Abstract
Oxidative stress is a major contributor to noise-induced hearing loss, the most common cause of hearing loss among military personnel and young adults. HK-2 is a potent, orally-active, multifunctional, redox-modulating drug that has been shown to protect against a wide range of neurological disorders with no observed side effects. HK-2 protected cochlear HEI-OC1 cells against various forms of experimentally-induced oxidative stressors similar to those observed during and after intense noise exposure. The mechanisms by which HK-2 protects cells is twofold, first by its ability to reduce oxidative stress generated by free radicals, and second, by its ability to complex biologically active transition metals such as Fe+2, thus reducing their availability to participate in the Fenton reaction where highly toxic hydroxyl radicals are generated. For the rat in vivo studies, HK-2 provided significant protection against noise-induced hearing loss and hair cell loss. Noise-induced hearing loss was induced by an 8–16 kHz octave band noises presented for 8 h/d for 21 days at an intensity of 95 dB SPL. In the Prevention study, HK-2 was administered orally beginning 5 days before the start of the noise and ending 10 days after the noise. Treatment with HK-2 dose-dependently reduced the amount of noise-induced hearing impairment, reflected in the cochlear compound action potential, and noise-induced hair cell loss. In a subsequent Rescue experiment in which HK-2 was administered for 10 days starting after the noise was turned off, HK-2 also significantly reduced the amount of hearing impairment, but the effect size was substantially less than in the Prevention studies. HK-2 alone did not adversely affect HEI-OC1 cell viability, nor did it cause any adverse changes in rat body weight, behavior, cochlear function or hair cell integrity. Thus, HK-2 is a novel, safe, orally-deliverable and highly effective otoprotective compound with considerable potential for preventing hearing loss from noise and other hearing disorders linked to excessive oxidative stress.
Keywords: noise exposure, hearing loss, hair cell loss, otoprotection, oxidative stress
Introduction
Noise-induced hearing loss (NIHL), which often gives rise to tinnitus, is the most common cause of hearing impairment among military personnel and young adults (Carroll et al., 2017; Helfer et al., 2011; Lindblad et al., 2011; Nelson et al., 2005). While hearing protection devices can reduce the risk of NIHL, they are seldom worn in combat and other dangerous situations where communication and situational awareness are of paramount importance. Therefore, developing effective pharmacologic interventions that address the biological bases of NIHL remains an important scientific and clinical goal. One of the major causes of NIHL, as well as other forms of cochlear hearing loss, is believed to be excessive oxidative stress (Jacono et al., 1998; Jamesdaniel et al., 2012; Yamashita et al., 2004). Intense noise can disrupt blood flow to the cochlea resulting in ischemia or hypoxia (Nuttall, 1999; Shi, 2009). The high metabolic demands placed on the cells within the cochlea (Cheng et al., 2008; Le Prell et al., 2007; Lotz et al., 1986) leads to oxidative stress and the generation of reactive oxygen species (ROS) which are toxic to hair cells, support cells, and spiral ganglion neurons (Henderson et al., 2006; Yamashita et al., 2004). ROS levels increase dramatically a few hours after intense noise exposure, remain elevated for hours or days following the exposure and spread from the site of injury and ROS generation to surrounding tissues (Ohlemiller and Dugan, 1999a; Ohlemiller et al., 1999b; Van Campen et al., 2002; Yamashita et al., 2004). High-intensity sounds increase the production of the superoxide radical (Yamane et al., 1995a; Yamane et al., 1995b) and the highly reactive hydroxyl radical (Ohlemiller et al., 1999b) to toxic levels. Superoxide levels increase shortly after an intense exposure (Yamane et al., 1995a) followed a few hours later by a significant rise in hydroxyl radicals (Ohlemiller and Dugan, 1998). Hydroxyl radicals are formed during the Fenton reaction by the reaction of hydrogen peroxide with iron and other bioactive transition metals. Deferoxamine, a chelator that binds to iron, suppresses the production of hydroxyl radicals and attenuates noise-induced cochlear damage (Clerici and Yang, 1996; Yamasoba et al., 1999). The cochlea’s endogenous antioxidant enzyme levels are affected by noise and have been shown to increase after moderate-intensity noise exposure. Prior “sound conditioning exposure”, which can upregulate the cochlea’s antioxidant defense system, have been shown to reduce the hearing loss and cochlear damage caused by more intense damaging sound exposures (Canlon, 1997; Harris et al., 2006; Jacono et al., 1998).
Many studies have attempted to reduce the risk of NIHL through the use of drugs that either scavenge ROS or enhance the cochlea’s antioxidant defense systems. Some have found that NIHL can be reduced by ROS scavengers and natural antioxidants such as glutathione (GSH), acetyl-L-carnitine, N-acetyl-L-cysteine, D-methionine, ebselen (SPI-1005), resveratrol, ascorbic acid, Src-PTK inhibitors, and coenzyme Q10 (Bielefeld et al., 2005b; Choi and Choi, 2015; Clifford et al., 2011; Coleman et al., 2007; Kil et al., 2007; Lo et al., 2013; Sha and Schacht, 2017; Wu et al., 2010). On the other hand, others have reported that many of these same compounds had either no protective effect or increased the risk of NIHL (Davis et al., 2010; Hamernik, 2013). Despite the conflicting evidence in the literature, the antioxidant N-acetyl-L-cysteine (NAC) advanced to a randomized clinical trial (Kopke et al., 2007). In this clinical investigation, treatment with NAC was found to be no better than placebo in preventing NIHL (Kopke et al., 2015). These negative findings are consistent with other clinical studies in which natural antioxidant supplements failed to ameliorate other health problems (Joshi, 2015; Moser and Chun, 2016; Ozben, 2015).
Because NIHL remains a significant health care problem especially among combat personnel and workers in noisy industries, we tested a new multifunctional antioxidant, HK-2, to assess is efficacy in reducing NIHL and oxidative stress. HK-2 is a member of a new class of synthetic multifunctional redox modulators (MFRMs) that uses the innovative therapeutic strategy of combining both metal-attenuating and redox-modulating/radical scavenging properties into one molecule. This new synthetic compound not only reduces oxidative damage in cells exposed to cellular oxidizers but also independently sequesters and redistributes free transition metals (Fe, Cu, Zn and Mn) that can participate in the Fenton generation of toxic hydroxyl radicals. Because HK-2 crosses the blood-brain barrier and blood-retinal barrier (Kawada et al., 2015b), it was expected to enter the cochlea, protect against NIHL and reduce oxidative stress. To test this hypothesis, we carried out an in vivo study of NIHL with Sprague-Dawley rats and a second in vitro study assessing oxidative stress using cochlear House Ear Institute-Organ of Corti 1 (HEI-OC1) cells. We found that HK-2 significantly reduced NIHL and hair cell loss (Experiment 1) and also significantly reduced cell loss and damaged caused by ROS and reactive nitrogen species (RNS) (Experiment 2).
Methods
Test Compounds
HK-2 (1-(5-hydroxypyrimidin-2-yl) pyrrolidine-2,5-dione) was synthesized according to Kawada (Kawada and Kador, 2015a; Kawada et al., 2015b) and evaluated to have a purity of >99% (determined by NMR, HPLC). Trolox (6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, Sigma-Aldrich; purity ≥97%) served as a positive control for the in vitro studies.
Experiment 1
Subjects
Ten month old Sprague–Dawley rats (Charles River Laboratories) were used for the NIHL studies. The rats were housed in the Laboratory Animal Facility at the University at Buffalo and given free access to food and water. The colony room was maintained at 22 °C with a 12-hour light-dark cycle. All procedures regarding the use and handling of animals were reviewed and approved by the Institutional Animal Care and Use Committee at the University at Buffalo.
