Abstract
Background and Aims
Benign gastric outlet obstruction (GOO) has typically been managed surgically. However, many patients are poor operative candidates due to comorbidities. EUS-guided gastroenterostomy (EUS-GE) using lumen apposing metal stents (LAMSs) has previously demonstrated efficacy as definitive treatment for benign and malignant GOO; however, limited data exist on use as a bridge to resolution of the obstruction in an attempt to avoid or delay definitive surgery.
Methods
Retrospective series of consecutive patients who underwent EUS-GE between January 2013 and July 2019 for benign GOO at a tertiary referral center. The primary outcome was rate of definitive surgery; secondary outcomes included technical success and rate of adverse events.
Results
During the study period, 22 patients with benign GOO underwent EUS-GE (40% female, mean age 54.2). Mean procedure time was 66 minutes and technical success was achieved in 21. Five patients developed recurrent GOO while the LAMS was in place after a mean dwell time of 228 days; one patient was converted to surgical GE. LAMSs were removed electively in 18 patients after GOO resolution and mean dwell time of 270 days; one patient developed a recurrent GOO after LAMS removal and was converted to surgical GE. Rate of recurrent GOO after LAMS removal was 5.6%. Three severe adverse events occurred in the cohort.
Conclusions
EUS-GE was able to prevent surgery for GOO in 83.3% of cases. LAMSs needed to stay in place for a mean of 8.5 months to allow resolution of GOO, and there was a low rate of recurrent GOO (5.6%) after LAMS removal. Prospective, randomized trials comparing surgical with endoscopic anastomoses are needed in patients with benign causes of GOO.
Keywords: Endoscopic ultrasound, gastroenterostomy, gastrojejunostomy
BACKGROUNDS AND AIMS
Until the 1970s, benign disease comprised the majority of cases of gastric outlet obstruction (GOO), with common etiologies including peptic ulcer disease, caustic ingestion, pancreatitis, and extraluminal fluid collections.1 In more recent years, malignant disease has become the dominant etiology of GOO. However, 20% to 50% of cases continue to be due to benign disease.2, Regardless of etiology, GOO leads to vomiting, dehydration, and malnutrition and as a result requires relief of obstruction.3
Benign GOO has typically been managed endoscopically with balloon dilation of stenotic lesions. Intraluminal stent placement is a poor option for benign disease as there are no commercially available stents for this indication, the lack of removability and lack of long-term patency when uncovered stents are used, and high rates of reintervention due to migration when covered stents are used. Many benign strictures respond well to balloon dilation, but require an average of three endoscopic sessions before clinical success is achieved.4 GOO from extrinsic compression, such as in pancreatic fluid collections are not amenable to dilation and require either drainage of the collection or supportive care while the collection resolves.5 Due to the poor quality of life and worse clinic outcomes associated with GOO, patients often require gastroenteric bypass and cannot wait the required time for multiple dilations or spontaneous resolution of the obstruction.6
Surgical gastroenterostomy alters the upper gastrointestinal tract anatomy in order to provide definitive management of GOO; however, it is associated with delayed onset of oral intake and extended length of hospital stay when compared to endoscopic treatment when used for patients with malignant GOO.7 Further, surgical management is associated with a significant morbidity and many patients are poor operative candidates due to medical comorbidities.8,9 Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) entails placing a lumen-apposing metal stent (LAMS) between the stomach and the small bowel, distal to the site of obstruction, thereby bypassing the obstruction and regaining antegrade enteral flow. The first experiences using EUS-GE in patients with GOO demonstrated a high technical and clinical success rate.10 Subsequent studies have focused on EUS-GE as the definitive therapy for the obstruction with no plans for LAMS removal unless stent malfunction occurred.11
Because many causes for benign GOO are reversible, both surgical gastroenterostomy and permanent placement of a LAMS may not be required. Use of EUS-GE as a temporizing method in order to provide sufficient time for the obstruction to resolve could allow patients to avoid surgery; however, limited data exist on this approach. The current study aims to assess the efficacy of EUS-GE as a bridge therapy in order to allow for resolution of benign GOO and thereby avoid surgical intervention.
