Abstract
Despite tremendous advances in neonatal intensive care over the past 20 years, prematurity carries a high burden of neurological morbidity lasting lifelong. The term encephalopathy of prematurity (EoP) coined by Volpe in 2009 encompasses all aspects of the now known effects of prematurity on the immature brain, including altered and disturbed development as well as specific lesional hallmarks. Understanding the way cells are damaged is crucial to design brain protective strategies, and in this purpose, preclinical models largely contribute to improve the comprehension of the cell death mechanisms. While neuronal cell death has been deeply investigated and characterized in (hypoxic–ischemic) encephalopathy of the newborn at term, little is known about the types of cell death occurring in preterm brain injury. Three main different morphological cell death types are observed in the immature brain, specifically in models of hypoxic–ischemic encephalopathy, namely, necrotic, apoptotic, and autophagic cell death. Features of all three types may be present in the same dying neuron. In preterm brain injury, description of cell death types is sparse, and cell loss primarily concerns immature oligodendrocytes and, infrequently, neurons. In the present review, we first shortly discuss the different main severe preterm brain injury conditions that have been reported to involve cell death, including periventricular leucomalacia (PVL), diffuse white matter injury (dWMI), and intraventricular hemorrhages, as well as potentially harmful iatrogenic conditions linked to premature birth (anesthesia and caffeine therapy). Then, we present an overview of current evidence concerning cell death in both clinical human tissue data and preclinical models by focusing on studies investigating the presence of cell death allowing discriminating between the types of cell death involved. We conclude that, to improve brain protective strategies, not only apoptosis but also other cell death (such as regulated necrotic and autophagic) pathways now need to be investigated together in order to consider all cell death mechanisms involved in the pathogenesis of preterm brain damage.
Keywords: autophagy, apoptosis, necrosis, neonatal, neuroprotection, autophagic cell death, periventricular leucomalacia
Introduction
Neurodevelopmental deficits are frequent among infants born prematurely, particularly those born before 32 weeks of gestational age (very preterm infants, VPT). Around 5–10% VPT exhibit severe impairments such as cerebral palsy and cognitive delay, which are linked frequently to cystic periventricular leucomalacia (cPVL) and intraventricular and parenchymatous hemorrhages grades III and IV, corresponding to the most severe brain lesions. Around 10–15% of VPT exhibit more moderate impairments, such as limited cognitive functions and motoric deficits, linked morphologically to diffuse white and gray matter lesions. Finally, around 30–40% of VPT exhibit impaired academic achievement and behavioral disorders (Pierrat et al., 2017), associated presumably with altered brain development, dysmaturational disorders, and connectivity dysfunctions.
The major substrate of human preterm brain injury is the encephalopathy of prematurity (EoP) that is characterized by gray and white matter lesions reflecting combined acquired insults, altered developmental trajectories, and reparative phenomena (Kinney and Volpe, 2012). This term was quoted by Volpe in 2009 to characterize the multiple hit hypothesis and heterogeneous WM and GM impairments (including pre-oligodendrocytes injury, axonal injury, thalamic injury, subplate injury, and migrating GABAergic neuronal injury) affecting key developmental pathways and resulting in adverse clinical outcomes of VPT (Van’t Hooft et al., 2015; Schneider et al., 2016; Synnes and Hicks, 2018). On one hand, EoP covers severe brain lesions occurring after cell death such as in cPVL or intraventricular–parenchymatous hemorrhages (IVH). However, less severe brain lesions as in non-cystic and diffuse WM injury (detected by advanced MRI techniques and characterized by astrogliosis and microgliosis) are now more frequently observed with some features of cell death. On the other hand, subtler brain alterations can reflect disturbances of brain development or delay in brain maturation and affect functional connectivity with no apparent cell death (Ball et al., 2013; Gozdas et al., 2018).
The mostly studied anatomical structures involved in preterm brain injury are the periventricular white matter (PWM), the germinal matrix (source of most intraventricular hemorrhages), and the subventricular zone (SVZ, source of migrational and maturational disorders). Although there is growing evidence of the GM involvement (cortex, thalamus, nucleus caudatus, and deep GM injury), literature is sparser (Kinney and Volpe, 2012). Recent studies have shown that cortical interneurons development can be disrupted (reduced number and morphological complexity) in preterm infants with non-cystic WM injury or in inflammatory conditions (Panda et al., 2018; Stolp et al., 2019). Moreover, constant improvement in imaging techniques allows to study and detect microstructural alterations not only in WM but also in GM related to neurodevelopmental disorders (Chau et al., 2013; Nossin-Manor et al., 2013; Kersbergen et al., 2016).
Therefore, it is mandatory to understand the underlying pathophysiological cellular mechanisms contributing to WM and GM damage and/or organization defects. It is now widely recognized that events that induce an inflammatory context sensitize preterm brain to injury (Hagberg et al., 2015). Beside the inflammatory, vascular, and maturational factors implicated in the origin of preterm brain injury and some of those specific lesions, the etiology of the cellular factors and the cell death mechanisms encountered, either during altered brain development or in relation with specific lesions, are complex and interrelated. Focusing only on studies using cell death markers on pathological tissues, the present review proposes to do an overview of what has been described so far in human autopsy tissue and preclinical models regarding the different types of cell death involved in preterm brain injury. We here considered only studies using classical known and widely accepted cell death markers allowing the discrimination between cell death types (i.e., excluding studies reporting loss of cells without clarification of the type of cell death involved). Therefore, according to our selection criteria, this review will be focused mainly on the most severe cases of preterm brain injury involving “anatomical” evidence of brain damage.
Since EoP includes diverse causes that cannot be reproduced in one unique model, our investigations addressed the main pathological conditions related to EoP such as inflammation, hypoxia–ischemia (HI)/excitotoxicity, and other cytototoxic conditions related to current therapies such as hyperoxia, caffeine, and anesthetics. We will then discuss the need to investigate more in detail and improve our understanding of the different oligodendrocyte and neuronal death pathways that can play, even if not massive in some conditions, a role in neurological deficits developed by both preterm humans and animal models.
Main Different Types of Cell Death
It is now globally accepted that three main morphological types of cell death exist as previously proposed by Peter Clarke in 1990 in physiological cell death conditions during development (Clarke, 1990): type 1 or apoptosis, type 2 or autophagic cell death, and type 3 or necrotic cell death (Figure 1). This classification is also valid for cell death involved in different pathologies, including brain injury. However, it has been described that, especially in the immature brain, hybrid morphological forms of cell death could occur in the same dying cell, reflecting the presence of interconnections between the molecular pathways controlling the execution of different cell death types (Portera-Cailliau et al., 1997; Puyal et al., 2013). The multiplicity of the cell death pathways and their positive/negative crosstalks that depend, moreover, on the cell type, their maturity, and the type of stress (intensity, duration, hypoxic, ischemic, inflammatory, etc.) represent then a major challenge for the design of neuroprotective strategies (Puyal et al., 2013). We will do here a very brief summary of the three main types of cell death (reviewed in Northington et al., 2011; Puyal et al., 2013).
FIGURE 1.
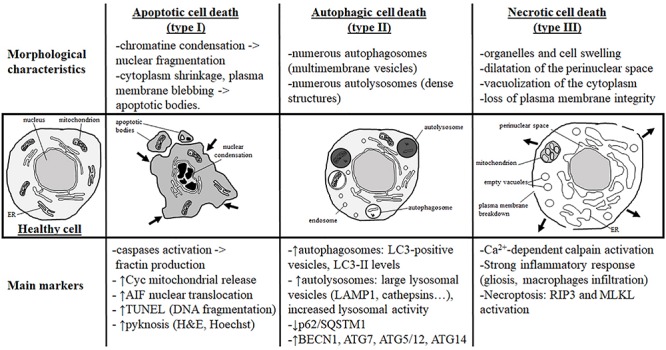
Morphological characteristics and most currently used markers of the three main types of cell death. Analysis of the dying cell ultrastructure is the most reliable method to identify the cell death type. The hallmark of apoptotic cell death (or type I) is a modification of the nuclear structure with presence of chromatin condensation and nuclear fragmentation. The cytoplasm shrinks and plasma membrane deforms until it forms apoptotic bodies that will be removed by phagocytic cells. Molecular markers of apoptosis are caspases (mainly caspase-3) and sometimes cytochrome c (Cyc) mitochondrial release in the cytosol. As a marker of caspase-independent apoptosis, nuclear translocation of AIF (Apoptosis-Inducing Factor) is mainly used. Pyknosis (condensation) of the nucleus shown by H&E (hematoxylin/eosin) or Hoechst stainings is also a main feature of apoptosis. TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) is a staining revealing DNA fragmentation used as a marker of apoptosis with caution if not complemented with a cleaved-CASP3 immunostaining. Autophagic cell death (or type II) is mainly characterized by the presence of numerous autophagosomes (multimembrane vesicles containing intact cytoplasmic material such organelles) and autolysosomes (electron dense structure due to material at different stage of degradation). To conclude on autophagy flux with biochemical markers, it is necessary to study both autophagosome formation and autolysosome degradation. In autophagic cell death, both processes have to be enhanced. Autophagosome presence is reflected by the number of LC3-positive vesicles or by LC3-II level of expression on immunoblot. When autophagic degradation activity is enhanced, vesicles positive for lysosomal markers [such as LAMP1 (lysosomal-associated membrane protein 1) or cathepsins] are increased in number and size since autolysosomes are larger than primary lysosomes. The decrease in p62/SQSTM1 (Sequestosome-1), an autophagosome cargo protein degraded selectively by autophagy, reveals enhanced autophagy. In some cases, the level of autophagy-related (ATG) proteins such as BECN1 (BECLIN1/ATG6), ATG7, ATG5/ATG12, or ATG14 is increased. In necrotic cell death (or type III), ultrastructural changes are mainly cytoplasmic with organelles and cell swelling and presence of empty vacuoles. Perinuclear space is also dilated. Plasma membrane integrity can be finally lost, inducing a strong inflammatory response. Very few tools are available to identify necrotic cell death and mainly indirect markers are used such as strong inflammation response or Ca2+-dependent activation of calpains [mainly suggested by the production of a 145- to 150-kDa calpain-dependent cleavage of spectrin (fodrin)]. Necroptosis type of necrosis could be investigated by RIP3/RIPK3 (Receptor-interacting serine/threonine-protein kinase) or MLKL (mixed lineage kinase domain-like) expression and activation.
Apoptotic Cell Death
Since about 50 years, apoptosis is largely the best-known and characterized form of programmed cell death, which has been proposed to be the most important mechanism of delayed cell death occurring in different physiological and pathological conditions, including the developing brain. The most obvious morphological changes occurring during apoptosis concern the nucleus. Chromatin is compacted and the nucleus condensates, leading to the characteristic apoptotic feature of pyknosis and finally nuclear fragmentation. The cytoplasm is also shrunk and plasma membrane is distorted, forming blebbs that will finally detach and then form apoptotic bodies (Figure 1).
At the molecular level, apoptosis has been extensively characterized and can occur through two different but convergent pathways: the intrinsic and the extrinsic pathways (Galluzzi et al., 2018).
The intrinsic apoptotic pathway, also called the mitochondrial pathway of apoptosis, involves mitochondria, which represent a reservoir of apoptotic factors (stored in the intermembrane space of mitochondria). The balance of pro- and anti-apoptotic proteins from the BCL2 protein family controls mitochondrial membrane integrity. These proteins are responsive to intracellular stress coming from mitochondria, endoplasmic reticulum (ER), cytoskeleton, or nucleus. Pro-apoptotic proteins are directly (BAX and BAK) or indirectly (BAD, PUMA, BIM) involved in mitochondrial membrane permeabilization. As anti-apoptotic proteins, BCL2, BCL-xL, or MCL1 prevents BAX and BAK-mediated permeabilization of the outer mitochondrial membrane and then the release of apoptotic factors in the cytosol. Among these factors, some, such as cytochrome c, SMAC/Diablo, and Omi/HtrA2, activate caspase-dependent pathways and others, such as AIF and endoG, will trigger DNA fragmentation independently of caspases.
Caspases, which are cysteine-aspartic proteases, are the main common effectors of both intrinsic and extrinsic apoptotic pathways. Caspases activation is then widely considered as a gold standard marker of apoptosis in many studies including those on human tissues. Initiator caspases (CASP), CASP2, -8, -9, -10, and -12, activate the executor caspases (CASP3, -6, and -7) (Riedl and Shi, 2004), which have as substrates many essential proteins such as structural proteins, enzymes involved in DNA repair, or transcription factors resulting in the morphological changes induced by apoptosis (cell shrinkage, membrane blebbing, chromatin condensation, and DNA fragmentation).
Whereas CASP9 is involved in the intrinsic pathway of apoptosis, CASP8 and 10 are the initiators of the extrinsic pathway. CASP8 and -10 activations depend on the binding of extracellular death signals (such as TNFα, FasL, and TRAIL) on “death” receptors, resulting in the formation of a death-inducing signaling complex (DISC) used as a platform for CASP8 autoactivation. CASP8 can then directly activate the executor caspases (CASP3, -6, and -7). However, CASP8 can also interfere with the intrinsic pathway of apoptosis by mediating the cleavage of BID and then producing a truncated form of BID (tBID) able to induce BAX anchoring to the mitochondrial membrane and subsequent membrane permeabilization.
Finally, the executor caspases (CASP3, -6, and -7) could also be activated by CASP12 in some ER stress conditions (oxydative stress, unfolded protein response, etc.).
Autophagic Cell Death
Autophagic cell death is morphologically characterized by the presence of numerous autophagosomes and autolysosomes in the cytoplasm of the dying cells, reflecting an excessive autophagy activity (Figures 1, 2).
FIGURE 2.
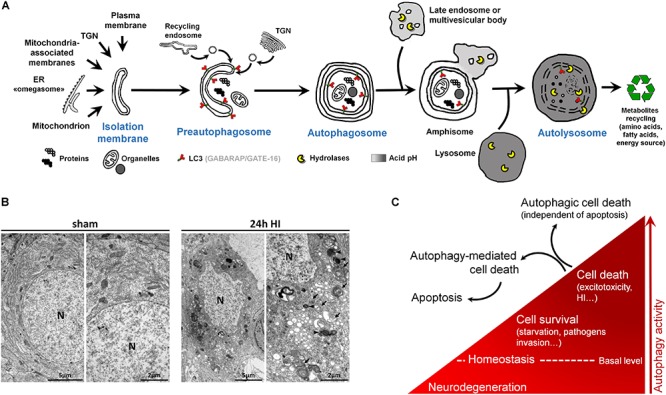
Autophagy and autophagic cell death. (A) Schematic illustration of (macro)autophagy degradation and recycling process showing the following: (1) isolation membrane formation from various possible intracellular membrane sources; (2) elongation and incurvation of the preautophagosome around the cytoplasmic material that has to be degraded (proteins, organelles); (3) closure end-to-end of the preautophagosome forming the autophagosome, a double-membrane vesicle containing undigested materials; (4) fusion of autophagosome with endosomes; (5) fusion of this amphisome with a lysosome to form a mature autophagosome able to degrade its content (autolysosome) thanks to lysosomal hydrolases. LC3 = Microtubule-associated protein 1A/1B-light chain 3 (and other family members). LC3 is the main marker of autophagosome. TGN = trans-Golgi network, ER = endoplasmic reticulum. (B) Electron micrographs showing CA3 neurons presenting features of autophagic cell death 24 h after perinatal hypoxia–ischemia (HI) in P7 rats (unilateral common carotid artery occlusion followed by 2 h of hypoxia at 8% of oxygen). Dying neurons displayed both numerous multimembrane vacuoles loaded with cytoplasmic material (autophagosomes, arrow) and electron dense vesicles containing digested material (autolysosomes, arrowhead). Note that nuclei (N) did not show chromatin condensation. Electron micrographs of CA3 neurons from sham-operated rat brain present healthy neurons. This study was carried out in accordance with the Swiss National Institutional Guidelines for Animal Experimentation. All experiments and methods were approved by the Veterinary Office of the Canton de Vaud. (C) Scheme illustrating the dual role of autophagy in neurons. At basal level, autophagy maintains cellular homeostasis. A reduction or impairment of autophagy can lead to neurodegeneration (such as for some proteinopathies). Autophagy can be activated, above its basal level, as a survival response to maintain energy level during a period of starvation, for example, or to eliminate invading bacteria or virus. However, in other stress conditions such as excitotoxicity, enhanced autophagy could lead to cell death as a mechanism of cell death by itself, independently of apoptosis or necrosis (autophagic cell death or type II), or as a mediator of another type of cell death, mainly apoptosis (autophagy-mediated cell death).
Macroautophagy, the main type of autophagy (beside microautophagy and chaperone-mediated autophagy) (Cuervo, 2004), is a physiological intracellular catabolic process using lysosomes for degradation. Macroautophagy (hereafter called autophagy) consists in the engulfment of some cytoplasmic material that has to be degraded (such as impaired long-lived proteins and organelles), in an intermediate compartment termed autophagosome. Autophagy can be divided into three major steps: (1) the induction phase, which consists in the isolation of a membrane termed the preautophagosome; (2) the autophagosome formation, which involves elongation, incurvation, and finally closure of the preautophagosome, resulting in multimembrane vesicles containing cytoplasmic material including organelles; and (3) the autophagosome maturation, which consists in the fusion of the autophagosome with a lysosome leading to the formation of the functional degradative compartment of autophagy, the autolysosome (Figure 2A).
Besides, multiple analyses are needed to characterize the presence of autophagy (Klionsky et al., 2016), and the analysis of the autophagosomal marker, microtubule-associated protein 1 light chain 3 (LC3), is an essential step (Figure 2A). This ATG protein (homologue of yeast ATG8) is subject to an important ubiquitylation-like modification resulting in LC3 conjugation to a phosphatidylethanolamine (PE), a membrane phospholipid that allows LC3 to be incorporated into the membrane of early and late autophagosomes. Despite the fact that other methods could be used (such as the gold standard electron microscopy), an increased number of LC3-positive dots combined with an increased lysosomal activity (e.g., monitored by p62/SQSTM1 degradation) is, in many cases, sufficient to conclude for enhanced autophagy.
The recent discovery (since the 1990s) of more than 30 evolutionarily conserved autophagy-related (ATG) genes has largely contributed to understand the roles of autophagy in cell survival and death (Levine and Kroemer, 2019). Due to the important roles of autophagy in maintaining cell homeostasis and in cell adaptation to stress conditions (such as starvation or pathogen invasion) (Marino et al., 2011), the existence of a type of cell death mediated exclusively by autophagy has long been controversial (Debnath et al., 2005; Kroemer and Levine, 2008; Clarke and Puyal, 2012). However, it is now accepted that in specific conditions, autophagic cell death can occur, independently of necrosis and apoptosis, such as during development (morphogenesis or metamorphosis) or in particular stressful conditions (Clarke, 1990; Liu et al., 2013; Ginet et al., 2014b) (Figures 2B,C). Despite this, pure autophagic cell death is rarely observed, and there is now growing evidence that autophagy-mediated cell death is more frequently involved, meaning situations where autophagy is acting upstream of another type of cell death (Kriel and Loos, 2019), especially apoptosis (Grishchuk et al., 2011; Puyal et al., 2013) (Figure 2C).
