Abstract
Exposure to fine particulate matter (PM2.5) is associated with increased mortality. Although epidemiology studies typically use outdoor PM2.5 concentrations as surrogates for exposure, the majority of PM2.5 exposure in the US occurs in microenvironments other than outdoors. We develop a framework for estimating the total US mortality burden attributable to exposure to PM2.5 of both indoor and outdoor origin in the primary non-smoking microenvironments in which people spend most of their time. The framework utilizes an exposure-response function combined with adjusted mortality effect estimates that account for underlying exposures to PM2.5 of outdoor origin that likely occurred in the original epidemiology populations from which effect estimates are derived. We demonstrate the framework using several different scenarios to estimate the potential magnitude and bounds of the US mortality burden attributable to total PM2.5 exposure across all non-smoking environments under a variety of assumptions. Our best estimates of the US mortality burden associated with total PM2.5 exposure in the year 2012 range from ~230,000 to ~300,000 deaths. Indoor exposure to PM2.5 of outdoor origin is typically the largest total exposure, accounting for ~40–60% of total mortality, followed by residential exposure to indoor PM2.5 sources, which also drives the majority of variability in each scenario.
Keywords: criteria pollutants, epidemiology, exposure modeling, inhalation exposure, particulate matter
Introduction
Elevated outdoor concentrations of fine particulate matter (i.e., the mass concentration of particles ≤ 2.5 µm in aerodynamic diameter; PM2.5) have been consistently associated with increased mortality in numerous epidemiology studies [1–9]. Although epidemiology studies typically use centrally monitored outdoor PM2.5 concentrations as surrogates for average human exposures to PM2.5 of outdoor origin, the majority of exposure to PM2.5 of outdoor origin in the US and other industrialized nations typically occurs in various other microenvironments, including inside residences, offices, schools, and vehicles [10–16]. This is because people spend the majority of their time in microenvironments other than outdoors [17, 18] and outdoor PM2.5 can infiltrate and persist into different microenvironments with varying efficiencies [19–24]. There are also many PM2.5 sources present in non-smoking indoor microenvironments, including cooking [25–27], burning incense and candles [28, 29], operating office equipment [30, 31], resuspension from settled dust from human activities such as walking and cleaning [32, 33], and secondary organic aerosols from oxidation reactions [34]. To date, the vast majority of air pollution epidemiology studies and quantitative risk assessments have not explicitly accounted for these varied microenvironmental exposures [35, 36].
The objective of this work is to develop a framework for estimating the total US mortality burden attributable to exposure to PM2.5 of both indoor and outdoor origin in the primary non-smoking microenvironments in which people spend most of their time. The framework primarily utilizes a modified version of an exposure-response function commonly used for air pollution risk assessment combined with adjusted mortality effect estimates that account for estimates of underlying microenvironmental exposures to PM2.5 of outdoor origin that likely occurred in prior epidemiology cohort studies. We demonstrate the utility of the framework by conducting several scenario analyses to estimate the likely magnitude and bounds of the US mortality burden associated with long-term PM2.5 exposures that result from both indoor and outdoor PM sources in each microenvironment. While no single model scenario is considered to be the definitive representation of the US mortality burden of microenvironmental PM2.5 exposures due to unique data limitations in each case, each model scenario offers insight into how the framework can be used with richer data sets in the future to refine nationwide mortality estimates and ultimately to inform policy decisions to reduce exposures in the microenvironments in which they most often occur.
Materials and methods
Selection of an appropriate exposure-response function
Integral to the model framework is the selection of an appropriate health impact function. A number of recent air pollution risk assessments have estimated mortality and/or morbidity associated with ambient PM2.5 exposure in various locations using different forms of health impact functions and associated effect estimates derived from epidemiology studies. Historically, most studies have used a variant of a generic exposure-response health impact function for ambient air pollution [37] to estimate a population’s change in health endpoint (Δyi) due to a change in the assumed population-average exposure to pollutant i (ΔEi) (e.g., Eq. 1).
| 1 |
where y0 is the annual baseline prevalence of illness (per person per year), βi is the health endpoint effect estimate for pollutant i resulting from prior epidemiology studies (e.g., per μg/m3 of pollutant i), ΔEi is the change in exposure concentration relative to an assumed baseline or threshold concentration (e.g., μg/m3 of pollutant i, typically assuming outdoor concentrations are surrogates for exposure), and Pop is size of the affected population. This approach has been used recently to estimate the mortality burden associated with outdoor PM2.5 concentrations in the US [38–42] and globally [43, 44]. For example, Fann et al. (2017) [42] used this approach with all-cause mortality effect estimates from Krewski et al. (2009) [5] to estimate that ~120,000 deaths (95% CI: 83,000–160,000) were associated with outdoor PM2.5 exposures in the in 2010. Fann et al. (2017) [42] also made another estimate of ~200,000 (95% CI: 43,000–1,100,000) deaths associated with outdoor PM2.5 using a different model form and effect estimates from Nasari et al. (2016) [45]. Similar approaches have also recently been extended to estimate the chronic health burden associated with long-term indoor PM exposures using effect estimates taken directly from the outdoor air epidemiology literature [46–50].
Another widely used approach to air pollution risk assessment is the Global Burden of Disease (GBD) study’s integrated exposure-response (IER) methodology [51–56], and its follow-up Global Exposure Mortality Model (GEMM) [57], which were developed in part because the generic expression in Eq. 1 is based on epidemiology cohort studies in the US and Europe with outdoor PM2.5 concentrations (typically below 30 µg/m3) that may not be representative for countries with much higher ambient air pollution levels [53] or for other, higher, PM2.5 exposures such as secondhand or active smoking. Here we primarily utilize a modified version of the generic exposure-response health impact function in Eq. 1 for the model framework because (a) it was developed for use with epidemiology studies with PM2.5 concentrations within the range of concern in non-smoking indoor and outdoor microenvironments in the US, (b) there is considerable uncertainty in the shape of the GBD IER function and its fitted parameters at lower PM2.5 concentrations most relevant to this study, and (c) it has been used successfully in other recent indoor microenvironmental exposure investigations. However, we also apply the IER model and evaluate its utility in the SI.
Modifying the exposure-response function
We modify the exposure-response function in Eq. 1 for PM2.5 in a manner similar to that in Logue et al. (2012) [48] to account for microenvironmental PM2.5 concentrations and exposures, albeit with a few additional modifications as shown in Eq. 2. First, we introduce a modified form of βi for ambient-generated PM2.5 (i.e., βPM2.5,AG,modified) to account for estimates of the underlying long-term average exposures to PM2.5 of outdoor origin that likely occurred in various microenvironments in the cohort populations used in the original epidemiology studies from which βPM2.5 was derived. This modification provides an adjusted effect estimate for outdoor PM2.5 based on estimates of long-term average microenvironmental exposures that can be more universally applied to other microenvironmental exposure estimates rather than using outdoor PM2.5 concentrations alone as a surrogate for exposure.
Second, we separately account for long-term average PM2.5 exposures above an assumed threshold concentration in each microenvironment j that result from ambient-generated sources (ΔCPM2.5,AG,j) and indoor-generated sources (ΔCPM2.5,IG,j). Third, tj accounts for the average fraction of time spent in a particular microenvironment j. Thus, the sums of ΔCPM2.5,AG,j × tj and ΔCPM2.5,IG,j × tj across all microenvironments more realistically account for total PM2.5 exposure (ΔEPM2.5) from both indoor and outdoor sources. Finally, we also allow for using different assumptions for modified mortality effect estimates for ambient-generated and indoor-generated PM2.5 (i.e., βPM2.5,AG,modified and βPM2.5,IG,modified, respectively). Although the framework can account for varying toxicity of ambient- and indoor-generated PM2.5, we assume equal toxicity here because of conflicting conclusions among the limited number of studies that have investigated differential toxicity using paired indoor, outdoor, and/or personal PM samples [58–63].
| 2 |
We consider four main microenvironments in which people are exposed to PM2.5 of both indoor and outdoor origin: (i) inside residences, (ii) inside indoor environments other than residences (e.g., schools, business, restaurants, etc.), (iii) inside vehicles, and (iv) outdoors. Equation 3 shows modified forms of the Σ(ΔCPM2.5,IG,j×tj) and Σ(ΔCPM2.5,AG,j×tj) terms in Eq. 2 that account for the long-term average PM2.5 concentrations resulting from both indoor and outdoor sources and the average fraction of time spent inside each of these four primary microenvironments.
| 3a |
| 3b |
where ΔCPM2.5,IG,residences and ΔCPM2.5,IG,other indoor are the differences in long-term average concentrations of indoor-generated PM2.5 in non-smoking residences and all other non-smoking indoor environments other than residences, respectively, both compared to a baseline value in which there are no indoor PM2.5 sources (μg/m3); ΔCPM2.5,AG,residences, ΔCPM2.5,AG,other indoor, and ΔCPM2.5,AG,vehicles are the differences in long-term average concentrations of ambient-generated PM2.5 in residences, indoor environments other than residences, and vehicles, respectively, compared to a baseline value (μg/m3); ΔCPM2.5,outdoor is the difference in long-term average outdoor PM2.5 concentrations also compared to a baseline value (μg/m3); and tresidences, tother indoor, tvehicles, and toutdoor are the long-term average fractions of time spent inside each microenvironment, respectively. Note that Eq. 3a assumes there are no indoor sources of PM2.5 inside vehicles, primarily because of a lack of comprehensive surveys of in-vehicle PM sources, although several studies have shown that in-vehicle PM2.5 exposures can be higher than the near-roadway exposures in some circumstances [64, 65].
