Abstract
Rationale and Objective
Poor inhibitory control is a well-established risk factor for Alcohol Use Disorder (AUD). Similarly, greater sensitivity to the stimulant effects and less sensitivity to the sedative effects of alcohol are also strongly linked to risk for AUD. Traditionally, these two risk factors have been considered to be orthogonal, and thus they have been studied independently. However, recent evidence from animal and human studies suggests they may be related. The current study examined the relationship between inhibitory control and subjective responses to alcohol in a sample of healthy young adults.
Methods
Moderate social drinkers (N=69) first completed the stop signal task to assess inhibitory control. They then participated in four sessions in which they received an oral dose of ethanol (0.8 g/kg) or placebo in alternating order, providing self-report measures of stimulation and sedation on the Biphasic Alcohol Effects Scale (BAES) at regular intervals.
Results
Linear mixed effects models showed that poor inhibitory control was associated with greater stimulation and less sedation following alcohol compared to placebo.
Conclusion
These findings provide the first direct evidence that individuals with poor inhibitory control experience greater sensitivity to the rewarding, stimulant effects of alcohol, and less sensitivity to the negative, sedative effects. These findings suggest that inhibition and subjective response to alcohol are not independent risk factors, and that together they constitute a heightened profile of risk for AUD.
Keywords: alcohol, stimulation, sedation, inhibitory control, reward
Introduction
To develop successful prevention and treatment efforts for Alcohol Use Disorder (AUD) among young adults, it is important to understand the risk factors that predispose individuals to problem drinking. Two well-established risk factors in this population are poor inhibitory control, i.e., difficulty stopping oneself from engaging in inappropriate or maladaptive behavior, and certain subjective responses, i.e., heightened stimulant responses and reduced sedative responses to alcohol. Traditionally, these risk factors have been considered orthogonal to each other. However, exciting new evidence suggests they may be related. The current study examined the relationship between inhibitory control and subjective responses to alcohol in a sample of healthy young adult drinkers.
Poor inhibitory control is a significant risk factor for AUD. Inhibitory control is typically measured using stop signal or go/no-go tasks which require participants to respond to ‘go’ targets but to inhibit responses on occasional signaled trials. Numerous cross-sectional studies show that heavier drinkers exhibit poorer inhibition than lighter drinkers (for reviews, see Perry and Carroll 2008; Weafer et al. 2015). More importantly for understanding risk, longitudinal studies show that poor inhibitory control prospectively predicts the onset and escalation of alcohol consumption and alcohol-related problems (Fernie et al. 2013; Rubio et al. 2008). Taken together, these studies suggest that poor inhibitory control is linked to problematic alcohol use, and may play a causal role in the development of AUD.
Subjective response to alcohol has also been linked to risk for AUD, although the direction of this link is subject to debate. Schuckit and colleagues have shown that lower levels of subjective response to alcohol are associated with greater risk for alcohol-related problems, perhaps due to a need to consume more alcohol to achieve the desired effects (Schuckit 1994; Tolentino et al. 2011). Importantly, they have conducted longitudinal research to show that a low level of response to alcohol predicts greater alcohol consumption and more negative alcohol-related outcomes twenty years later (Schuckit et al. 2011). On the other hand, there is also evidence linking greater sensitivity to the positive, stimulant effects of alcohol and lower sensitivity to the negative, sedative effects to increased risk for AUD (King et al. 2011; Quinn and Fromme 2011). Notably, longitudinal evidence has shown that this profile of subjective response predicts greater number and severity of AUD symptoms up to six years later (King et al. 2011; King et al. 2016; King et al. 2014). Researchers have attempted to resolve these apparent discrepancies, suggesting that initial stimulant effects and later sedative effects may both be predictive of future AUD.
