Abstract
The paradigm of central nervous system (CNS) drug discovery has mostly relied on traditional approaches of rodent models or cell-based in vitro models. Owing to the issues of species differences between humans and rodents, it is difficult to correlate the robustness of data for neurodevelopmental studies. With advances in the stem-cell field, 3D CNS organoids have been developed and explored owing to their resemblance to the human brain architecture and functions. Further, CNS organoids provide a unique opportunity to mimic the human brain physiology and serve as a modeling tool to study the normal versus pathological brain or the elucidation of mechanisms of neurological disorders. Here, we discuss the recent application of a CNS organoid explored for neurodevelopment disease or a screening tool for CNS drug development.
Teaser
3D cerebral organoids are a novel class of in vitro tool possessing a close resemblance to human brain architecture and function. They serve as a robust model to study early-stage neurodevelopment and CNS drug toxicity screening.
Keywords: Stem cells, 3D brain organoids, drug screening, disease modeling, neurodegenerative disorders
Introduction
Stem cells are unspecialized cells that can give rise to various specialized cells and can remain undifferentiated and self-renew themselves for long periods. These cells can be used to model human development and disease, in tissue replacement and for screening drug activity or toxicity. The various types of stem cells include embryonic stem cells (ESCs), somatic or adult stem cells (ASCs) and induced pluripotent stem cells (iPSCs) [1]. Stem cells, owing to their intrinsic ability, self-assemble into complex structures or organoids under suitable conditions. The growth factors modulate various signaling pathways and play an essential part in cell survival, proliferation and self-renewal; stem cells can be coaxed into forming organized clusters of cells or organoids [2]. Renewed interest in the field of pluripotent stem cells (PSCs) and near-physiological 3D organoid models has provided new prospects for studying organ and tissue development, regeneration and disease modeling and mechanisms.
Organoids are organ-like 3D multicellular structures that provide a more accurate representation of the natural microenvironment and thereby allow assessment and modeling of organ development, maintenance and repair of tissue structure ex vivo [3–5]. These organoid models provide the near-physiological system that can be efficiently used to model various diseases and study tissue responses to drugs, damage or mutation [3,6]. Organoids developed from disease-specific or mutated patient cells have helped understand the, previously unknown, underlying mechanism and pathophysiology in such cases. The development of organoids requires a detailed knowledge related to the induction of various germ-layer formation and subsequent lineage specification. Organoids can be derived not only from the PSCs, which include the ESCs and iPSCs, but also from organ-restricted ASCs [7]. Although PSCs can give rise to several different cell types, they are capable of generating cells characteristic of all three germ layers, and hence have the ability to proliferate into a number of organs. ASCs retain their organ identity and have the ability to self-renew and generate all the different cell types in that organ, thereby playing a key part in tissue homeostasis or repair [8]. Owing to the immense resemblance of the in vitro organoid system to the in vivo conditions, organoid cultures have become fundamental tools in research and clinical application [9].
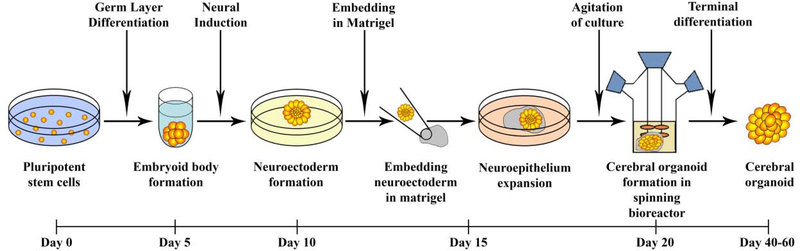
Organoids derived from PSCs have been generated for various organs from mouse and human cells. They have been developed for many different mammalian organs and parts, including the brain [10,11], lung [12,13], heart [14] and liver [15]. The complexity of the human brain has always fascinated scientists. There has always been limited access to human brain tissue for experimental studies owing to ethical and practical issues, especially in utero. Scientists have utilized indirect noninvasive techniques, including ultrasound imaging, aborted material and animal models for exploring neurological diseases but, owing to various limitations, these cannot be employed or are difficult to model [16]. In 2009, Sato and colleagues made a major breakthrough by using Matrigel® to develop epithelial intestinal organoids from mouse stem cells [17]. In 2012, Sasai and colleagues designed SFEBq which was a serum-free floating culture of the embryoid body-like aggregates for quick aggregation and reported that the PSCs differentiated and polarized to form a continuous neuroectoderm-like epithelium that subsequently generated stratified cortical tissues containing cortical progenitors and neurons [18]. Later, in 2013, Lancaster and Knoblich embedded embryoid bodies (EBs), which are 3D aggregates of stem cells in Matrigel®, and were able to develop these small 3D structures that resembled an organ and were referred to as cerebral or brain organoids [19]. Figure 1 highlights the most commonly used method for generating cerebral organoids.
Figure 1.
Schematic of cerebral organoid development. Organoids developed from the embryoid bodies using induced pluripotent stem cell (iPSC)-derived cells that were grown in neural induction medium to generate neuroectoderm that were embedded in Matrigel® and allowed to grow in a spinning bioreactor or orbital shaker for better diffusion to obtain the 3D cerebral organoids. On exposure to retinoic acid, cerebral organoids self-organize through self-patterning mechanisms to display diverse populations of neural progenitors including radial glia, which expand forming cerebral structures [24].
3D organoid method development
The inadequacy of stem-cell-derived monolayer or 2D cell culture systems encouraged efforts to develop a better 3D in vitro model that can reiterate the structural and functional intricacy of the human brain. Brain organoids are self-assembled 3D constructs generated from various neural and neuronal subtypes that resemble the human embryonic brain owing to their spatial organization and the ability to replicate the gene expression in vitro [16,20,21]. These mini-brains can summarize the cellular organization along with the epigenetic and transcriptional signature of the developing brain. Brain organoids are not the only 3D neural culture systems but other structures like neurospheres are also 3D clusters of the central nervous system (CNS) consisting of various cells like the neurons or glial cells, although they lack distinctive cytoarchitecture [22]. Brain organoids can mimic the cytoarchitecture and other developmental trajectories found in vivo owing to the ability of PSCs to self-organize. Hence, these organoids can be effectively used to study neurological diseases and defects, which are otherwise challenging to study in an animal model owing to the uniqueness of the human brain.
