Abstract
In this placebo-controlled randomized clinical trial, we examined the efficacy of 250mg d-cycloserine (DCS) for enhancing the effects of cognitive behavior therapy targeting anxiety sensitivity reduction in the context of smoking cessation treatment. We hypothesized that DCS would enhance treatment of our mechanistic targets—anxiety sensitivity and panic and related symptoms—and result in greater smoking abstinence. A total of 53 smokers were randomized to a 7-week integrated treatment and received study medication (DCS or placebo) prior to sessions 3–5; these sessions emphasized interoceptive exposure practice. Nicotine replacement therapy was initiated at session 5 (quit date). We found that DCS augmentation led to greater reductions of one (anxiety sensitivity) of two of our mechanistic targets at early but not late assessments, and that engaging that target predicted better smoking outcomes. However, there was no evidence of group (DCS vs. placebo) differences in smoking cessation success at treatment endpoint or follow-up evaluations. Hence, although we found that DCS can enhance treatment targeting a smoking maintaining factor, additional strategies appear to be needed to significantly affect smoking outcomes.
Keywords: smoking cessation, d-cycloserine, behavioral intervention, nicotine-replacement therapy, panic, anxiety sensitivity
1. Introduction
Panic attacks are more common among smokers than among the general non-psychiatric population (Lasser et al., 2000) and smokers who experience panic attacks evidence greater difficulty attaining smoking abstinence (Piper et al., 2010; Zvolensky et al., 2005b, 2005a). Indeed, in DSM-5, panic attacks were added as a specifier for all DSM-5 disorders, because panic attacks represent a severity marker and are associated with poorer outcomes for a range of co-occurring disorders (Asmundson et al., 2014; Craske et al., 2010). Accordingly, smokers who experience panic attacks may benefit from smoking cessation interventions that also target their panic attacks and underlying psychopathology. In fact, panic can contribute to the maintenance of smoking (Cosci et al., 2010; Zvolensky et al., 2005a). For example, smokers with a history of panic attacks relative to those without report higher levels of tobacco dependence (Piper et al., 2011; Vujanovic et al., 2010) and increased affect-regulatory smoking motivations (Farris et al., 2014). Panic attacks are also associated with more severe subjective nicotine withdrawal symptom severity (Farris et al., 2015), and lower success rates in quitting (Farris et al., 2015; Piper et al., 2011).
There is growing support for behavioral treatments that intervene on anxiety sensitivity as a means both to reduce panic attacks and to facilitate smoking cessation (Schmidt et al., 2016; Smits et al., 2016b; Zvolensky et al., 2018b, 2018a). Anxiety sensitivity, the fear of anxiety-related sensations (Reiss et al., 1986), represents one individual difference variable that has shown to maintain panic attacks (McNally, 2002) and is linked to a wide range of negative health behaviors (Otto et al., 2016a). Acting as an “amplifier” of emotions and bodily sensations, anxiety sensitivity is related to motivation to avoid or escape negative emotional and physical experiences (Otto et al., 2016a; Otto and Smits, 2018; Smits et al., 2018), which among smokers who attempt to quit, results in greater tobacco withdrawal symptoms and poorer success rates (Assayag et al., 2012; Bakhshaie et al., 2018, 2016; Brown et al., 2001). These data and related laboratory work suggest that anxiety sensitivity, independent of negative mood, is associated with internal dysregulation during smoking abstinence (Zvolensky et al., 2014b).
Zvolensky and colleagues have developed an integrated treatment program for anxiety sensitivity and smoking cessation (Zvolensky et al., 2018). The program orients patients to a model that emphasizes the relation between mental health and smoking and the importance of reducing anxiety sensitivity to achieve quit success. It utilizes cognitive-behavioral treatment (CBT) methods such as education, cognitive restructuring, and interoceptive exposure—i.e., repeated confrontation to anxiety-related sensations to achieve fear extinction—to target anxiety sensitivity (Smits et al., 2004, 2008b). It also employs standard CBT methods (e.g., self-monitoring, discussing past quit experiences, setting a target quit date, developing alternate coping strategies, seeking social support for quitting, stimulus control strategies, relapse prevention) to aid smokers in quitting. The program is delivered in combination with nicotine replacement therapy (NRT), which starts on the quit date. Following a series of smaller scale studies aimed at refining the treatment program (Bogiaizian et al., 2017; Feldner et al., 2008; Zvolensky et al., 2014a), a four-session version underwent testing in a large randomized controlled investigation involving 526 treatment-seeking smokers (Schmidt et al., 2016). The results supported the hypothesis that the integrated program reduces anxiety sensitivity and panic symptoms as well as related symptoms (Schmidt et al., 2016) and that anxiety sensitivity reduction also mediates the effects of the treatment on smoking abstinence (Zvolensky et al., 2018a). However, the between-group effect on anxiety sensitivity reduction, although statistically significant, was small (d = .18), and the intervention did not outperform the control condition in terms of short-term or long-term smoking abstinence rates.
To build upon this treatment development work, we designed the present pilot trial to evaluate a strategy for boosting anxiety sensitivity reduction in the context of smoking cessation treatment. We selected the medication d-cycloserine (DCS)—a partial agonist at the N-methyl-D-aspartate (NMDA) glutamatergic receptor—for this clinical application, because DCS has been shown to enhance the effects of exposure-based therapy across anxiety disorders, including panic disorder, when administered acutely before an exposure session (Mataix-Cols et al., 2017). The administration of DCS facilitates NMDA receptor function, which is critical for emotional learning and specifically for the consolidation or retention of fear extinction (Davis et al., 2006)—a core target of engagement for exposure therapy (Berry et al., 2009). Hence, administering DCS at interoceptive exposure sessions may facilitate reductions in anxiety sensitivity and panic related symptoms, which in turn, may lead to greater quit success. Accordingly, we enrolled treatment-seeking smokers with panic attacks in a pilot, double-blind, randomized smoking cessation clinical trial. They were enrolled in a 7-week version of the integrated intervention (Zvolensky et al., 2018), termed Panic Smoking Reduction Treatment (PSRT) for the current study, and received either DCS or placebo (PBO) medication. They were asked to make a quit attempt at Week 5. Measures of mechanistic targets and efficacy were administered at baseline (Week 0), weekly during the intervention period, and up to 6 months after the quit date (Week 29). We hypothesized that, relative to participants assigned to PBO, participants assigned to DCS would evidence (a) greater reductions in anxiety sensitivity and panic related symptoms (i.e., mechanistic target engagement) and (b) greater abstinence (i.e., clinical efficacy). We also predicted that target engagement would be related to efficacy, such that the effects of treatment on abstinence would be mediated by reductions in anxiety sensitivity and panic related symptoms, respectively.
2. Method
2.1. Participants
Participants were adult smokers recruited via community-based and online strategies (e.g., social media, Craigslist, flyers, newspaper advertisements) in the central Texas area. They were eligible if they (1) smoked a minimum of 8 cigarettes per day for at least one year; (2) were motivated to quit smoking (at least a 5 on a 10-point scale); (3) reported a history of at least one panic attack within the last year (indexed via the Structured Clinical Interview for DSM-IV; SCID-NP; (First et al., 2010); and (4) endorsed smoking as an emotion regulation strategy (indexed by a score of 78 or above on the Smoking Abstinence Expectancy Questionnaire; Abrams et al., 2011). Additionally, eligible participants had to pass a medical screening by the study physician to ensure it was safe for them to use DCS and nicotine patches, and endorse a willingness to attend all study sessions and adhere to protocol. The following exclusion criteria were employed: (1) current or past diagnosis of a psychotic, bipolar, or developmental disorder; (2) current suicidal or homicidal risk with intent or plan (3) active substance abuse or dependence (excluding nicotine) or eating disorder within the past 6 months; (4) use of other tobacco products (5) current use of nortriptyline, bupropion, or isoniazid psychotropic ethionamide compounds; (6) current use of any pharmacotherapy or psychotherapy for smoking cessation outside of the research study, concurrent psychotherapy initiated within three months of baseline, or ongoing psychotherapy specifically targeting treatment of anxiety or mood disorders other than general supportive therapy initiated at least 3 months prior to the study; (7) limited mental competency and the inability to give informed, voluntary, written consent to participate; (8) planned to move outside of immediate area in the next six months; (9) insufficient command of the English language; and (10) currently pregnant or breastfeeding, planned on becoming pregnant in the next year, or women of childbearing potential not using medically accepted forms of birth control.
