5%, respectively, whereas for tumors > 1.5 cm in diameter, the local tumor progression rates at 6 months, 1 year, and 2 years after RFA were 37%, 57.7%, and 66.4%, respectively. The cumulative local tumor progression rate was significantly lower for tumors ≤ 1.5 cm in diameter than tumors > 1.5 cm in diameter (p = 0.001) (Fig. 2).
Fig. 2. Cumulative local tumor progression rates after RFA of tumors ≤ 1.5 cm and > 1.5 cm in diameter.
Overall Survival
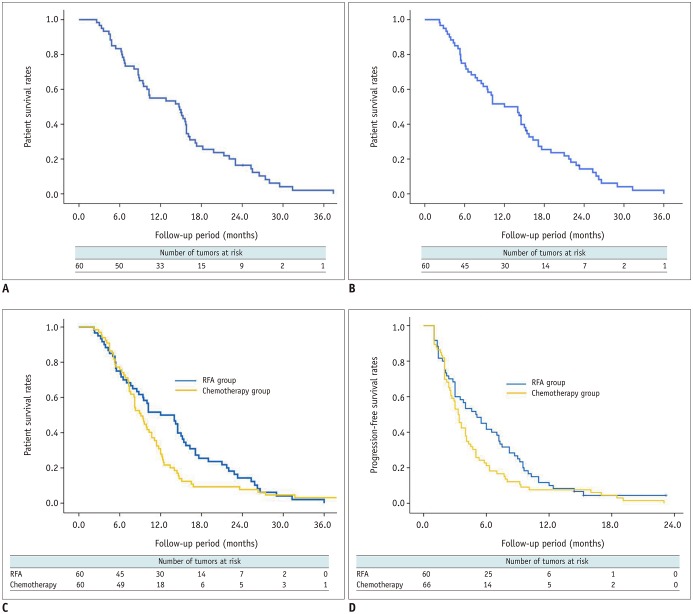
The median follow-up time of the 60 patients in the RFA group, and of the 66 patients in the chemotherapy group was 14.7 (interquartile range, 6.7–17.9 months), and 10.4 months (interquartile range, 7.6–13.4 months), respectively. As of October 2018, 57 of the 60 patients (95%) in the RFA group had died, and 3 (5%) remained alive. Sixty-four of the 66 patients (97%) in the chemotherapy group had died, and 2 (3%) remained alive. The median overall survival periods after RFA and from the time of diagnosis of hepatic metastasis were 12 and 14.7 months, respectively (Fig. 3A, B). The overall survival rates at 6 months, 1, 2, and 3 years after RFA were 75%, 50%, 14.3%, and 0%, respectively, whereas the overall survival rates at 6 months, 1, 2, and 3 years from the time of diagnosis of hepatic metastases in the RFA group were 83.3%, 55%, 16.4%, and 2.1%, respectively. Median overall survival from the initial treatment was higher in the RFA group (12 months) than in the chemotherapy group (9.1 months), but the difference was not statistically significant (p = 0.094) (Fig. 3C). Median progression-free survival from the initial treatment was also higher in the RFA group (5 months) than in the chemotherapy group (3.3 months), but the difference was only marginally significant (p = 0.068) (Fig. 3D).
Fig. 3. Survival rates between RFA group and chemotherapy group.
Overall survival from date of diagnosis of hepatic metastases (A) and from date of initial RFA (B) in 60 patients. C. Median overall survival from initial treatment was higher in RFA group (12 months) than in chemotherapy group (9.1 months), but difference was not statistically significant (p = 0.094). D. Median progression-free survival from initial treatment was higher in RFA group (5 months) than in chemotherapy group (3.3 months), but difference was marginally significant (p = 0.068).
Multivariate Cox regression analyses showed that the diameter of the largest tumor (hazard ratio [HR], 2.19; p = 0.007), TNM stage before curative surgery (HR, 2.73; p = 0.001), time between surgery and the development of recurrence (HR, 2.26; p = 0.016), and absence or presence of extrahepatic metastasis (HR, 3.37; p = 0.002) were independently associated with overall survival after RFA (Table 2).
