Abstract
Purpose:
To investigate the role of sex on retinal nerve fiber layer (RNFL) thickness at 768 circumpapillary locations based on OCT findings.
Design:
Population-based cross-sectional study.
Participants:
We investigated 5646 eyes of 5646 healthy participants from the Leipzig Research Centre for Civilization Diseases (LIFE)-Adult Study of a predominantly white population.
Methods:
All participants underwent standardized systemic assessments and ocular imaging. Circumpapillary RNFL (cRNFL) thickness was measured at 768 points equidistant from the optic nerve head using spectral-domain OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany). To control ocular magnification effects, the true scanning radius was estimated by scanning focus. Student t test was used to evaluate sex differences in cRNFL thickness globally and at each of the 768 locations. Multivariable linear regression and analysis of variance were used to evaluate individual contributions of various factors to cRNFL thickness variance.
Main Outcome Measures:
Difference in cRNFL thickness between males and females.
Results:
Our population consisted of 54.8% females. The global cRNFL thickness was 1 μm thicker in females (P < 0.001). However, detailed analysis at each of the 768 locations revealed substantial location specificity of the sex effects, with RNFL thickness difference ranging from −9.98 to +8.00 μm. Females showed significantly thicker RNFLs in the temporal, superotemporal, nasal, inferonasal, and inferotemporal regions (43.6% of 768 locations), whereas males showed significantly thicker RNFLs in the superior region (13.2%). The results were similar after adjusting for age, body height, and scanning radius. The superotemporal and inferotemporal RNFL peaks shifted temporally in females by 2.4° and 1.9°, respectively. On regions with significant sex effects, sex explained more RNFL thickness variance than age, whereas the major peak locations and interpeak angle explained most of the RNFL thickness variance unexplained by sex.
Conclusions:
Substantial sex effects on cRNFL thickness were found at 56.8% of all 768 circumpapillary locations, with specific patterns for different sectors. Over large regions, sex was at least as important in explaining the cRNFL thickness variance as was age, which is well established to have a substantial impact on cRNFL thickness. Including sex in the cRNFL thickness norm could therefore improve glaucoma diagnosis and monitoring.
Glaucoma is an optic neuropathy characterized by a progressive loss of retinal ganglion cells (RGCs) and their axons, which make up the retinal nerve fiber layer (RNFL), leading to increased cupping of the optic disc and eventual vision loss. Although structural evaluation of the optic disc is important for the diagnosis of glaucoma, it is limited by the interindividual variability of the optic nerve head (ONH) anatomic features.1 In addition, standard perimetry has limited usefulness early in the disease because it may detect visual field deficits only after significant RGC loss.2 However, the pattern of circumpapillary RNFL (cRNFL) thickness allows the detection of early changes in the RNFL and has been recognized as a hallmark for both diagnosing and monitoring progression in glaucomatous diseases.3–5
OCT is a high-resolution cross-sectional imaging technique that can measure RNFL thickness objectively.6 To facilitate glaucoma diagnostics, commercial OCT machines provide normative databases for RNFL thickness and flag areas in individual retinal scans that deviate from the population-based norm. In determining the norm, current databases take age into consideration, but do not consider other factors such as ethnicity, refractive error, or sex. It is well established that increased age is correlated with RNFL thinning.7,8 We and others have also shown the association of the cRNFL thickness profile with ocular magnification,9,10 ethnicity,11,12 and scanning circle displacement13 and radius.14 These studies support the notion that incorporating these other parameters into the normative cRNFL thickness models may improve glaucoma diagnostics and monitoring.
Sex is another parameter that is readily available to clinicians yet not included in the existing normative database for OCT. The observed sex variations in the prevalence of glaucoma15 suggest that physiologic differences may exist between males and females, particularly in their RNFL anatomic characteristics. Previous studies have investigated the association of sex with RNFL thickness with mixed findings. Wang et al,16 Khawaja et al,17 and Wang et al18 showed that male sex was associated significantly with decreased RNFL thickness in children16 and adults,17,18 whereas others have found no significant difference between RNFL thickness and sex.8,12,19 The variability of these results may be related to the different population sizes included in the studies, because the latter studies are based on fewer than 350 participants. However, another important limitation is the restriction of circumpapillary locations by coarsely defined sectors. Most of the current literature on sex differences compare the global RNFL thickness means between males and females and divide the locations into at most 6 sectors, neglecting the high location specificity of cRNFL thickness over the full scanning circle. A more detailed, comprehensive evaluation of sex differences with regard to RNFL thickness would help us to better understand the RNFL anatomic characteristics and evaluate the need to establish a sex-specific normative database.
In this study, we investigated the sex variations in the full cRNFL thickness profile measured by spectral-domain (SD) OCT at 768 circumpapillary locations using a large German population-based dataset. Additionally, we examined the effects of various systemic and ocular factors, including age, body height, estimated OCT scanning circle radius, and major RNFL thickness peak locations, to evaluate for their relative contributions to RNFL thickness variance.
Methods
Participants
This population-based study, the Leipzig Research Centre for Civilization Diseases (LIFE)-Adult Study, was conducted by the Leipzig Research Centre for Civilization Diseases at Leipzig University from August 2011 through November 2014.20 Ten thousand participants were selected randomly from half a million residents in the city of Leipzig in the eastern part of Germany. Signed consent forms were obtained for enrollment. The study was approved by the ethical committee at the Medical Faculty of Leipzig University and adhered to the tenets of the Declaration of Helsinki and all federal and state laws.
The LIFE-Adult Study recruitment occurred in an age- and sex-stratified manner, with a main focus on participants from 40 to 80 years of age. The overall population consisted of 400 participants between 19 and 39 years of age and 9600 participants between 40 and 80 years of age. Each age interval (by decade) was balanced with respect to the number of participants and sex. To this end, 6 of the 19-year-old participants were counted in the 20- to 30-year age group.