METHODS
This was a retrospective study of patients who underwent EUS-GE at a large tertiary referral center between January 2013 and January 2019, with follow-up data until July 2019. Consecutive patients who underwent EUS-GE for benign GOO were included. Exclusion criteria included age less than 18 years, malignant GOO, and lack of postprocedural follow-up. A total of 5 patients have been reported in prior publications.10,12 All patients provided written informed consent and were aware of off-label LAMS use. Data from electronic medical records were abstracted and included demographics, etiology of GOO, site of obstruction, prior endoscopic stent placement or dilation, procedure time, type, size and number of stent(s) used, technical and clinical success, postprocedure length of stay, need for reinterventions, and adverse events (AEs). The institutional review board approved this study and the retrospective review of this data.
Study Endpoints
The primary outcome of interest was rate of definitive surgery; secondary outcomes of interest included technical success rate, clinical success rate and AE rate and severity. Rate of definitive surgery was defined as need for conversion to a surgical gastroenterostomy on a per-patient basis. Technical success was defined as adequate positioning and deployment of the stent as determined by endoscopy and fluoroscopy. Clinical success was defined as the ability to tolerate at least a full liquid diet during follow-up. Severity of AEs was graded according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon.13
EUS-GE Technique
All procedures were performed by 1 of 2 therapeutic endoscopists experienced in therapeutic EUS and LAMS placement with or without trainee involvement. Patients were under general anesthesia with endotracheal intubation given risk of aspiration due to underlying GOO. A LAMS (Axios, Boston Scientific; Marlborough, Mass, USA) with or without a cautery tip was used in all cases. Several different approaches were used to identify the targeted jejunal loop: orojejunal tube-assisted,10 balloon-assisted,14 or fluid instillation followed by free-hand direct approach.15 Under fluoroscopic and EUS-guidance, an optimal area of a small bowel loop adjacent to the stomach was identified with a therapeutic linear echoendoscope (GF-UCT180; Olympus America, Center Valley, Pa, USA).
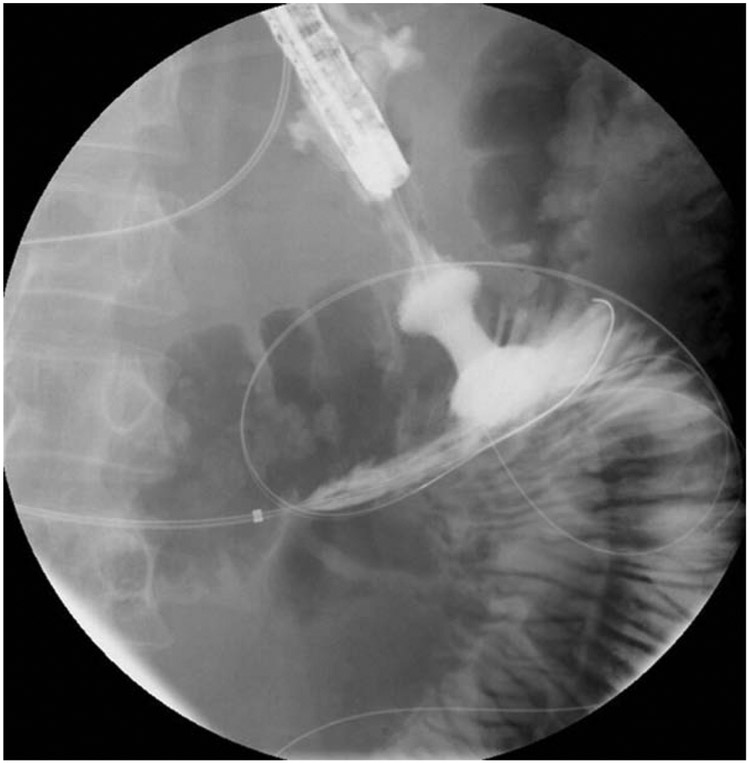
If “freehand” entry into the small bowel was not used, the small bowel was targeted using a 19-gauge needle to puncture the stomach wall and small bowel under EUS guidance. Using echosonographic and fluoroscopic guidance, we passed a 0.025-inch guidewire through the needle and into the small bowel Figure 1. Before the advent of electrocautery-enhanced LAMSs, the anastomotic tract required dilation in order to accommodate the stent. When the electrocautery system became available, dilation was not required before stent deployment and the device passed over the wire using pure cutting current.
Figure 1.
0.025” guidewire passing into the small bowel using echosonographic and fluoroscopic guidance.
Statistical Analysis
Continuous variables were reported as means with standard deviations (SD) or, for skewed data, medians with interquartile ranges (IQR). Comparison of linear variables was performed with the t-test and categorical variables by using the chi-square test. A level of significance of P <0.05 was adopted for all inferential testing. Statistical analysis was performed using STATA version 15.1 (StataCorp LLC; College Station, Tex, USA).
