Abstract
Purpose
International clinical practice guidelines call for initial volume resuscitation of at least 30 mL/kg body weight for patients with sepsis-induced hypotension or shock. Although not considered in the guidelines, pre-existing cardiac dysfunction may be an important factor clinicians weigh in deciding the quantity of volume resuscitation for patients with septic shock.
Methods
We conducted a multicenter survey of clinicians who routinely treat patients with sepsis to evaluate their beliefs, behaviors, knowledge, and perceived structural barriers regarding initial volume resuscitation for patients with sepsis and concomitant heart failure with reduced ejection fraction < 40% (HFrEF). Initial volume resuscitation preferences were captured as ordinal values and additional testing for volume resuscitation preferences was performed using McNemar’s and Wilcoxon signed rank tests as indicated. Univariable logistic regression models were used to identify significant predictors of ≥ 30 cc/kg fluid administration.
Results
A total of 317 clinicians at nine U.S. hospitals completed the survey (response rate 47.3%). Most respondents were specialists in either internal medicine or emergency medicine. Substantial heterogeneity was found regarding sepsis resuscitation preferences for patients with concomitant HFrEF. The belief that patients with septic shock and HFrEF should be exempt from current sepsis bundle initiatives was shared by 39.4% of respondents. A minimum fluid challenge of ~30 mL/kg or more was deemed appropriate in septic shock by only 56.4% of respondents for patients with concomitant HFrEF, compared to 89.1% of respondents for patients without HFrEF (p < 0.01). Emergency medicine physicians were most likely to feel that < 30 ml/kg was most appropriate in patients with septic shock and HFrEF.
Conclusions
Clinical equipoise exists regarding initial volume resuscitation for patients with sepsis-induced hypotension or shock and concomitant HFrEF. Future studies and clinical practice guidelines should explicitly address resuscitation in this subpopulation.
Keywords: Septic shock, resuscitation, crystalloid, fluid challenge, systolic heart failure
Background
The 2018 Surviving Sepsis Campaign guidelines recommend that patients with sepsis-induced hypotension or shock receive a rapid bolus of 30 ml/kg body weight of crystalloid fluid initiated within the first hour of presentation and completed within three hours [1]. Yet, aggressive volume resuscitation in early sepsis may not be tolerated equally well in all patients. While literature suggests that cumulative positive fluid balance is associated with worse outcomes in patients with septic shock [2, 3], two recent randomized trials completed in limited-resource settings in Africa raise concern that overly aggressive early volume resuscitation may also be harmful in some patients [4, 5]. Adherence to a minimum 30 mL/kg volume challenge was not associated with improved survival in a multicenter before-and-after sepsis bundle study targeting emergency care in New York [6]. In contrast, a recent multicenter observational before-and-after study in California found sepsis bundle implementation correlated with greater volume resuscitation and increased survival in patients with sepsis and concomitant heart failure [7], although causality could not be determined. Whether the net effect of aggressive early volume resuscitation is unclear [8], and some experts now advocate for a more conservative fluid resuscitation approach [9].
In our experience, pre-existing cardiac dysfunction is an important factor clinicians weigh in deciding the quantity of volume resuscitation that patients with severe sepsis or septic shock receive. The ideal resuscitation strategy, however, is uncertain. Risks of aggressive volume resuscitation, including volume overload and pulmonary edema, may be greater in patients with heart failure and reduced ejection fraction (HFrEF) than the general population. Moreover, pre-existing myocardial dysfunction may contribute to hemodynamic derangement and morbidity in infected patients [10]. Whether the role for early aggressive volume resuscitation in the general population extends to patients with HFrEF is controversial.
We conducted a multicenter survey of clinicians who routinely manage patients with sepsis to (1) identify practice habits, knowledge, and attitudes of physicians regarding fluid resuscitation of patients with HFrEF in severe sepsis/septic shock and (2) identify barriers to adherence to fluid resuscitation guidelines in these patients. We hypothesized that equipoise exists in self-reported clinical practice regarding volume resuscitation in septic shock with HFrEF that is unexplained by practice level or knowledge. We also hypothesized that provider concern and training regarding fluid resuscitation in patients with HFrEF are the principal barriers to the administration of 30 cc/kg.