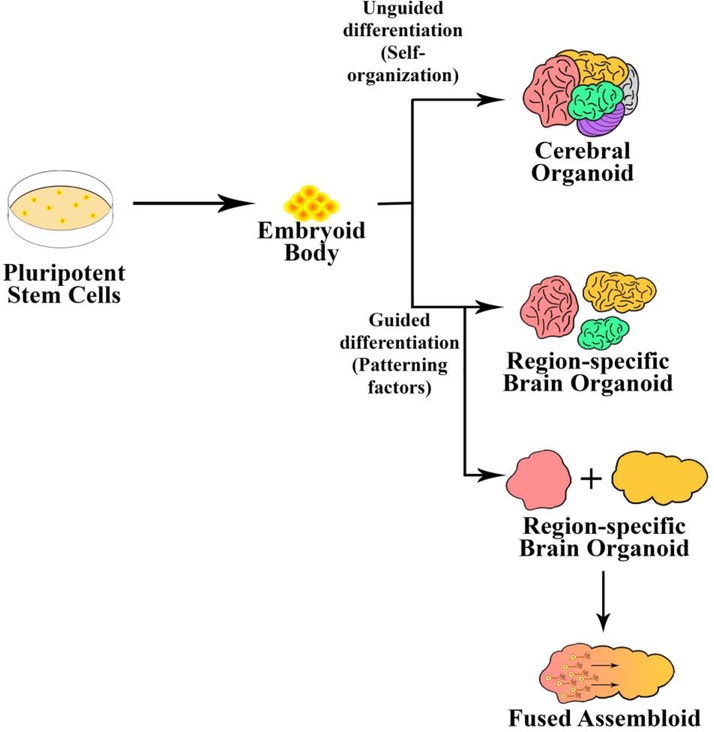
Brain organoids have been generated mainly by two different methodologies with one being unguided wherein the cells rely on spontaneous morphogenesis and intrinsic differentiation capacities within the stem-cell aggregates. In the case of unguided differentiation, the organoids are allowed to grow with minimum external interference and they exhibit various cell lineages including forebrain, midbrain, hindbrain, retina and choroid plexus [10,20,23]. The major challenge with organoids developed by this method is high variability and heterogeneity [20]. Also, human PSCs (hPSCs) are differentiated into desired lineages with the help of external patterning factors. Figure 2 highlights the difference between both methods. In these organoid structures, the PSCs are induced to form aggregates referred to as an embryoid body (EB) which, after ectoderm formation, is guided toward a neural or non-neural fate. In guided methods, the organoids developed are mostly brain-region-specific, depending on the various growth factors that are used during the differentiating process [11]. These organoids exhibit less batch-to-batch variation owing to directed differentiation, which is capable of generating specific cell types with relative proportions [20]. The guided organoids have been shown to contain neural progenitor cells, neurons and astrocytes among other brain cells and have shown directed differentiation to develop brain-region-specific organoids, including cerebral cortex [11], hippocampus [24], midbrain [5] and cerebellum [25], as shown in Figure 3.
Figure 2.
Approaches for developing organoids. (a) Unguided approach. Organoids can be developed by relying on the self-organizing properties of the stem-cell aggregates producing cerebral or whole organoids. The resulting cerebral organoids often contain heterogeneous tissues resembling various brain regions. (b) Guided approach. Organoids can be differentiated into brain-region-specific organoids by guided differentiation in the presence of external patterning factors. Two or more guided brain organoids can also be fused together to model the interaction between different brain regions.
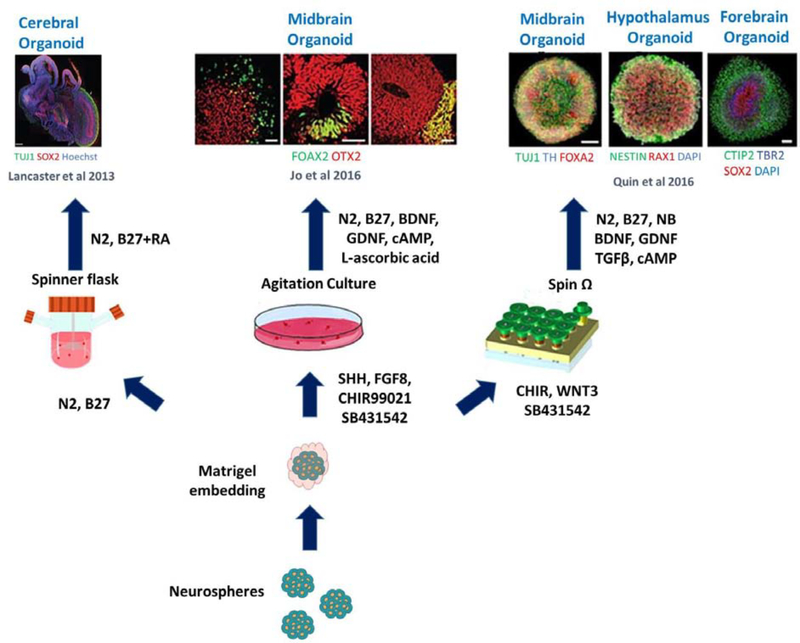
Figure 3.
Compilation of different methods used for developing different brain regions in central nervous system (CNS) organoids. The illustration represents the different modeling methods established to produce region specific CNS organoids. Reproduced, with permission, from [89].
Various groups have tried many different approaches for organoid development to study specific neurodevelopmental objectives. In the case of fused assembled organoids, different brain-region-specific organoids were developed separately and then fused together to generate organoids consisting of distinct regions, which improved interregional interaction modeling. Birey et al. developed fused ventral and dorsal brain organoids and demonstrated interneuron migration from one domain to another, which connected synaptically and formed microcircuits with the local excitatory neurons [26]. Lately, researchers have also tried to recapitulate the normal brain tissue more closely by incorporating six cell types to develop spheroids including major cell types like neurons, microglia, astrocytes and oligodendrocytes, to have a better insight into various tight junctions and cell markers of the blood–brain barrier (BBB), which could be used for drug neurotoxicity assessments. To get comprehensive details about the BBB-based organoids and their application, please refer to references [27–29]. In another study, researchers explored human-specific brain evolution by studying the cortical development and gene expression and compared this with cerebral organoids developed from PSCs derived from humans, macaques and chimpanzees to explore the unique evolutionary differences during development [30]. Various other groups have also studied organoids over extended periods of time, allowing the development of various features, including dendritic spine formation, Ca2+ surge and active neuronal networks [31]. Knoblich and colleagues also demonstrated the presence of active neurons that were electrically active and spiked action potential, which was observed using calcium imaging [10].
Recent developments in CNS disease modeling using cerebral organoids
Cerebral organoids have been widely explored for studying the mechanism and pathophysiology of many diseases, especially those affecting neurodevelopment. They have also been employed for testing drug efficacy and toxic effects on neural cells. Below, we discuss the important diseases and pathological conditions for which cerebral organoids have been explored and Table 1 highlights the organoid role for disease modeling and drug screening.