Necrotic Cell Death
Necrosis (or type 3B cell death) (Clarke, 1990) is usually considered as a passive form of cell death (unregulated and energy-independent) occuring rapidly and irreversibly in conditions precluding apoptotic and autophagic mechanisms (Golstein and Kroemer, 2007). Necrosis is characterized morphologically by the presence of dilated organelles (primarily mitochondria and ER), the vacuolization of the cytoplasm (empty vacuoles), and the swelling of the perinuclear space. The nucleus is first preserved; however, it ends up also swelling as the cell rounds up until plasma membrane ruptures (Figure 1), leading to the release of cellular contents [including damage-associated molecular patterns (DAMPs)] that promote and exacerbate the inflammatory response.
More recently, programmed forms of necrotic cell deaths have been identified, demonstrating that, in some cases, necrotic cell death could be regulated. One of the best characterized programmed form of necrosis is necroptosis. Necroptosis is initiated by the activation of death receptors (Kitanaka and Kuchino, 1999; Galluzzi et al., 2017) and is dependent on the RIPK1/3-MLKL pathway leading to the translocation of MLKL to intracellular and plasma membranes, and then inducing membrane permeabilization and rupture.
Encephalopathy of the Prematurity in Humans and Evidence of Cell Death
As mentioned in the Introduction, EoP that covers the different injury patterns related to premature birth can affect either the WM or GM but, depending on the nature and the severity of the lesion, the extent of brain lesions could vary strongly between the individuals. With new emerging MRI techniques, better characterization of EoP now became possible and opens new understanding, such as functional connectivity, diffusion tensor imaging, fractional anisotropy measures and fiber tractography, and automated segmentation techniques for volume determination and growth (Ball et al., 2013; Gozdas et al., 2018; Schneider et al., 2018; Duerden et al., 2019; Jakab et al., 2019). The most prominent findings of EoP in VPT are (1) decreased global brain volume due to white (WM) and gray matter reduction [(GM); cortical GM as well as deep nuclear GM], (2) hydrocephalus ex vacuo (CSF), and (3) connectivity impairment (Dubois et al., 2008; Ball et al., 2013; Chau et al., 2013; Kidokoro et al., 2013; Friedrichs-Maeder et al., 2017; Gozdas et al., 2018; Duerden et al., 2019; Jakab et al., 2019).
This new understanding facilitates the development of new preclinical animal models, but still implementation of multiple features of EoP seems difficult (see review from Kinney and Volpe, 2012) and specific features of cell death in particular models, either neuronal or glial cell death, are lacking.
While it is widely accepted that cell death could happen in those conditions, cell death evidence in preterm brain injury are sparse and controversial, notably because few studies on human preterm brain tissue have characterized and determined either the type of cell death or, more importantly, the nature of the dying cell type (Tables 1, 2). Another point to mention is that in the studies claiming that cell death is not involved, not all the different types of cell death have been investigated (most often only apoptotic cell death markers were considered) and one can speculate that cell death types other than apoptosis might be involved in EoP. Finally, when comparing the different cohorts in Tables 1, 2, it is important to keep in mind that some infants have experienced a re-direction of care (most European countries) whereas some infants have died reaching their natural life span (US and Canada) and that the extent of cell death might have been influenced by different ethical practices. Moreover, as shown in Table 1, most of the studies were performed with cohorts from the Boston Children’s Hospital, meaning that some data could come from the same tissue collection.
TABLE 1.
Cell death after WMI in human preterm brain from autopsy.
| Type of WMI | Country (years of sample collection) | GA | PNA | Pathological features | Cell death markers | + Cell types | ROIs | References |
| Diffuse PWMI | US | Not indicated PCA: 28 to 42 w | Not indicated | ↓ preOL (O4+), ↑ oxidative stress in O4+, no cortical neuronal loss | H&E, TUNEL c-CASP3, fractin IHC | O4 | DWM | Back et al., 2005 |
| Diffuse WMI | CA (1983–2000) | 25 to 39 w | < 1 to 22 w | ↓ immature OL, ↑ preOL (O4+ O1-); ↑ GFAP, CD44 and HA; ↑ Iba-1 necrotic foci (microcysts) 59% ↑ focal axonopathy | H&E c-CASP3 IHC axonopathy (β-APP, fractin) | n.d. | WM | Buser et al., 2012 |
| Diffuse WMI | US (2003-2010) | 22 to 42 w | < 1 to 9 w | ↑ GFAP, CD44 and HA; ↑ Iba-1 necrotic foci (microcysts) 36% ↑ Olig2, ↑ focal axonopathy | H&E, c-CASP3 IHC, axonopathy (β-APP, fractin, SMI-312 IHC) | n.d. | WM | Buser et al., 2012 |
| PVL Diffuse WMI | FR | -26 to 38 w -26 w | -0 d to 2.2 w -7 w | -cysts, ↑ Vimentin -↑ Vimentin | TUNEL | GFAP, Vimentin | CTX, PWM, SCWM | Gelot et al., 2009 |
| PVL | US (1997–1999) | 32.8 ± 3.1 w | 3.7 ± 4.1 w | ↑ WM and GM gliosis, focal necrosis; ↑ neuronal loss | H&E, GFAP IHC | n.d. | TH, Hipp, GP, cerebellar nuclei | Pierson et al., 2007 |
| PVL | United Kingdom | 24 to 28.9 w | 1 d to 5.1 w | ↑ GFAP, ↑ Iba-1, ↓ neurons, ↑ LC3-II | LC3-II IHC | n.d. | TH | Vontell et al., 2015 |
| PVL | US | 27 to 40 w | 0 to 64 w | ↑ gliosis (GFAP), ↑ CD68, ↑ protein nitration (O4+, O1+), ↑ oxidative stress (O4+, O1+ and GFAP+) | Hematoxylin, TUNEL, Oxydative stress | O4, O1 | WM | Haynes et al., 2003 |
| PVL | US | 25 to 40 w | 0 to 8 w | ↑12/15-LOX (O4+, O1+ and APC+) in diffuse PVL component ↑12/15-LOX (CD68+) in cyst | H&E, TUNEL, Oxydative stress | n.d. and 12/15-LOX+ | WM | Haynes and van Leyen, 2013 |
| PVL | US | 34.5 ± 1.1 w | 7.0 ± 3.5 w | Diffuse astrogliosis no overall differences in olig2, MBP, c-CASP3 and KI67 ↑ c-CASP3 in necrotic foci | H&E c-CASP3 IHC | n.d. | PWM | Billiards et al., 2008 |
| PVL | US(1993–2007) | 32.5 ± 4.8 w | 4.1 ± 6.6 w | ↑ gliosis (GFAP), ↑ CD68, ↓ neuronal density, ↑ axonal damage | H&E, fractin IHC | n.d. | TH | Ligam et al., 2009 |
| PVL | US | 36 ± 3 w | 7.5 ± 17 w | ↑ gliosis (GFAP), ↑ axonopathy, ↑ GM damage (TH31%, CTX15%) | H&E, β-APP IHC, fractin IHC | n.d. | WM, TH, CTX | Haynes et al., 2008 |
| PVL | US(1993–2007) | 33.9 ± 4.3 w | 5.9 ± 14.0 w | ↓ layer V cortical neurons (MAP2+) No difference in cortical thickness ↑ cortical gliosis (GFAP) | H&E, fractin IHC | MAP2 | CTX | Andiman et al., 2010 |
| PVL | US | 32.8 ± 4.1 w | 1.9 ± 2.3 w | ↓ granular neurons (MAP2+) | H&E | MAP2 | CWM, PWM, SP | Kinney et al., 2012 |
APC = adenomatous polyposis coli (mature OL); CA = Canada; CaR = calretinin; c-CASP = cleaved caspase; CTX = cerebral cortex; CWM = central white matter; d = day; DWM = deep white matter; FR = France; GA = gestational age at birth; GABA = g-aminobutyric acid; GAD = glutamic acid decarboxylase; GE = ganglionic eminence; GFAP = Glial fibrillary acidic protein; GM = gray matter; H&E = hematoxylin–eosin staining; Hipp = hippocampus; IHC = immunohistochemistry; LAMP1 = lysosomal-associated membrane protein 1; LC3 = microtubule-associated proteins 1A/1B light chain 3B; LOX = lipoxygenase; MAP2 = microtubule-associated protein 2; MBP = Myelin Basic Protein; n.d. = not defined; O1 = mature OL; O4 = immature preOL; OL = oligodendrocyte; PNA = postnatal age; PTL = perinatal telencephalic leukoencephalopathy; PVL = periventricular leukomalacia; PWM = periventricular white matter; PWMI = periventricular white matter injury; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter; SP = subplate; TH = thalamus; United Kingdom = United Kingdom; US = United States; w = weeks; WM = white matter; WMI = white matter injury; + = positive.
TABLE 2.
Cell death after IVH in human preterm brains from autopsy.
| Type of insult | Country (years of sample collection) | GA | PNA | Pathological features | Cell death markers | + Cell types | ROIs | References |
| IVH | DE + NL (1999–2006) | 23 to 30 w | 1 to 4 w | ↑ sFas in CSF | sFas in CSF | – | CSF | Schmitz et al., 2011 |
| CA | 18 to 28 w | 1 to 98 d | ↓ KI67+ cells, ↑ apoptosis | H&E, TUNEL, c-CASP3 IHC | n.d. | GE | Del Bigio, 2011 | |
| US | 23 to 27 w | 1 to 5 d | ↑ apoptosis, ↑ microglial infiltration (CD68+) | TUNEL, c-CASP3 IHC, Sytox green | n.d. | GM, PVZ | Georgiadis et al., 2008 | |
| DE (1999–2001) | 18 to 25 w | Not indicated | ↑ sFas in CSF, no differences in sFasL and c-CASP3 | sFas and sFasL c-CASP3 activity | – | CSF | Felderhoff-Mueser et al., 2003 | |
| US (1990–1998) | 26 ± 12 w | Severe IVH: 6 ± 6.5 d Mild IVH: 12 ± 5 d | ↑ TUNEL (astrocytes and OL) | H&E, TUNEL | n.d. | WM | Chamnanvanakij et al., 2002 | |
| HU (1993–1999) | 24 to 36 w | 3 d to 27 w | WM disorganization ↑ karyorrhexis | H&E, TUNEL | n.d. | WM, germinal layer | Hargitai et al., 2001 |
CA = Canada; c-CASP = cleaved caspase; CSF cerebrospinal fluid; d = day; DE = Germany; sFas = soluble Fas; sFasL = soluble fasL; GA = gestational age at birth; GE = ganglionic eminence; GM = gray matter; H&E = hematoxylin–eosin staining; HU = Hungary; IHC = immunohistochemistry; IVH = intraventricular hemorrhage; n.d. = not defined; NL = Netherlands; PNA = postnatal age in weeks; PVZ = periventricular zone; ROIs = regions of interest (i.e., where cell death is detected); US = United States; w = weeks; WM = white matter; + = positive.
WM Injuries and Periventricular Leucomalacia (PVL)
The most important type of WM injury (WMI) in EoP is the PVL (malacia: infarction and leucos: white), first described as focal coagulative necrosis occurring in deep hemispheric WM (Banker and Larroche, 1962). The cPVL is the most severe form of PVL, whose incidence has decreased fivefold in the last decade and which carries the highest burden of neurological morbidity, especially cerebral palsy. Depending on the centers, the incidence of cPVL is around 4–8% for the babies born before 32 weeks of gestation and around 6 to 12% for babies born before 27 weeks of gestation (McGowan and Vohr, 2019).
cPVL is characterized by the presence of large focal periventricular WM necrotic lesions (necrotic foci), where all the cells are destroyed without specificity regarding the cell type (pan-cell death). These necrotic foci (evolving in cyst) are surrounded by reactive glial and microglial cells localized in the neighboring WM site where delayed cell death has been proposed to occur. Histological observations have reported that cPVL cases are characterized, in addition to the presence of necrotic foci, by a massive loss not only of WM (i.e., evidenced by the thinning of corpus callosum and subcortical WM) but also of GM (strong volume reduction of either cortical and deep nuclear structures). Several studies have reported increasing presence of apoptosis (mainly evidenced by TUNEL staining and c-CASP-3 immunohistochemistry) in cPVL in WM and GM, but few of these studies have identified the cell type of these apoptotic dying cells (Hargitai et al., 2001; Chamnanvanakij et al., 2002; Back et al., 2005; Robinson et al., 2006; Pierson et al., 2007; Billiards et al., 2008; Haynes et al., 2008; Ligam et al., 2009; Andiman et al., 2010; Zubiaurre-Elorza et al., 2011; Buser et al., 2012; Kinney et al., 2012; Haynes and van Leyen, 2013; Vontell et al., 2015) (see Table 1). However, apoptosis has been reported to mediate cell death of surrounding necrotic foci pre-oligodendrocytes (O4+ cells) (Haynes et al., 2003; Back et al., 2005; Robinson et al., 2006; Haynes and van Leyen, 2013), astrocytes (Gelot et al., 2009), but also of neurons in different locations including WM, subplate, cortex, basal ganglia, thalamus, and hippocampus (Robinson et al., 2006; Pierson et al., 2007; Ligam et al., 2009; Andiman et al., 2010; Kinney et al., 2012; Haynes and van Leyen, 2013; Vontell et al., 2015). Interestingly, the density of a type of subcortical neurons, granular neurons (GAD67/65+), is significantly reduced not only in the periventricular and central WM, but also in the subplate region in the presence of PVL, suggesting that this particular subtype of neurons is more selectively sensitive to preterm brain injury (Robinson et al., 2006). Then, diffuse axonal injury (distant from necrotic foci) is also observed in the WM and positive for the apoptotic marker fractin (Haynes et al., 2008; Andiman et al., 2010; Buser et al., 2012). This widespread axonopathy in PVL is reflecting either secondary degeneration to GM damage (such as thalamus) or primary alteration following a direct injury to the axon.
Focal or diffuse nondestructive lesions characterize a milder form of WMI with an incidence up to 20 to 25% in VPT infants (Schneider and Miller, 2019). This form is more frequently seen in preterm neonates in recent years and has been histologically characterized by the absence of focal necrotic lesions and the presence of diffuse reactive astrocytes (Billiards et al., 2008; Buser et al., 2012) and activated microglia (without macrophages infiltration) maintained in a pro-inflammatory state (Haynes et al., 2003; Buser et al., 2012). These observations are mostly confirmed by human imaging studies (Kidokoro et al., 2014; Van’t Hooft et al., 2015; Schneider et al., 2016; Synnes and Hicks, 2018), with microstructural alterations measured on advanced magnetic resonance (MR) techniques such as diffusion-weighted imaging (DWI) and apparent diffusion coefficients (ADC), T1 maps, and fractional anisotropy vectors (FA) and interesting longitudinal observations.
Few studies on postmortem tissue of human preterm suggested the absence of neuronal and cortical injury in diffuse WMI (dWMI) (Back et al., 2005; Robinson et al., 2006; Pierson et al., 2007) (see Table 1). Moreover, the study of Verney et al. (2012) reported that there was no significant loss of the number of Olig2+ oligodendrocytes in diffuse lesions (near necrotic foci) in preterm non-cystic PWMI (Verney et al., 2012). Their conclusion was that axonal injury could occur without pre-oligodendrocytes death. Billiards et al. (2008) showed also no decrease in Olig2+ cell density. However, they observed Olig2+ cell proliferation and an increase in Olig2+ cell density in necrotic foci and they assumed that if some loss of pre-oligodendrocytes still occurs, it could be compensated by a subsequent proliferation of OL progenitors (Billiards et al., 2008). Anyway, even if pre-oligodendrocytes death did not occur, this would not necessarily indicate that there is no cell death in PWMI (especially in foci). Billiards et al. (2008) showed the presence of apoptotic cells (without identifying the cell type) in necrotic foci (microcysts) and reported also that Olig2+ cell (that “did not appear apoptotic”) density was not affected in periventricular necrotic foci or in the surrounding WM. The study of the group of Charriaut-Marlangue showed that the majority of astrocytes “in the penumbral WM” were apoptotic (TUNEL+) in human preterm cPVL and in one case of non-cystic PVL (Gelot et al., 2009). Regarding these two studies, one can hypothesize that astrocytes could be the apoptotic cells described by Billiards and colleagues (2008). Another hypothesis could be that pre-oligodendrocytes death, even if not massive, is occurring through an apoptotic-independent mechanism, i.e., an alternative cell death pathway.
Apart from few studies reporting the loss of pre-oligodendrocytes (preOL) (Haynes et al., 2003; Haynes and van Leyen, 2013), it appears that the main mechanism of the glial dysfunction in dWMI is not due to glial cell death, but more due to dysmaturation of the oligodendrocyte precursor cells (PreO4+) and the imbalance and implication of “bad” microglia (M1) and “good” (M2) microglia phenotypes (Billiards et al., 2008; Buser et al., 2012). This disequilibrium seems to be responsible for the dysmyelination and the gliosis observed for instance in dWMI. Evidence of increased oxidative stress has been reported in immature OLs in dWMI, suggesting that oxidative stress could be a key player of the dysmaturation of OLs supporting the formation of dWMI lesions (Haynes et al., 2003; Billiards et al., 2008; Buser et al., 2012; Haynes and van Leyen, 2013). Multiple preclinical and clinical studies have shown that preOL are less resistant to radical stress, certainly because of decreased expression and function of antioxidant enzymes (such as SOD and GN-oxidase) compared to more mature babies, such as term or even later age (Van’t Hooft et al., 2015).
In summary, cell death is rarely reported in human preterm WMI and PVL tissue from autopsy and cell death characterization (co-localization of cell death and cell type markers) is either missing or sparse.
Intraventricular and Intraparenchymatous Hemorrhages
Severe to moderate cerebral intraventricular hemorrhage (IVH grades III and IV or newly IPE) in preterm infants continues to be a major clinical problem and a neurological issue, occurring in about 5–10% of VPT. In contrast to cPVL, the incidence of IVH has remained stable over the last decade. Over 40% of surviving infants with IVH grade III develop post-hemorrhagic ventricular dilatation and about one third develop severe neurological impairment, such as cerebral palsy and mental retardation. To date, there is only little evidence for therapy to prevent infants from developing either hydrocephalus or serious neurological disability in this condition. There is one trial using a drainage and fibrinolytic approach (DRIFT Trial) that has shown some potential benefits on the neurological outcome, but data were inconclusive due to follow-up problems and other methodological issues (Luyt et al., 2019). There is also a management trial (ELVIS TRIAL) comparing early versus late approach in lumbar tapping and rickham insertion, which is underway (de Vries et al., 2019). It is known that blood rapidly accumulates within the ventricles following IVH, and this leads to disruption of normal cerebrospinal fluid flux and can cause obstruction and increased local tissue pressure as one of the potential etiological mechanisms (Klebe et al., 2020). As described in Table 2, reports about preterm human data, brain cell death, and IVH are rare, and consist in analyses of tissue from autopsy and clinical data in preterm infants, about apoptotic and necrotic markers detected in the cerebrospinal fluid (Felderhoff-Mueser et al., 2003; Schmitz et al., 2011), in the ganglionic eminence (Del Bigio, 2011), and in the periventricular zone (Hargitai et al., 2001; Chamnanvanakij et al., 2002; Georgiadis et al., 2008). However, to date, no study has systematically investigated the involvement of the different types of cell death and characterized the type of cell specifically affected by IVH.