Data Collection
Each participant underwent a standardized assessment including structured interviews, questionnaires, physical examinations, and blood and urine testing. As part of the ophthalmic assessment, SD OCT imaging (Spectralis; Heidelberg Engineering, Heidelberg, Germany) was performed, yielding cRNFL thickness scans with a resolution of 768 points equidistant from the ONH. The location of the cRNFL thickness circle and the coordinate system used in this study is illustrated in Figure 1. Additionally, fundus images (Nidek AFC-230; Oculus, Wetzlar, Germany) of undilated eyes were obtained from all participants. All images were read by 2 independent, ophthalmologically trained observers who classified retinal or optic nerve abnormalities based on current standards.
Figure 1.
Schematic diagram of the circumpapillary retinal nerve fiber layer thickness (cRNFLT) measurement circle around the optic nerve head (ONH), superimposed on a fundus image of a right eye. The coordinate system begins at the temporal (T) pole on the disc–fovea axis (0°) and traverses clockwise to the superior (S), nasal (N), and inferior (I) locations. The locations of the greatest RNFLT on the circle, that is, the superotemporal (ST) and inferotemporal (IT) peaks, are denoted by red asterisks at the intersections of the circle and the retinal nerve fiber bundle trajectories. The angle between these 2 peaks is referred to as the interpeak angle, marked by the dashed arrow arc.
Participant Inclusion and Exclusion Criteria
For the current analysis, the 10 000 baseline participants of the LIFE-Adult Study underwent the following exclusion process, as illustrated by the flowchart in Supplemental Material S1 (available at www.aaojournal.org). In the beginning phase of the LIFE-Adult Study, the Spectralis SD OCT machine was incapacitated by a hardware defect. Therefore, OCT imaging could not be performed for 471 participants while the device was being repaired. The hardware defect also affected the database of existing scans, and despite all efforts, several measurements of participants or single eyes were partially damaged and could not be exported from the machine. As a result, OCT measurements of 931 participants were entirely unavailable for analysis.
Of the remaining 17 974 eyes of 9069 participants, 2678 participants were excluded based on clinically significant findings on ophthalmic imaging. More specifically, OCT and fundus images were analyzed by 2 independent, experienced, and clinically trained observers. If there was a disagreement between the 2 observers, a consensus was reached to classify the participant’s eye. Clinical and subclinical ophthalmic findings were graded based on current ophthalmologic standards. Participants were excluded from the current analysis if disease of the posterior eye was present in at least 1 eye, for example, retinal detachment or hole, edema, bleeding, retinal pigment epithelium detachment, tumor, staphyloma, scarring, atrophy, fundus with disseminated white areas, cotton-wool spots, or fibrosis in cases of traction or puckering with foveal involvement. Within the macular region, the following additional exclusion criteria applied: age-related macular degeneration stage 3 and 4 and maculopathy unrelated to age-related macular edema (stage 5), as previously described by the Gutenberg Health Study20 and based on the Rotterdam classification.21,22 For the ONH, participants were further excluded if any of the following were present: excavation (suspected glaucoma [i.e., violation of the inferior-superior-nasal-temporal rule, vertically oval with cup-to-disc ratio >0.7], optic disc pit, or coloboma of the optic disc), bleeding, neovascularization, optic atrophy, sectoral paleness, ONH swelling, papilledema, or optic disc drusen. Furthermore, participants were excluded for any signs of vascular abnormalities, for example, vascular occlusion, ischemia, bleeding, aneurysm, tortuosity, or neovascularization.
Of the remaining 6319 participants, 613 were excluded because of a diagnosis of glaucoma reported in the interview, and a further 38 participants were excluded because of the presence of glaucoma medication among the medications taken by the participant at the time of the test. Furthermore, 94 participants were excluded because of unreliable OCT measurements. The SD OCT image reliability criteria were: B-scan number per location of 50 or more, signal-to-noise ratio of 20 dB or more, and missing or unreliable RNFL thickness A-scans of 5% or less. If both eyes of an included participant were reliable, 1 eye was selected randomly. Ultimately, 5646 eyes of 5646 participants remained for further analysis. More details regarding the data collection methods and inclusion and exclusion criteria can be found in our recent publication9 and the original description of the LIFE-Adult Study.23
Statistical Analysis
All statistical analyses were performed in R environment using version 3.4 or higher (R Foundation for Statistical Computing, Vienna, Austria). Student t tests were performed to evaluate for sex differences in the participants’ demographic background as well as in cRNFL thickness, both globally and at each of the 768 angular locations. The effect of sex was then corrected for age, scanning circle radius, and body height via multivariable linear regression. Scanning radius was included as an independent variable because eye size and lens effects have been observed to influence the RNFL thickness measurement.24,25 More specifically, the Spectralis OCT machine emits a fixed scanning circle with a radius of 6°, or approximately 1.75 mm, into the ONH region to measure the RNFL thickness. The ocular magnification effects of the eyes, such as corneal curvature, ametropia, and axial length, can affect the true circle size from the aforementioned theoretical value. A larger axial length, for example, would result in a larger scanning circle radius. A potential difference in eye size or lens anatomic features between males and females could therefore affect the RNFL thickness measurement. The true scanning circle radius (in millimeters) is estimated from the focus settings used by the Spectralis machine, according to a widely used model.26
For the purposes of comparing our results with the current standard 6-sectorial RNFL thickness report, the sex differences in cRNFL thickness were averaged over each of the 6 regions: temporal (T), superotemporal (ST), superonasal (SN), nasal (N), inferonasal (IN), and inferotemporal (IT). Univariable and multivariable linear regression analyses were carried out as above.
To investigate the relevance of sex in comparison with other parameters, we designed an analysis of variance (ANOVA) method to determine the respective part of variance explained by sex but unexplained by each of the other parameters, namely, age, scanning circle radius, body height, major RNFL thickness peak locations, and interpeak angle. More specifically, at each of the 768 locations, a linear regression model was calculated with sex and each of these variables as a regressor. Then, we calculated the ratio of the variance explained by sex relative to the total explained variance (i.e., the variance explained by both variables together). For instance, choosing age as an additional independent variable, we determined independently for each of the 768 circumpapillary locations the amount of variance explained by sex relative to the variance explained by age according to the standard ANOVA formula given in the following equation:
We assumed a significant impact of sex if the P value of the sex coefficient was smaller than 0.05. The false discovery rate method was used for P value correction for the 768 multiple comparisons. For calculating the average variance explained by sex but unexplained by each other variable, we averaged over all significant locations after correction for multiple comparisons.