2.2. Procedures
The study was funded by the National Institute of Drug Abuse (NIDA; R34DA034658) and is registered on clinicaltrials.gov (ID: ). The Institutional Review Board of the University of Texas at Austin approved the study and a Data Safety and Monitoring Board oversaw all procedures. Details of the protocol have been described by Smits and colleagues (Smits, Kauffman et al., 2016).
2.2.1. Eligibility Testing
Eligibility testing involved a four-step process: (1) online eligibility survey, (2) telephone screening, (3) comprehensive in-person or telephone interview which included a medical health history and the SCID-IV-NP (First et al. 2010).
2.2.2. Interventions
Panic and Smoking Reduction Treatment (PSRT).
All participants were enrolled into PSRT. PSRT is a manualized intervention that combines intensive standard smoking cessation counseling and pharmacotherapy (i.e., the nicotine patch) with procedures for reducing panic and anxiety sensitivity (Zvolensky et al., 2018). For this application, PSRT consisted of 90-minute sessions delivered in-person once-weekly over a 7-week period by masters level and doctoral students supervised and certified by JAJS, a licensed clinical psychologist. Components specifically targeting anxiety sensitivity included: (1) psychoeducation exercises developed for panic intervention programs; (2) cognitive restructuring; and (3) interoceptive exposure. In order to provide interoceptive exposure practice that was particularly relevant for smokers, we combined standard interoceptive exposure practice (e.g., head rolling, voluntary hyperventilation, straw breathing, running in place) with a “withdrawal challenge” during sessions 3–5. The withdrawal challenge involved asking participants to refrain from smoking two hours prior to the session to induce symptoms of nicotine withdrawal. In order to maximize the learning from exposure practice, participants were asked to also refrain from smoking in the two hours following the end of each session, thus mirroring response prevention that is a critical component to exposure therapy. “Quit day” took place on week 5 of the protocol. All participants were educated about the use of Nicoderm CQ®, 24-hour transdermal nicotine patches the week prior to the quit date (during the Week 4 session) and provided 8 weeks of patches. Study therapists assessed side effects associated with the nicotine patch.
DCS/Placebo.
The first two sessions of the treatment protocol did not involve exposure practice. Thus, since DCS was hypothesized to enhance response to exposure sessions, randomization to DCS treatment condition did not occur until session 3. As a result, the first 2 sessions were considered the open phase of the treatment. DCS (250mg) and PBO pill capsules were identical in appearance, and administered by study personnel blind to study condition 1 hour prior to sessions 3, 4, and 5. Group assignment was determined by the study biostatistician (DR) using variable-sized permuted block randomization.
2.3. Assessment
2.3.1. Screening and Baseline
Demographics.
Participants provided standard demographic information including gender, age, race/ethnicity, and level of education during the screening session.
Psychiatric Diagnosis.
Diagnostic exclusions, history of panic attacks within the last year, and lifetime prevalence of Axis I diagnoses were determined by the SCID-NP (First et al., 2010) during the screening visit by a trained and certified study assessor. All diagnostic interviews were supervised by JAJS.
Smoking History.
Smoking history was assessed with the Smoking History Questionnaire (SHQ), a 30-item measure that includes items pertaining to smoking rate, age of onset of initiation, and years of being a regular smoker (Brown et al., 2002).
Motivation to Quit Smoking.
Participants reported their motivation to quit on a 10-point scale at prescreen (Smits et al., 2016a, 2012).
Nicotine Dependence.
Nicotine dependence was assessed using the 6-item Fagerström Test for Cigarette Dependence (Fagerström, 2012).
Smoking Abstinence Expectancies.
Participants completed the Smoking Abstinence Expectancies Questionnaire (SAEQ; Abrams et al., 2011) at prescreen. The SEAQ is a psychometrically-sound, 28-item self-report measure of expected consequences as a result of 24-hours of smoking abstinence.
2.3.2. Intervention Targets
Anxiety Sensitivity.
Anxiety sensitivity was assessed using the Anxiety Sensitivity Index (ASI-3; (Taylor et al., 2007), an 18-item measure designed to assess fear of bodily sensations. Using a 5-point Likert scale (0 = very little to 4 = very much), participants rated their concern regarding the possible negative consequences of internal anxiety-related sensations. Participants completed the ASI-3 at baseline and all treatment sessions and follow-up visits. The ASI-3 demonstrates strong psychometric properties (Taylor et al., 2007). Cronbach’s alpha in the present study was .93.
Panic and Related Symptoms.
The Panic Disorder Severity Scale-Self Report (PDSS-SR; Houck et al., 2002)). The PDSS-SR is a 7-item measure assessing the severity of seven features of panic: frequency of panic attacks, distress during panic attacks, anticipatory anxiety, agoraphobic fear and avoidance, interoceptive fear and avoidance, impairment of work functioning, and impairment of social functioning. Items are rated on a five-point Likert scale ranging from 0 (none) to 4 (extreme), with a total possible score of 28 (Shear et al., 2001). Higher scores indicate greater severity and impairment. Participants completed the PDSS-SR prior to the baseline visit, at all treatment sessions, and at follow-up visits. The PDSS-SR is a psychometrically-sound instrument (Houck et al., 2002; Shear et al., 2001). Cronbach’s alpha in the present study was .78.
2.3.4. Efficacy
Smoking Status and Abstinence.
The timeline follow-back (TLFB) self-report procedure was completed at baseline, each treatment session, and at follow-up appointments to assess cigarette consumption at each day following the previous assessment. The TLFB has demonstrated good reliability and validity (Brown et al., 1998) when used previously among high anxiety sensitive smokers (McLeish et al., 2007; Smits et al., 2016b). Self-reported 7-day point-prevalence abstinence (PPA) was biochemically verified at every assessment by expired carbon monoxide (CO; ≤8 ppm) using a Bedfont Micro Smokerlyzer®. Additionally, self-reported abstinence assessed at 10, 16, and 24 weeks following quit day were verified with saliva cotinine (≤10 ng/mL). Abstinence was considered missing if neither CO nor cotinine levels were available for verification of abstinence at the assessment visit (Blankers et al., 2016).
2.3.5. Integrity and Safety
Treatment Adherence.
All PSRT sessions were videotaped and 10% were randomly selected for rating. Independent raters assessed for the presence or absence of overall protocol adherence and clinician competence. Overall protocol adherence assessed coverage of specific concepts and techniques assigned for each session and was represented as a total percentage (0%−100%).
Adverse Events.
Participants were assessed for adverse events (AE) at each treatment session and all follow-up visits. Specifically, participants were monitored for significant symptoms or adverse reactions to both DCS (assessed during Weeks 3–5) and nicotine patches (assessed during Weeks 5–13). The study physician reviewed all reported AEs to determine whether the medication or patches should be discontinued.
