Abstract
Objective:
To analyze the incidence rate of herpes zoster ophthalmicus (HZO) and differences by age, sex, race and region from 1994 to 2018.
Design:
Retrospective, observational cohort study.
Subjects:
Patients with a new International Classification of Disease (ICD) 9th or 10th code for herpes zoster (HZ) and HZO from January 1, 1994 to December 31, 2018 in the OptumLabs ® Data Warehouse.
Methods:
OLDW, a longitudinal, real-world data asset with de-identified administrative claims and electronic health record (EHR) data, was used to identify enrollees with continuous enrollment in the database for ≥365 days. Patients with no history of HZ or HZO and a new code for HZ and HZO were counted as incident cases. The incidence rate of HZO was calculated by year, 10-year age groups, sex, race, and geographic region.
Main Outcome Measures:
Differences in incidence rate from 1994 to 2018, by 10-year age groups and sex.
Results:
From 1994 to 2018, 633,474 cases of HZ were reported with 49,745 (7.9%) having HZO. The incidence of HZO increased from 1994 to 2018 by an estimated 1.1 cases per 100,000 person-years annually (95% CI 1.0 to 1.3, P<0.001). The estimated relative increase was 3.6% annually (95% CI: 3.0% to 4.1%). HZO incidence rates increased in all ages over 10 until 2007, then began declining in individuals younger than 21 and older than 60, stabilizing in individuals 21 to 30, and increasing more slowly among individuals 31 to 60. Men had an HZO incidence rate ratio (IRR) of 0.74 compared to women. Compared to Caucasians, the IRR were 0.70, 0.75, and 0.64 for Asians, African Americans, and Hispanics, respectively. Compared to the Northeast, the IRRs were 0.80, 0.71, and 0.77 for the Midwest, West, and South, respectively.
Conclusions:
The incidence of HZO has increased 3.6% per year from 1994 to 2018 in the United States. Since 2008, HZO incidence declined in individuals younger than 21 and older than 60 while increasing at a lower rate in middle-aged adults. Given the continued increase, greater efforts should be made to vaccinate eligible adults 50 and above. More research on earlier vaccination is warranted.
Precis:
The incidence rate of herpes zoster ophthalmicus increased overall from 1994 to 2018. Since 2008, rates have declined in adults ≤20 and >60 years old, with continued increases between 31 to 60 years old.
Introduction
Herpes zoster (HZ), also known as shingles, occurs in 1 in 3 individuals during their lifetime in the United States (US) and typically manifests as a painful dermatomal rash.1 Approximately 10 to 20% of patients with HZ will develop herpes zoster ophthalmicus (HZO), a form of HZ that occurs when varicella zoster virus reactivates along the ophthalmic branch of the trigeminal nerve.2 Of those individuals with HZO, 50 to 71% have ocular involvement with complications such as keratitis, uveitis, retinal necrosis and loss of vision, leading to significant pain, morbidity, and decreased quality of life.3–6 It is also now recognized that approximately 20% of patients with HZO may have a chronic course requiring ongoing treatment.7
The few studies on HZ incidence suggest that rates have been increasing in the two decades up to 2012 and a 2018 report from the Center for Disease Control (CDC) revealed that HZ has continued to rise in adults through 2016.8–10 However, these studies did not report on HZO incidence. There is no consensus on why HZ has been increasing although several theories do exist. One theory is that that the introduction of the varicella vaccine in 1996 lowered exposure to wild-type varicella infection in the population, decreasing immune boosting. Others have speculated that the rising HZ incidence may be due to an increase in immunocompromised conditions or changes in health-seeking behavior, leading to increased diagnosis of HZ.11–13 Another major factor that may be affecting the epidemiology is the introduction of vaccines for HZ: Zostavax® (ZVL; Merck & Co, Inc, Whitehouse Station, NJ) and Shingrix® (RZV; GlaxoSmithKline, Philadelphia, PA). Since the introduction of ZVL in 2006 and RZV in 2017, there have been few reports on the influence of these vaccines on HZ rates and even less that have looked at vaccine effect on HZO.8,9
Aside from a small population study that showed increasing HZO from 1980 to 2007, there has been limited information available on HZO epidemiology.4 A retrospective study of a single site tertiary care center suggested an increase in new HZO cases and an age shift towards younger individuals from 2007 to 2013; however, the referral bias inherent to this type of study makes it difficult to estimate if there has been a change in HZO over time.14 Overall, there is a lack of knowledge on HZO incidence rates and whether they are shifting.
