Abstract
Rationale.
Electrophysiological studies show that systemic nicotine narrows frequency receptive fields and increases gain in neural responses to characteristic-frequency stimuli. We postulated that nicotine enhances related auditory processing in humans.
Objectives.
The main hypothesis was that nicotine improves auditory performance. A secondary hypothesis was that the degree of nicotine-induced improvement depends on the individual’s baseline performance.
Methods.
Young (18–27 years old), normal-hearing, non-smokers received nicotine (Nicorette gum, 6 mg) or placebo gum in a single-blind, randomized, crossover design. Subjects performed four experiments involving tone-in-noise detection, temporal gap detection, spectral ripple discrimination and selective auditory attention before and after treatment. The perceptual differences between post-treatment nicotine and placebo conditions were measured and analyzed as a function of the pre-treatment baseline performance.
Results.
Nicotine significantly improved performance in the more difficult tasks of tone-in-noise detection and selective attention (effect size=−0.3) but had no effect on relatively easier tasks of temporal gap detection and spectral ripple discrimination. The two tasks showing significant nicotine effects further showed no baseline-dependent improvement.
Conclusions.
Nicotine improves auditory performance in difficult listening situations. The present results support future investigation of nicotine effects in clinical populations with auditory processing deficits or reduced cholinergic activation.
Introduction
Nicotine is known to affect muscular, neuronal, cardiovascular, gastrointestinal and other systems’ activities and functions. In the mouse auditory cortex, a systemic nicotine injection increases the gain and shortens latency near the center of a neuron’s receptive field while decreasing the gain at the edges of the receptive field (Askew et al. 2017; Intskirveli and Metherate 2012; Kawai et al. 2011). This “sharpening” in the receptive field by nicotine may also act as a ‘stimulus filter’ to enhance attentional gain to task-relevant stimuli while reducing the gain to task-irrelevant stimuli (Kassel 1997). Physiological studies in both the visual and auditory systems support this stimulus-filter model (Disney et al. 2007; Metherate et al. 2012).
In comparison, only a few human studies found enhanced selective attention by nicotine in non-smoking healthy human subjects (e.g., Behler et al. 2015; Heishman et al. 2010; Knott et al. 2009; Lawrence et al. 2002). The present study attempts to bridge the knowledge gap between perceptual effects of nicotine on sensory processing in humans and the established physiological effects of nicotine in animals. We designed four experiments to probe the effect of nicotine on four different aspects of auditory perception, including (1) central gain in tone-in-noise detection, (2) temporal resolution in gap detection, (3) spectral resolution in spectral ripple discrimination, and (4) reaction time in selective attention. A group of young healthy non-smokers participated in the study by consuming either 6-mg nicotine chewing gum or a non-nicotine placebo gum having the same flavor as the nicotine gum. Our main hypothesis was that compared with the placebo gum, the nicotine gum would improve auditory performance. A secondary hypothesis was that those with the lowest baseline performance would produce the most improvement from nicotine (Knott et al. 2014a; Knott et al. 2015; Newhouse et al. 2004).
Materials and Methods
Subjects
Eighteen individuals were recruited, and a total of 14 subjects participated in the study (age range=18–27 years, mean±std=21±3 years; 9 males; 12 right-handed). All subjects gave written informed consent approved by the University of California, Irvine’s Institutional Review Board. All subjects received monetary compensation for their participation. An online survey facilitated initial subject screening to ensure no known hearing dysfunction, medical or mental health illness including drug dependency, diabetes mellitus, renal failure, cardiovascular disease, neurological disease, psychiatric disorder, central nervous system disorder, or regular use of prescription medication (excluding oral contraceptives), and low nicotine dependence via use and exposure consisting of a score of 0–2 out of 10 maximum on the Fagerström index of smoking dependency (Bramer and Kallungal 2003; Heatherton et al. 1991). Twelve subjects had no smoking history, i.e., smoked no more than 100 cigarettes in their lifetime and none in the past year (Knott et al. 2014a), and two smoked socially, i.e., smoked no more than 1 cigarette per week or 4 per month. To avoid chemical interactions, all subjects were asked to abstain from the following prior to testing: (1) drug use for ≥3 days, (2) alcohol consumption for 24 hours, and (3) food consumption ≥1 hour. To avoid caffeine withdrawal in regular caffeine-consumers, one half cup of a caffeine-containing beverage ≥1 hour was permitted (Lawrence et al. 2002). At the beginning of each session, female subjects took a pregnancy test to confirm negative results for continued participation. Eligible subjects had audibility ≤20 dB HL (decibels Hearing Level) at octave frequencies between 0.125 and 8 kHz, bilaterally. Data from four individuals were excluded from further analysis due to elevated audibility (1 subject) or incomplete treatment sessions (3 subjects), leaving 14 subjects whose data were analyzed.