Iatrogenic Preterm Brain Injury
Iatrogenic conditions involved during prematurity are contributory factors changing the normal developmental trajectory of the brain, notably the impact of nutrition on growth and maturation (Beauport et al., 2017; Schneider et al., 2018) and the use of supplemental oxygen for instance. Further, some drugs such as caffeine as well as anesthetic and sedative drugs might influence physiological cerebral development. In regard to cell death types and iatrogenic factors, there are no direct reports in human tissue associating them with cell death types and cells involved, and only suppositions can be made based on preclinical studies as detailed extensively further.
Hyperoxia
When leaving the hypoxic intrauterine milieu, the preterm infant will be exposed to either diminished or excessive oxygen saturation and variation in cerebral blood flow, which are potentially harmful for the retina, the lung, but also the brain and the neurodevelopmental outcome at long term [Safe Boosc (Askie et al., 2011; Panfoli et al., 2018)]. Therefore, targeting specific oxygen saturation, and regional cerebral oxygenation levels through different methods such as near-infrared spectroscopy (NIRS) are widespread in the neonatal intensive care units, and several trials are still ongoing to confirm the detrimental effect of hypo/hyperoxia and free radical stress in preterm brain injury (Klebe et al., 2020). These data are supported by a tremendous work of body on preclinical models, which are described later.
Caffeine
Caffeine therapy for the apnea of prematurity syndrome (by stimulating the immature respiration center) is the most widespread medication used in premature infants. Babies start to be treated with caffeine between day of life 1 until they reach 34 weeks of corrected age and doses are relatively high (between 5 and 10 mg/kg/day 1 × daily). Clinical studies such as the CAP Trial (Caffeine for Apnea of Prematurity) have shown a protective effect of caffeine administrated in VPT infants, in terms of neurodevelopmental outcome and mortality (Schmidt et al., 2012, 2017; Panfoli et al., 2018). While the short-term data at 18 months was slightly convincing and significant, the effect about the neurodevelopmental outcome was ephemeral at 5 years of follow-up. In fact, a pilot study using high-dose caffeine therapy in premature infants showed increased cerebellar injury and neurobehavioral deficits when compared to low dose (McPherson et al., 2015). This controversy between preclinical worrying and clinical reassuring data is not really rational and raises many questions and doubts. There is probably an effect of the dose. Therapeutical levels preventing apnea consequences in babies are low and harmless, whereas higher doses and/or the multiplicity of drugs combined are linked to neurotoxicity, but this has still to be confirmed.
Anesthetics
When looking at clinical risk factors for neurodevelopmental outcome, surgery is a major and constant contributor for impaired neurological outcome, and these concerns are true for neonates at term and premature infants (Sun, 2010; Morriss et al., 2014; Sinner et al., 2014; Stolwijk et al., 2016; Walker et al., 2018). Premature infants may undergo several surgical procedures such as ductus ligation, laparotomies, and thoracotomies defined as major surgeries and herniotomies or endoscopic laryngoscopies defined as minor surgeries. It is still unclear whether the surgical stress, the pain perception, or the administrated drugs for anesthesia are neurotoxic (Broad et al., 2017; Walker et al., 2018), but the combination of both seems to play a major role. This also explains the development of several preclinical models in rodent and primate animals in order to answer the unsolved questions (Baud and Saint-Faust, 2019).
There are only very few clinical data on humans and associated secondary effects with anesthetics/or surgery. The group of Buonocuore measured oxidative stress (Stolwijk et al., 2017), using non-protein bound iron (NPBI) in neonates who underwent noncardiac surgery. These biomarkers were found to be predictive for brain injury post-operatively. Walker et al. (2018) in a cohort of United Kingdom Epicure in ELBW infants found an association of neonatal surgery in the volume of the amygdala and the somatosensory response and pain response in young adults ex-preterm. Filan et al. (2012) analyzed post hoc 30 babies exposed to surgery who had more evidence of WMI and smaller brain volumes, particularly in the deep GM. They could not find any difference in the neurodevelopmental outcome.
The most used anesthetic drugs in premature infants are GABA agonists such as thiopental and propofol, and NMDA antagonists (either ketamine or anesthetic gazes such as isoflurane or sevoflurane). Potential effects of anesthetic drugs on the brain development are driven from the multiplicity of preclinical studies, but very few are described on human data (Sun, 2010; Sinner et al., 2014).
Preclinical Models of Preterm Brain Injury and Evidence of Cell Death
Due to the multifactorial etiologies and the heterogeneity of the brain pathologies involved in EoP, the challenge was, over the last decades, to develop preclinical models reproducing as best as possible the pathophysiology of brain injury and/or brain development impairment observed in the premature human brain. However, the use of a single preclinical model of preterm brain injury cannot reproduce exactly the human situation, and in conclusion, each model presents advantages/disadvantages as reviewed by others (Back et al., 2012; Kinney and Volpe, 2012; Jantzie and Robinson, 2015).
Cell death has been investigated in preterm models in three main species: rodent (rat and mice), sheep, and non-human primate (macaque, baboon) (Tables 3–9). Few studies were undertaken in rabbit and pig. Human preterm neonates are highly exposed to WM injury between 23 and 32 weeks of gestation that corresponds in rodents to the postnatal period before 7 days (P1–P6), in sheep to gestational age around 95 days (90–120 days, term at 145 days), and in macaques to gestational age 125–145 days (term at around 160 days). Whereas substantial cell death can occur in severe models such as those of cPVL in rodents (Table 3), the identification of the presence of cell death frequently showed sparse cell death in specific sites both in WM and GM affecting mainly oligodendrocytes (OL) but also neurons. Animal models have contributed to hypothesize that, in preterm brain injury, a primary OL loss during the “acute” phase is followed by a regenerative process producing immature OL that leads to impaired myelination (Rousset et al., 2006; Segovia et al., 2008). Neuronal injury, including not only neuronal death but also deficits in dendrite and spine maturation (Balakrishnan et al., 2013), could be a direct consequence of primary GM injury as well as secondary through axonal deleterious factors linked to primary WM injury (Rousset et al., 2006; Segovia et al., 2008; Back and Volpe, 2018).
TABLE 3.
Cell death in preclinical models of preterm white matter injury following inflammation.
| Species | Time of the insult | Model | Cell death markers (time post insult) | + Cell types | ROIs | Neuroprotectant | References |
| Sheep | 91 d ga | LPS (150 ng/kg), i.v., over 3 d | c-CASP3 (7 d) | n.d. | SCWM | hUCB cells | Paton et al., 2018 |
| 93–96 d ga | LPS (100 ng), i.v. | TUNEL, c-CASP3 (3 d), Cysts (30%) | Olig2, CNPase | CBWM | – | Dean et al., 2009 | |
| 109 d ga | LPS (150 ng/kg), i.v., over 3 d | Pyknotic nuclei (114 d ga) | n.d. | PVWM | hAECs | Yawno et al., 2017b | |
| 5 h, 12 h, 24 h, 2 d, 4 d, 8 d, or 15 d before preterm delivery at 125 d ga | 1 × LPS (10 mg), intra-amniotic | c-CASP3 (2 d to 15 d) | n.d. | WM, Hipp, CX | – | Gussenhoven et al., 2018 | |
| Rat | E17, 6 h before preterm delivery | LPS (1 mg/kg), maternal i.p. | c-CASP3 (6 h) | n.d. | n.d. | Fingolimod | Yavuz et al., 2017 |
| E18 | LPS (1 mg/kg), maternal i.p. | TUNEL (E20) | PDGFαR | SZ | NAC | Paintlia et al., 2004 | |
| E18+E19 | LPS (0.5 mg/kg.), maternal i.p., LPS (0.3 mg/kg.), maternal i.p. | -TUNEL, c-CASP3 (P6) -TUNEL, c-CASP3 (P7) -Impaired autophagy (P1) | n.d. | -PVWM -ST, PVWM, GV -Whole brain | -APC, EPO, SPD – – | Kumral et al., 2007, 2017, Yesilirmak et al., 2007,Rousset et al., 2006 Carloni et al., 2016 | |
| P3 | LPS, i.c. -(10 μg) -(1 mg/kg) | -Fractin (4 d) -RIP1, RIP3 and MLKL RNAs (1 d) -Fractin (1 d) |
-SMI-312 | -ST, -ST, SCWM, CC -Whole hemisphere -CG, CC | – | Ginet et al., 2016, Lodygensky et al., 2014,Pierre et al., 2019 | |
| P3 | LPS (0.25 mg/kg), i.p. | -c-CASP3, TUNEL (2d) | n.d. | -CX, SCWM extracts | -MSC-EVs | Drommelschmidt et al., 2017 | |
| P5 | LPS (10 μg), i.c. | -TUNEL (1 d) -Cysts (30%, injection site) | n.d. | -SCWM (near injection) -CC | -Minocycline – | Fan et al., 2005a | |
| P5 | LPS (2 mg/kg), i.p. | TUNEL, c-CASP3 (1 d) | O4 | CG | Celecoxib | Fan et al., 2013 | |
| P5 | IL-1β (10 ng), i.c. | -TUNEL, c-CASP3 (1d) | Lectin, O4, O1, NF | -Sub-ventricular area, CC, outer layers of CTX | -NBQX | Cai et al., 2004 | |
| Rabbit | 24–30 d ga | LPS, uterine horn, + ceftriaxone (+24 h) | -TUNEL (2 d) -Cysts (∼25–23%) | n.d. | -PVWM, CA2 -PVWM | – | Debillon et al., 2003, Debillon et al., 2000 |
| Rhesus macaque | 130 d ga, 16 h before preterm delivery | LPS (1 mg), i.a. | c-CASP3 | n.d. | PVWM | – | Schmidt et al., 2016 |
APC = activated protein C; CA = cornu ammonis; CBWM = cerebellar WM; c-CASP3 = cleaved caspase-3; CNPase = 2′,3′-cyclic nucleotide 3′-phosphodiesterase; CTX = cortex; d = day; E = embryonic day; ga = gestational age; GV = germinative ventricular zone; hAECs = human amnion epithelial cells; h = hours; Hipp = hippocampus; i.a. = intra-amniotic; i.c. = intracerebral; i.p. = intraperitoneal; i.v. = intravenously; LPS = lipopolysaccharide; MSC-EVs = mesenchymal stem/stromal cells; NAC = N-acetyl cysteine; n.d. = not defined; NF = neurofilament; O4, O1 = oligodendrocyte markers O4, O1; Olig2 = oligodendrocyte transcription factor; P = postnatal day; PDGFαR = platelet-derived growth factor-α receptor; PVWM = periventricular WM; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter; SMI-312 = anti-neurofilament Marker (pan axonal, cocktail); SPD = surfactant protein; ST = striatum; SZ = subventricular zone; TH = thalamus; hUCB = human umbilical cord blood; WM = white matter; nd = not defined.
TABLE 9.
Cell death in preclinical models of iatrogenic preterm brain injury: caffeine and anesthetics.
| Species | Time of the insult | Model | Cell death markers(time post insult) | + Cell types | ROIs | Neuroprotectant | References |
| Rat | E20 | Propofol, maternal i.v. (8.0 mg/kg) + 1 h (1.2 mg/kg/min) | -c-CASP3 (6 h) | -NeuN | -CTX, TH, HTH | Dexmedetomidine | Li et al., 2016a |
| E14 | Sevoflurane, maternal (3.5%) for 2 h | -↑LC3-II, ↑BECLIN-1, ↓SQSTM1, ↓mTOR, TUNEL, ↓BCL2, (2 h, 12 h, 1 d, 2 d) |
-Nestin+ | Whole brain | 3-MA | Li X. et al., 2017 | |
| P3 | Caffeine, 1 × i.p. (80 mg/kg) | c-CASP3 (6 h) | n.d. |
Whole brain (Hipp, ST, TH, CTX) | – | Cabrera et al., 2017 | |
| P4 | Caffeine, 1 × /d i.p., for 3 d (10 mg/kg) | TUNEL (6 h, 12 h, 1 d) | n.d. | HTH, DG | – | Endesfelder et al., 2017a | |
| P6 | Caffeine, 1 × (10 mg/kg) | PARP-1, AIF (1 d or 2 d) | n.d. | Whole brain | – | Endesfelder et al., 2017b | |
| P3 | MgSO4, 1 × /h, for 3 h (4 × 250 mg/kg) | c-CASP3, apoptotic by EM | Neurons | CTX, STcn, STp, Hipp, TH, CB c | – | Dribben et al., 2009 | |
| E20 | Propofol, maternal i.v. (8.0 mg/kg) + 1 h (1.2 mg/kg/min) | -c-CASP3 (6 h) | -NeuN | -CTX, TH, HTH | Dexmedetomidine | Li et al., 2016a | |
| P4 |
Phenobarbital, 1 × /d i.p., for 3 d (50 mg/kg) |
-TUNEL (6 h, 12 h, 1 d) | -n.d. | Frontal CTX, retrosplenial CTX, HTH, TH, DG | – | Endesfelder et al., 2017a | |
| Mouse | E14 | Sevoflurane (2.5%), 2 h | -c-CASP3 | -n.d. | Whole brain | – | (Zheng et al., 2013) |
| P6 |
Sevoflurane (3%) or isoflurane (2%) or desflurane (4/8%), for 2 h/3 h or 6 h |
-c-CASP3, c-PARP, TUNEL (18 h) -c-CASP3, TUNEL (2 d) |
-n.d | -CX, Hipp, Am, TH, ST, CP -Hipp -Hipp |
- -Luteolin -Tanshinone IIA |
Kodama et al., 2011
Wang Y. et al., 2019 Xia et al., 2017 |
|
| P6 | Sevoflurane (3%), 1 × /d for 2 h, for 3 d | ↑LC3-II, ↓SQSTM1, | n.d | Hipp | 3-MA | Wang X. et al., 2019 | |
| P3 | -Midazolam, 1 × i.p. (6 mg/kg); ketamine, 1 × i.p. (40 mg/kg); fentanyl, 1 × i.p. (40 μg/kg) + caffeine (80 mg/kg) |
c-CASP3 (6 h) | n.d. | Whole brain (Hipp, ST, TH, CTX) | – | Cabrera et al., 2017 | |
| P5–P7 | Propofol, i.p. (50/100 mg/kg) | c-CASP3 | n.d. | STcn, CTX | – | Cattano et al., 2008 | |
| Rhesus macaque | 100–120 d ga |
Isoflurane (5 h) 3 h before preterm delivery |
c-CASP3 | MBP, NeuN | - ST, PT | – | Noguchi et al., 2018 |
| 120 d ga | Propofol, i.v. (5 h), 3 h before preterm delivery | c-CASP3 fractin (3 h) |
MBP, neurons | CTX, STcn, STp, Am, CB, infCOLL CSO, CC, iC |
– | Creeley et al., 2013 | |
| 120 d ga 100–120 d ga |
-Isoflurane, i.v. (0.7–1.5 vol%), 5 h, 3 h before preterm delivery - + caffeine (1 × 80 mg/kg, 1 × /d 20 mg/kg) |
-c-CASP3 (3 h) -↑ c-CASP3 |
-MBP, NeuN -MBP, NeuN |
CTX, STcn, STp, Am, CB, -CB, ST |
– – |
Creeley et al., 2014
Noguchi et al., 2018 |
|
| 120 d ga | Ketamine, i.v. (10 mg/kg) + 5 h (10–85 mg/kg/min) | c-CASP3 (3 h) | n.d. | CTX, STcn, STp, Am, GP, CB | – | Brambrink et al., 2012 | |
AIF = apoptosis-inducing factor; Am = amygdala, CB = cerebellum; CC = corpus callosum; c-CASP = cleaved caspase; CSO = centrum semiovale, CG = cingulum; CTX = cortex; Cyc = cytochrome c; d = day; DG = dentate gyrus; E = embryonic day; eC = external capsule; EM = electronic microscopy; EPO = erythropoietin; FB = forebrain; GP = globus pallidus; h = hours; Hipp = hippocampus; HTH = hypothalamus; iC = internal capsule, infCOLL = inferior colliculus NAcc = nucleus accumbens; n.d. = not defined; P = postnatal day; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter; ST = striatum; STcn = striatal caudate nucleus; STp = striatal putamen; TH = thalamus; WM = white matter.
The need to better understand preterm brain damage at the molecular level requires selecting the most appropriate species, age (at which the insult is done), and type of injury, as well as the severity of the insult according to the precise addressed question. Preterm birth is by itself a high-risk factor for brain injury but different deleterious events (alone or combined) can occur and aggravate brain development such as inflammation, hypoxia, ischemia, or hemorrhage. Preclinical models were developed to reproduce one or two of these factors. In the present review, we mainly focused on studies revealing the presence of a type of cell death in the different panels of preterm brain injury models using cell death markers (i.e., studies using more than classical histological observations of pyknosis and karyorrhexis or cell counting).
Preclinical Models of Periventricular Leucomalacia
Even if, thanks to the important progress in neonatal care, the incidence of cPVL has considerably decreased over the past decades, cPVL remains a major cause of cerebral palsy (Hielkema and Hadders-Algra, 2016; Pierrat et al., 2017). Some models were developed and found to reproduce the formation of cysts (or necrotic foci), i.e., macroscopic areas of massive cell death surrounded by activated microglia suggesting rapid necrotic mechanisms (Debillon et al., 2003).
In a rat inflammatory model, intracerebral injection of LPS formed cysts at the injection site in the corpus callosum with TUNEL+ cells surrounding the necrotic foci (Fan et al., 2013). Models of intrauterine infection in rabbit and sheep have also been shown to induce the formation of WM cysts (25–30% of survivors) accompanied by apoptotic cell death of WM oligodendrocytes (c-CASP3+) (Dean et al., 2009) and, in rabbit, cell death in the CA2 region of the hippocampus (TUNEL+) (Debillon et al., 2003) (Table 3). These models showed not only that a local strong inflammation can lead to cyst formation but also that fetal systemic inflammation can, for some treated animals, induce necrotic foci formation in the WM and/or apoptotic cell death in both oligodendrocytes and neurons. Many in vitro studies strongly suggested that activated microglia (M1 phenotype) directly contribute to oligodendrocyte and neuron apoptotic death by releasing reactive oxygen species or TNF-α (Dean et al., 2010; Baburamani et al., 2014).