Table 1.
Current application of cerebral organoids for the disease modeling and drug toxicity screening
| Disease state | Brain region | Organoid type | Cell line | Refs |
|---|---|---|---|---|
| Autosomal recessive primary microcephaly (MCPH) | Whole brain | Cerebral organoid | Single human induced pluripotent stem cell (iPSC) line (H9) and mouse A9 embryonic stem (ES) cells | [10] |
| MCPH | Whole brain | Cerebral organoids | WDR62-deficient iPSC and ES cells | [37] |
| Zika virus | Whole brain | Cerebral organoids | Two independent human iPSC lines | [39] |
| Zika virus | Hypothalamus | Hypothalamic organoids | Multiple iPSC lines | [5] |
| Zika virus | Forebrain | Forebrain organoids | Multiple iPSC lines | [5] |
| Zika virus | Midbrain | Midbrain organoid | Multiple iPSC lines | [5] |
| Zika virus | Forebrain | Forebrain-specific organoids | Human iPSC line | [42] |
| Schizophrenia | Whole brain | Cerebral organoids | Two HESC and seven iPSC lines derived from different control individuals and from individuals diagnosed with schizophrenia at different ages | [51] |
| Alzheimer’s disease | Whole brain | Cerebral organoids | Five iPSC lines derived from control individual and from individuals diagnosed with Alzheimer’s disease, Down syndrome and Creutzfeldt–Jakob disease | [53] |
| Alzheimer’s disease (AD) and frontotemporal dementia (FTD) | Whole brain | Cerebral organoids | FTD patient (tau P301L carrier) and related healthy individual | [58] |
| AD | Whole brain | Cerebral organoids | Six iPSC lines derived from control individual and from individuals diagnosed with familial AD of which two carried duplications in the gene for amyloid precursor protein | [54] |
| Glioblastoma | Whole brain | Cerebral organoids | Single ES cell line (H9) | [75] |
| Glioblastoma | Whole brain | Cerebral organoids | Two hESCs (H1 and H9) and single iPSC (H6) | [72] |
| Prenatal cocaine exposure | Neocortex | Neocortical organoid | Six hPSC lines including H1, H9, H14, CT2, NIH-i5 and NIH-i7 | [67] |
| Neonatal hypoxic stress | Whole brain | Cerebral organoids | Single hES cell line (H9) | [90] |
| Down syndrome | Forebrain | Ventral forebrain organoids | Six hiPSC lines including three from control and three derived from Down syndrome patient | [60] |
| Miller–Dieker syndrome (MDS) | Forebrain | Forebrain organoids | Two control and two MDS-patient-derived skin fibroblasts | [62] |
Modeling of early-stage neurotoxicity screening
Ethical and practical concerns limit access to human embryos, leading researchers to rely heavily on model systems, such as the mouse, as a surrogate to understanding human brain development and disease. The brain organoids (derived from human stem cells) offer an in vitro model system for studying early-stage human brain development and neuronal disorders. The developing brain in the fetus is highly vulnerable to chemical exposure, which can lead to alteration in neural development compared with an adult brain [32]. Developmental neurotoxicity (DNT) refers to adverse reactions that alter the normal development of the CNS or its function owing to exposure to toxic substances or chemicals or new drug molecules [14]. It is highly important to screen chemicals that can potentially lead to neurodevelopmental disorders; however, there is a lack of data established for such potential toxicants. Additionally, DNT studies are currently entirely based on in vivo animal studies, which show a lot of variability in data along with the other limitations [33]. As mentioned earlier, 3D brain organoids serve as a platform technology for drug screening, can be used to study the toxic effects of chemicals and can provide a broad horizon for better evaluation of developmental neurotoxicants. In one of the recent studies by Pamies and colleagues, it was demonstrated that rotenone, a commonly used plant pesticide, could potentially lead to DNT using brain spheroids. The authors demonstrated that rotenone treatment (2 weeks) led to higher levels of reactive oxygen species, impaired neurite outgrowth, abnormal calcium reabsorption and synaptogenesis [33]. Similarly, Liu et al. also studied the neurotoxic effects if vincristine (anti-brain-tumor drug) using cerebral organoids. They observed dose-dependent neurotoxicity and inhibited fibronectin and tubulin development in the cerebral organoid [34]. Placental exposure of heavy metals to a fetus can lead to various health effects such as neurological, developmental and endocrine disorders [35]. Yin and co-workers studied the effects of exposure to the heavy metal cadmium during prenatal stages, this has previously been shown to affect the developing brain in vivo owing to the long biological half-life of cadmium. The brain organoids were employed for studying the neural dysfunctions under cadmium exposure, which were engineered on array chips with octagon-shaped micropillars. Under exposure to the heavy metal, they observed increased cell death, impaired neurogenesis, skewed neural maturation and disturbed brain regionalization, suggesting impairment in the neurogenesis of fetal brain that could affect learning and cause behavioral defects that are generally observed after cadmium exposure [36].
Modeling of autosomal and Zika-associated microcephaly
Microcephaly is a birth abnormality where the baby’s head is smaller in circumference than normal. Autosomal recessive primary microcephaly (MCPH) is a neurodevelopmental disorder associated with a reduction in brain size along with nonprogressive mental retardation. Cerebral organoids have been extensively used for disease modeling and to study the drug efficacies for the MCPH. Scientists have used patient-derived iPSCs along with RNA interference for the formation of cerebral organoids, using them as a model tool to study the pathogenesis of MCPH [10]. Another group also studied MCPH using WDR62-deficient cerebral organoids and mutant mouse models, comparing the results regarding brain size. They observed the reduction in the size of organoid and mouse brain caused by the disruption in neural progenitor cells (NPCs). Based on these observations, it was hypothesized that the WDR62/CEP170/KIF2A protein pathway is responsible for microcephaly owing to the disassembly and disruption of the cilium (the key regulator for the formation of normal brain) [37].