The locations of the 2 greatest RNFL thicknesses on the OCT scanning circle, that is, the ST and IT peaks (Fig 1), were extracted using a custom developed algorithm. First, a moving average filter of ± 12° was applied at each of the 768 locations to remove random noise. The ST and IT peaks were then determined as the 2 local, smoothed RNFL thickness maxima on the temporal side.
Results
Demographic Information
A total of 5646 eyes from 5646 participants (3093 females and 2553 males) from the population-based LIFE-Adult Study were selected for analysis according to our criteria. The population was predominantly white, consisting of 5583 (98.9%) European persons, 13 Arabic persons, 10 Central Asian persons, 5 East Asian persons, 4 African persons, 5 Latin American persons, and 26 persons of mixed or other races. The ethnicity was defined based on the country of birth of both parents as reported by the participant. Of the selected eyes, 2825 (50%) were right eyes.
Figure 2 shows the histograms of age, body height, scanning radius, global RNFL thickness, and the IT and ST RNFL thickness peak locations by sex. Since the LIFE-Adult Study focused on participants between 40 and 80 years of age, as detailed in “Methods,” there were fewer participants younger than 40 years. Although the number of participants older than 40 years was evenly distributed in the original dataset, the number of selected participants in our study decreased with age after exclusion of retinal and optic nerve disease on imaging, self-reported diagnosis of glaucoma, and presence of glaucoma medications, because these findings were more common in the elderly groups.
Figure 2.
Histograms showing age, body height, scanning radius, retinal nerve fiber layer thickness (RNFLT) mean, and the locations of inferotemporal and superotemporal peaks in males (M; blue) and females (F; red). SD = standard deviation.
Table 1 summarizes the demographic, systemic, and ocular characteristics in both sex groups. The mean age was 55.30 ± 12.5 years for males and 54.48 ± 11.8 for years females (P = 0.012). Males were on average 12.8 cm taller than females (P < 2.2×10−16). The scanning circle radius was 0.006 mm longer in females compared with males (P = 0.002). The average cRNFL thickness over all 768 locations was 96.74 ± 9.58 mm in males and 97.74 ± 9.29 μm in females; the global RNFL thickness was 1.00 μm thicker in females compared with males (P = 7.66 × 10−5). Additionally, the RNFL thickness peak locations varied significantly by sex: the IT peaks of females were 1.9° more T than those of males (P = 1.18×10−9), and the ST peaks of females were 2.4° more T than those of males (P = 1.11 × 10−19).
Table 1.
Comparison of Demographic, Systemic, and Ocular Characteristics for Male and Female Particpants
| Male | Female | Male: Female | P Value | |
|---|---|---|---|---|
| Age (yrs) | 55.30 | 54.48 | 0.82 | 0.012 |
| Body height (cm) | 177.2 | 164.4 | 12.8 | <2.2×10−16 |
| Scanning radius (mm) | 1.751 | 1.757 | −0.006 | 0.002 |
| Mean RNFL thickness (μm) | 96.74 | 97.74 | −1.00 | 7.66×10−5 |
| Inferotemporal peak (°) | 286.9 | 288.8 | −1.9 | 1.18×10−9 |
| Superotemporal peak (°) | 72.61 | 70.18 | 2.43 | <2.2×10−16 |
RNFL = retinal nerve fiber layer.
Average Retinal Nerve Fiber Layer Thickness and Retinal Nerve Fiber Layer Thickness Difference at 768 Angular Locations by Sex
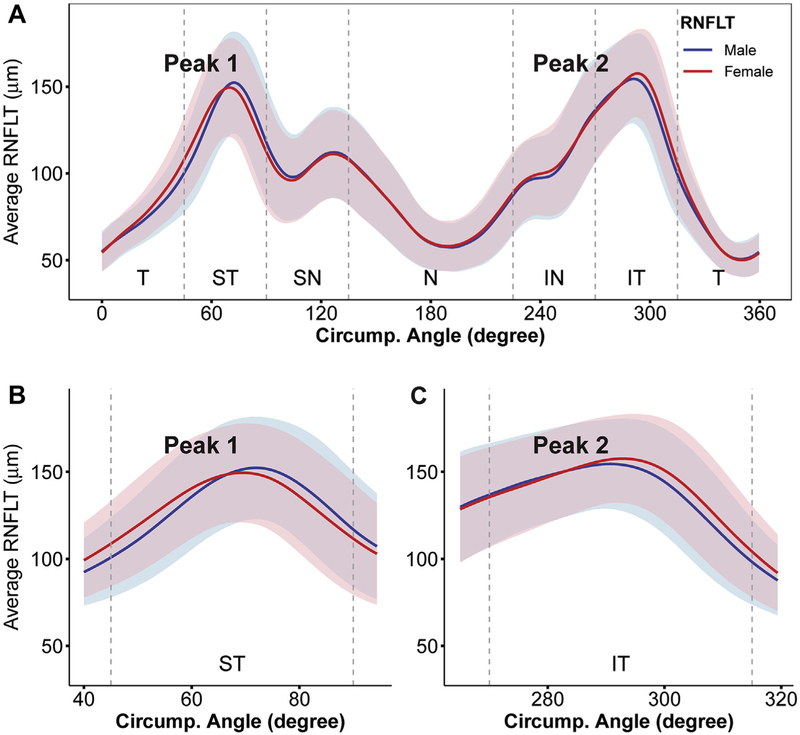
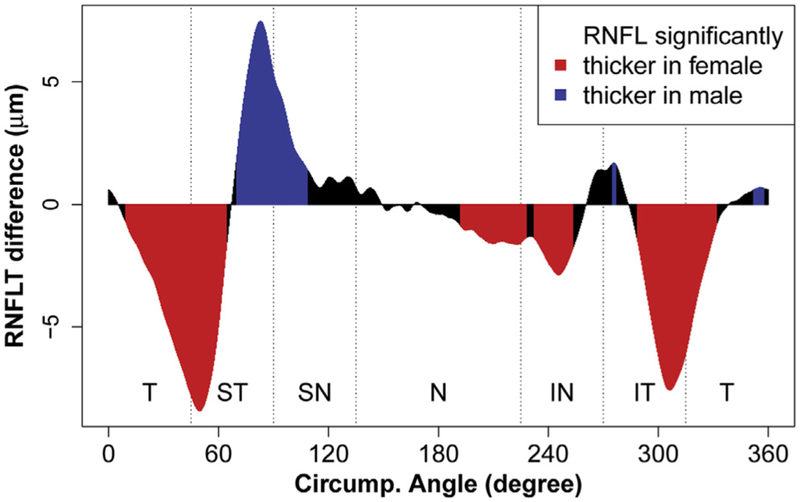
Next, a more detailed look at cRNFL thickness was obtained by analyzing the average cRNFL thickness at each of the 768 angular locations around the scanning circle. Figure 3 plots the mean±standard deviation curve for cRNFL thickness in both males and females. Two major RNFL peaks were observed in the ST and IT regions in a double-hump configuration, where the shift between males and females appeared to be most prominent. The pointwise difference in cRNFL thickness by sex was then calculated, as illustrated in Figure 4. Females showed a significantly greater RNFL thickness (P < 0.05; red) in the T, ST, N, IN, and IT regions (43.6% of all the 768 locations). Males showed a significantly greater RNFL thickness (P < 0.05; blue) primarily in the S region (13.2% of all locations). The RNFL thickness difference ranged from −8.42 μm (at the angular location of 49.7°) to +7.48 μm (at 82.5°), with a positive value corresponding to a thicker RNFL in males (data not shown).