Given the significant morbidity associated with HZO, studies on HZO epidemiology are crucial for informing public health policy and directing clinician efforts. In particular, the introduction of the vaccines for HZ emphasizes the need to understand who is at greatest risk of HZO and how these trends are changing over time. The objective of this study was to examine the incidence rate of HZO in the American population from 1994 to 2018 and to assess by age, race, sex, and geographic region.
Methods
A retrospective, observational cohort study was conducted on over 200 million records in the de-identified healthcare claims database, OptumLabs ® Data Warehouse15. OLDW is a real-world data asset that contains administrative claims and electronic health record (EHR) data for US patients enrolled in commercial insurance, Medicare Advantage, or Medicare Part D plans. Comparisons between the OLDW and US Census Bureau show that the age, sex, race, and geographic distributions of OLDW enrollees are similar to those of the US population for both the commercially insured and the Medicare Advantage groups.16 There is less representation in the West for Medicare Advantage enrollees in OLDW compared to US Census estimates with fairly comparable representation in all other regions.15
To be included in the study, enrollees must have been continuously enrolled for 365 or more days, and not have a history of HZ and HZO. Between January 1, 1994 and December 31, 2018, over 63 million unique people were represented in the OLDW. Cases of HZ and HZO were identified by using International Classification of Disease (ICD) 9th and 10th revision codes. Patients with one new code for HZ (ICD-9 053.XX; ICD-10 B02.XX) and HZO (ICD-9 0532.X; ICD-10 B023.X) were counted as an incident case. Information was extracted on the patients’ year of birth, sex, race (Asian, African American, Hispanic, Caucasian, Other), and geographic region (Midwest, Northeast, South, West, and Other). The exact date of birth is not available through OLDW. Therefore, age was approximated by year of birth (i.e. a patient categorized as 1 year of age in 1994 experienced their 1-year birthday in 1994). Data were not available for individuals born before 1930.
The time trend in the overall incidence was assessed through Huber robust regression of the estimated annual incidence rate on calendar year, using ten-year age-time intervals and broken-stick models of the time ranges from 1994 to 2007 and 2008 to 2018, adjusting for age.17 The specific time ranges were established to examine HZO incidence rates prior to and after the Advisory Committee on Immunization Practices’ (ACIP) recommendation for the ZVL vaccine in 2008.18 A sensitivity analysis using 2006 (FDA approval of ZVL) as the cut point was conducted. When comparing sex, race/ethnicity, and region, we calculated the incidence rate ratio using bootstrap resampling of years to compute approximate 95% confidence intervals (a highly conservative procedure which avoids relying on the very large number of person-years as a basis for inference).
Between 1994 and 2018, the incidence rate of HZO was calculated by year, 10-year age groups, 10-year birth cohorts, sex, race, and geographic region. Data were stratified into 10-year age groups starting at less than 1 year of age. Age groups were also aggregated into 10-year age groups to tabulate changes in the slope of HZO incidence overall and by sex from the periods 1994 to 2007 and 2008 to 2018. As a sensitivity analysis, we also standardized the incidence rates by age, sex, and race/ethnicity, using the average of the years 2014 to 2018 in the OLDW database. Standardized incidence rates were analyzed in the same way using broken-stick Huber robust regression with adjustments for age. Statistical significance testing was conducted at a level of 0.01.
All statistical analyses were conducted in R (Version 3.5, The R Project for Statistical Computing, Vienna, Austria, http:/www.r-project.org). Only de-identified data were available for analysis. The University of California San Francisco Institutional Review Board approval was obtained and the described research adheres to the tenets of the Declaration of Helsinki.