Modifying effect estimates for PM2.5 of outdoor origin
Data from the 1992–1994 National Human Activity Pattern Survey (NHAPS) showed that, on average, people in the US spent 68.7% of their time in residences, 18.2% of their time in indoor locations other than residences (e.g., offices, factories, bars, schools, and restaurants), 5.5% of their time in vehicles, and 7.6% of their time outdoors [17]. Therefore, historically observed associations between outdoor PM2.5 concentrations and adverse health outcomes can reasonably be expected to have indirectly accounted for the underlying exposures to PM2.5 of outdoor origin that infiltrates and persists in these various microenvironments [66]. Failing to account for these underlying exposures to PM2.5 of outdoor origin in different microenvironments can lead to exposure misclassification and errors in effect estimates [35, 67–80]. To account for this phenomenon, we developed a modified mortality effect estimate for PM2.5 of outdoor origin (i.e., βPM2.5,AG,modified) based on the average fraction of PM2.5 of outdoor origin that infiltrates and persists in each assumed microenvironment (i.e., the infiltration factor) combined with the average fraction of time spent in each microenvironment, as shown in Eq. 4.
| 4 |
where βPM2.5 is the mortality effect estimate for outdoor PM2.5 from epidemiology studies that used outdoor concentrations as surrogates for average population exposure to outdoor PM2.5, Fj is the average PM2.5 infiltration factor for microenvironment j, and tj is the fraction of time spent in each microenvironment j. ΣFj×tj is estimated using Eq. 5, which represents a weighted average of the product of the infiltration factors and fractional time spent in each of the four microenvironments used herein.
| 5 |
We estimate a mean value of ΣFj×tj to be ~0.60 for the US population using a number of data sources as described in the SI. Although there would be variability in this value for each individual in a particular population included in a cohort study, this value is assumed to be broadly applicable as a reasonable estimate of the population-average value.
Applying the model framework: scenario analyses
We apply the model framework using MATLAB to estimate the magnitude and bounds of the US mortality burden of long-term average total PM2.5 exposures that result from indoor and outdoor PM sources in all non-smoking microenvironments. We define two primary scenarios that involve different assumptions and data sources for key input parameters, including: (i) a nationwide estimate based primarily on data from field measurements (where possible) and nationwide distributions of model input parameters; and (ii) a nationwide estimate based primarily on regionally varying modeled microenvironmental PM2.5 concentrations and other regionally varying model input parameters (where possible). A third scenario involves an application of the GBD IER model for comparison purposes; methods and results are included in the SI (although we have limited confidence in the approach for a number of reasons as described in the SI). Each model scenario is constructed to yield insight into how the framework can be used to generate mortality estimates and attribute them to microenvironmental exposures, while also highlighting unique data limitations present within each set of scenario assumptions.
For both Scenario 1 and 2, we use a central pooled estimate of RR for the increase in long-term all-cause mortality associated with outdoor PM2.5 concentrations in the US of 7.3% per 10 µg/m3 (95% CI: 3.7–11%) as reported in a recent quantitative meta-analysis of outdoor PM2.5 C-R functions [39]. We convert the pooled RR estimate of 1.073 per 10 µg/m3 to an effect estimate (i.e., βPM2.5) of 0.0070 (95% CI: 0.0036–0.0104), where βPM2.5 = ln(RR)/10 [81]. We fit a Weibull distribution to these reported values, resulting in a mean (±SD) value of βPM2.5 = 0.0070 (± 0.0016) per µg/m3 with distribution shape factors of α = 0.765 and β = 4.95. A Weibull distribution was used because it yields a distribution that is very close to normal in shape, but does not produce any negative values. Moreover, we estimate βPM2.5,AG,modified to be ~0.0117 per µg/m3 using Eq. 4 (i.e., 0.0070 divided by 0.6) with a 95% CI of 0.0060–0.0174 per µg/m3. This modified effect estimate for all-cause mortality associated with outdoor PM2.5 represents a more generalizable effect estimate that accounts for the population-average locations and durations in which people are likely exposed to PM2.5 of outdoor origin.
Scenario 1: Nationwide estimate based primarily on prior field studies
In Scenario 1, we estimated the mortality burden for the adult population 35 years and older using nationwide distributions of model inputs. We assumed a national annual average outdoor PM2.5 concentration of 9.1 µg/m3 with 10th and 90th percentiles of 6.6 and 11.2 µg/m3, respectively, taken from the EPA’s nationwide monitoring network data for the year 2012 [82]. The year 2012 was chosen because it was the year for which we had the most comprehensive national (Scenario 1) and regional (Scenario 2) estimates for indoor and outdoor PM2.5 concentrations. We fit a lognormal distribution through the reported arithmetic mean and percentiles to construct a distribution from which to sample (GM = 8.84 µg/m3 and GSD = 1.246). We assumed a baseline (i.e., threshold) PM2.5 concentration of zero in each microenvironment, which is consistent with other recent applications of the core health impact function used in this scenario [42, 43] and with a number of studies that suggest there is no evidence of a population threshold in the relationship between long-term exposure to ambient PM2.5 and mortality [83–86]. We assumed that the 2012 nationwide population (Pop) and mortality rate (y0) for persons 35 years and older were 166,516,716 and 1.463 × 10−2 per person per year, respectively, using data from the CDC WONDER system [87].
We used Monte Carlo simulations with 10,000 iterations to sample from what we assumed for the purposes of Scenario 1 to be nationally representative distributions of every other model input parameter, including modified PM2.5 mortality effect estimates (described previously), time-activity patterns, and estimates of long-term average PM2.5 concentrations of both indoor and outdoor origin in each microenvironment taken largely from prior field measurements. There are three versions of Scenario 1, each of which involved sampling from different distributions to estimate residential PM2.5 concentrations of both indoor and outdoor origin. We sampled data from (i) the Relationship of Indoor, Outdoor and Personal Air (RIOPA) [13] and (ii) the Multi-Ethnic Study of the Atherosclerosis and Air Pollution (MESA Air) [18, 19] studies independently, as well as (iii) both RIOPA and MESA equally. Briefly, the RIOPA study measured indoor and outdoor PM2.5 concentrations concurrently for 48 h in 212 non-smoking residences in three US cities, while MESA Air measured indoor and outdoor PM2.5 concentrations concurrently over a 2-week period in 208 homes in warm seasons and 264 homes in cold seasons in seven US cities. Crucially, subsequent analyses of both data sets reported distributions of PM2.5 infiltration factors, which can be used to estimate the relative contributions of both indoor and outdoor sources to indoor PM2.5 concentrations in the sample residences. Although a few other studies have also explicitly measured indoor concentrations of PM2.5 in US residences resulting from indoor and outdoor sources, including a study of 294 inner-city homes of children with asthma in seven cities [27] and 68 smoking and non-smoking homes in six cities [88], we chose to rely on the RIOPA and MESA Air studies because they included large sample sizes of non-smoking homes occupied by adults in multiple US cities.
All relevant model inputs and data sources for Scenario 1 are summarized in full in the SI. Each model iteration represents a population-level estimate of total mortality summed across all microenvironmental exposures; thus, the central tendency of the model output provides the most likely estimate of the magnitude of the total mortality associated with PM2.5 exposure and the output range informs the likely bounds of that estimate. In all microenvironments, if a sampled value of a microenvironmental PM2.5 concentration was a negative value, it was replaced with zero.
Scenario 2: Nationwide estimate based on regional model outputs
In Scenario 2, we similarly applied the model framework to make a nationwide estimate of the total mortality burden attributable to microenvironmental PM2.5 exposures, albeit using regional assumptions for some input parameters for which regional data were available, including population demographics, baseline over-35 adult mortality rates, outdoor PM2.5 concentrations, and, importantly, residential indoor PM2.5 concentrations of both indoor and outdoor origin. We used the same nationwide distributions of time-activity patterns and all non-residential indoor microenvironmental PM2.5 concentrations from Scenario 1 because we are not aware of any robust regional data sets for these parameters. However, given that the Scenario 1 analysis demonstrated the sensitivity of the model to assumptions for residential exposures, and given that other air pollution risk assessments have shown the utility of using geographically varying population demographics and mortality rates [38, 42], we consider Scenario 2 a reasonable, albeit somewhat limited, attempt to construct a national mortality estimate using more granular input data.