Methods
Survey Design
The survey instrument was designed using previously validated techniques for identifying clinician practice habits in critically ill patients [11–13]. Survey questions were constructed by consensus from four investigators (G.W., R.E.S, R.L.O., J.R.B.). HFrEF was defined for the survey as a known prior diagnosis of left ventricular ejection fraction of less than 40%, consistent with international guidelines [14].
The survey consisted of 28 questions over three sections. The first section captured respondent characteristics, including level of training, duration of practice, practice type, primary specialty, and completion of a critical care fellowship.
The second section captured clinician beliefs, practice habits, perceived institutional/structural barriers, and knowledge regarding early volume resuscitation in sepsis with HFrEF. Questions were structured in both positive and negative language to minimize risk of acquiescence bias. Six-point Likert scale responses (strongly disagree, disagree, neutral, agree, strongly agree, uncertain) were used. Knowledge evaluation consisted of rating self-perceived knowledge and confirmatory testing of awareness of prior controversies now thoroughly addressed in the medical literature. Specifically, confirmatory testing evaluated if respondents recognized that measuring central venous pressure to determine intravascular volume or volume responsiveness in septic shock is not considered reliable [15–18]. Knowledge testing also asked if respondents were aware that multiple recent multicenter trials (PROCESS, PROMISE, and ARISE) failed to show a benefit to protocolized resuscitation versus usual care for septic shock [19–21].
The final section asked respondents to select the volume of fluid they would administer (0–0.5 liters, 0.6–1.0 liters, 1.1–2.0 liters, 2.1–4.0 liters, 4.1–6.0 liters, or 6.0 or more liters) to a theoretical 75-kilogram (kg) patient in septic shock with versus without concomitant HFrEF in order to determine whether the patient was volume non-responsive.
For all questions concerning patients with HFrEF, respondents were instructed to assume that the patient does not have significant signs of volume overload.
Questions were reviewed and tested by a group of attending physicians prior to release of the survey to determine the cognitive validity of self-reported responses and assess for overall completeness, appropriateness, and length of the questions based on previously published recommendations [22]. As questions were extensively reviewed by the study design team and based on previously validated methodology, no pilot validation study was completed. Study questions were not changed after distribution.
Survey Administration
The survey was distributed in accordance with guidelines on proper web-based administration [23]. It was conducted between January and July 2017 as a closed voluntary survey offered to select emergency medicine and critical care clinical units at nine university-affiliated hospitals across the United States (Table E1). Local investigators recruited participants at each respective site via email and in person at weekly or monthly conferences. All survey responses were anonymous. The survey software limited each email address to a single response. Institutional review board approval was obtained prior to distribution, and consent for participation was obtained from all respondents.
Data Analysis
Descriptive statistics were used to quantify respondent characteristics. Responses to Likert questions were reported in unadulterated form. Initial volume resuscitation preferences were captured as ranges of volume resuscitation thought appropriate before deeming a patient volume non-responsive (ordinal variable). Volume resuscitation preference for patients in septic shock with versus without concomitant HFrEF were compared using Wilcoxon signed rank test for paired data. Appropriateness of ~30 mL/kg or greater fluid challenge for patients with versus without HFrEF was compared using McNemar’s test, again comparing within-respondent differences in practice preference depending on presence of concomitant HFrEF diagnosis. Univariable logistic regression models were used to identify significant predictors of ≥ 30 cc/kg fluid administration. To minimize extreme responding bias, all Likert results were re-coded in binary format for entry into prediction models (online supplement). Respondent characteristics found to be significantly associated with differences in initial volume resuscitation were evaluated for their correlation with beliefs and behaviors regarding resuscitation practices. For all tests, a two-sided alpha threshold of 0.05 was used for statistical significance. Data were analyzed using SPSS Statistics, version 24 (SPSS, Armonk, NY) and SAS v9.3 (SAS Institute, Cary, NC).