RESULTS
During the study period, 53 patients underwent EUS-GE, 22 for benign disease. There were 9 women (mean age 54.2 ± 13.4 years). Four patients (18.2%) had surgically altered anatomy; 2 Roux-en-Y gastrojejunostomy, 1 Billroth I, and 1 Billroth II. American Society of Anesthesiologists (ASA) status was 2 (n=4, 18.2%), 3 (n=15, 68.2%), and 4 (n=3, 13.6%), and 6 patients (27.3%) were outpatients at the time of EUS-GE. Etiology of GOO was peptic stricture (n=5, 22.7%), anastomotic stricture (n=4, 18.2%), duodenal hematoma (n=3, 13.6%), recurrent acute pancreatitis (n=1, 4.5%), chronic pancreatitis (n=4, 18.2%), pancreatic pseudocyst (n=1, 4.5%) and walled off pancreatic necrosis (n=4, 18.2%). Eleven patients (50%) had failed prior endoscopic treatment, including endoscopic dilation alone (n=3, 13.6%), endoscopic dilation with stenting (n=2, 9.1%), pancreatic pseudocyst drainage or necrosectomy (n=5, 22.7%) and percutaneous endoscopic gastroenterostomy and jejunostomy tube placement (n=1, 4.5%). Patient demographic data are shown in Table 1.
Table 1.
Patient demographics
| EUS-gastroenterostomy in benign gastric outlet obstruction (n=22) | ||
|---|---|---|
| Age, mean ± SD | 54.2 | 13.4 |
| Women, no. (%) | 9 | 40.9% |
| Surgically altered anatomy | ||
| · Roux-en-Y Gastrojejunostomy | 2 | |
| · Billroth I | 1 | |
| · Billroth II | 1 | |
| Prior unsuccessful endoscopic treatment, no. (%) | 11 | 50.0% |
| Etiology of gastric outlet obstruction | ||
| · Peptic stricture | 5 | |
| · Anastomotic stricture | 4 | |
| · Duodenal hematoma | 3 | |
| · Recurrent acute pancreatitis | 1 | |
| · Chronic pancreatitis | 5 | |
| · Pancreatic pseudocyst | 1 | |
| · Walled-off pancreatic necrosis | 3 |
Procedure
The technique to target the small bowel was orojejunal tube-assisted water instillation in 5 (22.7%), balloon-assisted in 8 (36.4%), and fluid instillation with freehand puncture using electrocautery was performed in 9 (40.9%). Mean procedure time was 66 ± 33.5 minutes and technical success was achieved in 21 (95.4%). One technical failure occurred in a patient with duodenal stenosis from chronic pancreatitis. In this case, the small bowel was too far from stomach to allow needle access to the bowel; this patient proceeded to single balloon–assisted jejunal feeding tube placement. Anastomoses were gastroduodenal in 5 patients (23.8%) and gastrojejunal in 16 patients (76.2%). Stent diameters were 15 mm (16 patients, 76.2%), and 20 mm (5 patients, 23.8%). Two patients (9.5%) required a second overlapping stent to span the distance between stomach and small bowel. Of 6 outpatient procedures, 2 patients (33.3%) were admitted for monitoring after the procedure, and the other 4 (66.6%) remained as outpatients. Inpatients were hospitalized for a mean of 7.5 days after the procedure (SD ± 7.9).