Experimental protocol
All experiments took place in a double-walled, sound-attenuated booth. The tone-in-noise detection experiment measured the central gain of the stimulus-filter model, which would predict lower, or better tone detection thresholds with the nicotine treatment. We chose two pure tones at 2000 and 4000 Hz, and a pink noise as a masker, since it would produce a similar degree of masking between the tones (Fletcher 1938). The pink noise had a center frequency of 2828 Hz, a bandwidth of 5 octaves, and was fixed at 50 dB SPL. All stimuli had a duration of 500 ms, including 2.0-ms linear rise-fall times. A three-interval, three-alternative, forced-choice adaptive procedure was used to measure the tone-in-noise detection threshold. During each trial, two of the three intervals contained the noise alone, and a randomized interval contained the tone embedded in noise. Subjects had to select the interval in which they perceived the tone. The tone threshold was measured with two different starting levels at 45 and 70 dB SPL. A two-down, one-up decision rule was used to estimate the 71% correct performance level.
The gap detection experiment measured temporal resolution within the same perceptual channel or between two different channels (Phillips et al. 1997). For the within-channel condition, a temporal gap was marked by two tones of the same frequency (2 or 4-kHz). For the between-channel condition, the temporal gap was marked by two tones of different frequencies (2:4 kHz or 4:2 kHz). Gap detection thresholds were measured for tones presented at 45 and 70 dB SPL, or ~10 and 40 dB SL in the presence of 50 dB SPL pink noise. The pink noise was used to minimize spectral splatter and would not interfere with gap detection. The duration of the two tones varied between 125 and 250 ms, depending on the gap duration, to produce a total stimulus duration of 500 ms. The above-mentioned adaptive procedure was used to estimate the gap detection threshold.
The spectral-temporally modulated ripple test (Aronoff and Landsberger 2013) measured dynamic spectral resolution in terms of ripples per octave. The reference stimulus had 20 ripples per octave. The ripple test threshold was the number of ripples per octave that could be just discriminable from the reference stimulus. The modulation depth was set to 20 Hz and the ripple repetition rate was set to 5 Hz. All ripple stimuli had a duration of 500 ms with 100-ms linear ramps. The same adaptive procedure, except for a one-down, one-up decision rule, was used to estimate the ripple discrimination threshold.
The test of attention in listening measured the reaction time required to discriminate between two tones that were presented sequentially and had same or different frequencies or locations (Zhang et al. 2012). In the different frequency condition, the frequencies of the two tones were drawn randomly between 476 and 6188 Hz with the constraint that the two frequencies had to differ by ≥2.1 equivalent rectangular bandwidths. The tone could be located in either the left or the right ear. One test condition was to detect a frequency difference while the tone location served as the distractor, in which the subject heard two tones presented randomly to the left or right ear and had to indicate as quickly as possible whether the two tones had the same or different frequencies. The other condition was to detect a location difference while the tone frequency was the distractor, in which the subject heard two tones of same or different frequencies and had to indicated whether the two tones were presented to the same or different ears. Each condition had four possible stimulus combinations, same frequency same location, same frequency different location, different frequency same location, and different frequency different location, resulting in a total of eight data points for the subjects. The subjects could perform this selective attention task accurately with an average error rate of 5%. We also ran a control condition where neither frequency nor location was task-relevant, the subjects were instructed to press a button as soon as they heard the second tone. In all conditions, tone level varied between 70 to 85 dB SPL and tone duration varied between 100 and 300 ms. The silent interval between the two tones was fixed at 300 ms. All subjects used their dominant hand to press the response button. Before testing each experimental condition, subjects completed five practice trials with the option to repeat the practice as many times as needed to become familiar with the task. Reaction times longer than 2 s or shorter than 100 ms, which suggested lapsed attention and interrupted performance or premature responses, were excluded (~20% trials) from calculations.
