Abstract
Background
There are few studies that directly investigate disparities in outcome within the African diaspora in the US. We investigated the association between nativity of Black women diagnosed with breast cancer (Caribbean or USA place of birth) and ethnicity, age at diagnosis, treatment, tumor characteristics and outcome.
Methods
The data were obtained from the University of Miami Health System, and Jackson Health System. Individual-level data from 1132 cases was used to estimate hazard rations (HRs) of women born in the Caribbean (Caribbean Blacks, CB) or in the USA (US Black, USB) using Cox proportional hazards regression analysis for overall survival.
Results
The cohort contains data from 624 (54.9%) USB women and 507 (45%) CB women diagnosed with breast cancer between 2006 and 2017. Compared to CB patients, USB patients had more Estrogen Receptor negative (31.4% vs. 39.1%, P = 0.018) and triple negative breast cancers (19.6% vs. 27.9%, P = 0.003). CB women presented at more advanced stages III/IV (44.2% vs. 35.2%; P = 0.016). CB patients showed a better overall survival (hazard ratio, HR = 0.75; 95% CI 0.59–0.96; P = 0.024). Overall Black Hispanic patients had a better overall survival (HR = 0.51; 95% CI 0.28–0.93; P = 0.028) compared to non-Hispanic Black patients.
Conclusion
In conclusion the study found that CB immigrants diagnosed with breast cancer have an improved overall survival when compared with USB patients. This finding suggests that within the African diaspora in the USA, additional factors beyond race contribute to worse outcomes in African Americans.
Keywords: Breast cancer, Caribbean-born Black, US-born Black, Health disparities
Introduction
Breast cancer is a frequent cause of morbidity and mortality in women of African descent living in the US [1, 2]. Although African American women have a similar incidence of breast cancer compared to white women they experience worse outcomes [1–3]. Breast cancer is diagnosed at more advanced stages in Black women than in White women, and Black women are disproportionally diagnosed with the most aggressive forms of this disease [2–4].
The Black population living in the US is not monolithic. In the United States, the number of newly arrived immigrants from the Caribbean has grown, exceeding those from Europe [5]. One in ten Blacks living in the US is foreign born [6]. With the history of the Slave trade, then immigration of indentured laborers (East Indian and Chinese) the Caribbean is now very heterogeneous but the majority of the study participants self-identified as African descent with other ‘mixes’ indicating that individuals could not accurately report their precise ancestry. Caribbean nationals make up over 50% of the Non-Hispanic Black immigrant population in the US [7, 8]. The largest contributors to Caribbean Black (CB) immigration are Haiti, Jamaica, the Dominican Republic and Trinidad & Tobago [6].
In Florida, the CB population has a lower cancer mortality rate when compared to the US-born Black population [6, 9, 10]. New York and Florida, which have the two largest CB communities in the US, report the lowest mortality rate among the 17 states with black populations over 1 million [9]. Etiological studies of the cancer risks among Caribbean immigrant women have been sparse and limited [11–16] but Caribbean-born women living in the US and in their native country were shown to have differences in incidence and outcomes of breast cancer from that of African American women [9, 12]. Because a significant portion of Caribbean women self-classifies as African-American it is necessary to consider the unique characteristics of the Caribbean population when analyzing breast cancer in women of African descent living in the US. We performed a detailed review of 1132 self-identified Black patients, born in the United States or born in the Caribbean from 18 different countries to undertake a systematic analysis on the effect of nativity on breast cancer outcome in Black women. We aimed to determine associations between nativity, breast cancer characteristics, treatment and overall survival.
Methodology
Study cohort
This is a retrospective cohort study conducted with Institutional Review Board approval from the University of Miami (protocol #2016-0291). Patients were identified through the University of Miami Health System (UMHS) and Jackson Health System (JHS) institutional tumor registry. We collected data, through medical chart abstraction, from 1368 individuals diagnosed with breast cancer between the years 2006 and 2017 from the UMHS. UMHS is an academic medical center with a Comprehensive Cancer Center, and JHS, a non-profit safety net hospital. Standard of care at both sites are similar. All data were de-identified prior to analysis. The safety net hospital in our system provides care for all patients, regardless of citizenship status, as long as the patient resides within the county. Abstracted data included sociodemographic factors, genetic testing results, and treatment histories. Male patients were excluded from the survival analyses. The inclusion criteria encompassed: (1) males and females aged 18 years or older, (2) pathologically diagnosed with breast cancer, (3) born in the Caribbean or the US, (4) self-identify as African descent. Exclusion criteria included patients with unknown or any non-Caribbean/non-USA birthplaces. Eight hundred and twenty-nine patients from JHS and 538 patients from UMHS were included in this study.
