Abstract
Under physiological states, the nervous system and the kidneys communicate with each other to maintain normal body homeostasis. However, pathological states disrupt this interaction as seen in hypertension, and kidney damage can cause impaired renorenal reflex and sodium handling. In acute kidney injury (AKI) and chronic kidney disease (CKD), damaged kidneys can have a detrimental effect on the central nervous system. CKD is an independent risk factor for cerebrovascular disease and cognitive impairment, and many factors, including retention of uremic toxins and phosphate, have been proposed as CKD-specific factors responsible for structural and functional cerebral changes in patients with CKD. However, more studies are needed to determine the precise pathogenesis. Epidemiological studies have shown that AKI is associated with a subsequent risk for developing stroke and dementia. On the other hand, recent animal studies have shown that the renal nerve contributes to kidney inflammation and fibrosis, whereas activation of the cholinergic anti-inflammatory pathway, which involves the vagus nerve, splenic nerve, and immune cells in the spleen, has a significant renoprotective effect. Therefore, elucidating mechanisms of communication between the nervous system and the kidney enables us not only to develop new strategies to ameliorate neurological conditions associated with kidney disease, but also to design safe and effective clinical interventions for kidney disease utilizing the neural and neuroimmune control of kidney injury and disease.
Keywords: acute kidney injury, brain, ischemia reperfusion, macrophages, cytokines
Introduction
The nervous system and the kidneys interact to maintain normal body homeostasis. However, pathological states, such as hypertension and kidney damage, can disrupt this interaction, which further leads to loss of normal homeostasis.
Chronic kidney disease (CKD) is a critical health burden worldwide with an overall prevalence rate of 13.1% in the US (1). Neurological disorders, including cerebrovascular disease and cognitive impairment, are prevalent in patients with CKD. The annual incidence of stroke is 15.1% and 9.6% in patients on hemodialysis and patients with CKD, respectively, whereas the annual incidence of stroke is 2.6% in patients without CKD (United States Renal Data System 2006 Annual Report. Morbidity & mortality. Neuroepidemiology: Incident & prevalent stroke [online] https://www.usrds.org/2006/pdf/06_morb_morte_06.pdf (2006)). In patients with CKD stage 3–5, a graded association between kidney and cognitive function was observed independent of the vascular risk factors (2, 3). Acute kidney injury (AKI) is also an emerging health burden worldwide, particularly in critically ill patients, and has high morbidity and mortality (4). A meta-analysis involving more than 3 million patients demonstrated that 1 in 5 adults and 1 in 3 children worldwide experienced AKI (KDIGO definition) during hospitalization (5). AKI can lead to uremic encephalopathy (6); furthermore, epidemiological studies have shown that AKI is associated with a subsequent risk for developing stroke and dementia (7-9). Therefore, damaged kidneys can have a detrimental effect on the central nervous system (CNS), a consequence that cannot be underestimated because the prevalence of CKD and AKI are increasing, and neurological disorders are associated with higher morbidity/mortality, lower quality of life, and increased health care costs.
Recent studies have demonstrated that the nervous system can affect the course of AKI and CKD directly or indirectly via the immune system (neuroimmunomodulation). In this review, we discuss the interactions between the nervous system and the kidney, focusing on possible underlying mechanisms that contribute to neurological disorders in patients with CKD and AKI and neural/neuroimmune control of kidney disease, which is a novel and promising therapeutic target in patients with kidney disease.
Physiological cross-talk between the nervous system and the kidney
The nervous system and the kidney communicate in various ways to maintain a normal physiological state. For example, neuroendocrine/kidney interactions are responsible for regulation of blood osmolality by vasopressin (10-12). Systemic changes in osmolality are detected by osmoreceptors expressed in specific regions of the CNS. The neuronal activity in these regions regulates the secretion of vasopressin from the hypothalamus/posterior pituitary gland and also stimulates or inhibits thirst and sodium appetite. Circulating vasopressin acts on vasopressin 2 receptors expressed in the renal collecting duct, which increases the number of aquaporin-2 channels in the apical membrane and leads to enhanced water reabsorption by the kidney.
Another example is renal sensory afferent and sympathetic efferent nerves working together with the kidney to maintain sodium balance (13). Renal sensory nerves, which innervate predominantly the pelvic wall and to a lesser extent renal vessels and parenchyma (14), are excited by increased pelvic pressure and pelvic wall stretch (15, 16). In contrast, sympathetic nerves innervate the whole renal vasculature with the most dense innervation to the afferent arterioles; the tubules are also innervated by sympathetic nerves (17). Thus, norepinephrine released from sympathetic nerve terminals can directly act on the renal vasculature and the tubular epithelial cells. Increased efferent renal sympathetic nerve activity (ERSNA) increases renin secretion (juxtaglomerular granular cells), decreases urinary sodium excretion (tubular epithelial cells), and decreases renal blood flow (vascular smooth muscle cells). In normal rats, unilateral renal denervation (ablation of both sensory afferent and sympathetic efferent nerves) increased ipsilateral urinary sodium excretion (due to sympathetic ablation), and increased contralateral ERSNA decreased contralateral urinary sodium excretion (18). Stimulation of the renal sensory afferent nerves decreased contralateral ERSNA with an increase in contralateral urinary sodium excretion (19-21). These findings support the existence of the inhibitory renorenal reflex—an increase in afferent renal nerve activity (ARNA) suppresses ERSNA. Several studies have also shown that an increase in ERSNA increases ARNA (22-24), suggesting a negative feedback system between ERSNA and ARNA.
The inhibitory renorenal reflex is important in the control of sodium balance because the responsiveness of the renal sensory afferent nerve is modulated by the amount of sodium intake. Increasing pelvic pressure resulted in greater ipsilateral ARNA responses and contralateral urinary sodium excretion in rats fed a high-sodium diet compared with rats fed a low-sodium diet (25). That is, with a high sodium intake, augmented ARNA further suppresses ERSNA through the inhibitory renorenal reflex, resulting in an increase in urinary sodium excretion to prevent sodium retention. In contrast, with a low sodium intake, suppressed ARNA leads to an increased ERSNA and results in a decrease in urinary sodium excretion to prevent sodium loss. Further studies are warranted to understand the precise mechanism by which ARNA responses are affected by the amount of sodium intake, although the contribution of angiotensin II (22, 25, 26), norepinephrine (22), and endothelin (27, 28) has been suggested. In summary, under healthy conditions, renal sensory afferent/sympathetic efferent nerves and the kidney act together to maintain sodium balance in response to a range of dietary sodium intake through inhibitory renorenal reflex pathways.
Cross-talk between the nervous system and the kidney in pathological states
Impaired renorenal reflex under pathological conditions
Renal nerves play a critical role in the pathogenesis of hypertension in the spontaneously hypertensive rat (SHR) (29, 30). SHRs have increased ERSNA, decreased responsiveness of the renal sensory afferent nerves to increased pelvic pressure or pelvic administration of chemicals (31-33), and suppressed ARNA responses to ERSNA (34). In a backcross of SHR and Wistar-Kyoto rats, the degree of stimulation of ARNA elicited by increased pelvic pressure was inversely correlated with the level of basal mean arterial pressure (35). These findings may suggest that suppressed ARNA leads to an increased ERSNA, as observed with a low sodium intake, resulting in sodium retention and hypertension in SHR. Indeed, angiotensin II and norepinephrine were reported to contribute to the reduced responsiveness of the renal sensory afferent nerves in SHR as with a low sodium intake (34, 36). This impaired inhibitory renorenal reflex was also observed in various pathological states, such as diabetes (37), heart failure (38), hypoxia (39), ureteral obstruction (40), cirrhosis (41), and renal ischemia–reperfusion injury (IRI) (42).
In diseased kidneys, activation of renal sensory afferent nerves can evoke a reflex different from the inhibitory renorenal reflex. In two-kidney, one-clip hypertensive rats, denervation of the clipped kidney increased urinary sodium excretion from the intact kidney and from the ischemic kidney, which was accompanied by a decrease in contralateral ERSNA (43). These results may suggest that renal sensory afferent nerves in ischemic kidneys increase contralateral ERSNA. The mechanism of conversion from inhibitory to excitatory renorenal reflex in diseased kidneys has yet to be determined, although excitation of renal chemosensitive nerves by increased adenosine might play a role (44, 45). Thus, renal denervation seems to be a reasonable approach to treat hypertension. Renal denervation has been studied intensely in humans with refractory hypertension, although the efficacy is still controversial (46-49).