Study design
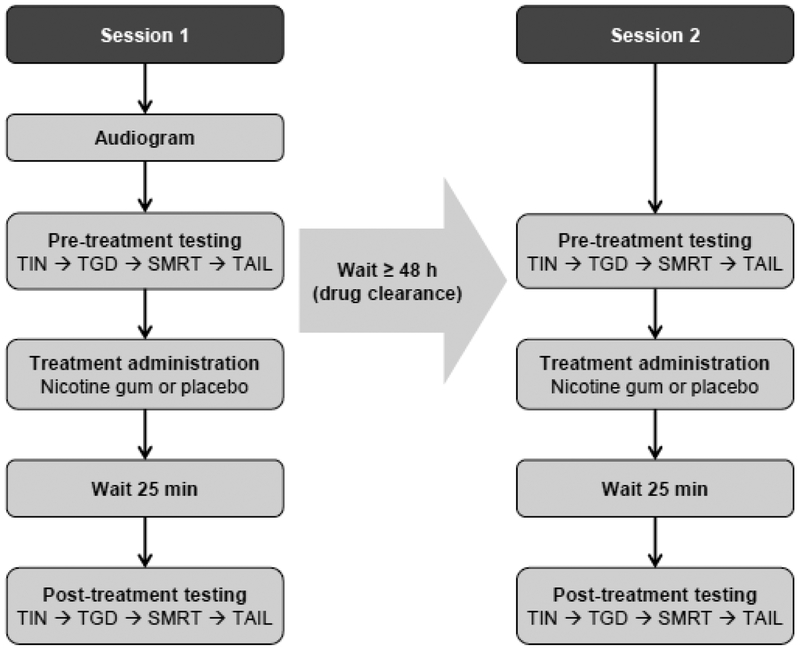
Figure 1 shows the study design in a flow chart. Sessions occurred between 8:30 am and 2:00 pm, with the majority starting before noon and taking place during a consistent time across sessions to avoid confounding arousal and attention effects. At least one day preceding each session, subjects were reminded of abstinence instruction, and verbal compliance was confirmed before testing commenced. In Session 1, audiograms were first measured, then the pre-treatment baseline performance in the four experiments was measured. After pre-treatment testing, subjects received either nicotine or placebo gum in a randomized design. The protocol was repeated with either nicotine or placebo treatment, adhering to a single-blind intra-subject design. In Session 2, ≥48 hours after Session 1 to allow for treatment clearance, the subjects completed the same tests, except for audiogram, in the same order as Session 1. Subjects participated in a minimum of two treatment options (nicotine and placebo) in one experiment, with the possibility of completing all four experiments. The order of drug administration was counterbalanced over subjects.
Figure 1.
Study design. In Session 1, subjects were first tested with audiogram, then completed pre-treatment testing in order of TIN (tone-in-noise detection), TGD (temporal gap detection), SMRT (spectral-temporally modulated ripples test), and TAIL (test of attention in listening). The subjects were then treated with either nicotine or placebo, and waited for 25 mins before the post-treatment testing in the same order. The four experiments usually took 0.5–1 h to complete. In Session 2, ≥48 h after Session 1 to allow for treatment clearance, the subjects completed the same tests, except for audiogram, in the same order as Session 1.