Beside inflammation, excitotoxicity is one of the most common deleterious mechanisms involved in the formation of cysts. Excitotoxicity consists in a massive intracellular increase of calcium initiated by overactivation of excitatory amino acids receptors (mainly glutamate) (Puyal et al., 2013). In the human preterm brain, the expression of N-methyl-D-aspartate (NMDA) glutamate receptors peaks in pre-oligodendrocytes, allowing the WM to be particularly vulnerable to excitotoxic insult (Jantzie et al., 2015b). Then, the model using intracerebral injection of the glutamate receptor agonist ibotenate (Table 6) has been widely used to reproduce some aspects of human preterm PVL, including WM cyst formation, ventriculomegaly, reduction in brain structure volume, and decrease of WM thickness and myelination (Descloux et al., 2018). Previous studies showed that ibotenate-induced excitotoxicity triggers an important inflammatory response involved in cyst formation (Tahraoui et al., 2001; Dommergues et al., 2003). Concerning cell death type, compiling evidence suggested that apoptosis is importantly involved in this model. One study mentioned the presence of c-CASP3+-positive cells in WM (Neubauer et al., 2016). Neurodegeneration of cortical neurons has also been demonstrated by Fluoro-Jade staining and evidence suggested that caspase-dependent (CASP3) and -independent apoptotic mechanisms (AIF nuclear translocation) were implicated in cortical damage without performing, in most of the studies, an identification of the type of dying cells. Astrocytic cell death has been reported to occur in the site of ibotenate injection (Tahraoui et al., 2001). More recently, we have shown that cortical neurons [(RBFOX3)/NeuN+] presented both activation of CASP3 and nucleus fragmentation (Descloux et al., 2018). Moreover, we have clearly demonstrated that apoptotic neurons displayed mixed morphological features of apoptosis (chromatin condensation) and intense autophagy (increased presence of autophagosomes and autolysosomes). Ibotenate increased both the number of autophagosomes (increased LC3-II and LC3+ dots) and autophagic degradation [p62/SQSTM1 reduction and increased number of autolysosomes (LAMP1- and CATHEPSIN B+ vesicles)]. Inhibition of autophagy with 3-methyladenin (3-MA) prevented CASP3 activation, suggesting a role of autophagy upstream of apoptosis as previously shown in other models (Puyal et al., 2009; Grishchuk et al., 2011; Xie et al., 2016). Interestingly, 3-MA afforded strong protection by reducing not only cortical lesion but also WM deficits, indicating that preventing primary neuronal death by autophagy inhibition could reduce WM damage (Descloux et al., 2018).
TABLE 6.
Cell death in preclinical models of excitotoxin-induced preterm brain injury.
| Species | Time of the insult | Model | Cell death markers (time post insult) | + Cell types | ROIs | Neuroprotectant | References |
| Rat | P5 | SCWM ibotenate injection (10 μg) | -c-CASP3, enhanced autophagy (1 d) -c-CASP3, Fluoro-Jade B, AIF (1 d), -cysts -c-CASP3, TUNEL (1 d) |
-NeuN -n.d. -n.d. -n.d. |
-CTX -CTX -PVWM -CTX |
-3-MA -HIP/HAP; S18986, -BDNF -BDNF |
Descloux et al., 2018
Destot-Wong et al., 2009; Haldipur et al., 2014 Husson et al., 2005 |
| Mouse | P5 | SCWM ibotenate injection (10 μg) | -c-CASP3, AIF (5 d) -c-CASP3, AIF, Fluoro-Jade B (1 d) -c-CASP3 (1 d, 2 d), cysts -c-CASP3 (1 d–30 d) -TUNEL -c-CASP3, TUNEL, Fluoro-Jade B (1 d) -TUNEL (1 d) |
-n.d. -n.d. -n.d. -n.d. -GFAP -n.d. -n.d. |
-CTX, SCWM -CTX -CTX -CTX -SCWM -CTX -CTX |
- -FBP -CASP2 KO -Substance P - -NAP -melatonin |
Griesmaier et al., 2010; Neubauer et al., 2016
Dommergues et al., 2003; Rogido et al., 2003; Wegleiter et al., 2014 Carlsson et al., 2011 Medja et al., 2006 Tahraoui et al., 2001 Sokolowska et al., 2011 Husson et al., 2002 |
AIF = apoptosis-inducing factor; c-CASP3 = cleaved caspase-3; CTX = cortex; d = day; FBP = fructose 1,6-biphosphate; h = hours; HIP/PAP = hepatocarcinoma intestine pancreas protein/pancreatitis-associated protein I; NAP = actif motif of ADNP (activity-dependent neuroprotective protein); 3MA = 3-methyladenine; P = postnatal day; PVWM = periventricular WM; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter.
Lesions mimicking human PVL were also observed in a sheep model of severe hypoxia–ischemia (Table 4) involving a double hit protocol and producing WM necrotic foci related to GM (CX and TH) damage (transient maternal hypoxia and brachiocephalic artery occlusion followed by a second hypoxia 6 h later) (Hagen et al., 2014).
TABLE 4.
Cell death in preclinical models of preterm brain injury following hypoxia/ischemia.
| Species | Time of the insult | Model | Cell death markers (time post insult) | + Cell types | ROIs | Neuroprotectant | References |
| Sheep | 91 d ga | biCCAO (45 min) | -TUNEL (1 d) -c-CASP3, fractin, pyknotic nuclei |
-O4,O1 -n.d. |
-PVWM, CTX, BG -PVWM |
– | Riddle et al., 2006 |
| 92 d ga | - Maternal Hx (10.5% O2, 30 min) + brachioc. AO (25 min) - + 2nd Hx (+6 d) |
-c-CASP3 (1 d) pyknotic nuclei (1 d, 4 w) -“necrotic” foci (H&E, Iba1+) |
- O4 - n.d. |
-PVWM, SP -PVWM |
– |
McClendon et al., 2017 Hagen et al., 2014 |
|
| 94–96 d ga 93–96 d ga |
UCO (25 min) | -TUNEL (3 d) -cysts (∼30%) |
-GFAP -n.d. |
-SCWM, PVWM, hipp, STcn - PVWM |
-Dopa – |
Brew et al., 2016 Mallard et al., 2003 |
|
| 98-99 d ga | UCO (25 min) | -c-CASP3 (3 d) -NeuN count (3 d) -c-CASP3, NeuN count (3 d) |
n.d. | -STcn, STp, CA4, DG, MN, MGN, SCWM -STcn, CA1/2, CA3, CA4, DG, MN -CA3 |
-Epo -Hypoth. – |
Wassink et al., 2017 Bennet et al., 2007 Dean et al., 2006 |
|
| 102 d ga | UCO (23-25 min) | -c-CASP3, TUNEL (10 d) -NeuN count (10 d) -TUNEL (10 d) |
n.d. | -SVZ, PVWM, SCWM, ST -CTX -iC |
-Melatonin – -UCB |
Yawno et al., 2017a Li J. et al., 2017 Li et al., 2016b |
|
| 125–133 d ga | Partial UCO (blood flow < 50%, 60 min) | -TUNEL (+3 h) -Necrotic-like neurons (H&E, Nissl) (0 h, 3 h), -TUNEL (3 h) -Annexin V and PI (0 h, 3 h) |
n.d. | -CTX, Hipp, BG, CB, BS -CB, pons, BG, mesencephalon -CTX, CB, Pons -CTX, CB, HTH, TH, Hipp |
-WIN 55, 212-2 – |
Alonso-Alconada et al., 2010 Goni-de-Cerio et al., 2007 |
|
| 132 d ga | UCO (10 min) | -“Necrotic”/ pyknotic cells (H&E) (1 d), -c-CASP3 (1 d) |
n.d. | CC, PVWM, eC, CTX, SVZ | – | Castillo-Melendez et al., 2013 | |
| Rat | E5–E19 | Maternal Hx (10% O2) | -Cysts (H&E), TUNEL (P0, P5) | n.d. | SCWM | – | Fontaine et al., 2008 |
| E18 | - Uterine AsO (60 min) (45 min) |
-TUNEL (P2) -c-CASP3 (P5, P9) -c-CASP3 (P2, P9) |
-NeuN -n.d. -NeuN, O4,O1,PDGFαR |
-SPN -PVWM -CTX, PVWM |
-EPO -EPO – |
Jantzie et al., 2015a Mazur et al., 2010 Robinson et al., 2005 |
|
| E21 | Uterine horn blood supply Occ (10 or 15 min) | TUNEL, c-CASP3 (1 d) |
n.d. | -Hipp, CTX | – | Huang et al., 2013 | |
| P1 | 100% N2 (25 min) | -TUNEL (1 d) -FJB (1 d) -Necrosis,↑ autophagy |
n.d. | -CA1, DG -CA2, CA3 -hipp |
– – – |
Takada et al., 2015; Ikebara et al., 2017 | |
| P1–P2 | UniCCAO+6%O2 - (3 h) - (1.5 h–2 h) |
-ISEL staining (12 h, 1 d) -c-CASP3 (1 d) |
n.d. | - SP, IZ, SVZ, neoCTX, TH -CTX |
– – |
McQuillen et al., 2003 Okusa et al., 2014 |
|
| P3 | UniCCAO+6%O2 - (30 min) - (3.5 h) |
-c-CASP3, fractin (1 d) -c-CASP3 (4 d) |
-n.d. -O4 |
-CTX -SCWM |
-Lf – |
van de Looij et al., 2014 Segovia et al., 2008 |
|
| P6 | uniCCAO+ 6%O2 (1 h) | TUNEL (1 d) | n.d. | periCCWM | MgSO4 (-30 min) | Seyama et al., 2018 | |
| Mouse | P3–P11 | 10.5% O2, for 8 d | -c-CASP3, PI (P11, P15, P18) | Olig, CC1 | -CC, CG, eC | – | Jablonska et al., 2012; Scafidi et al., 2014 |
| P2–P3 | UniCCAO+6%O2 (30 min) | TUNEL (1 d) | n.d. | CTX | Hypoth. | Kida et al., 2013 | |
| Pig | 35/60 d ga | 7% O2 (1 h) | BAD, BAX | n.d. | CTX | – | Abedin et al., 2005 |
| Rabbit | E22/E25 | Uterine ischemia (40 min) | -TUNEL (1 d) -c-CASP3 (1 d) |
-n.d. -NeuN, Ctip2 |
-CTX, BG, TH, CXP, STp, SCWM -CTX, Hipp, ST, TH |
– – |
Derrick et al., 2004; Buser et al., 2010 |
AO = artery occlusion; biCCAO = bilateral carotid artery occlusion; brachioc = brachiocephalic; BS = brainstem; BG = basal ganglia; CB = cerebellum; CBWM = cerebellar WM; CC = corpus callosum; c-CASP3 = cleaved caspase-3; CG = cingulum; CTX = cortex; CxP = cortical plate; d = day; DG = dentate gyrus; Dopa = dopamine; E = embryonic day; eC = external capsule; EPO = erythropoietin; ga = gestational age; GV = germinative ventricular zone; h = hours; H&E = hematoxylin–eosin staining, Hipp = hippocampus; HTH = hypothalamus; Hypoth. = hypothermia; Hx = hypoxia; iC = internal capsule; i.c. = intracerebral; i.p. = intraperitoneal; i.v. = intravenous; IZ = intermediate zone; Lf = lactoferrin; LPS = lipopolysaccharide; MGN = medial geniculate nucleus; MN = thalamic medial nucleus; n.d. = not defined; O = occlusion; P = postnatal day; periCCWM = pericallosal white matter; PI = propidium iodide; PVWM = periventricular WM; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter; SP = subplate; SPN = subplate neurons; ST = striatum; STcn = striatal caudate nucleus; STp = striatal putamen; SZ = subventricular zone; TH = thalamus; UCO = umbilical cord occlusion; uniCCAO = unilateral Common Carotid Artery Occlusion; w = weeks.
Preclinical Models of Diffuse White Matter Injury
While hypoxia/ischemia represents one of the major risk factors for neonatal encephalopathy in term babies, for preterm, however, inflammation has been of, in the last decade, growing importance in the field. The role of a hypoxic event in preterm brain injury has, indeed, no clear evidence. Argument that is more rigorous is needed today before claiming hypoxic–ischemic encephalopathy such as measurement of hypoxemia and metabolic acidosis (Graham et al., 2004; Gilles et al., 2017; Paneth, 2018). It is now proposed that inflammation could be a leading cause of preterm brain damage (Gilles et al., 2017). In human preterm babies, chorioamnionitis and especially postnatal sepsis are associated with WM injury and adverse neurodevelopmental outcomes (Shah et al., 2008; Anblagan et al., 2016; Bierstone et al., 2018; Heo et al., 2018). Moreover, inflammation is a common process in different preterm brain injuries either directly as in intrauterine infection/chorioamnionitis or secondarily because of a primary insult such as hemorrhage or hypoxia/ischemia. Despite the current questioning of the role of hypoxia in preterm brain damage, most of the studies investigating cell death mechanisms in diffuse WM injury have been performed in models involving both stimuli: inflammation (Table 3) and hypoxia/ischemia (HI) (Tables 4, 5) (Jantzie and Robinson, 2015).
TABLE 5.
Cell death in preclinical models of preterm LPS-sensitized HI brain injury.
| Species | Time of the insult | Model | Cell death markers (time post insult) | + Cell types | ROIs | Neuroprotectant | References |
| Mouse | P2 | LPS (0.05 μg/kg, -3 h), i.p. + uniCCAO + 6.5% O2 (90 min) | c-CASP3 (1 d) | O4, RECA | SCWM | JNK inhibition | Wang et al., 2010, 2012 |
| P3 | LPS (0.05 μg/kg, -2 h), i.p + uniCCAO + 8% O2 (20 min) | TUNEL, c-CASP3 (1 d) | n.d. | CTX | PINK1 KO | Zhu et al., 2016 |
c-CASP3 = cleaved caspase-3; CTX = cortex; d = day; h = hours; i.p. = intraperitoneal; LPS = lipopolysaccharide; P = postnatal day; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter; uniCCAO = unilateral Common Carotid Artery Occlusion; w = weeks.
Preclinical Inflammatory Models
Direct inflammatory lesions are induced in most of the preclinical models by an acute or chronic exposure to either gram-negative bacteria lipopolysaccharide (LPS) endotoxin or more recently by injection of interleukin-1β that leads to a persistent inflammatory response, astrogliosis, and myelination deficits (Dean et al., 2015). It has been shown that exposure to inflammation during the perinatal period could alter transiently or permanently neurodevelopmental outcome (learning and motor difficulties and development of psychiatric disease such as schizophrenia or autism) (Fan et al., 2005b; Boksa, 2010; Rousset et al., 2013; Schaafsma et al., 2017; Straley et al., 2017; Zhang et al., 2018). Cell death has been mostly investigated in models of fetal infection in rat, sheep, and macaques in which apoptotic cell death (c-CASP3) was found to be scattered, located essentially in WM, and observed mainly after days following inflammatory insult (Table 3). When investigated, cell type identification indicated that CASP3 activation occurred in oligodendrocyte lineage (Olig2, CNPase, and PDGFαR). However, injury of the GM was also described. A study in sheep involving intra-amniotic LPS injection also described CASP3 activation in the cortex and the hippocampus 8 days after LPS exposure (Gussenhoven et al., 2018). Another study involving i.p. LPS injections in rat dam (at E18 and E19) showed the presence of TUNEL and c-CASP3-positive cells in the periventricular striatum of pups 7 days after birth (Rousset et al., 2006).
An intracerebral injection of LPS consists of much more severe inflammatory models by mimicking late conditions of pathogen infiltration after the breakdown of the blood–brain barrier. In a model of LPS exposure in postnatal rat (P3), the production of the CASP-dependent fragment of β-actin, fractin, was observed in the subcortical WM (SCWM) as well as in axonal fascicles of striatum (Lodygensky et al., 2014; Ginet et al., 2016), suggesting a LPS-induced axonopathy (Sokolowski et al., 2014). The mechanisms involved in apoptosis induction could depend on the cell type. In fact, in a model of intracerebral injection of IL-1β in P5 rats inducing a global CASP3 activation, TUNEL staining was observed in Lectin+ microglia, in O4+ and O1+ oligodendrocyte precursors, and in some NF+ neurons 1 day after the insult (Cai et al., 2004). Inflammation-related cell death was partially associated with excitotoxicity since the AMPA/kainate receptors antagonist NBQX reduced the global number of TUNEL+ cells without protecting oligodendrocyte precursors. This could be explained by an age-dependent sensitivity of WM pre-oligodendrocytes to AMPA toxicity that occurs later at P7 (Follett et al., 2000).
A single study investigated autophagy in an inflammatory model (Carloni et al., 2016), showing that, when LPS was injected in the rat dam (at E18 and E19), autophagy appeared impaired (LC3-II and p62/SQSTM1 levels were increased in P1 brain rat pups). However, this study evaluated the overall autophagy response on whole brain extracts, a method that limits the conclusions concerning the effect on autophagy according to either WM, GM, or the cell type.
Very recently, an involvement of necroptosis has been proposed to occur after LPS-induced brain inflammation in P3 old rats by showing an increase in necroptotic gene expressions (RIPK1, RIPK3, and MLKL) 24 h after the insult (Pierre et al., 2019).
Hypoxic–Ischemic Models
Even if the role of HI has become a subject of debate (Gilles et al., 2017), it remains one of the most used models for studying preterm brain injury that induces both severe WM and GM injury and are then used mainly as a model of cPVL. Cell death has been investigated mainly in sheep and rodent (Table 4).
Sheep models consist in inducing a hypoxic–ischemic event in the fetus by either umbilical cord occlusion or by brachiocephalic arteries occlusion combined with maternal hypoxia. CASP3 activation, pyknotic nuclei, as well as TUNEL staining were described mainly in PVWM (periventricular and subcortical WM) and SCWM but also in cortex, basal ganglia (caudate nucleus and putamen), and less frequently in thalamus, hypothalamus, and hippocampus. One study investigating cell death within the first 3 h following HI showed that necrotic features are detected early, from time zero onwards, without signs of apoptosis in different cerebral regions (mesencephalon, cerebellum, pons, basal ganglia, cortex, hippocampus, thalamus, and hypothalamus) (Goni-de-Cerio et al., 2007). From 3 h onwards, apoptotic dying cells are detected and not restricted to cerebral regions where necrotic neurons were histologically observed at 0 h (mesencephalon, cerebellum, pons, and basal ganglia). Interestingly, some early necrotic regions will then become strongly apoptotic later (especially cortex, cerebellum, and pons), suggesting that different cell death processes are involved sequentially in the same damaged cerebral region. However, since all these observations are focused on unregulated necrosis, it could be speculated that regions displaying either apoptotic or necrotic features at later time points are regions where delayed cell death is prominent, including potentially not only apoptosis but also a regulated form of necrosis such as necroptosis.
The subplate zone is a transient layer of the developing cerebral cortex that plays a highly important role in the structural brain development and its plasticity. It contains different neuronal cells and especially early-generated subplate neurons whose cell bodies are located in developing WM. Subplate injury has been since years discussed in EoP and is becoming more and more important over the last decade. When sheep maternal hypoxia and fetal ischemia were induced early (92d GA), CASP3 activation was also present in the subplate but essentially in oligodendrocytes, suggesting a more important resistance of subplate neurons in sheep (McClendon et al., 2017). On the contrary, selective vulnerability of subplate neurons was described in the most widely used model of HI in P2–P3 rodents (uniCCAO followed by systemic hypoxia) (McQuillen et al., 2003; Mikhailova et al., 2017). However, another study could not confirm a specific vulnerability of subplate neurons compared to other deep layers or to the WM (Okusa et al., 2014). It is important to point out that these studies have evaluated only the density of neurons in the subplate and did not perform co-labeling with cell death markers, which is not sufficient to conclude for a specific neuron death in the subplate since an impairment in neuronal differentiation could also be involved. In a different rat model consisting in placental transient hypoxia–ischemia at E18 (uterine arteries occlusion), however, the presence of TUNEL+ neurons has been reported in the subplate at P2 (Jantzie et al., 2015a).