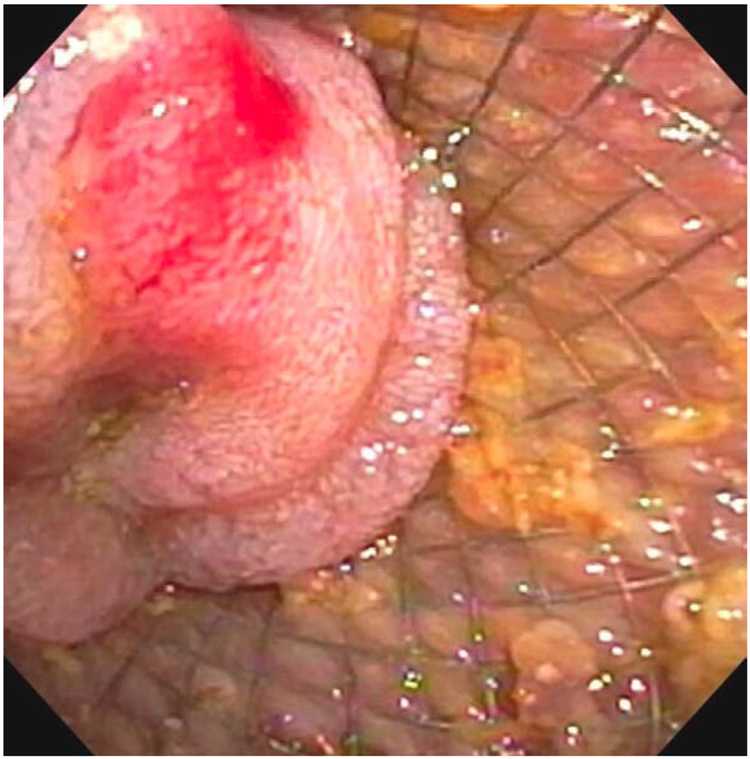
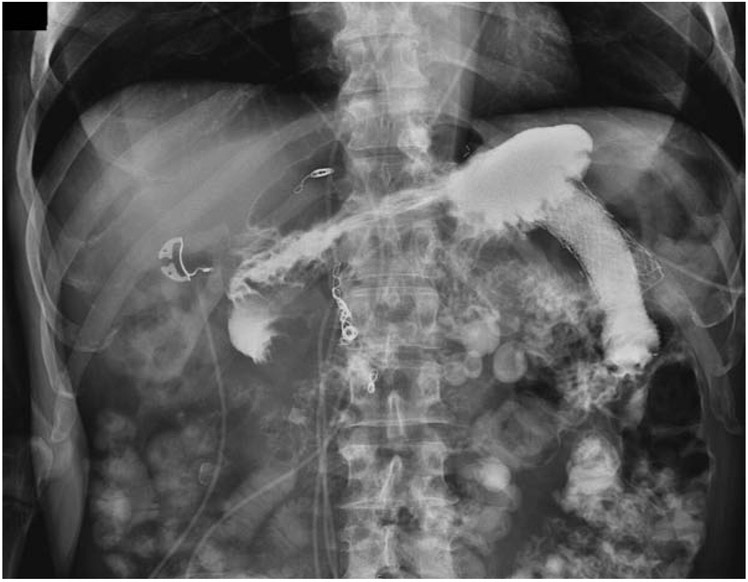
Four total AEs (19%) occurred in this cohort with one being mild abdominal pain that resolved without intervention and did not require hospitalization. Three severe AEs (14.3%) occurred in the cohort. One patient developed bleeding from a gastric ulcer at the anastomotic site on postprocedure day 2 Figure 2. This was managed with an ICU admission, endoscopy for treatment epinephrine injection and argon plasma coagulation of the bleeding tissue with ultimately a favorable outcome without need for stent removal. The second severe AE occurred one year after the procedure in a patient whose LAMS spontaneously migrated to the level of the proximal ileum, leading to a small-bowel obstruction. Attempts to remove this endoscopically were unsuccessful and ultimately required laparotomy for removal with of the stent; the GOO had resolved at this point and surgical bypass was not required. The third severe AE occurred in a patient with necrotizing pancreatitis requiring multiple necrosectomies. In this patient, the gastrojejunostomy stents were found to traverse from the stomach through the colon and into the jejunum. Despite this mispositioning, an upper GI series demonstrated no evidence of contrast leaking around the stent proximally or distally (Figure 3), and he tolerated a regular diet with plans for surgical revision at a later date, once optimized nutritionally. Procedural and outcomes data are shown in Table 2.
Figure 2.
Endoscopic view of a gastric ulcer that developed at the anastomotic site on postprocedure day 2 leading to hemorrhage.
Figure 3.
Fluoroscopic view of a malpositioned gastrojejunostomy stent traversing from the stomach through the colon and into jejunum. Despite this, an upper GI series demonstrated no evidence of contrast leaking around the stent proximally or distally.
Table 2.