Figure 3.
A, Graph showing average circumpapillary retinal nerve fiber layer thickness (RNFLT; in micrometers) at 768 angular locations for males (blue line) and females (red line). Standard deviations are denoted by the light blue shade for males and light pink shade for females. B, C, Magnified views of the superotemporal (ST) and inferotemporal (IT) peaks. IN = inferonasal; N = nasal; SN = superonasal; T = temporal.
Figure 4.
Graph showing the average circumpapillary retinal nerve fiber layer thickness (RNFLT) difference (in micrometers) between males and females at each of the 768 angular locations. The red regions indicate where RNFLT was significantly greater in females (P < 0.05), and the blue regions indicate where RNFLT was significantly greater in males (P < 0.05) after adjustment for multiple comparisons. IN = inferonasal; IT = inferotemporal; N = nasal; SN = superonasal; ST = superotemporal; T = temporal.
Multivariable Linear Regression Analysis Combining Sex, Age, Scanning Circle Radius, and Body Height
A multivariable linear regression analysis was carried out based on the following analyses. As shown in Figure 2 and Table 1, small but significant differences in age and scanning circle radius between males and females studied were evident in our population. Body height also differed significantly between males and females, as expected. Multivariable linear regression was used to account for the possible effects of these variables on the observed sex differences in cRNFL thickness. The regression coefficients for sex at each of the 768 locations are listed in Supplemental Material S2 (available at www.aaojournal.org), where a positive coefficient corresponds to a thicker RNFL thickness in males. After adjusting for age, scanning radius, and body height, females were found to show a significantly greater RNFL thickness at 41.5% of the locations, whereas males showed a significantly higher RNFL thickness at 8.7% of the locations (P < 0.05; Supplemental Material S3, available at www.aaojournal.org). The regional distribution of significance remained similar to that in Figure 4. After correction, the RNFL thickness difference ranged from −9.98 μm (at the angular location of 50.6°) to +8.00 μm (at 83.0°), with a positive value corresponding to a thicker RNFL in males (Supplemental Material S2).
Regression Analyses for 6-Sector Retinal Nerve Fiber Layer Thickness versus Sex
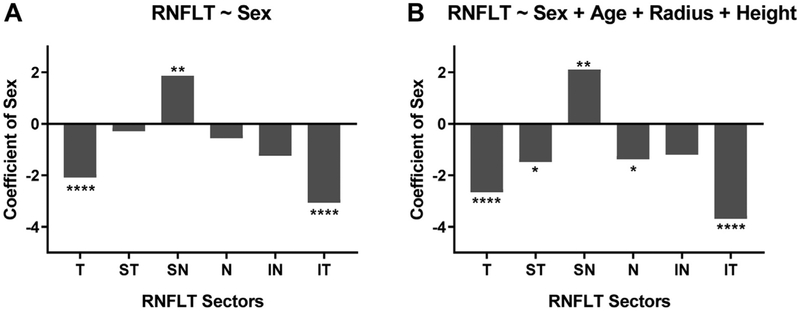
Furthermore, in keeping with the format of the current clinical RNFL thickness report, the average sex difference in RNFL thickness at each of the 6 sectors (T, ST, SN, N, IN, and IT) was analyzed using univariable and multivariable linear regression. When sex alone was treated as independent variable, females showed a significantly higher RNFL thickness in regions T and IT (P < 0.0001), whereas males showed a significantly higher RNFL thickness in region SN (P < 0.01; Fig 5A). When multivariable linear regression was applied to adjust for the effects of age, scanning radius, and body height, females were found to have a significantly higher RNFL thickness in regions ST and N (P < 0.05) in addition to regions T and IT (P < 0.0001); males continued to show a significantly higher RNFL thickness in SN (P < 0.01; Fig 5B).
Figure 5.
A, Graph showing linear regression between 6-sector retinal nerve fiber layer thickness (RNFLT) and sex. B, Graph showing multivariable linear regression for 6-sector RNFLT and sex with correction for the effect of age, scanning circle radius, and body height. In both graphs, males are coded as 1 and females are coded as 0. The y-axis denotes the regression coefficient for sex only. Significance level: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001. IN = inferonasal; IT = inferotemporal; N = nasal; SN = superonasal; ST = superotemporal; T = temporal.