Results
From January 1, 1994 to December 31, 2018, 633,474 cases of HZ were reported of which 49,745 (7.9%) included a code specific for HZO. The incidence rate of HZO increased from 1994 to 2018 by an estimated 1.1 cases per 100,000 person-years annually (95% CI 1.0 to 1.3, P<0.001). The estimated relative increase in the HZO incidence rate was 3.6% per year (95% CI: 3.0% to 4.1%). For individuals below 50 years of age with known race (representing 88% of the available person-years), the standardized incidence rate increased an average of 0.31 cases per 100,000 person-years (95% CI: 0.20 to 0.41) from 1994 to 2018. For individuals over the age of 70, the standardized incidence rate changed an average of −0.86 cases per 100,000 person-years (95% CI: −1.36 to −0.37) from 2010 to 2018.
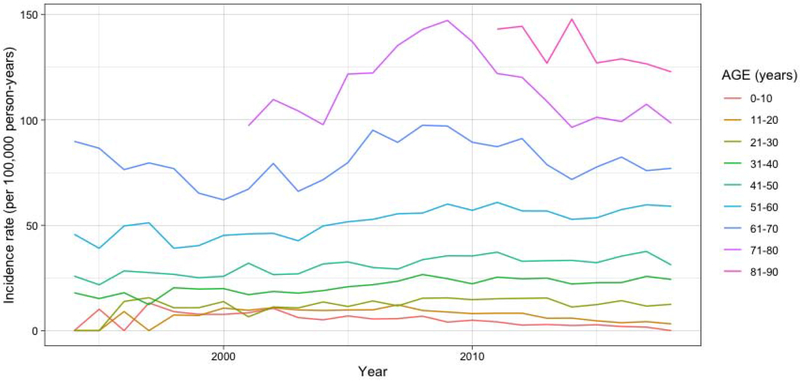
Figure 1 demonstrates the IR of HZO by 10-year age groups from 1994 to 2018. The age-specific IRs changed from 1994 to 2007 compared to 2008 and beyond, which is further explained in Table 1. Using broken-stick regression, the slope of the HZO incidence rate shifted from −0.24 (95% CI: −0.36 to −0.11) to −0.37 (95% CI: −0.52 to −0.22) per 100,000 person-years per year in individuals in the 0 to 10 age group, 0.74 (95% CI: 0.47 to 1.00) to 0.14 (95% CI: −0.17 to 0.46) in the 41 to 50 age group, and 6.23 (95% CI: 5.05 to 7.40) to −2.26 (95% CI: −2.96 to −1.57) in individuals aged 61 to 70 (Table 1). The changes in the slopes of HZO incidences among all age groups from 1994 to 2007 compared to 2008 to 2018 were statistically significant except for in children between 0 to 10 years old (P=0.56). Using 2006 as the cut point did not change the overall trends in HZO incidence rate. Results were similar based on the standardized incidence rates, except that there was no statistically significant evidence of a change in slope for the age group 31 to 40 years old (P=0.24).
Figure 1.
Incidence rate of HZO by ten-year age groups from 1994 to 2018
Table 1:
Slopes of Herpes Zoster Ophthalmicus (HZO) Incidence Rates per year from 1994 to 2018 by 10-year Age Groups
| Year Range | |||||||
|---|---|---|---|---|---|---|---|
| 1994-2007 | 2008-2018 | ||||||
| Age (years) | Slope of HZO incidence ratea | 95% CI | Slope of HZO incidence rate | 95% CI | P-valueb | ||
| 0-10 | −0.24 | −0.36 | −0.11 | −0.37 | −0.52 | −0.22 | 0.56 |
| 11-20 | 0.49 | 0.35 | 0.63 | −0.74 | −0.91 | −0.57 | <0.001 |
| 21-30 | 0.43 | 0.23 | 0.63 | −0.04 | −0.29 | 0.20 | 0.002 |
| 31-40 | 0.59 | 0.39 | 0.80 | 0.18 | −0.07 | 0.43 | 0.001 |
| 41-50 | 0.74 | 0.47 | 1.00 | 0.14 | −0.17 | 0.46 | <0.001 |
| 51-60 | 1.18 | 0.81 | 1.55 | 0.31 | −0.14 | 0.76 | <0.001 |
| 61-70 c | 6.23 | 5.05 | 7.40 | −2.26 | −2.96 | −1.57 | <0.001 |
| 71+ d | - | - | - | −3.76 | −4.95 | −2.56 | - |
Change in HZO incidence rate per 100,000 person-years per year.