Table 2. Results of Univariable and Multivariable Cox-Proportional Hazard Model for Evaluating Factors Associated with Overall Survival of 60 Patients after RFA.
| Variable | Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Adjusted Hazard Ratio | 95% CI | P | |
| Age (> 60.5 years, n = 30) | 1.03 | 0.61–1.74 | 0.912 | NA | NA | NA |
| Sex (male) | 1.34 | 0.78–2.29 | 0.291 | NA | NA | NA |
| TNM stage (≥ IIB) | 2.06 | 1.13–3.75 | 0.018 | 2.73 | 1.47–5.07 | 0.001 |
| Poorly differentiated | 2.30 | 1.10–4.81 | 0.027 | 2.10 | 0.99–4.44 | 0.052 |
| Tumor number (≥ 2) | 1.46 | 0.84–2.52 | 0.179 | NA | NA | NA |
| Tumor size (> 1.5 cm) | 2.16 | 1.25–3.73 | 0.006 | 2.19 | 1.24–3.89 | 0.007 |
| Period to recurrence (< 1 year) | 1.88 | 0.99–3.57 | 0.055 | 2.26 | 1.16–4.38 | 0.016 |
| Presence of extrahepatic metastasis | 3.81 | 1.79–8.07 | < 0.001 | 3.37 | 1.57–7.20 | 0.002 |
CI = confidence interval, NA = not applicable
During follow-up, 55 of the 60 patients (92%) developed new lesions at other liver sites and/or in distant areas. Of these 55 patients, 14 were treated with RFA, 20 with systemic chemotherapy, three with intra-arterial chemoinfusion, two with systemic chemotherapy and radiation therapy, and one with partial hepatectomy. The remaining 15 patients underwent supportive treatment only (pain or ascites control, biliary drainage).
DISCUSSION
The prognosis for patients with pancreatic adenocarcinoma remains dismal, with reported 5-year survival rates ranging from 0.4–8% (1,2). Despite treatment by curative resection, local recurrence, and/or liver metastases represent two frequent patterns of recurrence that are largely resistant to current treatments, including chemotherapy and radiation (9,29,30,31). Patients with hepatic metastases have a worse prognosis than those with local recurrence, with a shorter survival time (3 months vs. 7 months) (29). Therefore, effective control of hepatic metastasis is an important treatment goal for prolonging survival in such patients (32).
Hepatic resection of metastatic tumors arising from pancreatic adenocarcinoma is an alternative to RFA. However, although surgical resection has been known to be effective in patients with hepatic metastases arising from colorectal, breast, or neuroendocrine primary tumors, the role for hepatic resection in patients with hepatic metastases arising from pancreatic adenocarcinoma is less well defined (33). Hepatic resection of metastatic disease, even if confined to the liver, has been discouraged, largely because of the poor prognosis, and rapid progression of pancreatic adenocarcinoma (33).
To the best of our knowledge, the first application of RFA to the treatment of liver metastasis arising from pancreatic adenocarcinoma was described in a case report by Thomas et al. (20). In this report, percutaneous RFA was performed on one new liver metastasis in a 49-year-old female, who had previously undergone pancreaticoduodenectomy, followed by adjuvant chemoradiation to treat pancreatic adenocarcinoma. RFA was technically successful in this patient, but a large liver abscess occurred 3 weeks later. The abscess was successfully treated by percutaneous drainage, and the patient showed no evidence of either cancer or abscess recurrence 6 months later. This case suggested that RFA can be used to successfully treat hepatic metastases (of limited extent) in patients previously treated for pancreatic adenocarcinoma, but that the incidence of liver abscess is likely to be high because of bilioenteric anastomosis (20).
Park et al. (21) evaluated the clinical feasibility of RFA in 34 patients, with 28 having metachronous hepatic metastasis arising after curative resection of pancreatic adenocarcinoma, and six having a synchronous hepatic metastasis that was detected at the time of surgery. The RFA was performed intraoperatively in the six patients with synchronous hepatic metastasis. All 34 patients had no known metastasis other than to the liver. The median overall survival time from the date of diagnosis of hepatic metastasis was 14 months; however, the authors did not provide overall survival data from the time of RFA. In their multivariate analysis, the diameter of the largest hepatic metastasis (< 2 cm vs. ≥ 2 cm) and the pathology of pancreatic adenocarcinoma (well or moderate differentiation vs. poor differentiation) were significantly associated with overall patient survival from the time of diagnosis of hepatic metastasis. However, the authors did not provide the local tumor progression rate after RFA for each hepatic metastasis.