Variance Explained by Sex, But Unexplained by Other Parameters
As detailed in “Methods,” we carried out multivariable linear regression analyses of RNFL thickness = [independent variable] + sex and performed ANOVA to further investigate the contribution of each of the 6 factors (age, scanning radius, body height, superior and inferior peak locations, and interpeak angle) to the RNFL thickness variance in comparison with sex. Figure 6 shows the location-specific percent variance in RNFL thickness that was left unexplained by each independent variable. In a regression model of age and sex, age accounted for 46.4% and sex accounted for 53.6% of the explained RNFL thickness variance. That is, sex explained 7.2% more RNFL thickness variance overall compared with age. In a regression model of scanning radius and sex, scanning radius accounted for 66.5% and sex accounted for 33.5% of the explained RNFL thickness variance. In a regression model of body height and sex, body height accounted for 26.5% and sex accounted for 73.5% of the explained RNFL thickness variance. In regression models of peak locations and sex, the superior and inferior peak locations accounted for 94.9% and 93% of the explained RNFL thickness variance, respectively, and the interpeak angle accounted for 96% of the variance. In addition to the relative variance, we calculated the absolute percent variance of RNFL thickness explained by sex and by other parameters, which is shown in Supplemental Material S4 (available at www.aaojournal.org).
Figure 6.
Graphs showing the ratio of variance unexplained by each variable when fitting a linear regression model with sex to predict circumpapillary retinal nerve fiber layer thickness, as explained by the standard analysis of variance (ANOVA) formula in “Methods.” The 2 identical subplots in the first row were added as a spatial marker and illustrate the locations where retinal nerve fiber layer thickness (RNFLT) is significantly thicker in males (blue) or females (red). The other 6 subplots in rows 2 through 4 depict ANOVA results at these significant locations for age, scanning radius, body height, superotemporal peak location, inferotemporal peak location, and interpeak angle, respectively. The percent variance plots (i.e., the black shaded areas in each subplot) denote the percentages of variance explained by sex that was not already explained by the respective parameter. For instance, in the age subplot (second row, first column), a percent variance value of 60% means that for the respective circumpapillary location, 60% of the explained variance of the regression model RNFLT equals approximately age plus sex was explained by sex and 40% by age. Locations without significant sex effects (i.e., all locations shown in black in the top row) were omitted in this analysis. IN = inferonasal; Inf. = inferior; IT = inferotemporal; N = nasal; SN = superonasal; ST = superotemporal; Sup. = superior; T = temporal.
Discussion
In this large population-based sample of 5646 participants, we demonstrated substantial effects of sex on cRNFL thickness unexplained by a number of other parameters. Averaged over the entire scanning circle, global cRNFL thickness was significantly thicker in females compared with males. This observation is consistent with previous studies16–18 showing the association of male sex with a thinner RNFL. In a study of 1565 participants, Wang et al16 reported that the average RNFL thickness was 1.0 μm thicker in girls than boys, which is consistent with our finding that females showed on average 1.0-μm thicker RNFL than males. The European Prospective Investigation of Cancer (EPIC)-Norfolk Eye Study17 (11 030 eyes of 6309 participants) found that males on average showed 0.44-μm thinner global RNFL thickness compared with females; this study used scanning laser polarimetry (GDx VCC, Carl Zeiss, Meditec Inc, Dublin, CA) instead of OCT, which may explain the slight discrepancy in the magnitude of RNFL thickness difference. The Beijing Eye Study18 (n = 1654) also reported that female sex was associated significantly with greater RNFL thickness. Others have reported no significant sex differences in RNFL thickness8,12,19; however, these studies are limited by their small population sizes ranging from 92 to 300 participants.
Although global averaging allows a comparison with previous studies, our detailed data analysis on each of the 768 single locations reveals that this approach may yield misleading results, because we demonstrated a considerable location specificity of the sex effects, with specific areas where the effects were in opposite directions, so that global or large-sector averaging may reduce or even cancel out the relevant effects. In particular, females exhibited a significantly greater RNFL thickness compared with males at more than 40% of the retinal locations, primarily in the T, ST, N, IN, and IT regions. Males, however, showed a significantly greater RNFL thickness than females at a much smaller proportion of locations (13%), which fell mostly in the ST and SN regions.
Using an exceptionally high spatial resolution, we described for the first time the sex differences in RNFL thickness at each circumpapillary location, which could not be carried out adequately in previous studies focusing on global RNFL thickness or limited sectors. Although, to our best knowledge, no previous study operated with a spatial resolution as high as our 768 measurement points, Wang et al16 reported, using the 6-sector RNFL measurement, that girls showed a significantly thicker RNFL in the T, IT, N, and SN regions, but found no significant sex differences in the ST and IN regions. This regional distribution of significance is overall similar to, but does not entirely agree with, our results. The discrepancy may be because our study population focused on adults between 40 and 80 years of age, and the location-specific sex differences may change with age. More importantly, however, it demonstrates the limitation of analyzing RNFL thickness on coarsely defined sectors. For example, although we showed significant sex differences in the ST and IN regions using a spatial resolution of 768 points (Fig 4), our 6-sectorial univariable analysis did not reveal a significance in the ST or IN regions (Fig 5A) because it represents the average of all the points in those regions. Thus, if females harbored thicker RNFL in one part of the region and males in another part, as observed in regions ST and IN, the effects could cancel out one another and the true RNFL thickness difference would be missed.
In accordance with our criticism of global averaging detailed previously, we would like to emphasize the relative size of sex effects at single locations versus globally. The global RNFL thickness difference, although significant, was only 1 μm. However, at the angular location of 50.6°, the RNFL was 9.98 μm thicker in females compared with males (Supplemental Material S2). To put this number into perspective, we can compare it with the effect of age, which is well known to have a significant impact on RNFL thickness. Our previous work using the same dataset9 indicates that at this particular location, cRNFL thickness is expected to decrease by 0.32 μm per year. In the context of this finding, a difference of 9.98 μm would be equivalent to a difference of 31 years in age. This again highlights the value of using fine spatial resolution, which captures the magnitude of the sex effect in greater detail.