The P-value corresponds to comparison of slopes between the two time intervals.
The slope was computed from 2000 to 2007 given that the available data does not include individuals born prior to 1930 and the full age range could not be captured until 2000.
The slope was not available for ages 71+ for the first time period from 1994 to 2007 because the available data does not include individuals born prior to 1930.
Key: CI = Confidence Interval; HZO = Herpes Zoster Ophthalmicus.
Table 2 shows that HZO IR increased by 10-year age group, starting with an average of 4.8 cases per 100,000 person-years in children ages 1 to 10 and rising to 131.6 in ages 81 to 90 from 1994 to 2018. IR also increased by decade of birth, with the oldest cohort, born in 1930 to 1939, having the highest average IR of 115.7 cases per 100,000 person-years and individuals born in 2010 to 2019 having an IR of 2.1 cases per 100,000 person-years from 1994 to 2018.
Table 2:
Incidence Rates of Herpes Zoster Ophthalmicus by Age Group, Decade of Birth, Sex, Race and Region from 1994 to 2018
| Cases | Person-Years | Incidence Ratea | |
|---|---|---|---|
| Age Group (years) | |||
| 0-10 | 638 | 13,301,196 | 4.8 |
| 11-20 | 1,305 | 16,767,397 | 7.8 |
| 21-30 | 1,768 | 13,357,953 | 13.2 |
| 31-40 | 4,243 | 18,900,676 | 22.4 |
| 41-50 | 6,999 | 21,701,978 | 32.3 |
| 51-60 | 10,732 | 19,660,935 | 54.6 |
| 61-70 | 10,449 | 12,796,084 | 81.7 |
| 71-80 | 10,030 | 8,834,445 | 113.5 |
| 81-90 | 3,581 | 2,721,988 | 131.6 |
| Unknown | 0 | 2,195 | 0.0 |
| Decade of Birth | |||
| 1930-1939 | 12,021 | 10,390,005 | 115.7 |
| 1940-1949 | 11,652 | 14,780,355 | 78.8 |
| 1950-1959 | 10,653 | 20,624,339 | 51.7 |
| 1960-1969 | 7,485 | 21,497,818 | 34.8 |
| 1970-1979 | 3,903 | 17,258,873 | 22.6 |
| 1980-1989 | 2,178 | 15,020,071 | 14.5 |
| 1990-1999 | 1,420 | 16,063,776 | 8.8 |
| 2000-2009 | 391 | 10,447,890 | 3.7 |
| 2010-2019 | 42 | 1,959,525 | 2.1 |
| Unknown | 0 | 2,195 | 0.0 |
| Sex | |||
| Female | 28,937 | 65,077,059 | 44.5 |
| Male | 20,653 | 62,390,951 | 33.1 |
| Otherb | 155 | 576,837 | 26.9 |
| Race | |||
| Asian | 1,404 | 4,674,280 | 30.0 |
| African American | 3,456 | 10,739,351 | 32.2 |
| Hispanic | 2,958 | 10,748,465 | 27.5 |
| Caucasian | 35,089 | 81,371,550 | 43.1 |
| Otherc | 1,660 | 5,899,176 | 28.1 |
| Unknown | 5,178 | 14,612,025 | 35.4 |
| Region | |||
| Midwest | 15,034 | 37,808,500 | 39.8 |
| Northeast | 6,284 | 12,683,745 | 49.5 |
| South | 19,898 | 52,400,469 | 38.0 |
| West | 6,015 | 17,151,919 | 35.1 |
| Otherd | 2,514 | 8,000,214 | 31.4 |
Incidence rate is the number of cases per 100,000 person-years.
Other sex is defined as individuals that had both male and female or neither gender listed.
Other race is defined as individuals that were of a race that was not one of the four listed races.