Nicotine was delivered in the form of two pieces of mint-flavored polacrilex gum (4 mg and 2 mg; Nicorette®, Johnson & Johnson, Inc). The total 6-mg dose produced a nicotine plasma concentration of 15–30 ng/ml, i.e., the approximate blood concentration after smoking one, medium nicotine yield, cigarette (Hukkanen et al. 2005). This dose was selected based on previous studies with non-smokers showing drug tolerance without any significant adverse side effects resulting in terminated participation (Knott et al. 2014a; Knott et al. 2014b). The placebo administration consisted of two pieces of commercially available mint-flavored gum (Eclipse®), resembling the nicotine gum in size, shape, color, and texture. Subjects wore a blindfold during treatment administration to mask any potential visual differences between placebo and nicotine gum. A drop of Tabasco sauce was added to each gum piece to disguise taste bias (Thiel and Fink 2007). Pulse rate was measured via pulse oximetry before and after treatment (Choice MMed America Co; Thiel and Fink 2007). Mood and side effects were also monitored before and after treatment (Harkrider and Hedrick 2005; Lawrence et al. 2002; Parrott et al. 1996). To regulate drug administration and minimize side effects, subjects followed manufacturer guidelines to chew the gum for 25 min, biting twice per minute and ‘parking’ the gum between teeth and cheek between bites when cued by an auditory signal. Following 25 min and prior to blind fold removal, subjects removed the treatment gum and chewed a commercially available, cinnamon-flavored gum for 2 min at the same pace as before to mask any remaining taste differences between treatments (Knott et al. 2014a). This “wash” method disguised treatment in 7 out of 10 subjects who participated in multiple experiments and 30% of the time (9 out 32 times polled). In some subjects, however, changes in mood and/or side effects from nicotine could have biased the treatment administered. Post-treatment testing began 30 min from the beginning of treatment administration considering oral nicotine exhibits peak blood nicotine concentrations 30 min after nicotine gum chewing (Hukkanen et al. 2005). All experiment protocols could be completed in 30–60 min, which is well within the time course of the 120-min nicotine elimination half-life (Hukkanen et al. 2005).
Data analysis
To test our main hypothesis that nicotine improves auditory performance, we used a one-sample t-test to compare the difference in post-treatment performance between nicotine and placebo data. We would accept the hypothesis if the difference was significantly less than zero at the p<0.05 level. We also calculated the effect size in terms of dividing the mean difference between the post-treatment nicotine and placebo conditions by the standard deviation of their joint distribution. Furthermore, we used linear regression between the nicotine-placebo difference and the baseline performance to test whether those with the lowest baseline performance would benefit the most from the nicotine treatment (Knott et al. 2015; Newhouse et al. 2004). We would accept this secondary hypothesis if significant positive linear regression existed between the nicotine-placebo difference and the baseline performance at the p<0.05 level. The baseline performance was the average of the two sets of pre-treatment data from the nicotine and placebo conditions. The average was justified because no significant difference was found between these two pre-treatment conditions in any of the four experiments (the Kolmogorov-Smirnov test, p=0.57, 0.80, 0.73, and 0.80 for the tone-in-noise detection, gap detection, ripple discrimination, and selective attention experiment, respectively).
Results
Pulse oximetry
Nicotine increased the pulse rate from a pre-nicotine level of 73.2±1.5 (beats per min) to a post-nicotine level of 80.5±1.2 (n=10; paired t-test, p<0.01), whereas the placebo produced no significant change in the pulse rate (pre-placebo=72.3±1.8; post-placebo=72.5±1.6; n=10, p>0.05). The present nicotinic effect on pulse rate was consistent with previous reports using oral nicotine (Smucny et al. 2015; Thiel and Fink 2007), and provided evidence that nicotine had indeed entered the bloodstream during the experiments.
Mood changes and side effects
All subjects provided subjective, pre- and post-treatment ratings using a 9-category mood profile and a 5-point side-effects scale (Harkrider and Hedrick 2005). Ratings were averaged across all four experiments. While nicotine increased mood ratings of energy, contentedness and focus (p<0.05), placebo also increased ratings of relaxation, calmness, energy, alertness, and hunger (p<0.05). Subjects rated nicotine’s side effects on a scale from 1 = none (no symptoms) to 5 = severe (jittery, dull or pounding headache, nausea, vomiting) (Harkrider and Hedrick 2005). Side effects increased with nicotine (p<0.01), but not placebo (p>0.05). Although no subject reported nicotine side effects higher than 3 (jittery, dull headache), the significant side effects rating in the present non-smokers further verified nicotine entry into the blood. Of the four subjects that did not complete the study, only one subject decided to discontinue participation based on nicotine side effects.