Data variables
Data extracted includes: (1) patient demographics: weight and height; medical and family health history; hormone exposures: menopausal status, age at: menarche and menopause; age at diagnosis; date of diagnosis; smoking and alcohol habit, (2) tumor characteristics: tumor node metastasis (TNM) status, stage, ER, PR, HER2, grade; germline genetic testing results; type and date of surgery, use of neoadjuvant or adjuvant therapy, (3) survival data: date of death, dates of relapse and last contact.
Statistical analysis
The data were captured into REDCap, a secure web application, HIPPA compliant server at the University of Miami. For analysis purposes, the women were divided in two groups: US born Black (USB) and Caribbean-born Black (CB). The USB group was composed of Blacks born in the USA, the CB were born in the Caribbean (the countries included were: Anguilla, Antigua, Aruba, Bahamas, Barbados, Belize, British Virgin Islands, Cayman Islands, Cuba, Dominica, Dominican Republic, British Guyana, Haiti, Jamaica, St. Kitts and Nevis, St. Lucia, Trinidad Tobago, Turks and Caicos Islands, Suriname, US Virgin Islands and West Indies). We excluded patients where place of birth was listed as: unknown (211), other (6) and non-Caribbean, non-USA born (19). Statistical analyses were performed using SPSS (IBM SPSS Statistics Version 24) and STATA IC 14.2 (StataCorp, College Station, TX) (Supplement Table 1). All patients were included in the analyses, even when missing specific data points. Summary statistics were used to describe the patient cohort. Wilcoxon rank-sum was used for continuous variables in nonparametric distributions. Chi square testing (or Fisher’s Exact, when appropriate) was used to analyze associations between categorical variables. Univariable and multivariable Cox proportional hazards regression, the log-rank test, and the Kaplan–Meier method were utilized to assess survival outcomes. All patients were included in the survival analyses, and censored at date of last follow-up or date of death (all cause). Stepwise backwards multivariable regression analyses only included covariates with p values ≤0.05 from the univariable models (excluding triple negative breast cancer to remove redundancies with ER, PR and HER2). All tests were two-sided, with significance set at P < 0.05.
Results
Cohort characteristics
Table 1 shows the demographics of the cohort. The cohort was composed of 1131 Black individuals with breast cancer. There were 5 males, comprising 0.4% of the total sample, and 1126 women. There were 624 (55.1%) US-born Blacks (USB) patients and 507 (45%) Caribbean Black (CB) patient. The proportion of breast cancer patients born in the US or in the Caribbean are similar by treating institutions. The majority of the patients were seen at safety net hospital (USB = 69.5% and CB = 70.2%) while the private care health system had less than a third of the cohort (USB = 30.5% and CB = 29.8%; P = 0.78). The most frequent countries of birth for the Caribbean Black patient cohort were: Haiti (49.2%), Jamaica (17.5%), Bahamas (8.5%), Dominican Republic (7.5%), Cuba (7.5%) and Trinidad & Tobago (2.8%).
Table 1.