HK-2 Administration
The animals in the Noise groups and HK-2 control groups were administered standard laboratory rat chow containing either: (1) 0.025 wt.% of HK-2 corresponding to an oral dose of 16 mg/kg/day HK-2, (2) 0.1 wt. % of HK-2 corresponding to an oral dose of 40 mg/kg/day HK-2 or (3) 0.2 wt. % HK-2 corresponding to an oral dose of 125 mg/kg/day HK-2. The average daily dosage of HK-2 was determined by measuring the amount of HK-2-treated food consumed every 2 days over the duration of HK-2 treatment. Body weights were also measured every 2 days. The amount of HK-2 treated food given to each rat was adjusted every 2 days based on body weight.
Noise Exposure
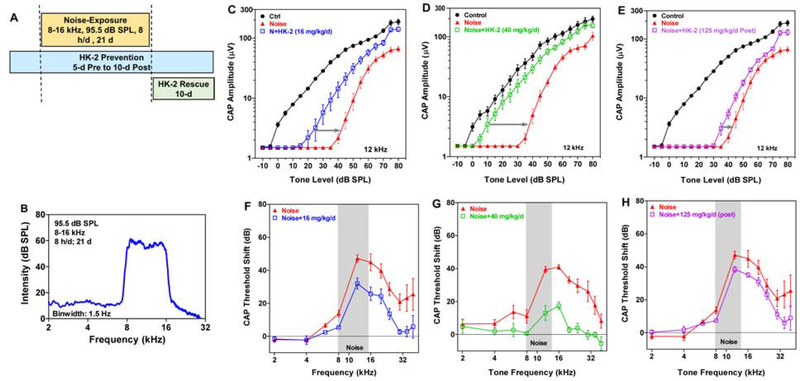
All noise-exposed rats were exposed to the noise in standard housing cages; the cages were located in a separate, dedicated noise-exposure room in the lab animal facility as previously described (Chen et al., 2014; Zhao et al., 2018). No other animals were housed in the noise-exposure room. The electrical signal for the 8–16 kHz octave band noise was generated digitally (Adobe Audition), routed to a soundcard (44 kHz sampling rate, 16 bits) on a personal computer. The analog signal was routed to a power amplifier (Amp 300, Audio Source Inc.) and presented through a loudspeaker (Fostex FT17H) suspended approximately 8 cm above the acoustically transparent wire mesh ceiling of the cage in which the rat was housed. Sound levels in the cage were measured at the height of animal’s ear using a half-inch condenser microphone (Larson Davis; 2450), preamplifier and power supply (Larson Davis, 2221). The output of the microphone was digitized (RME, model Babyface Pro) and the acoustic signal was analyzed using custom MATLAB software. The overall level of the noise was 95 dB sound pressure level (SPL) (+/− 2 dB SPL, Figure1B). The noise was presented for 8 h/d for 21 days (Figure 1A).
Figure 1:
HK-2 prevents NIHL. (A) Schematic of 21-d noise and schedule of dosing for HK-2 Prevention treatment (36 days) and HK-2 Rescue treatment (10 days post-exposure). (B) Spectrum of 95.5 dB SPL, 8–16 kHz (1.5 Hz/bin) noise presented 8 hours/day for 21 days. Mean (SEM) CAP I/O functions for 12 kHz (C-E) and 30 kHz F-H) CAP thresholds shifts as a function of frequency in Noise group vs. Noise+HK-2 group (see legend in each panel). Threshold shifts in HK-2 treated group significantly less than in Noise groups (see text for details).
Experimental Groups
Five groups of rats (n= 8/group) were used. The control groups included a sham control group (no noise, no HK-2) and an HK-2 control group (no noise, 40 mg/kg/d HK-2 for 36 days). The sham control group and the HK-2 control group were housed in a different room in the lab animal facility under standard housing conditions and ambient noise levels. As schematized in Figure 1A, two groups of rats in the Prevention study were noise exposed for 21 days and treated with HK-2 beginning 5 days before the start of the noise exposure and continuing for 10 days after the noise exposure (36-d treatment). One of the exposed groups in the Prevention study was treated with 16 mg/kg/d and the other group was treated with 40 mg/kg/d. The group of rats in the Rescue study (Figure 1A) was noise exposed for 21 days; after the exposure the rats were treated with 125 mg/kg/d of HK-2 for 10 days. All of the Noise+HK2 groups in the Prevention and Rescue studies were accompanied by its own yoked-Noise control group (noise exposure, but regular rat chow). This was done because we did not have enough equipment and cages equipped with a speaker to expose all of these groups at the same time.
Compound action potential (CAP)
The CAP, which reflects the gross neural output of the cochlea, was recorded from all animals approximately 2-months post-exposure using procedures described previously (Chen et al., 2010). Tone bursts (10 ms, 1 ms rise/fall time, cosine-gated, 2–65 kHz) were generated by using the TDT RX6 Multifunction Processor (200 kHz sampling rate, Tucker-Davis Technologies, Alachua, FL). The electrical tone burst signal was amplified and delivered to a transducer assembly (ACO ½” microphone) inserted into the ear canal. The transducer was calibrated in a cavity approximating the volume of the ear canal using a ½” microphone (model 2540, Larson Davis) and microphone preamplifier (Model 2221, Larson Davis).
Each rat was anesthetized with a cocktail of ketamine and xylazine (50 mg/kg; 6 mg/kg, i.m., respectively) and then transferred to a custom designed head-holder. Body temperature was maintained at 37 °C using a homoeothermic heating blanket (Harvard Apparatus). The right cochlea was exposed ventrolaterally. A Teflon-coated gold wire electrode (Cat# 751000, A-M Systems Inc.) was placed on the round window membrane and a silver chloride ground electrode was placed in the neck muscle. Tone-evoked responses were amplified with a DAM-50 preamplifier (WPI, 1000x, 0.1 Hz – 10 kHz), digitized (100 kHz sampling rate) and averaged 100 times (TDT RX6 Multifunction Processor) using custom written data acquisition and analysis software (MATLAB 6.1). The CAP amplitude was defined as the voltage difference between the first negative peak and the subsequent positive peak. CAP amplitude was plotted as a function of intensity to generate an input/output (I/O) function at each test frequency. Threshold was defined as the intensity needed to produce a CAP amplitude of 3 μV.
Cochleograms
After collecting the electrophysiological data, the anesthetized animal was euthanized and the cochleae removed, fixed in 10% buffered formalin and stained with Ehrlich’s hematoxylin solution as described previously (Jamesdaniel et al., 2008). The basilar membrane containing the organ of Corti was dissected out as a flat surface preparation, mounted in glycerin on a glass slide and the sensory hair cells examined with a light microscope (Zeiss Standard, 400X magnification). A hair cell was counted as present if both the cuticular plated and nucleus were clearly visible and considered missing if either were absent. Outer hair cells (OHC) and inner hair cells (IHC) were counted along the entire basilar membrane from base to apex, and a cochleogram was constructed showing the percentages of missing OHC and IHC as a function of percent distance from the apex of the cochlea; cochlear location was related to frequency using a rat tonotopic map (Müller, 1991).
Experiment 2
Experiment 2 was conducted to determine if HK-2 was toxic to HEI-OC1 cells derived from the cochlea. Additional studies were conducted to determine if HK-2 could protect HEI-OC1 cells against damage caused by ROS and / or RNS. To put the results into perspective, the HK-2 results were compared to Trolox, a cell-permeable, water-soluble derivative of vitamin E that protects against ROS and RNS.