Even if widespread CASP3 activation or TUNEL labeling were shown in models of HI in P1 to P6 rodents in both GM (cortex, thalamus) and WM (Segovia et al., 2008; Jablonska et al., 2012; Scafidi et al., 2014; Seyama et al., 2018), few studies characterized the type of dying cells. In a mouse model of chronic hypoxia (8 days at 10.5% O2 from P3), oligodendrocytes were shown to undergo CASP3-dependent apoptosis even 1 week after the end of hypoxic treatment (Jablonska et al., 2012). In the model of HI in P3 rats pups, Segovia and colleagues (Segovia et al., 2008) showed that, in addition to pre-oligodendrocyte maturation arrest, O4+ cells underwent mainly caspase-independent cell death mechanisms (c-CASP3 negative but morphological characteristics of degeneration) 24 h after the insult, whereas 4 days later, they died mostly through caspase-dependent pathways, suggesting that different forms of cell death are involved in acute or delayed cell death of oligodendrocytes (Segovia et al., 2008).
In a model of anoxia in P1 rat pups, damaged hippocampus showed cell death with EM mixed morphological features of apoptosis, necrosis, and enhanced autophagy (Takada et al., 2015), suggesting that more than one cell death type could be involved in hypoxic–ischemic brain damage with a continuum of cell death that could include the three cell death types as it has been proposed in models of HI in more mature P7 brain (Portera-Cailliau et al., 1997; Ginet et al., 2009a; Northington et al., 2011). Interestingly, this study showed that whereas CA2/CA3 presented a neurodegeneration revealed by both Fluoro-Jade B+ staining and EM ultrastructure study, this hippocampal region was TUNEL negative and presented no more CASP3 activation than in control. This result points out the necessity to study cell death with alternative markers of cell death, different from CASP3 activation (that is moreover developmentally expressed in the immature brain) and TUNEL staining.
In summary, in inflammatory and/or HI premature models, cell death has been investigated in different models and species, and is present in cortical and deep GM neurons and in different glial cells (OL and microglia) with different cell death features reported (necrosis, apoptosis, and autophagic cell death).
Preclinical Models of Intraventricular and Intraparenchymatous Hemorrhages
Very few studies have investigated cell death occurring after IVH in preclinical preterm models (Table 7). Cell death has been studied in two main models reproducing the conditions of germinal matrix hemorrhage (GMH) (germinal matrix ruptures through the ependyma into the lateral ventricle) leading to IVH in preterm human brains. The first approach consists of applying periventricular or intracerebroventricular injections of blood (from dam or autologous) in rodent pups (P1 to P4). The second approach is to perform an i.p. injection of glycerol in rabbit fetus (29 days GA) that produces an intracranial pressure through profound osmotic changes followed by a reperfusion leading to GMH that can extend to the lateral ventricle (Table 7). In both models, pups developed severe IVH associated with progressive post-hemorrhagic hydrocephalus (PHH) compatible with grade 3 or grade 4 IVH in humans, even if the percentage of induced PHH is variable. The IVH pups displayed impaired sensorimotor functions, inflammation, defects in myelination, and increased levels of inflammatory cytokines in the CSF.
TABLE 7.
Cell death in preclinical models of preterm brain injury following intracerebral hemorrhage.
| Species | Time of the insult | Model | Cell death markers (time post insult) | + Cell types | ROIs | Neuroprotectant | References |
| Mouse | P1 | i.c. autologous blood (15 μl) | -TUNEL (2 d) |
-n.d. | -ST, germinal matrix | - | Xue et al., 2003 |
| P5 | i.c.v. autologous blood (20 μl) | -TUNEL (P23) | -n.d. | -PV SCWM | -UC-MSCs | Mukai et al., 2017 | |
| Rat | P4 | dam’s blood (100 μl) i.c.v. | -TUNEL (P32) | -n.d. | -PVZ | -Human UC-MSCs | Ahn et al., 2013 |
| Rabbit | 29 d ga, (2 h, 3 h, or 4 h after preterm delivery | Glycerol (6.5 g/kg), i.p | -TUNEL -TUNEL -c-CASP3 (1 d) -TUNEL, Fluoro-Jade B, CASP 3/7 activity (3 d) -TUNEL, Fluoro-Jade B (1-3 d) |
-O4 -O4 -n.d. -n.d. -n.d. |
-CR, CC -CR, CC -Choroid plexus -PVA, CTX -PVA, CTX |
-NBQX -NOG – -Celecoxib, SC-51089, etanercept -Apocynin |
Dohare et al., 2016 Dummula et al., 2011 Gram et al., 2014 Vinukonda et al., 2010 Georgiadis et al., 2008; Zia et al., 2009 |
BG = basal ganglia; CC = corpus callosum; CASP3 = cleaved caspase-3; CG = cingulum; CTX = cortex; CR = corona radiate; d = day; ga = gestational age; h = hours; i.c. = intracerebral; i.c.v. = intracerebroventricular; NOG = Noggin; P = postnatal day; PI = propidium iodide; PV = periventricular; PVA = periventricular area including germinal matrix, caudate nucleus, corona radiata, and corpus callosum; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter; ST = striatum; UC-MSCs = umbilical cord-derived mesenchymal stromal cells.
Increased cell death and apoptosis have been evidenced by TUNEL staining and CASP3 activation in the periventricular WM (Dummula et al., 2011; Ahn et al., 2013; Gram et al., 2014; Dohare et al., 2016; Mukai et al., 2017) and adjacent GM [striatum (Xue et al., 2003) or cortex (Georgiadis et al., 2008; Zia et al., 2009; Vinukonda et al., 2010)]. However, the identification of the dying cells has not been determined except in some studies showing that cell death (Fluoro-Jade+ cells) could affect O4+ pre-oligodendrocytes or neurons.
Preclinical Models of Iatrogenic Preterm Brain Injury
Hyperoxia
The involvement of cell death and apoptosis in preterm brain injury is well documented in preterm models of hyperoxia in postnatal rodent (from P0 to P6) (Table 8). Hyperoxia was shown in those models to affect both WM and GM (cortex, striatum, thalamus, and hippocampus) by promoting cell death of oligodendrocytes (Gerstner et al., 2006, 2007; Schmitz et al., 2014; Hoeber et al., 2016) and neurons (Yis et al., 2008a, b; Endesfelder et al., 2014). The involvement of apoptosis as a major cell death process was demonstrated by the implication of not only CASP3 but also numerous molecular markers of both intrinsic (Cyc, AIF, and APAF-1) and extrinsic (Fas, FasL, FADD, CASP8, and BID) apoptotic pathways inducing caspase-dependent (CASP1, 2, 3, and 8) and independent (AIF) mechanisms.
TABLE 8.
Cell death in preclinical models of iatrogenic preterm brain injury: hyperoxia.
| Species | Time of the insult | Model | Cell death markers (time post insult) | + Cell types | ROIs | Neuroprotectant | References |
| Rat | E21 | 80% O2 (8 d) | -TUNEL (P3) | -n.d. | -WM | -iNO | Pham et al., 2014 |
| E21 | 60% O2 (8 d) | -TUNEL (P3) | -n.d. | -WM | - | Vottier et al., 2011 | |
| P0 | 80% O2 (5 d) | -TUNEL, cell count (5 d) | -Neurons -Neurons |
-CA1, CA3 -CA1, DG, prefrontal/ parietal/ retrosplenial CTX, subiculum |
-Uridine -EPO -Topiramate |
Goren et al., 2017 Yis et al., 2008a Yis et al., 2008b Kurul et al., 2009 |
|
| P2 | 95% O2 (5 d) | -ELISA DNA fragm. | -n.d. | -CB, basal FB, frontal CTX, Hipp | -BSO | Taglialatela et al., 1998 | |
| 80% O2 -(1 d) |
-TUNEL, AIF (5 d) -c-CASP3 (1 d) -TUNEL -c-CASP3 -CASP2, 3, 8; silver staining -TUNEL (P7) |
-CC1 -n.d. -n.d. -MBP -n.d. -Olig2 |
-CC, CG, eC -CTX, WM -frontal/ retrosplenial CTX, HTH, TH -CC -CTX, STcn, NAcc, CC and adjacent WM, TH, Hipp, HTH - CC, deep cortical WM, CTX, TH |
-Minocycline -Dextromethorphan -Dexmedetomidine -17β-estradiol -EPO -EPO |
Schmitz et al., 2014 Posod et al., 2014 Sifringer et al., 2015 Gerstner et al., 2006 Gerstner et al., 2007 Kaindl et al., 2008 Hoeber et al., 2016 |
||
| P6 | -(1 d or 2 d) | -TUNEL, neuronal density -PARP-1, AIF, c-CASP3 |
-Nestin, DCX, NeuN -n.d. |
-Frontal/ retrosplenial/parietal CTX, TH, HTH -Whole brain |
-Caffeine -Caffeine |
Endesfelder et al., 2014 Endesfelder et al., 2017b |
|
| -(12 h or 1 d) | -CASP2, Cyc, APAF-1, AIF, BCL2, BID, c-CASP3, Fluoro-Jade B -BECN1, ATG3, ATG5, ATG12, LC3 |
-Neurons and n.d. -n.d. |
-TH -Whole brain |
-Caspase inhibitor (TRP601) -EPO |
Sifringer et al., 2012 Bendix et al., 2012 |
||
| -(2 h, 6 h, 12 h, 1 d, 2 d, and 3 d) | -c-CASP8, c-CASP3, Fas, FasL, FADD -Fluoro-Jade B, silver staining, CASP1 |
-n.d. -n.d. |
-CG, parietal CTX, CC, ST, TH | -CASP8 inhibitor (TRP801) -Recombinant IL-18BP |
Dzietko et al., 2008 Felderhoff-Mueser et al., 2005 |
||
| Mouse | P6 | 80% O2 -(2 h, 6 h, 12 h, 1 d, 2 d) -(1 d) |
-Fluoro-Jade B, silver staining -CASP2, CASP3, CASP8, silver staining |
-n.d. -n.d. |
-CG, parietal CTX, CC, ST, TH -CTX, STcn, NAcc, CC and adjacent WM, TH, Hipp, HTH |
-KO IRAK-4 -EPO |
Felderhoff-Mueser et al., 2005 Kaindl et al., 2008 |
| P6 | FasL + 80% O2 (1 d) | -c-CASP8, c-CASP3 | -n.d. | -Cingulate/parietal CTX, CC, ST, TH | -CASP8 inhibitor (TRP801) | Dzietko et al., 2008 | |
AIF = apoptosis-inducing factor; BSO = buthionine sulfoxide; CC = corpus callosum; c-CASP = cleaved caspase; CG = cingulum; CTX = cortex; Cyc = cytochrome c; d = day; DG = dentate gyrus; E = embryonic day; eC = external capsule; EPO = erythropoietin; FB = forebrain; h = hours; Hipp = hippocampus; HTH = hypothalamus; iNO = inhaled nitric oxide; KO IRAK-4 = mice deficient in IL-1 receptor–associated kinase 4 (IRAK-4); NAcc = nucleus accumbens; n.d. = not defined; P = postnatal day; recombinant IL-18BP = Recombinant interleukin–18 binding protein; ROIs = regions of interest (i.e., where cell death is detected); SCWM = subcortical white matter; ST = striatum; STcn = striatal caudate nucleus; TH = thalamus; WM = white matter.
Furthermore, Bendix and colleagues (Bendix et al., 2012) showed an effect of hyperoxia on autophagy during the first 24 h of hyperoxia in the developing rat preterm brain. Some important autophagy-related genes (atg) for autophagosome formation were upregulated (BECLIN1, ATG3, ATG5, ATG12, and LC3-II) (Bendix et al., 2012) at 12 h after hyperoxia treatment. However, we cannot conclude on the effect of hyperoxia on autophagic flux (active autophagic process of degradation) since efficiency of the degradative part of autophagy was not investigated. Increased autophagosome presence could result from enhanced autophagic flux as well as impaired lysosomal degradation (Puyal et al., 2013; Klionsky et al., 2016). While this study showed that autophagy is certainly affected by hyperoxia, more investigations (assessing the state of the flux and its function) are needed before to conclude on the role of autophagy in hyperoxia-mediated cell death.
Caffeine
It has been shown that caffeine treatment (10 to 80 mg/kg, i.p.) activated apoptosis (CASP3, AIF) and increased TUNEL staining in the brain of rat pups (P3, P6) (Table 9) (Cabrera et al., 2017; Endesfelder et al., 2017a, b). Recently, Noguchi and colleagues showed that caffeine treatment in fetal macaque (110–120 days GA) anesthetized with isoflurane strongly sensitized brain (CX, ST, and CB) against isoflurane-induced apoptosis, especially NeuN-positive neurons, more than MBP-positive oligodendrocytes (Noguchi et al., 2018).
Anesthetics
Different studies demonstrated pro-apoptotic effects of anesthetics [especially propofol, ketamine, and ethers (isoflurane, sevoflurane)] on immature brain of rodents and macaques (Table 9). Combination of anesthetic with caffeine is a powerful aggravating factor (Noguchi et al., 2018). These anesthetics have been shown to trigger widespread CASP3 activation or to increase TUNEL labeling in neurons and oligodendrocytes (Cattano et al., 2008; Dribben et al., 2009; Brambrink et al., 2010, 2012; Creeley et al., 2013; Li et al., 2016a; Xiong et al., 2016) associated with some cognitive deficits. In two studies using sevoflurane, autophagy was shown to be enhanced, in addition to apoptosis, in immature brains, and autophagy inhibition using 3-MA was both anti-apoptotic and neuroprotective (Li X. et al., 2017; Wang X. et al., 2019).
Conclusion and Future Prospects
In conclusion, common and converging observations from animal models and human brains from autopsy suggested that cell death mechanisms in preterm brain injuries mainly involve apoptotic mechanisms. Although most of the studies missed to identify the type of cells subjected to cell death, some suggested that different cell types are affected depending not only on the severity and the type of the insult but also on the time point investigated. In mild preterm injuries, the cell death appeared to be diffuse and restricted (when detected) to preOL, whereas in more severe cases, cell death could affect almost all cell types including neurons.
Nevertheless, it appears that, although cell death is an ineluctable event occurring in severe preterm brain injuries such as cPVL and IVH, but not necessarily in dWMI, cell death has not been deeply investigated and characterized either in preclinical models or in tissues from human preterm neonates. The importance of apoptosis versus other cell death pathways (such as regulated necrotic and autophagic) is certainly overestimated since almost all the studies used only apoptotic (such as c-CASP3 and TUNEL) markers as indicators of cell death. Moreover, by using only c-CASP3 and TUNEL markers, cell death occurrence can be simply underestimated or wrongly declared absent since cell death types independent of CASP3 and not inducing (at least in an early stage) DNA fragmentation exist. In the model of anoxia in P1 rat pups, damaged hippocampal CA2/CA3 showed, for example, cell death mechanisms mainly independent of CASP3, not positive for TUNEL and with EM morphological features of necrosis and enhanced autophagy (Takada et al., 2015). We have previously clearly demonstrated the presence and the prodeath role of enhanced neuronal autophagy in models of both term (Ginet et al., 2009b; Puyal et al., 2009; Ginet et al., 2014a; Xie et al., 2016) and more recently preterm (Descloux et al., 2018) brain injury. We showed that, after neonatal cerebral HI, rat hippocampal dying CA3 neurons displayed high autophagic features without apoptosis activation, i.e., TUNEL and CASP3 negative (Ginet et al., 2009b). In addition, the discovery of programmed forms of necrosis and some of its molecular actors will allow one to investigate the implication of regulated necrosis. Necroptosis is especially concerned since this type of cell death is known to be activated by inflammatory signals.
Since “we find what we are looking for”, one could speculate that most of the studies missed to identify cell death mechanisms involved in the pathogenesis of the studied preterm brain damage. The increasing advances made to understand the biochemical mechanisms of the different cell death pathways and the recognition of regulated necrosis and autophagic cell death lead to switch, over the last 10 years, in the identification of the cell death type from a purely morphological to a molecular analysis (Galluzzi et al., 2012). Today, many markers of the different types of cell death could be used in human samples to systematically evaluate the presence not only of apoptosis but also of other forms of cell death including regulated necrosis and autophagic or autophagy-mediated cell death. Expression and activation of RIPK3 and MLKL could be investigated as markers for necroptosis. Concerning autophagic or autophagy-mediated cell death, an increase in autophagosome presence alone is not enough to demonstrate an increased autophagic flux since it could result from a lysosomal degradation failure. Then, an increase in punctate LC3 immunolabeling (marker of autophagosomes) together with an increase lysosomal activity, evaluated by either a decrease in p62/SQSTM1 labeling (selectively degraded by autophagy) or an increase in the size and number of vesicles labeled by lysosomal markers such as LAMP1 or different cathepsins (presumably autolysosomes), should be used to detect enhanced autophagy. Moreover, since increased autophagy could be either a prosurvival or a prodeath mechanism, conclusions on the role of enhanced autophagy in cell death has to be taken with caution due to the duality of autophagy in cell survival (protective vs. destructive) depending the conditions and cell type.
Future studies designed to provide a more precise characterization of the molecular cell death mechanisms and also identification of dying cell types will allow a better understanding of the preterm brain pathogenesis and will certainly participate to develop new and efficient protective strategies to prevent or treat injuries of the preterm human brain.
Author Contributions
AT, VG, and JP conceived, wrote the manuscript, drew the tables and figures, and critically revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- 3-MA
3-methyladenin
- ADC
apparent diffusion coefficients
- ATG
autophagy-related gene
- CASP
caspase
- c-CASP
cleaved caspase
- cPVL
cystic periventricular leucomalacia
- CSF
cerebrospinal fluid
- dWMI
diffuse white matter injury
- EM
electronic microscopy
- EoP
encephalopathy of prematurity
- ER
endoplasmic reticulum
- FA
fractional anisotropy
- GA
gestational age
- GM
gray matter reduction
- GMH
germinal matrix hemorrhage
- HI
hypoxia–ischemia
- IVH
intraventricular hemorrhage
- LC3
microtubule-associated protein 1 light chain 3
- LPS
lipopolysaccharide
- MRI
magnetic resonance imaging
- OL
oligodendrocytes
- PE
phosphatidylethanolamine
- PHH
post-hemorrhagic hydrocephalus
- preOL
pre-oligodendrocyte
- PVL
periventricular leucomalacia
- PWM
periventricular white matter
- SCWM
subcortical WM
- SVZ
subventricular zone
- VPT
very preterm infants
- WM
white matter
- WMI
white matter injury.
Footnotes
Funding. This work was supported by the grants from the Swiss National Science Foundation (310030-163064 and 310030-182332) and by the Fondation Paralysie Cérébrale.