Other region is defined as individuals that had two or more regions or neither region listed.
Compared to females, males had a lower overall HZO incidence rate ratio (IRR) of 0.74 (95% CI: 0.72 to 0.76). For both sexes over the age of 10, the HZO IR slopes increased from 1994 to 2007 and shifted among different age groups from 2008 to 2018 (Table 3). Compared to Caucasians, Asians had an IRR of 0.70 (95% CI: 0.67 to 0.73), African-Americans had an IRR of 0.75 (95% CI: 0.72 to 0.79), and Hispanics had an IRR of 0.64 (95% CI: 0.60 to 0.67). Compared to the Northeast, the Midwest had an IRR of 0.80 (95% CI: 0.75 to 0.85), the West had an IRR of 0.71 (95% CI: 0.66 to 0.76), and the South had an IRR of 0.77 (95% CI: 0.71 to 0.81).
Table 3:
Slopes of Herpes Zoster Ophthalmicus Incidence Rates per year from 1994 to 2018 by 10-year Age Groups and Sex
| Year Range | |||||||
|---|---|---|---|---|---|---|---|
| 1994-2007 | 2008-2018 | ||||||
| Gender | Age (years) | Slope of HZO incidence ratea | 95% CI | Slope of HZO incidence rate | 95% CI | ||
| Male | 0-10 | −0.01 | −0.18 | 0.15 | −0.47 | −0.67 | −0.27 |
| 11-20 | 0.60 | 0.41 | 0.78 | −0.80 | −1.03 | −0.58 | |
| 21-30 | 0.57 | 0.30 | 0.83 | 0.04 | −0.28 | 0.35 | |
| 31-40 | 0.42 | 0.15 | 0.70 | 0.15 | −0.18 | 0.48 | |
| 41-50 | 0.79 | 0.45 | 1.13 | 0.03 | −0.39 | 0.44 | |
| 51-60 | 1.42 | 1.01 | 1.83 | 0.05 | −0.44 | 0.55 | |
| 61-70b | 4.69 | 3.31 | 6.06 | −1.87 | −2.69 | −1.05 | |
| 71+ c | − | − | − | −4.35 | −6.00 | −2.69 | |
| Female | 0-10 | −0.06 | −0.20 | 0.09 | −0.29 | −0.47 | −0.12 |
| 11-20 | 0.63 | 0.45 | 0.81 | −0.72 | −0.93 | −0.50 | |
| 21-30 | 0.78 | 0.51 | 1.04 | −0.24 | −0.56 | 0.08 | |
| 31-40 | 0.91 | 0.61 | 1.21 | 0.19 | −0.17 | 0.55 | |
| 41-50 | 0.78 | 0.38 | 1.17 | 0.21 | −0.26 | 0.69 | |
| 51-60 | 1.14 | 0.56 | 1.72 | 0.59 | −0.11 | 1.29 | |
| 61-70b | 7.69 | 5.91 | 9.46 | −2.69 | −3.75 | −1.63 | |
| 71+ c | - | - | - | −3.26 | −5.02 | −1.50 | |
Change in HZO incidence rate per 100,000 person-years per year.
The slope was computed from 2000 to 2007 given that the available data does not include individuals born prior to 1930 and the full age range could not be captured until 2000.
The slope was not available for ages 71+ for the first time period from 1994 to 2007 for both men and women because the available data does not include individuals born prior to 1930.
Key: CI = Confidence Interval; HZO = Herpes Zoster Ophthalmicus.
Discussion
From 1994 to 2018, the HZO incidence rate increased 3.6% per year in this study using a large US administrative real world data set. Prior to the ACIP recommendation for ZVL in 2008, HZO incidence rates were decreasing in children between 0 to 10 years of age and increasing in all older age groups. However, from 2008 and beyond, HZO incidence rates began to significantly decline among individuals younger than 21 and older than 60 years old. HZO incidence rates began stabilizing in 21 to 30-year olds and continued to increase, but at a less significant rate among individuals between 31 to 60 years of age.