Cell Incubation Procedure
HEI-OC1 cells were generously provided by Dr. Federico Kalinec, David Geffen School of Medicine, University of California, Los Angeles, CA (Kalinec et al., 2003). Cells were grown under permissive conditions (33 °C under 10% CO2), which induces expression of an immortalizing gene that triggers de-differentiation and accelerates proliferation. Cells (four passages or less) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, MediaTech Inc., Manassas, VA) supplemented with 10% fetal bovine serum (FBS, ThermoFisher, Waltham, MA) in 250 mL plastic cell culture flasks as previously described (Kalinec et al., 2016). A uniform population of adhering, growing cells was obtained by performing multiple “slap-and-wash” cycles where the flask was smacked to detach all non-adhesive cells. Upon reaching 80% confluence, the attached cells were detached with Accutase and seeded onto 96-well clear flat-bottom plastic plates (200 μL per well; density of 2.0 × 105 cells/mL, ThermoFisher, Waltham, MA). One day of growth in standard media resulted in about 80% confluence in each well. All studies were conducted in triplicate, at a minimum.
Compound Toxicology
To test for compound toxicity, media containing 1, 10, 100, or 1000 μM of HK-2 in 1.0 v/v% DMSO was added to the HEI-OC1 seeded on 96-well plates. After 24 h, the media was removed by aspiration, the cells were washed three times with PBS, and the media was replaced with Cell Titer 96® AQueous One Solution Cell Proliferation Assay (MTS, Promega, Madison, WI) and incubated for 1 h according to manufacturer’s protocol. Absorbance was recorded at 490 nm using a 96-well plate reader. The results were normalized to blank control cells (100%).
Exposure to Hydrogen Peroxide (H2O2)
Prior to H2O2 exposure, the growth medium was aspirated, and the cells were washed with PBS. The cells were then pre-treated with FBS-free medium with/without 1 mM of HK-2 or Trolox in 1.0 v/v% DMSO. After 1 h incubation, the media was removed, and after washing with PBS, the cells were exposed for 2 h to FBS-free media containing 1 mM H2O2. After H2O2 exposure, the cells were again washed with PBS and cultured with MTS proliferation solution for 1 h according to manufacturer’s protocol. Absorbance was recorded at 490 nm using a 96-well plate reader, and results were normalized to blank control cells (100%) that were not treated with H2O2.
Exposure to Fenton Reagent
Prior to Fenton reagent exposure, the cells were aspirated of growth media and washed with PBS. Cells were then pre-treated with FBS-free media with/without 1 mM HK-2 or Trolox in 1.0 v/v% DMSO for 1 h, the solution was aspirated, cells washed with PBS, and then exposed for 2 h to oxidizing conditions in FBS-free media containing 1 mM Fenton reagent (1 mM H2O2 and 1 mM Fe2+). After aspirating the Fenton reagent, the cells were washed with PBS and the solution replaced with MTS proliferation media and then incubated for 1 h according to manufacturer’s protocol. Absorbance was recorded at 490 nm using a 96-well plate reader, and the results were normalized to blank control cells (100%) untreated with Fenton reagent. A dose-dependent protection study of HEI-OC1 cells exposed to 1 mM Fenton reagent was conducted as above except that each of the seeded 96 wells was pre-treated for 1 h with FBS-free media with/without 1, 10, 100, 1000 μM of HK-2, or 1, 10, 100, 1000 μM of Trolox in 1.0 v/v% DMSO prior to the addition of 1 mM Fenton reagent.
Exposure to Superoxide
Prior to superoxide exposure, the cells were aspirated of growth media and washed with PBS. Cells were then pre-treated with FBS-free media with/without 1 mM of HK-2 or 1 mM Trolox in 1.0 v/v% DMSO or Superoxide Dismutase (SOD, Thermo Fisher, Waltham, MA) at a concentration of ~100 μM for 1 h. After 1 h of pre-treatment, the cells were then exposed to Xanthine Oxidase (Roche Diagnostics, Indianapolis, IN) at a concentration generating ~100 μM superoxide/h. After 1 h exposure to superoxide generated by xanthine oxidase, the cells were washed with PBS and media was replaced with MitoSOX Red reagent (100 μL at 1 μM), a mitochondrial superoxide indicator, and incubated for 2 h according to manufacturer’s protocol (Thermo Fisher, Waltham, MA). The fluorescence of each well was measured using a fluorescent microplate reader at excitation/emission (Ex/Em) of 510/580 nm. The relative fluorescence intensity was normalized to blank control cells (100%) not treated with xanthine oxidase.
Nitrogen Radical Exposure
To investigate the role of the nitrogen radical, 3-Morpholinosydnonimine (SIN-1, Toronto Research Center, Toronto, ON, Canada), which generates precursors of peroxynitrite, nitric oxide and superoxide, was added to cells. SIN-1 in solution spontaneously decomposes in the presence of oxygen, releasing nitric oxide and superoxide under physiological conditions. Since nitric oxide and superoxide react to form peroxynitrite, SIN-1 can produce peroxynitrite under physiological conditions (Ischiropoulos et al., 1995). The appropriate concentration of SIN-1 was determined for this study using a protocol similar to Meij (Meij et al., 2004). Briefly, cells were pre-treated with FBS-free media and exposed to either 1, 10, 100, 1000, and 3000 μM concentrations of SIN-1 for 24 h. The cells were washed with PBS and the medium was replaced with MTS proliferation solution for 1 h according to manufacturer’s protocol. Absorbance was recorded at 490 nm using a 96-well plate reader and results normalized to blank (untreated) control cells (100%).
Exposure to Peroxynitrite
Prior to SIN-1 exposure, cells were pre-treated with FBS-free media with/without 1 mM compounds HK-2 or Trolox in 1.0 v/v% DMSO for 2 h. Cells were washed with PBS and treated for 24 h with 3 mM SIN-1 that generates a flux of ONOO- at a rate of 30 μM min−1 (Ischiropoulos et al., 1995). Cells were washed with PBS and the media was replaced with a MTS proliferation media for 1 h according to manufacturer’s protocol. Absorbance was recorded at 490 nm using a 96-well plate reader and results normalized to blank (untreated) control cells (100%).
Statistics
Data were analyzed with a one-way or two-way analysis of variance (ANOVA) using Graph-Pad Prism (version 5.01) or Sigma Stat (version 3.5). When appropriate, a repeated-measures analysis was used.
Results
Experiment 1: NIHL in vivo
To identify potential side effects of HK-2, we compared body weights, CAP responses and cochleograms in the control group (n=8) to the HK-2 sham control group (n=8) that received 40 mg/kg/d of HK-2 for 36 days; this group received the largest total amount of HK-2. The body weights, CAP responses and cochleograms of the control group and HK-2 sham control group were nearly identical (Data not shown). In addition, we did not observe any adverse behavioral effects in the HK-2 sham group or the control group. In addition, no adverse effects were observed in the Prevention group that received 16 mg/kg/d of HK-2 for 36 days or the Rescue group that received 125 mg/kg/d of HK-2 for 10 days.