The finding that females in our population have a thicker RNFL on average is consistent with epidemiologic data that females have a lower incidence of primary open-angle glaucoma compared with males until 80 years of age.15 Interestingly, evidence suggests that estrogen exposure protects against the development of glaucoma, and this seems to be at least in part the result of estrogen’s protective effect on the RNFL.27 In animal models of glaucoma, the neuroprotective effect of estrogen is thought to be mediated through estrogen receptors on RGCs, because administration of estrogen preserved RGCs and retinal nerve fibers.28,29 Furthermore, postmenopausal females who used hormone replacement therapy demonstrated greater RNFL thickness compared with females who did not,30 providing further evidence that estrogen exposure protects against loss of RNFL and slows the aging of the optic nerve. Taken together, these data suggest a potential biological explanation for the observed anatomic differences in RNFL between males and females in our study.
Several factors could potentially affect the RNFL thickness as confounding variables. Age and scanning radius, for example, have been shown to affect cRNFL thickness.9,14 Body height is another factor that has been associated with RNFL thickness,17 and given the obvious sex differences in height, one might assume that this could explain the observed RNFL thickness difference. However, even after adjusting for these variables via multivariable linear regression, sex was still noted to exert a significant effect on RNFL thickness, and the regional distribution of significance remained very similar to that before correction (Fig 4; Supplemental Material S2). The areas of significance did decrease slightly after adjusting for these variables; females showed significantly thicker RNFL at 2.1% fewer locations, and males showed significantly thicker RNFL at 4.5% fewer locations. These small variations likely represent areas where the sex difference in RNFL thickness was accounted for by age, scanning radius, body height, or a combination thereof.
These findings are in line with the ANOVA results, which measured the individual contributions of various factors to the RNFL thickness variance in comparison with that of sex. We compared sex with age, scanning radius, and body height one at a time and found that the mean variance in RNFL thickness that was unexplained by these variables was rather large (Fig 6), indicating that these factors did not play a major role in the observed RNFL thickness variance.
The current work is of high relevance for both scientists and OCT manufacturers because it provides insight into improving the existing RNFL thickness norms. Currently, age is well established to have a substantial impact on RNFL thickness and is therefore the only factor included in the RNFL thickness norm used by commercial OCT machines. However, our results show that overall, sex explains more RNFL thickness variance (53.6%), as does age (46.4%). In some circumpapillary retinal locations (e.g., S and N regions), the ratio of variance explained by sex to that explained by age is much higher (Fig 6), suggesting that sex matters substantially more than age in predicting the RNFL thickness in these locations. Overall, a large amount of RNFL thickness variance can be explained by sex that is unexplained by age. These findings, coupled with the fact that sex is a readily available parameter to clinicians, justify the notion that in addition to age, sex could be included in the RNFL thickness norm to diagnose and monitor progression of glaucoma in both sex groups more accurately.
Interestingly, we found that the major RNFL thickness peak locations differed significantly in males and females, with females having ST and IT peaks temporally shifted by 2.4° and 1.9°, respectively. This led to a significantly smaller interpeak angle in females. The peak locations and interpeak angle each explained more than 90% of the relative variance when predicting RNFL thickness together with sex (Fig 6). Furthermore, they explained a substantial amount of the absolute RNFL thickness variance (Supplemental Material S4). These findings are important because they provide a direction for future research: they suggest that peak measures may serve as even better parameters for RNFL thickness norms compared with sex. However, one shortcoming of using peak locations is that with glaucomatous change and RNFL thinning, the locations of physiologic peaks become unreliable. A potential solution may be to use the major retinal blood vessel locations in lieu of peak locations. Prior works have demonstrated the relationship between blood vessel locations and RNFL thickness profiles.31,32 We also previously showed that the major retinal vessel locations likely remain stable regardless of glaucoma severity.33 Thus, future work could investigate the relationship between blood vessel locations and sex, as well as the feasibility of including blood vessel location as a parameter in normative RNFL thickness databases in place of sex.
It is known that ocular magnification effects resulting from axial length, specific lens properties, or both have a substantial impact on OCT RNFL thickness measurements. Magnification effects, that is, the true scanning radius of the OCT scan, are not directly accessible but can be approximated by parameters like axial length or, as in our study, the scanning focus. Various previous studies showed that increased axial length is correlated with thinner RNFL, which has been explained by a larger true scanning circle, causing the measurement to be obtained from retinal locations more distant from the optic nerve, where the RNFL is naturally thinner.10,34 Consistent with this, in the LIFE-Adult Study, we previously showed a strong relationship between thinner RNFL and larger scanning radius estimated by focus.9 Therefore, it is of particular interest if the sex-specific effects on RNFL thickness shown herein are affected by sex-related magnification differences. Because of the substantial sex-related differences in body height, one might assume a larger ocular axial length in males, and therefore, because increased axial length is correlated with magnification, a larger scanning radius in males. Because RNFL is naturally thinner at retinal locations more distant from the optic nerve, one therefore might expect thicker (uncorrected) RNFL in females compared with males.
Results from previous population-based studies seem to support inconsistent conclusions. Some studies indeed report larger axial lengths in males (see Table 2 in Chen et al35 for a recent overview). However, these differences are typically small and are not significant in other studies when combined with further systemic parameters in multiple regressions.36,37 Moreover, ocular magnification is a combination of axial length and lens-related effects, and several previous population-based studies showed significantly higher rates of myopia in females.38–43 Based on this, and contrary to the expectations based on axial length noted previously, one alternatively might assume that females harbor larger ocular magnification (i.e., larger scanning radius) compared with males. Consistent with the latter, we found a slightly (but significantly) larger scanning radius in females. If magnification effects were to impact sex-specific RNFL thickness differences, we therefore would expect slightly thinner RNFL in females. However, interestingly, even when uncorrected for magnification effects, females showed significantly thicker RNFL in our study. At the same time, magnification effects by themselves (regardless of sex) were considerable, and larger scanning radius was strongly associated with thinner global RNFL in our study population.9 In total in the present study, magnification effects explained substantially more RNFL thickness variance than sex (66.5% vs. 33.5% of the explained variance; see “Variance Explained by Sex, But Unexplained by Other Parameters,” above). After correcting for these magnification effects, RNFL thickness remained substantially larger in females. To summarize, first, consistent with previous works, larger scanning circle was strongly associated with thinner global RNFL in our study population. Second, we found slightly larger scanning radii in females compared with males. Third, we also found significantly thicker RNFL in females. This means that although the association between scanning radius and RNFL thickness observed in our population clearly reflects well-known ocular magnification effects, the presence of thicker RNFL in females, even when uncorrected for magnification, indicates sex-related physiologic differences in RNFL thickness that work in the opposite direction from the effects of ocular magnification on RNFL thickness.