Summary of the breast cancer in Black women cohort
| Variable | Caribbean-born (n = 507) | USA-born (n = 624) | P value |
|---|---|---|---|
| Age at diagnosis, mean (95% CI)a | 55.7 (54.7–56.8) | 57.6 (56.4–58.7) | 0.001 |
| BMI, mean (95% CI)a | 29.6 (28.9–30.3) | 30.9 (30.1–31.7) | 0.015 |
| Genetic testing no. (%) | |||
| Tested | 39/507 (7.7) | 40/624 (6.4) | 0.414 |
| Genetic mutation no. (%) | |||
| Positive | 8/39 (20.5) | 9/40 (22.5) | 0.83 |
| HER-2 no. (%) | |||
| Positive | 93/406 (22.9) | 78/441 (17.7) | 0.061 |
| Negative | 313/406 (77.1) | 363/441 (82.3) | |
| ER no. (%) | |||
| Positive | 276/402 (68.7) | 288/472 (61.0) | 0.019 |
| Negative | 126/402 (31.3) | 184/472 (39.0) | |
| PR no. (%) | |||
| Positive | 238/408 (58.3) | 231/458 (50.4) | 0.020 |
| Negative | 170/408 (41.7) | 227/458 (49.6) | |
| Triple-negative no. (%) | 84/426 (19.7) | 133/478 (27.8) | 0.004 |
| Ductal histology no. (%) | |||
| Yes | 342/459 (74.5) | 404/566 (71.4) | 0.254 |
| No | 117/459 (25.5) | 162/566 (28.6) | |
| Stage at diagnosis no. (%) | |||
| I | 100/384 (26.0) | 144/490 (29.4) | – |
| II | 116/384 (30.2) | 173/490 (35.3) | |
| III | 90/384 (23.4) | 94/490 (19.2) | |
| IV | 78/384 (20.3) | 79/490 (16.1) | |
| Stage at diagnosis no. (%) | |||
| Early: I and II | 218/385 (56.6) | 317/490 (64.7) | 0.016 |
| Advanced: III and IV | 167/385 (43.4) | 173/490 (35.3) | |
| Current alcohol consumers no. (%) | 49/442 (11.9) | 102/512 (19.9) | < 0.001 |
| Current smoker no. (%) | 7/441 (1.6) | 37/515 (7.2) | < 0.001 |
| Surgery performed no. (%) | |||
| Mastectomy/lumpectomy | 345/504 (68.5) | 454/623 (72.9) | 0.011 |
| No surgery | 159/504 (31.5) | 169/623 (27.1) | |
| Radiation treatment no. (%) | |||
| Yes | 182/507 (35.2) | 175/624 (28) | 0.010 |
| No | 326/507 (64.7) | 449/624 (72) | |
| Chemotherapy treatment no. (%) | |||
| Yes | 235/507 (46.4) | 248/624 (39.7) | 0.027 |
| No | 272/507 (53.6) | 376/624 (60.3) | |
| Hormone antagonist therapy no. (%) | |||
| Administered | 171/507 (33.8) | 177/624 (28.4) | 0.063 |
| Not administered | 336/507 (66.2) | 337/624 (66.3) | |
| Treatment type (%) | |||
| Adjuvant | 205/283 (72.4) | 193/299 (64.5) | 0.041 |
| Neoadjuvant | 78/283 (27.6) | 106/299 (35.5) | |
| Ethnicity no. (%) | |||
| Hispanic | 76/507 (15) | 7/615 (1.1) | < 0.001 |
| Non-Hispanic | 431/507 (85) | 608/615 (98.9) | |
| Treating institution | |||
| Safety Net Hospital | 355/507 (70.0) | 434/624 (69.6) | 0.848 |
| Comprehensive Cancer Center | 152/507 (29.9) | 190/624 (30.4) |
Comparison by independent t test. All other comparisons are by Chi squared correlation
The mean age of diagnosis of the entire cohort was 56.7 years (sd. 13.1 years). The mean age at diagnosis in CB patients was 55.7 (sd. 12.1) years while it was 57.6 (sd. 13.9) for USB patients (P = 0.020). The mean age of Hispanic Black (HB) patients was 56.6 years (sd. 10.6) and that of non-Hispanic Blacks (NHB) patients was 56.7 years (sd. 13.4). USB patients had a Higher Body Mass Index (BMI), (30.9) when compared to CB patients (29.6; P = 0.015). The USB patients had a higher use of tobacco (7.2% vs. 1.6%, P < 0.001) and alcohol (19.9% vs. 11.9%; P < 0.001) as compared to CB patients.
CB patients had more ER-positive and PR-positive tumors, (ER = 68.7% vs. 61%; P = 0.019 and PR = 58.3% vs. 50.4%; P = 0.02) than the USB breast cancer patients. The USB breast cancer patients had more triple-negative breast cancer (TNBC) subtypes (27.8% compared to 19.7%; P = 0.004). The USB patients underwent surgery more often (72.9% vs. 68.5%; P = 0.011). More CB breast cancer patients received radiation treatment (35.2% vs. 28.1%; P = 0.05) and chemotherapy (48.5% vs. 42.7%; P = 0.016), compared to USB patients. The CB breast cancer patients had more stage III and IV disease at presentation (44.2% vs. 35.2%; P = 0.016), when compared with USB patients. Genetic germline testing was offered to 79 patients (7%). The proportions of tested CB (n = 39; 7.7%) compared to USB (n = 40; 6.4%) showed no significant difference (P = 0.414). Of those tested, there was no significant differences in inherited germline mutations between USB (21.9%) and CB patients (20.5%) (P = 0.83).