Scenario 2 uses regional estimates of residential indoor PM2.5 concentrations of indoor origin and ambient infiltration factors recently made using a nationally representative set of combined residential energy and indoor air quality (REIAQ) models for non-smoking US residences [89]. Briefly, the REIAQ model set combined building energy models with dynamic pollutant mass balance models to estimate the hourly concentrations of a number of pollutants of indoor and outdoor origin, including PM2.5, in a total of 3971 individual home models in 19 cities that are estimated to represent ~80% of the US housing stock as of approximately the early 2000s. The model set assumed cooking was the primary indoor PM2.5 source and assumed the same generation rates and cooking frequency for all homes. The model set also accounted for historical outdoor PM2.5 concentrations and modeled infiltration air exchange rates, window opening behaviors, and forced air heating and cooling system runtimes based on historical outdoor environmental conditions combined with a building physics model. We used these modeled results for the regionally varying annual average residential indoor PM2.5 concentrations of indoor origin (i.e., ΔCPM2.5,IG,residences) in conjunction with regional distributions of ambient PM2.5 infiltration factors combined with regional distributions of outdoor PM2.5 concentrations for the year 2012 from EPA [82] to generate estimates of ΔCPM2.5,AG,residences in each of the 19 modeled cities. We used the infiltration factor approach (rather than using values of ΔCPM2.5,AG,residences directly from REIAQ) because the model set is weighted more heavily toward homes in cities with higher ambient PM2.5 concentrations than rural areas, while the EPA outdoor concentration data are more broadly applicable to the rest of the population.
We grouped the REIAQ model outputs for each of the 3971 home models into nine US census divisions and calculated a population-weighted annual average and SD for ΔCPM2.5,IG,residences and infiltration factors (Finf) across all homes in each division (Table 1). We fit beta and lognormal distributions to summary statistics of infiltration factors and indoor PM2.5 concentration of indoor origin, respectively, for Monte Carlo sampling from each division. For PM2.5 of ambient origin, we used annual average (and 10th and 90th percentiles) outdoor PM2.5 concentration data for nine US regions reported by EPA [82]. Because the nine EPA regions group states differently than the nine US census divisions, we regrouped the EPA data by assuming that every state in an EPA region had the same annual outdoor PM2.5 concentration summary statistics as other states in that region. We estimated the annual average (and 10th and 90th percentile) outdoor PM2.5 concentration in each census division by weighting each assumed state-level summary statistic by the population in each census division. We fit lognormal distributions to the resulting estimates of annual outdoor PM2.5 summary statistics (means and 10th and 90th percentiles) in each division for subsequent Monte Carlo sampling.
Table 1.
Summary of estimates for key input parameters made for each US census division for the regional analysis in Scenario 2
| Census division | Mean (SD) residential indoor PM2.5 concentration resulting from indoor sources (µg/m3) [90] | Mean (SD) residential PM2.5 infiltration factor [90] | Mean (10th–90th percentile) outdoor PM2.5 concentration (µg/m3) in 2012 [83] | Baseline adult mortality rate in 2012, y0 (per 100,000) [88] | Over-35 adult population in 2012 (Pop) [88] |
|---|---|---|---|---|---|
| New England | 5.78 (2.14) | 0.50 (0.08) | 9.22 (7.25–11.2) | 1429.5 | 8,209,960 |
| Middle Atlantic | 5.84 (2.01) | 0.50 (0.08) | 9.22 (7.25–11.2) | 1475.6 | 22,655,120 |
| East North Central | 5.66 (1.91) | 0.46 (0.09) | 9.98 (8.74–11.43) | 1576.8 | 25,115,038 |
| West North Central | 5.69 (1.86) | 0.45 (0.09) | 9.40 (7.74–11.16) | 1566.0 | 10,965,126 |
| South Atlantic | 6.47 (2.07) | 0.40 (0.08) | 8.86 (6.90–10.66) | 1481.7 | 33,363,675 |
| East South Central | 6.81 (1.94) | 0.40 (0.08) | 9.82 (8.34–11.37) | 1759.6 | 9,999,343 |
| West South Central | 4.80 (1.54) | 0.46 (0.09) | 9.61 (8.01–11.14) | 1449.3 | 18,513,908 |
| Mountain | 5.38 (1.82) | 0.49 (0.09) | 7.95 (6.03–10.25) | 1332.1 | 11,481,569 |
| Pacific | 6.30 (2.03) | 0.46 (0.08) | 9.06 (6.01–12.88) | 1241.2 | 26,212,977 |
We then ran the 10,000 iteration Monte Carlo analysis 9 times—one for each census division—with over-35 adult mortality rates and populations [87] (also shown in Table 1) to yield estimates of total mortality and distributions of the different microenvironmental exposure contributions in each division. We summed the median total mortality estimates from each census division to generate an estimate of the national mortality burden associated with total PM2.5 exposure. We estimated the mortality burden attributable to each microenvironment and source type using the average fractional exposure contributions multiplied by the best estimate (i.e., median) total mortality, similar to Scenario 1.
Results and Discussion
Scenario 1: Nationwide estimates based primarily on prior field studies
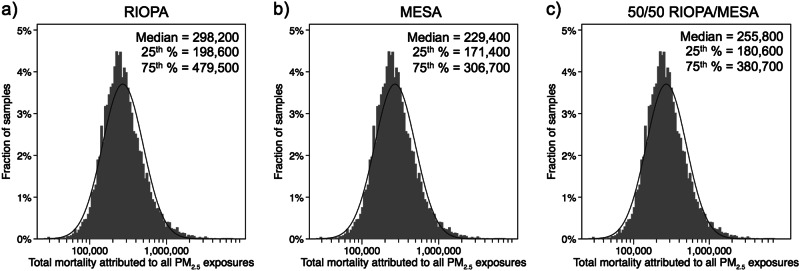
The resulting distributions of estimates of the annual US mortality burden of total PM2.5 exposure in 2012 attributable to both indoor and outdoor sources in all microenvironments combined using assumptions in Scenario 1 are shown in Fig. 1. Results for all three RIOPA and MESA sampling approaches were approximately lognormally distributed with a Shapiro–Wilk test statistic (W) > 0.98 and p < 0.00001 on the log-transformed values for each case. We consider the median values as our most likely estimate of the total mortality burden of all PM2.5 exposures for each scenario, with an interquartile range (IQR, or 25th to 75th percentiles) serving as a measure of the most reasonable bounds of the central estimate. The median (IQR) estimate of the total mortality associated with all PM2.5 exposures for each scenario were ~298,200 (198,600–479,500), ~229,400 (171,400–306,700), and ~255,800 (180,600–380,700) deaths for the 100% RIOPA, 100% MESA, and 50%/50% RIOPA/MESA scenarios, respectively. These estimates would mean that aggregate PM2.5 exposures accounted for between 9 and 12% of the total number of adult deaths over the age of 35 in 2012.
Fig. 1.
Frequency distributions of the total annual US PM2.5 mortality burden estimated by Monte Carlo simulations of microenvironmental exposures to PM2.5 of both indoor and outdoor origin using three cases in Scenario 1, including sampling residential indoor concentrations from: a RIOPA-only, b MESA-only, and c from RIOPA and MESA equally (i.e., 50/50 RIOPA/MESA). The approximate curve fit is a lognormal distribution and summary statistics (median and interquartile range) are provided in units of deaths per year
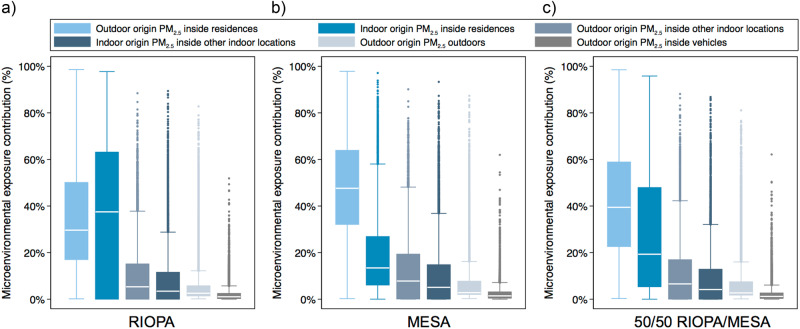
Distributions of the estimated fractional exposure contributions from indoor and outdoor sources in each microenvironment modeled in Scenario 1 are shown in Fig. 2. In each of the three RIOPA/MESA cases, residential PM2.5 exposure to indoor and outdoor sources combined was the dominant exposure, accounting for 70% of the total PM2.5 exposure across all three scenarios, on average. Residential exposure accounted for an average of ~67% of the total exposure to PM2.5 of outdoor origin across the three scenarios, followed by an average of ~17% of outdoor origin exposure attributed to other indoor environments, with direct outdoor exposure accounting for only ~12% of all outdoor-origin exposure, on average.