Poor inhibitory control and subjective response to alcohol have traditionally been thought of as independent risk factors for alcohol abuse, and as such they are typically studied separately. However, evidence from animal and human studies suggests that they may be related. In laboratory animals, drug-naïve mice and rats bred to be high alcohol preferring exhibit poorer response inhibition compared to non-alcohol preferring lines (Beckwith and Czachowski 2016; Bowers and Wehner 2001; Logue et al. 1998; Wilhelm et al. 2007), suggesting a relationship between sensitivity to alcohol reward and poor inhibitory control. In humans, poor inhibitory control is associated with greater subjective reward and stimulation following amphetamine (Weafer and de Wit 2013; Weafer et al. 2017), providing preliminary data for an association between poor inhibitory control and drug reward sensitivity in humans. Finally, self-report measures of impulsivity have been associated with subjective responses to alcohol. Hendershot et al (2015) reported that individuals high on a self-report measure of ADHD symptoms experienced greater stimulation, but not sedation, following intravenous alcohol. Other findings have shown that high impulsivity as assessed by the Barratt Impulsiveness Scale is associated with greater stimulation (Berey et al. 2019; Leeman et al. 2014) and reduced sedation (Berey et al. 2017; Leeman et al. 2014) following alcohol. However, the association between behavioral measures of inhibition and subjective response to alcohol has not yet been tested.
The current study investigated associations between poor inhibitory control and subjective response to alcohol in healthy young adult moderate drinkers. Inhibitory control was measured using the stop signal task (Logan et al. 1997), a behavioral measure of the time required to inhibit a response (stop signal reaction time; SSRT). Subjective response to alcohol was measured using the Biphasic Alcohol Effects Scale (Martin et al. 1993), a measure of subjective stimulant and sedative effects, following acute oral doses of alcohol and placebo. We hypothesized that individuals with poorer inhibitory control (longer SSRT) would report greater stimulation and less sedation following alcohol relative to placebo.
Methods
Design
Healthy volunteers completed a behavioral measure of inhibitory control in a drug-free state, followed by a four-session alcohol challenge to assess their subjective responses to oral ethanol (0.8 g/kg) or placebo. Participants received both ethanol and placebo twice, in alternating order, with drug administered first (ethanol or placebo) randomly assigned, under double blind conditions. We tested the degree to which individual differences in inhibitory control were associated with ratings of stimulation and sedation after alcohol. The Institutional Review Board of the University of Chicago approved the study, and it was carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent for participation.
Participants
Male and female moderate social drinkers (n=95) were recruited through online and printed advertisements. Volunteers were eligible to participate if they consumed an average of 7 to 30 standard drinks per week (e.g., 12 oz beer or 1.5 oz liquor), with at least one heavy drinking episode (i.e., 4 or more drinks in one sitting for women, 5 or more for men) in the past month. These drinking criteria ensured that participants could tolerate the alcohol dose, and that the sample consisted of moderate drinkers who occasionally engaged in heavy drinking, thus putting them at risk for developing AUD. Additional inclusion criteria were: age 21–29, BMI 19–26, at least a high school education, and fluency in English. Exclusion criteria were: past year DSM-V diagnosis, lifetime history of substance dependence or ADHD, serious medical conditions, night shift work, smoking >5 cigarettes/day, use of medications other than birth control, or pregnancy, lactation, or plans to become pregnant in the next 3 months.
Measures
Stop signal task (Logan et al. 1997). This task provided a behavioral measure of inhibitory control. Participants were instructed to respond as quickly as possible to go signals (an X or O presented on the computer screen), and to inhibit responses on trials in which a stop signal (auditory tone) occurred. The duration of the stop signal delay was adjusted to target a 50% successful inhibition rate. Specifically, the stop signal delay increased by 50ms following successful inhibition trials and decreased by 50ms following failed inhibition trials. Stop signal reaction time (SSRT) was calculated by subtracting the final mean stop signal delay from the mean go RT. The task consisted of 144 go and 48 stop trials. Task data were considered valid if the following criteria were met: inhibition rate between 40–60%, go accuracy >80%, and mean go RT < 800ms.
Biphasic Alcohol Effects Scale (BAES; Martin et al. 1993). The BAES is a measure of subjective stimulation (e.g., talkative, elated) and sedation (e.g., sedated, sluggish) responses to alcohol. Responses for the 7 individual stimulation items and 7 individual sedation items are reported on a Likert scale (0 – 10), and stimulation and sedation item scores are summed separately to provide a total score for each (score range = 0 – 70).
Procedure
Participants abstained from drugs, including alcohol, for 24 hours prior to each session, as verified by self-report, breath alcohol, and urine screens. Participants first attended an orientation session in which they provided informed consent and were familiarized with laboratory procedures and study protocol. They then completed the stop signal task to assess drug-free levels of inhibitory control.