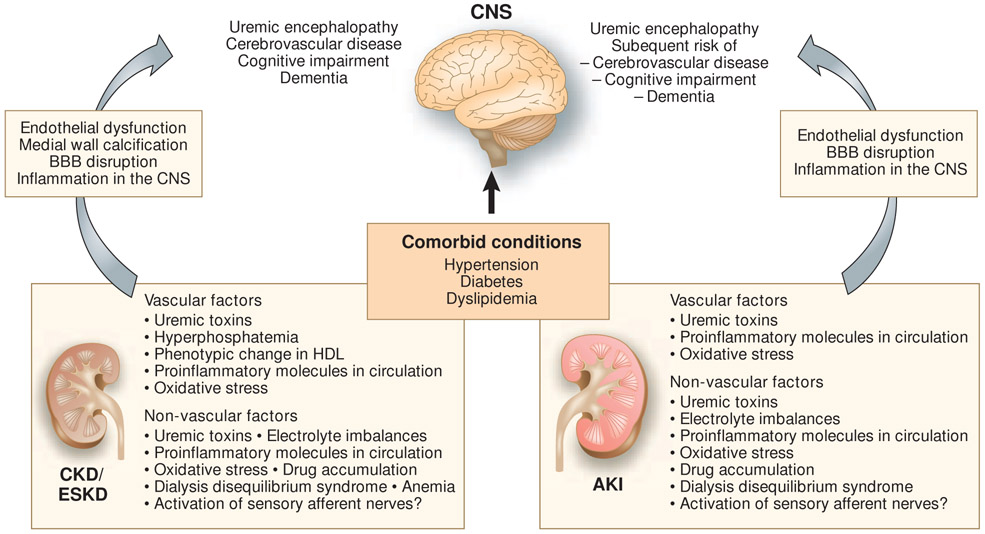
The effects of CKD on the CNS (Figure 1)
Figure 1.
Structural and functional cerebral damage caused by CKD/ESKD and AKI. Kidney damage-specific factors that likely contribute to cerebral dysfunction are described. Those factors are thought to be pathogenic along with comorbid conditions, such as hypertension, diabetes, and dyslipidemia, leading to cerebral damage. AKI, acute kidney injury; BBB, blood–brain barrier; CKD, chronic kidney disease; CNS, central nervous system; ESKD, end-stage kidney disease; HDL, high-density lipoprotein.
The kidney and brain require sTab high blood flow and share anatomical characteristics in their arterial vasculature. Both organs have “strain vessels” (afferent arteriole in the kidney and perforating arteriole in the brain), which are short and small arterioles branched out from much larger arteries that autoregulate tissue perfusion (50). The vasculatures of these two organs are susceptible to traditional risk factors for atherosclerosis, such as old age, hypertension, diabetes, dyslipidemia, and smoking, which is consistent with the fact that strain vessels are exposed to a large pulsatile pressure and are directly influenced by the stiffness and hemodynamics of large arteries. Thus, one could argue that deterioration of kidney and cerebrovascular function simply reflect common vascular injury. However, many epidemiological studies have shown that CKD per se is a risk factor for cerebrovascular disease independent of the traditional risk factors for atherosclerosis (51-54). - Leukoaraiosis is a pathological appearance of the brain white matter, which is thought to be due to perfusion abnormalities within the arterioles that perforate through the deep brain structures. It is recognized as periventricular and white matter hyperintensities in T2-weighted or FLAIR MRI, and is pathologically characterized by neuronal loss, demyelination, and gliosis. Leukoaraiosis is thought to represent ischemia and is associated with a higher risk of stroke and dementia (55). Patients with CKD have a high prevalence of leukoaraiosis (56, 57). CKD is also a risk factor for cognitive impairment (2, 57, 58), which is explained, at least in part, by subclinical small vessel disease.
Several possibilities have been proposed as non-traditional CKD-specific causes of structural and functional cerebral changes, although many of these are currently just correlative, and more experimental and clinical studies are warranted to prove causality. Uremic toxins, which may accumulate in CKD, seem to have a direct impact on cerebrovascular disease and cognitive functions. An extensive review of relevant reports on uremic toxins and cerebrorenal interactions suggested that uric acid, indoxyl sulfate, p-cresyl sulfate, interleukin 1β, interleukin 6, tumor necrosis factor α (TNFα), and parathyroid hormone are likely to have an impact on the CNS, although the mechanisms are yet to be determined (59). Guanidine compounds have also been investigated as potential uremic neurotoxins (60). Guanidinosuccinate evoked inward whole-cell currents in primary murine spinal cord neurons owing to specific interaction with voltage- and ligand-gated Ca2+ channels, suggesting the potential for calcium-induced neurotoxicity (61). Asymmetric dimethylarginine (ADMA), which is another uremic toxin and an endogenous nitric oxide inhibitor, may affect cerebral blood flow. Infusion of a subpressor dose of ADMA to healthy volunteers significantly decreased cerebral perfusion, which was accompanied by an increase in arterial stiffness (62). Infusion of ADMA to normal rats significantly decreased exploratory behavior and locomotion; these effects were also observed in 5/6 nephrectomized rats, an animal model of CKD (63). Indeed, ADMA was independently associated with cerebral small vessel disease and positively correlated with the severity of leukoaraiosis in humans (64).
Excessive phosphate has also attracted attention as a cause of vascular damage in patients with CKD. In a mouse model of CKD, high phosphate levels caused endothelial dysfunction and increased VCAM-1/ICAM-1 expression in endothelial cells (65). Elevated phosphate levels directly cause arterial medial calcification by inducing an osteogenic phenotypic change of vascular smooth muscle cells. Aortic smooth muscle cells under elevated phosphate conditions showed increases in mineral deposition with expression of bone-forming transcription factors (e.g., Runx2) and procalcification proteins, such as osteocalcin, osteopontin, and alkaline phosphatase (66, 67). Notably, this phenotypic change of vascular smooth muscle cells was confirmed by lineage-tracing experiments in mice (68), and calcified human arterial samples also showed expression of Runx2, osteocalcin, and alkaline phosphatase (69). These changes induced by a high level of phosphate are mediated by sodium-dependent phosphate cotransporters, PiT-1 and PiT-2, through which phosphates can enter vascular smooth muscle cells (70, 71). Fibroblast growth factor 23, which is often upregulated in patients with CKD and induces phosphaturia, might also directly contribute to cerebrovascular disease (72-74). Indoxyl sulfate induced oxidative stress and increased expression levels of osteoblast markers in human aortic smooth muscle cells (75) and in the arcuate aorta of hypertensive rats (76). Both phosphate and indoxyl sulfate were also shown to induce reactive oxygen species production and decrease cell viability in cerebral endothelial cells (77). Studies in CKD animal models demonstrated accumulation of nitrotyrosine in the cerebral cortex and an increased number of pyknotic neuronal cells with accumulation of 8-hydroxy-2-deoxyguanosine in the hippocampus and that administration of antioxidants reversed these pathological changes in the brain and improved cognitive impairment without affecting renal function, thus providing additional evidence for the role of reactive oxygen species (78, 79).