Fig. 2.
Distributions of the estimated contributions of microenvironmental exposures to PM2.5 of indoor and outdoor origin to total PM2.5 exposures across the US population using the three Scenario 1 cases: sampling residential indoor concentrations from a RIOPA-only, b MESA only, and c RIOPA and MESA equally (i.e., 50/50 RIOPA/MESA). Boxes represent 25th and 75th percentile values (i.e., interquartile range, or IQR); horizontal line represents median values; whiskers represent upper and lower adjacent values (i.e., 50% beyond the IQR)
In both the MESA-only and the combined RIOPA/MESA 50/50 scenarios, residential exposure to PM2.5 of outdoor origin dominated total exposure, accounting for an average of 48 and 42% of total exposure in the MESA-only and 50/50 combined scenarios, respectively. Residential exposure to PM2.5 of indoor origin was the second largest contributor to total exposure in these two scenarios, ranging from an average of 19 to 28% of total exposure in the MESA-only and 50/50 combined scenarios, respectively. Conversely, the largest contributor to total exposure in the RIOPA-only scenario was residential exposure to PM2.5 of indoor origin (average of 37%) followed by residential exposure to PM2.5 of outdoor origin (average of 36%). Given the wide ranges of exposure contributions generated by sampling from RIOPA and MESA separately, and given the large differences in the two study designs and findings, we expect the combined 50/50 RIOPA/MESA sampling approach to yield the most plausible nationwide exposure estimates of the three approaches in Scenario 1. Thus, we use only the combined 50/50 RIOPA/MESA study results from Figs. 1 and 2 to estimate the likely mortality burden associated with microenvironmental exposure to PM2.5 of indoor and outdoor origin in Scenario 1 (Table 2).
Table 2.
Mean, standard deviation (SD), and interquartile range (IQR: 25th to 75th percentiles) of the estimated contributions of indoor and outdoor sources in each microenvironment to total PM2.5 exposures and the estimated associated US mortality burden in Scenario 1 (50/50 RIOPA/MESA)
| Outdoor or indoor sources | Microenvironment | Mean fraction of total PM2.5 exposure ± SD [IQR] | Mean estimate of annual deaths attributed to total PM2.5 exposure ± SD [IQR] |
|---|---|---|---|
| Due to PM2.5 of outdoor origin | Residences | 42.1 ± 23.8% | 107,700 ± 61,000 |
| [22.6–58.9%] | [57,800–150,600] | ||
| Other indoor locations | 10.9 ± 12.9% | 28,000 ± 33,000 | |
| [0–16.9%] | [100–43,300] | ||
| Vehicles | 2.4 ± 3.9% | 6100 ± 9900 | |
| [0.3–2.6%] | [900–6700] | ||
| Outdoor | 7.3 ± 11.1% | 18,800 ± 28,600 | |
| [1.8–7.5%] | [4,500–19,100] | ||
| Total outdoor contribution | 62.7 ± 25.2% |
160,500 ± 40,400 [63,300–219,600] |
|
| Due to PM2.5 of indoor origin | Residences | 28.1 ± 26.3% | 72,000 ± 67,300 |
| [5.3–47.9%] | [13,700–122,600] | ||
| Other indoor locations | 9.1 ± 12.6% | 23,300 ± 32,200 | |
| [0–12.8%] | [0–32,800] | ||
| Vehicles | n/a | n/a | |
| n/a | n/a | ||
| Outdoor | n/a | n/a | |
| n/a | n/a | ||
| Total indoor contribution | 37.3 ± 25.2% |
95,300 ± 24,000 [13,700–155,400] |
|
| Total contribution | 100% |
255,800 [77,000–375,000] |
Mortality burden estimates are based on the ‘best estimate’ of the median total mortality burden resulting from Monte Carlo sampling of the RIOPA and MESA studies combined (i.e., each sampled equally: 50/50 RIOPA/MESA)
We estimate the mortality burden associated with PM2.5 exposure in each microenvironment by multiplying the mean fractional exposure contribution (from Fig. 2) by the median total mortality burden of ~255,800 deaths per year for the combined 50/50 RIOPA/MESA scenario (from Fig. 1). Using this approach, we estimate that exposure to PM2.5 of outdoor origin across all microenvironments accounted for ~160,500 deaths in 2012 (IQR of ~63,300 to ~219,600 deaths), while exposure to PM2.5 of indoor origin across all microenvironments accounted for ~95,300 deaths (IQR of ~13,700 to ~155,400). Our estimate of the mortality burden attributable to outdoor sources is between the ~120,000 and ~200,000 deaths in 2010 estimated by Fann et al. (2017) [42] using RR estimates and response functions from Krewski et al. (2009) [5] and Nasari et al. (2016) [45], respectively. However, our estimate is almost twice the ~88,400 deaths in 2015 estimated by Cohen et al. (2017) [55] largely because of the threshold concentration used (i.e., zero compared to a uniform distribution between 2.4 and 5.8 µg/m3) and also because of the use of a different model form and associated effect estimates that are not modified to account for microenvironmental exposure to outdoor-origin PM2.5. Both issues are explored in more detail in Scenario 3 in the SI.
In the combined 50/50 RIOPA/MESA scenario, we estimate that the largest contributor to PM2.5-associated mortality is residential indoor exposure to PM2.5 of outdoor origin, accounting for an estimated ~107,700 deaths annually (IQR of ~57,800 to ~150,600). The next largest contributor is residential indoor exposure to PM2.5 of indoor origin, accounting for an estimated ~72,000 deaths annually (IQR of ~13,700 to ~122,600). Indoor exposure to PM2.5 of indoor and outdoor origin in other indoor locations is estimated to account for ~23,300 (IQR of ~0 to ~32,800) and ~28,000 (IQR of ~100 to ~43,300) deaths annually, respectively. Finally, outdoor exposure to PM2.5 of outdoor origin is estimated to account for only ~18,800 (IQR of ~4,500 to ~19,100) deaths annually. Overall, these results demonstrate the importance of indoor environments, and particularly residential indoor environments, in governing human exposure to PM2.5 of both indoor and outdoor origin, and provide novel estimates of the potential magnitude of the nationwide mortality burden associated with these exposures.
Scenario 2: Nationwide estimate based on regional model outputs
Table 3 shows estimates of regional and total mortality associated with microenvironmental PM2.5 exposures resulting from the regional model application (Scenario 2). The median (IQR) estimate of the total mortality associated with all PM2.5 exposures across all microenvironments and sources was ~281,800 (159,700–359,300), which places Scenario 2 approximately between the RIOPA-only and 50/50 RIOPA/MESA cases from Scenario 1. Exposure to PM2.5 of outdoor and indoor origin in all microenvironments was estimated to account for ~139,500 deaths (IQR of ~69,600 to ~177,900) and ~142,300 deaths (IQR of ~90,100 to ~181,400) in 2012, respectively. The relative contributions of indoor and outdoor PM2.5 sources to total mortality are approximately equal, largely because of the use of relatively high indoor concentrations (similar to the RIOPA-only approach in Scenario 1) and relatively low residential infiltration factors that were estimated in the REIAQ model set. Accordingly, residential indoor PM2.5 of indoor origin is estimated to be the single dominant contributor to the total mortality burden in Scenario 2, followed by residential indoor PM2.5 of outdoor origin.
Table 3.