In children less than 10 years old, the incidence of HZO cases was decreasing from 1994 to 2007 but dropped more precipitously from 2008 to 2018. The decreasing incidence rates may be secondary to the introduction of the varicella vaccine in 1996 and the continued rapid decline in the past decade may be associated with the implementation of the two-dose varicella vaccination program in 2006. Varicella vaccination is widespread with over 80% of children over 7 years old having two-dose coverage in 2012.9 This decline in HZO in young children is similar to the HZ trends published in a 2018 study by the CDC that found declining rates of HZ among children less than 18 years old from 1998 to 2016.9 In a multi-database study from 2003 to 2014 looking at varicella vaccination, researchers found that HZ incidence declined by 72% among 0 to 17 year olds.19 The decline in HZO incidence since 2008 in individuals older than 60 years of age raises the question of whether the zoster vaccine is having an impact in this age group. The CDC report noted a deceleration in HZ cases among a similar older age group.8 Zoster vaccine coverage is thought to be low, with an upper estimate of 31 % in 2015, but at this level, it may be having an impact on incidence in the age group that is vaccine eligible.20
HZO incidence in the 31 to 60-year-old groups has continued to increase since 2008, although at a slower rate than prior to that time. The decreasing HZO incidence rates among the younger and older population suggests that a larger proportion of new HZO cases may be occurring in individuals between 31 to 60 years old since the overall rate continues to increase. Although changes in incidence are difficult to determine from retrospective studies from single centers, one study reported that HZO cases were increasing among individuals younger than 50 years of age, with the percentage of all HZO cases going from 16.2% between 1996 to 2004 to 29.6% between 2005 to 2012 in this age group.12 Another study from a tertiary care center found that the average age of HZO onset went from 61 years old in 2007 to 56 years old in 2013.14 The increase in HZO incidence among the middle age groups parallels the recent CDC report that found a continued increase in HZ rates among those between 35 to 55 years of age.8
It is not possible to attribute the changing HZ and HZO epidemiology to a particular cause with an observational study, but there are several potential explanations. One theory is that intermittent exposure to wild-type varicella helped boost one’s natural immunity and that universal varicella vaccination has decreased such exposure, leading to waning immunity and reactivation of the varicella virus at a younger age.2,11,21,22 Yet, studies in Canada and the United Kingdom from a time when there was no comprehensive varicella vaccination program in those countries demonstrated an increase in overall HZ incidence, suggesting that there may be additional factors in effect.23 Others have theorized that the live virus within the varicella vaccine has led to increasing cases of HZ but this has been proven unlikely as the IRs for HZ were similar prior to and after the introduction of the vaccine.13
The continued rise in HZO in the 31 to 60-year-old groups raises questions about the age recommendations for HZ vaccination. In 2017, RZV was approved by the FDA for adults without contraindications who are 50 years of age or older. Soon after, the ACIP adopted this more efficacious vaccine as the preferred one for this age group.24 The American Academy of Ophthalmology (AAO) also recommends RZV in accordance with the FDA approval.25 ZVL coverage has been historically low with only 0.9% to 31.8% coverage by state in 2014 and 31% nationwide in 2015.20,26 Coverage data for RZV is not yet known due to its recent approval. Given the changing epidemiological trends and risks associated with HZO, it is crucial to continue advocating for HZ vaccination in eligible adults.27 In addition, there is a need to further study whether HZ vaccination is indicated in individuals younger than 50.
This study demonstrated that females and Caucasians are at higher risk of developing HZO. Through 2007, HZO incidence rates were increasing for both males and females greater than 10. Since 2008, the incidence rates have been declining in the youngest and oldest age groups for both males and females. A previous study from Hawaii failed to find evidence of a difference in HZO incidence by sex, but did not stratify by age. The same study also showed that HZO was more common among non-Pacific Islanders but did not further differentiate by race and ethnicity.28 The risk of HZO by sex and race aligns with studies on HZ that found that women and Caucasians are at higher risk.13,29,30
HZO rates were found to be highest in the Northeastern United States and lowest in the West, which may have some relation to ZVL coverage. A 2014 analysis demonstrated that vaccination rates were the second lowest in the Northeast at 30.3% and highest in the West at 37.4%.26 There is little information available on the role of geographic location and risk of HZ or HZO, although some reports have indicated a possible association between ultraviolet exposure and HZ risk.31 The correlation between HZO and geographic region seen in this study is hypothesis-generating and requires further study.