It is interesting that high-density lipoprotein (HDL), which is considered to be antiatherogenic, can have an altered phenotype in the CKD milieu. HDL was isolated from 82 children with stage 2–5 CKD who were free of concomitant diseases that affect HDL function and its effects on human aortic endothelial cells were examined (80). Compared with HDL isolated from controls, HDL isolated from children with CKD inhibited nitric oxide production and increased superoxide production and VCAM-1 expression; the detrimental effects on endothelial cells strongly correlated with CKD stage, with the most remarkable effects induced by HDL isolated from children on dialysis. Importantly, a decreased nitric oxide production ex vivo was significantly associated with increased arterial stiffness due to increased aortic pulse wave velocity and carotid intima–media thickness in children with CKD. Furthermore, a longitudinal follow-up of children with CKD undergoing kidney transplantation demonstrated that the deleterious effects of HDL on endothelial cells were lower when HDL was isolated from patients 3 months after transplantation. These findings are consistent with an observation that HDL cholesterol levels were not associated with all-cause or cardiovascular mortality in patients with eGFR < 90 ml/min (81) and suggest that the CKD milieu per se induces a modified HDL phenotype that is detrimental to endothelial cells and atherogenic. Little is known about the mechanism of the phenotypic change of HDL in patients with CKD, although accumulated symmetric dimethylarginine (82) in and altered protein composition (83) of the HDL particles in patients with CKD might play a role.
In summary, many CKD-specific and nonspecific factors interact with each other and contribute to structural and functional cerebral changes in patients with CKD. However, it is yet to be determined to what extent these CKD-specific factors contribute to cerebrovascular diseases and cognitive impairment and whether specific interventions targeting these factors are beneficial in patients with CKD.
The effects of AKI on the CNS (Figure 1)
Accumulated uremic toxins in the setting of renal dysfunction, especially untreated renal failure, can lead to uremic encephalopathy, although its pathogenesis is poorly understood. Patients exhibit irritability, attention deficits, altered mental status, seizures, and death (6). Patients with AKI are more susceptible to encephalopathy compared with patients with CKD probably due to inadequate time for adaptation to uremic toxins that accumulate following AKI.
Recent epidemiological studies have also shown that AKI is associated with a risk of subsequent stroke and dementia (7-9). A nationwide observational study in Taiwan showed that dialysis-requiring AKI was associated with a higher risk and higher severity of subsequent stroke events even after adjustment for progression to CKD, and its impact was similar to that of diabetes (7). Another study in elderly patients who received intensive care during their hospitalization and survived to hospital discharge showed that dialysis-requiring AKI was associated with a higher risk of dementia after the adjustment for other risk factors (8). AKI was also significantly associated with a higher incidence of dementia (1.88-fold increased risk) during a 12-year follow-up period in a broader population (not limited to dialysis-requiring AKI or elderly patients) (9).
Pathological changes occurring in the brain during AKI may explain a higher risk of subsequent stroke and dementia. Renal IRI in mice increased neuronal pyknosis and the number of activated microglial cells in the hippocampus after 24 h (84). Brains from AKI mice also had increased levels of inflammatory cytokines both in the cerebral cortex and the hippocampus, as well as astrocyte activation in the cortex and corpus callosum, and those mice had reduced locomotor activity. Renal IRI in rats also resulted in motor and locomotor activity disturbances that were accompanied by significant upregulation of Toll-like receptor 4 (TLR4) in the hippocampus and striatum (85), suggesting that TLR4 plays an important role; this was also shown in kidney–lung interaction in AKI (86). Another study showed that dopamine turnover was decreased in the striatum, mesencephalon, and hypothalamus, which was accompanied by decreased spontaneous motor activity in rats with renal IRI (87).
The mechanism of those pathological changes in the brain during AKI is mostly unknown. Notably, an increased neuronal pyknosis or astrocyte activation was not observed in liver IRI (84), suggesting that the effects on the brain depend on the organ injured and that those brain changes might be specific for AKI and are not just associated with generalized inflammation following any organ injury. However, factors other than just acute decline of renal function and accumulation of uremic toxins may play a role considering that bilateral renal IRI induced functional and transcriptional changes in the lung distinguishable from those induced by bilateral nephrectomy (88, 89). Animal studies have suggested that inflammation in the brain plays an important role in the post-AKI brain changes; these changes were accompanied by increased vascular permeability in the brain (84, 85), suggesting a disruption of the blood–brain barrier (BBB). BBB disruption can lead to infiltration of inflammatory molecules, such as cytokines and chemokines released from the kidney after ischemic AKI (90), and toxic metabolites into the brain. Taken together, these findings support the concept that the AKI-induced inflammatory milieu causes BBB disruption, which exposes the brain to circulating inflammatory molecules and toxic metabolites leading to inflammation and pathological changes in the brain. It is also possible that sensory afferent nerves innervating the periphery, including the kidney, are activated by danger-associated molecular patterns or cytokines in AKI and directly affect the brain.
Contribution of the renal nerve to kidney inflammation and fibrosis
It is well known that the renal nerve is critically involved in the pathogenesis of hypertension. Recent studies suggested that the renal nerve can also enhance kidney inflammation and fibrosis in mice (91, 92). Ipsilateral renal denervation 2 days before unilateral ureteral obstruction (UUO) surgery markedly suppressed immune cell infiltration and fibrosis in the kidney, suggesting that the renal nerve enhances renal inflammation and fibrosis (91). The authors further investigated which neurotransmitters released by renal sensory afferent and sympathetic efferent nerves are responsible for the enhancement of renal damage. Continuous infusion of calcitonin gene-related peptide (CGRP) or norepinephrine into the denervated kidney dose-dependently abrogated the protective effect of renal denervation, but infusion of neuropeptide Y or substance P did not affect renal outcomes. Consistent with these results, blocking CGRP receptors and α2-adrenergic receptors expressed in tubular epithelial cells also conferred protection against kidney inflammation and fibrosis. Similar results were found in a unilateral kidney IRI model (92). Renal denervation after disease induction (UUO and IRI surgeries) did not significantly ameliorate kidney fibrosis, which suggests that the renal nerve plays an important role during the acute phase of injury. Renal denervation performed just before IRI surgery also ameliorated AKI in rats (93, 94). The protective effect of renal denervation has also been shown in other kidney injury models, such as anti-Thy-1.1 nephritis (95) and lupus nephritis (96). Taken together, these findings indicate that the local activity of sensory afferent and sympathetic efferent nerves in the kidney contributes to kidney inflammation and fibrosis and that renal denervation is possibly effective in suppressing those changes.
Neuroimmune interaction in regulating inflammatory diseases
The vagus nerve and the cholinergic anti-inflammatory pathway (CAP)
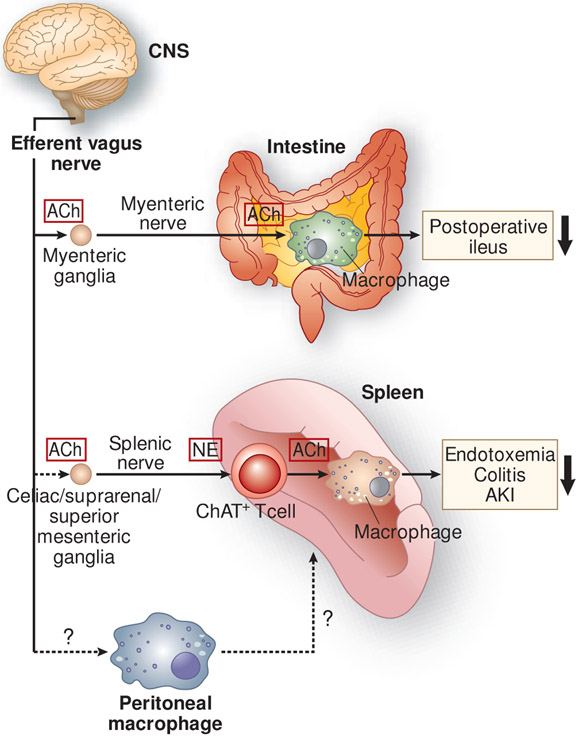
The vagus nerve (10th cranial nerve) is a bilateral nerve bundle that is composed of axons of both efferent and afferent neurons. The efferent neurons provide input to most organs in the periphery and some skeletal muscles, and the afferent neurons transmit sensory information from visceral organs to the CNS. In 2002, Kevin Tracey found that a small amount of a potent anti-inflammatory agent (CNI-1493) administered via the intracerebroventricular route dramatically decreased the level of plasma tumor necrosis factor (TNF), which is released mostly from the spleen, in rats treated with lipopolysaccharide (LPS) (97). Although CNI-1493 is able to inhibit macrophage activation in the periphery and systemic administration of CNI-1493 ameliorated AKI-induced lung injury (98), the decreased level of plasma TNF was caused not through a direct effect on macrophages but through neural connections to the spleen. This conclusion was drawn because of the negligible systemic concentrations of CNI-1493 (97). Transecting the vagus nerve canceled the decrease in plasma TNF, and electrical stimulation of the vagus nerve was sufficient to decrease plasma TNF. These findings suggested that an anti-inflammatory signal descends from the CNS through the vagus nerve to the spleen to attenuate peripheral inflammation. Thereafter, this pathway has been intensively explored; now it is known as the cholinergic anti-inflammatory pathway (CAP) as described below (Figure 2).