Estimates of regional and total mortality associated with microenvironmental exposures to PM2.5 of indoor and outdoor origin in 2012 resulting from the regional Monte Carlo procedure (Scenario 2)
| Census division | Percentile | Total mortality | Mortality associated with PM2.5 of ambient origin | Mortality associated with PM2.5 of indoor origin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inside residences | Other indoors | Vehicles | Outdoor | Total | Inside residences | Other indoors | Total | |||
| New England | Median | 13,900 | 4500 | 1400 | 300 | 1000 | 7200 | 5500 | 1200 | 6700 |
| 25th % | 7900 | 3400 | 0 | 100 | 200 | 3700 | 4200 | 0 | 4200 | |
| 75th % | 17,600 | 5600 | 2200 | 300 | 1000 | 9100 | 6900 | 1600 | 8600 | |
| Middle Atlantic | Median | 39,600 | 12,800 | 4100 | 900 | 2600 | 20,400 | 15,900 | 3300 | 19,300 |
| 25th % | 22,700 | 9800 | 0 | 100 | 700 | 10,600 | 12,200 | 0 | 12,200 | |
| 75th % | 50,300 | 15,800 | 6300 | 1000 | 2600 | 25,700 | 19,900 | 4600 | 24,500 | |
| East North Central | Median | 47,100 | 15,100 | 5200 | 1100 | 3300 | 24,800 | 18,300 | 4000 | 22,300 |
| 25th % | 26,200 | 11,300 | 0 | 200 | 900 | 12,500 | 13,800 | 0 | 13,800 | |
| 75th % | 60,200 | 18,800 | 8200 | 1200 | 3400 | 31,700 | 23,000 | 5500 | 28,500 | |
| West North Central | Median | 19,700 | 6100 | 2100 | 400 | 1300 | 9900 | 8100 | 1700 | 9800 |
| 25th % | 11,100 | 4600 | 0 | 100 | 400 | 5000 | 6100 | 0 | 6100 | |
| 75th % | 25,100 | 7500 | 3300 | 500 | 1300 | 12,600 | 10,100 | 2400 | 12,500 | |
| South Atlantic | Median | 56,200 | 14,600 | 5700 | 1200 | 3700 | 25,300 | 26,100 | 4800 | 30,900 |
| 25th % | 32,400 | 10,800 | 0 | 200 | 1000 | 12,000 | 20,400 | 0 | 20,400 | |
| 75th % | 71,600 | 18,200 | 9000 | 1400 | 3800 | 32,500 | 32,400 | 6700 | 39,200 | |
| East South Central | Median | 21,600 | 5800 | 2300 | 500 | 1500 | 10,000 | 9800 | 1800 | 11,600 |
| 25th % | 12,500 | 4400 | 0 | 100 | 400 | 4900 | 7700 | 0 | 7700 | |
| 75th % | 27,500 | 7100 | 3700 | 600 | 1500 | 12,800 | 12,100 | 2500 | 14,600 | |
| West South Central | Median | 29,300 | 9800 | 3300 | 700 | 2100 | 16,000 | 10,600 | 2700 | 13,300 |
| 25th % | 15,800 | 7300 | 0 | 100 | 600 | 8000 | 7800 | 0 | 7800 | |
| 75th % | 37,700 | 12,400 | 5300 | 800 | 2200 | 20,600 | 13,400 | 3700 | 17,100 | |
| Mountain | Median | 16,000 | 4800 | 1600 | 300 | 1000 | 7800 | 6700 | 1500 | 8200 |
| 25th % | 9000 | 3700 | 0 | 100 | 300 | 4000 | 5100 | 0 | 5100 | |
| 75th % | 20,400 | 6000 | 2500 | 400 | 1000 | 9800 | 8400 | 2100 | 10,500 | |
| Pacific | Median | 38,500 | 11,100 | 3800 | 800 | 2500 | 18,200 | 17,000 | 3300 | 20,300 |
| 25th % | 22,000 | 8300 | 0 | 100 | 600 | 9000 | 13,000 | 0 | 13,000 | |
| 75th % | 48,900 | 13,800 | 5900 | 900 | 2400 | 23,000 | 21,300 | 4600 | 25,900 | |
| Total | Median | 281,800 | 84,700 | 29,500 | 6200 | 19,100 | 139,500 | 118,000 | 24,400 | 142,300 |
| 25th % | 159,700 | 63,500 | 0 | 1100 | 5000 | 69,600 | 90,100 | 0 | 90,100 | |
| 75th % | 359,300 | 105,200 | 46,400 | 7100 | 19,200 | 177,900 | 147,600 | 33,800 | 181,400 | |
Total mortality in Scenario 2 is driven largely by PM2.5 exposures in the most populated census divisions: South Atlantic, East North Central, Middle Atlantic, and Pacific. The East South Central census division had the highest estimated mortality associated with PM2.5 per capita because of relatively high residential indoor concentrations resulting form indoor sources combined with the highest baseline adult mortality rate in 2012. The lowest per capita mortality estimate was in the Mountain census division, with moderate residential indoor PM2.5 concentrations and a moderate baseline mortality rate. Regional differences in ΔCPM2.5,IG,residences were driven variations in air exchange rates [89] and system runtimes (which primarily affects particle filtration [90]).
Best estimates of the total mortality burden associated with PM2.5 exposure in the US made using the assumptions in Scenarios 1 and 2, as well as the contribution of each microenvironmental and source-specific exposure, are shown in Fig. 3 for direct comparison. Although the magnitude of total mortality varies in each scenario, best estimates consistently range from ~230,000 to ~300,000 deaths in 2012. Residential exposures to PM2.5 from indoor sources drive the vast majority of variability in each case, suggesting that a better understanding of the nationwide contribution of indoor sources to total exposure are needed, as is a better understanding of the toxicity of indoor sources.
Fig. 3.
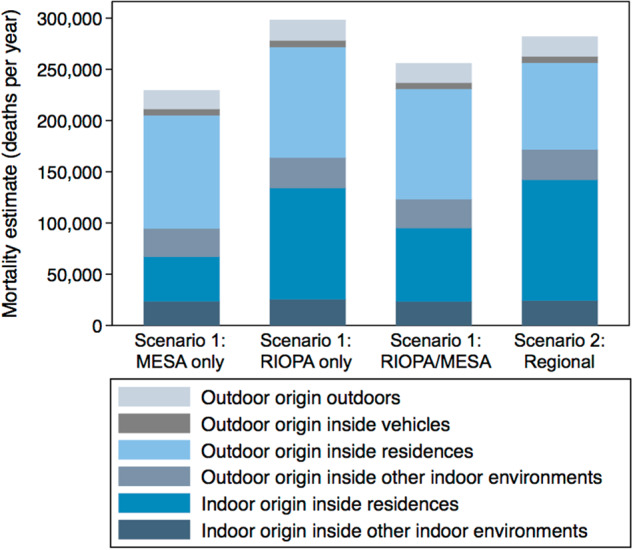
Best estimates of the number of annual deaths in the US associated with exposure to PM2.5 of indoor and outdoor origin in each microenvironment in Scenarios 1 and 2
Limitations
One obvious assumption in this work is that the observed relationships between outdoor PM2.5 concentrations and mortality in the epidemiology literature are indeed causal and that the underlying exposure-response functions and effect estimates accurately reflect a causal and quantifiable relationship [91–94]. Further, the framework assumes that the exposure-response function in Eq. 1 (i) has no threshold PM2.5 concentration below which additional mortality does not occur [83–86] and (ii) appropriately describes the shape of the observed mortality responses from prior epidemiology studies [95]. Additionally, we do not make any modifications to the exposure-response function and effect estimates based on the magnitude of PM2.5 exposure concentrations or varying chemical constituents of PM2.5, although there is some evidence that both of these adjustments may be warranted [96–100]. Moreover, the framework assumes that there is no double counting of the health effects of indoor PM2.5 sources. We consider this a reasonable assumption because most studies have reported relatively low correlations between personal and ambient PM2.5 concentrations (i.e., R2 < 0.3) [13, 69], but the potential for ambient PM2.5 mortality effect estimates resulting from epidemiology cohort studies including an inherent but un-quantified indoor contribution remains.
Another obvious assumption and potential limitation in this work is that we assume that the modified exposure-response endpoint effect estimates for mortality associated with PM2.5 from both indoor and outdoor sources are the same, and that there are no changes in PM2.5 toxicity that occur due to size-resolved aerosol dynamics that govern the particle infiltration and persistence process. Although some studies have suggested that particles of outdoor origin may be more harmful than indoor-generated particles [59, 60], other studies have shown that indoor-generated fine particulate matter is at least as toxic as outdoor particulate matter [61], if not more [62]. However, there is a tremendous lack of data to support or reject either assumption at this time. Given the lack of data on mortality endpoints from various indoor and outdoor PM2.5 sources, we consider this a reasonable assumption for this exploratory analysis. This same assumption also has precedent in a number of other recent studies in the literature that have evaluated mortality endpoints associated with indoor and outdoor PM2.5 sources [46–48]. Additionally, there is mounting evidence from air filter intervention studies in homes that reducing indoor PM2.5 concentrations (comprising a mixture of both indoor and outdoor sources) can lead to improvements in some biomarkers and other clinical measures that are associated with both short-term and long-term cardiovascular health endpoints [101–107].
There are also several assumptions implicit in our approach to modifying health endpoint effect estimates (β) to account for the underlying exposures to PM2.5 of outdoor origin that likely occurred in the original epidemiology populations from which effect estimates are derived. First, we assumed that the distributions of activity patterns and residential building characteristics (i.e., infiltration factors) that we used match both the general population and the epidemiology cohort populations, although this may not be true. For example, elderly populations who are more susceptible to adverse effects associated with PM2.5 exposure tend to spend more time indoors than the general population. Second, we did not consider some potential non-linear effects of various parameters including potential covariance of infiltration factors and ambient PM2.5 as well as occupancy and indoor particle generation. Third, we assumed that the human activity patterns reported in NHAPS [17] are still valid in 2012, even though data were collected in 1992–1994.