Survival analysis
As expected for the entire cohort, worse outcomes were observed when the breast cancer was triple-negative subtype (HR = 1.37, 95% CI 1.02–1.84; P = 0.039) or diagnosed at advanced stages: III and IV (HR=4.41; 95% CI 3.31–5.90; P < 0.001). Hormone receptor expression of ER (HR = 0.68, 95% CI 0.51–0.90; P = 0.007) and PR (HR=0.63, 95% CI 0.48–0.83; P=0.001), were protective factors in overall survival. Treatment by surgical intervention, (HR = 0.23, 95% CI 0.18–0.30; P < 0.001), hormone antagonism (HR = 0.59, 95% CI 0.44–0.77; P < 0.001) and radiotherapy (HR = 0.46, 95% CI 0.35–0.62; P < 0.001), were favorable factors consistent with the current literature [17]. There was no association between overall survival and BMI.
During a median follow-up of 144 months, there was a significant difference in outcomes by nativity. In a univariate model, Caribbean-born patients had a reduction of 25% in risk of death compared with the US-born cohort (HR = 0.75, 95% CI 0.59–0.96; P = 0.024) (Table 2; Fig. 1). Caribbean-born patients had significantly better median overall survival compared to US-born Black patients [1–47.9 months (95% CI 118.8–177.1) vs. 98.6 months (95% CI 82.1–115.2 months] (Log-Rank, Mantel-Cox P = 0.02). In the multivariate Cox proportional hazards regression model adjusting for ER, PR, surgery, hormonal treatment, radiation and stage, Caribbean-born Blacks continue to have a significantly better outcome (HR=0.68, 95% CI 0.49–0.94, P=0.018).
Table 2.
Univariate Cox proportional hazards model for overall survival
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Place of birth | |||
| USA (referent) | 0.75 | 0.59–0.96 | 0.024 |
| Caribbean | |||
| Ethnicity (all) | |||
| Non-Hispanic (referent) | 0.51 | 0.28-0.93 | 0.028 |
| Hispanic | |||
| Ethnicity among Caribbean | |||
| Hispanic (referent) | 1.98 | 1.00-3.94 | 0.048 |
| Caribbean non-Hispanic | |||
| BMI (continuous)—any increase in weight | 0.99 | 0.98-1.01 | 0.55 |
| Age at diagnosis (continuous) | 1.01 | 1.00-1.02 | 0.015 |
| Estrogen receptor (ER) | |||
| Negative (referent) | 0.68 | 0.51-0.90 | 0.007 |
| Positive | |||
| Progesterone receptor (PR) | |||
| Negative (referent) | 0.63 | 0.48-0.83 | 0.001 |
| Positive | |||
| HER2/neu | |||
| Negative (referent) | 1.01 | 0.72-1.42 | 0.95 |
| Positive | |||
| Triple negative | |||
| No (referent) | 1.37 | 1.02-1.84 | 0.039 |
| Yes | |||
| Genetic mutation | |||
| No (referent) | 2.04 | 0.57-7.25 | 0.27 |
| Yes | |||
| Surgery | |||
| No (referent) | 0.23 | 0.18-0.30 | < 0.001 |
| Yes | |||
| Hormone antagonism | |||
| No (referent) | 0.59 | 0.44-0.77 | < 0.001 |
| Yes | |||
| Chemotherapy | |||
| No (referent) | 1.07 | 0.84-1.36 | 0.57 |
| Yes | |||
| Radiation | |||
| No (referent) | 0.46 | 0.35-0.62 | < 0.001 |
| Yes | |||
| Stage of disease | |||
| Stage I and II (referent) | 4.41 | 3.31-5.90 | < 0.001 |
| Stage III and IV | |||
| Histology | |||
| Non-ductal (referent) | 1.03 | 0.78-1.36 | 0.85 |
| Ductal |
Fig. 1.