Figure 2.
Anti-inflammatory neural circuits elicited by the stimulation of the efferent vagus nerve. In the cholinergic anti-inflammatory pathway, stimulation of the efferent vagus nerve appears to transmit a signal to the splenic nerve, although a direct connection between the efferent vagus and splenic nerves remains controversial. Norepinephrine (NE), released from splenic nerve terminals, interacts with β2-adrenergic receptors expressed on choline acetyltransferase (ChAT)-positive (acetylcholine-producing) T cells in the spleen, which leads to the release of acetylcholine (ACh) from these cells. Subsequently, ACh binds to α7 nicotinic acetylcholine receptors (α7nAChRs) expressed on macrophages, resulting in the suppressed production of pro-inflammatory cytokines by macrophages and suppressed inflammation. This pathway has been demonstrated in many inflammatory disease models, including endotoxemia, colitis, and acute kidney injury (AKI). Another anti-inflammatory circuit involves a direct interaction between ACh released from the myenteric nerve and α7nAChRs expressed on muscularis macrophages of the intestine in postoperative ileus. Vagus nerve stimulation (VNS) causes phenotypic changes in peritoneal macrophages toward anti-inflammation, and adoptive transfer of these macrophages confers protection from AKI, which requires the spleen. The mechanism underlying changes in the phenotype of peritoneal macrophages after VNS and interaction of peritoneal macrophages with immune cells in the spleen remains unclear. CNS, central nervous system.
The CAP begins with the activation of the efferent vagus nerve and transmission of the signal to the splenic nerve (99), probably via the celiac/suprarenal/superior mesenteric ganglia (100-104), although it is still controversial whether there is a direct connection between the efferent vagus nerve and the splenic nerve (105, 106). Norepinephrine is released from splenic nerve terminals and interacts with β2-adrenergic receptors expressed on a choline acetyltransferase-positive CD4+ CD44high CD62Llow memory T cell subpopulation in the spleen, which leads to the release of acetylcholine from these cells (107). Acetylcholine binds to α7 nicotinic acetylcholine receptors (α7nAChRs) expressed on macrophages, resulting in the suppressed production of pro-inflammatory cytokines, such as TNFα, by macrophages, and suppressed inflammation (99, 108). This anti-inflammatory efferent vagus nerve–spleen axis has been demonstrated in many inflammatory disease models, including endotoxemia (99, 107, 108), colitis (109), and renal IRI (110) (discussed below). In addition, another anti-inflammatory pathway mediated by the efferent vagus nerve has been described (Figure 2) (111). In this pathway the efferent vagus nerve synapses on cholinergic myenteric neurons that are in close contact with muscularis macrophages expressing α7nAChRs in the intestine. Vagus nerve stimulation (VNS) ameliorated surgery-induced intestinal inflammation and improved postoperative intestinal transit (111). Thus, VNS produces an anti-inflammatory effect that requires α7nAChRs but may or may not require the spleen and T cells, depending on the disease.
CAP activation to ameliorate AKI
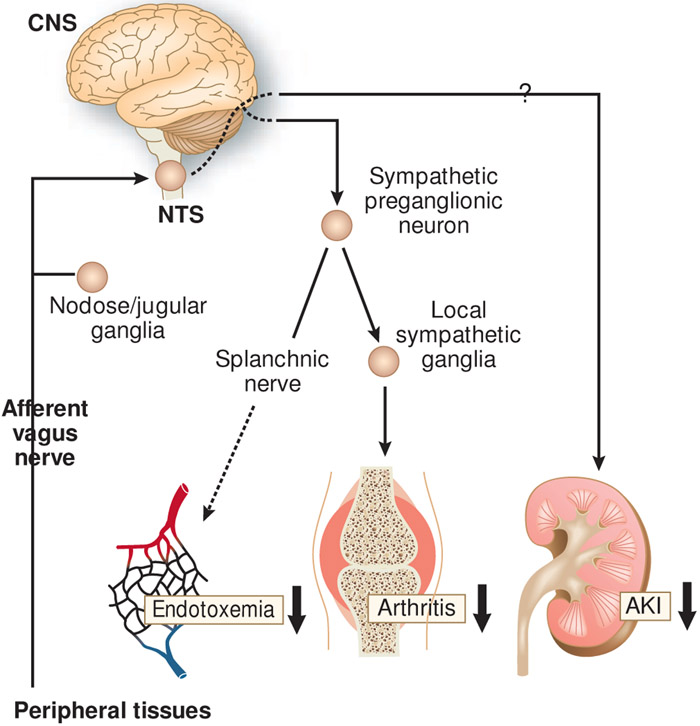
The protective effect of CAP activation in AKI was explored in a mouse model of bilateral renal IRI (110). Electrical stimulation of the left cervical vagus nerve 24 h prior to IRI markedly ameliorated AKI. VNS was not protective in α7nAChRmice or splenectomized mice, suggesting that CAP activation was responsible for the protection. Stimulation of the peripheral end of the transected vagus nerve also protected the kidney, which is consistent with activation of the efferent vagus nerve of the CAP. It is interesting that renoprotection was also observed when the central end of the transected vagus nerve was stimulated. This finding indicates that “afferent VNS” protects the kidney through a pathway different from the canonical efferent vagus nerve of the CAP, which clearly merits further investigation (Figure 3). In a similar manner, afferent VNS was also shown to be protective against experimental arthritis (112). The proposed mechanism was that afferent VNS activates sympathetic efferent neurons through the CNS, which increases the release of norepinephrine from local sympathetic nerve terminals within inflamed joints resulting in the local suppression of the innate immune response (Figure 3). Stimulation of abdominal vagal afferent fibers also suppressed systemic inflammation in endotoxemia, and the efferent arm of this pathway was suggested to be in the splanchnic nerves (Figure 3) (113). VNS was also effective in renal transplantation (114, 115). VNS in brain-dead donor rats reduced inflammation in the donors and immune cell infiltration to the recipient kidneys, which led to improved long-term kidney function and recipient survival.
Figure 3.
Anti-inflammatory neural circuits elicited by the stimulation of the afferent vagus nerve. The signal from the afferent vagus nerve is transmitted to the nucleus tractus solitarius (NTS), a region in the brain. It was suggested that the sympathetic nerve is the efferent pathway from the central nervous system (CNS) that exerts an anti-inflammatory effect in arthritis and endotoxemia models. An efferent pathway has not been identified in the context of protection against acute kidney injury (AKI).
Pulsed ultrasound was protective against AKI and thought to activate the CAP (116, 117). Ultrasound application with a clinical machine 24 h before bilateral renal IRI ameliorated kidney injury in mice. Splenectomy, splenic sympathectomy, genetic deletion of T and B cells (Rag1−/− mice) or α7nAChR (Chrna7−/− mice), or treatment with an α7nAChR antagonist abolished the protective effect. Furthermore, bone marrow chimera experiments suggested that the protection required α7nAChR expression in hematopoietic cells. Protection by ultrasound was also demonstrated in a cecal ligation and puncture model of sepsis. Although the mechanism by which ultrasound protects the kidney from AKI needs further investigation, recent findings suggest that the direct target of ultrasound could be the vagus nerve (118) or the spleen, including the splenic nerve (119, 120).