Despite the large uncertainties associated with this work, the exposure attribution and mortality burden estimates clearly demonstrate the importance of considering indoor microenvironments in PM2.5 exposure assessments and epidemiology studies. They also illustrate the potential magnitude and reasonable bounds of the mortality burden potentially associated with microenvironmental exposures to PM2.5 of both indoor and outdoor origin. Results also demonstrate that efforts to reduce the US PM2.5 associated mortality burden should at least consider indoor pollutant control in addition to controlling outdoor sources. This model framework can also be used for high-level policy analysis of the costs and benefits of reducing exposures to PM2.5 of indoor and outdoor origin through various interventions (e.g., source control, air purifiers, changing infiltration/ventilation across the building stock, etc.).
This work intentionally focuses solely on non-smoking homes; further model applications could include incorporating data on smoking rates and contributions to indoor PM2.5 concentrations. This work also highlights the need for several areas of research to improve these estimates and reduce uncertainty. For example, a better understanding of how outdoor PM2.5 infiltration factors vary geographically and by different building types is needed to more accurately characterize outdoor PM2.5 exposures for epidemiology studies. Additionally, a better understanding of the toxicity of both indoor and outdoor origin PM2.5 is needed, including characterizing the toxicity of a wide variety of typical indoor sources and also characterizing how the size-resolved dynamics of the outdoor PM2.5 infiltration process may affect the toxicity of PM2.5 of outdoor origin in indoor environments.
Electronic supplementary material
Acknowledgements
This work was supported by the US Environmental Protection Agency, Office of Radiation and Indoor Air, Indoor Environments Division. The authors were also supported in part by an ASHRAE New Investigator Award and in part by the US Environmental Protection Agency under Assistance Agreement No. #83575001 awarded to Illinois Institute of Technology. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication. The authors would like to acknowledge Torkan Fazli for providing outputs from her REIAQ model data set.
Funding
This work was supported by the US Environmental Protection Agency, Office of Radiation and Indoor Air, Indoor Environments Division. BS and PA were also supported in part by an ASHRAE New Investigator Award and in part by the US Environmental Protection Agency under Assistance Agreement No. #83575001 awarded to Illinois Institute of Technology. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary material
The online version of this article (10.1038/s41370-018-0103-4) contains supplementary material, which is available to authorized users.
References
- 1.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air pollution and mortality in the medicare population. N Engl J Med. 2017;376:2513–22. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 4.Gharibvand L, Shavlik D, Ghamsary M, Beeson WL, Soret S, Knutsen R, et al. The association between ambient fine particulate air pollution and lung cancer incidence: results from the AHSMOG-2 study. Environ Health Perspect. 2017;125:378–84. doi: 10.1289/EHP124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 2009;5–114; discussion 115–36. [PubMed]
- 6.Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA J Am Med Assoc. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–42. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 8.Shi L, Zanobetti A, Kloog I, Coull BA, Koutrakis P, Melly SJ, et al. Low-concentration PM2.5 and mortality: estimating acute and chronic effects in a population-based study. Environ Health Perspect. 2015;124. 10.1289/ehp.1409111. [DOI] [PMC free article] [PubMed]
- 9.US EPA. Integrated science assessment for particulate matter. Research Triangle Park, NC: National Center for Environmental Assessment; 2009. [Google Scholar]
- 10.Brown KW, Sarnat JA, Koutrakis P. Concentrations of PM2.5 mass and components in residential and non-residential indoor microenvironments: The Sources and Composition of Particulate Exposures study. J Expo Sci Environ Epidemiol. 2012;22:161–72. doi: 10.1038/jes.2011.41. [DOI] [PubMed] [Google Scholar]
- 11.Burke JM, Zufall MJ, Ozkaynak H. A population exposure model for particulate matter: case study results for PM(2.5) in Philadelphia, PA. J Expo Anal Environ Epidemiol. 2001;11:470–89. doi: 10.1038/sj.jea.7500188. [DOI] [PubMed] [Google Scholar]
- 12.Clayton CA, Perritt RL, Pellizzari ED, Thomas KW, Whitmore RW, Wallace LA, et al. Particle Total Exposure Assessment Methodology (PTEAM) study: distributions of aerosol and elemental concentrations in personal, indoor, and outdoor air samples in a southern California community. J Expo Anal Environ Epidemiol. 1993;3:227–50. [PubMed] [Google Scholar]
- 13.Meng QY, Turpin BJ, Korn L, Weisel CP, Morandi M, Colome S, et al. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: Analyses of RIOPA data. J Expo Anal Environ Epidemiol. 2005;15:17–28. doi: 10.1038/sj.jea.7500378. [DOI] [PubMed] [Google Scholar]
- 14.Van Ryswyk K, Wheeler AJ, Wallace L, Kearney J, You H, Kulka R, et al. Impact of microenvironments and personal activities on personal PM2.5 exposures among asthmatic children. J Expo Sci Environ Epidemiol. 2014;24:260–8. doi: 10.1038/jes.2013.20. [DOI] [PubMed] [Google Scholar]
- 15.Wallace L. Indoor particles: a review. J Air Waste Manag Assoc. 1996;46:98–126. doi: 10.1080/10473289.1996.10467451. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Frey HC. Geographic differences in inter-individual variability of human exposure to fine particulate matter. Atmos Environ. 2011;45:5684–91. doi: 10.1016/j.atmosenv.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–52. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 18.Spalt EW, Curl CL, Allen RW, Cohen M, Williams K, Hirsch JA, et al. Factors influencing time-location patterns and their impact on estimates of exposure: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) J Expo Sci Environ Epidemiol. 2016;26:341–8. doi: 10.1038/jes.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen RW, Adar SD, Avol E, Cohen M, Curl CL, Larson T, et al. Modeling the residential infiltration of outdoor PM2.5 in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Health Perspect. 2012;120:824–30. doi: 10.1289/ehp.1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Zhao B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos Environ. 2011;45:275–88. doi: 10.1016/j.atmosenv.2010.09.048. [DOI] [Google Scholar]
- 21.Long CM, Suh HH, Catalano PJ, Koutrakis P. Using time- and size-resolved particulate data to quantify indoor penetration and deposition behavior. Environ Sci Technol. 2001;35:2089–99. doi: 10.1021/es001477d. [DOI] [PubMed] [Google Scholar]
- 22.MacNeill M, Kearney J, Wallace L, Gibson M, Héroux ME, Kuchta J, et al. Quantifying the contribution of ambient and indoor-generated fine particles to indoor air in residential environments. Indoor Air. 2014;24:362–75. doi: 10.1111/ina.12084. [DOI] [PubMed] [Google Scholar]
- 23.MacNeill M, Wallace L, Kearney J, Allen RW, Van Ryswyk K, Judek S, et al. Factors influencing variability in the infiltration of PM2.5 mass and its components. Atmos Environ. 2012;61:518–32. doi: 10.1016/j.atmosenv.2012.07.005. [DOI] [Google Scholar]
- 24.Wallace L, Williams R. Use of personal-indoor-outdoor sulfur concentrations to estimate the infiltration factor and outdoor exposure factor for individual homes and persons. Environ Sci Technol. 2005;39:1707–14. doi: 10.1021/es049547u. [DOI] [PubMed] [Google Scholar]
- 25.Wallace L. Indoor sources of ultrafine and accumulation mode particles: size distributions, size-resolved concentrations, and source strengths. Aerosol Sci Technol. 2006;40:348–60. doi: 10.1080/02786820600612250. [DOI] [Google Scholar]
- 26.Wallace LA, Emmerich SJ, Howard-Reed C. Source strengths of ultrafine and fine particles due to cooking with a gas stove. Environ Sci Technol. 2004;38:2304–11. doi: 10.1021/es0306260. [DOI] [PubMed] [Google Scholar]
- 27.Wallace LA, Mitchell H, O’Connor GT, Neas L, Lippmann M, Kattan M, et al. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111:1265–72. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afshari A, Matson U, Ekberg LE. Characterization of indoor sources of fine and ultrafine particles: a study conducted in a full-scale chamber. Indoor Air. 2005;15:141–50. doi: 10.1111/j.1600-0668.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 29.Ott WR, Siegmann HC. Using multiple continuous fine particle monitors to characterize tobacco, incense, candle, cooking, wood burning, and vehicular sources in indoor, outdoor, and in-transit settings. Atmos Environ. 2006;40:821–43. doi: 10.1016/j.atmosenv.2005.08.020. [DOI] [Google Scholar]