Results
A total of 669 surveys were distributed to nine academic centers; 317 completed the survey, yielding a 47.3% completion rate. Excluding respondent characteristics, missing values constituted on average 1.4 responses (0.4%) per survey question, range 0 to 5 missing values per question (0% to 1.6%).
Respondent Characteristics
Characteristics of the respondents appear in Table 1. Nearly all respondents were physicians (95.6%), with the remainder nurse practitioners or physician assistants. Approximately half of respondents (48.6%) were attending physicians, 14.2% were fellows, and one-third (32.5%) were residents. The primary specialty of most participants was internal medicine (43.5%), emergency medicine (33.8%), or anesthesiology (12.6%). Nearly all internal medicine and anesthesiology attending physician respondents (94.4%) completed critical care fellowship training.
Table 1.
Characteristics of Survey Respondents
| Characteristics | Number (%) of Respondents (n = 317) |
|---|---|
| Primary specialtya | |
| Internal medicine | 138 (43.5%) |
| Anesthesiology | 40 (12.6%) |
| Surgery | 17 (5.4%) |
| Emergency medicine | 107 (33.8%) |
| Unknown | 16 (5.0%) |
| Completed critical care fellowship | 109 (34.4%) |
| Training level | |
| Attending physician | 154 (48.6%) |
| Fellow physician | 45 (14.2%) |
| Resident physician | 103 (32.5%) |
| Nurse practitioner or physician assistant | 13 (4.1%) |
| Unknown | 2 (0.6%) |
| Years of experience after completing training program | |
| 0–5 years | 62 (19.6%) |
| 6–10 years | 38 (12.0%) |
| 11–15 years | 17 (5.4%) |
| More than 15 years | 44 (13.9%) |
| In training | 148 (46.7%) |
| Unknown | 8 (2.5%) |
| Practice primarily in teaching hospital | 303 (95.6%) |
| Hospital type | |
| University hospital | 205 (64.7%) |
| Public hospital | 49 (15.5%) |
| Other nonprofit or private hospital | 59 (18.6%) |
| Not reported | 4 (1.3%) |
One respondent was dual trained in emergency medicine and internal medicine, and therefore both were listed as that respondent’s primary specialty.
Beliefs and Behavior in Early Volume Resuscitation for Septic Shock
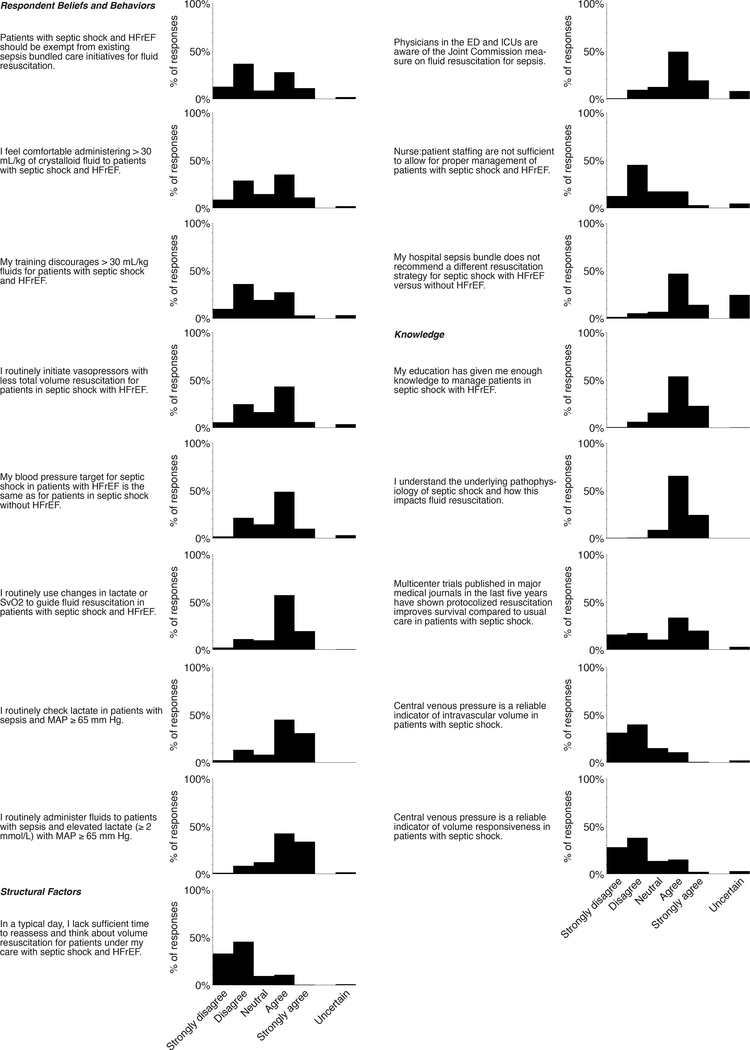