Procedural and outcomes data
| EUS-gastroenterostomy in benign gastric outlet obstruction (n=22) | ||
|---|---|---|
| Procedure time, mean ± SD minutes | 66 | 33.5 |
| Technical success, no. (%) | 21 | 95.4% |
| Anastomosis | ||
| · Gastroduodenal | 5 | |
| · Gastrojejunal | 16 | |
| Cases requiring second overlapping stent | 2 | |
| Clinical outcome | ||
| Outpatient cases, no. (%) | 6 | 27.2% |
| Adverse events (severity grade) | ||
| · Abdominal pain, mild | 1 | |
| · Bleeding, severe | 1 | |
| · LAMS migration and impaction, severe | 1 | |
| · EUS-GJ LAMS traversed through colon, severe | 1 | |
| Outpatients discharged to home, no. (%) | 4 | 66.6% |
| Recurrent GOO while LAMS in place | 5 | |
| LAMS dwell time before recurrent GOO, mean ± SD days | 228 | 242 |
| Recurrent GOO with LAMS in place requiring conversion to surgery | 2 | |
| Patients with elective stent removal, no. (%) | 15 | 83.3% |
| Length of time stent left in place, mean ± SD days | 270 | 273 |
| Length of follow-up, median (range), days | 465.5 | 82 - 1263 |
Clinical Outcomes
All 22 patients with technically successful stent placement were able to tolerate at least a full liquid diet. Five patients (23.8%) developed recurrent GOO while LAMSs were in place after a mean LAMS dwell time of 228 days; 3 (14.3%) were due to stent occlusion from food residue and were managed with endoscopic clearance of debris from the stent. One patient with GOO secondary to recurrent pancreatitis represented with failure to thrive and concern for recurrent GOO; on endoscopic evaluation, the LAMS was patent but the patient was converted to a venting percutaneous endoscopic gastrostomy and feeding jejunostomy followed by LAMS removal. He ultimately regained the weight he had previously lost.
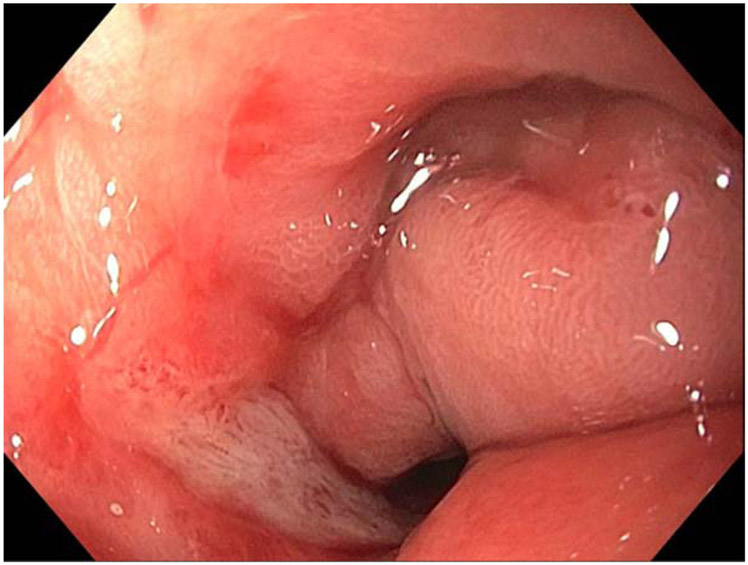
One patient with NSAID-associated pyloric stenosis developed recurrence of GOO due to food residue occluding the stent. This occurred 185 days after the LAMS was first placed, and after 3 separate endoscopic balloon dilations of the stenotic pylorus without improvement the anastomosis (Figure 4) was ultimately converted to a surgical gastroenterostomy at a separate site after the EUS anastomotic site was closed.
Figure 4.
Endoscopic view of a patient with NSAID-induced pyloric stenosis that failed to respond to 3 separate endoscopic balloon dilations. This patient’s anastomosis was ultimately converted to a surgical gastroenterostomy at a separate site after the EUS anastomotic site was closed.
LAMSs were removed electively in 18 out of 21 patients (85.7%) after GOO resolution and a mean dwell time of 270± 273 days. Two of these patients had their LAMS exchanged in setting of persistent GOO in order to continue use of this as a definitive enteral bypass after they were deemed poor operative candidates by surgical consultation.
One patient with peptic ulcer disease developed recurrent GOO after stent removal and was converted to a surgical gastrojejunostomy 577 days after stent removal. There are 3 patients (14.3%) awaiting stent retrieval at the time of this manuscript; 2 with walled-off pancreatic necrosis, 1 mentioned above with misposition of the LAMS through the colon, and a third with a stenosed Billroth I. Excluding the 3 patients awaiting stent removal, only 3 of 18 patients (16.6%) in the cohort required conversion to surgical anastomoses for treatment of GOO after a mean follow-up length of 564 days (SD ± 381). No patient deaths occurred during the follow-up period.