Participants completed four beverage administration sessions, in which they consumed beverages containing either ethanol or placebo in alternating order. Ethanol and placebo were administered twice each to minimize the influence of day-to-day variability (Rhodes and Hawk 2016). The beverage administration sessions took place from 3:00pm to 8:00pm, and were separated by 2–7 days. Participants were tested individually in comfortably furnished rooms, designed to resemble a living room environment. Participants were instructed to not eat for 4 hours prior to each session in order to reduce variability in alcohol absorption rates and trajectory of breath alcohol concentration (BrAC), both between participants and across sessions. To minimize drug expectancies, they were told they could receive one of the following: stimulant, sedative, alcohol, or placebo. Participants first completed a baseline (pre-drug) BAES measure. At 3:30, participants consumed beverages containing 0.8 g/kg ethanol (divided into four servings of 0.2 g/kg each) or a matching placebo, to be consumed within 15 minutes. They then completed the BAES and provided breath samples to assess BrAC (Alco-sensor IV; Intoximeters, St. Louis, MO) every 30 min following beverage administration for 2.5 hours. Between assessments, participants were allowed to read or watch movies, but they were not allowed to work, access the internet, use their phones, or sleep. Sessions ended at 8:00pm, after confirmation that BrAC had fallen below 40mg/100ml. Upon completion of all sessions, participants were debriefed and compensated for their time.
Beverage administration
Ethanol and placebo were administered orally. The ethanol dose was 0.8 g/kg for men and 0.7 g/kg for women to achieve equivalent BrACs across sex (Fillmore 2001; Mulvihill et al. 1997). This dose was chosen to produce peak BrACs of 80mg/100ml. This BrAC models a binge level of intoxication and has been shown to produce reliable increases in stimulation and sedation (Weafer et al. 2016). Each dose was split into four equal servings. Ethanol beverages were served in a 10% solution by volume with the subjects’ preferred fruit juice flavor. The placebo beverage consisted of the fruit juice plus 3ml ethanol added as a taste mask. All beverages were sprayed with an alcoholic mist to provide a strong alcoholic scent. Beverages were served in opaque cups, and participants were given a total of 15 minutes to consume the four servings.
Data analyses
Associations between SSRT and subjective response to alcohol were analyzed using linear mixed effects models for repeated measures (Hedeker and Gibbons 2006). These models tested the degree to which SSRT interacted with beverage (ethanol vs. placebo) and time to predict measures of stimulation and sedation. The models were built by treating observations as nested within subjects and allowing for random subject intercepts, beverage, and time effects to allow for individual differences in alcohol response and time trends, and to account for the correlation between repeated measurements. Beverage order (ethanol or placebo administered first) and sex were included as model covariates. Data from all four sessions were included in the model, and effects were estimated across sessions. The effects of interest were the three-way interactions among SSRT, beverage (ethanol vs. placebo), and time. For visualization purposes, we also calculated area under the curve (AUC) scores for Stimulation and Sedation on each session. AUC was calculated by multiplying the average of each pair of consecutive observations by the corresponding time interval (.5 hours) and then summing all values starting with the first time point and ending with the last, as described in Matthews et al. (1990).
Results
Sample characteristics
Of the 95 participants who completed this study, 70 had valid stop signal data. Of those without valid data, 12 had inhibition rates either <40% or >60%, 9 had go RTs >800ms, one had an SSRT that was >3 sd’s above the mean, and three were missing stop signal data entirely. One additional participant was determined to be an outlier in terms of baseline subjective response data, and thus was excluded from analyses. Table 1 presents demographic and substance use data for the final sample of n = 69. Men and women did not differ on any demographic or substance use measure (ps > 0.07).
Table 1.