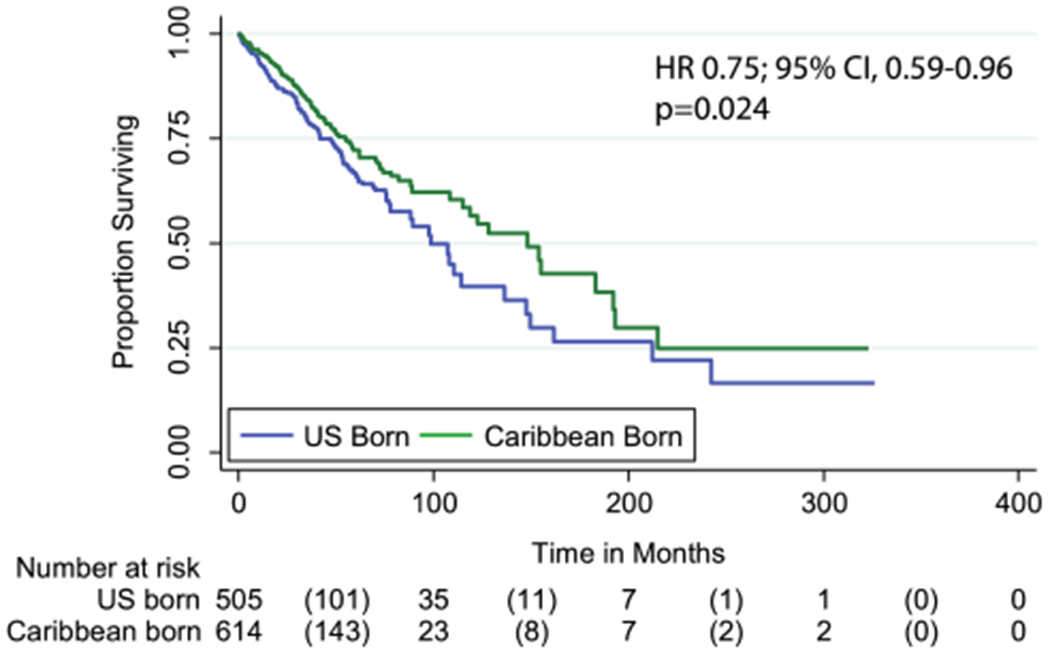
Kaplan–Meier graph of overall survival (months) stratified by Stage1/2 at diagnosis of Caribbean-born Blacks and US-born Blacks. USB (median 98.6 months) have worse overall survival than CB patients (median 1–47.9 months), Log-Rank Mantel Cox, P = 0.020
We then performed a comparison of overall survival stratified by treating institution by the Kaplan-Meier to rule out confounding variables such as socioeconomic status. Caribbean-born patients continued to show a higher median overall survival of 115 months (95% CI 84.6–145.3) compared to 88.3 months (95% CI 75.08–101.5 months) (Log-Rank, Mantel-Cox P = 0.05) at the JMS treated group. CB patients had a median overall survival of 183.2 months (95% CI not available) compared to 149.3 months (95% CI 86–212.7) (Log-Rank, Mantel-Cox P = 0.07) when treated at UMHS.
Further analysis of matched ER, triple negative and stage were performed to determine if the difference in overall survival and better outcomes in the CB patient group was attributable to ER, TNBC and stage. CB patients maintained their overall survival outcome advantage with a median 58.8 months (95% CI 37.4–80.2 months) over USB, median 47.3 months (95% CI 35.9–58.9 months), Log-Rank P = 0.026 when controlled for ER, TNBC and stage (Supplement Table 3, Supplement Fig. 1A, B). We performed a second hazard regression model comparing ethnicity and clinical characteristics by nativity with the aim of identifying which factors had a significant effect on outcome for the two groups (Table 3). Variables that had a favorable effect on outcome for both CB and USB patients were PR expression (CB HR = 0.64, 95% CI 0.43–0.96; P = 0.032 and USB HR = 0.64, 95% CI 0.43–0.95; P = 0.028), surgical intervention (HR = 0.19, 95% CI 0.13–0.27; P < 0.001 and USB HR = 0.26, 95% CI 0.19–0.36; P < 0.001) and radiotherapy (CB HR = 0.47, 95% CI 0.31–0.71; P < 0.001 and USB HR=0.48, 95% CI 0.33–0.70; P = < 0.001). Advanced stage at diagnosis remained a factor related to worse outcomes in both CB (HR = 4.48, 95% CI 2.84–7.09; P < 0.001) and USB groups (HR = 4.66, 95% CI 3.20–6.78; P < 0.001). In this analysis the effect of hormonal antagonism was only a significant variable in the USB patient population (HR = 0.5, 95% CI 0.36–0.78; P = 0.001), indicating potential tumor characteristics amongst the groups.
Table 3.