DISCUSSION
Benign GOO presents a unique challenge for patients and clinicians. Surgical bypass is a permanent, anatomy-altering procedure with associated morbidity. In contrast endoscopic therapy of intraluminal strictures often requires multiple sessions over several months before the patient can return to a normal diet. Endoscopy may be preferred if the GOO is partial and the patient is able to maintain a liquid or soft diet during the requisite time for resolution, but this is not possible if the GOO is complete. A method of relieving the obstruction that is not permanent and with a low associated morbidity would allow for sufficient time to treat intrinsic stenosis or allow for spontaneous resolution of external compression.
EUS-GE was first described in 2012 by Binmoeller et al16 using a porcine model16 and has demonstrated considerable efficacy in palliating malignant GOO in patients unfit for surgical bypass.17 There have been reports of EUS-GE for benign GOO; however, most studies have focused on short-term outcomes immediately after stent placement.18 EUS-GE offers a unique solution to the problem of benign GOO by offering a temporizing solution to a time-limited problem. There are no previous studies demonstrating the use of EUS-GE as a bridge to resolution of the GOO.
In the present study we found a high technical success rate and low adverse event rate. EUS-GE was able to prevent surgery for GOO in 83.3% of cases with LAMS needing to stay in place for a mean of 8.5 months to allow resolution of GOO. After LAMS removal, there was a low rate of GOO recurrence (5.6%), suggesting this temporizing treatment option is effective. Though a small percentage of the patients in our cohort were ultimately converted to surgical anastomoses, this occurred a mean of 270 days after EUS-GE creation. In those that did require surgery, the prolonged period of oral feeding while the stent was in place may have led to more favorable surgical outcomes due to improved nutritional status. Data suggest that enteral feeding is superior to parenteral nutrition in the preoperative period before surgical treatment of GOO.19 Jejunal tube placement is an option in the preoperative period, and may be the preferred modality if local expertise does not allow for EUS-GE. Anecdotally patients prefer oral feedings rather than tube feedings when given both options.
LAMSs used for EUS-GE are off-label. Permanent LAMS placement is not advised in other LAMS applications such as pancreatic fluid collection drainage due to the risk of bleeding and buried stent.20 The need to remove LAMSs after EUS-GE is highlighted by one of our AEs, wherein the LAMS spontaneously migrated, leading to a small-bowel obstruction. We recommend removing the stent as soon as there is evidence that the GOO has resolved, via cross-sectional imaging, upper GI series, or endoscopy. This recommendation for removal does not apply to cases of malignant obstruction where the LAMS can serve as destination therapy.
Our study has a few notable limitations including the retrospective design, which limits generalizability. Additionally, EUS-GE is a procedure that requires significant technical expertise. In order to obtain this expertise, one has to already be highly skilled in other forms of therapeutic EUS and have the patience to approach the learning curve for this complex procedure. Finally, 3 patients are awaiting stent removal, which limits our complete knowledge of their experience.
In conclusion, this study of patients undergoing EUS-GE at a large tertiary referral center for benign gastric outlet obstruction found a low rate of conversion to a surgical gastroenterostomy. We found a high technical success rate with only one technical failure in the cohort and a low rate of adverse events with good patient tolerance of the procedure. Future studies on this topic should be conducted in a prospective, randomized fashion comparing surgical anastomoses with EUS-GE.
Acknowledgments
Funding declaration: Dr. T.W. James receives research and training support by a grant from the NIH (T32DK007634). All other authors declare no relevant funding for this work.
