Abstract
Treatment with fludarabine phosphate (9-β-D-arabinofuranosyl-2-F-adenine 5′-phosphate, F-araAMP) leads to regressions and cures of human tumor xenografts that express Escherichia coli purine nucleoside phosphorylase (EcPNP). This occurs despite the fact that fludarabine (F-araA) is a relatively poor substrate for EcPNP, and is cleaved to liberate 2-fluoroadenine at a rate only 0.3% that of the natural E. coli PNP substrate, adenosine. In this study, we investigated a panel of naturally occurring PNPs to identify more efficient enzymes that may be suitable for metabolizing F-araA as part of experimental cancer therapy. We show that Trichomonas vaginalis PNP (TvPNP) cleaves F-araA with a catalytic efficiency 25-fold greater than the prototypic E. coli enzyme. Cellular extracts from human glioma cells (D54) transduced with lentivirus stably expressing TvPNP (D54/TvPNP) were found to cleave F-araA at a rate similar to extracts from D54 cells expressing EcPNP, although much less enzyme was expressed per cell in the TvPNP transduced condition. As a test of safety and efficacy using TvPNP, human head and neck squamous cell carcinoma (FaDu) xenografts expressing TvPNP were studied in nude mice and shown to exhibit robust tumor regressions, albeit with partial weight loss that resolved post-therapy. F-araAMP was also a very effective treatment for mice bearing D54/TvPNP xenografts in which approximately 10% of tumor cells expressed the enzyme, indicating pronounced ability to kill non-transduced tumor cells (high bystander activity). Moreover, F-araAMP demonstrated activity against D54 tumors injected with an E1, E3 deleted adenoviral vector encoding TvPNP. In that setting, despite higher F-araA cleavage activity using TvPNP, tumor responses were similar to those obtained with EcPNP, indicating factors other than F-Ade production may limit regressions of the D54 murine xenograft model. Our results establish that TvPNP is a favorable enzyme for activating F-araA, and support further studies in combination with F-araAMP for difficult-to-treat human cancers
Introduction
The use of Esherichia coli purine nucleoside phosphorylase (EcPNP) to activate deoxyadenosine analogs has demonstrated impressive efficacy against human tumor xenografts in mice (1–10), including cancers of brain, prostate, pancreatic, and bladder, among others. The strategy differs fundamentally from herpes simplex virus thymidine kinase, cytosine deaminase, and other prototypic tumor sensitization genes based on the ability to ablate non-cycling malignant cells (11) (including the putative tumor stem cell compartment), robust bystander activity (12, 13) and a unique mechanism of action that disrupts both protein and RNA synthesis (11). Because preclinical findings have indicated that fludarabine phosphate (9-β-D-arabinofuranosyl-2-F-adenine 5′-monophosphate, F-araAMP) is the best available prodrug for use with EcPNP (14), phase I clinical testing was utilized to evaluate this agent together with intratumoral administration of an E1, E3 deleted adenoviral vector expressing the E. coli enzyme (: IND 14271). In human subjects with head and neck squamous cell carcinoma or other locoregional tumors, strong anticancer activity was observed using the approach in vivo (15).
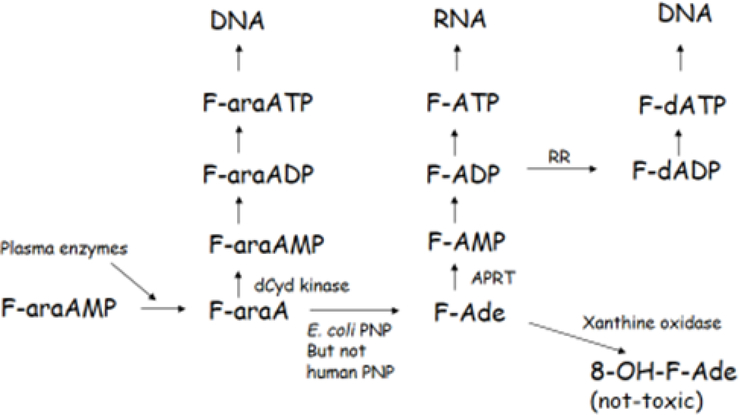
F-araAMP is clinically approved for treatment of chronic lymphocytic leukemia and is a soluble precursor of the nucleoside F-araA, which is the primary circulating form of this compound and a substrate for EcPNP. EcPNP cleaves the glycosidic bond of F-araA, liberating 2-fluoroadenine (F-Ade), a potent cytotoxic adenine analog (11). Human PNP does not recognize adenine containing nucleosides (including F-araA) as substrates (1), and therefore F-Ade is not normally produced in human cells following administration of F-araA. Expression of EcPNP generates F-Ade from F-araA in malignant target tissue, leading to activation of F-Ade by human enzymes primarily to 2-fluoroadenosine 5’-triphosphate (F-ATP), with subsequent RNA incorporation (Figure 1). The ultimate target of F-Ade and its metabolites is not known, but it is clear that unlike most antimetabolites, F-Ade potently inhibits both protein and RNA synthesis (11) and is therefore active against both proliferating and nonproliferating tumor tissue. Importantly, F-Ade freely diffuses across plasma membranes and can destroy neighboring malignant cells that do not express the enzyme, accounting for a strong bystander effect (12, 13). In vivo murine studies with EcPNP and F-araAMP have demonstrated excellent antitumor activity even when < 3% of tumor cells express EcPNP (2). Because F-Ade is effective against the non-proliferating compartment of solid malignancies, this approach is expected to be useful against human tumors with a low mitotic index that are otherwise refractory to conventional chemo- and radiotherapy. Importantly, the toxicity of F-araAMP is not increased in mice bearing cancers that express high levels of EcPNP (2,14,16), indicating very little F-Ade generated within a tumor mass circulates systemically due to slow release from dying cancer cells and inactivation by xanthine oxidase (17). Moreover, F-Ade released from tumor tissue is significantly diluted throughout a murine or human host. In clinical studies, F-Ade could not be detected in serum using a sensitive HPLC-based assay (15). Findings with regard to overall safety are also compatible with the prolonged intratumoral half-life of F-Ade nucleotides (3). F-araAMP has been found superior to other in vivo EcPNP substrates due to relatively low toxicity (2), prolonged circulating half-life (3), and additional pharmacodynamic parameters (14). F-araAMP has the further advantage that it is already clinically approved, a feature that greatly facilitates patient testing.
Figure 1. Metabolism of F-araAMP.
F-araAMP is converted by plasma enzymes to F-araA, which is activated by deoxycytidine kinase (dCyd kinase) in host cells not expressing EcPNP to F-araA nucleotides. F-araATP is a potent inhibitor of DNA polymerases, resulting in inhibition of cell growth. In cells expressing EcPNP, F-araA is also cleaved to F-Ade, which is activated by adenine phosphoribosyl transferase (APRT) to 2-fluoro-adenosine 5’-monophosphate (F-AMP) and subsequently converted to both F-ATP and F-dATP by human enzymes. F-ATP is incorporated into RNA and inhibits both RNA and protein synthesis. Although F-Ade is also converted to deoxynucleotides via ribonucleotide reductase (RR) and incorporated into DNA, cytotoxicity resulting from the incorporation into DNA is secondary to that resulting from production of F-ATP and its disruptive effects on RNA.