HK-2 Prevention Study
Two doses of HK-2 were tested to assess its effectiveness in preventing NIHL from the 21-d noise exposure (8 h/d; 8–16 kHz, 95 dB SPL). In both Prevention studies, HK-2 treatment began 5 days prior to the start of the noise exposure and continued until 10-d post-exposure (Fig. 1B). To illustrate the otoprotective effects of HK-2, we present the CAP I/O functions at 12 kHz, a test frequency located within the 8–16 noise exposure (Fig. 1C–D). The effects of the 16 mg/kg/d dose of HK-2 on 12 kHz CAP I/O functions are presented in Figure 1C. The Noise CAP I/O function was shifted to the right of its yoked Control group ~40 dB. The rightward shift of the I/O function in the Noise+16 mg/kg/d HK-2 group was reduced to ~20 dB when compared to its yoked-Noise group (Figure 1C, gray line). Because the Noise+16 mg/kg/d HK-2 group was evaluated in parallel with its yoked-Control group and its yoked-Noise group, a separate one-way ANOVA repeated measure (dB SPL) was used to test for group differences in overall amplitude. CAP amplitudes differed significantly among groups (F=13.97(2,18 df), p<0.0001). Overall CAP amplitudes in the Noise group and Noise+16 mg/kg/d HK-2 group were significantly less than in Controls. The overall amplitudes in the Noise group were significantly less than in the Noise+16 mg/kg/d HK-2 group (p<0.05, Bonferroni Multiple comparison). (Note: We did not test for level-dependent (i.e., dB SPL) group differences because of difficulties associated with interpreting the results due to the spread of excitation).
The 40 mg/kg/d dose of HK-2 provided even greater protection as illustrated by the 12 kHz CAP I/O functions (Figure 1D). The I/O function of the yoked-Noise group was again shifted to the right of the yoked-Control group ~40 dB, while the rightward shift of the Noise+40 mg/kg/d HK-2 group was only shifted ~10 dB to the right of the yoked-Control group. Thus, the high dose of HK-2 reduced the threshold shift by 30 dB (Figure 1D, gray line) versus 20 dB for the low dose. Because the Noise+40 mg/kg/d HK-2 group was evaluated in parallel with its yoked-Control group and its yoked-Noise group, a separate one-way ANOVA repeated measure (dB SPL) was used to test for group differences in overall amplitude. CAP amplitudes differed significantly among groups (F=22.98 (2,18 df), p<0.0001). Overall CAP amplitudes in the yoked-Noise group and the Noise+40 mg/kg/d HK-2 group were significantly less than in the yoked-Control group. The overall amplitudes in the yoked-Noise group were significantly less than in the Noise+40 mg/kg/d HK-2 group (p<0.05, Bonferroni Multiple comparison). Thus, the 36-d treatment with HK-2 dose-dependently prevented NIHL. (Note: We did not test for level-dependent group differences).
HK-2 Rescue
To determine if HK-2 could reduce the hearing loss if delivered after a noise exposure, rats were given 125 mg/kg/d of HK-2 for 10 days immediately following the 21-d exposure. Post-exposure administration of HK-2 had a modest rescue effect on the 12 kHz CAP I/O function. The I/O of the Noise group was shifted to the right of the Control group approximately ~40 dB; post-exposure treatment with 125 mg/kg/d reduced the rightward shift approximately 7 dB compared to Noise alone (Figure 1E). Because the Noise+125 mg/kg/d HK-2 group was evaluated in parallel with its yoked-Control group and its yoked-Noise group, a separate one-way ANOVA repeated measure (dB SPL) was used to test for group differences in overall amplitude. There were significant differences in CAP amplitudes among the groups (F=23.18 (2, 18 df), p<0.0001). The overall amplitude in the Noise+125 mg/kg/d HK-2 group was slightly larger than those in the yoked-Noise group; however, this difference was not statistically significant. The overall amplitudes in the yoked-Noise group and Noise+125 mg/kg/d HK-2 group were both significantly less than in the yoked-Control group (p<0.05, Bonferroni multiple comparison). (Note: We did not test for level-dependent group differences). The 12 kHz CAP I/O results presented here are representative of those seen at other frequencies within and above the 8–16 kHz noise exposure band.
CAP Threshold Shifts
CAP thresholds were measured at each test frequency and used to calculate the CAP threshold shifts relative to the Control group for the Noise group and Noise+HK-2 group. In the Prevention study, CAP threshold shifts in the Noise+16 mg/kg/d HK-2 group were significantly lower than in the Noise group (two-way ANOVA repeated measure, F=11.78 (1,14df), p<0.004); Bonferroni post-hoc comparisons revealed significant difference at 35 and 40 kHz (p<0.05) (Figure 1F). Threshold shifts in the Noise+40 mg/kg/d HK-2 group were also significantly less than in the Noise group (two-way ANOVA repeated measure, F=48.17 (1,10 df), p<0.0001); Bonferroni post-hoc analysis revealed significant reduction in thresholds shifts from 12–35 kHz (p<0.05) (Figure 1G). Mean CAP threshold shifts were less for the 40 mg/kg/d HK-2 group than for the 16 mg/kg/d HK-2 group. When HK-2 was administered after the noise exposure, mean CAP threshold shifts in the Rescue experiment were overall significantly less in Noise+125 mg/kg/d HK-2 (post) group than in the Noise group (two-way ANOVA repeated measure, F=4.94 (1,14 df), p<0.043) (Figure 1H), but a post-hoc analysis did not reveal a frequency specific effect. However, the magnitude of the HK-2 protective effect in the Rescue experiments was much less than in the Prevention studies.
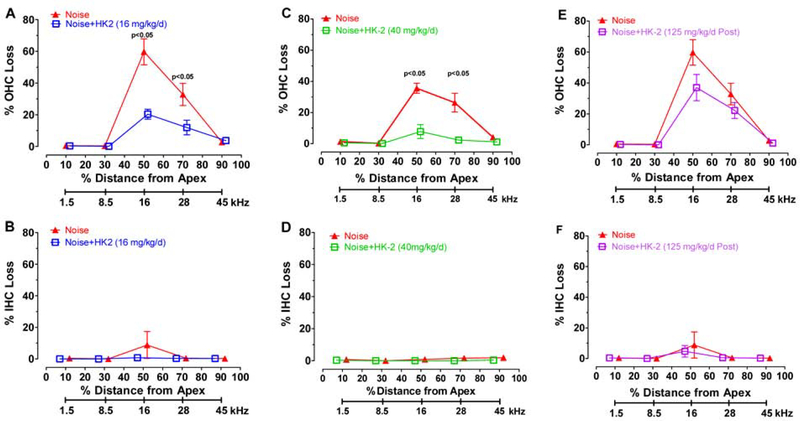
HK-2 Prevents OHC Loss
Mean (± SEM) cochleograms were prepared showing the percent OHC and IHC loss in 20% intervals along the length of the cochlea for each of the three HK-2 treated groups and its paired noise-exposure group. The frequency corresponding to the cochlear locations are shown beneath the cochleograms (Figure 2) (Müller, 1991). In the Prevention study, the OHC lesion in the Noise group treated with 16 mg/kg/d HK-2 (Figure 2A) significantly small than in the Noise group contemporaneously exposed the same noise (two-way repeated measure ANOVA, F=15.09 (1, 14 df), p<0.0016); the OHC lesions at the 50% (16 kHz) and 70% (28 kHz) cochlear locations were significantly less in HK-2 treated group than the Noise group (Bonferroni post-hoc comparison, p<0.05). In the Prevention study, the OHC lesion in the Noise group treated with 40 mg/kg/d was significantly less than in the Noise group (Figure 2C, F=40.03 (1, 14 df), p<0.0001); the OHC lesions at the 50% (16 kHz) and 70% (28 kHz) locations were significantly less in the Noise group treated with 40 mg/kg/d of HK-2 than Noise alone group (Bonferroni post-hoc, p<0.05). In the Rescue study, the OHC lesions in the Noise group treated with 125 mg/kg/d were slightly less than in the Noise alone group; however, the between group differences did not quite reach statistical significance (p=0.061). There was little evidence of noise-induced IHC loss in any of the groups and no differences between groups (Figure 2B, D, F).