Respondents’ views and self-reported practices regarding volume resuscitation for septic shock with HFrEF demonstrated substantial heterogeneity. Four in ten respondents (39.4%) felt patients with septic shock and HFrEF should be exempt from current sepsis bundle initiatives. A similar proportion (37.5%) reported being uncomfortable administering > 30 mL/kg crystalloid to patients with septic shock and HFrEF. One-third of respondents (30.5%) indicated their training discourages administering > 30 mL/kg fluids for patients with septic shock and HFrEF. Responses to these three questions were highly co-linear, demonstrating internal consistency. Beliefs and behaviors in other aspects of sepsis resuscitation did not exhibit such heterogeneity among respondents (Figure 1).
Figure 1: Responses to Likert questions regarding beliefs, behaviors, perceived structural barriers, and knowledge of early volume resuscitation in sepsis.
Structural Factors
Awareness of sepsis resuscitation quality initiatives was high: just 10.1% of survey respondents reported emergency department and ICU physicians were unfamiliar with the Joint Commission measure on early fluid resuscitation for patients with sepsis (Figure 1). Only 7.0% of respondents indicated their hospital sepsis care bundle recommends a different volume resuscitation strategy for septic shock with HFrEF compared to without HFrEF.
Knowledge Regarding Sepsis Resuscitation
Most respondents perceived having sufficient training and understanding of sepsis pathophysiology and volume resuscitation (Figure 1). Confirmatory knowledge testing indicated most respondents did not believe central venous pressure was a reliable indicator of intravascular volume or volume responsiveness in septic shock, beliefs consistent with the existing body of literature [15, 18, 24]. However, most respondents were unaware that multiple recent multicenter trials have not shown a benefit to protocolized resuscitation versus usual care for septic shock [19–21].
Appropriate Volume Challenge for Septic Shock by Diagnosis of HFrEF
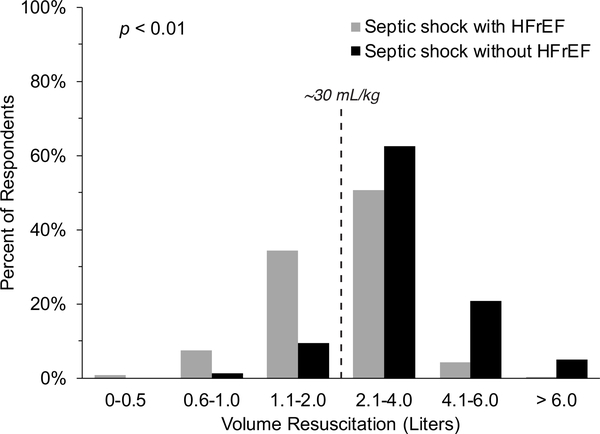
The majority of respondents (56.1%) indicated they would give significantly less fluid in the first six hours for patients in septic shock with HFrEF, compared to those without HFrEF, before deeming the patient volume non-responsive (Figure 2). Nearly 90% of respondents felt that septic shock patients without HFrEF should receive a fluid challenge of ~30 ml/kg or more before determining volume non-responsiveness, but only 56.4% felt that volume appropriate for patients in septic shock with concomitant HFrEF (p < 0.01) (Figure 2).
Figure 2: Respondents’ views regarding appropriate initial volume resuscitation for septic shock, with or without concomitant HFrEF, needed before deeming the patient non-responsive to fluid challenge.