Stimulation of neurons in a specific brain region, C1 neurons, can also activate the CAP and protect the kidney against AKI (121). C1 neurons residing in the medulla oblongata mediate autonomic responses to a variety of stresses, such as inflammation, hypoxia, and hypotension (122). Selective optogenetic stimulation of C1 neurons protected mouse kidneys from IRI, and the protection required the spleen, α7nAChRs, and β2-adrenergic receptors. The investigators also showed that the sympathetic nerve, not the efferent vagus nerve, was the efferent neural circuit from the C1 neurons to the periphery leading to CAP activation and kidney protection. The terminal fields of the sympathetic nerve mediating the kidney protection have not yet been determined.
Studies showed that CAP activation can lead to kidney protection in AKI. Adoptive transfer of splenocytes isolated from VNS-treated (110) or ultrasound-treated (116) mice to non-treated mice was sufficient to protect recipient mouse kidneys from AKI. These findings indicate that CAP activation can alter the phenotype of splenocytes, which contributes to the kidney protection. The interaction between the spleen and the kidney in AKI seems to be complex (123, 124). AKI induced by epinephrine infusion (125, 126) or bilateral renal artery occlusion (127, 128) in dogs was ameliorated by splenectomy, whereas splenectomized mice showed worse kidney injury in mild renal IRI (116). AKI can also affect the phenotype of splenocytes; T-cell deficient mice receiving splenocytes from wild-type mice that had bilateral renal IRI 5 days before had reduced renal IRI compared with those receiving splenocytes from sham-operated, wild-type mice (129). Another study also suggested that the phenotype of splenic lymphocytes can be altered 6 weeks after severe unilateral renal IRI (130). Notably, the interaction between the nervous system including the vagus/splenic nerves and the spleen seems to be context-dependent. In an angiotensin II infusion model, T cells egressed from the spleen and infiltrated into the kidney and aorta, leading to inflammation and hypertension, and these steps required the vagus nerve and the splenic nerve (131, 132). The importance of renal sympathetic nerve activity was also shown in the same model by another group (133). A detrimental effect of the spleen and splenic nerve was also reported in atherosclerosis (134). Increased splenic sympathetic activity was important in myeloid progenitor proliferation and differentiation in the spleen, which contributed to the subsequent formation of atherosclerotic plaque in diabetes. Thus, it is important to delineate how the spleen interacts with the kidney in the context of CAP activation and amelioration of AKI.
Peritoneal macrophages might also be involved in CAP activation (Figure 2) (135). VNS or ultrasound application caused phenotypic changes in peritoneal macrophages toward anti-inflammation, and adoptive transfer of these macrophages conferred protection from kidney IRI. It is still unclear how VNS and ultrasound change the phenotype of peritoneal macrophages. Splenectomy or genetic deletion of Rag1 or α7nAChR nullified the protection, suggesting that there might be an interaction between peritoneal macrophages and immune cells in the spleen. RNA sequencing of nicotine/LPS-treated peritoneal macrophages isolated from wild type and α7nAChR−/− mice identified hairy and enhancer of split-1 (Hes1) as a key downstream molecule to activate the CAP. Hes1, which is a transcriptional repressor, was reported to suppress production of inflammatory cytokines and chemokines in macrophages (136, 137). Thus, α7nAChR-positive peritoneal macrophages are involved in a non-canonical CAP and Hes1 plays an important role in activating the CAP to protect the kidneys from injury.
Clinical application of CAP activation to treat AKI
Many clinical trials of VNS are ongoing to translate the beneficial effect of VNS into the treatment of patients with various inflammatory diseases. A small pilot clinical study was conducted to investigate the effect of VNS in patients with Crohn’s disease (138), on the basis of animal studies showing that VNS was protective in an experimental model of Crohn’s disease (139, 140). After VNS (through an implanted stimulator) for 6 months, five out of seven patients with active Crohn’s disease achieved clinical remission, judged by decreased disease activity index and improved endoscopic findings. The first clinical trial using an implanted stimulator in patients with refractory rheumatoid arthritis, which was conducted following a positive animal study (141), was also promising (142). VNS significantly suppressed TNF production and improved disease severity for up to 12 weeks. Carefully controlled clinical trials with a larger number of patients are warranted to confirm the efficacy of VNS in these diseases.
Before conducting clinical trials of VNS in patients with AKI, a risk–efficacy ratio should be carefully evaluated. Electrical stimulation of the cervical vagus nerve has been shown to be effective in renal IRI in mice, but it requires an invasive surgery. Ultrasound may be more easily applied in clinical trials because it is noninvasive, although it is still unknown how ultrasound activates the CAP. Another alternative to invasive surgeries is a transcutaneous vagus nerve stimulator, which the U.S. Food and Drug Administration recently approved for the treatment of migraine pain and episodic cluster headache pain. Evaluation of the efficacy of a noninvasive transcutaneous (including auricular) vagus nerve stimulator for AKI is needed. Pharmacological activation of the CAP by GTS-21, an agonist of α7nAChR, or nicotine is another strategy, although pharmacological approaches generally lead to less specificity and more off-target effects. In animal studies, GTS-21 or nicotine was protective against renal IRI (143), cisplatin-induced AKI (144), and LPS-induced AKI (145).
Much work needs to be done to delineate the precise mechanism of renoprotection by VNS, ultrasound, and other strategies to activate the CAP. Defining precise neural circuits (e.g., downstream pathways of afferent VNS) is an important task. Emerging techniques, such as optogenetics, which enable us to selectively stimulate or inhibit target neurons (146), should help us in dissecting neural circuits. Another unanswered question is the interaction between the spleen and the kidney in the context of CAP activation and amelioration of AKI. Determining the precise mechanism of renoprotection will lead to better prediction of adverse effects in the present strategies and identification of more focused therapeutic targets.
Conclusions
Under pathological conditions, the interaction between the nervous system and the kidney is disrupted, which impairs normal body homeostasis (e.g., hypertension and kidney damage can result in impaired reno-renal reflex and sodium handling). Both AKI and CKD have a direct detrimental effect on the CNS, and the mechanism is multifactorial and needs further investigation. Increasing evidence suggests that the nervous system, including the renal, vagus, and splenic nerves (the latter two are involved in CAP activation), modulate the course of AKI and CKD. The significance of these interactions between the nervous system and the kidney is increasing, considering the high prevalence of AKI and CKD. Determination of the mechanism by which AKI and CKD cause neurological disorders and development of appropriate interventions should reduce morbidity and mortality in patients with AKI and CKD. The clinical application of the neural and neuroimmune control of kidney disease (e.g., VNS) is promising; however, elucidating the underlying mechanisms, including precise neural circuits and interaction between the spleen and the kidney, is critical for safe and effective clinical application.
Table 1.
Glossary of Neuroscience Terms.