3. Results
3.1. Participant Flow and Sample Characteristics
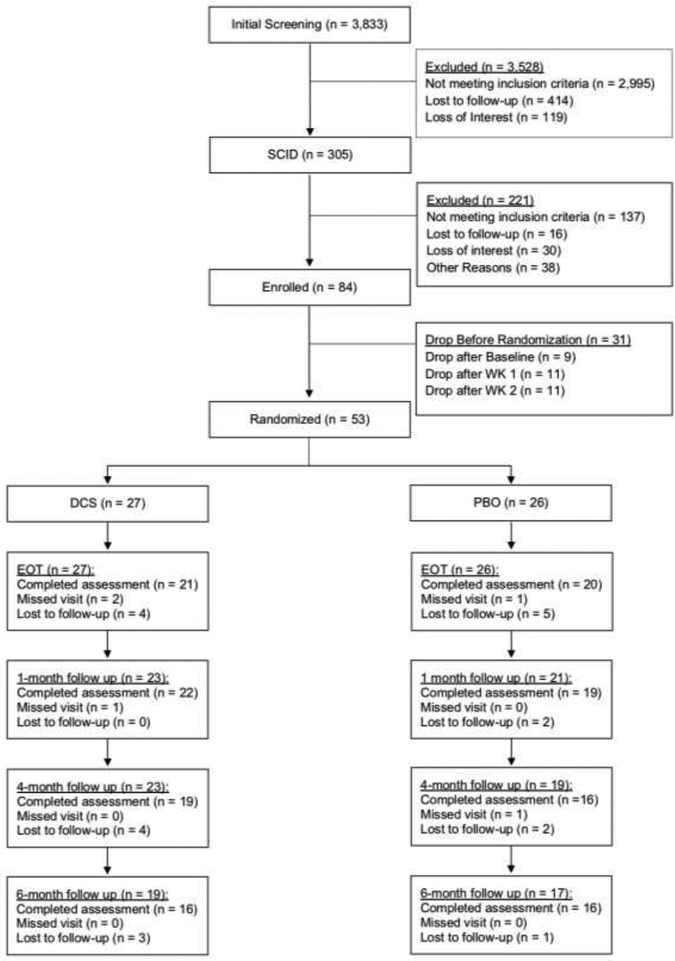
Figure 1 shows participant flow. Of the 84 individuals were deemed eligible and completed baseline assessment, only 75 came to the first session. An additional 22 individuals were lost before randomization, resulting in a sample of 53 for the study (DCSn = 27; PBOn = 26). Analyses indicated that the 3 groups (DCS, PBO, and the 31 not who were not randomized) did not differ on any of the demographic or study variables at baseline, including sex, age, ethnicity, race, education, relationship status, anxiety sensitivity, panic symptom severity, cigarette dependence, withdrawal symptoms, number of cigarettes smoked per week, and carbon monoxide (ps>.104 on all of the 12 variables).
Figure 1.
CONSORT diagram
As can be seen in Table 1, the mean age among study participants (i.e., those who were randomized) was 35.90 (SD = 12.50) years. Most participants were female and non-Hispanic, and approximately half were married. The study sample reported low to medium nicotine dependence, smoking on average 14.22 cigarettes per day (SD = 6.01). Participants reported high levels of anxiety sensitivity and moderate levels of panic and related symptoms. Finally, the mean number of CBT sessions attended was 5.81 of 7 (SD = 1.73) and 5.96 of 7 (SD = 1.24) for DCS and PBO conditions, respectively. Attendance of the major end point assessments declined over time with 77.77% of DCS participants and 76.92% PBO participants attending the final treatment visit and 59.25% of DCS participants and 61.53% PBO participants attending the 6-month follow-up. Participants with missing data did not differ from participants with complete data on any of the demographic or baseline clinical characteristics.
Table 1.
Sample characteristics
| DCS (n = 27) M (sd) or n (%) | PBO (n = 26) M (sd) or n (%) | |
|---|---|---|
| Demographics Characteristics | ||
| age | 35.19 (12.37) | 36.64 (12.85) |
| female | 19 (70.37%) | 18 (69.23%) |
| Hispanic or Latino | 0 (0.00%) | 3 (11.54%) |
| race | ||
| White | 26 (96.30%) | 23 (88.46%) |
| Black or African American | 0 (0.00%) | 1 (3.85%) |
| Asian | 1 (3.70%) | 1 (3.85%) |
| Native American/Alaska Native | 0 (0.00%) | 1 (3.85%) |
| Marital status | ||
| Single | 15 (55.56%) | 12 (46.15%) |
| Living with Partner | 2 (7.41%) | 2 (7.69%) |
| Married | 4 (14.81%) | 8 (30.77%) |
| Divorced | 5 (18.52%) | 2 (7.69%) |
| Widowed | 1 (3.70%) | 0 (0.00%) |
| Education | ||
| Graduate School | 3 (11.11%) | 4 (15.38%) |
| College Graduate | 8 (29.63%) | 6 (23.08%) |
| Partial College | 13 (48.15%) | 12 (46.15%) |
| High School Graduate | 3 (11.11%) | 4 (15.38%) |
| Clinical Characteristics at Screen | ||
| Cigarettes per day | 15.51 (6.64) | 12.91 (5.06) |
| CO reading (ppm) | 27.15 (12.66) | 19.92 (13.47) |
| FTND total | 3.82 (1.37) | 4.35 (1.43) |
| ASI-3 total | 27.85 (16.34) | 28.72 (13.42) |
| PDSS total | 10.67 (3.50) | 10.54 (4.23) |
3.2. Integrity and Safety
Treatment Adherence.
The overall integrity of treatment sessions was high, with protocol adherence averaging 84.70% (SD = 19.04).
Adverse Events.
Reported adverse events were low (11.11% of DCS participants and 7.69% of PBO participants) and did not differ significantly across conditions (Fisher Exact Test p = 1). One participant, randomized to PBO, reported elevated anxiety symptoms following the first drug administration. This AE was deemed possibly related to study procedures and further drug administration was discontinued. All other AEs (i.e., chipped bone in back, Pericarditis, anemia, migraine) were reported in the follow-up period and deemed unrelated to study procedures by the study physician. No adverse events related to nicotine patch use were reported.
3.3. Target Engagement
Data Analytic Approach.
Multilevel modeling (MLM) was used to analyze the data from the multiple assessments of anxiety sensitivity and panic related symptoms over time (Table 3 reports the unadjusted means for these measures). MLM is intent-to-treat and includes all participants, regardless of missing data, as long as the participants have at least one assessment. Consistent with previous work (Hofmann et al., 2013; OʼCleirigh et al., 2018; Smits et al., 2016b), we modeled the growth curve of outcomes from time of randomization, which, in this case, occurred at the beginning of session 3, through the 6-month follow-up (Week 29). The study was comprised of three phases after randomization: (1) the post-randomization, pre-quit, treatment phase (sessions 3–5), (2) the post-quit treatment phase (after session 5 to session 7, [session 7 was end-of-treatment; EOT]), and (3) the follow-up phase (after session 7 through Week 29 or the 6-month follow-up). Thus, our initial analysis for each outcome was a three-phase piecewise growth model, allowing quadratic change during each phase. However, the quadratic term was not significant for any phase for any of the outcome variables, so it was dropped in all analyses. We also tested whether a two-phase growth model (treatment phase and follow-up phase) fit the data better than the three-phase model for each outcome, using AIC and BIC to compare model fit. Because the two-phase model had lower AIC and BIC, we employed a two-phase model for the psychological outcomes (ASI-3 and PDSS-SR). Since previous research showed that baseline severity may moderate improvement (participants with higher baseline severity are expected to improve more than those with low baseline severity), pre-randomization severity on the each variable (ASI-3 and PDSS-SR, respectively) was included as a moderator of the “Time” variables for each phase of the growth curve model (GCM). Finally, since baseline smoking “severity” may also impact response to treatment, pre-randomization CO level and number of cigarettes smoked per week were also included as covariates and moderators of the “Time” variable. Non-significant baseline severity effects were dropped from analyses. Since there are no generally accepted effect size measures for MLM effects, we used the t-to-d conversion to calculate approximate effect sizes for each significant effect reported. Power analyses, using the program RMASS2, indicated that we had greater than .80 power to detect a medium effect size (d = .50) for the MLM analyses of ASI-3 and PDSS-SR.