Multivariate Cox proportional hazards model for overall survival in each group
| Variablea | Caribbean born | USA born |
|---|---|---|
| Ethnicity (all) | ||
| Non-Hispanic (referent) | – | – |
| Hispanic | 0.46 (0.24–0.92), P = 0.028 | 2.75 (0.68–11.12), P = 0.16 |
| BMI (continuous) | 0.99 (0.96–1.03), P = 0.68 | 0.99 (0.97–1.02), P = 0.58 |
| Age at diagnosis (continuous) | 1.00 (0.99–1.02), P = 0.37 | 1.01 (1.00–1.03), P = 0.025 |
| Estrogen receptor (ER) | ||
| Negative (referent) | – | – |
| Positive | 0.71 (0.47–1.08), P = 0.11 | 0.70 (0.48–1.02), P = 0.06 |
| Progesterone receptor (PR) | ||
| Negative (referent) | – | – |
| Positive | 0.64 (0.43–0.96), P = 0.032 | 0.64 (0.43–0.95), P = 0.028 |
| HER2/neu | ||
| Negative (referent) | – | – |
| Positive | 0.99 (0.61–1.62), P = 0.99 | 1.08 (0.67–1.74), P = 0.76 |
| Triple negative | ||
| No (referent) | – | – |
| Yes | 1.17 (0.73–1.89), P = 0.51 | 1.41 (0.96–2.07), P = 0.08 |
| Genetic mutation | ||
| No (referent) | – | – |
| Yes | 3.62 (0.51–25.9), P = 0.20 | 0.91 (0.16–5.23), P = 0.92 |
| Surgery | ||
| No (referent) | – | – |
| Yes | 0.19 (0.13–0.27), P < 0.001 | 0.26 (0.19–0.36), P < 0.001 |
| Hormone antagonism | ||
| No (referent) | – | – |
| Yes | 0.69 (0.46–1.04), P = 0.07 | 0.53 (0.36–0.78), P = 0.001 |
| Chemotherapy | ||
| No (referent) | – | – |
| Yes | 0.99 (0.68–1.45), P = 0.98 | 1.19 (0.87–1.65), P = 0.27 |
| Radiation | ||
| No (referent) | – | – |
| Yes | 0.47 (0.31–0.71), P < 0.001 | 0.48 (0.33–0.70), P < 0.001 |
| Tobacco use | ||
| Never/former (referent) | – | – |
| Current | 0.62 (0.09–4.49), P = 0.64 | 1.23 (0.68–2.24), P = 0.48 |
| Alcohol use | ||
| Never/former (referent) | – | – |
| Current | 0.17 (0.04–0.69), P = 0.013 | 0.79 (0.52–1.21), P = 0.29 |
| Stage of disease | ||
| Stage I and II (referent) | – | – |
| Stage III and IV | 4.48 (2.84–7.09), P < 0.001 | 4.66 (3.20–6.78), P < 0.001 |
| Histology | ||
| Non-ductal (referent) | – | – |
| Ductal | 1.26 (0.80–1.97), P = 0.32 | 0.88 (0.62–1.26), P = 0.50 |
Referent variable listed first. Result expressed in the following order: HR (95% CI), P-value. The following variables were included in the model: stage, radiation, hormone antagonism, surgery, ER, PR, age at diagnosis and place of birth
In univariate analysis, all Black Hispanic patients independent of region of birth had a 49% reduced risk of death (HR = 0.51, 95% CI 0.28–0.93; P = 0.028) while Caribbean non-Hispanic Blacks had an increased risk of death compared to Caribbean Hispanic Black patients (HR = 1.98, 95% CI 1.00–3.94; P = 0.048). A multivariate analysis model adjusting for similar factors did not reach significance for the relationship between Hispanic ethnicity and place of birth (HR = 1.82, 95% CI 0.73–4.60; P = 0.20) (Supplement Table 2). Only 1.1% of the USB patient population self-identified as Hispanic and/or were indicated in their medical records, therefore, determining the positive effect on outcomes in the USB population is probably understated and restricted by sample size.
Discussion
This study is a comprehensive analysis of a large cohort of Black women diagnosed with breast cancer in the US with attention to Caribbean nativity. Our study included women diagnosed and treated within both a safety net hospital and an academic comprehensive cancer center. The proportion of US-born and Caribbean-born Black patients from each institution is similar, therefore, the outcome differences seen should be attributable to factors independent of treating institution. Prior studies looking at USB and CB breast cancer patients showed that US-born Blacks had favorable outcomes compared to Caribbean-born Blacks [12, 18]. However, these studies compared breast cancer patients across different health systems and in different countries which have substantial differences in gross domestic products (GDP) and access to healthcare.