Although excellent in vivo antitumor activity has been observed during preclinical xenograft trials with F-araAMP and EcPNP, F-araA is a comparatively poor substrate for EcPNP (3). F-araA is cleaved with a catalytic efficiency only 0.05 or 0.03% that of 6-methylpurine-2′-deoxyriboside (MeP-dR) or 2-F-2′-deoxyadenosine (F-dAdo), two EcPNP substrates that have demonstrated robust in vivo efficacy against EcPNP-expressing human tumor xenografts in mice (3, 18). Prior in vivo studies (18, 2, 3) with tumor cells that stably express EcPNP indicated that enhanced expression of EcPNP resulted in improved anticancer activity of F-araAMP without increasing toxicity, and suggested more efficient conversion of F-araA to F-Ade (on a per cell basis) might result in enhanced antitumor effectiveness. It is reasonable to imagine that PNP activity, itself, could be the major determinant of antitumor activity in certain clinical settings. Part of the rationale for the present study, therefore, was to identify an enzyme that more efficiently cleaves F-araA—as a means to better understand and address limiting features of the overall approach.
Accordingly, we tested PNPs from various prokaryotic organisms to identify enzymes that most effectively convert F-araA to F-Ade. We found that cleavage of F-araA was not improved above that seen with EcPNP for many of the enzymes tested. However, we discovered that Trichomonas vaginalis PNP (TvPNP) was able to cleave F-araA with a catalytical efficiency 25-fold greater than EcPNP. We pursued this observation by characterizing TvPNP biochemically, developing human glioma and head/neck cancer xenografts stably transduced with the enzyme, demonstrating robust antitumor activity of TvPNP in combination with systemic F-araAMP, showing strong bystander killing mediated by the strategy, and testing a replication deficient adenovirus as a delivery vehicle for tumor treatment. Because previous studies suggest that PNP/F-araAMP may be limited by F-araA delivery (16), we also evaluated intratumoral administration of F-araAMP with TvPNP and showed that this route led to more efficient tumor regressions. Our findings indicate very strong anticancer activity mediated by TvPNP, but that for a glioma xenograft model, factors such as fludarabine delivery and/or intratumoral F-Ade distribution are prominent features that limit tumor remission in vivo.
Materials and Methods
Generation of D54 (human glioma) cells expressing TvPNP
D54 tumor cells stably transduced with EcPNP were prepared as described previously, and cell lines encoding TvPNP (D54, human glioma; FaDu, human head and neck squamous cell carcinoma (HNSCC)) were established by published methods (2). T. vaginalis genomic DNA was a gift of Dr. C. Rivers (University of Alabama at Birmingham (UAB)). The TvPNP gene was amplified by PCR using specific primers and AccuPrime Pfx supermix (Life Technologies, CA). Primers were designed based on the TvPNP sequence available at Genbank (XM_001323400); TvPNP-F: 5′ GTTAACGGATCCATGGCAACACCCCATAACTCTGCT 3′; TvPNP-R: 5′ TCTAGAGTTAACGTCCTTATAATTTGATTGCTGCTTC 3′; TvPNP-R1: 5′ ATAGTTTAGATCCGAGGACCAATCAT 3′. First round PCR was performed using TvPNP-F and TvPNP-R1 primers. Nested PCR (2nd round) was conducted using the product from the 1st round of amplification and primers TvPNP-F and TvPNP-R. PCR product was cloned into pCR4Blunt-Topo vector (Invitrogen) and sequenced to ensure identity (pCRBlunt-TvPNP). Lentivirus encoding TvPNP was generated as described (2) based on protocols developed by Trono and colleagues. Replication deficient lentivirus encoding TvPNP was collected from tissue culture supernatant and concentrated by sucrose cushion centrifugation. D54 or FaDu tumor cells were transduced with recombinant lentiviral TvPNP, followed by clonal cell selection and testing for PNP activity.
Generation of an E1E3 deleted adenoviral vector expressing TvPNP (Ad/TvPNP)
To establish adenovirus expressing TvPNP, pCRBlunt-TvPNP was digested with EcoRI and XbaI and cloned into EcoRI and XbaI sites of the pACCMV.pLpA adenovirus transfer vector (gift of Dr. R. Gerard). The pACCMV-TvPNP was cotransfected with pJM17 (Microbix, Ontario, Canada) to obtain recombinant Ad/TvPNP via homologous recombination in 293 cells (19). The resulting Ad/TvPNP was verified by TvPNP DNA specific PCR and TvPNP enzymatic activity. Large scale adenovirus stock was prepared as described previously (20).
Studies of human tumor xenografts in mice
All experiments were performed according to National Institutes of Health (NIH) guidelines and regulations. The Institutional Animal Care and Use Committees (IACUC) of Emory University or Southern Research Institute approved all experimental protocols and procedures. Animals were maintained under barrier conditions at room temperature (21–25° C) with a 12-hour light/dark cycle. Food and water were provided ad libitum.
Parental and TvPNP expressing D54 tumor cells (2 × 107 cells in 0.2 ml) were injected subcutaneously into the flanks of female nude mice (nu/nu) purchased from Charles River Laboratories. Tumors were measured with calipers and an estimate of weight calculated using the equation (length × width2)/2 = mm3 and converted to mg assuming unit density. Mice were monitored daily and body weights and tumor dimensions collected twice weekly. Treatment with recombinant adenoviral vectors and F-araAMP were performed as described in the figures when tumors were 250 – 500 mg. Tumor growth delay (T-C) was determined as the difference in median days to 2 doublings (median days to 2000 mg for the study using intratumoral injections of F-araAMP) between drug-treated and vehicle-treated groups. The time to the evaluation point for each animal was used as the endpoint for Student’s t-test, Mann-Whitney rank sum test, or a life table analysis in order to statistically compare growth data between treatment groups. For parental (FaDu) and transduced (FaDu-PNP) cell lines methods were as above, except 5 X 106 tumor cells in 100 μl volumes were implanted on both flanks of athymic nude mice. Animals were grouped for intraperitoneal injections of saline or fludarabine at ~ 4 weeks, when tumors were 200–300 mg in size. F-araAMP was obtained from Schering A.-G. (Berlin, Germany). F-Ade was obtained from General Intermediates of Canada, Inc. (Edmonton, Alberta Canada).
Measurement of PNP activity
Purified TvPNP was obtained from Dr. CC Wang (University of California, San Francisco) and EcPNP protein prepared as described previously (3). PNP activity was measured at 25° C in 200 μl volumes containing 50 mM potassium phosphate, 100 mM HEPES buffer (pH 7.4), either 100 μM MeP-dR or F-araA, and an amount of enzyme providing a linear increase in product formation during the incubation period. Reactions were stopped by boiling and precipitated proteins removed by filtration (0.2 μm). Formation of product (MeP or F-Ade) was monitored using reverse phase HPLC as described (3). One unit of PNP activity was defined as the amount of extract necessary to cleave 1 nmole of substrate (MeP-dR or F-araA) per mg protein in a one hour period. TvPNP and EcPNP activities were also measured in transduced cells and tumor tissue removed from mice on the first day of treatment with F-araAMP. Cell pellets and tumors were homogenized in 10 mM HEPES buffer (pH 7.4), centrifuged at 100,000 x g, and the supernatant dialyzed against 100 mM HEPES (pH 7.4) buffer containing 20% glycerol and 1 mM dithiothreitol. PNP activity was then determined for extracts as above.