| Afferent nerve (neuron, fiber) | A nerve (neuron, fiber) that transmits sensory information from the periphery to the central nervous system |
| ARNA | Afferent renal nerve activity |
| Astrocyte | A non-neuronal cell in the central nervous system that has many functions including provision of nutrients to neurons and support of the blood–brain barrier |
| CAP | Cholinergic anti-inflammatory pathway |
| Calcitonin gene-related peptide (CGRP) | A neuropeptide that is released from sensory afferent neurons when they are stimulated and has immunomodulatory functions |
| Celiac/suprarenal/superior mesenteric ganglia | Clusters of nerve cells in the abdomen that provide sympathetic innervation to many abdominal organs |
| Corpus callosum | A bundle of commissural fibers connecting the two cerebral hemispheres |
| C1 neurons | Neurons residing in the medulla oblongata that regulate the hypothalamic pituitary axis and the autonomic nervous system |
| Efferent nerve (neuron, fiber) | A nerve (neuron, fiber) that transmits signals from the central nervous system to the periphery |
| ERSNA | Efferent renal sympathetic nerve activity |
| Hippocampus | A small, curved formation in the brain that plays a critical role in memory consolidation |
| Leukoaraiosis | A pathological appearance of the periventricular area and white matter that is thought to be related to small vessel disease and perfusion disturbances |
| Medulla oblongata | The lowest portion of the brainstem |
| Mesencephalon | The uppermost portion of the brainstem |
| Microglial cell | A specialized macrophage in the central nervous system |
| Myenteric neuron/ganglion/nerve | A neuron/ganglion/nerve residing in the muscle layers and innervating the smooth muscle cells in the intestine |
| Neuropeptide Y | A neuropeptide that is released from sympathetic neurons simultaneously with norepinephrine when they are stimulated and has immunomodulatory functions |
| Nodose/jugular ganglia | Ganglia where the cell bodies of vagus afferent neurons reside |
| Nucleus tractus solitarius (NTS) | A group of neuronal cell bodies in the medulla oblongata that form an integrative center for sensory information from several cranial nerves including the vagus |
| Splanchnic nerve | A nerve providing afferent and efferent autonomic (primarily sympathetic) innervation to many abdominal organs |
| Striatum | A structure with a striped appearance that plays a critical role in the motor and reward systems |
| Substance P | A neuropeptide that is released from sensory afferent neurons when they are stimulated and has immunomodulatory functions |
| Sympathetic preganglionic neuron | A neuron residing in the spinal cord that projects to sympathetic ganglia (e.g., celiac ganglion) |
Acknowledgments
The authors wish to thank members of the Okusa laboratory for their contributions as well as Drs. Diane L. Rosin, Patrice Guyenet, Ruth Stornetta and Stephen Abbott, Department of Pharmacology, University of Virginia, for their contributions on studies of neural circuitry and the kidney and Dr. John Hossack, Department of Biomedical Engineering, University of Virginia, for expertise in ultrasound. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) under award numbers R01DK085259, R01DK062324, T32 DK0792922 and U18 EB021787 (MDO), and by the Uehara Memorial Foundation Research Fellowship and Japan Society for the Promotion of Science Overseas Research Fellowships (ST). The authors have declared that no conflict of interest exists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298(17):2038–47. [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127–33. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2010;58(2):338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. [DOI] [PubMed] [Google Scholar]
- 5.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81(10):942–8. [DOI] [PubMed] [Google Scholar]
- 7.Wu VC, Wu PC, Wu CH, Huang TM, Chang CH, Tsai PR, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerra C, Linde-Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care. 2012;16(6):R233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai HH, Yen RF, Lin CL, Kao CH. Increased risk of dementia in patients hospitalized with acute kidney injury: A nationwide population-based cohort study. PLoS One. 2017;12(2):e0171671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson GL. Physiology of ADH secretion. Kidney Int Suppl. 1987;21:S20–6. [PubMed] [Google Scholar]
- 11.Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84(1):169–208. [DOI] [PubMed] [Google Scholar]
- 12.Agre P. Homer W. Smith award lecture. Aquaporin water channels in kidney. J Am Soc Nephrol. 2000;11(4):764–77. [DOI] [PubMed] [Google Scholar]
- 13.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308(2):R79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol. 1991;311(3):389–404. [DOI] [PubMed] [Google Scholar]
- 15.Genovesi S, Pieruzzi F, Wijnmaalen P, Centonza L, Golin R, Zanchetti A, et al. Renal afferents signaling diuretic activity in the cat. Circ Res. 1993;73(5):906–13. [DOI] [PubMed] [Google Scholar]
- 16.Kopp UC, Smith LA, Pence AL. Na(+)-K(+)-ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. Am J Physiol. 1994;267(4 Pt 2):R1109–17. [DOI] [PubMed] [Google Scholar]
- 17.Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol. 1992;70(5):735–49. [DOI] [PubMed] [Google Scholar]
- 18.Colindres RE, Spielman WS, Moss NG, Harrington WW, Gottschalk CW. Functional evidence for renorenal reflexes in the rat. Am J Physiol. 1980;239(3):F265–70. [DOI] [PubMed] [Google Scholar]
- 19.Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol. 1984;246(1 Pt 2):F67–77. [DOI] [PubMed] [Google Scholar]
- 20.Kopp UC, Smith LA. Inhibitory renorenal reflexes: a role for substance P or other capsaicin-sensitive neurons. Am J Physiol. 1991;260(1 Pt 2):R232–9. [DOI] [PubMed] [Google Scholar]
- 21.Kopp UC, Smith LA. Role of prostaglandins in renal sensory receptor activation by substance P and bradykinin. Am J Physiol. 1993;265(3 Pt 2):R544–51. [DOI] [PubMed] [Google Scholar]
- 22.Kopp UC, Cicha MZ, Smith LA, Ruohonen S, Scheinin M, Fritz N, et al. Dietary sodium modulates the interaction between efferent and afferent renal nerve activity by altering activation of alpha2-adrenoceptors on renal sensory nerves. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopp UC, Cicha MZ, Smith LA, Mulder J, Hokfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of alpha1- and alpha2- adrenoceptors on renal sensory nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1561–72. [DOI] [PubMed] [Google Scholar]
- 24.Niijima A. The effect of efferent discharges in renal nerves on the activity of arterial mechanoreceptors in the kidney in rabbit. The Journal of physiology. 1972;222(2):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopp UC, Cicha MZ, Smith LA. Endogenous angiotensin modulates PGE(2)-mediated release of substance P from renal mechanosensory nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2002;282(1):R19–30. [DOI] [PubMed] [Google Scholar]
- 26.Kopp UC, Cicha MZ, Smith LA. Angiotensin blocks substance P release from renal sensory nerves by inhibiting PGE2-mediated activation of cAMP. Am J Physiol Renal Physiol. 2003;285(3):F472–83. [DOI] [PubMed] [Google Scholar]
- 27.Kopp UC, Grisk O, Cicha MZ, Smith LA, Steinbach A, Schluter T, et al. Dietary sodium modulates the interaction between efferent renal sympathetic nerve activity and afferent renal nerve activity: role of endothelin. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopp UC, Cicha MZ, Smith LA. Differential effects of endothelin on activation of renal mechanosensory nerves: stimulatory in high-sodium diet and inhibitory in low-sodium diet. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1545–56. [DOI] [PubMed] [Google Scholar]
- 29.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90(2):513–57. [DOI] [PubMed] [Google Scholar]
- 30.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1(2):731–67. [DOI] [PubMed] [Google Scholar]
- 31.Kopp UC, Cicha MZ, Farley DM, Smith LA, Dixon BS. Renal substance P-containing neurons and substance P receptors impaired in hypertension. Hypertension. 1998;31(3):815–22. [DOI] [PubMed] [Google Scholar]
- 32.Kopp UC, Smith LA. Bradykinin and protein kinase C activation fail to stimulate renal sensory neurons in hypertensive rats. Hypertension. 1996;27(3 Pt 2):607–12. [DOI] [PubMed] [Google Scholar]
- 33.Kopp UC, Smith LA, DiBona GF. Impaired renorenal reflexes in spontaneously hypertensive rats. Hypertension. 1987;9(1):69–75. [DOI] [PubMed] [Google Scholar]
- 34.Kopp UC, Cicha MZ, Smith LA. Impaired interaction between efferent and afferent renal nerve activity in SHR involves increased activation of alpha2-adrenoceptors. Hypertension. 2011;57(3):640–7. [DOI] [PubMed] [Google Scholar]
- 35.DiBona GF, Jones SY, Kopp UC. Renal mechanoreceptor dysfunction: an intermediate phenotype in spontaneously hypertensive rats. Hypertension. 1999;33(1 Pt 2):472–5. [DOI] [PubMed] [Google Scholar]