In our study, Caribbean-born Blacks were more likely to have ER/PR-positive breast cancers, had more advanced stages of breast cancer at presentation and underwent chemotherapy treatment and radiation treatment more often than USB women with breast cancer. The USB patients presented with more early-stage breast cancer, underwent surgery more often, were more likely to be overweight and obese, and used alcohol and tobacco at higher rates than CB women with breast cancer. There was no difference in the percent of germline mutation carriers between USB and CB patients. In our previous work, we demonstrated that Caribbean natives diagnosed with breast and ovarian cancer, had higher than expected rates of hereditary breast cancer with a deleterious mutation seen in 25% of Bahamian women [19, 20] and 12% of women from Trinidad & Tobago [21, 22]. It is important to highlight that 5–10% of all women diagnosed with breast cancer in the USA are reported to have deleterious breast cancer gene mutation, 12% of Black women in general [23] and 20% of Black women with triple negative breast cancer [24]. Our current data show that Black women in South Florida are severely undertested. This undertesting should have a disproportionate effect on the CB women with breast cancer.
There is limited information on Hispanic Blacks, which constitute an important part of the Caribbean population. Recent data from Surveillance, Epidemiology and End Results Program (SEER) showed that the incidence of breast cancer in Hispanic women is increasing [25]. However, they have lower incidence compared to non-Hispanic White women (NHW) and non-Hispanic Black (NHB) [25]. There is conflicting data on Hispanic women’s risk of breast cancer specific-mortality compared to NHW women. The scarce data available show worse outcomes to Hispanic Whites (HW) but lower or similar risk of breast cancer mortality to NHB, however, the reasons behind these findings have not yet been explored [26–29]. The majority of the Hispanic Black women in our cohort were born in Cuba and Dominican Republic. In our cohort, we observed that Hispanic Black women had better outcomes than non-Hispanic Black women and more so in the Caribbean-born Black patient cohort.
The present study has some limitations. First, this is a retrospective/database review and as such there is a possibility of misclassification or information bias. Two, our cohort represents a curated cohort of women diagnosed with breast cancer within a health system in South Florida. It is feasible that Caribbean-born immigrants in other parts of the United States have different overall outcomes due to specific local factors such as environment, health care access, stigma and discrimination. Nonetheless, our cohort has similar findings to improved cancer related outcomes in immigrant populations [9, 30]. Third, in this study, we grouped all breast cancer patients by region and not by individual country of origin. It is likely that there are subregional differences across the patient populations depending on country of origin as observed with Hispanic Black patients. Fourth, as a cofounder to nativity as a factor driving outcome, second generation Caribbean ancestry could not be taken into consideration. Finally, the study was unable to determine length of time in the US by the Caribbean immigrant population, and therefore, add time as a variable in hazard ratio calculations. This limitation provides an opportunity to conduct prospective studies to determine whether and how length of time in the US affects outcomes.
In conclusion, Black women diagnosed with breast cancer have worse outcomes compared to all other racial groups. Although US-born and Caribbean-born Black women are racially similar, there are significant differences in breast cancer presentation, type of breast cancer and overall outcomes. These differences may be due to differences in healthcare utilization, systemic barriers to accessing care, psychosocial factors such as chronic stress exposure, cultural norms, environmental exposures (native country and relocated environment) and differences in genomic ancestral diversity. These sub-racial disparities in breast cancer outcomes highlighted in this study, have identified opportunities to address immigrant and native specific risk factors to improve the health and quality of life of women diagnosed with breast cancer.
Supplementary Material
Acknowledgments
Funding Research reported in this publication was supported by funds from Sylvester Comprehensive Cancer Center. SG is supported by DOD OCRP W81XWH1810072.
Role of the Funder/Sponsor The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US government.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-019-05403-9) contains supplementary material, which is available to authorized users.
Conflict of interest There are no conflicts of interest.
Research involving human and animal participants This study did not involve animals.
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent This was a retrospective study; informed consent was not sought.