Results
Evaluation of TvPNP enzyme activity
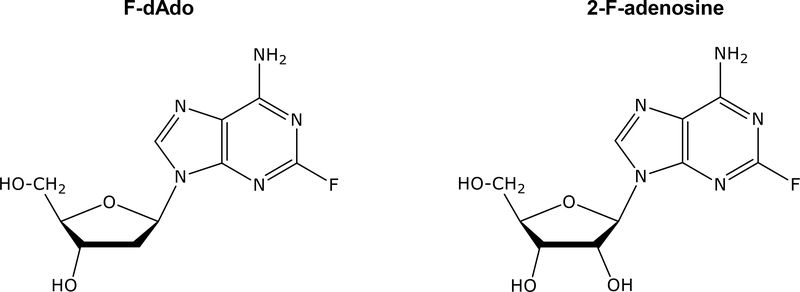
Purified EcPNP or TvPNP were incubated with 100 μM of each substrate shown in Table 1 and rates of each reaction determined. EcPNP and TvPNP cleaved adenosine with similar efficiency. The rate of cleavage of adenosine by TvPNP was 501,000 units, whereas EcPNP exhibited 398,000 units. Similar cleavage rates between EcPNP and TvPNP were also observed with other adenine ribose or deoxyribose nucleoside analogs (2-F-adenosine, F-dAdo, 6-methylpurine riboside, and MeP-dR), although cleavage of 2-Cl-2’-deoxyadenosine (cladribine) by TvPNP was approximately 10-fold greater than EcPNP. Importantly, TvPNP cleaved arabinofuranosyl adenine analogs at rates that were 25 to 61-fold greater than observed with EcPNP. The rate of F-araA cleavage by TvPNP was 7% that seen with MeP-dR, whereas with EcPNP it was 0.3% that of MeP-dR, which further supports F-araA as a superior substrate for TvPNP. F-araA is an isomer of 2-F-adenosine (Figure 2), and the only structural difference in these compounds is the orientation of the hydroxyl group at the 2’ position of the molecule.
Table 1.
Comparison of substrate activity of TvPNP and EcPNP
| Substrate | TvPNP | EcPNP |
|---|---|---|
| nmoles/mg-h | ||
| Adenosine | 501,000 | 398,000 |
| 9-β-D-arabinofuranosyl adenine | 38,000 | 620 |
| 2-F-adenosine | 185,000 | 215,000 |
| 2-F-2′-deoxyadenosine (F-dAdo) | 400,000 | 435,000 |
| 9-β-D-arabinofuranosyl-2-F-adenine (F-araA, fludarabine) | 32,000 | 1,300 |
| 2-Cl-2′-deoxyadenosine (cladribine) | 350,000 | 39,000 |
| Inosine | 154,000 | 342,000 |
| 2′-deoxyinosine | 660,000 | 664,000 |
| 9-β-D-arabinofuranosyl-hypoxanthine | 48 | 61 |
| Guanosine | 14,000 | 156,000 |
| 9-β-D-arabinofuranosyl-guanine | 16 | 310 |
| 9-β-D-ribofuranosyl-6-methylpurine | 155,000 | 92,000 |
| 9-[2-deoxy-β-D-ribofuranosyl]-6-methylpurine (MeP-dR) | 484,000 | 461,000 |
| 9-β-D-arabinofuranosyl-6-methylpurine | 570 | 14 |
Enzymes were incubated with 100 μM of each compound and cleavage rate determined using reverse phase HPLC to separate base from nucleoside. The results with EcPNP have been reported previously [23] except for guanosine, 9-β-D-arabinofuranosyl-guanine, and 9-β-D-arabinofuranosyl-6-methylpurine. Each value is the mean of at least two determinations which exhibited good agreement.
Figure 2.
Structures of 2-F-adenosine, F-dAdo, and F-araA.
Interestingly, cleavage by arabinofuranosyl hypoxanthine (araHx) or arabinofuranosyl guanine (araG) using TvPNP was not more efficient than with EcPNP, indicating arabinofuranosyl nucleosides are not uniformly superior substrates. Although 2′-deoxyinosine cleavage by the two enzymes was similar, the TvPNP cleaved guanosine at a rate <10% of EcPNP. The ratio of araG/guanosine cleavage activity was similar for the two enzymes (0.0011 vs 0.0020), suggesting that poor enzymatic activity for arabinofuranosyl guanine by TvPNP was primarily due to poor activity against guanine nucleosides, which would not be an issue with regard to adenine arabinofuranosyl nucleosides, such as F-araA.
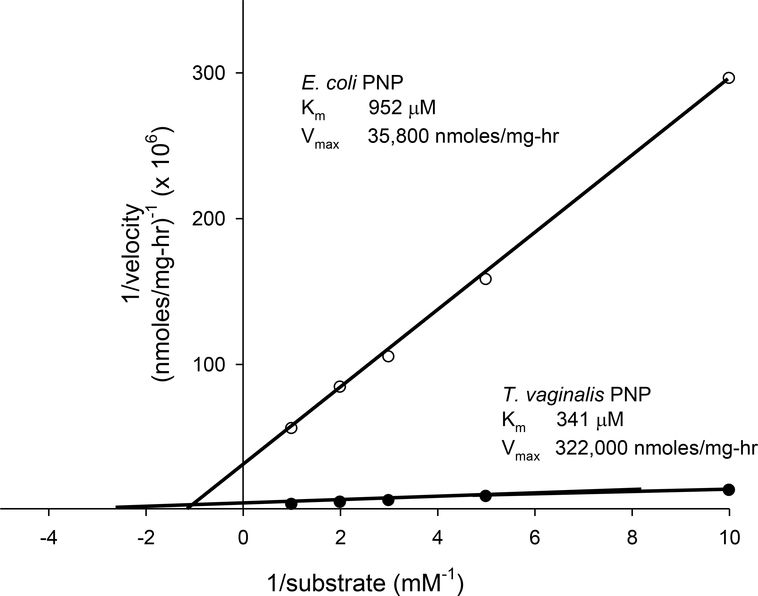
Measured Km for F-araA with TvPNP was determined to be ~ 340 μM, whereas with EcPNP it was ~ 950 μM (Figure 3). The Vmax of F-araA with TvPNP was approximately 320,000 units, versus 36,000 units for EcPNP. These results indicate that the catalytic efficiency (Vmax/Km) of TvPNP using F-araA was 25-fold greater than EcPNP, and suggests use of the T. vaginalis enzyme would be efficacious as part of in vivo prodrug activation. We therefore generated tools for evaluating TvPNP in conjunction with F-araAMP against solid tumor models in mice.
Figure 3. Michaelis-Menten plot of 1/(velocity of the reaction) vs. 1/(F-araA concentration) obtained for EcPNP or TvPNP.