This study has several limitations. First, the study population consisted mainly of white (European) participants, and the number of nonwhite participants was negligible. Therefore, the findings may not be generalizable to populations of different ethnicities. Second, only one type of OCT machine, the Spectralis, was used to obtain the RNFL scans. However, it is worth noting that similar trends were found in other large-population studies consisting of various ethnic groups and based on different imaging methods.16–18 Another limitation is that the analyzed LIFE-Adult Study dataset did not include refractive error or axial length, factors that not only may influence RNFL thickness peaks but have also been used to estimate ocular magnification effects in some previous studies. Therefore, we used the true scanning radius, estimated from the OCT focus settings, as an approximation of magnification effects. The focus, which is readily available from the OCT machine, combines the effects of axial length and possible lens-related effects. It remains unclear how the focus as a surrogate for magnification compares with the previously used surrogates, namely axial length and refractive error, which are known to be closely related.24 We assume that the focus may offer an advantage over using axial length alone, because it immediately includes lens-related effects, but further studies are needed to compare these parameters directly in how they approximate OCT magnification effects.
To highlight the timely nature of this research, the current findings can be linked to an ongoing discussion on recent machine learning results by which sex could be predicted from fundus photographs with an area under the receiver operating characteristic curve (AUC) of 0.97,44 indicating general sex-related anatomic retinal differences. Although our data are not fundus images, but rather results of retinal layer segmentations in depth, and although we focus on only a very small part of the retina, namely, a single circle around the ONH, our findings indicate that such sex-specific retinal differences are also at least partially reflected in cRNFL thickness. We additionally performed a logistic regression of our 768 circumpapillary locations to predict sex statistically and received an area under the receiver operating characteristic curve of 0.77. It is likely that an appropriate deep learning algorithm for RNFL thickness on a larger part of the retina would improve the area under the receiver operating characteristic curve substantially. Future work using deep learning to predict sex from RNFL thickness and other anatomic markers, such as blood vessel locations, may improve our understanding of the sex-specific differences in the structure of the eye.
In conclusion, we described the differences in cRNFL anatomic features between females and males at an unprecedented level of detail using a large, predominantly white, population-based dataset with high spatial resolution. We demonstrated that, although females have a 1-μm thicker global average RNFL than males, the sex impacts are very location specific, so that global or large-sector averaging may cancel out the relevant effects. When compared with age, sex was found to explain more of the RNFL thickness variance overall at a number of retinal locations, suggesting that current RNFL thickness norms may benefit from incorporating sex into the database. Because the major RNFL thickness peak locations were temporally shifted in females and explained most of the RNFL thickness variance, future work can focus on the feasibility of including peak locations, or the closely related retinal blood vessel locations, in the normative database in place of sex.
Supplementary Material
Acknowledgments
The authors thank the LIFE-Adult Study participants for their time and the LIFE-Adult Study team for their commitment to the eye investigation and corresponding examinations that made this analysis possible.
Financial Disclosure(s):
The author(s) have made the following disclosure(s): M.W.: Patent - 036770-571001WO, 036770-572001WO, No. 62/804,903.
T.E.: Patent - 036770-571001WO, 036770-572001WO, No. 62/804,903.
Supported by the LIFE Leipzig Research Center for Civilization Diseases, Leipzig University, Leipzig, Germany (LIFE is funded by the European Union, the European Social Fund, the European Regional Development Fund, and Free State Saxony’s excellence initiative); Lions Foundation, Massachusetts; Grimshaw-Gudewicz Foundation, Fall River, Massachusetts; Research to Prevent Blindness, Inc., New York, New York; Bright-Focus Foundation, Clarksburg, Maryland; Alice Adler Fellowship, Boston, Massachusetts; the National Institutes of Health, Bethesda, Maryland (grant nos.: K99EY028631, R21 EY030142, R21EY030631, R01EY030575, and core grant P30EY003790); and Federal Ministry of Education and Research (BMBF), Bonn, Germany; i:DSem-Integrative Data Semantics in Systems Medicine (grant no.: 031L0026).