Another form of microcephaly results from Zika virus (ZIKV) infection (disease caused by virus transmission by Aedes mosquitoes). This viral infection during pregnancy can pass to the fetus, leading to microcephaly, other congenital or neurological malformations and birth defects in the infants [38]. Because cerebral organoids represent the fetal brain physiology, they provide an excellent opportunity to model Zika-virus-based microcephaly and study the underlying mechanisms. Gabriel et al. showed that two isolated strains of Zika virus readily infected the human iPSC-derived brain organoid and the early stage of infection triggered premature differentiation of NPCs, which led to a depletion of the progenitor cells and centrosomal structural defects [39]. Another study using cerebral organoids by Cugola et al. correlated the link between Zika virus with increased congenital brain malformations. They demonstrated that Zika virus infection led to the depletion of proliferative zones and disruptions in the cortical layers. They also recapitulated their organoid results in mice and showed that the virus was able to infect fetuses and cause intrauterine growth restrictions along with increased cell death owing to the virus infecting the human cortical progenitor cells. This study indicates that Zika virus can cross the placenta, inducing cell death by autophagy and apoptosis, causing microcephaly by targeting cortical progenitor cells and impairing neurodevelopment [40]. Similarly, Dang et al. also studied the relationship between Zika virus and microcephaly using hESC-derived cerebral organoids. They showed that Zika virus prototype strain MR766 efficiently infected the organoids and decreased the overall size of the organoid along with upregulation of innate immune receptor Toll-like receptor 3 (TLR3) after Zika virus infection of human organoids. Their findings showed a link between Zika-virus-mediated TLR3 activation, perturbed cell fate and a reduction in organoid volume, which is reminiscent of microcephaly [41].
To overcome the limitations of high cost, variability and tissue heterogeneity, Qian et al. developed forebrain-specific organoids, midbrain and hypothalamic organoids using miniaturized spinning bioreactors. They further used forebrain organoids to model Zika virus exposure during various stages of pregnancy and the observed results were consistent with the clinical findings with exposure to early-stage organoids leading to many defects including features mimicking microcephaly [5]. Cerebral organoids were also used to test drug efficacy (repurposing or new drugs) and toxicity screening for Zika virus microencephaly drugs. Xu and colleagues have repurposed the drug niclosamide (an FDA-approved anthelmintic drug that inhibits Zika virus replication) and Emricasan (a pan-caspase inhibitor), against the protected hNPCs they reduce caspase-3 activity. They have shown that their drug combination results in a reduction in Zika virus replication via the cyclin-dependent kinase pathway targeting and providing neuroprotection to the neuroprogenitor cells and astrocytes [42]. Another investigation by Li et al. established that, in response to Zika virus infection, cholesterol-25-hydroxylase (CH25H) was released and its enzymatic product: 25-hydroxycholesterol (25HC), had a protective effect against Zika virus for a diverse population (i.e., human neuronal cells, human cortical organoids, mice and macaques). Cerebral organoid treatment with 25HC suppressed Zika virus infection and reduced tissue damage and associated malfunctions (microcephaly); thus, it could be used as a natural antiviral agent for Zika virus treatment [43].
Modeling of neurodevelopmental and neurodegenerative disorders
Developing reliable, predictive animal models for complex neurodevelopmental and psychiatric disorders is essential to increase our understanding of the neurobiological basis of the disorder and for the development of novel drugs with improved therapeutic efficacy [44]. Autism spectrum disorder (ASD) is a brain developmental disorder associated with challenges related to social interaction, communication and behavior [45]. Mariani et al. studied neurodevelopmental alterations in patients with severe idiopathic ASD using patient- and related control-derived telencephalic organoids. They observed an accelerated cell cycle and synaptic growth in patient-derived organoids along with overexpression of GABAergic neurons [46]. Periventricular hypertropia (PH) is another neurodevelopmental disorder where the neurons fail to migrate to their proper position during cortical development from the ventricular zone to the cerebral cortex and form clusters around ventricles, leading to recurrent seizures along with mild intellectual disability [19,47]. Klaus et al. analyzed cerebral organoids for PH, which were derived from the iPSCs of patients with mutated cadherin-receptor–ligand pair for DCHS1, FAT4 and isogenic knockout lines and suggested that an altered progenitor cell morphology and navigation system for neurons were the cause for PH. They demonstrated a changed morphology of neural progenitor cells resulted in a defect of the neuronal migration dynamics and cortical heterotopia that could be linked to PH. They also showed that a small number of mutated neurons along with the dysregulated genes were linked to the axon guidance, neuronal migration and patterning using single-cell RNA-sequencing data [48].
Schizophrenia is a chronic major psychotic disorder associated with cognitive impairment [49]. Although the etiology of schizophrenia remains contentious, it is a multifactorial neurodevelopmental disorder influenced by genetic and environmental factors that have been studied using in vitro or in utero cerebral tissue to overcome the shortcoming of existing animal models and improve disease understanding [50]. Stachowiak and colleagues modeled the first trimester of in utero brain development using cerebral organoids derived from control and schizophrenia patients. They studied the early stages of telencephalic development and observed abnormal dispersion of neural progenitor cells from the ventricular to cortical zones. They also observed that altered fibroblast growth factor receptor (FGFR)1 signaling pathways led to the progression of cortical malformation in schizophrenia [51]. Further, to understand the role disrupted in schizophrenia 1 (DISC1) and nuclear distribution element-like 1 (Ndel1) – key scaffolding proteins that regulate many distinct signaling pathways in cortical and hippocampal neurodevelopment – in vitro, Ye et al. developed schizophrenia patient-derived forebrain organoids with a DISC1 mutation and explored its role in the development of psychiatric disorders. They have observed that DISC1 mutation disrupted its interaction with Ndel1 and also showed the potential mechanistic understanding of how DISC1 mutation with its C-terminal deletion can affect neural developmental processes and how genetic insults can contribute to psychiatric disorders [52].
Alzheimer’s disease (AD) is a progressive disease that worsens gradually and is the most common cause of dementia. Despite the development of many animal and in vitro models for AD, there is a lack of an experimental approach that fully recapitulates essential aspects of the disease in human cells. Recently, Gonzalez et al. modeled AD using the organoids produced from iPSCs derived from patients affected by familial AD and have shown that organoids showed all the pathological hallmarks of AD (including accumulation of amyloid plaques and neurofibrillary tangles) [53]. Padmanabhan et al. explored a similar approach to model age-related neurodegeneration for studying the AD-like pathology in an age-dependent manner using neural organoids. They also reported that treatment with β- and γ-secretase inhibitors on these organoids reduced the amyloid and tau levels [54]. Park and colleagues developed the 3D co-culture model of a brain cell (e.g., neurons, astrocytes and microglia) in a 3D microfluidic pattern to model AD. In another study, this group developed 3D neurospheroids using the microfluidic chip for inducing a constant flow of fluid to closely recapitulate the in vivo brain microenvironment. These researchers studied the effects of amyloid-β (control vs treatment channel containing neurospheroids) using a parallel channel chip configuration for AD progression [55,56]. Pavoni et al. studied amyloid-β level fluctuations associated with AD using neuroectodermal organoids and demonstrated that these organoids displayed an increased Aβ42:Aβ40 ratio, after chemical induction with Aftin-5 (an Aβ42 inducer), which is similar to AD patient etiology and can be helpful to model the AD-associated phenotypes [57]. Further, to explore the potential of the CRISPR/Cas9 genome-editing technique, Seo et al. generated cerebral organoids using reprogrammed iPSC lines of a frontotemporal dementia patient to study AD. They have shown that tau phosphorylation could be reduced with blockage of p25 and an increase in expression of synaptophysin can be increased using CRISPR gene-editing technology [56,58]. Thus, the above-mentioned investigation showed that the cerebral organoid technology could be applied to study healthy and diseased populations or could be explored to provide the mechanistic insight of the diverse neurodegenerative disorder pathologies at more-physiological levels, which is not possible with the pre-existing animal models and offers a new platform for discovery of novel targets and screening of drugs for therapeutic intervention. Furthermore, this technology could be extended to produce relevant models for other neurodegenerative diseases.