Enzymes were incubated with increasing concentrations of F-araA (0.1 to 1 mM) in triplicate and conversion to F-Ade determined using reverse phase HPLC. The rate of reaction for each concentration was determined and the reciprocal of reaction velocity vs. reciprocal of substrate concentration plotted. The amount of F-Ade produced was less than 2% of total F-araA in all cases and standard deviation of each measurement was less than 13% of the mean. Linear regression analysis of the data resulted in r values greater than 0.992.
Tumor regression of head and neck squamous cell carcinoma mediated by TvPNP
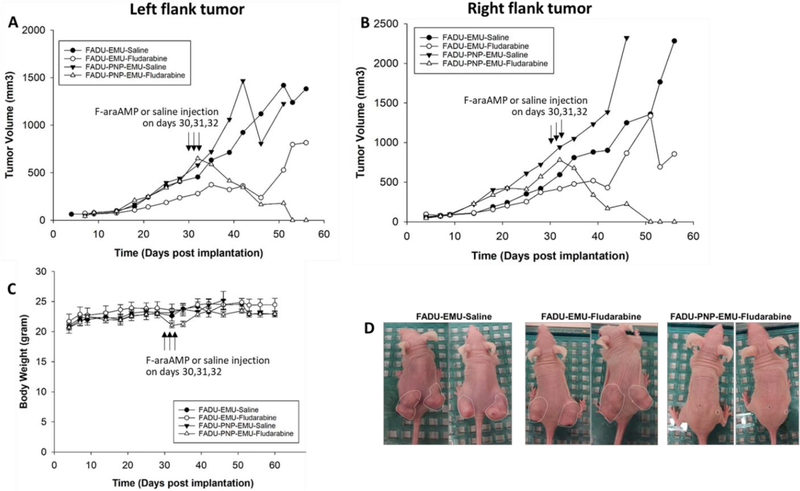
Head and neck tumors have been utilized both in animal models and the clinical setting for intratumoral viral administration, including an EcPNP clinical trial completed by our laboratory (21). We therefore tested an HNSCC cancer model in the present studies. TvPNP was anticipated to generate significantly greater levels of F-Ade (in comparison to EcPNP) based on catalytic efficiency data shown above. However, because F-Ade ablates both dividing and non-dividing cells, a highly active enzyme could engender substantial risk of host toxicity. Accordingly, we applied a stringent protocol in which 100% of FaDu HNSCC tumor cells expressed recombinant TvPNP in human tumor xenografts. Results in Figure 4 demonstrate durable and pronounced regressions with substantial prolongation of life against otherwise lethal tumors. Modest (10 – 12 %) weight loss was observed in the TvPNP/fludarabine treatment group, which resolved following treatment. TvPNP activity on the day of F-araAMP treatment was > 800 PNP units, which is compatible with other studies showing strong anticancer activity in vivo using tumors expressing EcPNP (2, 14, 16, 18).
Figure 4. Tumor regressions of human head and neck squamous cell carcinoma transduced with TvPNP.
FaDu cells (a model of human HNSCC) were transduced with TvPNP using a retroviral construct. As a stringent test of safety, tumor masses containing 100% transduced cells were established bilaterally and compared to parental tumors (no PNP expression). Strong and durable anti-cancer activity was observed specifically following TvPNP transduction with a brief (3 day) course of F-araAMP (167 mg/kg given three times a day for 3 days beginning on day 30, Panels A and B). Parental and PNP tumors without fludarabine administration were studied as controls. Animals in all groups tolerated treatment well, with minor weight loss (Panel C) that resolved post-therapy and no treatment-related deaths (n = 5 animals per cohort). A photomicrograph showing representative animals is provided in Panel D. Growth rate of tumors treated with TvPNP and fludarabine was significantly different from other groups (p < 0.0001).
F-araA cleavage in D54 cells that stably express TvPNP
We next evaluated human glioma cell lines expressing TvPNP. Human glioma comprise a second tumor type well suited for intratumoral PNP administration, and have provided a basis for previous studies of EcPNP in vitro and in vivo (1, 2, 16, 18, 22). We therefore applied the D54 model to a series of experiments with TvPNP. Cell culture extracts from D54/TvPNP or D54 cells expressing EcPNP (D54/EcPNP) were first assayed for ability to cleave MeP-dR or F-araA. Parental cells (without PNP expression) exhibit no conversion of either substrate (2). The rate of cleavage of F-araA by cell extracts from the two lines was similar (360 ± 8 vs 350 ± 6 nmoles/mg-h). However, extracts from D54/EcPNP cells cleaved MeP-dR at a rate 12-fold greater than D54/TvPNP cells (150,000 ± 10,000 vs 13,000 ± 500 units). Since EcPNP and TvPNP cleave MeP-dR with similar efficiency (Table 1), this indicated approximately 12-fold increased functional enzyme in D54/EcPNP on a per cell basis. The relative rates of cleavage of F-araA and MeP-dR (using transduced tumor cell lysates) are consistent with results obtained from purified PNP enzymes (Table 1 and Figure 3) and support the conclusion that F-araA is a superior substrate for TvPNP compared to EcPNP.
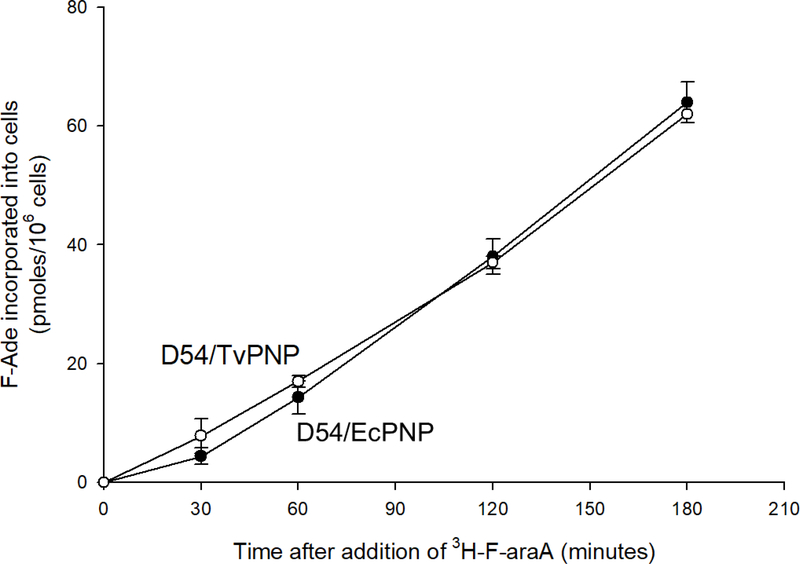
To verify F-araA cleavage by the two models, cells expressing either EcPNP or TvPNP were incubated with [3H]F-araA, and the amount of F-Ade produced and converted to F-Ade metabolites was investigated. Results shown in Figure 5 demonstrate that F-Ade incorporation into cells was similar for both recombinant cell lines. Approximately 50% of administered F-araA was cleaved in either cell culture following 180-minute incubation, and approximately 60% of F-Ade produced by cleavage of F-araA remained associated with cells (40% released to the medium). Previous experiments have shown that concentrations of F-araA in medium overlying parental (non-PNP expressing) D54 cultures do not decrease over a 180 minute incubation (14). Disappearance of radioactivity from tissue culture supernatant is therefore attributable to cleavage of F-araA by PNP, followed by activation of F-Ade to nucleotide metabolites within tumor cells. Similar metabolism of F-araA by the two cell models complements enzyme assay results using cell extracts, and indicates F-araA cleavage by the lines is similar despite > 10-fold higher levels of EcPNP activity as judged by enzymatic assay on a per milligram tissue basis and known kinetics for MeP-dR versus F-araA.