References
- Abedin N., Ashraf Q., Mishra O. P., Delivoria-Papadopoulos M. (2005). Effect of hypoxia on the expression of pro- and anti-apoptotic proteins in neuronal nuclei of the guinea pig fetus during gestation. Brain Res. Dev. Brain Res. 156 32–37. 10.1016/j.devbrainres.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Ahn S. Y., Chang Y. S., Sung D. K., Sung S. I., Yoo H. S., Lee J. H., et al. (2013). Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 44 497–504. 10.1161/STROKEAHA.112.679092 [DOI] [PubMed] [Google Scholar]
- Alonso-Alconada D., Alvarez F. J., Alvarez A., Mielgo V. E., Goni-de-Cerio F., Rey-Santano M. C., et al. (2010). The cannabinoid receptor agonist WIN 55,212-2 reduces the initial cerebral damage after hypoxic-ischemic injury in fetal lambs. Brain Res. 1362 150–159. 10.1016/j.brainres.2010.09.050 [DOI] [PubMed] [Google Scholar]
- Anblagan D., Pataky R., Evans M. J., Telford E. J., Serag A., Sparrow S., et al. (2016). Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci. Rep. 6:37932. 10.1038/srep37932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiman S. E., Haynes R. L., Trachtenberg F. L., Billiards S. S., Folkerth R. D., Volpe J. J., et al. (2010). The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol. 20 803–814. 10.1111/j.1750-3639.2010.00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askie L. M., Brocklehurst P., Darlow B. A., Finer N., Schmidt B., Tarnow-Mordi W., et al. (2011). NeOProM: neonatal oxygenation prospective meta-analysis collaboration study protocol. BMC Pediatr. 11:6. 10.1186/1471-2431-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baburamani A. A., Supramaniam V. G., Hagberg H., Mallard C. (2014). Microglia toxicity in preterm brain injury. Reprod. Toxicol. 48 106–112. 10.1016/j.reprotox.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S. A., Luo N. L., Mallinson R. A., O’Malley J. P., Wallen L. D., Frei B., et al. (2005). Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann. Neurol. 58 108–120. 10.1002/ana.20530 [DOI] [PubMed] [Google Scholar]
- Back S. A., Riddle A., Dean J., Hohimer A. R. (2012). The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics 9 359–370. 10.1007/s13311-012-0108-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S. A., Volpe J. J. (2018). “Encephalopathy of prematurity: pathophysiology. pathophysiology,” in Volpe’s Neurology of the Newborn, (Amsterdam: Elsevier; ), 405.e8–424.e8. [Google Scholar]
- Balakrishnan B., Dai H., Janisse J., Romero R., Kannan S. (2013). Maternal endotoxin exposure results in abnormal neuronal architecture in the newborn rabbit. Dev. Neurosci. 35 396–405. 10.1159/000353156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G., Boardman J. P., Aljabar P., Pandit A., Arichi T., Merchant N., et al. (2013). The influence of preterm birth on the developing thalamocortical connectome. Cortex 49 1711–1721. 10.1016/j.cortex.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Banker B. Q., Larroche J. C. (1962). Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch. Neurol. 7 386–410. [DOI] [PubMed] [Google Scholar]
- Baud O., Saint-Faust M. (2019). Neuroinflammation in the developing brain: risk factors. involvement of microglial cells, and implication for early anesthesia. Anesth. Analg. 128 718–725. 10.1213/ANE.0000000000004032 [DOI] [PubMed] [Google Scholar]
- Beauport L., Schneider J., Faouzi M., Hagmann P., Huppi P. S., Tolsa J. F., et al. (2017). Impact of early nutritional intake on preterm brain: a magnetic resonance imaging study. J. Pediatr. 181:e21. 10.1016/j.jpeds.2016.09.073 [DOI] [PubMed] [Google Scholar]
- Bendix I., Schulze C., Haefen C., Gellhaus A., Endesfelder S., Heumann R., et al. (2012). Erythropoietin modulates autophagy signaling in the developing rat brain in an in vivo model of oxygen-toxicity. Int. J. Mol. Sci. 13 12939–12951. 10.3390/ijms131012939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet L., Roelfsema V., George S., Dean J. M., Emerald B. S., Gunn A. J. (2007). The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J. Physiol. 578(Pt 2), 491–506. 10.1113/jphysiol.2006.119602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierstone D., Wagenaar N., Gano D. L., Guo T., Georgio G., Groenendaal F., et al. (2018). Association of histologic chorioamnionitis with perinatal brain injury and early childhood neurodevelopmental outcomes among preterm neonates. JAMA Pediatr. 172 534–541. 10.1001/jamapediatrics.2018.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiards S. S., Haynes R. L., Folkerth R. D., Borenstein N. S., Trachtenberg F. L., Rowitch D. H., et al. (2008). Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18 153–163. 10.1111/j.1750-3639.2007.00107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. (2010). Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav. Immun. 24 881–897. 10.1016/j.bbi.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Brambrink A. M., Evers A. S., Avidan M. S., Farber N. B., Smith D. J., Martin L. D., et al. (2012). Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology 116 372–384. 10.1097/ALN.0b013e318242b2cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink A. M., Evers A. S., Avidan M. S., Farber N. B., Smith D. J., Zhang X., et al. (2010). Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 112 834–841. 10.1097/ALN.0b013e3181d049cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew N., Azhan A., den Heijer I., Boomgardt M., Davies G. I., Nitsos I., et al. (2016). Dopamine treatment during acute hypoxia is neuroprotective in the developing sheep brain. Neuroscience 316 82–93. 10.1016/j.neuroscience.2015.12.022 [DOI] [PubMed] [Google Scholar]
- Broad K. D., Kawano G., Fierens I., Rocha-Ferreira E., Hristova M., Ezzati M., et al. (2017). Surgery increases cell death and induces changes in gene expression compared with anesthesia alone in the developing piglet brain. PLoS One 12:e0173413. 10.1371/journal.pone.0173413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser J. R., Maire J., Riddle A., Gong X., Nguyen T., Nelson K., et al. (2012). Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71 93–109. 10.1002/ana.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser J. R., Segovia K. N., Dean J. M., Nelson K., Beardsley D., Gong X., et al. (2010). Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia. J. Cereb. Blood Flow Metab. 30 1053–1065. 10.1038/jcbfm.2009.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O. H., O’Connor S. D., Swiney B. S., Salinas-Contreras P., Manzella F. M., Taylor G. T., et al. (2017). Caffeine combined with sedative/anesthetic drugs triggers widespread neuroapoptosis in a mouse model of prematurity. J. Matern. Fetal. Neonatal. Med. 30 2734–2741. 10.1080/14767058.2016.1261400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z., Lin S., Pang Y., Rhodes P. G. (2004). Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr. Res. 56 377–384. 10.1203/01.PDR.0000134249.92944.14 [DOI] [PubMed] [Google Scholar]
- Carloni S., Favrais G., Saliba E., Albertini M. C., Chalon S., Longini M., et al. (2016). Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J. Pineal. Res. 61 370–380. 10.1111/jpi.12354 [DOI] [PubMed] [Google Scholar]
- Carlsson Y., Schwendimann L., Vontell R., Rousset C. I., Wang X., Lebon S., et al. (2011). Genetic inhibition of caspase-2 reduces hypoxic-ischemic and excitotoxic neonatal brain injury. Ann. Neurol. 70 781–789. 10.1002/ana.22431 [DOI] [PubMed] [Google Scholar]
- Castillo-Melendez M., Baburamani A. A., Cabalag C., Yawno T., Witjaksono A., Miller S. L., et al. (2013). Experimental modelling of the consequences of brief late gestation asphyxia on newborn lamb behaviour and brain structure. PLoS One 8:e77377. 10.1371/journal.pone.0077377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattano D., Young C., Straiko M. M., Olney J. W. (2008). Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth. Analg. 106 1712–1714. 10.1213/ane.0b013e318172ba0a [DOI] [PubMed] [Google Scholar]
- Chamnanvanakij S., Margraf L. R., Burns D., Perlman J. M. (2002). Apoptosis and white matter injury in preterm infants. Pediatr. Dev. Pathol. 5 184–189. 10.1007/s10024-001-0205-0 [DOI] [PubMed] [Google Scholar]
- Chau V., Synnes A., Grunau R. E., Poskitt K. J., Brant R., Miller S. P. (2013). Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81 2082–2089. 10.1212/01.wnl.0000437298.43688.b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. G. (1990). Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. 181 195–213. [DOI] [PubMed] [Google Scholar]
- Clarke P. G., Puyal J. (2012). Autophagic cell death exists. Autophagy 8 867–869. 10.4161/auto.20380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley C., Dikranian K., Dissen G., Martin L., Olney J., Brambrink A. (2013). Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br. J. Anaesth. 110(Suppl. 1), i29–i38. 10.1093/bja/aet173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley C. E., Dikranian K. T., Dissen G. A., Back S. A., Olney J. W., Brambrink A. M. (2014). Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology 120 626–638. 10.1097/ALN.0000000000000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A. M. (2004). Autophagy: many paths to the same end. Mol. Cell Biochem. 263 55–72. 10.1023/B:MCBI.0000041848.57020.57 [DOI] [PubMed] [Google Scholar]
- de Vries L. S., Groenendaal F., Liem K. D., Heep A., Brouwer A. J., van ’t Verlaat E., et al. (2019). Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch. Dis. Child Fetal. Neonatal. Ed. 104 F70–F75. 10.1136/archdischild-2017-314206 [DOI] [PubMed] [Google Scholar]
- Dean J. M., Farrag D., Zahkouk S. A., El Zawahry E. Y., Hagberg H., Kjellmer I., et al. (2009). Cerebellar white matter injury following systemic endotoxemia in preterm fetal sheep. Neuroscience 160 606–615. 10.1016/j.neuroscience.2009.02.071 [DOI] [PubMed] [Google Scholar]
- Dean J. M., Gunn A. J., Wassink G., George S., Bennet L. (2006). Endogenous alpha2-adrenergic receptor-mediated neuroprotection after severe hypoxia in preterm fetal sheep. Neuroscience 142 615–628. 10.1016/j.neuroscience.2006.06.066 [DOI] [PubMed] [Google Scholar]
- Dean J. M., Shi Z., Fleiss B., Gunn K. C., Groenendaal F., van Bel F., et al. (2015). A Critical Review of Models of Perinatal Infection. Dev. Neurosci. 37 289–304. 10.1159/000370309 [DOI] [PubMed] [Google Scholar]
- Dean J. M., Wang X., Kaindl A. M., Gressens P., Fleiss B., Hagberg H., et al. (2010). Microglial MyD88 signaling regulates acute neuronal toxicity of LPS-stimulated microglia in vitro. Brain Behav. Immun. 24 776–783. 10.1016/j.bbi.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Debillon T., Gras-Leguen C., Leroy S., Caillon J., Rozé J. C., Gressens P. (2003). Patterns of cerebral inflammatory response in a rabbit model of intrauterine infection-mediated brain lesion. Dev. Brain Res. 145 39–48. 10.1016/s0165-3806(03)00193-7 [DOI] [PubMed] [Google Scholar]
- Debillon T., Gras-Leguen C., Verielle V., Winer N., Caillon J., Roze J. C., et al. (2000). Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr. Res. 47 736–742. 10.1203/00006450-200006000-00009 [DOI] [PubMed] [Google Scholar]
- Debnath J., Baehrecke E. H., Kroemer G. (2005). Does autophagy contribute to cell death? Autophagy 1 66–74. 10.4161/auto.1.2.1738 [DOI] [PubMed] [Google Scholar]
- Del Bigio M. R. (2011). Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain 134(Pt 5), 1344–1361. 10.1093/brain/awr052 [DOI] [PubMed] [Google Scholar]
- Derrick M., Luo N. L., Bregman J. C., Jilling T., Ji X., Fisher K., et al. (2004). Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J. Neurosci. 24 24–34. 10.1523/JNEUROSCI.2816-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descloux C., Ginet V., Rummel C., Truttmann A. C., Puyal J. (2018). Enhanced autophagy contributes to excitotoxic lesions in a rat model of preterm brain injury. Cell Death Dis. 9:853. 10.1038/s41419-018-0916-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destot-Wong K. D., Liang K., Gupta S. K., Favrais G., Schwendimann L., Pansiot J., et al. (2009). The AMPA receptor positive allosteric modulator, S18986, is neuroprotective against neonatal excitotoxic and inflammatory brain damage through BDNF synthesis. Neuropharmacology 57 277–286. 10.1016/j.neuropharm.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Dohare P., Zia M. T., Ahmed E., Ahmed A., Yadala V., Schober A. L., et al. (2016). AMPA-kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. J. Neurosci. 36 3363–3377. 10.1523/JNEUROSCI.4329-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommergues M. A., Plaisant F., Verney C., Gressens P. (2003). Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience 121 619–628. 10.1016/s0306-4522(03)00558-x [DOI] [PubMed] [Google Scholar]
- Dribben W. H., Creeley C. E., Wang H. H., Smith D. J., Farber N. B., Olney J. W. (2009). High dose magnesium sulfate exposure induces apoptotic cell death in the developing neonatal mouse brain. Neonatology 96 23–32. 10.1159/000201327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drommelschmidt K., Serdar M., Bendix I., Herz J., Bertling F., Prager S., et al. (2017). Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 60 220–232. 10.1016/j.bbi.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Dubois J., Benders M., Borradori-Tolsa C., Cachia A., Lazeyras F., Ha-Vinh Leuchter R., et al. (2008). Primary cortical folding in the human newborn: an early marker of later functional development. Brain 131(Pt 8), 2028–2041. 10.1093/brain/awn137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden E. G., Halani S., Ng K., Guo T., Foong J., Glass T. J. A., et al. (2019). White matter injury predicts disrupted functional connectivity and microstructure in very preterm born neonates. Neuroimage Clin. 21:101596. 10.1016/j.nicl.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummula K., Vinukonda G., Chu P., Xing Y., Hu F., Mailk S., et al. (2011). Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. J. Neurosci. 31 12068–12082. 10.1523/JNEUROSCI.0013-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzietko M., Boos V., Sifringer M., Polley O., Gerstner B., Genz K., et al. (2008). A critical role for Fas/CD-95 dependent signaling pathways in the pathogenesis of hyperoxia-induced brain injury. Ann. Neurol. 64 664–673. 10.1002/ana.21516 [DOI] [PubMed] [Google Scholar]
- Endesfelder S., Weichelt U., Schiller C., Sifringer M., Bendix I., Buhrer C. (2017a). Caffeine protects against anticonvulsant-induced neurotoxicity in the developing rat brain. Neurotox. Res. 32 460–472. 10.1007/s12640-017-9768-z [DOI] [PubMed] [Google Scholar]
- Endesfelder S., Weichelt U., Strauss E., Schlor A., Sifringer M., Scheuer T., et al. (2017b). Neuroprotection by caffeine in hyperoxia-induced neonatal brain injury. Int. J. Mol. Sci. 18 E187. 10.3390/ijms18010187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endesfelder S., Zaak I., Weichelt U., Buhrer C., Schmitz T. (2014). Caffeine protects neuronal cells against injury caused by hyperoxia in the immature brain. Free Radic. Biol. Med. 67 221–234. 10.1016/j.freeradbiomed.2013.09.026 [DOI] [PubMed] [Google Scholar]
- Fan L. W., Kaizaki A., Tien L. T., Pang Y., Tanaka S., Numazawa S., et al. (2013). Celecoxib attenuates systemic lipopolysaccharide-induced brain inflammation and white matter injury in the neonatal rats. Neuroscience 240 27–38. 10.1016/j.neuroscience.2013.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L. W., Pang Y., Lin S., Rhodes P. G., Cai Z. (2005a). Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neurosci 133 159–168. 10.1111/j.1460-9568.2006.04918.x [DOI] [PubMed] [Google Scholar]
- Fan L. W., Pang Y., Lin S., Tien L. T., Ma T., Rhodes P. G., et al. (2005b). Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 82 71–82. 10.1002/jnr.20623 [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U., Buhrer C., Groneck P., Obladen M., Bartmann P., Heep A. (2003). Soluble Fas (CD95/Apo-1), soluble Fas ligand, and activated caspase 3 in the cerebrospinal fluid of infants with posthemorrhagic and nonhemorrhagic hydrocephalus. Pediatr. Res. 54 659–664. 10.1203/01.PDR.0000084114.83724.65 [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U., Sifringer M., Polley O., Dzietko M., Leineweber B., Mahler L., et al. (2005). Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. Ann. Neurol. 57 50–59. 10.1002/ana.20322 [DOI] [PubMed] [Google Scholar]
- Filan P. M., Hunt R. W., Anderson P. J., Doyle L. W., Inder T. E. (2012). Neurologic outcomes in very preterm infants undergoing surgery. J. Pediatr. 160 409–414. 10.1016/j.jpeds.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Follett P. L., Rosenberg P. A., Volpe J. J., Jensen F. E. (2000). NBQX attenuates excitotoxic injury in developing white matter. J. Neurosci. 20 9235–9241. 10.1523/jneurosci.20-24-09235.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine R. H., Olivier P., Massonneau V., Leroux P., Degos V., Lebon S., et al. (2008). Vulnerability of white matter towards antenatal hypoxia is linked to a species-dependent regulation of glutamate receptor subunits. Proc. Natl. Acad. Sci. U.S.A. 105 16779–16784. 10.1073/pnas.0803004105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs-Maeder C. L., Griffa A., Schneider J., Huppi P. S., Truttmann A., Hagmann P. (2017). Exploring the role of white matter connectivity in cortex maturation. PLoS One 12:e0177466. 10.1371/journal.pone.0177466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Kepp O., Chan F. K., Kroemer G. (2017). Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. 12 103–130. 10.1146/annurev-pathol-052016-100247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Aaronson S. A., Abrams J. M., Adam D., Agostinis P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25 486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Abrams J. M., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., et al. (2012). Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 19 107–120. 10.1038/cdd.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelot A., Villapol S., Billette, de Villemeur T., Renolleau S., Charriaut-Marlangue C. (2009). Astrocytic demise in the developing rat and human brain after hypoxic-ischemic damage. Dev. Neurosci. 31 459–470. 10.1159/000232564 [DOI] [PubMed] [Google Scholar]
- Georgiadis P., Xu H., Chua C., Hu F., Collins L., Huynh C., et al. (2008). Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke 39 3378–3388. 10.1161/STROKEAHA.107.510883 [DOI] [PubMed] [Google Scholar]
- Gerstner B., Buhrer C., Rheinlander C., Polley O., Schuller A., Berns M., et al. (2006). Maturation-dependent oligodendrocyte apoptosis caused by hyperoxia. J. Neurosci. Res. 84 306–315. 10.1002/jnr.20880 [DOI] [PubMed] [Google Scholar]
- Gerstner B., Sifringer M., Dzietko M., Schuller A., Lee J., Simons S., et al. (2007). Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann. Neurol. 61 562–573. 10.1002/ana.21118 [DOI] [PubMed] [Google Scholar]