Tone-in-noise detection
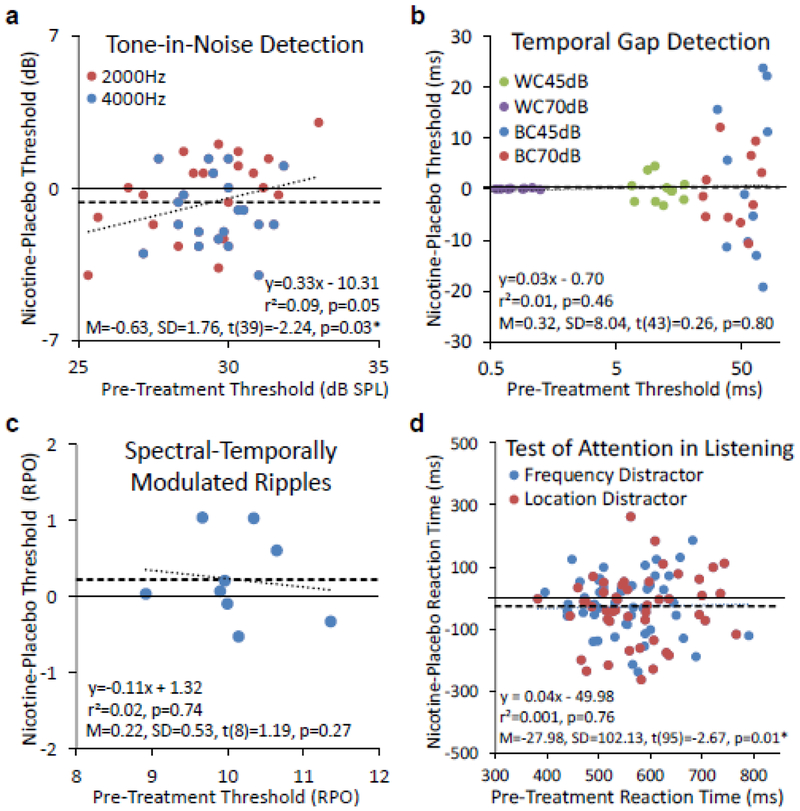
Ten subjects participated in the tone-in-noise detection experiment. Fig. 2a shows the individual subjects’ threshold difference between the nicotine and placebo post-treatment performance as a function of the pre-treatment baseline performance (the individual data being displayed as circles: red for 2000 Hz and blue for 4000 Hz). The mean difference (thick dashed horizontal line) was −0.63 dB, which was significantly lower from the 0-dB effect (thin solid horizontal line; p=0.03) and consistent with the main hypothesis that nicotine improved tone-in-noise detection. Dividing the −0.63 dB mean difference by the 2.01 dB standard deviation produced a small-to-medium effect size of −0.32. Linear regression (thick dotted line) just missed the significance level (r2=0.09; p=0.05), providing only a trend in support of the secondary hypothesis.
Figure 2.
Post-treatment placebo and nicotine difference as a function of pre-treatment baseline performance in four auditory experiments. a. Tone-in-noise detection. Individual data are represented by circles (red for 2000 Hz and blue for 4000 Hz). The mean difference is represented by the thick dashed horizontal line. The regression line is represented by the dotted line. The text box shows the linear regression equation (top), r2 and p value (middle), and the mean difference, standard deviation, and the one-sample t-test result (bottom). The same convention is applicable to panel b, c, and d. b. Temporal gap detection. WC=Within-Channel, BC=Between-Channel. c. Spectral-Temporally modulated ripple discrimination. RPO=Ripples Per Octave. d. Selective attention.
Temporal gap detection
Eleven subjects participated in the gap detection experiment. Fig. 2b shows both the individual (circles) and mean (thick dashed horizontal line) nicotine-placebo difference data as a function of pre-treatment baseline performance. Consistent with the previous result (Phillips et al. 1997), the nicotine-placebo variability increased from the within-channel (WC) to the between-channel (BC) condition and decreased with the stimulus level. We found neither a significant nicotine effect (mean=0.32 ms; effect size=0.01; p=0.80) nor a significant regression (r2=0.01; p=0.46; the regression line virtually overlaps with the mean difference line).
Spectral-temporally modulated ripples
Nine subjects participated in this spectral ripple discrimination experiment. Fig. 2c shows both the individual (circles) and mean (thick dashed horizontal line) nicotine-placebo difference data as a function of pre-treatment baseline performance. Again, we found neither a significant nicotine effect (mean=0.22 ripples per octave; effect size=0.29; p=0.27) nor a significant regression (r2=0.02; p=0.74; the dotted line).