Figure 5. Cleavage and utilization of F-araA by D54/EcPNP or D54/TvPNP cells.
D54 tumor cells transduced with either EcPNP (D54/EcPNP, filled circles) or TvPNP (D54/TvPNP, open circles) were incubated in triplicate with 10 μM [3H]F-araA. A portion of the culture medium was removed at various time points following addition, and analyzed for F-araA or F-Ade by reverse phase HPLC (3). The amount of F-araA converted to F-Ade and activated to F-Ade nucleotides intracellularly was determined by measuring loss of radioactivity from the medium. At the end of each experiment, cells were harvested using Tryple Express (Gibco, Grand Island NY) and cell numbers determined using a Coulter Counter. Each value represents the mean and standard deviation of three measurements.
Treatment of D54 tumors that stably express TvPNP
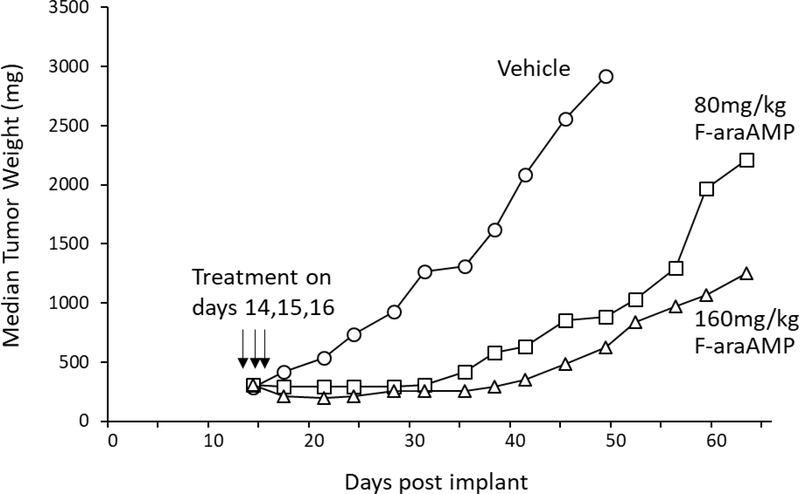
D54/TvPNP cells were next used to generate subcutaneous tumors on flanks of mice in which 10% of malignant cells expressed TvPNP. We have shown previously that systemically administered F-araAMP does not affect growth of parental D54 (non PNP expressing) tumors in mice (1, 2, 14, 16, see also Figure 8, below). However, when mice with TvPNP bearing tumors were treated intraperitoneally with either 80 or 160 mg/kg F-araAMP for three consecutive days, excellent antitumor activity was observed (Figure 6), a finding compatible with studies of EcPNP using this otherwise refractory tumor model (2, 14, 16, 18).
Figure 8. Effect of intratumoral F-araAMP plus Ad/TvPNP or Ad/EcPNP on D54 tumor growth.
Parental D54 human glioma tumors were treated intratumorally with vehicle (open circles), Ad/EcPNP (open squares), Ad/TvPNP (open diamonds), F-araAMP (filled circles), Ad/EcPNP plus F-araAMP (filled squares), or Ad/TvPNP plus F-araAMP (filled diamonds). Adenoviral vectors (2 × 1011 VP) were injected into the tumors twice a day (at 6-hour intervals) for 3 consecutive days starting on day 20 post implant. Eighteen mg F-araAMP dissolved in 0.15 ml DMSO was injected into tumors once per day for 3 consecutive days starting on day 25 post implant. F-araAMP (0.15 ml), adenoviral vectors (0.2 ml), or PBS/3% sucrose control (0.2 ml) were injected into D54 tumors using 8 separate injections of approximately equal volume in an effort to evenly distribute the administered agents. Cleavage rates of MeP-dR and F-araA (on day 25) by EcPNP in the D54 tumors treated with Ad/EcPNP were 1,200 ± 900 units and 5 ± 4 units, respectively. Cleavage of MeP-dR and F-araA (on day 25) by TvPNP in D54 tumors treated with Ad/TvPNP were 2,200 ± 700 units and 150 ± 50 units, respectively. Each treatment arm contained 8 mice. There were no deaths or significant weight loss in any of the treatment groups. The growth of tumors in mice treated with F-araAMP plus Ad/EcPNP or Ad/TvPNP was significantly different than vehicle treated controls (P< 0.001) and was also significantly different than in mice treated with F-araAMP alone (P = 0.037 and 0.011, respectively).
Figure 6. Effect of F-araAMP on D54 tumors in which 10% of the cells express T. vaginalis PNP.
Wild-type D54 tumor cells (parental) were mixed with D54 cells transduced using the T. vaginalis PNP gene so that 10% of the mixture expressed the transgene. Tumors were established sc in the flanks of nude mice and allowed to reach a size of approximately 300 mg. Mice were then administered either 80 or 160 mg/kg F-araAMP (dissolved in saline) in the peritoneal cavity (0.2ml/10 g of body weight) three times per day (every 4 hours) for 3 consecutive days beginning on day 14 post implant. Cleavage rates of MeP-dR and F-araA in tumors on day 14 were 430 ± 30 units and 16 ± 1 units, respectively (N=3). Each treatment arm contained 6 mice, and no animals died in any treatment group. (6% weight loss in mice treated with 80 mg/kg F-araAMP and 14% weight loss following 160 mg/kg F-araAMP were noted that resolved post therapy.) The growth of tumors in mice administered F-araAMP was significantly different than controls (P< 0.001).