Genetic neurodevelopmental disease modeling
Down syndrome (also known as human trisomy 21) is a genetic neurodevelopmental disorder that occurs owing to a partial or full extra copy of chromosome 21, leading to intellectual disability and physical features [59]. Xu et al. demonstrated the overproduction of OLIG2+ forebrain neural progenitor in iPSCs derived from a down syndrome patient’s cerebral organoids. There was also excessive production of GABAergic interneurons observed in these organoids, along with memory impairment in chimeric mice [60].
Lissencephaly is another rare gene-linked neurological disorder caused by heterozygous deletion of chromosome 17p13.3, characterized by the absence of cortical folding with Miller–Dieker syndrome being its most severe form [18,21,26]. Bershteyn et al. analyzed the mutations associated with lissencephaly on biological processes and cell types using cerebral organoids to comprehend the first trimester of cortical development. They revealed cell-type-specific defects over various stages of neuroepithelial cell expansion and migration of neurons during cortical development, which could be linked to lissencephaly [61]. In another study, Iefremova and colleagues also developed forebrain organoids to study the MDS-associated changes. They observed a reduced expansion rate along with switching of cell division from symmetric to asymmetric for ventricular radial glial cells [62].
Rett syndrome is a rare postnatal neurodevelopmental disorder caused by X-linked mutations on methyl-CpG-binding protein 2 (MECP2) gene, mostly affecting females [63]. Mellios et al. investigated the functions of MeCP2 on prenatal brain development using control and MeCP2-deficient cerebral organoids. They observed significant alterations in neuronal development, neurogenesis and neuronal migration. They also hypothesized a novel miRNA-mediated molecular cascade downstream of MeCP2 based on two miRNAs, miR-199 and miR-214, which were found to be increased during early brain development [64].
Timothy syndrome is also a rare genetic disorder caused by a missense mutation in the L-type calcium channel which is linked with developmental delay and autism and can affect the heart, digits (fingers and toes) and the nervous system [65]. Birey et al. developed human forebrain spheroids, which had functionally integrated glutamatergic and GABAergic neurons to study Timothy syndrome. They demonstrated interneuron migration in organoids developed from patient-derived cells, where they moved more frequently and less efficiently leading to abnormal cortical development [66].
Modeling of prenatal alcohol and drug abuse as well as nicotine exposure
It is known that alcohol-related neurodevelopmental disorder (ARND) and alcohol-related birth defects (ARBD) are very common in the prenatal alcohol-exposed fetus (known as fetal alcohol spectrum disorders; FASD) [67]. Zhu et al. explored the 3D brain organoid model to examine the underlying mechanisms for neural dysfunctions in prenatal alcohol exposure. They observed the attenuated neurite outgrowth and skewed neural maturation in the ethanol-exposed brain organoids along with alteration in genes (GSX2, RSPO2) and various pathways (Hippo signaling pathway) important for the development of the healthy brain (i.e., neuronal differentiation, brain regionalization or cortical organization) [68].
Prenatal cocaine exposure leads to neurological and behavioral changes along with impaired attention in the infants. It also leads to premature birth, reduced bodyweight and increased seizure risk [69]. Lee et al. explored the 3D neocortical organoids to study the effect of cocaine exposure on neurodevelopment and showed an increase in reactive oxygen species, interruption in the development of neural tissue and induction of premature neuronal differentiation [67]. Another study by Wang et al. used the organoid-on-a-chip system to model neurodevelopmental disorders under early stages of prenatal nicotine exposure. The organoids were developed by the self-organized controlled approach under continuous perfused cultures. The study revealed that nicotine exposure leads to premature neuronal differentiation, expression of neuronal marker TUJ1 and disruption in cortical and brain regionalization development. Their data suggested that prenatal nicotine exposure leads to impaired neurogenesis and human brain organoids provided a platform for investigating effects of abused substances or alcohol on CNS development [70].
Modeling of brain tumor
Despite a lot of research, brain tumors are still is one of the deadliest forms of cancer with metastatic brain tumors affecting almost 150 000 people a year, which nearly accounts for one in four people detected with cancer in the USA (according to the American Association of Neurological Surgeons; AANS) [71]. It is very difficult to generate a robust brain tumor model and another challenge is the cellular and genetic heterogeneity or transcriptional heterogeneity within or between the tumor models. Owing to the unique human cerebral microenvironment, 3D cerebral organoids have been explored to study tumor biology and to overcome the challenges and limitations posed by the current preclinical mouse cancer models. The brain tumor glioblastoma multiforme (GBM) is considered a dreadful form of cancer, which needs to be further studied in detail to determine the factors leading to its cause. Linkous et al. developed a cerebral organoid glioma (GLICO) model system for high-throughput drug screening and to overcome the limitations of the present preclinical model systems for GBM by retro-engineering patient-specific GBMs using patient-derived glioma stem cells (GSCs) or cerebral organoids derived from hESCs. They demonstrated that the GSCs from the tumor in the cerebral organoid closely resemble the patient’s GBMs, by deeply invading and proliferating within the host tissue with the support of invasion by the interconnected network of tumor microtubles [72]. In another study, Bian et al. recapitulated tumorigenesis in the brain by introducing oncogenic mutations via transposon (DNA sequence that can change its position within a genomek) and CRISPR/Cas9-mediated mutagenesis in a 3D in vitro model referred to as neoCOR (neoplastic cerebral organoid). They also reported that neoCOR could serve as a suitable model for preclinical investigations for studying the tumor biology owing to the presence of the tumor and normal cell populations within the same culture. This model is very useful for examining the interactions between transformed and nontransformed cells or for drug screening (i.e., analysis of antitumor effects in the same system along with a biosafety test) [73]. da Silva et al. also established hybrid organoids using human GBM spheroids, which could infiltrate into early-stage cerebral organoids. This model could serve as a basis for modeling and quantification of GBM infiltration and be used for identification of anti-GBM invasion strategies. Their investigation showed that these organoids had an invasive tumor phenotype, which was distinct from noncancerous (adult neural progenitor derived spheroid) in the cerebral organoid [74]. Ogawa and colleagues developed cerebral organoids with genetic modification using CRISPR/Cas9 technology, which showed invasive features within the organoids and were transplanted into immunodeficient mice. These mice exhibited an invasive phenotype along with disease pathology [75]. Thus, all these studies showed that the cerebral organoid model is an innovative tool to study brain tumor biology similar to the human brain environment ex vivo and could serve as a high-throughput platform for drug screening.