Sample characteristics (n=69)
| Mean (SD) | |
|---|---|
| Sex (M:F) | 34:35 |
| Age | 24.3 (2.6) |
| Education (years) | 15.6 (1.6) |
| Race | |
| Caucasian | 39 |
| African-American | 13 |
| More than one race | 12 |
| Asian | 3 |
| American Indian/Alaskan | 1 |
| Not reported | 1 |
| Alcohol use (past month) | |
| Number of drinking days | 15.2 (4.8) |
| Number of binge days* | 4.2 (2.6) |
| Drinks per week | 12.1 (4.5) |
Note.
binge day defined as 4/5+ in one drinking episode for men/women
SSRT
Mean SSRT during stop signal performance was 252.0 ms (SD = 25.1; range = 205.5 – 311.5). Participants successfully inhibited on 50.4% of stop signal trials. Mean accuracy on go trials was 98.3% (SD = 0.2), and mean go RT was 470.1 ms (SD = 95.8).
Breath Alcohol Concentration (BrAC)
Mean peak BrAC for the sample was 79 (SD = 12.0) mg/100 ml at 90 minutes. Men and women did not differ in peak BrAC (p = 0.63), and peak BrAC was not related to individual differences in SSRT (r = 0.06, p = 0.62).
Associations between SSRT and stimulation following ethanol
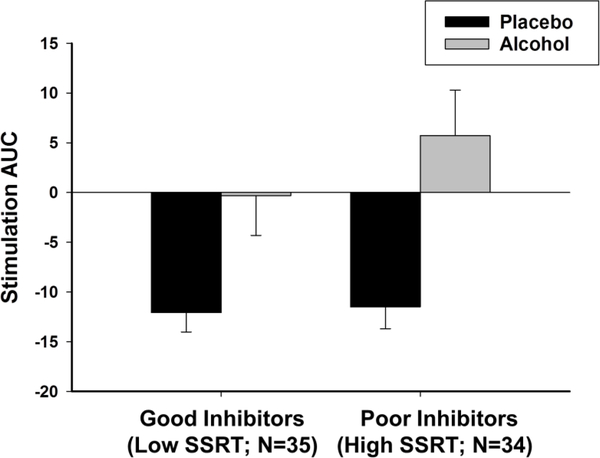
Table 2 (top panel) presents results from the linear mixed effects model testing the degree to which SSRT interacted with beverage and time to predict ratings of stimulation. Consistent with previous reports and as shown in Table 3, ethanol increased stimulation relative to placebo, as indicated by the positive interaction between beverage and linear effect of time. Moreover, the magnitude of ethanol effect differed according to individual differences in SSRT, as indicated by a significant beverage x time x SSRT interaction. The positive parameter estimate for this interaction indicates that individuals with longer SSRT (i.e., poor inhibitory control) reported greater ethanol-induced increase in stimulation relative to individuals with shorter SSRT (i.e., good inhibitory control). For visualization purposes, we divided the sample according to a median split of SSRT and plotted mean area under the curve (AUC) stimulation scores following ethanol and placebo for good inhibitors (low SSRT) and poor inhibitors (high SSRT). Figure 1 shows that stimulation AUC scores following placebo were similar for both groups. The negative AUC values indicate that stimulation decreased from baseline throughout the placebo sessions. By contrast, stimulation AUC scores following ethanol were greater in individuals with poor inhibitory control compared to those with good inhibitory control.
Table 2.