This study has certain limitations. The observational nature of this analysis complicates any measures of causation from being made in relation to the trends seen in HZO incidence, given potential unmeasured confounders. We are not able to compare HZO incidence between the time periods before and after the introduction of the varicella vaccine in 1996, which would have provided additional clarification on changing HZO patterns. Analyses were conducted at the level of aggregate data and cannot be used to infer individual level changes in risk. Different choices of cut point for the broken-stick regression or the use of more general polynomial trend models would result in modest differences in estimated HZO incidence rate slopes. Statistical estimates of trends in the youngest age groups are less reliable, due to the small numbers of cases. Due to the focus on patients in commercial healthcare programs, Medicare Part D and Medicare Advantage, the presented data may underestimate the true incidence of HZO if some patients left the healthcare plan at age 65. However, this would have been the case throughout the study period and would not affect the ability to detect a change in incidence. The database also does not account for individuals that are either uninsured or have other forms of insurance. However, OLDW is relatively generalizable to the US population given the demographic similarities to the US Census. Coding for HZO diagnosis has been found to be highly accurate, but undercoding for HZO or changes in health-seeking behavior could affect results.32 This study counted a single code for HZO as an incident diagnosis if a patient met the enrollment eligibility criteria. This criterion was utilized in order to include patients who may not have returned for a subsequent evaluation after their initial diagnosis of HZO, which may occur particularly when there is no intraocular involvement. Overall, such limitations would be unlikely to fully account for the magnitude of change seen in the HZO incidence rate.
Conclusions
Following the ACIP recommendation for ZVL in 2008, HZO rates have declined in the youngest and oldest age groups while continuing to increase among individuals between 31 to 60 years of age. Given the potential shift in HZO burden towards middle-aged individuals, it is crucial for clinicians to support vaccination efforts for individuals 50 years of age and older. These results also raise the question of whether HZ vaccine recommendations should be re-evaluated for individuals in younger age groups.
Acknowledgements:
The authors wish to thank Nina Veeravalli from OptumLabs for her extensive help with review of the data.
Financial Support: National Institutes of Health (NIH), Bethesda, Maryland; NIH Grants 1R01 EY028739-01, 5R01 EY028739-02. The NIH had no role in the design or conduct of this research. OptumLabs Warehouse research credit through OptumLabs ®, Cambridge, Massachussetts, OptumLabs Visiting Fellow. OptumLabs participated in the review of the manuscript.
Abbreviations and Acronyms:
- ACIP
Advisory Committee on Immunization Practices
- CDC
Centers for Disease Control
- FDA
Food and Drug Administration
- HZ
herpes zoster
- HZO
herpes zoster ophthalmicus
- ICD
International Classification of Disease
- IR
incidence rate
- OLDW
Optum Labs Data Warehouse
- RZV
recombinant subunit vaccine
- US
United States
- ZVL
zoster vaccine live
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Ocular Microbiology and Immunology Group Meeting, San Francisco, 2019.