Ethical concerns and current challenges of CNS organoid development
The brain organoid has emerged as a powerful tool for studying human brain development but it has also raised ethical concerns and sociolegal concerns [76,77]. The major concerns relating to organoid generation relate to their potential to become sentient by attaining sensory inputs or the cultivation of cognitive processes owing to their human origin and their ability to recapitulate the human brain [76]. Another concern is the generation of human–animal chimeras following the transplantation in the animals, which has been an ongoing topic for discussion for a long time. For example, studies from Mansour et al. have presented evidence of anatomic integration generating mature and functional brain tissues after transplantation of brain organoids into an adult mouse [78]. In the case of brain organoids, the major points for discussion include which animals can be subjected to transplantation (which also involves sociolegal concerns), to what level can the animal’s brain be enhanced and the societal place for the developed chimeras including research protection as a result of enhanced brain function [76,79]. Classical ethical issues pertaining to stem cell use including the type of donor consent, storage and feedback to the patient on the relevant clinical findings remain to be discussed [76].
Along with the diverse advantages, organoids still possess several limitations, which include the absence of all physiological neural cell types (especially microglia), crosstalk with other tissues in the developing embryo, organoid-to-organoid variability and lack of reproducibility. The major limitation with organoids is lack of vascularization, which is mainly the result of lack of nutrient supply. Organoids only grow up to a few millimeters in diameter. Lack of vasculature leads to inadequate diffusion through the dense tissues, which leads to necrosis at the organoid core and limits the viable thickness [20,80]. However, recently, many groups have tried to overcome this by using various approaches. Lancaster et al. used spinning bioreactors to improve nutrient exchange [10] and Park and colleagues overcame the issue by inducing the expression of hETV2 (a gene associated with the formation of vasculature in the developing embryo) within hESC-derived organoids. Notably, the presence of vasculature-like structures led to improved functional maturation of the organoids [81]. In another study, Giandomenico et al. demonstrated that improving oxygen supply by generating the air–liquid interface in cerebral organoids can lead to better neuronal survival and generation of active neuronal networks [82]. Alternatively, it was also shown that implanting the brain organoids into an adult mouse brain can lead to vascularization in vivo and organoids able to show functional neuronal networks, synaptic connectivity and blood vessels in the graft [78].
Another issue with current cerebral or other body type organoids is the high variability in developing organoids. Upon examining the organoids at various stages of development, inconsistent neural induction, or neural ectoderm formation, was observed. It has also been hypothesized that this variability can be linked to the low surface-area:volume ratio during organoid development. For improving tissue architecture and increasing the reproducibility, various groups have developed novel strategies, for example Lancaster and colleagues have generated microfilament-engineered cerebral organoids consisting of poly(lactide-co-glycolide) (PLGA) fiber microfilaments to generate elongated embryoid bodies, which showed improved cortical development and increased neuroectoderm formation [83]. It was assumed that cerebral organoids mainly consisted of differentiated cells from neuroectodermal lineages (which included neurons, oligodendrocytes and astrocytes) and lacked cells derived from other lineages such as microglia [84,85]. However, Ormel et al. demonstrated that microglia were also innately developed within a cerebral organoid [86].
Concluding remarks and future perspectives
Development of brain organoids has revolutionized the study of human brain evolution, disease and development. The use of patient-specific iPSCs or genetically modified cells has helped in exploring diseases that could previously not be modeled, or the mechanisms that were not clear, and has also helped in developing potential therapeutic strategies. Current brain organoid protocols only model neurological defects during the early stages of fetal development. However, organoids that could model aging neurons might provide an insight into neurological diseases like AD and Parkinson’s disease, which have a late onset. Using brain organoids for testing drug efficacy and toxicity would help in screening for safe and effective treatment options and reduce the number of animals required for the development of CNS therapeutics. During the early stages of screening, the drugs showing toxic or undesirable effects could be removed. Cerebral organoids can also provide an opportunity for exploring the possibility of repurposing the already existing drugs. The use of a patient’s own cells can derive organoids to provide new prospects for drug testing and genetic screening to aid in identifying the target drugs or their combination for precision therapy [87]. These individual-derived organoids provide opportunities to develop patient-specific therapeutic strategies, which can be employed to test efficacy or resolve issues associated with the proposed therapy and provide the most suitable treatment options for the patient. Genetic modification using genetic-engineering techniques helps in exploring the mutations, analyzing cellular phenotypes and repairing the risk alleles from the target gene to develop effective therapeutic strategies [88].
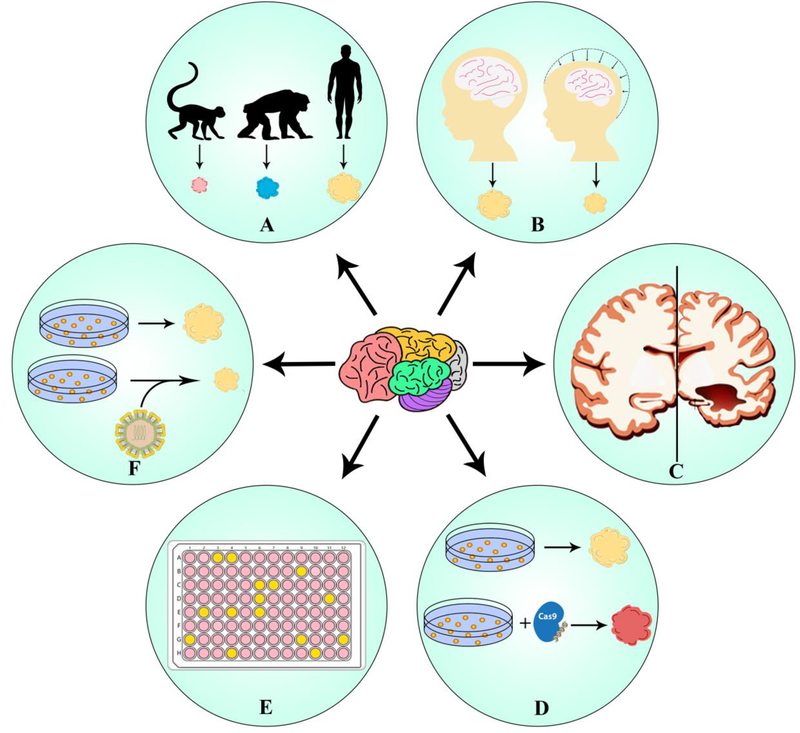
Figure 4.