Test of attention in listening
Twelve subjects participated in the selective attention experiment. In the control condition where the subjects did not have to pay any attention to sound frequency or location but simply pressed the response button as soon as they heard the second tone, there was no significant difference between the post-treatment nicotine and placebo performance (290±73 vs. 282±67 ms; p=0.53). Fig. 2d shows both the individual (circles) and mean (thick dashed horizontal line) nicotine-placebo difference data as a function of pre-treatment baseline performance. First, compared with the control condition, the attention task, regardless of treatment, significantly slowed the reaction time (566±83 ms; p<0.0001). Second, compared with the placebo treatment, the nicotine treatment significantly shortened the reaction time by 28 ms (effect size=−0.31; p=0.01). Third, there was no significant regression between the nicotine-placebo difference and the baseline performance (r2=0.001; p=0.76; the regression line virtually overlaps with the mean difference line).
Discussion
The present study tested the hypotheses that nicotine improves auditory processing in terms of (1) central gain, (2) temporal resolution, (3) spectral resolution, and (4) selective attention. Our results partially supported this hypothesis by showing significantly improved nicotine over placebo performance in the central gain and selective attention experiments but not in temporal and spectral resolution experiments. We found minimal evidence for the secondary hypothesis that those with lower baseline performance would benefit more from the nicotine treatment, with only a statistical trend in the central gain results.
Comparisons with previous studies
Relative to extensive animal literature, nicotine studies on human auditory processing are scarce. Harkrider and colleagues (Harkrider and Champlin 2001a; b; Harkrider et al. 2001) found that nicotine administered via a transdermal patch (7mg/24h) to non-smokers produced no effect on otoacoustic emissions but enhanced auditory brainstem and cortical responses. In a combined behavioral and electrophysiological study involving both four smokers and 10 non-smokers, Harkrider and Hedrick (2005) found nicotine produced no symptoms in one third of these smokers and non-smokers but a variety of symptoms from itchiness in the patch area to headache and nausea correlated in the remaining two thirds of the subjects. They also found a task-dependent result, showing that not only did the severity of nicotine symptoms correlate with consonant-vowel discrimination in quiet for non-smokers, but nicotine improved consonant-vowel discrimination in noise for both smokers and non-smokers.
The previous results were partially consistent with the present study, showing improved performance by nicotine in the tone-in-noise and selective attention tasks but no effect on the more basic temporal and spectral resolution measurements. The discrepancy between the previous and present results cannot be explained by participant characteristics, as both studies tested young, normal-hearing non-smokers. Instead, this discrepancy may be related to differences in task difficulty, suggesting that nicotine produces a significant effect in difficult listening situation only.
Peripheral and central mechanisms
The previous and present findings are likely a result of central rather than peripheral mechanisms. Physiologically, Harkrider et al. (2001) found no nicotine effect on otoacoustic emissions, a peripheral phenomenon related to outer hair cells in the cochlea. Instead, nicotine effects were observed in the auditory midbrain and cortex that may reflect enhanced receptive field (Askew et al. 2017). Similarly, human imaging studies have shown that nicotine enhances neural responses in hippocampus, sensory and motor cortices in healthy, non-smokers (Smucny et al. 2015) while decreasing hyperactivity in such brain areas in schizophrenic patients (Smucny et al. 2016). Although significant nicotine effects are observed in the brain, relationship of these physiological effects to perceptual effects remains unclear and understudied (Hong et al. 2011). Finally, the baseline-dependent nicotinic effect is likely due to central mechanisms (Baschnagel and Hawk 2008; Knott et al. 2014a; Knott et al. 2015; Knott et al. 2014b; Newhouse et al. 2004). However, we found minimal evidence for the baseline-dependent nicotinic effect on the present auditory processing experiments.