In the present study, we evaluated the safety and efficacy of percutaneous RFA in 60 patients with 94 metachronous hepatic metastasis, arising after curative resection of pancreatic adenocarcinoma. We found that RFA led to complete tumor necrosis of all 94 tumors (median diameter, 1.5 cm) after one (n = 89) or two (n = 5) sessions of RFA, without any procedure-related mortality. The local tumor progression rate during follow-up after RFA was 38.3%. The cumulative local tumor progression rates at 6 months, 1 year, and 2 years were 23.3%, 41.2%, and 47.3%, respectively. In agreement with previous findings showing that tumor size was significantly associated with local tumor progression after the use of RFA to treat hepatic malignancies (13,15,19,34), we found that the local tumor progression rate was significantly lower for tumors ≤ 1.5 cm in diameter than for those > 1.5 cm in diameter (p = 0.001).
We also found overall median survival and 3-year survival rates from the time of initial RFA of 12 months and 0%, respectively, while they were 14.7 months and 2.1%, respectively, from the first diagnosis of liver metastasis. The 14.7 months median survival period we observed (from time of diagnosis) was substantially longer than the 3 months median survival reported for patients conservatively managed for hepatic recurrence, though it is hard to compare directly (29,35). In addition, the median overall survival from the initial treatment was also longer in the RFA group (12 months) than in the chemotherapy group (9.1 months) in our study, but the difference was not statistically significant (p = 0.094). However, the present study hinted that a larger study might also find better overall survival in the RFA group than in the chemotherapy group.
In our study, multivariate analysis showed that a large tumor diameter of > 1.5 cm, TNM stage before curative surgery ≥ IIB, a time between surgery and development of tumor recurrence of < 1 year, and the presence of extrahepatic metastasis were all associated with poor overall patient survival from the time of initial RFA. According to our current results, patients with a tumor of small diameter (≤ 1.5 cm), relatively early TNM stage (≤ IIA) before the time of curative surgery, late hepatic recurrence (≥ 1 year after curative resection), and liver-only metastasis benefited most from RFA treatment.
Theoretically, RFA can result in thermal injury to the bile ducts, and can form an inadvertent connection between the biliary tree and the ablation zone. Such ablation zones may become contaminated with enteric bacteria, arriving through bilioenteric anastomoses, thereby resulting in liver abscesses (36). Although we found that liver abscesses after RFA occurred more frequently in patients with (13.2%, 5/38) than without (4.5%, 1/22) bilioenteric anastomoses, the difference failed to reach statistical significance (p = 0.399), probably due to small sample size of our study patients. Thus, monitoring for the possibility of liver abscesses caused by bilioenteric anastomoses must be performed following RFA treatment of hepatic tumors. Further, an aggressive antibiotic prophylaxis regimen in conjunction with routine pre-procedure bowel preparation may provide protection against liver abscesses after locoregional therapy in patients with bilioenteric anastomosis (19,37). This issue should be further evaluated in a future study.
This study's limitations include its retrospective nature, which causes it to be vulnerable to a variety of potential biases. Nevertheless, we believe that our results indicate that RFA may play a potential role in the treatment of liver metastases arising from pancreatic cancer, and our data provides support for prospective investigations.