Modern applications of cerebral organoids. (a) Interspecies evolution. Organoids have provided much opportunity to study human brain evolution in comparison with other species such as apes, where very limited brain models are available. (b) Congenital brain deformation. They have also provided a unique opportunity to model disorders with cerebral malformations such as microcephaly caused by infectious diseases or genetic alterations. (c) Neurodegenerative disease. The current organoid model only recapitulates fetal brain, various groups have also used them to model neurodegenerative disorders like Alzheimer’s disease and schizophrenia. (d) Gene editing. Researchers have developed organoids from reprogrammed induced pluripotent stem cells (iPSCs) using genome-editing techniques such as CRISPR/Cas9 to model neurodegenerative disorders, disorders with genetic defect or to study brain tumors by inducing mutations. (e) Drug compound screening. Organoids have also been used for high-throughput drug screening to carry out drug efficacy and toxicity studies to evaluate drug exposure defects on fetal brain. (f) Disease pathology. Organoids have also been used to study infectious diseases such as Zika virus and its associated fetal brain defects.
Highlights.
3D CNS organoids share architecture and functions resembling the human brain
Recapitulating key features of the early-stage brain under a controlled microenvironment
Modeling tool to study CNS drug development, toxicity and personalized screening
Mimicking the human brain physiology to study unique neurodevelopmental disorders
Acknowledgments
R.D.J. would like to acknowledge the financial support from NIH (R03DA044877), The Campbell Foundation (Florida, USA) and SOP-Office of Science, Basic Science Pilot funding and start-up funds from the Department of Pharmaceutical Sciences, School of Pharmacy, Texas Tech University Health Sciences Center (TTUHSC), TX, USA. M.P. acknowledges Prof. Steven Dubinett and Prof. Brigitte Gomperts for providing the continuous financial support.
Footnotes
Conflicts of interest
The authors report no conflicts of interest in relation to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stern L and Gautier R (1922) II. Les rapports entre le liquide Céphalo-Rachidien et les éléments nerveux de l’axe cerebrospinal. Arch. Internationales de Physiologie 17, 391–448 (in French) [Google Scholar]
- 2.Völkner M et al. (2016) Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Rep. 6, 525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin X et al. (2016) Engineering stem cell organoids. Cell Stem Cell 18, 25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcez PP et al. (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818 [DOI] [PubMed] [Google Scholar]
- 5.Qian X et al. (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harkness L et al. (2019) Fibronectin-conjugated thermoresponsive nanobridges generate three dimensional human pluripotent stem cell cultures for differentiation towards the neural lineages. Stem Cell Res. 38, 101441. [DOI] [PubMed] [Google Scholar]
- 7.Clevers H (2016) Modeling development and disease with organoids. Cell 165, 1586–1597 [DOI] [PubMed] [Google Scholar]
- 8.Drost J and Clevers H (2017) Translational applications of adult stem cell-derived organoids. Development 144, 968–975 [DOI] [PubMed] [Google Scholar]
- 9.Schutgens F et al. (2016) Pluripotent stem cell-derived kidney organoids: an in vivo-like in vitro technology. Eur. J. Pharmacol 790, 12–20 [DOI] [PubMed] [Google Scholar]
- 10.Lancaster MA et al. (2013) Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paşca AM et al. (2015) Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson DC et al. (2017) Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl. Med 6, 622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sucre JMS et al. (2016) A three-dimensional human model of the fibroblast activation that accompanies bronchopulmonary dysplasia identifies Notch-mediated pathophysiology. Am. J. Physiol. Lung Cell. Mol. Physiol 310, L889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voges HK et al. (2017) Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 144, 1118–1127 [DOI] [PubMed] [Google Scholar]
- 15.Broutier L et al. (2016) Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc 11, 1724–1743 [DOI] [PubMed] [Google Scholar]
- 16.Lancaster MA and Knoblich JA (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- 17.Sato T et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 18.Nakano T et al. (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 [DOI] [PubMed] [Google Scholar]
- 19.Lancaster MA and Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc 9, 2329–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian X et al. (2019) Brain organoids: advances, applications and challenges. Development 146, dev166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iefremova V et al. (2017) An organoid-based model of cortical development identifies non-cell-autonomous defects in Wnt signaling contributing to Miller–Dieker syndrome. Cell Rep. 19, 50–59 [DOI] [PubMed] [Google Scholar]
- 22.Pamies D (2017) A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34, 362–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H (2018) Modeling neurological diseases with human brain organoids. Front. Synaptic Neurosci 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi H et al. (2015) Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun 6, 8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muguruma K et al. (2015) Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537–550 [DOI] [PubMed] [Google Scholar]
- 26.Birey F et al. (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nzou G et al. (2018) Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Sci. Rep 8, 7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin ND and Paşca SP (2018) Building models of brain disorders with three-dimensional organoids. Neuron 100, 389–405 [DOI] [PubMed] [Google Scholar]
- 29.Kamal K and Waldau B (2019) Bioengineering an artificial human blood–brain barrier in rodents. Bioengineering 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollen AA et al. (2019) Establishing cerebral organoids as models of human-specific brain evolution. Cell 176, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quadrato G et al. (2017) Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smirnova L (2014) Developmental neurotoxicity – challenges in the 21st century and in vitro opportunities. ALTEX 31, 129–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pamies D et al. (2018) Rotenone exerts developmental neurotoxicity in a human brain spheroid model. Toxicol. Appl. Pharmacol 354, 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F et al. (2019) Vincristine impairs microtubules and causes neurotoxicity in cerebral organoids. Neuroscience 404, 530–540 [DOI] [PubMed] [Google Scholar]
- 35.Caserta D et al. (2013) Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur. Rev. Med. Pharmacol. Sci 17, 2198–2206 [PubMed] [Google Scholar]
- 36.Yin F et al. (2018) Engineering brain organoids to probe impaired neurogenesis induced by cadmium. ACS Biomater. Sci. Eng 4, 1908–1915 [DOI] [PubMed] [Google Scholar]
- 37.Zhang W et al. (2019) Modeling microcephaly with cerebral organoids reveals a WDR62–CEP170–KIF2A pathway promoting cilium disassembly in neural progenitors. Nat. Commun 10, 2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mlakar J et al. (2016) Zika virus associated with microcephaly. N. Eng. J. Med 374, 951–958 [DOI] [PubMed] [Google Scholar]
- 39.Gabriel E et al. (2017) Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20, 397–406 [DOI] [PubMed] [Google Scholar]