Gray bars indicate HFrEF. Black bars indicate no HFrEF. HFrEF indicates heart failure with reduced ejection fraction.
Factors Associated with Volume Challenge of at least ~30 mL/kg in Septic Shock with HFrEF
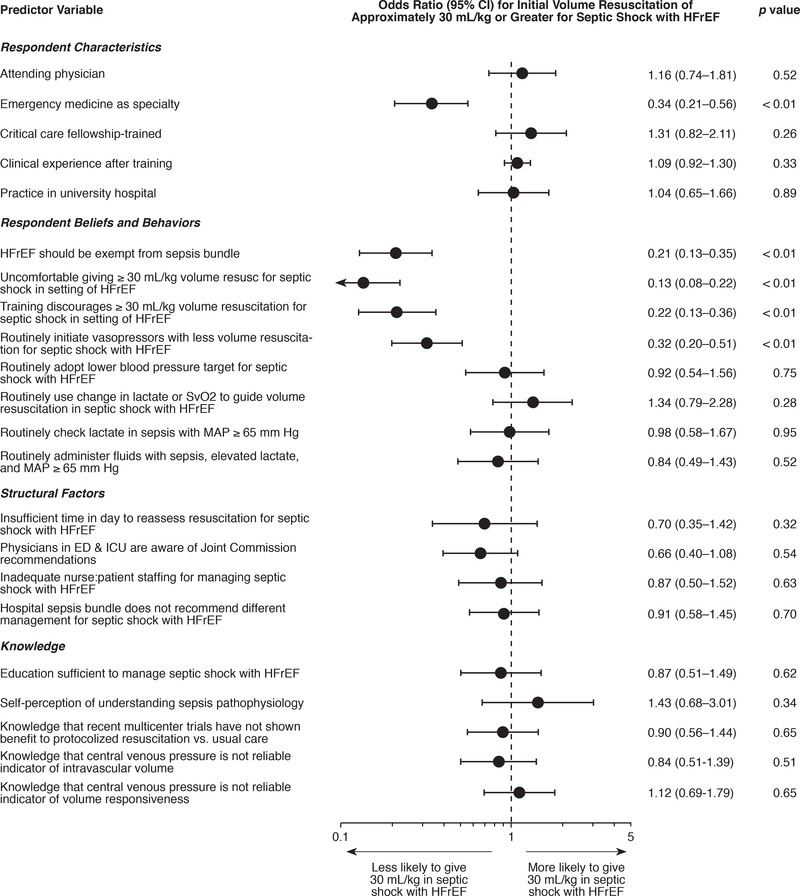
Specialty was associated with the belief that a ~30 ml/kg or greater volume challenge was appropriate in septic shock with HFrEF (p < 0.01). In particular, compared to all other respondents, emergency medicine clinicians were significantly less likely to consider a volume challenge of at least ~30 mL/kg appropriate (odds ratio (OR) 0.34, 95% confidence interval (CI) 0.21–0.56; p < 0.01) (Figure 3). This association of emergency medicine with preferred volume challenge < 30 mL/kg for septic shock with HFrEF remained statistically significant when analysis was restricted to attending physicians only (OR 0.49, 95% CI 0.25-.97; p = 0.04), and also when analysis was restricted to physicians in training programs only, i.e. internship, residency, or fellowship (OR 0.23, 95% CI 0.11–0.48; p < 0.01). When compared only to internal medicine respondents, the association between emergency medicine and volume challenge threshold lower than ~30 mL/kg also remained significant (OR 0.31, 95% CI 0.18–0.53; p < 0.01).
Figure 3: Factors associated with belief that patients with septic shock and HFrEF should receive approximately 30 mL/kg or greater initial volume resuscitation within the first six hours before being labeled fluid non-responsive.
Odds ratios and p-values were calculated from univariable logistic regression models. HFrEF indicates heart failure with reduced ejection fraction.
Respondents’ belief that HFrEF should be exempt from the sepsis bundle, discomfort administering 30 mL/kg volume challenge in HFrEF, lower threshold for initiating vasopressors, and prior training recommending a lower challenge volume threshold for HFrEF also all were associated with the position that a volume challenge < ~30 mL/kg was appropriate for management of patients in septic shock with HFrEF (Figure 3). Each of these beliefs and behaviors were highly correlated with training in emergency medicine (p < 0.01).