Table 3.
Unadjusted, raw means for ASI-3 and PDSS-SR at each time point beginning at randomization.
| ASI-3 | PDSS | |||
|---|---|---|---|---|
| DCS | PBO | DCS | PBO | |
| week | mean (SD) | mean (SD) | mean (SD) | mean (SD) |
| 3 | 22.63 (14.33) | 22.26 (13.68) | 8.63 (4.06) | 7.48 (5.93) |
| 4 | 20.88 (14.99) | 17.73 (11.18) | 7.29 (5.00) | 7.41 (4.96) |
| 5 | 20.80 (13.47) | 19.78 (14.04) | 6.55 (5.03) | 6.87 (6.38) |
| 6 | 17.00 (11.07) | 16.58 (13.27) | 5.79 (5.28) | 6.16 (6.40) |
| 7 | 15.37 (10.38) | 17.80 (13.77) | 5.32 (5.14) | 6.65 (6.91) |
| 9 | 16.00 (10.71) | 15.32 (13.21) | 4.56 (4.90) | 5.89 (5.83) |
| 13 | 16.64 (8.87) | 18.00 (13.99) | 6.82 (4.26) | 4.73 (6.35) |
| 15 | 14.45 (8.95) | 17.24 (14.03) | 5.55 (3.47) | 7.06 (6.51) |
| 21 | 17.00 (10.06) | 16.15 (11.17) | 5.80 (4.66) | 6.23 (7.42) |
| 29 | 16.43 (11.01) | 17.89 (14.43) | 5.36 (6.96) | 5.40 (5.93) |
Note: When ASI-3 and PDSS were assessed at a treatment session (weeks 3–7), the assessment took place at the beginning of the session.
Anxiety Sensitivity.
The final two-phase MLM model for anxiety sensitivity was:
In this model, ASIij was the ASI-3 score for individual i at timepoint j, TRTi was the treatment condition for individual i (0 = DCS, 1 = PBO). TimeP1 was the time variable which coded the slope during phase 1 of the model (sessions 3–7), and TimeP2 was the time variable which coded the slope during phase 2 (after session 7 [EOT] through 6-month follow-up).
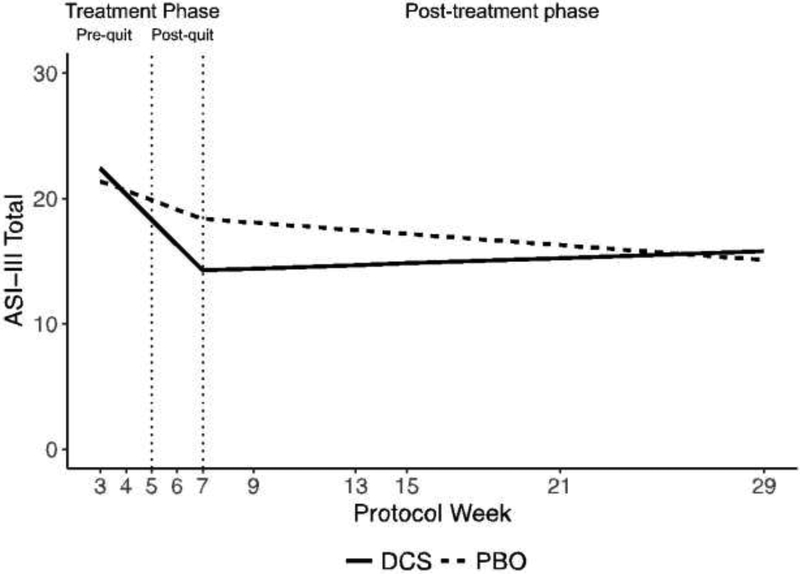
The two-phase growth curve model for anxiety sensitivity showed that participants receiving DCS reported lower ASI-3 scores (4.10 points lower) at post-treatment than participants receiving PBO, b = 4.10, 95%CI:[.25, 7.94], t(26) = 2.19, p= .038, d= .86 (see Figure 2). Further, DCS participants improved faster during the treatment phase than PBO participants, b= 1.28, 95%CI:[.17, 2.39], t(23) = 2.38, p= .026, d= .99. However, by the 6-month follow-up, DCS and PBO participants did not differ on ASI-3, b= −.71, 95%CI:[−6.50, 5.07],t(26) = −.25, p= .802. Finally, across the two treatment conditions, participants with higher baseline ASI-3 severity improved faster during the treatment phase than those with low baseline ASI-3 severity, b= −1.92, 95%CI:[−2.53, −1.31],t(23) = −6.52, p< .001, d = 2.72.
Figure 2.
Model estimated ASI-3 score in the treatment and post-treatment phase
Panic and Related Symptoms.
The final two-phase MLM model for panic and related symptoms was the same as the MLM model for anxiety sensitivity, except baseline CO and baseline number of cigarettes smoked were significant covariates, so they were retained in the model.
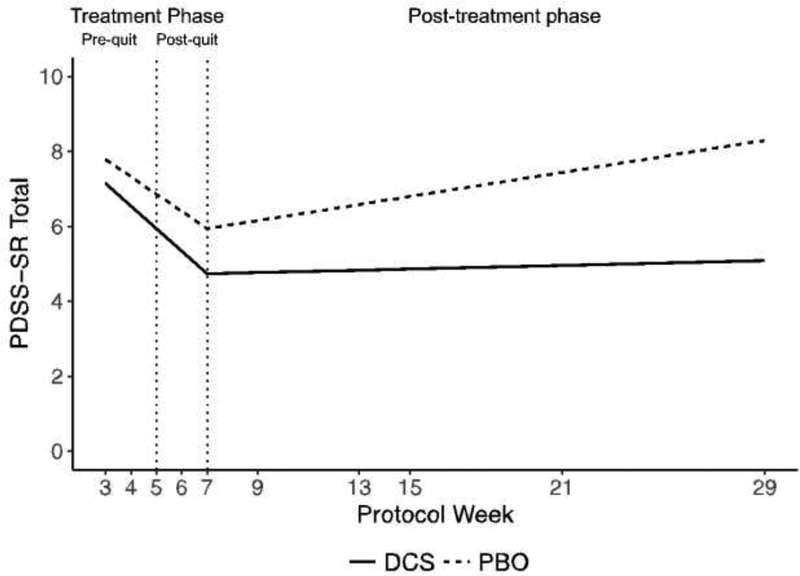
PDSS level was not significantly different between conditions at post-treatment. However, at the 6-month follow-up, participants receiving DCS reported significantly lower PDSS-SR scores than did participants receiving PBO, b= 3.21, 95%CI:[1.15, 5.26],t(27) = 3.21, p= .003, d= 1.24 (see Figure 3). In addition to these treatment effects, the analysis also showed that a higher pre-randomization PDSS-SR score was related to faster decreases in PDSS-SR scores during treatment, b= .31, 95%CI:[.14, .49], t(18) = 3.84 p< .001, d= 1.81. Also, higher pre-randomization CO and higher pre-randomization cigarettes smoked per week were associated with higher PDSS-SR scores across the study, b= 1.19, 95%CI:[.29, 2.08], t(45) = 2.68, p= .010, d= .80, and b= 1.15, 95%CI:[.32, 1.98], t(41) = 2.79, p= .008, d= .87.
Figure 3.
Model estimated PDSS-SR score in the treatment and post-treatment phase
3.4. Efficacy
Data Analytic Approach.