In conclusion, RFA is safe and may offer successful local tumor control in patients with hepatic metastases arising from pancreatic adenocarcinoma. Patients with a tumor of small diameter (≤ 1.5 cm), early TNM stage (≤ IIA) before the time of curative surgery, late hepatic recurrence (≥ 1 year after curative resection), and liver-only metastasis, benefited most from RFA treatment. In our study, RFA provided better survival outcomes than chemotherapy for this specific group with borderline statistical difference.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg. 1995;82:111–115. doi: 10.1002/bjs.1800820137. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005;128:1642–1654. doi: 10.1053/j.gastro.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 6.Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Choi EK, Yoon HK, Ko GY, Sung KB, Gwon DI. Transcatheter arterial chemoembolization for hepatic recurrence after curative resection of pancreatic adenocarcinoma. Gut Liver. 2010;4:384–388. doi: 10.5009/gnl.2010.4.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 11.Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 12.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 13.Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT., Jr Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology. 2009;253:861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park IJ, Kim HC, Yu CS, Kim PN, Won HJ, Kim JC. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol. 2008;15:227–232. doi: 10.1245/s10434-007-9625-z. [DOI] [PubMed] [Google Scholar]
- 15.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 16.Lee CW, Kim JH, Won HJ, Shin YM, Ko HK, Kim PN, et al. Percutaneous radiofrequency ablation of hepatic metastases from gastric adenocarcinoma after gastrectomy. J Vasc Interv Radiol. 2015;26:1172–1179. doi: 10.1016/j.jvir.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Babawale SN, Jensen TM, Frøkjær JB. Long-term survival following radiofrequency ablation of colorectal liver metastases: a retrospective study. World J Gastrointest Surg. 2015;7:33–38. doi: 10.4240/wjgs.v7.i3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai XM, Yang W, Zhang ZY, Jiang AN, Wu W, Lee JC, et al. Long-term outcomes and prognostic analysis of percutaneous radiofrequency ablation in liver metastasis from breast cancer. Int J Hyperthermia. 2019;35:183–193. doi: 10.1080/02656736.2018.1488279. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, Kim JH, Won HJ, Shin YM, Kim PN. Radiofrequency ablation of hepatic metastases after curative resection of extrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;197:W1129–W1134. doi: 10.2214/AJR.11.6420. [DOI] [PubMed] [Google Scholar]
- 20.Thomas KT, Bream PR, Jr, Berlin J, Meranze SG, Wright JK, Chari RS. Use of percutaneous drainage to treat hepatic abscess after radiofrequency ablation of metastatic pancreatic adenocarcinoma. Am Surg. 2004;70:496–499. [PubMed] [Google Scholar]
- 21.Park JB, Kim YH, Kim J, Chang HM, Kim TW, Kim SC, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol. 2012;23:635–641. doi: 10.1016/j.jvir.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 22.Hua YQ, Wang P, Zhu XY, Shen YH, Wang K, Shi WD, et al. Radiofrequency ablation for hepatic oligometastatic pancreatic cancer: an analysis of safety and efficacy. Pancreatology. 2017;17:967–973. doi: 10.1016/j.pan.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 23.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 24.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes--a 10-year experience at a single center. Radiology. 2016;278:601–611. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon HM, Kim JH, Shin YM, Won HJ, Kim PN. Percutaneous radiofrequency ablation using internally cooled wet electrodes for treatment of colorectal liver metastases. Clin Radiol. 2012;67:122–127. doi: 10.1016/j.crad.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25:1691–1705.e4. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad ED, Katz A. Progression-free survival and time to progression as primary end points in advanced breast cancer: often used, sometimes loosely defined. Ann Oncol. 2009;20:460–464. doi: 10.1093/annonc/mdn670. [DOI] [PubMed] [Google Scholar]
- 28.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 30.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Katsumata K, Tomioka H, Sumi T, Yamasaki T, Takagi M, Kato F, et al. Liver metastasis of pancreatic cancer managed by intra-arterial infusion chemotherapy combined with degradable starch microspheres. Int J Clin Oncol. 2003;8:110–112. doi: 10.1007/s101470300019. [DOI] [PubMed] [Google Scholar]
- 32.Kandel P, Wallace MB, Stauffer J, Bolan C, Raimondo M, Woodward TA, et al. Survival of patients with oligometastatic pancreatic ductal adenocarcinoma treated with combined modality treatment including surgical resection: a pilot study. J Pancreat Cancer. 2018;4:88–94. doi: 10.1089/pancan.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleisner AL, Assumpcao L, Cameron JL, Wolfgang CL, Choti MA, Herman JM, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified. Cancer. 2007;110:2484–2492. doi: 10.1002/cncr.23074. [DOI] [PubMed] [Google Scholar]
- 34.Lee BC, Lee HG, Park IJ, Kim SY, Kim KH, Lee JH, et al. The role of radiofrequency ablation for treatment of metachronous isolated hepatic metastasis from colorectal cancer. Medicine. 2016;95:e4999. doi: 10.1097/MD.0000000000004999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gbolahan OB, Tong Y, Sehdev A, O'Neil B, Shahda S. Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study. BMC Cancer. 2019;19:468. doi: 10.1186/s12885-019-5630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319–2329. doi: 10.1245/s10434-006-9220-8. [DOI] [PubMed] [Google Scholar]
- 37.De Jong MC, Farnell MB, Sclabas G, Cunningham SC, Cameron JL, Geschwind JF, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg. 2010;252:142–148. doi: 10.1097/SLA.0b013e3181dbb7a7. [DOI] [PubMed] [Google Scholar]