Other sepsis resuscitation practices were not associated with HFrEF volume challenge threshold, including routine use of screening lactate for non-hypotensive cryptogenic shock and use of lactate/central venous oxygen saturation to help guide volume resuscitation. Neither perceived knowledge nor confirmatory knowledge testing responses were associated with a ~30 mL/kg volume challenge threshold for septic shock with HFrEF.
Discussion
Early volume resuscitation is a cornerstone of therapy for severe sepsis and septic shock. Current international guidelines strongly recommend administering 30 mL/kg of crystalloid within the first three hours of presentation for patients with sepsis and either hypotension or elevated lactate. However, there has been some controversy regarding the appropriateness of the 30 mL/kg fluid bolus for such patients and there is some evidence that this quantity of fluid maybe harmful [4, 25]. Our multicenter survey of clinicians who routinely care for such patients reveals strikingly divergent views regarding appropriateness of the recommended 30 mL/kg volume challenge for patients with septic shock who have concomitant HFrEF. Nearly half (43.6%) of respondents believed a volume challenge less than the recommended 30 mL/kg was more appropriate in patients with septic shock and concomitant HFrEF without signs of volume overload at initial presentation, and a similar proportion believed patients with HFrEF should be exempt from sepsis bundle initiatives. In contrast, near consensus existed for this volume threshold in patients without HFrEF.
This survey provides a current, large-scale evaluation of clinician practice patterns, knowledge, and perceptions on early volume resuscitation for patients with septic shock and HFrEF. Other surveys of sepsis resuscitation have not considered patient subgroups, such as those with concomitant HFrEF, in whom clinician beliefs and behaviors may differ [26, 27]. Prior research has identified several potential contributors toward physician non-adherence to practice guidelines, including lack of awareness and familiarity, lack of agreement, presence of external barriers, inability to overcome inertia of previous practice, and lack of motivation [11, 12]. Critical care providers have identified previously that patient complexity, guideline complexity, and lack of necessary time to follow recommendations are common reasons for guideline non-adherence [28]. In contrast, our data reveal that widely held concerns regarding potential harm of volume overload in HFrEF compete directly with sepsis resuscitation initiatives, are engrained in clinician training, and influence self-reported practice preferences for septic shock, particularly among emergency medicine specialists. Disagreement with appropriateness of international guidelines for patients with concomitant HFrEF may impede adherence to the recommended minimum volume resuscitation.
The optimal fluid resuscitation strategy in patients with sepsis-associated hypoperfusion and concomitant HFrEF is uncertain and not well studied. Current guidelines do not account for HFrEF or other high-risk subpopulations and yet note the poor quality of evidence supporting initial volume resuscitation recommendations for sepsis generally. Observational data have yielded conflicting findings on the potential benefit of initial resuscitation with 30 cc/kg [6, 7, 29–32]. To date, no prospective trials have been published to guide practice in the subpopulation of patients with HFrEF. The ongoing Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial (NCT03434028) is designed to determine the impact of a conservative versus liberal fluid resuscitation strategy in patients with sepsis-induced hypotension and does not mandate 30 mL/kg initial resuscitation. CLOVERS may offer higher evidence to guide risk/benefit of aggressive early volume resuscitation for sepsis generally, but applicability of findings to patients with concomitant HFrEF may remain uncertain if not explicitly tested. Pending results of CLOVERS, additional studies are merited to determine the ideal fluid resuscitation strategy in subpopulations with unique risk/benefit profiles for fluid overload and vasopressors.