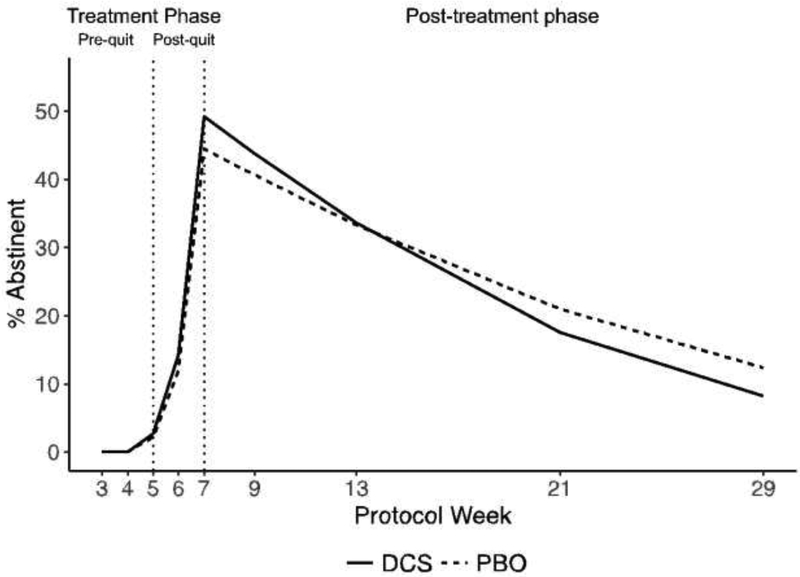
The same piecewise growth curve model approach was applied to the abstinence data (PPA), but because abstinence was dichotomous, the analysis included a logistic linking function and a binomial distribution (a generalized linear mixed model [GLMM]). For PPA, the three-phase growth curve model had lower AIC and BIC, so, for PPA, a three-phase model was used (see Figure 4). Because all participants were smoking at baseline, we could not use baseline PPA as a “baseline severity” measure. Instead, our proxy measures of baseline severity for PPA were our baseline measures of CO and number of cigarettes smoked per week. They were added as moderators of the “time” variables for each phase. Non-significant covariate terms were dropped.
Figure 4.
Model estimated abstinence rate in the treatment and post-treatment phase.
We performed a post-hoc power analysis to determine our power to detect treatment condition differences in abstinence rates at the 6-month follow-up given the number of actual participants in the study (N=53) and the abstinence rate actually obtained in the placebo condition (10%). Using an attrition rate of 40% (the attrition rate in the present study), we performed a Monte Carlo study to determine the smallest difference in abstinence rates between conditions that was detectable with .80 power. The Monte Carlo study showed that we had .80 power to detect a significant difference between treatment conditions if that difference was 17% or greater (equivalent to an abstinence rate in the DCS condition of 27% or higher in our study in which the abstinence rate in the placebo condition was 10%).
Abstinence.
The final model for abstinence was:
In this model, PPAij was abstinence (0=abstinent, 1=smoking) for individual i at time point j, TRTi was the treatment condition for individual i (0=DCS, 1=PBO). TimeP1 is the time variable coding slope during phase 1 of this model (sessions 3–5), TimeP2 is time for phase 2 of this model (after session 5-session 7), and TimeP3 is time for phase 3 (after session 7 to 6-month follow-up). COi is the z-scored baseline CO level for individual i.
The three-phase piecewise growth model for abstinence (Figure 4) showed that the change in abstinence over the course of the study did not vary by treatment condition. Specifically, abstinence rates were not different between treatments at either end of treatment or at the 6-month follow-up, and between-group effect sizes were small (Table 2). Abstinence rates in both treatments peaked at the end of treatment at around 50% for both groups but then returned to about 10% in both groups by the 6-month follow-up. The analysis did show that participants with higher baseline CO had slower rates of improvement in abstinence from quit date to end-of-treatment, and had lower abstinence rates throughout the follow-up period. Baseline number of cigarettes smoked per week was not significantly related to abstinence over and above baseline CO.
Table 2.
Abstinence estimates at major end points
| DCS | PBO | Cohen’s h | |
|---|---|---|---|
| EOT | 49.17% | 44.49% | .094 |
| 1-month Follow-up | 43.80% | 40.63% | .064 |
| 4-Month Follow-up | 17.58% | 20.96% | −.086 |
| 6-Month Follow-up | 8.25% | 12.35% | −.136 |
Note. According to Cohen (1988)(Cohen, 1988), the rule of thumb for interpreting a Cohen’s h effect size is: .20 is a small effect, .50 is a medium effect, and .80 is a large effect.
3.5. Mediation
Data Analytic Approach.
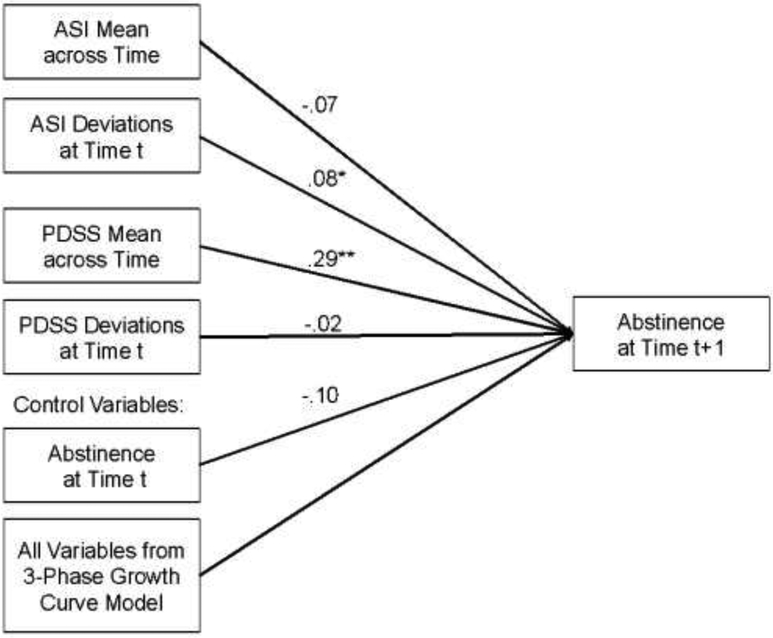
Since analyses indicated that there were no treatment condition differences in abstinence, we did not perform a typical mediation analysis to test whether changes in anxiety sensitivity or panic and related symptoms mediated treatment effects on abstinence. Instead, we examined whether these targets were related to abstinence in a longitudinal cross-lag analysis. In this analysis, ASI-3 and PDSS-SR were added as time-varying predictors (TVPs) of abstinence at the next assessment, controlling for abstinence at the previous assessment, in the three-phase growth curve model for abstinence shown above. Since TVPs conflate the between-subjects and within-subjects relation between the TVP and outcome, we first disaggregated ASI-3 and PDSS-SR for each person at each timepoint into their between-subjects average level and their within-subjects deviations from their average level (Hedeker and Gibbons, 2006; Wang and Maxwell, 2015). Such longitudinal cross lag analyses using disaggregated predictors can show quasi-causal relations between the predictors and outcome (Hamaker et al., 2015), especially when controlling for other putative predictors. Thus, both disaggregated predictors (ASI-3 and PDSS-SR), along with abstinence, were included as additional predictors of abstinence at the next time point in the three-phase GCM for abstinence (see Figure 5). Power analyses using the program PinT (Power in Two-level Models) indicated that we would have greater than .90 power to detect a medium effect size (d= .50) in the cross lag analyses.
Figure 5.
Cross lag panel model for anxiety sensitivity and panic and related symptoms predicting abstinence at the next assessment.
Relation between Target Engagement and Abstinence.
Results indicated that within-person deviations from their mean level of ASI-3 predicted smoking abstinence at the next time point. In particular, when participants had lower ASI-3 than their own average, they were more likely to be abstinent at the next time point than when their ASI-3 was higher than their own average, b = −.08, 95%CI:[−.010, −.144], t(178) = 2.26 p = .025, d = .34. This analysis also showed that between-subjects differences in PDSS-SR were related to abstinence. Specifically, participants with lower mean levels of PDSS-SR over the course of the study were more likely to be abstinent on average across the study, b = .29, 95%CI:[.100,.488], t(34) = 3.08 p = .004, d = 1.06.