Development of an adenoviral vector expressing TvPNP
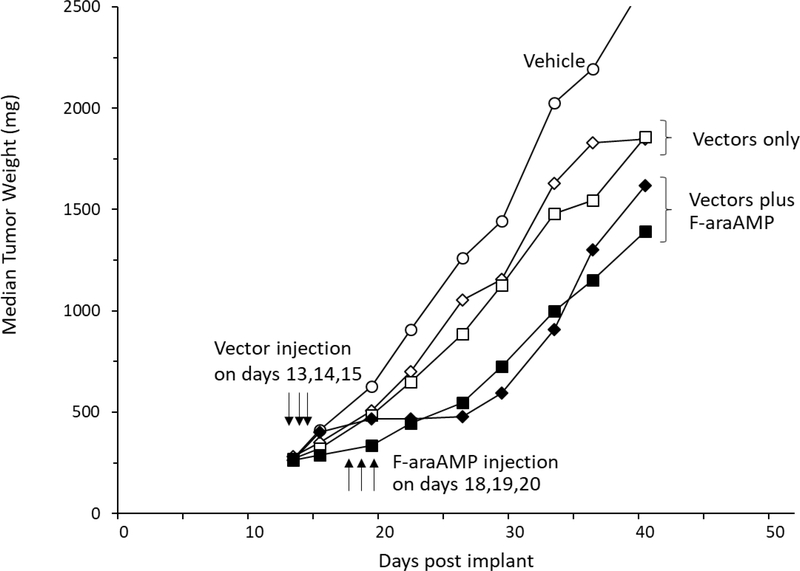
Earlier findings have indicated that treatment with F-araAMP results in strong antitumor activity in D54 tumors injected with an adenoviral vector expressing EcPNP followed by systemic F-araAMP (Ad/EcPNP (2)). To test a similar approach using the more active TvPNP enzyme, an adenovirus was generated. Systemic treatment of mice with 120 mg/kg F-araAMP inhibited growth of tumors that had been injected with Ad/TvPNP (T-C value of 12 days, Figure 7). The slowing of tumor growth was similar to that seen in D54 tumors inoculated with Ad/EcPNP and treated intraperitoneally with 120 mg/kg F-araAMP (T-C value of 10 days). Representative tumors were removed from three mice on the first day of F-araAMP treatment and levels of MeP-dR and F-araA cleavage determined using tumor extracts. MeP-dR cleavage activity in tumors injected with Ad/EcPNP or Ad/TvPNP was similar, indicating both vectors expressed comparable amounts of enzymatic activity.
Figure 7. Effect of systemic F-araAMP plus Ad/TvPNP or Ad/EcPNP on D54 tumor growth.
Parental D54 tumor cells (no PNP expression) were established subcutaneously on flanks of nude mice. Thirteen days following implantation, tumors were injected with 2 × 1011 virus particles of Ad/EcPNP (squares) or Ad/TvPNP (diamonds) once a day for 3 consecutive days (i.e., days 13, 14, and 15). Each administration of adenoviral vector was in a 150 μl volume by 8 separate injections of approximately 20 μl each in an effort to evenly distribute transgene expression throughout tumor tissue. On day 18, adenovirus treated mice were given 120 mg/kg F-araAMP (dissolved in saline) IP (0.2 ml/10 g of body weight) three times per day (every 4 hours) on days 18, 19, and 20) (filled squares and diamonds) or vehicle (open squares and diamonds). Tumors in the no-treatment group (open circles) were injected with 150 μl saline intratumorally once a day for 3 days (13, 14, 15), and subsequently injected IP (0.2 ml/10 g of body weight) with saline three times a day for 3 consecutive days (18, 19, 20). The rates of cleavage of MeP-dR and F-araA in Ad/TvPNP treated tumors on day 18 were 2,900 ± 600 units and 130 ± 40 units, respectively; in tumors treated with Ad/EcPNP, rates of cleavage were 3,100 ± 1,500 units and 26 ± 10 units, respectively (N=3). Each treatment arm contained 8 mice. Five percent weight loss was noted in mice treated with Ad/EcPNP plus F-araAMP, with 13% weight loss in mice treated with Ad/TvPNP plus F-araAMP, which resolved. One mouse died in the Ad/TvPNP plus F-araAMP treatment group, which could be attributable to F-araAMP or combination treatment. No weight loss or deaths were noted in other treatment groups. Growth rates of tumors treated with F-araAMP plus Ad/EcPNP or Ad/TvPNP were significantly different than vehicle treated controls (P< 0.001).
In previous experiments, we demonstrated antitumor activity when both vector and F-araAMP were injected directly into a tumor mass (16). The approach allows increased prodrug delivery to tumor parenchyma as a means to overcome suboptimal levels of F-araA within malignant tissue. Strong inhibition of tumor growth was observed by applying this protocol using TvPNP plus IT F-araAMP (Figure 8). It is important to note that antitumor activity was observed here when F-araAMP was injected into tumors of approximately 1 gram (roughly 5% of total animal weight), and that regressions of large xenografts such as these represent a very stringent test for efficacy. Tumor mass in these experiments decreased from 1 gram to approximately 500 mg after a single three day course of treatment with prodrug. Antitumor activity with Ad/TvPNP was again similar to Ad/EcPNP, presumably because F-araA cleavage following intratumoral prodrug administration is not rate limiting in studies such as these (see also below).
Discussion
In the current report we identify a non-mammalian enzyme (TvPNP) suitable for improved cleavage of F-araA. In contrast to previous studies based on EcPNP or other purine nucleoside phosphorylases, F-araA can serve as a superior substrate for TvPNP (Table 1 and Figure 3); i.e., catalytic efficiency of TvPNP with F-araA was 25-fold greater than the prototypic enzyme characterized previously (EcPNP). The current study also demonstrates robust in vivo antitumor activity mediated by TvPNP plus F-araA, with use of this enzyme resulting in strong bystander effect (Figures 6–8). Our findings show growth inhibition mediated by stably expressed TvPNP, even when levels of F-araA cleavage activity are markedly less than following adenoviral gene transfer (16 units versus 130 units; compare Figures 6 and 7). Antitumor activity was comparable in these studies, a finding likely due to more diffuse distribution of PNP using transduced cell lines (as opposed to adenoviral gene delivery, which results in compartmentalized gene expression primarily along needle tracks) (2).
Although the TvPNP enzyme was dramatically superior to EcPNP in vitro, studies in vivo showed strong but comparable antitumor activity with either enzyme. We note that since EcPNP or TvPNP following injection of an adenoviral vector are limited to isolated pockets of tumor cells within a malignant mass, enhanced enzymatic activity on a “per cell” basis may not confer a prominent effect on overall D54 cancer regression. For example, the amount of F-Ade produced by an EcPNP treated tumor may be sufficient to kill all or most of the neoplastic cells in the region expressing the transgene. If 50% of susceptible tumor cells surrounding an injection site are destroyed using Ad/EcPNP, a 25-fold increase in enzyme activity (and F-Ade production) with Ad/TvPNP vector would only result in (at best) a 2-fold increase in overall cell killing. In other words, our findings suggest PNP enzymatic activity is not rate limiting for tumor regression in studies such as those shown here. Physical limits to bystander activity (due to inefficient PNP delivery by adenoviral vectors) and distribution of transgene may therefore be more important determinants of antitumor activity for in vivo models than the amount of toxin being produced.
By the same token, if delivery of F-araA to tumor parenchyma is rate limiting for anticancer efficacy, it becomes important to consider the possibility that all or most F-araA reaching tumor cells expressing either EcPNP or TvPNP is cleaved (irrespective of underlying enzyme activity), so that use of a superior enzyme such as TvPNP might not enhance efficacy (i.e., comparable amounts of F-Ade would be liberated in either case despite use of TvPNP for in vivo xenograft studies of this type). In that scenario, improved methods for delivery of F-araA to tumor parenchyma would be expected to optimize antitumor activity. This interpretation is supported by strong efficacy noted following direct (intratumoral) F-araAMP inoculation (Figure 8), previous publications using EcPNP, and recent evidence of enhanced efficacy using conventional chemotherapy delivered intratumorally (https://intensitytherapeutics.com/products/lead-product-int230–6/).