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Med. 1982;61(5):310–316. [DOI] [PubMed] [Google Scholar]
- 2.Liesegang TJ. Herpes zoster ophthalmicus: natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 suppl.):3–12. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg JM. Herpes zoster: Epidemiology, natural history, and common complications. J Am Acad Dermatol. 2007;57(6 suppl):130–135. [DOI] [PubMed] [Google Scholar]
- 4.Yawn BP, Wollan PC St. Sauver JL, Butterfield LC. Herpes zoster--eye complications: rates and trends. PLoS One. 2013;32(7):736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nithyanandam S, Stephen J, Joseph M, Dabir S. Factors affecting visual outcome in herpes zoster ophthalmicus: a prospective study. Clin Exp Ophthalmol. 2010;38(9):845–850. [DOI] [PubMed] [Google Scholar]
- 6.Vrcek I, Choudhury E, Durairaj V. Herpes zoster ophthalmicus: a review for the internist. Am J Med. 2017;130(1):21–26. [DOI] [PubMed] [Google Scholar]
- 7.Tran KD, Falcone MM, Choi DS, et al. Epidemiology of herpes zoster ophthalmicus recurrence and chronicity. Ophthalmology. 2016;123(7):1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis. 2018;(404). [DOI] [PubMed] [Google Scholar]
- 9.Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among children. Clin Infect Dis. 2018;(404). [DOI] [PubMed] [Google Scholar]
- 10.Wolfson LJ, Daniels VJ, Altland A, Black W, Huang W, Ou W. The impact of varicella vaccination on the incidence of varicella and herpes zoster in the United States: updated evidence from observational databases, 1991–2016 [published online ahead of print, April 24, 2019]. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz305. [DOI] [PubMed] [Google Scholar]
- 11.Goldman GS. Cost-benefit analysis of universal varicella vaccination in the U.S. taking into account the closely related herpes-zoster epidemiology. Vaccine. 2005;23(25):3349–3355. [DOI] [PubMed] [Google Scholar]
- 12.Chan AY, Conrady CD, Ding K, Dvorak JD, Stone DU. Factors associated with age of onset of herpes zoster ophthalmicus. 2012;34(5):535–540. [DOI] [PubMed] [Google Scholar]
- 13.Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis. 2016;63(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies EC, Pavan-Langston D, Chodosh J. Herpes zoster ophthalmicus: declining age at presentation. Br J Ophthalmol. 2016;100(3):312–314. [DOI] [PubMed] [Google Scholar]
- 15.OptumLabs. OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation 2018. Edition. Cambridge, MA: OptumLabs. [Google Scholar]
- 16.OptumLabs. Optum Research Data Assets. Cambridge, MA: OptumLabs; https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf Published 2014. Accessed September 20, 2019. [Google Scholar]
- 17.Sen A, Srivastava M. Regression Analysis: Theory, Methods and Applications. Berlin, Heidelberg: Springer; 1990. [Google Scholar]
- 18.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2008;57(RR-5):1–43. [PubMed] [Google Scholar]
- 19.Weinmann S, Naleway AL, Koppolu P, et al. Incidence of herpes zoster among children: 2003-2014. Pediatrics. 2019;144(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations-United States, 2015. MMWR Surveill Summ. 2017;66(11):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28(11):954–959. [DOI] [PubMed] [Google Scholar]
- 22.Donahue JG, Kieke BA, Gargiullo PM, et al. Herpes zoster and exposure to the varicella zoster virus in an era of varicella vaccination. Am J Public Health. 2010;100(6):1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds MA, Chaves SS, Harpaz R, Lopez AS, Seward JF. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. J Infect Dis. 2008; 197(s2):S224–S227. [DOI] [PubMed] [Google Scholar]
- 24.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Policy statement: recommendations for herpes zoster vaccine for patients 50 years of age and older. Ophthalmology. 2018;125(11):1813–1816. [Google Scholar]
- 26.Lu PJ, O’Halloran A, Williams WW, Harpaz R. National and state-specific shingles vaccination among adults aged >60 years. Am J Prev Med. 2017;52(3):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen EJ. Prevention of herpes zoster: we need to do better. JAMA Ophthalmol. 2013;131 (3):396–398. [DOI] [PubMed] [Google Scholar]
- 28.Borkar DS, Tham VM, Esterberg E, et al. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. PLoS One. 2017;32(7):736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yawn BP, Saddier P, Wollan PC St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. [DOI] [PubMed] [Google Scholar]
- 31.Zak-Prelich M, Borkowski JL, Alexander F, Norval M. The role of solar ultraviolet irradiation in zoster. Epidemiol Infect. 2002;129(3):593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pimentel MA, Browne EN, Janardhana PM, et al. Assessment of the accuracy of using ICD-9 codes to identify uveitis, herpes zoster ophthalmicus, scleritis, and episcleritis. JAMA Ophthalmol. 2016;134(9):1001–1006. [DOI] [PubMed] [Google Scholar]