References
- 1.DeSantis CE et al. (2016) Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin 66(1):31–42 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE et al. (2016) Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin 66(4):290–308 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30 [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34 [DOI] [PubMed] [Google Scholar]
- 5.Pew Research Center (2015) Modern immigration wave brings 59 million to U.S., driving population growth and change through 2065: views of immigration’s impact on U.S. society mixed. Pew Research Center, Washington, DC [Google Scholar]
- 6.Anderson M, Lopez G (2018) Key facts about black immigrants in the U.S. Pew Research Center 1:1–5 [Google Scholar]
- 7.Zong J, Batalova J (2016) Caribbean immigrants in the United States. Migration Policy Institute; https://www.migrationpolicy.org/article/caribbean-immigrants-united-states [Google Scholar]
- 8.US Census Bureau (2010) US Census Bureau 2010. https://www.census.gov/programs-surveys/decennial-census/data/tools.html
- 9.Pinheiro PS et al. (2016) Black heterogeneity in cancer mortality: US-Blacks, Haitians, and Jamaicans. Cancer Control 23(4):347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh GK, Siahpush M (2001) All-cause and cause-specific mortality of immigrants and native born in the United States. Am J Public Health 91(3):392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camacho-Rivera M et al. (2015) Breast cancer clinical characteristics and outcomes in Trinidad and Tobago. J Immigr Minor Health 17(3):765–772 [DOI] [PubMed] [Google Scholar]
- 12.Taioli E et al. (2010) Breast cancer survival in women of African descent living in the US and in the Caribbean: effect of place of birth. Breast Cancer Res Treat 122(2):515–520 [DOI] [PubMed] [Google Scholar]
- 13.DeGennaro V Jr et al. (2016) Development of a breast cancer treatment program in Port-au-Prince, Haiti: experiences from the field. J Glob Oncol 2(1):9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez A et al. (2017) Presentation, treatment, and outcomes of Haitian women with breast cancer in Miami and Haiti: disparities in breast cancer—a retrospective cohort study. J Glob Oncol 3(4):389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ragin C et al. (2018) Breast cancer research in the Caribbean: analysis of reports from 1975 to 2017. J Glob Oncol 4:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlumbrecht M et al. (2019) Endometrial cancer outcomes among non-Hispanic US born and Caribbean born black women. Int J Gynecol Cancer 29:897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pertschuk LP et al. (1990) Immunocytochemical estrogen and progestin receptor assays in breast cancer with monoclonal antibodies. Histopathologic, demographic, and biochemical correlations and relationship to endocrine response and survival. Cancer 66(8):1663–1670 [DOI] [PubMed] [Google Scholar]
- 18.Consedine NS et al. (2015) Beyond the black box: a systematic review of breast, prostate, colorectal, and cervical screening among native and immigrant African-descent Caribbean populations. J Immigr Minor Health 17(3):905–924 [DOI] [PubMed] [Google Scholar]
- 19.Donenberg T et al. (2011) A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat 125(2):591–596 [DOI] [PubMed] [Google Scholar]
- 20.Akbari MR et al. (2014) The spectrum of BRCA1 and BRCA2 mutations in breast cancer patients in the Bahamas. Clin Genet 85(1):64–67 [DOI] [PubMed] [Google Scholar]
- 21.Donenberg T et al. (2016) A survey of BRCA1, BRCA2, and PALB2 mutations in women with breast cancer in Trinidad and Tobago. Breast Cancer Res Treat 159(1):131–138 [DOI] [PubMed] [Google Scholar]
- 22.Donenberg T et al. (2018) A clinically structured and partnered approach to genetic testing in Trinidadian women with breast cancer and their families. Breast Cancer Res Treat 174(2):469–477. 10.1007/s10549-018-5045-y [DOI] [PubMed] [Google Scholar]
- 23.Pal T et al. (2015) A high frequency of BRCA mutations in young black women with breast cancer residing in Florida. Cancer 121(23):4173–4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenup R et al. (2013) Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol 20(10):3254–3258 [DOI] [PubMed] [Google Scholar]
- 25.Miller KD et al. (2018) Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin 68(6):425–445 [DOI] [PubMed] [Google Scholar]
- 26.Khan HM et al. (2014) Inferential statistics from Black Hispanic breast cancer survival data. Sci World J 2014:604581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banegas MP, Li CI (2012) Breast cancer characteristics and outcomes among Hispanic Black and Hispanic White women. Breast Cancer Res Treat 134(3):1297–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trapido EJ et al. (1994) Cancer in south Florida Hispanic women. A 9-year assessment. Arch Intern Med 154(10):1083–1088 [PubMed] [Google Scholar]
- 29.McCoy CB et al. (2004) A community-based breast cancer screening program for medically underserved women: its effect on disease stage at diagnosis and on hazard of death. Rev Panam Salud Publica 15(3):160–167 [DOI] [PubMed] [Google Scholar]
- 30.Medhanie GA et al. (2017) Cancer incidence profile in sub-Saharan African-born blacks in the United States: similarities and differences with US-born non-Hispanic blacks. Cancer 123(16):3116–3124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