Our results with tumors that express high level EcPNP indicate that F-araAMP is superior to F-dAdo as a mediator of tumor regressions (1), despite the finding that F-dAdo is a much better substrate for EcPNP (Table 1). The catalytic activity of EcPNP with F-araA is 0.04% that of F-dAdo. The superior antitumor activity of F-araAMP in vivo can at least partially be attributed to the fact that mice tolerate 5 times more F-araAMP than F-dAdo (2,14) and that the plasma half-life (3) of F-araA in mice (50 minutes) is much longer than that of F-dAdo (7 minutes). In addition, we have shown that F-araA is cleaved in cells expressing high levels of EcPNP with much greater efficiency than expected based solely on kinetic parameters of the enzyme (14). Such observations suggest that improved substrate activity with the PNP enzyme is not a primary criteria for antitumor efficacy, and underscore importance of pharmacodynamic parameters in this approach to cancer treatment.
Mammalian PNP does not recognize F-araA as a substrate and therefore F-Ade is not produced in humans following treatment with F-araAMP (i.e., in the absence of EcPNP). The rate limiting step for production of F-Ade metabolites is production of F-Ade by EcPNP (Figure 1). Antitumor activity using gene-based systems such as EcPNP depend on numerous features, and the most important contributing mechanism(s) are not well defined. For example, we have shown previously that at a given level of intratumoral EcPNP expression, regressions of tumors exhibit strong dose dependence on the amount of F-araAMP administered systemically (2). We have further established that improving intratumoral F-araA delivery by intratumoral inoculation can augment efficacy (16). These results suggest F-araA dose intensity limits anticancer effect—and raises the possibility that insufficient F-araA delivery, itself (rather than level of PNP enzyme) represents a key obstacle. In addition, as described above, earlier findings indicate the potential importance of F-Ade diffusion to neighboring (non-PNP expressing) tumor cells as a means to optimize tumor regression (2). It is not known which of these factors (PNP activity, F-araA delivery, F-Ade partitioning, or other aspects) predominates as a barrier to PNP based treatment. In the present study, availability of a well characterized TvPNP enzyme has allowed a novel test of this question—and suggests EcPNP activity at currently achievable levels is not rate limiting in D54 xenografts.
F-araAMP is the preferred prodrug (in comparison to F-dAdo or MeP-dR) when high level enzyme is established in tumor cells. This includes stably transduced cancer lines generated with lentivirus encoding E. coli PNP (D54/EcPNP), or when tumors are injected with adenovirus expressing the E. coli enzyme (2, 14). However, when cells express comparatively lower levels of PNP transgene, F-araAMP has been found significantly less active than either MeP-dR or F-dAdo (18). TvPNP may be of particular value, therefore, in the setting of vectors with transgene expression lower than achieved in the present studies (Figures 4 and 6–8). For example, when F-araAMP is given in combination with vector systems that do not result in robust transgene expression (such as herpes viral constructs (22)), TvPNP should result in much better activation of F-araAMP than EcPNP. The same may be true for gene transfer trials in the clinic, where the ability to transduce a large proportion of tumor cells can be limiting. From this perspective, and in contrast to use of EcPNP in murine models in vivo, TvPNP may represent an optimal enzyme for testing in patients, since humans tolerate much less F-araAMP than mice (25 mg/m2, Q1Dx5 (human) vs 300 mg/m2, Q2Hx5 Q1Dx3 (murine)). The plasma concentration of F-araA following systemic administration of F-araAMP to humans (1 to 2 μM) is much lower than plasma F-araA following intraperitoneal injection of F-araAMP in mice (~100 μM), although the plasma half-life of F-araA in humans (10 to 20 hours) is much longer than in murine circulation (50 minutes), a feature that likely compensates for lower plasma levels. Based on a markedly prolonged circulation half-life of F-araA in humans and lower serum levels of the compound, the use of an enzyme with higher catalytic efficiency (lower Km and higher Vmax) could lead to greater amounts of F-Ade generated in PNP-based clinical trials.
In published studies, toxicity of F-araAMP was not affected in mice across a 2 – 3 log range of enhanced intratumoral EcPNP activity (2, 18). Similarly, weight loss or other evidence of toxicity due to a more efficiently cleaved substrate, F-dAdo, was not increased in mice bearing tumors expressing EcPNP. Toxic sequelae are important features that must be carefully balanced against increased cleavage activity in experimental systems such as those described here. In a clinical trial that tested EcPNP (15), negligible toxicity was observed despite significant antitumor effect. We believe the relatively low toxicity of F-Ade results in part from a prolonged half-life in mammalian tumor tissue following generation by EcPNP (> 24 hours, reference 3), much higher concentrations of F-Ade within a malignant mass (where the active compound is generated) compared to systemic levels (i.e., F-Ade released from tumor is diluted several orders of magnitude throughout a mouse or human), and rapid elimination by xanthine oxidase (unpublished observation). As predicted, serum F-Ade in human subjects receiving EcPNP plus F-araAMP was not detected using a MS/HPLC assay sensitive to < 1 ng/ml. In this context, Figure 4 shows a modest decrease of weight (10 – 12 %) for animals bearing TvPNP tumors treated with F-araAMP. The observed weight loss resolved spontaneously and returned to normal after cessation of therapy, indicating treatment was well tolerated. However, since enhancement of F-araAMP toxicity has not been observed in mice bearing tumors expressing EcPNP, these results suggest increased F-Ade production by TvPNP may confer loss of weight beyond that noted previously with EcPNP/fludarabine. Further studies will be necessary to evaluate this possibility.
In summary, data presented here describes in vitro cleavage activity for several TvPNP substrates, evidence for a 25-fold enhancement of F-araA cleavage by TvPNP based on both purified enzyme and recombinant overexpression, analysis of lentiviral or adenoviral tumor transduction, and studies of in vivo tumor regression. Future experiments will be needed to more fully elucidate why enhanced enzyme activity does not significantly augment D54 xenograft regressions in mice. However, we believe TvPNP may be of value in human subjects where much less F-araAMP can be administered, gene delivery is less efficient, and tumors have a lower proliferative index (doubling times of months instead of days)—and are therefore more difficult to treat. While conclusions regarding the potential for human therapeutic benefit must be tempered in light of significant differences between murine xenografts and solid tumors in man, evaluation of an alternative PNP enzyme, including enzymatic behavior in vitro and in vivo, provides important new information in this regard.
Acknowledgments
We thank Jan Tindall for help with preparation and revision of the manuscript. The research reported in this publication was supported in part by the Cancer Animal Models shared resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The work was funded by NIH grant CA119170, R01DE026941, 5T32CA160040, and the Georgia Research Alliance.