Linear mixed effects models testing associations between SSRT and subjective response to alcohol
| Estimate | SE | t | p | |
|---|---|---|---|---|
| BAES Stimulation | ||||
| Order | 2.92 | 2.33 | 1.23 | 0.215 |
| Sex | 3.87 | 2.30 | 1.69 | 0.096 |
| SSRT | 0.03 | 0.06 | 0.53 | 0.600 |
| Beverage | 1.69 | 1.06 | 1.59 | 0.113 |
| Time (Linear) | −3.51 | 0.53 | 6.66 | 0.000 |
| Time2 (Quadratic) | 0.42 | 0.10 | 4.19 | 0.000 |
| SSRT×Beverage | −0.06 | 0.04 | 1.41 | 0.160 |
| SSRT×Time | < −0.01 | 0.02 | 0.43 | 0.665 |
| SSRT×Time2 | < 0.01 | < 0.01 | 0.34 | 0.736 |
| Beverage×Time | 5.38 | 0.75 | 7.29 | < 0.001 |
| Beverage× Time2 | −1.15 | 0.14 | 8.10 | < 0.001 |
| SSRT×Beverage×Time | 0.06 | 0.03 | 2.19 | 0.029 |
| SSRT×Beverage×Time2 | −0.01 | < 0.01 | 1.77 | 0.077 |
| BAES Sedation | ||||
| Order | 1.47 | 1.31 | 1.11 | 0.270 |
| Sex | 0.97 | 1.30 | 0.75 | 0.459 |
| SSRT | < −0.01 | 0.03 | 0.23 | 0.820 |
| Beverage | −0.11 | 0.90 | 0.12 | 0.907 |
| Time (Linear) | 1.39 | 0.46 | 3.01 | 0.003 |
| Time2 (Quadratic) | −0.26 | 0.09 | 3.06 | 0.002 |
| SSRT×Beverage | 0.05 | 0.04 | 1.30 | 0.194 |
| SSRT×Time | 0.02 | 0.02 | 1.00 | 0.318 |
| SSRT×Time2 | < −0.01 | < 0.01 | 0.50 | 0.617 |
| Beverage×Time | 1.89 | 0.63 | 3.00 | 0.003 |
| Beverage× Time2 | −0.18 | 0.12 | 1.50 | 0.133 |
| SSRT×Beverage×Time | −0.06 | 0.03 | 2.55 | 0.011 |
| SSRT×Beverage×Time2 | < −0.01 | < 0.01 | 1.81 | 0.071 |
Note. Significant effects are indicated in a bold font. SSRT = stop signal reaction time
Table 3.
Mean (SD) BAES Stimulation and Sedation scores across sessions
| Stimulation | Sedation | |||
|---|---|---|---|---|
| Timepoint | Placebo | Alcohol | Placebo | Alcohol |
| Baseline | 17.9 (12.0) | 17.3 (12.9) | 6.0 (6.0) | 5.4 (5.5) |
| 30 min | 15.8 (12.2) | 24.6 (14.7) | 6.3 (6.7) | 9.6 (7.4) |
| 60 min | 12.6 (10.8) | 20.3 (14.6) | 8.4 (9.0) | 10.7 (8.3) |
| 90 min | 11.0 (9.9) | 16.8 (13.8) | 7.6 (8.3) | 11.3 (8.7) |
| 120 min | 10.9 (9.9) | 14.6 (13.0) | 7.3 (8.5) | 11.6 (8.9) |
| 150 min | 10.9 (10.1) | 11.9 (11.1) | 6.5 (8.1) | 11.3 (9.7) |
Note. BAES = Biphasic Alcohol Effects Scale
Fig 1.
Mean area under the curve (AUC) stimulation scores across the two placebo and the two alcohol sessions for good inhibitors (low SSRT) and poor inhibitors (high SSRT). Capped vertical lines represent standard error of the mean (SEM)
Associations between SSRT and sedation following ethanol
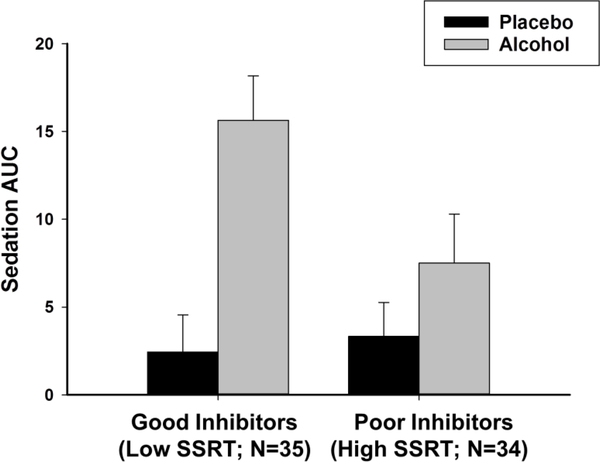
Table 2 (bottom panel) presents results from the linear mixed effects model testing the degree to which SSRT interacted with beverage and time to predict ratings of sedation. As expected, and as shown in Table 3, ethanol increased sedation relative to placebo throughout the session, as indicated by the positive interaction between beverage and time. Again, the magnitude of ethanol effect differed according to individual differences in SSRT. Here, the negative parameter estimate for the beverage x time x SSRT interaction indicates that individuals with high SSRT (poor inhibitory control) reported less sedation in response to alcohol than did individuals with low SSRT (good inhibitory control). We plotted AUC sedation scores following ethanol and placebo according to a median split of SSRT (Figure 2). The figure shows that the groups were similar in sedation levels following placebo. By contrast, individuals with poor inhibitory control reported markedly less sedation following ethanol than did those with good inhibitory control.