Limitations and future directions
First, the present study limited testing to a small number of healthy, normal-hearing young adults. The inclusion of both female and male subjects in the present study likely increased data variability and further reduced the power because sex hormones influence nicotine metabolism (Benowitz et al. 2006). In addition, the present study did not test other populations such as children, elderly, hearing-impaired individuals, or clinical patients. Future nicotine treatment could, instead, be given to special populations whose acetylcholinergic systems are either impaired or underdeveloped, for example, in Alzheimer’s patients (Levin et al. 2006; Sarter et al. 2009) or in children (Dwyer et al. 2009). Second, the present study limited testing to basic auditory processing tasks, requiring relatively low cognitive demand. Future studies should employ tasks varying in cognitive load, e.g., using an active three-stimulus auditory oddball paradigm (Knott et al. 2014a). Third, the present acute study using a single dosage had a severe time constraint which precluded a sensitive measure of within-subject variability (Zhang et al. 2012). This time constraint was related to oral nicotine administration, which might have produced insufficient nicotine serum concentration to significantly change perception as reflected by mild, self-reported side effects. Additionally, the absolute absorption rate of nicotine from gum administration may be subjected to greater variability across individuals as compared to other routes of administration, such as the transdermal patch. With oral administration, a quantity of nicotine can be swallowed and subject to first-pass metabolism rather than becoming fully absorbed via oral mucosa. Also, some of the drug may be retained within the gum instead of entering the subject’s blood stream (Hukkanen et al. 2005). To potentially produce a higher nicotine concentration or retain the drug’s effects over a longer time course while minimizing nicotinic side effects, future studies may consider varying the nicotine dosage, altering the administration route (e.g., gum, patch or inhaler), or introducing a chronic treatment condition (Myers et al. 2008; Newhouse et al. 2012).
Conclusion
The present study assessed the acute effect of oral nicotine administration on auditory processing in a group of young-adult, normal-hearing non-smokers. Compared with the placebo result, we found that nicotine significantly improved performance in tone-in-noise detection and selective attention tasks but produced no effect on relatively easy temporal and spectral resolution tasks. In the two tasks showing significant nicotinic improvement, we found little evidence for the baseline-dependent improvement. The present result supports the previous hypothesis that nicotine enhances auditory gating function, especially in difficult listening conditions. The present result suggests that future studies be conducted in younger, older, or clinical populations, where nicotine treatment could be used to target deficits in their acetylcholinergic systems relative to healthy young adults.
Acknowledgements
The authors thank Barbara Bodenhoefer, Sahara George, and Zekiye Onsan for subject recruitment assistance, Dr. Thomas Lu for technical assistance, Dr. Jonathan Venezia for statistical assistance, and the reviewers for helpful suggestions. This research was supported by grants from the National Institutes of Health to FGZ (5R01DC015587), to RM (4R01-DC013200) and a pre-doctoral fellowship to CQP (UL1-TR000153).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest
F.G.Z. owns stock in Axonics, Nurotron, Syntiant and Velox Biosystems. The other authors declare no competing interests.
References
- Aronoff JM, Landsberger DM (2013) The development of a modified spectral ripple test. J Acoust Soc Am 134: EL217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew C, Intskirveli I, Metherate R (2017) Systemic Nicotine Increases Gain and Narrows Receptive Fields in A1 via Integrated Cortical and Subcortical Actions. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschnagel JS, Hawk LW Jr. (2008) The effects of nicotine on the attentional modification of the acoustic startle response in nonsmokers. Psychopharmacology (Berl) 198: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behler O, Breckel TP, Thiel CM (2015) Nicotine reduces distraction under low perceptual load. Psychopharmacology (Berl) 232: 1269–77. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P 3rd (2006) Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther 79: 480–8. [DOI] [PubMed] [Google Scholar]
- Bramer SL, Kallungal BA (2003) Clinical considerations in study designs that use cotinine as a biomarker. Biomarkers 8: 187–203. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ (2007) Gain modulation by nicotine in macaque v1. Neuron 56: 701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM (2009) The dynamic effects of nicotine on the developing brain. Pharmacol Ther 122: 125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher H (1938) The mechanism of hearing as revealed through experiment of the masking effect of thermal noise. Proc Natl Acad Sci U S A 24: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkrider AW, Champlin CA (2001a) Acute effect of nicotine on non-smokers: II. MLRs and 40-Hz responses. Hear Res 160: 89–98. [DOI] [PubMed] [Google Scholar]