- Gilles F., Gressens P., Dammann O., Leviton A. (2017). Hypoxia-ischemia is not an antecedent of most preterm brain damage: the illusion of validity. Dev. Med. Child Neurol. 60 120–125. 10.1111/dmcn.13483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginet V., Pittet M. P., Rummel C., Osterheld M. C., Meuli R., Clarke P. G., et al. (2014a). Dying neurons in thalamus of asphyxiated term newborns and rats are autophagic. Ann. Neurol. 76 695–711. 10.1002/ana.24257 [DOI] [PubMed] [Google Scholar]
- Ginet V., Puyal J., Clarke P. G., Truttmann A. C. (2009a). Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am. J. Pathol. 175 1962–1974. 10.2353/ajpath.2009.090463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginet V., Puyal J., Magnin G., Clarke P. G., Truttmann A. C. (2009b). Limited role of the c-Jun N-terminal kinase pathway in a neonatal rat model of cerebral hypoxia-ischemia. J. Neurochem. 108 552–562. 10.1111/j.1471-4159.2008.05797.x [DOI] [PubMed] [Google Scholar]
- Ginet V., Spiehlmann A., Rummel C., Rudinskiy N., Grishchuk Y., Luthi-Carter R., et al. (2014b). Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy 10 846–860. 10.4161/auto.28264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginet V., van de Looij Y., Petrenko V., Toulotte A., Kiss J., Huppi P. S., et al. (2016). Lactoferrin during lactation reduces lipopolysaccharide-induced brain injury. Biofactors 42 323–336. 10.1002/biof.1278 [DOI] [PubMed] [Google Scholar]
- Golstein P., Kroemer G. (2007). Cell death by necrosis: towards a molecular definition. Trends Biochem. Sci. 32 37–43. 10.1016/j.tibs.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Goni-de-Cerio F., Alvarez A., Caballero A., Mielgo V. E., Alvarez F. J., Rey-Santano M. C., et al. (2007). Early cell death in the brain of fetal preterm lambs after hypoxic-ischemic injury. Brain Res. 1151 161–171. 10.1016/j.brainres.2007.03.013 [DOI] [PubMed] [Google Scholar]
- Goren B., Cakir A., Sevinc C., Serter Kocoglu S., Ocalan B., Oy C., et al. (2017). Uridine treatment protects against neonatal brain damage and long-term cognitive deficits caused by hyperoxia. Brain Res. 1676 57–68. 10.1016/j.brainres.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Gozdas E., Parikh N. A., Merhar S. L., Tkach J. A., He L., Holland S. K. (2018). Altered functional network connectivity in preterm infants: antecedents of cognitive and motor impairments? Brain Struct. Funct. 223 3665–3680. 10.1007/s00429-018-1707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E. M., Holcroft C. J., Rai K. K., Donohue P. K., Allen M. C. (2004). Neonatal cerebral white matter injury in preterm infants is associated with culture positive infections and only rarely with metabolic acidosis. Am. J. Obstet. Gynecol. 191 1305–1310. 10.1016/j.ajog.2004.06.058 [DOI] [PubMed] [Google Scholar]
- Gram M., Sveinsdottir S., Cinthio M., Sveinsdottir K., Hansson S. R., Morgelin M., et al. (2014). Extracellular hemoglobin - mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J. Neuroinflammation 11:200. 10.1186/s12974-014-0200-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesmaier E., Schlager G., Wegleiter K., Hermann M., Urbanek M., Simbruner G., et al. (2010). Role of p75NTR in NMDAR-mediated excitotoxic brain injury in neonatal mice. Brain Res. 1355 31–40. 10.1016/j.brainres.2010.07.095 [DOI] [PubMed] [Google Scholar]
- Grishchuk Y., Ginet V., Truttmann A. C., Clarke P. G., Puyal J. (2011). Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy 7 1115–1131. 10.4161/auto.7.10.16608 [DOI] [PubMed] [Google Scholar]
- Gussenhoven R., Westerlaken R. J. J., Ophelders D., Jobe A. H., Kemp M. W., Kallapur S. G., et al. (2018). Chorioamnionitis, neuroinflammation, and injury: timing is key in the preterm ovine fetus. J. Neuroinflammation 15:113. 10.1186/s12974-018-1149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H., Mallard C., Ferriero D. M., Vannucci S. J., Levison S. W., Vexler Z. S., et al. (2015). The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 11 192–208. 10.1038/nrneurol.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen M. W., Riddle A., McClendon E., Gong X., Shaver D., Srivastava T., et al. (2014). Role of recurrent hypoxia-ischemia in preterm white matter injury severity. PLoS One 9:e112800. 10.1371/journal.pone.0112800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P., Dupuis N., Degos V., Moniaux N., Chhor V., Rasika S., et al. (2014). HIP/PAP prevents excitotoxic neuronal death and promotes plasticity. Ann. Clin. Transl. Neurol. 1 739–754. 10.1002/acn3.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargitai B., Szabo V., Hajdu J., Harmath A., Pataki M., Farid P., et al. (2001). Apoptosis in various organs of preterm infants: histopathologic study of lung, kidney, liver, and brain of ventilated infants. Pediatr. Res. 50 110–114. 10.1203/00006450-200107000-00020 [DOI] [PubMed] [Google Scholar]
- Haynes R. L., Billiards S. S., Borenstein N. S., Volpe J. J., Kinney H. C. (2008). Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr. Res. 63 656–661. 10.1203/PDR.0b013e31816c825c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R. L., Folkerth R. D., Keefe R. J., Sung I., Swzeda L. I., Rosenberg P. A., et al. (2003). Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J. Neuropathol. Exp. Neurol. 62 441–450. 10.1093/jnen/62.5.441 [DOI] [PubMed] [Google Scholar]
- Haynes R. L., van Leyen K. (2013). 12/15-lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia. Dev. Neurosci. 35 140–154. 10.1159/000350230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. S., Kim E. K., Choi Y. H., Shin S. H., Sohn J. A., Cheon J. E., et al. (2018). Timing of sepsis is an important risk factor for white matter abnormality in extremely premature infants with sepsis. Pediatr. Neonatol. 59 77–84. 10.1016/j.pedneo.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Hielkema T., Hadders-Algra M. (2016). Motor and cognitive outcome after specific early lesions of the brain - a systematic review. Dev. Med. Child Neurol. 58(Suppl. 4), 46–52. 10.1111/dmcn.13047 [DOI] [PubMed] [Google Scholar]
- Hoeber D., Sifringer M., van de Looij Y., Herz J., Sizonenko S. V., Kempe K., et al. (2016). Erythropoietin restores long-term neurocognitive function involving mechanisms of neuronal plasticity in a model of hyperoxia-induced preterm brain injury. Oxid. Med. Cell Longev. 2016:9247493. 10.1155/2016/9247493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Lai H., Xu H., Wu W., Lai X., Ho G., et al. (2013). Impact of perinatal systemic hypoxic-ischemic injury on the brain of male offspring rats: an improved model of neonatal hypoxic-ischemic encephalopathy in early preterm newborns. PLoS One 8:e82502. 10.1371/journal.pone.0082502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson I., Mesplès B., Bac P., Vamecq J., Evrard P., Gressens P. (2002). Melatoninergic neuroprotection of the murine periventricular white matter against neonatal excitotoxic challenge. Ann. Neurol. 51 82–92. 10.1002/ana.10072 [DOI] [PubMed] [Google Scholar]
- Husson I., Rangon C. M., Lelievre V., Bemelmans A. P., Sachs P., Mallet J., et al. (2005). BDNF-induced white matter neuroprotection and stage-dependent neuronal survival following a neonatal excitotoxic challenge. Cereb. Cortex 15 250–261. 10.1093/cercor/bhh127 [DOI] [PubMed] [Google Scholar]
- Ikebara J. M., Takada S. H., Cardoso D. S., Dias N. M., de Campos B. C., Bretherick T. A., et al. (2017). Functional role of intracellular calcium receptor inositol 1,4,5-trisphosphate type 1 in rat hippocampus after neonatal anoxia. PLoS One 12:e0169861. 10.1371/journal.pone.0169861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska B., Scafidi J., Aguirre A., Vaccarino F., Nguyen V., Borok E., et al. (2012). Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J. Neurosci. 32 14775–14793. 10.1523/JNEUROSCI.2060-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab A., Ruegger C., Bucher H. U., Makki M., Huppi P. S., Tuura R., et al. (2019). Network based statistics reveals trophic and neuroprotective effect of early high dose erythropoetin on brain connectivity in very preterm infants. Neuroimage Clin. 22:101806. 10.1016/j.nicl.2019.101806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie L. L., Corbett C. J., Firl D. J., Robinson S. (2015a). Postnatal erythropoietin mitigates impaired cerebral cortical development following subplate loss from prenatal hypoxia-ischemia. Cereb. Cortex 25 2683–2695. 10.1093/cercor/bhu066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie L. L., Robinson S. (2015). Preclinical models of encephalopathy of prematurity. Dev. Neurosci. 37 277–288. 10.1159/000371721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie L. L., Talos D. M., Jackson M. C., Park H. K., Graham D. A., Lechpammer M., et al. (2015b). Developmental expression of N-methyl-D-aspartate (n.d.) receptor subunits in human white and gray matter: potential mechanism of increased vulnerability in the immature brain. Cereb. Cortex 25 482–495. 10.1093/cercor/bht246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaindl A. M., Sifringer M., Koppelstaetter A., Genz K., Loeber R., Boerner C., et al. (2008). Erythropoietin protects the developing brain from hyperoxia-induced cell death and proteome changes. Ann. Neurol. 64 523–534. 10.1002/ana.21471 [DOI] [PubMed] [Google Scholar]
- Kersbergen K. J., Leroy F., Isgum I., Groenendaal F., de Vries L. S., Claessens N. H. P., et al. (2016). Relation between clinical risk factors, early cortical changes, and neurodevelopmental outcome in preterm infants. Neuroimage 142 301–310. 10.1016/j.neuroimage.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Kida H., Nomura S., Shinoyama M., Ideguchi M., Owada Y., Suzuki M. (2013). The effect of hypothermia therapy on cortical laminar disruption following ischemic injury in neonatal mice. PLoS One 8:e68877. 10.1371/journal.pone.0068877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro H., Anderson P. J., Doyle L. W., Woodward L. J., Neil J. J., Inder T. E. (2014). Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134 e444–e453. 10.1542/peds.2013-2336 [DOI] [PubMed] [Google Scholar]
- Kidokoro H., Neil J. J., Inder T. E. (2013). New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am. J. Neuroradiol. 34 2208–2214. 10.3174/ajnr.A3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H. C., Haynes R. L., Xu G., Andiman S. E., Folkerth R. D., Sleeper L. A., et al. (2012). Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann. Neurol. 71 397–406. 10.1002/ana.22612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H. C., Volpe J. J. (2012). Modeling the encephalopathy of prematurity in animals: the important role of translational research. Neurol. Res. Int. 2012:295389. 10.1155/2012/295389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka C., Kuchino Y. (1999). Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ. 6 508–515. 10.1038/sj.cdd.4400526 [DOI] [PubMed] [Google Scholar]
- Klebe D., McBride D., Krafft P. R., Flores J. J., Tang J., Zhang J. H. (2020). Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: established mechanisms and proposed pathways. J. Neurosci. Res. 98 105–120. 10.1002/jnr.24394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Abdelmohsen K., Abe A., Abedin M. J., Abeliovich H., Acevedo Arozena A., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12 1–222. 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M., Satoh Y., Otsubo Y., Araki Y., Yonamine R., Masui K., et al. (2011). Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology 115 979–991. 10.1097/ALN.0b013e318234228b [DOI] [PubMed] [Google Scholar]
- Kriel J., Loos B. (2019). The good, the bad and the autophagosome: exploring unanswered questions of autophagy-dependent cell death. Cell Death Differ. 26 640–652. 10.1038/s41418-018-0267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Levine B. (2008). Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 9 1004–1010. 10.1038/nrm2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral A., Baskin H., Yesilirmak D. C., Ergur B. U., Aykan S., Genc S., et al. (2007). Erythropoietin attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neonatology 92 269–278. 10.1159/000105493 [DOI] [PubMed] [Google Scholar]
- Kumral A., Iscan B., Engur D., Tuzun F., Ozbal S., Ergur B. U., et al. (2017). Intranasal surfactant protein D as neuroprotective rescue in a neonatal rat model of periventricular leukomalacia. J. Matern. Fetal. Neonatal. Med. 30 446–451. 10.1080/14767058.2016.1174996 [DOI] [PubMed] [Google Scholar]
- Kurul S. H., Yis U., Kumral A., Tugyan K., Cilaker S., Kolatan E., et al. (2009). Protective effects of topiramate against hyperoxic brain injury in the developing brain. Neuropediatrics 40 22–27. 10.1055/s-0029-1224101 [DOI] [PubMed] [Google Scholar]
- Levine B., Kroemer G. (2019). Biological functions of autophagy genes: a disease perspective. Cell 176 11–42. 10.1016/j.cell.2018.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xiong M., Nadavaluru P. R., Zuo W., Ye J. H., Eloy J. D., et al. (2016a). Dexmedetomidine attenuates neurotoxicity induced by prenatal propofol exposure. J. Neurosurg. Anesthesiol. 28 51–64. 10.1097/ANA.0000000000000181 [DOI] [PubMed] [Google Scholar]
- Li J., Yawno T., Sutherland A., Loose J., Nitsos I., Allison B. J., et al. (2017). Term vs. preterm cord blood cells for the prevention of preterm brain injury. Pediatr. Res. 82 1030–1038. 10.1038/pr.2017.170 [DOI] [PubMed] [Google Scholar]
- Li J., Yawno T., Sutherland A., Loose J., Nitsos I., Bischof R., et al. (2016b). Preterm white matter brain injury is prevented by early administration of umbilical cord blood cells. Exp. Neurol. 283(Pt A), 179–187. 10.1016/j.expneurol.2016.06.017 [DOI] [PubMed] [Google Scholar]
- Li X., Wu Z., Zhang Y., Xu Y., Han G., Zhao P. (2017). Activation of autophagy contributes to sevoflurane-induced neurotoxicity in fetal rats. Front. Mol. Neurosci. 10:432. 10.3389/fnmol.2017.00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligam P., Haynes R. L., Folkerth R. D., Liu L., Yang M., Volpe J. J., et al. (2009). Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr. Res. 65 524–529. 10.1203/PDR.0b013e3181998baf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Shoji-Kawata S., Sumpter R. M., Jr., Wei Y., Ginet V., Zhang L., et al. (2013). Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. U.S.A. 110 20364–20371. 10.1073/pnas.1319661110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodygensky G. A., Kunz N., Perroud E., Somm E., Mlynarik V., Huppi P. S., et al. (2014). Definition and quantification of acute inflammatory white matter injury in the immature brain by MRI/MRS at high magnetic field. Pediatr. Res. 75 415–423. 10.1038/pr.2013.242 [DOI] [PubMed] [Google Scholar]
- Luyt K., Jary S., Lea C., Young G. J., Odd D., Miller H., et al. (2019). Ten-year follow-up of a randomised trial of drainage, irrigation and fibrinolytic therapy (DRIFT) in infants with post-haemorrhagic ventricular dilatation. Health Technol. Assess. 23 1–116. 10.3310/hta23040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard C., Welin A. K., Peebles D., Hagberg H., Kjellmer I. (2003). White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem. Res. 28 215–223. 10.1023/a:1022368915400 [DOI] [PubMed] [Google Scholar]
- Marino G., Madeo F., Kroemer G. (2011). Autophagy for tissue homeostasis and neuroprotection. Curr. Opin. Cell Biol. 23 198–206. 10.1016/j.ceb.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Mazur M., Miller R. H., Robinson S. (2010). Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. J. Neurosurg. Pediatr. 6 206–221. 10.3171/2010.5.PEDS1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon E., Shaver D. C., Degener-O’Brien K., Gong X., Nguyen T., Hoerder-Suabedissen A., et al. (2017). Transient hypoxemia chronically disrupts maturation of preterm fetal ovine subplate neuron arborization and activity. J. Neurosci. 37 11912–11929. 10.1523/JNEUROSCI.2396-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan E. C., Vohr B. R. (2019). Neurodevelopmental follow-up of preterm infants: what is new? Pediatr. Clin. North Am. 66 509–523. 10.1016/j.pcl.2018.12.015 [DOI] [PubMed] [Google Scholar]
- McPherson C., Neil J. J., Tjoeng T. H., Pineda R., Inder T. E. (2015). A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr. Res. 78 198–204. 10.1038/pr.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen P. S., Sheldon R. A., Shatz C. J., Ferriero D. M. (2003). Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J. Neurosci. 23 3308–3315. 10.1523/jneurosci.23-08-03308.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medja F., Lelievre V., Fontaine R. H., Lebas F., Leroux P., Ouimet T., et al. (2006). Thiorphan, a neutral endopeptidase inhibitor used for diarrhoea, is neuroprotective in newborn mice. Brain 129(Pt 12), 3209–3223. 10.1093/brain/awl239 [DOI] [PubMed] [Google Scholar]
- Mikhailova A., Sunkara N., McQuillen P. S. (2017). Unbiased quantification of subplate neuron loss following neonatal hypoxia-ischemia in a rat model. Dev. Neurosci. 39 171–181. 10.1159/000460815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss F. H., Jr., Saha S., Bell E. F., Colaizy T. T., Stoll B. J., Hintz S. R., et al. (2014). Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatr. 168 746–754. 10.1001/jamapediatrics.2014.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai T., Mori Y., Shimazu T., Takahashi A., Tsunoda H., Yamaguchi S., et al. (2017). Intravenous injection of umbilical cord-derived mesenchymal stromal cells attenuates reactive gliosis and hypomyelination in a neonatal intraventricular hemorrhage model. Neuroscience 355 175–187. 10.1016/j.neuroscience.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Neubauer V., Wegleiter K., Posod A., Urbanek M., Wechselberger K., Kiechl-Kohlendorfer U., et al. (2016). Delayed application of the haematopoietic growth factors G-CSF/SCF and FL reduces neonatal excitotoxic brain injury. Brain Res. 1634 94–103. 10.1016/j.brainres.2015.12.058 [DOI] [PubMed] [Google Scholar]
- Noguchi K. K., Johnson S. A., Manzella F. M., Masuoka K. L., Williams S. L., Martin L. D., et al. (2018). Caffeine augments anesthesia neurotoxicity in the fetal macaque brain. Sci. Rep. 8:5302. 10.1038/s41598-018-23560-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington F. J., Chavez-Valdez R., Martin L. J. (2011). Neuronal cell death in neonatal hypoxia-ischemia. Ann. Neurol. 69 743–758. 10.1002/ana.22419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossin-Manor R., Card D., Morris D., Noormohamed S., Shroff M. M., Whyte H. E., et al. (2013). Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T(1) imaging. Neuroimage 64 505–516. 10.1016/j.neuroimage.2012.08.086 [DOI] [PubMed] [Google Scholar]
- Okusa C., Oeschger F., Ginet V., Wang W. Z., Hoerder-Suabedissen A., Matsuyama T., et al. (2014). Subplate in a rat model of preterm hypoxia-ischemia. Ann. Clin. Transl. Neurol. 1 679–691. 10.1002/acn3.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintlia M. K., Paintlia A. S., Barbosa E., Singh I., Singh A. K. (2004). N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J. Neurosci. Res. 78 347–361. 10.1002/jnr.20261 [DOI] [PubMed] [Google Scholar]