Fig 2.
Mean area under the curve (AUC) sedation scores across the two placebo and the two alcohol sessions for good inhibitors (low SSRT) and poor inhibitors (high SSRT). Capped vertical lines represent standard error of the mean (SEM)
Associations between SSRT and demographic and drinking habit measures
No significant associations were observed between SSRT and any demographic or drinking habit measures (ps > .16).
Discussion
This study investigated associations between poor inhibitory control and subjective response to alcohol. Consistent with past reports, alcohol increased both stimulation and sedation within the sample. Moreover, as hypothesized, the magnitude of stimulant and sedative effects differed according to individual differences in inhibitory control. Individuals with poor inhibition reported greater stimulation and less sedation overall compared to those with good inhibition. Taken together, these findings provide the first evidence of a link between inhibition and subjective response to alcohol in humans.
The observed associations between poor inhibition and both greater stimulation and less sedation have important implications for understanding risk for AUD in young adults. The stimulant effects of alcohol are considered to represent the positive rewarding effects of the drug (Martin et al. 1993). Individuals who experience these effects may be more motivated to continue drinking, in order to prolong these positive feelings. Indeed, among young adults, heavy drinkers report greater alcohol-induced stimulation (King et al. 2002; Marczinski et al. 2007), and greater stimulation predicts the development of AUD later in life (King et al. 2011; King et al. 2016; King et al. 2014). By contrast, the sedative effects of alcohol are thought to represent negative effects, and individuals who experience these effects are less likely to continue a drinking episode. Accordingly, among young adults, heavy drinkers tend to report less alcohol-induced sedation than light drinkers, and less sedation is associated with more AUD symptoms later in life (King et al. 2011; King et al. 2014). Thus, our findings that individuals with poor inhibitory control report both greater stimulant effects and less sedative effects suggest an important set of risk factors for these individuals. That is, in addition to having difficulty controlling behavior (including alcohol consumption), these individuals also experience stronger rewarding effects from alcohol and weaker negative effects. Together, this could produce a ‘triple whammy’ that contributes to heightened risk for problematic alcohol consumption and alcohol-related problems in these individuals.
Our findings are consistent with previous reports from both animal and human studies linking poor inhibition and sensitivity to the rewarding effects of another class of abused drugs: stimulants. In animals, rats with poor response inhibition self-administer cocaine at higher rates than do animals with good response inhibition (Belin et al. 2008; Dalley et al. 2007). In humans, healthy young adults with poor inhibitory control report greater euphoria and stimulation following amphetamine relative to placebo (Weafer and de Wit 2013; Weafer et al. 2017). Additionally, individuals who score high on measures of impulsive personality (which include items related to inhibitory control) report greater subjective reward following both alcohol and amphetamine (Kirkpatrick et al. 2013; Leeman et al. 2014). Taken together, these findings provide compelling evidence linking poor inhibitory control and both alcohol and stimulant drug reward. Given that alcohol and stimulants are often co-abused (Furr et al. 2000; Heil et al. 2001), such an association could provide important insight into risk for poly-drug abuse in young adults with poor inhibitory control.
The behavioral link observed here between inhibitory control and subjective response to alcohol lays the groundwork for future studies to investigate common neurobiological mechanisms underlying this association. One candidate mechanism is the D2 receptor system, which is linked to both poor inhibitory control and drug reward (Jentsch and Pennington 2014). Specifically, in animals, low D2 receptor availability is associated with both poor inhibitory control and greater cocaine self-administration (Dalley et al. 2007). In humans, low D2 receptor availability is associated with poorer inhibition, and greater subjective response to amphetamine (Ghahremani et al. 2012; Robertson et al. 2015; Volkow et al. 1999; Volkow et al. 2002). Additionally, we have shown that less right frontal brain activity during inhibition is associated with greater subjective response to amphetamine, potentially due to less ‘top-down’ frontal regulation of striatal reward responses (Weafer et al. 2017). We have also shown that striatal activity during alcohol intoxication is directly linked to the drug’s stimulating effects (Weafer et al. 2018). Future studies are needed to test the degree to which reduced frontal activation during inhibition is associated with alcohol-induced stimulation and sedation, as well as the degree to which such associations might be mediated by D2 receptor number and function.