- Harkrider AW, Champlin CA (2001b) Acute effect of nicotine on non-smokers: III. LLRs and EEGs. Hear Res 160: 99–110. [DOI] [PubMed] [Google Scholar]
- Harkrider AW, Champlin CA, McFadden D (2001) Acute effect of nicotine on non-smokers: I. OAEs and ABRs. Hear Res 160: 73–88. [DOI] [PubMed] [Google Scholar]
- Harkrider AW, Hedrick MS (2005) Acute effect of nicotine on auditory gating in smokers and non-smokers. Hear Res 202: 114–28. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86: 1119–27. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 210: 453–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, Thaker GK, Stein EA (2011) Nicotine Enhances but Does Not Normalize Visual Sustained Attention and the Associated Brain Network in Schizophrenia. Schizophr Bull 37: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P 3rd, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57: 79–115. [DOI] [PubMed] [Google Scholar]
- Intskirveli I, Metherate R (2012) Nicotinic neuromodulation in auditory cortex requires MAPK activation in thalamocortical and intracortical circuits. J Neurophysiol 107: 2782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD (1997) Smoking and attention: a review and reformulation of the stimulus-filter hypothesis. Clin Psychol Rev 17: 451–78. [DOI] [PubMed] [Google Scholar]
- Kawai HD, Kang HA, Metherate R (2011) Heightened nicotinic regulation of auditory cortex during adolescence. J Neurosci 31: 14367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V, Choueiry J, Dort H, Smith D, Impey D, de la Salle S, Philippe T (2014a) Baseline-dependent modulating effects of nicotine on voluntary and involuntary attention measured with brain event-related P3 potentials. Pharmacol Biochem Behav 122: 107–17. [DOI] [PubMed] [Google Scholar]
- Knott V, de la Salle S, Choueiry J, Impey D, Smith D, Smith M, Beaudry E, Saghir S, Ilivitsky V, Labelle A (2015) Neurocognitive effects of acute choline supplementation in low, medium and high performer healthy volunteers. Pharmacol Biochem Behav 131: 119–29. [DOI] [PubMed] [Google Scholar]
- Knott V, Smith D, de la Salle S, Impey D, Choueiry J, Beaudry E, Smith M, Saghir S, Ilivitsky V, Labelle A (2014b) CDP-choline: effects of the procholine supplement on sensory gating and executive function in healthy volunteers stratified for low, medium and high P50 suppression. J Psychopharmacol 28: 1095–108. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Bolton K, Heenan A, Shah D, Fisher DJ, Villeneuve C (2009) Effects of acute nicotine on event-related potential and performance indices of auditory distraction in nonsmokers. Nicotine Tob Res 11: 519–30. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA (2002) Cognitive mechanisms of nicotine on visual attention. Neuron 36: 539–48. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184: 523–39. [DOI] [PubMed] [Google Scholar]
- Metherate R, Intskirveli I, Kawai HD (2012) Nicotinic filtering of sensory processing in auditory cortex. Front Behav Neurosci 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ (2008) Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology 33: 588–98. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Kellar K, Aisen P, White H, Wesnes K, Coderre E, Pfaff A, Wilkins H, Howard D, Levin ED (2012) Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology 78: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A (2004) Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol 4: 36–46. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ, Wesnes K, Pincock C (1996) Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharm Clin 11: 391–400. [Google Scholar]
- Phillips DP, Taylor TL, Hall SE, Carr MM, Mossop JE (1997) Detection of silent intervals between noises activating different perceptual channels: Some properties of “central” auditory gap detection. J Acoust Soc Am 101: 3694–3705. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM (2009) nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol 78: 658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LS, Tregellas JR (2015) Neuronal effects of nicotine during auditory selective attention. Psychopharmacology (Berl) 232: 2017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Rojas DC, Tregellas JR (2016) Neuronal effects of nicotine during auditory selective attention in schizophrenia. Hum Brain Mapp 37: 410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Fink GR (2007) Visual and auditory alertness: Modality-specific and supramodal neural mechanisms and their modulation by nicotine. J Neurophysiol 97: 2758–2768. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Barry JG, Moore DR, Amitay S (2012) A new test of attention in listening (TAIL) predicts auditory performance. PLoS One 7: e53502. [DOI] [PMC free article] [PubMed] [Google Scholar]