Abbreviations and Acronyms:
- ANOVA
analysis of variance
- cRNFL
circumpapillary retinal nerve fiber layer
- IT
inferotemporal
- LIFE
Leipzig Research Centre for Civilization Diseases
- N
nasal
- IN
inferonasal
- ONH
optic nerve head
- RGC
retinal ganglion cell
- RNFL
retinal nerve fiber layer
- SD
spectral-domain
- SN
superonasal
- ST
superotemporal
- T
temporal
Footnotes
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Leipzig University approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
References
- 1.Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992;99(2):215–221. [DOI] [PubMed] [Google Scholar]
- 2.Kuang TM, Zhang C, Zangwill LM, et al. Estimating lead time gained by optical coherence tomography in detecting glaucoma before development of visual field defects. Ophthalmology. 2015;122(10):2002–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109(1):77–83. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. Arch Ophthalmol. 1982;100(1): 135–146. [DOI] [PubMed] [Google Scholar]
- 5.Guedes V, Schuman JS, Hertzmark E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology. 2003;110(1):177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendschneider D, Tornow RP, Horn FK, et al. Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. J Glaucoma. 2010;19(7):475–482. [DOI] [PubMed] [Google Scholar]
- 8.Bowd C, Zangwill LM, Blumenthal EZ, et al. Imaging of the optic disc and retinal nerve fiber layer: the effects of age, optic disc area, refractive error, and gender. J Opt Soc Am A. 2002;19(1):197–207. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Elze T, Li D, et al. Age, ocular magnification, and circumpapillary retinal nerve fiber layer thickness. J Biomed Opt. 2017;22(12):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jun J-H, Lee S-Y. The effects of optic disc factors on retinal nerve fiber layer thickness measurement in children. Korean J Ophthalmol. 2008;22(2):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho H, Tham Y-C, Chee ML, et al. Retinal nerve fiber layer thickness in a multiethnic normal Asian population: the Singapore Epidemiology of Eye Diseases Study. Ophthalmology. 2019;126(5):702–711. [DOI] [PubMed] [Google Scholar]
- 12.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114(6):1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabriele ML, Ishikawa H, Wollstein G, et al. Optical coherence tomography scan circle location and mean retinal nerve fiber layer measurement variability. Invest Opthalmol Vis Sci. 2008;49(6):2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savini G, Barboni P, Carbonelli M, Zanini M. The effect of scan diameter on retinal nerve fiber layer thickness measurement using Stratus optic coherence tomography. Arch Ophthalmol. 2007;125(7):901–905. [DOI] [PubMed] [Google Scholar]
- 15.Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002;109(6):1047–1051. [DOI] [PubMed] [Google Scholar]
- 16.Wang C-Y, Zheng Y-F, Liu B, et al. Retinal nerve fiber layer thickness in children: the Gobi Desert Children Eye Study. Invest Ophthalmol Vis Sci. 2018;59(12):5285–5291. [DOI] [PubMed] [Google Scholar]
- 17.Khawaja AP, Chan MPY, Garway-Heath DF, et al. Associations with retinal nerve fiber layer measures in the EPIC-Norfolk Eye Study. Invest Opthalmol Vis Sci. 2013;54(7): 5028–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YX, Pan Z, Zhao L, et al. Retinal nerve fiber layer thickness. The Beijing Eye Study 2011. PLoS One. 2013;8(6): e66763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salchow DJ, Oleynikov YS, Chiang MF, et al. Retinal nerve fiber layer thickness in normal children measured with optical coherence tomography. Ophthalmology. 2006;113(5): 786–791. [DOI] [PubMed] [Google Scholar]
- 20.Korb CA, Kottler UB, Wolfram C, et al. Prevalence of age-related macular degeneration in a large European cohort: results from the population-based Gutenberg Health Study. Graefes Arch Clin Exp Ophthalmol. 2014;252(9): 1403–1411. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen R, Klaver CCW, Vingerling JR, et al. The risk and natural course of age-related maculopathy. Arch Ophthalmol. 2003;121(4):519. [DOI] [PubMed] [Google Scholar]
- 22.Klaver CCW, Assink JJM, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42(10): 2237–2241. [PubMed] [Google Scholar]
- 23.Loeffler M, Engel C, Ahnert P, et al. The LIFE-Adult-Study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health. 2015;15:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang SH, Hong SW, Im SK, et al. Effect of myopia on the thickness of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(8):4075–4083. [DOI] [PubMed] [Google Scholar]
- 25.Hirasawa K, Shoji N, Yoshii Y, Haraguchi S. Determination of axial length requiring adjustment of measured circumpapillary retinal nerve fiber layer thickness for ocular magnification. PLoS One. 2014;9(9):e107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garway-Heath DF, Rudnicka AR, Lowe T, et al. Measurement of optic disc size: equivalence of methods to correct for ocular magnification. Br J Ophthalmol. 1998;82(6): 643LP–649LP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuzzi R, Scalabrin S, Becco A, Panzica G. Sex hormones and optic nerve disorders: a review. Front Neurosci. 2019;13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo R, Cavaliere F, Watanabe C, et al. 17b-Estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res. 2008;173:583–590. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Li F, Ge J, et al. Retinal ganglion cell protection by 17-b-estradiol in a mouse model of inherited glaucoma. Dev Neurobiol. 2007;67(5):603–616. [DOI] [PubMed] [Google Scholar]
- 30.Deschênes MC, Descovich D, Moreau M, et al. Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Invest Opthalmol Vis Sci. 2010;51(5):2587. [DOI] [PubMed] [Google Scholar]
- 31.Hood DC, Salant JA, Arthur SN, et al. The location of the inferior and superior temporal blood vessels and interindividual variability of the retinal nerve fiber layer thickness. J Glaucoma. 2010;19(3):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hood DC, Fortune B, Arthur SN, et al. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma. 2008;17(7): 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Jin Q, Wang H, et al. The interrelationship between refractive error, blood vessel anatomy, and glaucomatous visual field loss. Transl Vis Sci Technol. 2018;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung CK-S, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: interpreting the RNFL maps in healthy myopic eyes. Invest Opthalmol Vis Sci. 2012;53(11):7194. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Lin H, Lin Z, et al. Distribution of axial length, anterior chamber depth, and corneal curvature in an aged population in South China. BMC Ophthalmol. 2016; 16(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bikbov MM, Kazakbaeva GM, Gilmanshin TR, et al. Axial length and its associations in a Russian population: the Ural Eye and Medical Study. PLoS One. 2019;14(2):e0211186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin G, Wang YX, Zheng ZY, et al. Ocular axial length and its associations in Chinese: the Beijing Eye Study. PLoS One. 2012;7(8):e43172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matamoros E, Ingrand P, Pelen F, et al. Prevalence of myopia in France: a cross-sectional analysis. Medicine (Balt). 2015;94(45):e1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu C, Pan C, Zhao C, et al. Prevalence and risk factors for myopia in older adult east Chinese population. BMC Ophthalmol. 2017;17(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin LL, Shih YF, Tsai CB, et al. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci. 1999;76(5):275–281. [DOI] [PubMed] [Google Scholar]
- 41.Quek TPL, Chua CG, Chong CS, et al. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic Physiol Opt. 2004;24(1):47–55. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994;35(13):4344–4347. [PubMed] [Google Scholar]
- 43.Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983;101(3): 405–407. [DOI] [PubMed] [Google Scholar]
- 44.Poplin R, Varadarajan AV, Blumer K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2(3):158–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.