- 36.Kopp UC, Cicha MZ. Impaired substance P release from renal sensory nerves in SHR involves a pertussis toxin-sensitive mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R326–33. [DOI] [PubMed] [Google Scholar]
- 37.Kopp UC, Cicha MZ, Yorek MA. Impaired responsiveness of renal sensory nerves in streptozotocin-treated rats and obese Zucker diabetic fatty rats: role of angiotensin. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R858–66. [DOI] [PubMed] [Google Scholar]
- 38.Kopp UC, Cicha MZ, Smith LA. Impaired responsiveness of renal mechanosensory nerves in heart failure: role of endogenous angiotensin. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R116–24. [DOI] [PubMed] [Google Scholar]
- 39.Chien CT, Fu TC, Wu MS, Chen CF. Attenuated response of renal mechanoreceptors to volume expansion in chronically hypoxic rats. Am J Physiol. 1997;273(5):F712–7. [DOI] [PubMed] [Google Scholar]
- 40.Ma MC, Huang HS, Chen CF. Impaired renal sensory responses after unilateral ureteral obstruction in the rat. J Am Soc Nephrol. 2002;13(4):1008–16. [DOI] [PubMed] [Google Scholar]
- 41.Ma MC, Huang HS, Chien CT, Wu MS, Chen CF. Temporal decrease in renal sensory responses in rats after chronic ligation of the bile duct. Am J Physiol Renal Physiol. 2002;283(1):F164–72. [DOI] [PubMed] [Google Scholar]
- 42.Ma MC, Huang HS, Wu MS, Chien CT, Chen CF. Impaired renal sensory responses after renal ischemia in the rat. J Am Soc Nephrol. 2002;13(7):1872–83. [DOI] [PubMed] [Google Scholar]
- 43.Kopp UC, Buckley-Bleiler RL. Impaired renorenal reflexes in two-kidney, one clip hypertensive rats. Hypertension. 1989;14(4):445–52. [DOI] [PubMed] [Google Scholar]
- 44.Katholi RE, Hageman GR, Whitlow PL, Woods WT. Hemodynamic and afferent renal nerve responses to intrarenal adenosine in the dog. Hypertension. 1983;5(2 Pt 2):I149–54. [DOI] [PubMed] [Google Scholar]
- 45.Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens. 1984;2(4):349–59. [PubMed] [Google Scholar]
- 46.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. The New England journal of medicine. 2014;370(15):1393–401. [DOI] [PubMed] [Google Scholar]
- 47.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA, et al. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976–82. [DOI] [PubMed] [Google Scholar]
- 48.Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36(4):219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–7. [DOI] [PubMed] [Google Scholar]
- 50.Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32(2):115–21. [DOI] [PubMed] [Google Scholar]
- 51.Chen YC, Su YC, Lee CC, Huang YS, Hwang SJ. Chronic kidney disease itself is a causal risk factor for stroke beyond traditional cardiovascular risk factors: a nationwide cohort study in Taiwan. PLoS One. 2012;7(4):e36332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koren-Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology. 2006;67(2):224–8. [DOI] [PubMed] [Google Scholar]
- 53.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ninomiya T, Perkovic V, Verdon C, Barzi F, Cass A, Gallagher M, et al. Proteinuria and stroke: a meta-analysis of cohort studies. Am J Kidney Dis. 2009;53(3):417–25. [DOI] [PubMed] [Google Scholar]
- 55.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel G, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke. 2007;38(12):3121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiner DE, Bartolomei K, Scott T, Price LL, Griffith JL, Rosenberg I, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009;53(3):438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seliger SL, Siscovick DS, Stehman-Breen CO, Gillen DL, Fitzpatrick A, Bleyer A, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15(7):1904–11. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe K, Watanabe T, Nakayama M. Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology. 2014;44:184–93. [DOI] [PubMed] [Google Scholar]
- 60.De Deyn PP, Vanholder R, D'Hooge R. Nitric oxide in uremia: effects of several potentially toxic guanidino compounds. Kidney Int Suppl. 2003(84):S25–8. [DOI] [PubMed] [Google Scholar]
- 61.D'Hooge R, Van de Vijver G, Van Bogaert PP, Marescau B, Vanholder R, De Deyn PP. Involvement of voltage- and ligand-gated Ca2+ channels in the neuroexcitatory and synergistic effects of putative uremic neurotoxins. Kidney Int. 2003;63(5):1764–75. [DOI] [PubMed] [Google Scholar]
- 62.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, et al. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006;37(8):2024–9. [DOI] [PubMed] [Google Scholar]
- 63.Kielstein H, Suntharalingam M, Perthel R, Song R, Schneider SM, Martens-Lobenhoffer J, et al. Role of the endogenous nitric oxide inhibitor asymmetric dimethylarginine (ADMA) and brain-derived neurotrophic factor (BDNF) in depression and behavioural changes: clinical and preclinical data in chronic kidney disease. Nephrol Dial Transplant. 2015;30(10):1699–705. [DOI] [PubMed] [Google Scholar]
- 64.Khan U, Hassan A, Vallance P, Markus HS. Asymmetric dimethylarginine in cerebral small vessel disease. Stroke. 2007;38(2):411–3. [DOI] [PubMed] [Google Scholar]
- 65.Six I, Maizel J, Barreto FC, Rangrez AY, Dupont S, Slama M, et al. Effects of phosphate on vascular function under normal conditions and influence of the uraemic state. Cardiovasc Res. 2012;96(1):130–9. [DOI] [PubMed] [Google Scholar]
- 66.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87(7):E10–7. [DOI] [PubMed] [Google Scholar]
- 67.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89(12):1147–54. [DOI] [PubMed] [Google Scholar]
- 68.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104(6):733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23(3):489–94. [DOI] [PubMed] [Google Scholar]
- 70.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98(7):905–12. [DOI] [PubMed] [Google Scholar]
- 71.Crouthamel MH, Lau WL, Leaf EM, Chavkin NW, Wallingford MC, Peterson DF, et al. Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: redundant roles for PiT-1 and PiT-2. Arterioscler Thromb Vasc Biol. 2013;33(11):2625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marebwa BK, Adams RJ, Magwood GS, Kindy M, Wilmskoetter J, Wolf M, et al. Fibroblast growth factor23 is associated with axonal integrity and neural network architecture in the human frontal lobes. PLoS One. 2018;13(9):e0203460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wright CB, Dong C, Stark M, Silverberg S, Rundek T, Elkind MS, et al. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS). Neurology. 2014;82(19):1700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wright CB, Shah NH, Mendez AJ, DeRosa JT, Yoshita M, Elkind MS, et al. Fibroblast Growth Factor 23 Is Associated With Subclinical Cerebrovascular Damage: The Northern Manhattan Study. Stroke. 2016;47(4):923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant. 2009;24(7):2051–8. [DOI] [PubMed] [Google Scholar]
- 76.Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant. 2008;23(6):1892–901. [DOI] [PubMed] [Google Scholar]
- 77.Stinghen AE, Chillon JM, Massy ZA, Boullier A. Differential effects of indoxyl sulfate and inorganic phosphate in a murine cerebral endothelial cell line (bEnd.3). Toxins (Basel). 2014;6(6):1742–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujisaki K, Tsuruya K, Yamato M, Toyonaga J, Noguchi H, Nakano T, et al. Cerebral oxidative stress induces spatial working memory dysfunction in uremic mice: neuroprotective effect of tempol. Nephrol Dial Transplant. 2014;29(3):529–38. [DOI] [PubMed] [Google Scholar]
- 79.Deng G, Vaziri ND, Jabbari B, Ni Z, Yan XX. Increased tyrosine nitration of the brain in chronic renal insufficiency: reversal by antioxidant therapy and angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2001;12(9):1892–9. [DOI] [PubMed] [Google Scholar]
- 80.Shroff R, Speer T, Colin S, Charakida M, Zewinger S, Staels B, et al. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol. 2014;25(11):2658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, et al. HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol. 2014;25(5):1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 2013;38(4):754–68. [DOI] [PubMed] [Google Scholar]
- 83.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, et al. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22(9):1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salama M, Farrag SM, abulasrar S, Amin MM, Ali AA, Sheashaa H, et al. Up-regulation of TLR-4 in the brain after ischemic kidney-induced encephalopathy in the rat. CNS Neurol Disord Drug Targets. 2013;12(5):583–6. [DOI] [PubMed] [Google Scholar]
- 86.Doi K, Ishizu T, Tsukamoto-Sumida M, Hiruma T, Yamashita T, Ogasawara E, et al. The high-mobility group protein B1-Toll-like receptor 4 pathway contributes to the acute lung injury induced by bilateral nephrectomy. Kidney Int. 2014;86(2):316–26. [DOI] [PubMed] [Google Scholar]
- 87.Adachi N, Lei B, Deshpande G, Seyfried FJ, Shimizu I, Nagaro T, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001;27(10):1655–60. [DOI] [PubMed] [Google Scholar]