Figure 2:
HK-2 prevents hair cell loss. Mean ± SEM percent outer hair cell (OHC) loss (top row) and inner hair cell (IHC) loss (bottom row) plotted as function of percent distance from apex of cochlea; cochlear frequency-place map shown below. (A-B) Noise group vs. Noise + HK-2 (16 mg/kg/d group). OHC losses in 16 mg/kg/d HK-2 treated group significantly less (p<0.0001) than is paired Noise group; significant differences at 50% and 70% locations (p<0.05, see text for details). (C-D) Noise group vs. Noise + HK-2 (40 mg/kg/d) group. OHC losses in 40 mg/kg/d HK-2 group significantly less (p<0.0001) than its paired Noise group; significant differences at 50% and 70% locations (p<0.05 see text for details) . (E-F) Noise group vs. Noise + 125 mg/kg/d (post-noise) group.
Experiment 2: Oxidative Stress in vitro
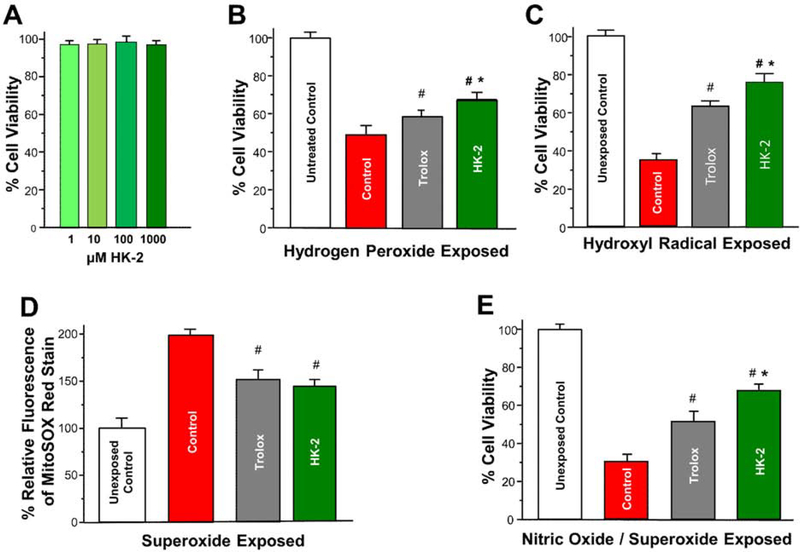
To determine if HK-2 was toxic to cells derived from the cochlea, we cultured HEI-OC1 cells for 24 h with 1,10, 100, or 1000 μM of HK-2. As shown in Figure 3A, HK-2 had no adverse effect on HEI-OC1 cell viability at any concentration.
Figure 3:
Protective effect of HK-2 on mean ± SD percent cell viability or mean ± SD percent relative MitoSOX fluorescence on HEI-OC1 cells during ROS and RNS stress. (A) HEI-OC1 cell viability is not adversely affected by 24 h exposure to HK-2 concentrations up to 1000 μM. (B) Untreated Control HEI-OC1 cells (white) and Control HEI-OC1 cells (red) compared to HEI-OC1 cells preloaded for 1 h with 1 mM Trolox (gray) or 1 mM HK-2 (green). Control, Trolox and HK-2 groups exposed for 2 h to 1 mM hydrogen peroxide. Cell viability in Control group treated with hydrogen peroxide significantly less than in Untreated Control group (p<0.001, see text for details). Cell viability with Trolox or HK-2 significantly greater than Control group (#, p<0.05). Cell viability with HK-2 significantly greater than Trolox (*, p<0.05). (C) Untreated Control HEI-OC1 cells (white) and Control HEI-OC1 cells (red) compared to HEI-OC1 cells preloaded for 1 h with 1 mM Trolox (gray) or 1 mM HK-2 (green). Control, Trolox and HK-2 groups then treated for 2 h ofwith hydroxyl radical generating reagent. Cell viability of Control group significantly less than Untreated Control group after hydroxyl radical exposure (p<0.001, see text for details). Cell viability with Trolox and HK-2 significantly greater than Control group (#, p<0.05). Cell viability with HK-2 significantly greater than Trolox (*, p<0.05). (D) Unexposed Control HEI-OC1 cells (white) and Control HEI-OC1 cells (red) compared to HEI-OC1 cells preloaded for 1 h with 1 mM Trolox (gray) or 1 mM HK-2 (green). Control, Trolox and HK-2 groups exposed for 1 h to superoxide radicals (~100 μM/h) generated for by xanthine oxidase. Percent relative MitoSOX fluorescence in Control group significantly greater than Unexposed Control group (p<0.001, see text for details). Percent relative fluorescence in Trolox group and HK-2 group significantly less than Control group (p<0.05). (E) Untreated Control HEI-OC1 cells (white) and Control HEI-OC1 cells (red) compared to HEI-OC1 cells preloaded for 3 h with 1 mM Trolox (gray) or 1 mM HK-2 (green). Control, Trolox and HK-2 groups then exposed for 24 h to 3 mM of SIN-1 which generates the precursors of peroxynitrite, nitric oxide and superoxide. Cell viability of the Control group was significantly less than in the Untreated Control group (p<0.001, see text for details). Cell viability in Trolox and HK-2 groups significantly greater than Control group (#, p<0.05). Cell viability in HK-2 group significantly greater in HK-2 group than Trolox group (*, p<0.05).
Hydrogen Peroxide Damage
To determine the extent to which HK-2 could protect against hydrogen peroxide, equal numbers of HEI-OC1 cells were seeded into separate wells containing media and cultured overnight to 80% confluence. Prior to peroxide exposure the growth media in all cells was replaced by FEBS free media with one of the four groups additionally treated with 1 mM HK-2 and a second group with 1 mM Trolox. After 1 h the media in all groups was again removed and the cells washed in PBS. Again, FEBS free media was added to all groups and the HK-2, Trolox and Untreated Control group were exposed for 2 h to 1 mM hydrogen peroxide. Cell viability in the Control group treated with 1 mM hydrogen peroxide was significantly decreased to ~50% relative to Untreated Control group (Fig. 3B) (one-way ANOVA, F (1, 3) =663.05, p<0.001, n=4/group). Pre-treatment with Trolox significantly increased cell viability relative to the Control group to ~60% (Tukey-Kramer post-hoc, #, p<0.05). Treatment with HK-2 increased cell viability to ~70%; the difference in cell viability between the HK-2 group and Control group was significant (Tukey-Kramer post-hoc, #, p<0.05) and the difference in cell viability between the HK-2 group and Trolox group was also significant (Tukey-Kramer post-hoc, *, p<0.05). These results suggest HK-2 is more effective than Trolox at reducing hydrogen peroxide-induced free radical damage. An alternative explanation is that HK-2 may be more effective than Trolox in entering cells to protect against oxidative stress.
Hydroxyl Damage
To test the beneficial effect of HK-2’s metal binding activity, HEI-OC1 cells were exposed to a Fenton reagent that generates the damaging hydroxyl radical. During a 1-h pre-loading period, one of the four groups was treated with 1 mM HK-2 and a second group was treated with 1 mM Trolox. The Untreated Control group and the Control group were maintained in culture media during this time. Afterwards, the cells in Control group, HK-2 group and Trolox group were exposed for 2 h to 1 mM of Fenton reagent that generates hydroxyl radicals. Cell viability in the Control group exposed to hydroxyl radicals was reduced significantly to ~40% (one-way ANOVA, F (1, 3 df) =1826.82, p<0.001, n=4/group) compared to the Untreated Control group (Fig. 3C). Pre-treatment with Trolox and HK-2 significantly increased cell viability compared to the Control group (Tukey-Kramer post-hoc, #, p<0.05). Cell viability in the HK-2 group (~75%) was significantly greater than viability (~60%) in the Trolox group (Tukey-Kramer post-hoc, *, p<0.05). These results suggest HK-2 is more effective than Trolox at reducing hydroxyl radial damage; however, the relative permeability of these two compounds could be another contributing factor.