- 40.Cugola FR et al. (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dang J et al. (2016) Zika Virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19, 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu M et al. (2016) Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med 22, 1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C et al. (2017) 25-Hydroxycholesterol protects host against Zika virus infection and its associated microcephaly in a mouse model. Immunity 46, 446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones C et al. (2011) Animal models of schizophrenia. Br. J. Pharmacol 164, 1162–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Concheiro M et al. (2010) Maternal buprenorphine dose, placenta buprenorphine and metabolite concentrations and neonatal outcomes. Ther. Drug Monitor. 32, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mariani J et al. (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fry AE et al. (2013) Neuropsychiatric disease in patients with periventricular heterotopia. J. Neuropsych. Clin. Neurosci 25, 26–31 [DOI] [PubMed] [Google Scholar]
- 48.Klaus J et al. (2019) Altered neuronal migratory trajectories in human cerebral organoids derived from individuals with neuronal heterotopia. Nat. Med 25, 561–568 [DOI] [PubMed] [Google Scholar]
- 49.Brennand KJ et al. (2011) Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Os J et al. (2010) The environment and schizophrenia. Nature 468, 203. [DOI] [PubMed] [Google Scholar]
- 51.Stachowiak EK et al. (2017) Cerebral organoids reveal early cortical maldevelopment in schizophrenia – computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye F et al. (2017) DISC1 regulates neurogenesis via modulating kinetochore attachment of Ndel1/Nde1 during mitosis. Neuron 96, 1041–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez C et al. (2018) Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry 23, 2363–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padmanabhan J et al. (2016) Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. Plos One 11, e0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park J et al. (2018) A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci 21, 941–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J et al. (2015) Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip 15, 141–150 [DOI] [PubMed] [Google Scholar]
- 57.Pavoni S et al. (2018) Small-molecule induction of Aβ−42 peptide production in human cerebral organoids to model Alzheimer’s disease associated phenotypes. Plos One 13, e0209150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seo J et al. (2017) Inhibition of p25/Cdk5 attenuates Tauopathy in mouse and iPSC models of frontotemporal dementia. J. Neurosci 37, 9917–9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jayant R and Nair M (2016) Role of biosensing technology for neuroAIDS management. J. Biosens. Bioelectron 7, e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu R et al. (2019) OLIG2 drives abnormal neurodevelopmental phenotypes in human iPSC-based organoid and chimeric mouse models of down syndrome. Cell Stem Cell 24, 908–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bershteyn M et al. (2017) Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iefremova V et al. (2017) An organoid-based model of cortical development identifies non-cell-autonomous defects in Wnt signaling contributing to Miller–Dieker syndrome. Cell Rep. 19, 50–59 [DOI] [PubMed] [Google Scholar]
- 63.Gold WA et al. (2017) Rett syndrome: a genetic update and clinical review focusing on comorbidities. ACS Chem. Neurosci 9, 167–176 [DOI] [PubMed] [Google Scholar]
- 64.Mellios N et al. (2017) MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol. Psychiatry 23, 1051–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasca SP et al. (2011) Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med 17, 1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birey F et al. (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee C-T. et al. (2016) CYP3A5 mediates effects of cocaine on human neocorticogenesis: studies using an in vitro 3D self-organized hPSC model with a single cortex-like unit. Neuropsychopharmacology 42, 774–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Y et al. (2017) Probing impaired neurogenesis in human brain organoids exposed to alcohol. Integrative Biology 9, 968–978 [DOI] [PubMed] [Google Scholar]
- 69.Keller RW and Snyder-Keller A (2006) Prenatal cocaine exposure. Annal. N. Y. Acad. Sci 909, 217–232 [DOI] [PubMed] [Google Scholar]
- 70.Wang Y et al. (2018) Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 18, 851–860 [DOI] [PubMed] [Google Scholar]
- 71. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Brain-Tumors.
- 72.Linkous A et al. (2019) Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 26, 3203–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bian S et al. (2018) Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 15, 631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.da Silva B et al. (2018) Spontaneous glioblastoma spheroid infiltration of early-stage cerebral organoids models brain tumor invasion. Advancing Life Sciences R&D 23, 862–868 [DOI] [PubMed] [Google Scholar]
- 75.Ogawa J et al. (2018) Glioblastoma model using human cerebral organoids. Cell Rep. 23, 1220–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavazza A and Massimini M (2018) Cerebral organoids: ethical issues and consciousness assessment. J. Medical Ethics 44, 606–610 [DOI] [PubMed] [Google Scholar]
- 77.Chen HI et al. (2019) Applications of human brain organoids to clinical problems. Developmental Dynamics 248, 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mansour AA et al. (2018) An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol 36, 432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen HI et al. (2019) Transplantation of human brain organoids: revisiting the science and ethics of brain chimeras. Cell Stem Cell 25, 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karzbrun E and Reiner O (2019) Brain organoids – a bottom-up approach for studying human neurodevelopment. Bioengineering 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cakir B et al. (2019) Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giandomenico SL et al. (2019) Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci 22, 669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lancaster MA et al. (2017) Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol 35, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dezonne RS et al. (2017) Derivation of functional human astrocytes from cerebral organoids. Sci. Rep 7, 45091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sloan SA et al. (2017) Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ormel PR et al. (2018) Microglia innately develop within cerebral organoids. Nat. Commun 9, 4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelava I and Lancaster MA (2016) Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev. Biol 420, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quadrato G et al. (2016) The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med 22, 1220–1228 [DOI] [PubMed] [Google Scholar]
- 89.Griffin BA et al. (2019) In utero exposure to norbuprenorphine, a major metabolite of buprenorphine, induces fetal opioid dependence and leads to neonatal opioid withdrawal syndrome. J. Pharmacol. Exp. Ther 370, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boisvert EM et al. (2019) Minocycline mitigates the effect of neonatal hypoxic insult on human brain organoids. Cell Death Dis. 10, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]