These findings raise an interesting question regarding potential associations between acute alcohol effects on inhibitory control and alcohol-induced stimulation and sedation. It is well-known that acute alcohol consumption impairs inhibitory control (de Wit 2009; Fillmore and Weafer 2013). Just as the current findings show that baseline levels of inhibitory control predict stimulation and sedation, alcohol-induced disinhibition may also be related to acute subjective responses to the drug. Indeed, there is preliminary evidence to suggest that individuals who show greater impairment of inhibitory control on a go/no-go task following a moderate dose of alcohol report greater stimulant (but not sedative) effects of alcohol (Quinn and Fromme 2016). It will be important for future studies to further test these potential associations with additional measures of inhibitory control, such as the stop signal task used in the current study, as well as multiple doses of alcohol.
This study had several limitations. First, we administered a single dose of alcohol. It will be important for future studies to test the degree to which poor inhibitory control predicts stimulation and sedation at lower and higher doses of alcohol. Second, participants consumed the alcohol dose in a relatively short time period, calling into question the ecological validity of the drinking procedures. Similarly, participants were alone in the testing rooms throughout the session, with minimal social interaction. While this controlled environment is beneficial for minimizing potentially confounding variables, it further limits the ecological validity of the drinking procedures. It will be important for future studies to replicate these findings in more ‘real-world’ drinking settings. Additionally, a relatively large number of subjects were excluded from analyses due to invalid stop signal data. Although this percentage of data loss is comparable with previous studies using this task (e.g., Weafer and de Wit 2013; Weafer et al. 2017), this should be considered a study limitation. Further, the sample size was not large enough to test the effects of potential moderating variables, including sex and typical alcohol consumption, on the relationship between inhibition and response to alcohol. Future studies with larger samples will be needed to investigate these associations. Finally, we recruited a sample of moderate drinkers who occasionally engage in heavy drinking. It is important to note that the findings may not generalize to individuals with heavier or lighter drinking patterns.
In sum, this is the first study to show that individuals with poor inhibitory control experience greater sensitivity to the rewarding, stimulant effects of alcohol, and less sensitivity to the negative, sedative effects. These findings suggest that inhibition and subjective response to alcohol are not independent risk factors. Instead, they are related at a behavioral level, and together form a heightened profile of risk for AUD, particularly in young adults. It will be important for future studies to determine the neurobiological mechanisms underlying this relationship, in order to provide both behavioral and neural targets for prevention and treatment efforts aimed at reducing AUD in this vulnerable population.
Acknowledgement of funding
This research was supported by National Institute on Drug Abuse Grants R21 DA037642 (HdW), R01 DA002812 (HdW, KLP), and National Institute on Alcohol Abuse and Alcoholism Grant K01 AA024519 (JW).