Superoxide Damage
Superoxide is generated during NIHL (Bielefeld et al., 2005a). To determine the extent to which HK-2 could suppress superoxide toxicity, wells containing equal numbers of HEI-OC1 cells were treated with FEBS free media with for 1 h with one of the groups additionally treated with 1 mM HK-2 and a second group with 1 mM Trolox. After 1 h the media in all groups was again removed and the cells washed in PBS followed by FEBS-free media. Afterwards, the Control group, HK-2 group and Trolox group were exposed for 1 h to superoxide radicals (~100 μM/h) generated by xanthine oxidase. MitoSOX, a fluorescent mitochondrial superoxide indicator, was used to assess relative superoxide levels in the four groups. Percent relative fluorescence of MitoSOX measured in the Control group (Fig. 3D) was significantly higher than in the Untreated Control group (one-way ANOVA, F (1, 3 df) =208.83, p< 0.001). The relative fluorescence values of MitoSOX in the Trolox group and HK-2 group were significantly less than in the Control group (Tukey-Kramer post-hoc, p<0.05). These results indicate that both Trolox and HK-2 are extremely effective in suppressing mitochondrial superoxide in HEI-OC1 cells.
Peroxynitrite Damage
Peroxynitrite and nitric oxide have been implicated in NIHL and cochlear damage (Mohrle et al., 2017; Ohinata et al., 2003; Shi et al., 2002; Yamasoba et al., 2005). To determine if HK-2 could protect HEI-OC1 cells against RNS damage, SIN-1 was used to generate nitric oxide and superoxide radicals, which are the precursors of peroxynitrite (Scarpato et al., 2011). To determine the appropriate amount of SIN-1 to use for these studies, the viability of HEI-OC1 was examined after being exposed for 24 h to RNS generated by 1, 10, 100, 1000, or 3000 μM of SIN-1. In a preliminary study, we found that HEI-OC1 cell viability decreased by ~35%, ~50% and ~75% with 100, 1000 and 3000 μM of SIN-1 respectively. Therefore, 3000 mM of SIN-1, which generates a peroxynitrite flux of 30 μM min−1 (Ischiropoulos et al., 1995), was used for subsequent studies with HK-2 and Trolox. To determine the extent to which HK-2 could protect against SIN-1-induced peroxynitrite damage, equal numbers of HEI-OC1 cells were cultured for 2 h in FEBS free media; the Control group was untreated, one group was treated with 1 mM HK-2 and a second group was treated with 1 mM Trolox. After 2 h the media was aspirated, the cells washed in PBS and new FEBS free media containing 3 mM of SIN-1 was added to the Control group, HK-2 group and Trolox group. After 24 h cultured in the presence of 3 mM of SIN-1, cell viability in the Control group (Fig. 3E) was significantly less than in the Untreated Control group (one-way ANOVA, F (1, 3 df) = 894.67, p<0.001). Cell viability in the Trolox group and the HK-2 group were significantly greater than the Control group (Tukey-Kramer post-hoc, #, p<0.05). In addition, cell viability in HK-2 group was significantly greater than in the Trolox group (Tukey-Kramer post-hoc, *, p<0.05).
Collectively, this series of in vitro experiments indicate that 1 mM of HK-2 is non-toxic to HEI-OC1 cells and provides significant protection against both ROS and RNS damage through its ability to both scavenge free radicals and attenuate redox-active metal activity. Overall, the protective activity of HK-2, a MFRM, was consistently superior to Trolox.
Discussion
NIHL is the most common cause of hearing impairment among military personnel and young adults (Carroll et al., 2017; Helfer et al., 2011; Nelson et al., 2005). Because personal hearing protection cannot be worn in many noisy circumstances, such as combat where communication and situational awareness are critically important, there is a need to develop effective pharmacotherapies to prevent NIHL. In the Prevention studies, orally administered HK-2 dose-dependently reduced all three metrics of noise damage, CAP amplitude loss (Fig. 1C–D), CAP threshold shifts (Fig. 1F–G) and hair cell loss (Fig. 2A–D). The 40 mg/kg/d dose of HK-2 almost completely prevented the noise-induced CAP functional deficits (Figure 1D, G) and the OHC lesion (Fig. 2C) suggesting that this dose was nearly optimal. Importantly, the 16 mg/kg/d dose, which was 60% less than the 40 mg/kg/d dose, also provided significant protection indicating that even much lower doses of HK-2 are quite effective. While these results are extremely promising, further experiments employing higher and lower HK-2 doses are needed to identify the upper and lower limits of protection and the optimal time and duration of treatment.
The body weight of the rats treated with HK-2 for 36 days increased normally during the study and no adverse effects on behavior, cochlear potentials or cochlear hair cells were noted, consistent with earlier observations (Kawada et al., 2015a; Kawada et al., 2015b). HK-2 also provided significant protection against NIHL and hair cell loss in the Prevention studies; the magnitude of the protective effects was greater with the 40 mg/kg/d dose than the 16 mg/kg/d dose. HK-2 also provided significant, but less, protection against NIHL in the Rescue study in which 125 mg/kg/d of HK-2 was administered for 10 days following the exposure. These results are consistent with other in vivo and in vitro studies in which HK-2 provided significant protection against various neurodegenerative disorders (Kawada et al., 2015a; Kawada et al., 2015b). In studies with mice, HK-2 was observed to be well-tolerated at doses of up to 1800 mg/kg/d indicating that HK-2 has a high margin of safety (Kawada, 2013).
Delivery of HK-2 in rat chow allows for convenient, long-term drug delivery throughout the day. However, our estimates of food intake using this approach are less precise than other methods because some chow likely fell to the floor of the cage as the foot pellets were being consumed. Therefore, the HK-2 doses reported here likely overestimate the true daily consumption. Daily delivery of HK-2 by gavage would allow for more precise dosing, but would likely introduce large fluctuations in the level of HK-2 in blood stream throughout the day. Regardless of drug-delivery method employed, pharmacokinetic studies need to be conducted to determine the levels of HK-2 in the blood, brain and cochlea with various drug dosing regimens.
In the Prevention studies, HK-2 delivery began 5 days prior to the start of the noise in order to allow sufficient time for the drug to achieve relatively high levels in the cochlea prior to the 21-d noise exposure. In the Rescue study, HK-2 treatment began after the noise was turned off. This raises several important questions. How long does it take for HK-2 to rise to a quasi-steady state level, and how stable are the levels during the 21-d exposure? In addition, how long does it take for HK-2 to be eliminated from the cochlea? Answers to these questions have important clinical implications?
In the Prevention study, HK-2 was administered from 5 days before to 10 days after the noise exposure. This treatment schedule was designed to optimize the protective effect by providing antioxidant therapy during and after the noise exposure. In future experiments, it would be important to dissect out the amount of protection that would occur if HK-2 was only administered during the 8 h/d daily noise exposures. One important question that could be addressed by daily dosing and daily testing is whether HK-2 is able to reduce the amount of temporary threshold shift during the exposure as well as permanent threshold shift months after the exposure. If HK-2 were to reduce the temporary threshold shift during a worker’s daily exposure, then workers could benefit by taking HK-2 prior to coming to work and/or during the workday.