- 88.Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol. 2007;293(1):F30–40. [DOI] [PubMed] [Google Scholar]
- 89.Karimi Z, Ketabchi F, Alebrahimdehkordi N, Fatemikia H, Owji SM, Moosavi SM. Renal ischemia/reperfusion against nephrectomy for induction of acute lung injury in rats. Ren Fail. 2016;38(9):1503–15. [DOI] [PubMed] [Google Scholar]
- 90.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013;24(2):229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015;87(2):350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujii T, Kurata H, Takaoka M, Muraoka T, Fujisawa Y, Shokoji T, et al. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur J Pharmacol. 2003;481(2–3):241–8. [DOI] [PubMed] [Google Scholar]
- 94.Ogawa T, Mimura Y, Kaminishi M. Renal denervation abolishes the protective effects of ischaemic preconditioning on function and haemodynamics in ischaemia-reperfused rat kidneys. Acta Physiol Scand. 2002;174(3):291–7. [DOI] [PubMed] [Google Scholar]
- 95.Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, et al. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol. 2008;19(7): 1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathis KW, Venegas-Pont M, Flynn ER, Williams JM, Maric-Bilkan C, Dwyer TM, et al. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol. 2013;305(7):R711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195(6):781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55(6):2362–7. [DOI] [PubMed] [Google Scholar]
- 99.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105(31):11008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain, behavior, and immunity. 1989;3(4):291–311. [DOI] [PubMed] [Google Scholar]
- 101.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42(2):153–69. [DOI] [PubMed] [Google Scholar]
- 102.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35(1):80–6. [DOI] [PubMed] [Google Scholar]
- 103.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci. 2010;154(1–2):66–73. [DOI] [PubMed] [Google Scholar]
- 104.Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989;3(4):281–90. [DOI] [PubMed] [Google Scholar]
- 105.Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97(11):1180–5. [DOI] [PubMed] [Google Scholar]
- 106.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–9. [DOI] [PubMed] [Google Scholar]
- 107.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8. [DOI] [PubMed] [Google Scholar]
- 109.Meroni E, Stakenborg N, Gomez-Pinilla PJ, De Hertogh G, Goverse G, Matteoli G, et al. Functional characterization of oxazolone-induced colitis and survival improvement by vagus nerve stimulation. PLoS One. 2018;13(5):e0197487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alphα7nAChR+ splenocytes. J Clin Invest. 2016;126(5):1939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63(6):938–48. [DOI] [PubMed] [Google Scholar]
- 112.Bassi GS, Dias DPM, Franchin M, Talbot J, Reis DG, Menezes GB, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017;64:330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komegae EN, Farmer DGS, Brooks VL, McKinley MJ, McAllen RM, Martelli D. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun. 2018;73:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoeger S, Bergstraesser C, Selhorst J, Fontana J, Birck R, Waldherr R, et al. Modulation of brain dead induced inflammation by vagus nerve stimulation. Am J Transplant. 2010;10(3):477–89. [DOI] [PubMed] [Google Scholar]
- 115.Hoeger S, Fontana J, Jarczyk J, Selhorst J, Waldherr R, Kramer BK, et al. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrol Dial Transplant. 2014;29(3):544–9. [DOI] [PubMed] [Google Scholar]
- 116.Gigliotti JC, Huang L, Bajwa A, Ye H, Mace EH, Hossack JA, et al. Ultrasound Modulates the Splenic Neuroimmune Axis in Attenuating AKI. J Am Soc Nephrol. 2015;26(10):2470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol. 2013;24(9):1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wasilczuk KM, Bayer KC, Somann JP, Albors GO, Sturgis J, Lyle LT, et al. Modulating the Inflammatory Reflex in Rats Using Low-Intensity Focused Ultrasound Stimulation of the Vagus Nerve. Ultrasound Med Biol. 2018. [DOI] [PubMed] [Google Scholar]
- 119.Zachs DP, Offutt SJ, Graham RS, Kim Y, Mueller J, Auger JL, et al. Noninvasive ultrasound stimulation of the spleen to treat inflammatory arthritis. Nat Commun. 2019;10(1):951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cotero V, Fan Y, Tsaava T, Kressel AM, Hancu I, Fitzgerald P, et al. Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nat Commun. 2019;10(1):952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci. 2017;20(5):700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R187–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int. 2016;89(3):555–64. [DOI] [PubMed] [Google Scholar]
- 124.Gigliotti JC, Okusa MD. The spleen: the forgotten organ in acute kidney injury of critical illness. Nephron Clin Pract. 2014;127(1–4):153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mandal AK, Haygood CC, Bell RD, Sethney T, James TM, Nordquist JA, et al. Effects of acute and chronic splenectomy on experimental acute renal tubular lesions. J Lab Clin Med. 1978;92(5):698–711. [PubMed] [Google Scholar]
- 126.Mandal AK. The spleen and acute renal failure: mechanisms of renal protection by splenectomy. Involvement of prostaglandins. Prostaglandins Leukot Med. 1982;9(1):85–107. [DOI] [PubMed] [Google Scholar]
- 127.Mandal AK, Taylor CA, Bell RD, Hillman NM, Jarnot MD, Cunningham JD, et al. Erythrocyte deformation in ischemic acute tubular necrosis and amelioration by splenectomy in the dog. Lab Invest. 1991;65(5):566–76. [PubMed] [Google Scholar]
- 128.Bell RD, Mandal AK. Increased hematocrit mitigates ischemic renal damage in the splenectomized dog. Am J Med Sci. 1989;297(3):169–73. [DOI] [PubMed] [Google Scholar]
- 129.Burne-Taney MJ, Liu M, Baldwin WM, Racusen L, Rabb H. Decreased capacity of immune cells to cause tissue injury mediates kidney ischemic preconditioning. J Immunol. 2006;176(11):7015–20. [DOI] [PubMed] [Google Scholar]
- 130.Burne-Taney MJ, Liu M, Ascon D, Molls RR, Racusen L, Rabb H. Transfer of lymphocytes from mice with renal ischemia can induce albuminuria in naive mice: a possible mechanism linking early injury and progressive renal disease? Am J Physiol Renal Physiol. 2006;291(5):F981–6. [DOI] [PubMed] [Google Scholar]
- 131.Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity. 2014;41(5):737–52. [DOI] [PubMed] [Google Scholar]
- 132.Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, Fardella S, et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat Commun. 2016;7:13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, et al. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II-Induced Hypertension. Circ Res. 2015;117(6):547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vasamsetti SB, Florentin J, Coppin E, Stiekema LCA, Zheng KH, Nisar MU, et al. Sympathetic Neuronal Activation Triggers Myeloid Progenitor Proliferation and Differentiation. Immunity. 2018;49(1):93–106 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Inoue T, Abe C, Kohro T, Tanaka S, Huang L, Yao J, et al. Non-canonical cholinergic anti-inflammatory pathway-mediated activation of peritoneal macrophages induces Hes1 and blocks ischemia/reperfusion injury in the kidney. Kidney Int. 2019;95(3):563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji JD, et al. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008;29(5):691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shang Y, Coppo M, He T, Ning F, Yu L, Kang L, et al. The transcriptional repressor Hes1 attenuates inflammation by regulating transcription elongation. Nat Immunol. 2016;17(8):930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bonaz B, Sinniger V, Hoffmann D, Clarencon D, Mathieu N, Dantzer C, et al. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28(6):948–53. [DOI] [PubMed] [Google Scholar]
- 139.Meregnani J, Clarencon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160(1–2):82–9. [DOI] [PubMed] [Google Scholar]
- 140.Sun P, Zhou K, Wang S, Li P, Chen S, Lin G, et al. Involvement of MAPK/NF-kappaB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One. 2013;8(8):e69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, Zitnik R, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One. 2014;9(8):e104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113(29):8284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74(1):62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chatterjee PK, Yeboah MM, Solanki MH, Kumar G, Xue X, Pavlov VA, et al. Activation of the cholinergic anti-inflammatory pathway by GTS-21 attenuates cisplatin-induced acute kidney injury in mice. PLoS One. 2017;12(11):e0188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y, et al. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7(5):e35361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanaka S, Okusa MD. Optogenetics in Understanding Mechanisms of Acute Kidney Injury. Nephron. 2018;140(2):152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]