Limitations
We acknowledge several potential limitations to our study. Self-reported clinician behaviors may not accurately reflect true practice management. Social desirability bias would risk over-reporting toward guideline adherence, an effect that anonymization of responses was intended to alleviate. Still, equipoise in clinician beliefs and self-reported behaviors was found. The moderate response rate to our survey raises the possibility of non-responder bias in precision of effect estimates, but the degree of heterogeneity in clinician beliefs and behaviors captured in our study suggests at least a substantial proportion of clinicians share dissimilar views and practice behaviors. Missing responses among those who completed the survey was low. Our study included nine university-affiliated hospitals in the U.S., potentially limiting generalizability to clinicians in other practice environments. The survey instrument did not address other relevant features of HFrEF patients (e.g. NYHA classification, extremely low ejection fraction, presence of ventricular assist device, pertinent exam findings) that might influence early hemodynamic management in septic shock. Finally, we focused the survey on patients with HFrEF, but similar considerations may exist in other subpopulations. Risks of aggressive volume resuscitation may be greatest in patients most susceptible to volume overload (e.g. HFrEF, cirrhosis, severe kidney dysfunction) and those in whom pulmonary edema threatens greater morbidity (e.g. respiratory insufficiency at risk of failing current level of breathing support). Our study suggests such patient-specific factors are important determinants of clinicians’ preferred volume resuscitation strategy, but we did not explore morbidities other than HFrEF.
Conclusions
In conclusion, equipoise exists among clinicians in beliefs and behaviors regarding appropriate initial volume resuscitation in patients with septic shock and HFrEF. Nearly half of clinician respondents held that a volume challenge less than the 30 mL/kg was appropriate for determining volume responsiveness in patients with septic shock and concomitant HFrEF, contrary to international guidelines. Future studies are merited to determine the role for individualizing initial volume resuscitation for septic shock in patients with concomitant HFrEF and other high-risk comorbidities.
Supplementary Material
Acknowledgments
Funding: Dr. Beitler has the following grant NIH K23 HL133489, which in part supported this research. Dr. Owens has received consulting fees from Novartis (unrelated to the current research) and has the following grant NIH/NHLBI R01 HL142114-01, which in part funded this research. Dr Montesi has grant funding from the Parker B. Francis Foundation and the Scleroderma Foundation (unrelated to current research). Dr. Bose has received funding from the Department of Defence, unrelated to the current research. Dr. Rahaghi has the following grant NIH/NHLI K23 HL136905. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Abbreviations
- HFrEF
heart failure with reduced ejection fraction
- OR
Odds ratio
- CI
Confidence interval
Footnotes
Competing interests
Dr. Wardi is on the editorial board of PEER and has received a speaker’s fee from Thermo Fisher Scientific (unrelated to current research). Dr. Owens has received consulting fees from Novartis (unrelated to the current research). Dr. Tolia has received funding from West Health and consulting fees from Roche and Change Healthcare, although unrelated to this manuscript.
Declarations
Institutional review board approval (IRB # 161260) was obtained prior to distribution, and consent for participation was obtained from all respondents.
Consent for publication: not applicable.
Availability of data: Data is not freely available at this time however are available from Dr. Wardi on reasonable request.
References
- 1.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44(6):925–8. [DOI] [PubMed] [Google Scholar]
- 2.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–65. [DOI] [PubMed] [Google Scholar]
- 3.Neyra JA, Li X, Canepa-Escaro F, Adams-Huet B, Toto RD, Yee J, et al. Cumulative Fluid Balance and Mortality in Septic Patients With or Without Acute Kidney Injury and Chronic Kidney Disease. Crit Care Med. 2016;44(10):1891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA. 2017;318(13):1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitland KKS, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B. Mortality after fluid bolus in African children with severe infection. New England Journal of Medicine. 2011;364(26):2483–95. [DOI] [PubMed] [Google Scholar]