Most patients seek treatment for hearing loss after a noise exposure, not before. Therefore, the results of Rescue experiments are clinically relevant because oxidative stress can continue for days or weeks following a noise exposure. To optimize our chances of reducing oxidative stress in the post-exposure period, we increased the dose of HK-2 to 125 mg/kg/d for 10 days. The total dose was selected because it was nearly equal (~87%) to the 40 mg/kg/d dose administered for 36 days in the Prevention study. However, the magnitude of the protective effect in the Rescue experiment was much less than in the 40 mg/kg/d Prevention study. The magnitude of the protective effect in the Rescue experiment was also much less than the 16 mg/kg/d Prevention study even though the total HK-2 dose in the Rescue study was almost eight times as great. The limited efficacy of HK-2 in the Rescue study most likely occurred because much of the damage may have already occurred during the preceding 21 days of the noise-exposure. A more realistic assessment of HK-2 Rescue efficacy could be achieved by delivering a full dose of HK-2 by gavage after each 8-h daily exposure during the full 21-d exposure. Alternatively, HK-2 could be delivered in chow for several days or weeks following a single, intense noise exposure, as might occur in combat, or after a rock concert or firecracker exposure.
HEI-OC1 cells display hair cell biomarkers such as math1, myosin7a and prestin making it a biologically useful cell line in which to investigate the mechanisms of hair cell survival, and cell death (Kalinec et al., 2016; Tavanai and Mohammadkhani, 2017). Because oxidative stress is believed to play a major causal role in noise-induced hair cell loss (Lynch and Kil, 2005; Sha et al., 2017), we hypothesized that HK-2 would enhance the survival of HEI-OC1 cells exposed to various RNS and ROS. HK-2 by itself was not toxic to cochlear HEI-OC1 cells, confirming its safety in a cochlear cell line. HK-2 also provided significant protection of HEI-OC1 cells when challenged by hydrogen peroxide, hydroxyl radicals, superoxide and nitric oxide. In all cases, HK-2 provided greater protection against oxidative stress than equal molar concentration of Trolox. Our previous studies with monofunctional analogs such as HK-1 and HK-10 indicate that the mechanisms by which HK-2 protect HEI-OC1 cells occurs in two fold. First, HK-2 reduces oxidative stress generated by free radicals and second, its ability to complex Fe+2 reduces the availability ROS to participate in the Fenton reaction thereby reducing the generation of the highly toxic hydroxyl radical.
In summary, HK-2 is a novel neuroprotectant that provides significant protection against NIHL and hair cell loss in vivo and significant protection of cochlear HEI-OC1 cells against oxidative stress. Importantly, this synthetic compound is orally available and, based on our results, appears to be capable of reaching the cochlea at sufficient levels to protect the cochlea against cellular redox changes associated with oxidative stress. Therefore, HK-2 represents a novel class of compounds which appear to be promising clinical candidates for the prevention of NIHL that merit further development.
Highlight.
Oxidative stress is a major contributor to noise-induced hearing loss
HK-2 is an orally available multifunctional redox modulating neuroprotective drug
HK-2 dose-dependently prevented noise-induced hearing loss and hair cell loss
HK-2 protected HEI-OC1 cells against various forms of oxidative stress in vitro
Acknowledgments
We sincerely thank Dr. Federico Kalinec, David Geffen School of Medicine, University of California, Los Angeles, CA for the gift of their HEI-OC1 cells. Supported by NIH 1R43DC016766–01 to Therapeutic Vision, Inc.
Competing Interest Statement: Peter Kador is the President of Therapeutic Vision and hold the patent on HK-2. Dr. Salvi was the PI on a subaward on an NIH grant (1R43DC016766–01) to Therapeutic Vision to test the efficacy of HK-2.
List of Abbreviations
- ANOVA
analysis of variance
- CAP
compound action potential
- HEI-OC1
House Ear Institute-Organ of Corti 1
- HK-2
1-(5-hydroxypyrimidin-2-yl) pyrrolidine-2,5-dione
- IHC
inner hair cell
- I/O
input/output
- MFRM
multifunctional redox modulators
- NAC
N-Acetyl-L-cysteine
- NIHL
noise-induced hearing loss
- OHC
outer hair cell
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bielefeld EC, Hu BH, Harris KC, Henderson D 2005a. Damage and threshold shift resulting from cochlear exposure to paraquat-generated superoxide. Hear Res 207, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld EC, Hynes S, Pryznosch D, Liu J, Coleman JK, Henderson D 2005b. A comparison of the protective effects of systemic administration of a pro-glutathione drug and a Src-PTK inhibitor against noise-induced hearing loss. Noise Health 7, 24–30. [DOI] [PubMed] [Google Scholar]
- Canlon B 1997. Protection against noise trauma by sound conditioning. [Review] [23 refs]. Ear, Nose, and Throat Journal 76, 248–50, 253–5. [PubMed] [Google Scholar]
- Carroll YI., Eichwald J., Scinicariello F., Hoffman HJ., Deitchman S., Radke MS., Themann CL., Breysse P. 2017. Vital Signs: Noise-induced hearing loss amount adults-United States 2011–2012 Morbidity and Mortality Weekly Report 66, 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Decker B, Krishnan Muthaiah VP, Sheppard A, Salvi R 2014. Prolonged noise exposure-induced auditory threshold shifts in rats. Hear Res 317, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R 2010. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265, 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PW, Liu SH, Young YH, Hsu CJ, Lin-Shiau SY 2008. Protection from noise-induced temporary threshold shift by D-methionine is associated with preservation of ATPase activities. Ear Hear 29, 65–75. [DOI] [PubMed] [Google Scholar]
- Choi SH, Choi CH 2015. Noise-Induced Neural Degeneration and Therapeutic Effect of Antioxidant Drugs. J Audiol Otol 19, 111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici WJ, Yang L 1996. Direct effects of intraperilymphatic reactive oxygen species generation on cochlear function. Hear Res 101, 14–22. [DOI] [PubMed] [Google Scholar]
- Clifford RE, Coleman JK, Balough BJ, Liu J, Kopke RD, Jackson RL 2011. Low-dose D-methionine and N-acetyl-L-cysteine for protection from permanent noise-induced hearing loss in chinchillas. Otolaryngol Head Neck Surg 145, 999–1006. [DOI] [PubMed] [Google Scholar]
- Coleman JK, Kopke RD, Liu J, Ge X, Harper EA, Jones GE, Cater TL, Jackson RL 2007. Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-L-carnitine. Hear Res 226, 104–13. [DOI] [PubMed] [Google Scholar]
- Davis RR., Custer DA., Krieg E., Alagramam K. 2010. N-Acetyl L-Cysteine does not protect mouse ears from the effects of noise*. Journal of Occupational Medicine and Toxicology 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamernik RP 2013. The Use of Drugs to Reduce Hearing Loss Following Acute Acoustic Trauma In: Command U.S.A.M.R.a.M., (Ed.), Ft. Detrick, MD 21702–5012: pp. 1–174. [Google Scholar]
- Harris KC, Bielefeld E, Hu BH, Henderson D 2006. Increased resistance to free radical damage induced by low-level sound conditioning. Hear Res 213, 118–29. [DOI] [PubMed] [Google Scholar]
- Helfer TM, Jordan NN, Lee RB, Pietrusiak P, Cave K, Schairer K 2011. Noise-induced hearing injury and comorbidities among postdeployment U.S. Army soldiers: April 2003-June 2009. Am J Audiol 20, 33–41. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH 2006. The role of oxidative stress in noise-induced hearing loss. Ear Hear 27, 1–19. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Duran D, Horwitz J 1995. Peroxynitrite-mediated inhibition of DOPA synthesis in PC12 cells. J Neurochem 65, 2366–72. [DOI] [PubMed] [Google Scholar]
- Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM 1998. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res 117, 31–8. [DOI] [PubMed] [Google Scholar]
- Jamesdaniel S, Manohar S, Hinduja S 2012. Is S-nitrosylation of cochlear proteins a critical factor in cisplatin-induced ototoxicity? Antioxid Redox Signal 17, 929–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S, Ding D, Kermany MH, Davidson BA, Knight PR 3rd, Salvi R, Coling DE 2008. Proteomic analysis of the balance between survival and cell death responses in cisplatin-mediated ototoxicity. Journal of Proteome Research 7, 3516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S 2015. Vitamin Supplementation in the Elderly. Clinics in Geriatric Medicine 31, 355–66. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F 2003. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol 8, 177–89. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Park C, Thein P, Kalinec F 2016. Working with Auditory HEI-OC1 Cells. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H 2013. Multi-Functional Antioxidants Targeting Neurodegenerative Diseases, University of Nebraska Medical Center, Omaha, NE. [Google Scholar]
- Kawada H, Kador PF 2015a. Orally Bioavailable Metal Chelators and Radical Scavengers: Multifunctional Antioxidants for the Coadjutant Treatment of Neurodegenerative Diseases. Journal of Medicinal Chemistry 58, 8796–805. [DOI] [PubMed] [Google Scholar]
- Kawada H., Blessing K., Kiyota T., Woolman T., Winchester L., Kador PF. 2015b. Effects of multifunctional antioxidants on mitochondrial dysfunction and amyloid-beta metal dyshomeostasis. J Alzheimers Dis 44, 297–307. [DOI] [PubMed] [Google Scholar]
- Kil J, Pierce C, Tran H, Gu R, Lynch ED 2007. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear Res 226, 44–51. [DOI] [PubMed] [Google Scholar]
- Kopke R, Slade MD, Jackson R, Hammill T, Fausti S, Lonsbury-Martin B, Sanderson A, Dreisbach L, Rabinowitz P, Torre P 3rd, Balough B 2015. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear Res 323, 40–50. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Jackson RL, Coleman JK, Liu J, Bielefeld EC, Balough BJ 2007. NAC for noise: from the bench top to the clinic. Hear Res 226, 114–25. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM 2007. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res 226, 22–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad AC, Hagerman B, Rosenhall U 2011. Noise-induced tinnitus: a comparison between four clinical groups without apparent hearing loss. Noise Health 13, 423–31. [DOI] [PubMed] [Google Scholar]
- Lo WC, Liao LJ, Wang CT, Young YH, Chang YL, Cheng PW 2013. Dose-dependent effects of D-methionine for rescuing noise-induced permanent threshold shift in guinea-pigs. Neuroscience 254, 222–9. [DOI] [PubMed] [Google Scholar]
- Lotz P, Posse D, Haberland EJ, Kuhl KD, Ernst A 1986. The metabolic reaction of the cochlea to unphysiological noise exposure. Acta Otolaryngol 102, 20–6. [DOI] [PubMed] [Google Scholar]
- Lynch ED, Kil J 2005. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today 10, 1291–8. [DOI] [PubMed] [Google Scholar]
- Meij JT., Haselton CL., Hillman KL., Muralikrishnan D., Ebadi M., Yu L. 2004. Differential mechanisms of nitric oxide- and peroxynitrite-induced cell death. Mol Pharmacol 66, 1043–53. [DOI] [PubMed] [Google Scholar]
- Mohrle D, Reimann K, Wolter S, Wolters M, Varakina K, Mergia E, Eichert N, Geisler HS, Sandner P, Ruth P, Friebe A, Feil R, Zimmermann U, Koesling D, Knipper M, Ruttiger L 2017. NO-Sensitive Guanylate Cyclase Isoforms NO-GC1 and NO-GC2 Contribute to Noise-Induced Inner Hair Cell Synaptopathy. Mol Pharmacol 92, 375–388. [DOI] [PubMed] [Google Scholar]
- Moser MA, Chun OK 2016. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. International Journal of Molecular Sciences 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M 1991. Frequency representation in the rat cochlea. Hear Res 51, 247–54. [DOI] [PubMed] [Google Scholar]
- Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M 2005. The global burden of occupational noise-induced hearing loss. Am J Ind Med 48, 446–58. [DOI] [PubMed] [Google Scholar]
- Nuttall AL 1999. Sound-Induced Cochlear Ischemia/Hypoxia as a Mechanism of Hearing Loss. Noise Health 2, 17–32. [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Schacht J 2003. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res 966, 265–73. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Dugan LL 1998. In vivo measurement of cochlear reactive oxygen species (ROS) in mice: effects of noise exposure and cochlear ischemia, Abr 21, Assoc. Res. Otolaryngol pp. 518. [Google Scholar]
- Ohlemiller KK, Dugan LL 1999a. Elevation of reactive oxygen species following ischemia-reperfusion in mouse cochlea observed in vivo. Audiol Neurootol 4, 219–28. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL 1999b. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol 4, 229–36. [DOI] [PubMed] [Google Scholar]
- Ozben TT 2015. Antioxidant supplementation on cancer risk and during cancer therapy: an update. Current Topics in Medicinal Chemistry 15, 170–178. [PubMed] [Google Scholar]
- Scarpato R., Gambacciani C., Svezia B., Chimenti D., Turchi G. 2011. Cytotoxicity and genotoxicity studies of two free-radical generators (AAPH and SIN-1) in human microvascular endothelial cells (HMEC-1) and human peripheral lymphocytes. Mutation Research 722, 69–77. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J 2017. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs 26, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X 2009. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol 174, 1692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Ren T, Nuttall AL 2002. The electrochemical and fluorescence detection of nitric oxide in the cochlea and its increase following loud sound. Hear Res 164, 49–58. [DOI] [PubMed] [Google Scholar]
- Tavanai E, Mohammadkhani G 2017. Role of antioxidants in prevention of age-related hearing loss: a review of literature. Eur Arch Otorhinolaryngol 274, 1821–1834. [DOI] [PubMed] [Google Scholar]
- Van Campen LE, Murphy WJ, Franks JR, Mathias PI, Toraason MA 2002. Oxidative DNA damage is associated with intense noise exposure in the rat. Hear Res 164, 29–38. [DOI] [PubMed] [Google Scholar]
- Wu HP, Hsu CJ, Cheng TJ, Guo YL 2010. N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hear Res 267, 71–7. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A 1995a. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol 252, 504–8. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Konishi K, Iguchi H, Nakagawa T, Shibata S, Kato A, Sunami K, Kawakatsu C 1995b. The emergence of free radicals after acoustic trauma and strial blood flow. Acta Otolaryngol Suppl 519, 87–92. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, Miller JM 2004. Delayed production of free radicals following noise exposure. Brain Res 1019, 201–9. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Schacht J, Shoji F, Miller JM 1999. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res 815, 317–25. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Pourbakht A, Sakamoto T, Suzuki M 2005. Ebselen prevents noise-induced excitotoxicity and temporary threshold shift. Neuroscience Letters 380, 234–8. [DOI] [PubMed] [Google Scholar]
- Zhao DL, Sheppard A, Ralli M, Liu X, Salvi R 2018. Prolonged low-level noise exposure reduces rat distortion product otoacoustic emissions above a critical level. Hear Res. [DOI] [PMC free article] [PubMed] [Google Scholar]