- 30.He C, Morawska L, Taplin L. Particle emission characteristics of office printers. Environ Sci Technol. 2007;41:6039–45. doi: 10.1021/es063049z. [DOI] [PubMed] [Google Scholar]
- 31.He C, Morawska L, Wang H, Jayaratne R, McGarry P, Richard Johnson G, et al. Quantification of the relationship between fuser roller temperature and laser printer emissions. J Aerosol Sci. 2010;41:523–30. doi: 10.1016/j.jaerosci.2010.02.015. [DOI] [Google Scholar]
- 32.Ferro AR, Kopperud RJ, Hildemann LM. Source strengths for indoor human activities that resuspend particulate matter. Environ Sci Technol. 2004;38:1759–64. doi: 10.1021/es0263893. [DOI] [PubMed] [Google Scholar]
- 33.Qian J, Ferro AR. Resuspension of dust particles in a chamber and associated environmental factors. Aerosol Sci Technol. 2008;42:566–78. doi: 10.1080/02786820802220274. [DOI] [Google Scholar]
- 34.Waring MS. Secondary organic aerosol in residences: predicting its fraction of fine particle mass and determinants of formation strength. Indoor Air. 2014;24:376–89. doi: 10.1111/ina.12092. [DOI] [PubMed] [Google Scholar]
- 35.Hänninen O, Rumrich I, Asikainen A. Challenges in estimating health effects of indoor exposures to outdoor particles: Considerations for regional differences. Sci Total Environ. 2017;589:130–5. doi: 10.1016/j.scitotenv.2017.02.228. [DOI] [PubMed] [Google Scholar]
- 36.Yeh S, Small MJ. Incorporating exposure models in probabilistic assessment of the risks of premature mortality from particulate matter. J Expo Anal Environ Epidemiol. 2002;12:389–403. doi: 10.1038/sj.jea.7500240. [DOI] [PubMed] [Google Scholar]
- 37.IEc. Health and Welfare Benefits Analyses to Support the Second Section 812 Benefit-Cost Analysis of the Clean Air Act. Cambridge, MA: Industrial Economics, Inc.; 2011. [Google Scholar]
- 38.Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal. 2012;32:81–95. doi: 10.1111/j.1539-6924.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 39.Fann N, Gilmore EA, Walker K. Characterizing the long-term PM 2.5 concentration-response function: comparing the strengths and weaknesses of research synthesis approaches: characterizing long-term PM 2.5 concentration-response function. Risk Anal. 2016;36:1693–707. doi: 10.1111/risa.12435. [DOI] [PubMed] [Google Scholar]
- 40.Fann N, Fulcher CM, Baker K. The recent and future health burden of air pollution apportioned across U.S. sectors. Environ Sci Technol. 2013;47:3580–9. doi: 10.1021/es304831q. [DOI] [PubMed] [Google Scholar]
- 41.Penn SL, Arunachalam S, Woody M, Heiger-Bernays W, Tripodis Y, Levy JI. Estimating state-specific contributions to PM2.5- and O3-related health burden from residential combustion and electricity generating unit emissions in the United States. Environ Health Perspect. 2016;125. 10.1289/EHP550. [DOI] [PMC free article] [PubMed]
- 42.Fann N, Kim S-Y, Olives C, Sheppard L. Estimated changes in life expectancy and adult mortality resulting from declining PM2.5 exposures in the contiguous United States: 1980–2010. Environ Health Perspect. 2017;125. 10.1289/EHP507. [DOI] [PMC free article] [PubMed]
- 43.Lelieveld J, Barlas C, Giannadaki D, Pozzer A. Model calculated global, regional and megacity premature mortality due to air pollution. Atmos Chem Phys. 2013;13:7023–37. doi: 10.5194/acp-13-7023-2013. [DOI] [Google Scholar]
- 44.Anenberg SC, Horowitz LW, Tong DQ, West JJ. An Estimate of the Global Burden of Anthropogenic Ozone and Fine Particulate Matter on Premature Human Mortality Using Atmospheric Modeling. Environ Health Perspect. 2010;118:1189–95. doi: 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasari MM, Szyszkowicz M, Chen H, Crouse D, Turner MC, Jerrett M, et al. A class of non-linear exposure-response models suitable for health impact assessment applicable to large cohort studies of ambient air pollution. Air Qual Atmosphere Health. 2016;9:961–72. doi: 10.1007/s11869-016-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan WR, Parthasarathy S, Fisk WJ, McKone TE. Estimated effect of ventilation and filtration on chronic health risks in U.S. offices, schools, and retail stores. Indoor Air. 2016;26:331–43. doi: 10.1111/ina.12189. [DOI] [PubMed] [Google Scholar]
- 47.Fisk WJ, Chan WR Effectiveness and cost of reducing particle-related mortality with particle filtration. Indoor Air 2017. 10.1111/ina.12371. [DOI] [PubMed]
- 48.Logue JM, Price PN, Sherman MH, Singer BC. A method to estimate the chronic health impact of air pollutants in U.S. residences. Environ Health Perspect. 2012;120:216–22. doi: 10.1289/ehp.1104035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montgomery JF, Reynolds CCO, Rogak SN, Green SI. Financial implications of modifications to building filtration systems. Build Environ. 2015;85:17–28. doi: 10.1016/j.buildenv.2014.11.005. [DOI] [Google Scholar]
- 50.Zhao D, Azimi P, Stephens B. Evaluating the long-term health and economic impacts of central residential air filtration for reducing premature mortality associated with indoor fine particulate matter (PM2.5) of outdoor origin. Int J Environ Res Public Health. 2015;12:8448–79. doi: 10.3390/ijerph120708448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnett RT, Pope CA III, Ezzati M, Olives C, Lim SS, Mehta S, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014. 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed]
- 52.Apte JS, Marshall JD, Cohen AJ, Brauer M. Addressing global mortality from ambient PM2.5. Environ Sci Technol. 2015;49:8057–66. doi: 10.1021/acs.est.5b01236. [DOI] [PubMed] [Google Scholar]
- 53.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–71. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 54.Rohde RA, Muller RA. Air pollution in China: mapping of concentrations and sources. PLoS ONE. 2015;10:e0135749. doi: 10.1371/journal.pone.0135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–18. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhury S, Dey S, Smith KR Ambient PM2.5 exposure and expected premature mortality to 2100 in India under climate change scenarios. Nat Commun 2018; 9. 10.1038/s41467-017-02755-y. [DOI] [PMC free article] [PubMed]
- 57.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci. 2018;115:9592–9597. [DOI] [PMC free article] [PubMed]
- 58.Ebelt ST, Wilson WE, Brauer M. Exposure to ambient and nonambient components of particulate matter: a comparison of health effects. Epidemiology. 2005;16:396–405. doi: 10.1097/01.ede.0000158918.57071.3e. [DOI] [PubMed] [Google Scholar]
- 59.Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113:499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monn C, Becker S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10–2.5) in outdoor and indoor air. Toxicol Appl Pharmacol. 1999;155:245–52. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- 61.Long CM, Suh HH, Kobzik L, Catalano PJ, Ning YY, Koutrakis P. A pilot investigation of the relative toxicity of indoor and outdoor fine particles: in vitro effects of endotoxin and other particulate properties. Environ Health Perspect. 2001;109:1019–26. doi: 10.1289/ehp.011091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebelt ST, Petkau AJ, Vedal S, Fisher TV, Brauer M. Exposure of chronic obstructive pulmonary disease patients to particulate matter: relationships between personal and ambient air concentrations. J Air Waste Manag Assoc. 2000;50:1081–94. doi: 10.1080/10473289.2000.10464166. [DOI] [PubMed] [Google Scholar]
- 63.Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, et al. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68:224–30. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- 64.Adams HS, Nieuwenhuijsen MJ, Colvile RN, McMullen MAS, Khandelwal P. Fine particle (PM2.5) personal exposure levels in transport microenvironments, London, UK. Sci Total Environ. 2001;279:29–44. doi: 10.1016/S0048-9697(01)00723-9. [DOI] [PubMed] [Google Scholar]
- 65.Kingham S, Meaton J, Sheard A, Lawrenson O. Assessment of exposure to traffic-related fumes during the journey to work. Transp Res Part Transp Environ. 1998;3:271–4. doi: 10.1016/S1361-9209(98)00005-4. [DOI] [Google Scholar]
- 66.Butler DA, Madhavan G, Alper J. Health risks of indoor exposure to particulate matter: workshop summary. Washington, D.C.: National Academies Press; 2016. 10.17226/23531. [PubMed]
- 67.Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL, et al. Estimating error in using residential outdoor PM2.5 concentrations as proxies for personal exposures: a meta-analysis. Environ Health Perspect. 2010;118:673–8. doi: 10.1289/ehp.0901158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL, et al. Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures: a review. Epidemiology. 2010;21:215–23. doi: 10.1097/EDE.0b013e3181cb41f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baxter LK, Crooks JL, Sacks JD. Influence of exposure differences on city-to-city heterogeneity in PM2.5-mortality associations in US cities. Environ Health 2017;16. 10.1186/s12940-016-0208-y. [DOI] [PMC free article] [PubMed]