Footnotes
Conflict of Interest Statement:
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Beckwith SW, Czachowski CL (2016) Alcohol-preferring P rats exhibit elevated motor impulsivity concomitant with operant responding and self-administration of alcohol. Alcohol Clin Exp Res 40: 1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320: 1352–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berey BL, Leeman RF, Chavarria J, King AC (2019) Relationships between generalized impulsivity and subjective stimulant and sedative responses following alcohol administration. Psychology of Addictive Behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berey BL, Leeman RF, Pittman B, O’Malley SS (2017) Relationships of impulsivity and subjective response to alcohol use and related problems. Journal of Studies on Alcohol and Drugs 78: 835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, Wehner JM (2001) Ethanol consumption and behavioral impulsivity are increased in protein kinase Cgamma null mutant mice. J Neurosci 21: RC180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14: 22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, Field M (2013) Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction 108: 1916–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT (2001) Cognitive preoccupation with alcohol and binge drinking in college students: alcohol-induced priming of the motivation to drink. Psychol Addict Behav 15: 325–32 [PubMed] [Google Scholar]
- Fillmore MT, Weafer J (2013) Behavioral inhibition and addiction. In The Wiley-Blackwell Handbook of Addiction Psychopharmacology, Mackillop J and de Wit H (Eds) pp 135–164 [Google Scholar]
- Furr CDM, Delva J, Anthony JC (2000) The suspected association between methamphetamine (‘ice’) smoking and frequent episodes of alcohol intoxication: data from the 1993 National Household Survey on Drug Abuse. Drug and Alcohol Dependence 59: 89–93 [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED (2012) Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci 32: 7316–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD (2006) Longitudinal Data Analysis. Wiley: Hoboken, NJ, USA [Google Scholar]
- Heil SH, Badger GJ, Higgins ST (2001) Alcohol dependence among cocaine-dependent outpatients: Demographics, drug use, treatment outcome and other characteristics. Journal of Studies on Alcohol 62: 14–22 [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Strang NM. Markovich MSD, Claus ED, Ramchandani VA (2015) Application of an alcohol clamp paradigm to examine inhibitory control, subjective responses and acute tolerance in late adolescence. Experimental and Clinical Psychopharmacology 23: 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Pennington ZT (2014) Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology 76: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao DC (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68: 389–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao DC (2016) A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry 79: 489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26: 827–35 [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao DC (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry 75: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Johanson CE, de Wit H (2013) Personality and the acute subjective effects of d-amphetamine in humans. Journal of Psychopharmacology 27: 256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Ralevski E, Limoncelli D, Pittman B, O’Malley SS, Petrakis IL (2014) Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology 231: 2867–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R (1997) Impulsivity and inhibitory control. Psychological Science 8: 60–64 [Google Scholar]
- Logue SF, Swartz RJ, Wehner JM (1998) Genetic correlation between performance on an appetitive-signaled nosepoke task and voluntary ethanol consumption. Alcohol Clin Exp Res 22: 1912–20 [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT (2007) Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav 21: 346–54 [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993) Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17: 140–6 [DOI] [PubMed] [Google Scholar]
- Matthews JNS, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. Br Med J 300: 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M (1997) Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol 58: 600–5 [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME (2008) The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 200: 1–26 [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K (2011) Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res 35: 1759–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K (2016) Individual differences in subjective alcohol responses and alcohol-related disinhibition. Exp Clin Psychopharmacology 24: 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JD, Hawk LW (2016) Smoke and mirrors: The overnight abstinence paradigm as an index of disrupted cognitive function. Psychopharmacology 233: 1395–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, Bilder R, Cannon T, Borg J, London ED (2015) Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci 35: 5990–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, Jimenez-Arriero MA, Ponce G, Palomo T (2008) The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res 32: 1681–7 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1994) Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151: 184–9 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Allen RC, Fukukura T, Knight EE, Cesario EM, Kreikebaum SA (2011) A prospective evaluation of how a low level of response to alcohol predicts later heavy drinking and alcohol problems. American Journal of Drug and Alcohol Abuse 37: 479–486 [DOI] [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, Smith TL, Schuckit MA (2011) Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res 35: 1034–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N (1999) Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D-2 receptor levels. Am J Psychiatry 156: 1440–1443 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos P, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N (2002) Brain DA D2 receptors predict reinforcing effects of stimulants in humans: Replication study. Synapse 46: 79–82 [DOI] [PubMed] [Google Scholar]
- Weafer J, de Wit H (2013) Inattention, impulsive action, and subjective response to d-amphetamine. Drug Alcohol Depend 133: 127–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Gallo DA, de Wit H (2016) Effect of alcohol on encoding and consolidation of memory for alcohol-related images. Alcohol Clin Exp Res 40: 1540–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Gorka SM, Hedeker D, Dzemidzic M, Kareken DA, Phan KL, de Wit H (2017) Associations between behavioral and neural correlates of inhibitory control and amphetamine reward sensitivity. Neuropsychopharmacology 42: 1905–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Mitchell SH, de Wit H (2015) Recent translational findings on impulsivity in relation to drug abuse. Curr Addict Rep 1: 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Ross TJ, O’Connor S, Stein EA, de Wit H, Childs E (2018) Striatal activity correlates with stimulant-like effects of alcohol in healthy volunteers. Neuropsychopharmacology 43: 2532–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH (2007) Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res 31: 1839–45 [DOI] [PubMed] [Google Scholar]