Footnotes
Disclosure statement:
Dr. Parker and Dr. Sorscher have ownership interests in PNP Therapeutics and serve on the Board of Directors for the company, which develops products used in research described by the paper. Drs. Parker and Sorscher are also inventors of technology being evaluated in studies described by this report. The terms of this arrangement for Dr. Sorscher have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Paula Allan and Jeong Hong also have minor equity interest in this company.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Parker WB, Sorscher EJ (2017) Use of E. coli purine nucleoside phosphorylase in the treatment of solid tumors. Current Pharmaceutical Design 23: 7003–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong JS, Waud WR, Levasseur DN, Townes TM, Wen H, McPherson SA, Moore BA, Bebok Z, Allan PW, Secrist JA III, Parker WB, and Sorscher EJ (2004) Excellent in vivo bystander activity of fludarabine phosphate against human glioma xenografts that express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res. 64: 6610–6615. [DOI] [PubMed] [Google Scholar]
- 3.Parker WB, Allan PW, Hassan AEA, Secrist JA III, Sorscher EJ, and Waud WR (2003) Anti-tumor activity of 2-fluoro-2’-deoxyadenosine against tumors that express E. coli purine nucleoside phosphorylase. Cancer Gene Therapy 10: 23–29. [DOI] [PubMed] [Google Scholar]
- 4.Voeks D, Martiniello-Wilks R, Madden V, Smith K, Bennetts E, Both GW, and Russell PJ (2002) Gene therapy for prostate cancer delivered by ovine adenovirus and mediated by purine nucleoside phosphorylase and fludarabine in mouse models. Gene Therapy 9: 759–768. [DOI] [PubMed] [Google Scholar]
- 5.Martiniello-Wilks R, Wang XY, Voeks DJ, Dane A, Shaw JM, Mortensen E, Both GW, and Russell PJ (2004) Purine nucleoside phosphorylase and fludarabine phosphate gene-directed enzyme prodrug therapy suppresses primary tumour growth and pseudo-metastases in a mouse model of prostate cancer. J Gene Med. 6: 1343–1357. [DOI] [PubMed] [Google Scholar]
- 6.Deharvengt S, Wack S, Aprahamian M, Hajri A. (2005) Transcriptional tumor-selective promoter targeting of E. coli purine nucleoside phosphorylase for pancreatic cancer suicide gene therapy. J Gene Med. 7: 672–680. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi E, Menendez S, Ozu C, Ohori M, Cordon-Cardo C, Logg CR, Kasahara N, Bochner BH (2007) Delivery of replication-competent retrovirus expressing Escherichia coli purine nucleoside phosphorylase increases the metabolism of the prodrug, fludarabine phosphate and suppresses the growth of bladder tumor xenografts. Cancer Gene Ther. 2007 14: 279–86. [DOI] [PubMed] [Google Scholar]
- 8.Ungerechts G, Springfield C., Frenzke ME, Lampe J, Johnston P, Parker WB, Sorscher EJ, Cattaneo R (2007) Lymphoma chemovirotherapy: CD20-targeted and convertasearmed measles virus can synergize with fludarabine. Cancer Res. 67: 10939–10947. [DOI] [PubMed] [Google Scholar]
- 9.Bossow S, Grossardt C, Temme A, Leber MF, Sawall S, Rieber EP, Cattaneo R, von Kalle C, Ungerechts G (2011) Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Therapy 18: 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie X, Guo J, Kong Y, Xie GX, Li L, Lv N, Xiao X, Tang J, Wang X, Liu P, Yang M, Xie Z, Wei W, Xie X (2011) Targeted expression of E. coli purine nucleoside phosphorylase and Fludara for prostate cancer therapy. J Gene Med. 13: 680–691. [DOI] [PubMed] [Google Scholar]
- 11.Parker WB, Allan PW, Shaddix SC, Rose LM, Speegle HF, Gillespie GY, and Bennett LL Jr (1998) Metabolism and metabolic actions of 6-methylpurine and 2-fluoroadenine in human cells. Biochem. Pharmacol 55: 1673–1681. [DOI] [PubMed] [Google Scholar]
- 12.Hughes BW, Wells AH, Bebok Z, Gadi VK, Garver RI Jr, Parker WB, and Sorscher EJ (1995) Bystander killing of melanoma cells using the human tyrosinase promoter to express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res. 55: 3339–3345. [PubMed] [Google Scholar]
- 13.Hughes BW, King SA, Allan PW, Parker WB, and Sorscher EJ (1998) Cell to cell contact is not required for bystander cell killing by E. coli purine nucleoside phosphorylase. J. Biol. Chem 273: 2322–2328. [DOI] [PubMed] [Google Scholar]
- 14.Parker WB, Allan PW, Waud WR, Hong JS, Sorscher EJ (2011) Effect of expression of adenine phosphoribosyltransferase on the in vivo anti-tumor activity of prodrugs activated by E. coli purine nucleoside phosphorylase. Cancer Gene Therapy 18: 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal EL, Chung TK, Parker WB, Allan PW, Clemons L, Lowman D, Hong J, Hung FR, Richman J, Conry RM, Mannion K, Carroll WR, Nabell L, Sorscher EJ (2015) Phase I dose-escalating trial of Escherichia coli purine nucleoside phosphorylase and fludarabine gene therapy for advanced solid tumors. Ann Oncol 26(7): 1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorscher EJ, Hong JS, Allan PW, Waud WR, Parker WB (2012) In vivo antitumor activity of intratumoral fludarabine phosphate in refractory tumors expressing E. coli purine nucleoside phosphorylase. Cancer Chemother Pharmacol. 70: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott RB, Brown GB (1962) The action of xanthine oxidase on some 2-substituted adenines. Journal of Biological Chemistry 237: 3215–3216. [PubMed] [Google Scholar]
- 18.Parker WB, King SA, Allan PW, Bennett LL Jr, Secrist JA III, Montgomery JA, Gilbert KS, Waud WR, Wells AH, Gillespie GY, and Sorscher EJ (1997) In vivo gene therapy of cancer with E. coli purine nucleoside phosphorylase. Human Gene Therapy 8: 1637–1644. [DOI] [PubMed] [Google Scholar]
- 19.Becker TC, Noel RJ, Coats WS, Gomez-Foix AM, Alam T, Gerard RD, Newgard CB (1994) Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol, 43: 161–89 [DOI] [PubMed] [Google Scholar]
- 20.Adenovirus methods and protocols Wold WSM. eds. Methods in molecular medicine, 1999; vol. 21: Humana Press; Totowa, New Jersey [Google Scholar]
- 21.Behbahani TE, Rosenthal EL, Parker WB, Sorscher EJ (2019) Intratumoral generation of 2-fluoroadenine to treat solid malignancies of the head and neck. Head & Neck 41: 1979–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharara B, Sorscher EJ, Gillespie GY, Lindsey JR, Hong JS, Curlee KV, Allan PW, Gadi VK, Alexander SA, Secrist JA III, Parker WB, and Waud WR (2005) Antibiotic-mediated chemoprotection enhances adaptation of E. coli PNP for herpes simplex virus based glioma therapy. Human Gene Therapy 16: 339–347. [DOI] [PubMed] [Google Scholar]
- 23.Secrist JA III, Parker WB, Allan PW, Bennett LL Jr, Waud WR, Truss JW, Fowler AT, Montgomery JA, Ealick SE, Wells AH, Gillespie GY, Gadi VK, and Sorscher EJ (1999) Gene therapy of cancer: Activation of nucleoside prodrugs with E. coli purine nucleoside phosphorylase. Nucleosides Nucleotides 18: 745–757. [DOI] [PubMed] [Google Scholar]