- 70.Baxter LK, Wright RJ, Paciorek CJ, Laden F, Suh HH, Levy JI. Effects of exposure measurement error in the analysis of health effects from traffic-related air pollution. J Expo Sci Environ Epidemiol. 2010;20:101–11. doi: 10.1038/jes.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baxter LK, Franklin M, Özkaynak H, Schultz BD, Neas LM. The use of improved exposure factors in the interpretation of fine particulate matter epidemiological results. Air Qual Atmosphere Health. 2013;6:195–204. doi: 10.1007/s11869-011-0160-5. [DOI] [Google Scholar]
- 72.Baxter LK, Sacks JD. Clustering cities with similar fine particulate matter exposure characteristics based on residential infiltration and in-vehicle commuting factors. Sci Total Environ. 2014;470–471:631–8. doi: 10.1016/j.scitotenv.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 73.Hodas N, Meng Q, Lunden MM, Rich DQ, Özkaynak H, Baxter LK, et al. Variability in the fraction of ambient fine particulate matter found indoors and observed heterogeneity in health effect estimates. J Expo Sci Environ Epidemiol. 2012;22:448–54. doi: 10.1038/jes.2012.34. [DOI] [PubMed] [Google Scholar]
- 74.Hodas N, Turpin BJ, Lunden MM, Baxter LK, Özkaynak H, Burke J, et al. Refined ambient PM2.5 exposure surrogates and the risk of myocardial infarction. J Expo Sci Environ Epidemiol. 2013;23:573–80. doi: 10.1038/jes.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. 2013;12. 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed]
- 76.Meng QY, Turpin BJ, Polidori A, Lee JH, Weisel C, Morandi M, et al. PM2.5 of ambient origin: estimates and exposure errors relevant to PM epidemiology. Environ Sci Technol. 2005;39:5105–12. doi: 10.1021/es048226f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarnat JA, Sarnat SE, Flanders WD, Chang HH, Mulholland J, Baxter L, et al. Spatiotemporally resolved air exchange rate as a modifier of acute air pollution-related morbidity in Atlanta. J Expo Sci Environ Epidemiol. 2013;23:606–15. doi: 10.1038/jes.2013.32. [DOI] [PubMed] [Google Scholar]
- 78.Sheppard L, Burnett RT, Szpiro AA, Kim SY, Jerrett M, Pope CA, et al. Confounding and exposure measurement error in air pollution epidemiology. Air Qual Atmosphere Health. 2012;5:203–16. doi: 10.1007/s11869-011-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones RR, Özkaynak H, Nayak SG, Garcia V, Hwang SA, Lin S. Associations between summertime ambient pollutants and respiratory morbidity in New York City: comparison of results using ambient concentrations versus predicted exposures. J Expo Sci Environ Epidemiol. 2013;23:616–26. doi: 10.1038/jes.2013.44. [DOI] [PubMed] [Google Scholar]
- 80.Mannshardt E, Sucic K, Jiao W, Dominici F, Frey HC, Reich B, et al. Comparing exposure metrics for the effects of fine particulate matter on emergency hospital admissions. J Expo Sci Environ Epidemiol. 2013;23:627–36. doi: 10.1038/jes.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rackes A, Ben-David T, Waring MS. Outcome-based ventilation: a framework for assessing performance, health, and energy impacts to inform office building ventilation decisions. Indoor Air. 2018. 10.1111/ina.12466. [DOI] [PubMed]
- 82.U.S. EPA. Air trends: particulate matter (PM2.5) trends. 2016. https://www.epa.gov/air-trends/particulate-matter-pm25-trends.
- 83.Roman HA, Walker KD, Walsh TL, Conner L, Richmond HM, Hubbell BJ, et al. Expert judgment assessment of the mortality impact of changes in ambient fine particulate matter in the U.S. Environ Sci Technol. 2008;42:2268–74. doi: 10.1021/es0713882. [DOI] [PubMed] [Google Scholar]
- 84.Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. 2012;120:708–14. doi: 10.1289/ehp.1104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwartz J, Coull B, Laden F, Ryan L. The effect of dose and timing of dose on the association between airborne particles and survival. Environ Health Perspect. 2007;116:64–69. doi: 10.1289/ehp.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinault L, Tjepkema M, Crouse DL, Weichenthal S, van Donkelaar A, Martin RV, et al. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ Health. 2016;15. 10.1186/s12940-016-0111-6. [DOI] [PMC free article] [PubMed]
- 87.CDC. National Center for Health Statistics WONDER Online Database: Compressed Mortality File 1999–2016 Series 20, No. 2V. CDC WONDER. 2017. https://wonder.cdc.gov/cmf-icd10.htm (accessed 22 May 2018).
- 88.Dockery DW, Spengler JD. Indoor-outdoor relationships of respirable sulfates and particles. Atmos Environ 1967. 1981;15:335–43. [Google Scholar]
- 89.Fazli T, Stephens B. Development of a nationally representative set of combined building energy and indoor air quality models for U.S. residences. Build Environ. 2018;136:198–212. doi: 10.1016/j.buildenv.2018.03.047. [DOI] [Google Scholar]
- 90.Touchie MF, Siegel JA. Residential HVAC runtime from smart thermostats: characterization, comparison, and impacts. Indoor Air. 2018. 10.1111/ina.12496. [DOI] [PubMed]
- 91.Cox L. Rethinking the meaning of concentration-response functions and the estimated burden of adverse health effects attributed to exposure concentrations: invited commentary. Risk Anal. 2016;36:1770–9. doi: 10.1111/risa.12670. [DOI] [PubMed] [Google Scholar]
- 92.Dominici F, Greenstone M, Sunstein CR. Particulate matter matters. Science. 2014;344:257–9. doi: 10.1126/science.1247348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frey HC. Dose-response models are conditional on determination of causality: invited commentary. Risk Anal. 2016;36:1751–4. doi: 10.1111/risa.12672. [DOI] [PubMed] [Google Scholar]
- 94.McClellan RO. Providing context for ambient particulate matter and estimates of attributable mortality: invited commentary. Risk Anal. 2016;36:1755–65. doi: 10.1111/risa.12674. [DOI] [PubMed] [Google Scholar]
- 95.Pope CA, Cropper M, Coggins J, Cohen A. Health benefits of air pollution abatement policy: Role of the shape of the concentration–response function. J Air Waste Manag Assoc. 2015;65:516–22. doi: 10.1080/10962247.2014.993004. [DOI] [PubMed] [Google Scholar]
- 96.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19:680–9. doi: 10.1097/EDE.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118:363–9. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2006;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin HH, Cohen AJ, Pope CA, Ezzati M, Lim SS, Hubbell BJ, et al. Meta-analysis methods to estimate the shape and uncertainty in the association between long-term exposure to ambient fine particulate matter and cause-specific mortality over the global concentration range: global particle pollution risks and their uncertainty. Risk Anal. 2016;36:1813–25. doi: 10.1111/risa.12421. [DOI] [PubMed] [Google Scholar]
- 100.Vodonos A, Awad YA, Schwartz J. The concentration-response between long-term PM 2.5 exposure and mortality; A meta-regression approach. Environ Res. 2018;166:677–89. doi: 10.1016/j.envres.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 101.Allen RW, Carlsten C, Karlen B, Leckie S, Eeden S, van, Vedal S, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011;183:1222–30. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- 102.Bräuner EV, Forchhammer L, Moller P, Barregard L, Gunnarsen L, Afshari A, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177:419–25. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- 103.Chuang HC, Ho KF, Lin LY, Chang TY, Hong GB, Ma CM, et al. Long-term indoor air conditioner filtration and cardiovascular health: A randomized crossover intervention study. Environ Int. 2017;106:91–96. doi: 10.1016/j.envint.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Kajbafzadeh M, Brauer M, Karlen B, Carlsten C, van Eeden S, Allen RW. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: a HEPA filter intervention study. Occup Environ Med. 2015;72:394–400. doi: 10.1136/oemed-2014-102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karottki DG, Spilak M, Frederiksen M, Gunnarsen L, Brauner EV, Kolarik B, et al. An indoor air filtration study in homes of elderly: cardiovascular and respiratory effects of exposure to particulate matter. Environ Health. 2013;12. 10.1186/1476-069X-12-116. [DOI] [PMC free article] [PubMed]
- 106.Lin LY, Chen HW, Su TL, Hong GB, Huang LC, Chuang KJ. The effects of indoor particle exposure on blood pressure and heart rate among young adults: an air filtration-based intervention study. Atmos Environ. 2011;45:5540–4. doi: 10.1016/j.atmosenv.2011.05.014. [DOI] [Google Scholar]
- 107.Weichenthal S, Mallach G, Kulka R, Black A, Wheeler A, You H, et al. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air. 2013;23:175–84. doi: 10.1111/ina.12019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.