- 6.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017;376(23):2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu VX, Morehouse JW, Marelich GP, Soule J, Russell T, Skeath M, et al. Multicenter Implementation of a Treatment Bundle for Patients with Sepsis and Intermediate Lactate Values. Am J Respir Crit Care Med. 2016;193(11):1264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B, Strait KM, Dharmarajan K, Li SX, Mody P, Partovian C, et al. Intravenous fluids in acute decompensated heart failure. JACC Heart Fail. 2015;3(2):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marik P, Bellomo R. A rational approach to fluid therapy in sepsis. Br J Anaesth. 2016;116(3):339–49. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen RW, Kasatpibal N, Riis A, Norgaard M, Sorensen HT. The impact of pre-existing heart failure on pneumonia prognosis: population-based cohort study. J Gen Intern Med. 2008;23(9):1407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennison CR, Mendez-Tellez PA, Wang W, Pronovost PJ, Needham DM. Barriers to low tidal volume ventilation in acute respiratory distress syndrome: survey development, validation, and results. Crit Care Med. 2007;35(12):2747–54. [DOI] [PubMed] [Google Scholar]
- 12.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PAC, & Rubin HR Why don’t physicians follow clinical practice guidelines?: A framework for improvement. Journal of the American Medical Association. 1999;282(15):1458–65. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox SR, Seigel TA, Strout TD, Schneider JI, Mitchell PM, Marcolini EG, et al. Emergency medicine residents’ knowledge of mechanical ventilation. The Journal of emergency medicine. 2015;48(4):481–91. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary. Journal of the American College of Cardiology. 2013;62(16):1495–539. [DOI] [PubMed] [Google Scholar]
- 15.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774–81. [DOI] [PubMed] [Google Scholar]
- 16.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskesen TG, Wetterslev M, Perner A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2016;42(3):324–32. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Critical Care Medicine. 2004;32(3):691–9. [DOI] [PubMed] [Google Scholar]
- 19.Investigators A, Group ACT, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–506. [DOI] [PubMed] [Google Scholar]
- 20.Pro CI, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–11. [DOI] [PubMed] [Google Scholar]
- 22.Karabenick SA, Woolley ME, Friedel JM, Ammon BV, Blazevski J, Bonney CR, et al. Cognitive Processing of Self-Report Items in Educational Research: Do They Think What We Mean? Educational Psychologist. 2007;42(3):139–51. [Google Scholar]
- 23.Eysenbach G Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shippy C, Appel PL, Shoemaker WC. Reliability of clinical monitoring to assess blood volume in critically ill patients. Critical Care Medicine. 1984;12(2):107–12. [DOI] [PubMed] [Google Scholar]
- 25.Klompas M, Rhee C. Current Sepsis Mandates Are Overly Prescriptive, and Some Aspects May Be Harmful. Critical care medicine. 2018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.McIntyre LA, Hébert PC, Fergusson D, Cook DJ, Aziz A, Group tCCCT. A survey of Canadian intensivists’ resuscitation practices in early septic shock. Critical Care. 2007;11(4):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djurkovic SBJ, Guerra JA, Sartorius J, Haupt MT. A survey of clinicians addressing the approach to the management of severe sepsis and septic shock in the United States. Journal of Critical Care. 2010;25(4):658e1–e6. [DOI] [PubMed] [Google Scholar]
- 28.Sinuff T, Cook D, Giacomini M, Heyland D, Dodek P. Facilitating clinician adherence to guidelines in the intensive care unit: A multicenter, qualitative study. Critical Care Medicine. 2007;35(9):2083–9. [DOI] [PubMed] [Google Scholar]
- 29.Ouellette DR, Shah SZ. Comparison of outcomes from sepsis between patients with and without pre-existing left ventricular dysfunction: a case-control analysis. Crit Care. 2014;18(2):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duttuluri MRK, Shapiro J, Mathew J, Jean R, Kurtz S, Mckenna K, Salonia J, Khouli H. Fluid resuscitation dilemma in patients with congestive heart failure presenting with severe sepsis/septic shock. InD45. Critical care: circulatory hemodymanics, shock, cardiovascular disease, and fluid management. American Thoracic Society. 2016:A7048. [Google Scholar]
- 31.Singh HIM, Sachdev S, Simmons B, Rabines A, Hassen GW. . The Effect of Initial Volume Resuscitation for Sepsis in Patients with Congestive Heart Failure: Is it Associated with Higher Mortality. Journal of Cardiac Failure 2016;1(22):S54–5. [Google Scholar]
- 32.Wardi GWA, Sell R, Malhotra A, Beitler J. Impact Of Fluid Resuscitation On Septic Patients With Systolic Heart Failure. Critical Care Medicine. 2016;44(12):446.26771791 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.