4. Discussion
The aim of the present study was to test, in a pilot randomized controlled trial, whether DCS can boost the effects of an exposure-based smoking cessation intervention for smokers with panic attacks in terms of (1) target engagement, namely reduction in anxiety sensitivity and panic symptoms, and (2) efficacy, namely increased abstinence rates (Smits et al., 2016a). Consistent with the work driving the development of DCS for augmenting fear extinction-based interventions (Davis et al., 2006; Otto et al., 2016b, 2016c), we found that administering DCS prior to interoceptive exposure sessions sped up the reduction in anxiety sensitivity and thus led to lower levels of anxiety sensitivity at the end of weekly counseling sessions. These between-group effects were strong, as evidenced by large effect sizes (Cohen’s d’s > .80), but waned over the course of the follow-up period. This general effect, an acceleration in treatment response but no advantage at follow-up, mirror the results when DCS was applied to the treatment of panic disorder in a large-scale trial (Otto et al., 2016c). Whereas a speeded treatment effect may have minimal effects for the overall treatment of panic disorder, it has the potential for stronger effects for smoking cessation, where early lapse is common, is linked to anxiety sensitivity, and is predictive of full relapse (Brown et al., 2001; Shiffman et al., 1997). Hence, early reduction in anxiety sensitivity has the potential to forestall lapse and relapse. Accordingly, we found that lowered anxiety sensitivity promoted abstinence, supporting theoretical models positing a causal relation between anxiety sensitivity reduction and quit success (Leventhal and Zvolensky, 2015; Otto et al., 2016a).
The DCS augmentation effect on panic and related symptoms was less strong, and between-group differences did not emerge until well after the quit week. Also, although panic and related symptoms were related to lower abstinence over the course of the study protocol, we did not observe the causal relation between panic and related symptoms and abstinence that we found for the anxiety sensitivity. This finding is generally consistent with past work that highlights a mechanistic role of anxiety sensitivity between emotional disorders and smoking (Leventhal and Zvolensky, 2015).
Importantly, despite the fact that DCS facilitated engagement of one of the putative targets (i.e., anxiety sensitivity), which in turn was related to greater abstinence, participants who received the DCS-augmented intervention did not evidence greater abstinence rates at any of the follow-up assessments. This pilot study aimed to obtain initial effect sizes for the hypothesized between-group effect on abstinence, recognizing that we would be underpowered to detect significant effects for the efficacy outcome. The between-group effect sizes we observed were Cohen’s h = .094, .064, −.086, −.126 at end of treatment, and 1-, 4- and 6-month post-quit follow-ups, respectively (all these effects were smaller than a small effect size, which is Cohen’s h = .20). These disappointing results mirror and extend those of previous work. Indeed, an adequately powered test of the integrated anxiety sensitivity and smoking cessation intervention also yielded support for (1) target engagement (i.e., anxiety sensitivity reduction), albeit less strong than in the present study and (2) a relation between target engagement and abstinence, but no evidence of efficacy (Schmidt et al., 2016; Zvolensky et al., 2018a). Interestingly, other research from our group focused on the treatment of panic vulnerable/high anxiety sensitive smokers showed that exercise, which can serve as an interoceptive exposure tactic (Smits et al., 2008a, 2007; Smits and Zvolensky, 2006), can significantly boost anxiety sensitivity reduction and abstinence (Smits et al., 2016b). The analyses of mechanisms of action revealed that, although anxiety sensitivity reduction emerged as mediator, so did reductions in dysphoria (Zvolensky et al., 2018b). Hence, it is possible that interventions that successfully reduce anxiety sensitivity will only help optimize smoking cessation outcomes if they also engage other related and complementary mechanisms. This finding is generally consistent with research that suggests multiple transdiagnostic factors may be involved in relapse (Schlam et al., 2019).
The findings of this pilot study add to a body of research supporting DCS as a clinical strategy for improving the outcomes of exposure-based interventions for anxiety (Mataix-Cols et al., 2017), in this case among anxious adults seeking treatment for smoking cessation. Initial evidence from a recent study also points to the potential of DCS to augment exposure based interventions focused specifically on cigarette craving (Otto et al., 2019). As opposed to targeting anxiety sensitivity, this study utilized smoking-related cue exposure treatment (CET) targeting the learned associations between smoking-related cues and tobacco use (Conklin and Tiffany, 2002). When delivered to treatment-seeking smokers who have achieved initial abstinence, two sessions of CET yielded significantly greater reductions in cravings when combined with DCS than when combined with PBO (Otto et al., 2019). As such, there is a model for the application of DCS to enhance CET for smoking that does not rely on a high anxiety sensitivity sample; replication in a larger study is needed to fully validate this promising approach.
The current study has a number of limitations. First, our test of the DCS augmentation strategy warranted random assignment at the first exposure session (session 3), which resulted in a loss of 31 of the 84 eligible individuals. Hence, it is possible that our observed effect of DCS on anxiety sensitivity and panic-related symptoms may not generalize to all individuals who meet inclusion criteria, but rather, may only generalize to those who will stay in treatment for at least 3 sessions. Second, our sample size, although typical for a treatment development study, was small and thus limited the detection of small effects. Also, the sample involved mostly females and lacked diversity in terms of racial and ethnic background. It is important to test whether the findings observed here depend on these demographic variables. Third, we used a 7-session version of the integrated anxiety sensitivity and smoking cessation intervention, and administered 250 mg of DCS one hour prior to three of the sessions. It is possible that different combinations of these intervention parameters might result in different outcomes.
In sum, the current findings underscore the validity of anxiety sensitivity, and, to a lesser extent, panic and related symptoms as predictors of smoking cessation failure. We found that an integrated intervention can yield significant reductions in these maintaining factors and that DCS can aid this process, but that doing so yields little benefit for achieving abstinence. Accordingly, additional strategies appear to be needed to complement the early and anxiety sensitivity-specific benefits of the current treatment approach.
Highlights.
We tested if d-cycloserine (DCS) would enhance treatment of two putative mechanistic targets—anxiety sensitivity and panic and related symptoms—and result in greater smoking abstinence > We found that DCS augmentation led to greater reductions of one (anxiety sensitivity) of two of our mechanistic targets and that engaging that target predicted better smoking outcomes. > There was no evidence of group (DCS vs. placebo) differences in smoking cessation success >
7. Funding/support
This study was funded as R34 grant (R34DA034658; ClinicalTrials.gov identifier: ) by the National Institute on Drug Abuse (Principal Investigators: JAJS, MWO, and MJZ).
8. Role of the funders/sponsors
The funding organizations had no influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest and financial disclosures
Dr. Smits reports receiving support from the National Institutes of Health. He also receives compensation for his work as a consultant to Big Health and his work as editor for Elsevier and the American Psychological Association. He also receives royalties from various book publishers. Dr. Otto reports current and past support from National Institutes of Health. In addition, Dr. Otto reports serving, in the last three years, as a paid speaker and Scientific Advisory Board chair for Big Health, and receiving book royalties from Oxford University Press, Routledge, and Springer. Mr. Papini reports receiving support from the National Institutes of Health and the Donald D. Harrington Foundation.