Glossary
- AE
Adverse event
- GOO
gastric outlet obstruction
- EUS
endoscopic ultrasound
- GE
gastroenterostomy
- LAMS
lumen apposing metal stent
- NSAID
non-steroidal anti-inflammatory drug
- SD
standard deviation
- IQR
interquartile range
Footnotes
Conflicts of interest: Dr. Baron is a consultant and speaker for Boston Scientific, W.L. Gore, Cook Endoscopy and Olympus America. Dr. Grimm is a consultant for Boston Scientific.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. The American journal of gastroenterology. 1996. ;91:1679. [PubMed] [Google Scholar]
- 2.Johnson CD. Gastric outlet obstruction malignant until proved otherwise. The American journal of gastroenterology. 1995. ;90:1740. [PubMed] [Google Scholar]
- 3.Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. The American journal of gastroenterology. 2002. ;97:72. [DOI] [PubMed] [Google Scholar]
- 4.Kochhar R, Malik S, Gupta P, Reddy YR, Dhaka N, Sinha SK, Gupta V, Noor MT, Mallick B. Etiological spectrum and response to endoscopic balloon dilation in patients with benign gastric outlet obstruction. Gastrointestinal endoscopy. 2018. ;88:899–908. [DOI] [PubMed] [Google Scholar]
- 5.Rana SS. An overview of walled-off pancreatic necrosis for clinicians. Expert review of gastroenterology & hepatology. 2019. ;13:331–43. [DOI] [PubMed] [Google Scholar]
- 6.Fujitani K, Ando M, Sakamaki K, Terashima M, Kawabata R, Ito Y, Yoshikawa T, Kondo M, Kodera Y, Yoshida K. Multicentre observational study of quality of life after surgical palliation of malignant gastric outlet obstruction for gastric cancer. BJS open. 2017. ;1:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD, Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointestinal endoscopy. 2010. ;71:490–9. [DOI] [PubMed] [Google Scholar]
- 8.Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Nagao J. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004. ;36:73–8. [DOI] [PubMed] [Google Scholar]
- 9.Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice?. Gastrointestinal endoscopy. 2004. ;60:1010–7. [DOI] [PubMed] [Google Scholar]
- 10.Khashab MA, Kumbhari V, Grimm IS, Ngamruengphong S, Aguila G, El Zein M, Kalloo AN, Baron TH. EUS-guided gastroenterostomy: the first US clinical experience (with video). Gastrointestinal endoscopy. 2015. ;82:932–8. [DOI] [PubMed] [Google Scholar]
- 11.Kerdsirichairat T, Irani S, Yang J, Gutierrez OI, Moran R, Sanaei O, Dbouk M, Kumbhari V, Singh VK, Kalloo AN, Khashab MA. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endoscopy international open. 2019. ;7:E144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, Peñas I, de la Serna C, Shah J, Binmoeller K, Gaidhane M, Grimm I, Baron T, Kahaleh M. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endoscopy international open. 2016. ;4:E276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL, Pike IM. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointestinal endoscopy. 2010. ;71:446–54. [DOI] [PubMed] [Google Scholar]
- 14.Ngamruengphong S, Kumbhari V, Tieu AH, Chavez YH, Bukhari M, Hajiyeva G, Ismail A, Aguila GL, Chen YI, Khashab MA. A Novel “Balloon/Snare Apparatus” Technique To Facilitate Easy Creation of Fistula Tract During EUS-Guided Gastroenterostomy (EUS-GE) [Abstract]. Gastrointestinal endoscopy. 2016. ;83:AB636. [DOI] [PubMed] [Google Scholar]
- 15.Chen YI, Kunda R, Storm AC, Aridi HD, Thompson CC, Nieto J, James T, Irani S, Bukhari M, Gutierrez OB, Agarwal A. EUS-guided gastroenterostomy: a multicenter study comparing the direct and balloon-assisted techniques. Gastrointestinal endoscopy. 2018. ;87:1215–21. [DOI] [PubMed] [Google Scholar]
- 16.Binmoeller KF, Shah JN. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: a porcine study. Endoscopy. 2012. ;44:499–503. [DOI] [PubMed] [Google Scholar]
- 17.Phillip SG, Young JY, Dong W, Thompson CC. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surgical endoscopy. 2019. January 1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YI, Kunda R, Storm AC, Aridi HD, Thompson CC, Nieto J, James TW, Irani S, Bukhari M, Gutierrez OB, Agarwal A. EUS-guided gastroenterostomy: a multicenter study comparing the direct and balloon-assisted techniques. Gastrointestinal endoscopy. 2018;87:1215–21. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZH, Lin SY, Dai QB, Hua J, Chen SQ. The effects of pre-operative enteral nutrition from nasal feeding tubes on gastric outlet obstruction. Nutrients. 2017;9:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, Perbtani YB, Mramba LK, Kerdsirichairat T, Prabhu A, Manvar A, Ho S, Pannu D, Keswani RN, Strand DS, Wang AY. Safety and rate of delayed adverse events with lumen-apposing metal stents (LAMS) for pancreatic fluid collections: a multicenter study. Endoscopy international open. 2018. ;6:E1267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]