- Panda S., Dohare P., Jain S., Parikh N., Singla P., Mehdizadeh R., et al. (2018). Estrogen treatment reverses prematurity-induced disruption in cortical interneuron population. J. Neurosci. 38 7378–7391. 10.1523/JNEUROSCI.0478-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneth N. (2018). Hypoxia-ischemia and brain injury in infants born preterm. Dev. Med. Child Neurol. 60:115. 10.1111/dmcn.13642 [DOI] [PubMed] [Google Scholar]
- Panfoli I., Candiano G., Malova M., De Angelis L., Cardiello V., Buonocore G., et al. (2018). Oxidative stress as a primary risk factor for brain damage in preterm newborns. Front. Pediatr. 6:369. 10.3389/fped.2018.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton M. C. B., Allison B. J., Li J., Fahey M. C., Sutherland A. E., Nitsos I., et al. (2018). Human umbilical cord blood therapy protects cerebral white matter from systemic LPS exposure in preterm fetal sheep. Dev. Neurosci. 40 258–270. 10.1159/000490943 [DOI] [PubMed] [Google Scholar]
- Pham H., Vottier G., Pansiot J., Duong-Quy S., Bollen B., Dalous J., et al. (2014). Inhaled NO prevents hyperoxia-induced white matter damage in neonatal rats. Exp. Neurol. 252 114–123. 10.1016/j.expneurol.2013.11.025 [DOI] [PubMed] [Google Scholar]
- Pierrat V., Marchand-Martin L., Arnaud C., Kaminski M., Resche-Rigon M., Lebeaux C., et al. (2017). Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 358:j3448. 10.1136/bmj.j3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre W. C., Akakpo L., Londono I., Pouliot P., Chemtob S., Lesage F., et al. (2019). Assessing therapeutic response non-invasively in a neonatal rat model of acute inflammatory white matter injury using high-field MRI. Brain Behav. Immun. 81 348–360. 10.1016/j.bbi.2019.06.032 [DOI] [PubMed] [Google Scholar]
- Pierson C. R., Folkerth R. D., Billiards S. S., Trachtenberg F. L., Drinkwater M. E., Volpe J. J., et al. (2007). Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 114 619–631. 10.1007/s00401-007-0295-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C., Price D. L., Martin L. J. (1997). Excitotoxic neuronal death in the immature brain is an apoptosis-necrosis morphological continuum. J. Comp. Neurol. 378 70–87. [PubMed] [Google Scholar]
- Posod A., Pinzer K., Urbanek M., Wegleiter K., Keller M., Kiechl-Kohlendorfer U., et al. (2014). The common antitussive agent dextromethorphan protects against hyperoxia-induced cell death in established in vivo and in vitro models of neonatal brain injury. Neuroscience 274 260–272. 10.1016/j.neuroscience.2014.05.059 [DOI] [PubMed] [Google Scholar]
- Puyal J., Ginet V., Clarke P. G. (2013). Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog. Neurobiol. 105 24–48. 10.1016/j.pneurobio.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Puyal J., Vaslin A., Mottier V., Clarke P. G. (2009). Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann. Neurol. 66 378–389. 10.1002/ana.21714 [DOI] [PubMed] [Google Scholar]
- Riddle A., Luo N. L., Manese M., Beardsley D. J., Green L., Rorvik D. A., et al. (2006). Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J. Neurosci. 26 3045–3055. 10.1523/JNEUROSCI.5200-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S. J., Shi Y. (2004). Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5 897–907. 10.1038/nrm1496 [DOI] [PubMed] [Google Scholar]
- Robinson S., Li Q., Dechant A., Cohen M. L. (2006). Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J. Neurosurg/ 104 6(Suppl.), 396–408. 10.3171/ped.2006.104.6.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S., Petelenz K., Li Q., Cohen M. L., Dechant A., Tabrizi N., et al. (2005). Developmental changes induced by graded prenatal systemic hypoxic-ischemic insults in rats. Neurobiol. Dis. 18 568–581. 10.1016/j.nbd.2004.10.024 [DOI] [PubMed] [Google Scholar]
- Rogido M., Husson I., Bonnier C., Lallemand M. C., Merienne C., Gregory G. A., et al. (2003). Fructose-1,6-biphosphate prevents excitotoxic neuronal cell death in the neonatal mouse brain. Brain Res. Dev. Brain Res. 140 287–297. 10.1016/s0165-3806(02)00615-6 [DOI] [PubMed] [Google Scholar]
- Rousset C. I., Chalon S., Cantagrel S., Bodard S., Andres C., Gressens P., et al. (2006). Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr. Res. 59 428–433. 10.1203/01.pdr.0000199905.08848.55 [DOI] [PubMed] [Google Scholar]
- Rousset C. I., Kassem J., Aubert A., Planchenault D., Gressens P., Chalon S., et al. (2013). Maternal exposure to lipopolysaccharide leads to transient motor dysfunction in neonatal rats. Dev. Neurosci. 35 172–181. 10.1159/000346579 [DOI] [PubMed] [Google Scholar]
- Scafidi J., Hammond T. R., Scafidi S., Ritter J., Jablonska B., Roncal M., et al. (2014). Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 506 230–234. 10.1038/nature12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma W., Basterra L. B., Jacobs S., Brouwer N., Meerlo P., Schaafsma A., et al. (2017). Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol. Dis. 106 291–300. 10.1016/j.nbd.2017.07.017 [DOI] [PubMed] [Google Scholar]
- Schmidt A. F., Kannan P. S., Chougnet C. A., Danzer S. C., Miller L. A., Jobe A. H., et al. (2016). Intra-amniotic LPS causes acute neuroinflammation in preterm rhesus macaques. J. Neuroinflammation 13:238. 10.1186/s12974-016-0706-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B., Anderson P. J., Doyle L. W., Dewey D., Grunau R. E., Asztalos E. V., et al. (2012). Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307 275–282. 10.1001/jama.2011.2024 [DOI] [PubMed] [Google Scholar]
- Schmidt B., Roberts R. S., Anderson P. J., Asztalos E. V., Costantini L., Davis P. G., et al. (2017). Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr. 171 564–572. 10.1001/jamapediatrics.2017.0238 [DOI] [PubMed] [Google Scholar]
- Schmitz T., Felderhoff-Mueser U., Sifringer M., Groenendaal F., Kampmann S., Heep A. (2011). Expression of soluble Fas in the cerebrospinal fluid of preterm infants with posthemorrhagic hydrocephalus and cystic white matter damage. J. Perinat. Med. 39 83–88. 10.1515/JPM.2010.125 [DOI] [PubMed] [Google Scholar]
- Schmitz T., Krabbe G., Weikert G., Scheuer T., Matheus F., Wang Y., et al. (2014). Minocycline protects the immature white matter against hyperoxia. Exp. Neurol. 254 153–165. 10.1016/j.expneurol.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Schneider J., Fischer Fumeaux C. J., Duerden E. G., Guo T., Foong J., Graz M. B., et al. (2018). Nutrient Intake in the first two weeks of life and brain growth in preterm neonates. Pediatrics 141 e20172169. 10.1542/peds.2017-2169 [DOI] [PubMed] [Google Scholar]
- Schneider J., Kober T., Bickle Graz M., Meuli R., Huppi P. S., Hagmann P., et al. (2016). Evolution of T1 relaxation, ADC, and fractional anisotropy during early brain maturation: a serial imaging study on preterm infants. AJNR Am. J. Neuroradiol. 37 155–162. 10.3174/ajnr.A4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Miller S. P. (2019). Preterm brain Injury: white matter injury. Handb. Clin. Neurol. 162 155–172. 10.1016/B978-0-444-64029-1.00007-2 [DOI] [PubMed] [Google Scholar]
- Segovia K. N., McClure M., Moravec M., Luo N. L., Wan Y., Gong X., et al. (2008). Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann. Neurol. 63 520–530. 10.1002/ana.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama T., Kamei Y., Iriyama T., Imada S., Ichinose M., Toshimitsu M., et al. (2018). Pretreatment with magnesium sulfate attenuates white matter damage by preventing cell death of developing oligodendrocytes. J. Obstet. Gynaecol. Res. 44 601–607. 10.1111/jog.13568 [DOI] [PubMed] [Google Scholar]
- Shah D. K., Doyle L. W., Anderson P. J., Bear M., Daley A. J., Hunt R. W., et al. (2008). Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J. Pediatr. 153:175.e1–171.e1. 10.1016/j.jpeds.2008.02.033 [DOI] [PubMed] [Google Scholar]
- Sifringer M., Bendix I., Borner C., Endesfelder S., von Haefen C., Kalb A., et al. (2012). Prevention of neonatal oxygen-induced brain damage by reduction of intrinsic apoptosis. Cell Death Dis. 3:e250. 10.1038/cddis.2011.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifringer M., von Haefen C., Krain M., Paeschke N., Bendix I., Buhrer C., et al. (2015). Neuroprotective effect of dexmedetomidine on hyperoxia-induced toxicity in the neonatal rat brain. Oxid. Med. Cell Longev. 2015:530371. 10.1155/2015/530371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner B., Becke K., Engelhard K. (2014). General anaesthetics and the developing brain: an overview. Anaesthesia 69 1009–1022. 10.1111/anae.12637 [DOI] [PubMed] [Google Scholar]
- Sokolowska P., Passemard S., Mok A., Schwendimann L., Gozes I., Gressens P. (2011). Neuroprotective effects of NAP against excitotoxic brain damage in the newborn mice: implications for cerebral palsy. Neuroscience 173 156–168. 10.1016/j.neuroscience.2010.10.074 [DOI] [PubMed] [Google Scholar]
- Sokolowski J. D., Gamage K. K., Heffron D. S., Leblanc A. C., Deppmann C. D., Mandell J. W. (2014). Caspase-mediated cleavage of actin and tubulin is a common feature and sensitive marker of axonal degeneration in neural development and injury. Acta Neuropathol. Commun. 2:16. 10.1186/2051-5960-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp H. B., Fleiss B., Arai Y., Supramaniam V., Vontell R., Birtles S., et al. (2019). Interneuron development is disrupted in preterm brains with diffuse white matter injury: observations in mouse and human. Front. Physiol. 10:955. 10.3389/fphys.2019.00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolwijk L. J., Lemmers P. M., Harmsen M., Groenendaal F., de Vries L. S., van der Zee D. C., et al. (2016). Neurodevelopmental outcomes after neonatal surgery for major noncardiac anomalies. Pediatrics 137:e20151728. 10.1542/peds.2015-1728 [DOI] [PubMed] [Google Scholar]
- Stolwijk L. J., Lemmers P. M. A., van Herwaarden M. Y. A., van der Zee D. C., van Bel F., Groenendaal F., et al. (2017). Predictive role of F2-isoprostanes as biomarkers for brain damage after neonatal surgery. Dis. Markers 2017:2728103. 10.1155/2017/2728103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley M. E., Van Oeffelen W., Theze S., Sullivan A. M., O’Mahony S. M., Cryan J. F., et al. (2017). Distinct alterations in motor & reward seeking behavior are dependent on the gestational age of exposure to LPS-induced maternal immune activation. Brain Behav. Immun. 63 21–34. 10.1016/j.bbi.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Sun L. (2010). Early childhood general anaesthesia exposure and neurocognitive development. Br. J. Anaesth. 105(Suppl. 1), i61–i68. 10.1093/bja/aeq302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnes A., Hicks M. (2018). Neurodevelopmental outcomes of preterm children at school age and beyond. Clin. Perinatol. 45 393–408. 10.1016/j.clp.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Taglialatela G., Perez-Polo J. R., Rassin D. K. (1998). Induction of apoptosis in the CNS during development by the combination of hyperoxia and inhibition of glutathione synthesis. Free Radic Biol. Med 25 936–942. 10.1016/s0891-5849(98)00131-2 [DOI] [PubMed] [Google Scholar]
- Tahraoui S. L., Marret S., Bodenant C., Leroux P., Dommergues M. A., Evrard P., et al. (2001). Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol. 11 56–71. 10.1111/j.1750-3639.2001.tb00381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S. H., dos Santos Haemmerle C. A., Motta-Teixeira L. C., Machado-Nils A. V., Lee V. Y., Takase L. F., et al. (2015). Neonatal anoxia in rats: hippocampal cellular and subcellular changes related to cell death and spatial memory. Neuroscience 284 247–259. 10.1016/j.neuroscience.2014.08.054 [DOI] [PubMed] [Google Scholar]
- van de Looij Y., Ginet V., Chatagner A., Toulotte A., Somm E., Huppi P. S., et al. (2014). Lactoferrin during lactation protects the immature hypoxic-ischemic rat brain. Ann. Clin. Transl. Neurol. 1 955–967. 10.1002/acn3.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Hooft J., van der Lee J. H., Opmeer B. C., Aarnoudse-Moens C. S., Leenders A. G., Mol B. W., et al. (2015). Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst. Rev. 4:71. 10.1186/s13643-015-0058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C., Pogledic I., Biran V., Adle-Biassette H., Fallet-Bianco C., Gressens P. (2012). Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J. Neuropathol. Exp. Neurol. 71 251–264. 10.1097/NEN.0b013e3182496429 [DOI] [PubMed] [Google Scholar]
- Vinukonda G., Csiszar A., Hu F., Dummula K., Pandey N. K., Zia M. T., et al. (2010). Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-alpha inhibition. Brain 133(Pt 8), 2264–2280. 10.1093/brain/awq107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontell R., Supramaniam V., Wyatt-Ashmead J., Gressens P., Rutherford M., Hagberg H., et al. (2015). Cellular mechanisms of toll-like receptor-3 activation in the thalamus are associated with white matter injury in the developing brain. J. Neuropathol. Exp. Neurol. 74 273–285. 10.1097/NEN.0000000000000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vottier G., Pham H., Pansiot J., Biran V., Gressens P., Charriaut-Marlangue C., et al. (2011). Deleterious effect of hyperoxia at birth on white matter damage in the newborn rat. Dev. Neurosci. 33 261–269. 10.1159/000327245) [DOI] [PubMed] [Google Scholar]
- Walker S. M., Melbourne A., O’Reilly H., Beckmann J., Eaton-Rosen Z., Ourselin S., et al. (2018). Somatosensory function and pain in extremely preterm young adults from the UK EPICure cohort: sex-dependent differences and impact of neonatal surgery. Br. J. Anaesth. 121 623–635. 10.1016/j.bja.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. W., Chang Y. C., Lin C. Y., Hong J. S., Huang C. C. (2010). Low-dose lipopolysaccharide selectively sensitizes hypoxic ischemia-induced white matter injury in the immature brain. Pediatr. Res. 68 41–47. 10.1203/00006450-201011001-00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. W., Tu Y. F., Huang C. C., Ho C. J. (2012). JNK signaling is the shared pathway linking neuroinflammation, blood-brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflammation 9:175. 10.1186/1742-2094-9-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Dong Y., Zhang Y., Li T., Xie Z. (2019). Sevoflurane induces cognitive impairment in young mice via autophagy. PLoS One 14:e0216372. 10.1371/journal.pone.0216372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang C., Zhang Y., Wang G., Yang H. (2019). Pre-administration of luteoline attenuates neonatal sevoflurane-induced neurotoxicity in mice. Acta Histochem. 121 500–507. 10.1016/j.acthis.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Wassink G., Davidson J. O., Dhillon S. K., Fraser M., Galinsky R., Bennet L., et al. (2017). Partial white and grey matter protection with prolonged infusion of recombinant human erythropoietin after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 37 1080–1094. 10.1177/0271678X16650455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegleiter K., Hermann M., Posod A., Wechselberger K., Stanika R. I., Obermair G. J., et al. (2014). The sigma-1 receptor agonist 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) protects against newborn excitotoxic brain injury by stabilizing the mitochondrial membrane potential in vitro and inhibiting microglial activation in vivo. Exp. Neurol. 261 501–509. 10.1016/j.expneurol.2014.07.022 [DOI] [PubMed] [Google Scholar]
- Xia Y., Xu H., Jia C., Hu X., Kang Y., Yang X., et al. (2017). Tanshinone IIA Attenuates Sevoflurane Neurotoxicity in Neonatal Mice. Anesth. Analg. 124 1244–1252. 10.1213/ANE.0000000000001942 [DOI] [PubMed] [Google Scholar]
- Xie C., Ginet V., Sun Y., Koike M., Zhou K., Li T., et al. (2016). Neuroprotection by selective neuronal deletion of Atg7 in neonatal brain injury. Autophagy 12 410–423. 10.1080/15548627.2015.1132134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M., Zhang L., Li J., Eloy J., Ye J. H., Bekker A. (2016). Propofol-induced neurotoxicity in the fetal animal brain and developments in modifying these effects-an updated review of propofol fetal exposure in laboratory animal studies. Brain Sci. 6:E11. 10.3390/brainsci6020011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Balasubramaniam J., Buist R. J., Peeling J., Del Bigio M. R. (2003). Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J. Neuropathol. Exp. Neurol. 62 1154–1165. 10.1093/jnen/62.11.1154 [DOI] [PubMed] [Google Scholar]
- Yavuz A., Sezik M., Ozmen O., Asci H. (2017). Fingolimod against endotoxin-induced fetal brain injury in a rat model. J. Obstet. Gynaecol. Res. 43 1708–1713. 10.1111/jog.13444 [DOI] [PubMed] [Google Scholar]
- Yawno T., Mahen M., Li J., Fahey M. C., Jenkin G., Miller S. L. (2017a). The beneficial effects of melatonin administration following hypoxia-ischemia in preterm fetal sheep. Front. Cell Neurosci. 11:296. 10.3389/fncel.2017.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawno T., Sabaretnam T., Li J., McDonald C., Lim R., Jenkin G., et al. (2017b). Human amnion epithelial cells protect against white matter brain injury after repeated endotoxin exposure in the preterm ovine fetus. Cell Transplant. 26 541–553. 10.3727/096368916X693572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilirmak D. C., Kumral A., Baskin H., Ergur B. U., Aykan S., Genc S., et al. (2007). Activated protein C reduces endotoxin-induced white matter injury in the developing rat brain. Brain Res. 1164 14–23. 10.1016/j.brainres.2007.04.083 [DOI] [PubMed] [Google Scholar]
- Yis U., Kurul S. H., Kumral A., Cilaker S., Tugyan K., Genc S., et al. (2008a). Hyperoxic exposure leads to cell death in the developing brain. Brain Dev. 30 556–562. 10.1016/j.braindev.2008.01.010 [DOI] [PubMed] [Google Scholar]
- Yis U., Kurul S. H., Kumral A., Tugyan K., Cilaker S., Yilmaz O., et al. (2008b). Effect of erythropoietin on oxygen-induced brain injury in the newborn rat. Neurosci. Lett. 448 245–249. 10.1016/j.neulet.2008.10.060 [DOI] [PubMed] [Google Scholar]
- Zheng H., Dong Y., Xu Z., Crosby G., Culley D.J., Zhang Y., et al. (2013). Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology 118 516–526. 10.1097/ALN.0b013e3182834d5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Narayan S., Su L., Al-Alawyat H., Liu J., Kannan S. (2018). Cerebellar injury and impaired function in a rabbit model of maternal inflammation induced neonatal brain injury. Neurobiol. Learn. Mem. 165:106901. 10.1016/j.nlm.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Qu Y., Lin Z., Zhao F., Zhang L., Huang Y., et al. (2016). Loss of PINK1 inhibits apoptosis by upregulating alpha-synuclein in inflammation-sensitized hypoxic-ischemic injury in the immature brains. Brain Res. 1653 14–22. 10.1016/j.brainres.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Zia M. T., Csiszar A., Labinskyy N., Hu F., Vinukonda G., LaGamma E. F., et al. (2009). Oxidative-nitrosative stress in a rabbit pup model of germinal matrix hemorrhage: role of NAD(P)H oxidase. Stroke 40 2191–2198. 10.1161/STROKEAHA.108.544759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L., Soria-Pastor S., Junque C., Segarra D., Bargallo N., Mayolas N., et al. (2011). Gray matter volume decrements in preterm children with periventricular leukomalacia. Pediatr. Res. 69 554–560. 10.1203/PDR.0b013e3182182366 [DOI] [PubMed] [Google Scholar]