References
- Abrams K, Zvolensky MJ, Dorman L, Gonzalez A, Mayer M, 2011. Development and validation of the smoking abstinence expectancies questionnaire. Nicotine Tob. Res 13, 1296–1304. 10.1093/ntr/ntr184 [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Taylor S, Smits JAJ, 2014. Panic disorder and agoraphobia: an overview and commentary on DSM-5 changes. Depress. Anxiety 31, 480–486. 10.1002/da.22277 [DOI] [PubMed] [Google Scholar]
- Assayag Y, Bernstein A, Zvolensky MJ, Steeves D, Stewart SS, 2012. Nature and role of change in anxiety sensitivity during NRT-aided cognitive-behavioral smoking cessation treatment. Cogn. Behav. Ther 41, 51–62. 10.1080/16506073.2011.632437 [DOI] [PubMed] [Google Scholar]
- Bakhshaie J, Kulesz PA, Garey L, Langdon KJ, Businelle MS, Leventhal AM, Gallagher MW, Schmidt NB, Manning K, Goodwin R, Zvolensky MJ, 2018. A prospective investigation of the synergistic effect of change in anxiety sensitivity and dysphoria on tobacco withdrawal. J. Consult. Clin. Psychol 86, 69–80. 10.1037/ccp0000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshaie J, Zvolensky MJ, Langdon KJ, Leventhal AM, Smits JAJ, Allan N, Schmidt NB, 2016. Anxiety sensitivity class membership moderates the effects of pre-quit reduction in anxiety sensitivity on quit-day tobacco craving. J. Anxiety Disord. 39, 79–87. 10.1016/j.janxdis.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AC, Rosenfield D, Smits JAJ, 2009. Extinction retention predicts improvement in social anxiety symptoms following exposure therapy. Depress. Anxiety 26, 22–27. 10.1002/da.20511 [DOI] [PubMed] [Google Scholar]
- Blankers M, Smit ES, van der Pol P, de Vries H, Hoving C, van Laar M, 2016. The missing=smoking assumption: a fallacy in internet-based smoking cessation trials? Nicotine Tob. Res 18, 25–33. 10.1093/ntr/ntv055 [DOI] [PubMed] [Google Scholar]
- Bogiaizian D, Zvolensky MJ, Solari A, Paulus DJ, Bakhshaie J, Salazar PL, 2017. Tratamiento de cesación tabáquica, reducción de la sensibilidad ansiosa y manejo de la regulación emocional:: un estudio de caso. Rev. Argent. Clínica Psicológica 26, 356–365. [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Matthew D, Miller IW, 1998. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav 12, 101–112. 10.1037/0893-164X.12.2.101 [DOI] [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE, 2001. Anxiety sensitivity: relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addict Behav. 26, 887–899. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, 2002. Distress tolerance and duration of past smoking cessation attempts. J. Abnorm. Psychol 111, 180–185. 10.1037/0021-843X.111.1.180 [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST, 2002. Applying extinction research and theory to cue-exposure addiction treatments. Addict 97, 155–167. [DOI] [PubMed] [Google Scholar]
- Cosci F, Knuts IJE, Abrams K, Griez EJL, Schruers KRJ, 2010. Cigarette smoking and panic: a critical review of the literature. J. Clin. Psychiatry 71, 606–615. 10.4088/JCP.08r04523blu [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Epstein A, Wittchen H-U, Pine DS, Lewis-Fernández R, Hinton D, DSM V Anxiety OC Spectrum, Posttraumatic and Dissociative Disorder Work Group, 2010. Panic disorder: a review of DSM-IV panic disorder and proposals for DSM-V. Depress. Anxiety 27, 93–112. 10.1002/da.20654 [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R, 2006. Effects of D-Cycloserine on Extinction: Translation From Preclinical to Clinical Work. Biol. Psychiatry 60, 369–375. 10.1016/j.biopsych.2006.03.084 [DOI] [PubMed] [Google Scholar]
- Fagerström K, 2012. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob. Res 14, 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, Blalock JA, Schmidt NB, 2014. Negative affect and smoking motives sequentially mediate the effect of panic attacks on tobacco-relevant processes. Am. J. Drug Alcohol Abuse 40, 230–239. 10.3109/00952990.2014.891038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, Otto MW, Leyro TM, 2015. The role of distress intolerance for panic and nicotine withdrawal symptoms during a biological challenge. J. Psychopharmacol 29, 783–791. 10.1177/0269881115575536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Zvolensky MJ, Babson K, Leen-Feldner EW, Schmidt NB, 2008. An integrated approach to panic prevention targeting the empirically supported risk factors of smoking and anxiety sensitivity: Theoretical basis and evidence from a pilot project evaluating feasibility and short-term efficacy. J. Anxiety Disord 22, 1227–1243. 10.1016/j.janxdis.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J, 2010. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Hamaker EL, Kuiper RM, Grasman RPPP, 2015. A critique of the cross-lagged panel model. Psychol. Methods 20, 102–116. 10.1037/a0038889 [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD, 2006. Longitudinal Data Analysis. John Wiley & Sons. [Google Scholar]
- Hofmann SG, Smits JAJ, Rosenfield D, Simon N, Otto MW, Meuret AE, Marques L, Fang A, Tart C, Pollack MH, 2013. d-Cycloserine as an Augmentation Strategy With Cognitive-Behavioral Therapy for Social Anxiety Disorder. Am. J. Psychiatry 170, 751–758. 10.1176/appi.ajp.2013.12070974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck PR, Spiegel DA, Shear MK, Rucci P, 2002. Reliability of the self-report version of the Panic Disorder Severity Scale. Depress. Anxiety 15, 183–185. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH, 2000. Smoking and mental illness: A population-based prevalence study. JAMA 284, 2606–2610. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ, 2015. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol. Bull 141, 176–212. 10.1037/bul0000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Cruz L, Monzani B, Rosenfield D, Andersson E, Pérez-Vigil A, Frumento P, de Kleine RA, Difede J, Dunlop BW, Farrell LJ, Geller D, Gerardi M, Guastella AJ, Hofmann SG, Hendriks G-J, Kushner MG, Lee FS, Lenze EJ, Levinson CA, McConnell H, Otto MW, Plag J, Pollack MH, Ressler KJ, Rodebaugh TL, Rothbaum BO, Scheeringa MS, Siewert-Siegmund A, Smits JAJ, Storch EA, Ströhle A, Tart CD, Tolin DF, van Minnen A, Waters AM, Weems CF, Wilhelm S, Wyka K, Davis M, Rück C, and the DCS Anxiety Consortium, Altemus M, Anderson P, Cukor J, Finck C, Geffken GR, Golfels F, Goodman WK, Gutner C, Heyman I, Jovanovic T, Lewin AB, McNamara JP, Murphy TK, Norrholm S, Thuras P, 2017. D-Cycloserine Augmentation of Exposure-Based Cognitive Behavior Therapy for Anxiety, Obsessive-Compulsive, and Posttraumatic Stress Disorders: A Systematic Review and Meta-analysis of Individual Participant Data. JAMA Psychiatry 74, 501–510. 10.1001/jamapsychiatry.2016.3955 [DOI] [PubMed] [Google Scholar]
- McLeish AC, Zvolensky MJ, Bucossi MM, 2007. Interaction between smoking rate and anxiety sensitivity: Relation to anticipatory anxiety and panic-relevant avoidance among daily smokers. J Anxiety Disord 21, 849–859. [DOI] [PubMed] [Google Scholar]
- McNally RJ, 2002. Anxiety sensitivity and panic disorder. Biol. Psychiatry 52, 938–946. [DOI] [PubMed] [Google Scholar]
- Otto MW, Eastman A, Lo S, Hearon BA, Bickel WK, Zvolensky M, Smits JAJ, Doan SN, 2016a. Anxiety sensitivity and working memory capacity: Risk factors and targets for health behavior promotion. Clin. Psychol. Rev 49, 67–78. 10.1016/j.cpr.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Otto MW, Kredlow MA, Smits JAJ, Hofmann SG, Tolin DF, de Kleine RA, van Minnen A, Evins AE, Pollack MH, 2016b. Enhancement of Psychosocial Treatment With d-Cycloserine: Models, Moderators, and Future Directions. Biol. Psychiatry 80, 274–283. 10.1016/j.biopsych.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Pachas GN, Cather C, Hoeppner SS, Moshier SJ, Hearon BA, Ward HB, Laffer AB, Smits JAJ, Evins AE, 2019. A placebo-controlled randomized trial of D-cycloserine augmentation of cue exposure therapy for smoking cessation. Cogn. Behav. Ther 48, 65–76. 10.1080/16506073.2018.1476908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Pollack MH, Dowd SM, Hofmann SG, Pearlson G, Szuhany KL, Gueorguieva R, Krystal, John H, Simon NM, Tolin., 2016c. Randomized Trial of D-Cycloserine Enhancement of Cognitive-Behavioral Therapy for Panic Disorder. Depress. Anxiety 33, 737–745. 10.1002/da.22531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Smits JAJ, 2018. Anxiety Sensitivity, Health Behaviors, and the Prevention and Treatment of Medical Illness. Clin. Psychol 25 10.1111/cpsp.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OʼCleirigh C, Zvolensky MJ, Smits JAJ, Labbe AK, Coleman JN, Wilner JG, Stanton AM, Gonzalez A, Garey L, Regenauer KS, Rosenfield D, 2018. Integrated Treatment for Smoking Cessation, Anxiety, and Depressed Mood in People Living With HIV: A Randomized Controlled Trial. J. Acquir. Immune Defic. Syndr 79, 261–268. 10.1097/QAI.0000000000001787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB, 2011. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addict. 106, 418–427. 10.1111/j.1360-0443.2010.03173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, Leitzke CJ, Zehner ME, Fiore MC, Baker TB, 2010. Psychiatric disorders in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. J. Consult. Clin. Psychol 78, 13–23. 10.1037/a0018065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ, 1986. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav. Res. Ther 24, 1–8. [DOI] [PubMed] [Google Scholar]
- Schlam TR, Baker TB, Smith SS, Cook JW, Piper ME, 2019. Anxiety Sensitivity and Distress Tolerance in Smokers: Relations with Tobacco Dependence, Withdrawal, and Quitting Success. Nicotine Tob. Res 10.1093/ntr/ntz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NB, Raines AM, Allan NP, Zvolensky MJ, 2016. Anxiety sensitivity risk reduction in smokers: A randomized control trial examining effects on panic. Behav. Res. Ther 77, 138–146. 10.1016/j.brat.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V, Vander Bilt J, Wang T, 2001. Reliability and validity of the Panic Disorder Severity Scale: Replication and extension. J. Psychiatr. Res 35, 293–296. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, Kassel JD, 1997. Individual differences in the context of smoking lapse episodes. Addict. Behav., Smoking Cessation: Clinical and Research Direction 22, 797–811. 10.1016/S0306-4603(97)00063-4 [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW, 2008a. Reducing anxiety sensitivity with exercise. Depress. Anxiety 25, 689–699. 10.1002/da.20411 [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Berry AC, Tart CD, Powers MB, 2008b. The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: a meta-analytic review. Behav. Res. Ther 46, 1047–1054. 10.1016/j.brat.2008.06.010 [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Kauffman BY, Lee-Furman E, Zvolensky MJ, Otto MW, Piper ME, Powers MB, Rosenfield D, 2016a. Enhancing panic and smoking reduction treatment with dcycloserine: Study protocol for a randomized controlled trial. Contemp. Clin. Trials 48, 46–51. 10.1016/j.cct.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Powers MB, Berry AC, Otto MW, 2007. Translating empirically supported strategies into accessible interventions: The potential utility of exercise for the treatment of panic disorder. Cogn. Behav. Pract 14, 364–374. 10.1016/j.cbpra.2006.07.005 [DOI] [Google Scholar]
- Smits JAJ, Powers MB, Cho Y, Telch MJ, 2004. Mechanism of Change in Cognitive-Behavioral Treatment of Panic Disorder: Evidence for the Fear of Fear Mediational Hypothesis. J. Consult. Clin. Psychol 72, 646–652. 10.1037/0022-006X.72.4.646 [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Zvolenksy MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, Powers MB, Frierson GM, Otto MW, Hopkins LB, Baird SO, 2016b. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: A randomized controlled trial. Psychosom. Med 78, 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Zvolensky MJ, 2006. Emotional vulnerability as a function of physical activity among individuals with panic disorder. Depress. Anxiety 23, 102–106. 10.1002/da.20146 [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Zvolensky MJ, Rosenfield D, Marcus BH, Church TS, Frierson GM, Powers MB, Otto MW, Davis ML, DeBoer LB, Briceno NF, 2012. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with elevated anxiety sensitivity: study protocol for a randomized controlled trial. Trials 13, 207–221. 10.1186/1745-6215-13-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Otto MW, Powers MB, Baird SO, 2018. Anxiety sensitivity as a transdiagnostic treatment target, in: Smits JAJ, Otto MW, Powers MB, Baird SO (Eds.), The Clinician’s Guide to Anxiety Sensitivity Treatment and Assessment. Academic Press, San Diego, C.A. [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Abramowitz JS, Holaway RM, Sandin B, Stewart SH, Coles M, Eng W, Daly ES, Arrindell WA, Bouvard M, Cardenas SJ, 2007. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol. Assess 19, 176–188. 10.1037/1040-3590.19.2.176 [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Marshall EC, Gibson LE, Zvolensky MJ, 2010. Cognitive-affective characteristics of smokers with and without posttraumatic stress disorder and panic psychopathology. Addict. Behav 35, 419–425. 10.1016/j.addbeh.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Maxwell SE, 2015. On disaggregating between-person and within-person effects with longitudinal data using multilevel models. Psychol. Methods 20, 63–83. 10.1037/met0000030 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakhshaie J, 2014a. An Anxiety Sensitivity Reduction Smoking-Cessation Program for Spanish-Speaking Smokers (Argentina). Cogn. Behav. Pract 21, 350–363. 10.1016/j.cbpra.2013.10.005 [DOI] [Google Scholar]
- Zvolensky MJ, Farris SG, Guillot CR, Leventhal AM, 2014b. Anxiety sensitivity as an amplifier of subjective and behavioral tobacco abstinence effects. Drug Alcohol Depend. 142, 224–230. 10.1016/j.drugalcdep.2014.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner EW, McLeish AC, 2005a. Smoking and panic attacks, panic disorder, and agoraphobia: A review of the empirical literature. Clin. Psychol. Rev 25, 761–789. 10.1016/j.cpr.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Garey L, Allan NP, Farris SG, Raines AM, Smits JAJ, Kauffman BY, Manning K, Schmidt NB, 2018a. Effects of anxiety sensitivity reduction on smoking abstinence: An analysis from a panic prevention program. J. Consult. Clin. Psychol 86, 474–485. 10.1037/ccp0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Garey L, Kauffman BY, Manning K, 2018. Integrative treatment program for anxiety sensitivity and smoking cessation., in: Smits JAJ, Otto MW, Powers MB, Baird SO (Eds.), The Clinician’s Guide to Anxiety Sensitivity Treatment and Assessment. Academic Press, San Diego, C.A. [Google Scholar]
- Zvolensky MJ, Rosenfield D, Garey L, Kauffman BY, Langdon KJ, Powers MB, Otto MW, Davis ML, Marcus BH, Church TS, Frierson GM, Hopkins LB, Paulus DJ, Baird SO, Smits JAJ, 2018b. Does exercise aid smoking cessation through reductions in anxiety sensitivity and dysphoria? Health Psychol. 37, 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, Antony MM, McCabe RE, Forsyth JP, Feldner MT, Leen-Feldner E, Karekla M, Kahler CW, 2005b. Evaluating the role of panic disorder in emotional sensitivity processes involved with smoking. J. Anxiety Disord 19, 673–686. 10.1016/j.janxdis.2004.07.001 [DOI] [PubMed] [Google Scholar]