Abstract
Competition for resources within a population can lead to niche partitioning between sexes, throughout ontogeny and among individuals, allowing con-specifics to co-exist. We aimed to quantify such partitioning in Antarctic fur seals, Arctocephalus gazella, breeding at South Georgia, which hosts ~95% of the world’s population. Whiskers were collected from 20 adult males and 20 adult females and stable isotope ratios were quantified every 5 mm along the length of each whisker. Nitrogen isotope ratios (δ15N) were used as proxies for trophic position and carbon isotope ratios (δ13C) indicated foraging habitat. Sexual segregation was evident: δ13C values were significantly lower in males than females, indicating males spent more time foraging south of the Polar Front in maritime Antarctica. In males δ13C values declined with age, suggesting males spent more time foraging south throughout ontogeny. In females δ13C values revealed two main foraging strategies: 70% of females spent most time foraging south of the Polar Front and had similar δ15N values to males, while 30% of females spent most time foraging north of the Polar Front and had significantly higher δ15N values. This niche partitioning may relax competition and ultimately elevate population carrying capacity with implications for ecology, evolution and conservation.
Subject terms: Biological techniques, Ecology, Zoology
Introduction
Competition for resources within a natural population can lead to diversification in resource use, ultimately allowing con-specifics to co-exist1. The ecological niche is positioned within an n-dimensional hypervolume2, generally composed of spatial, temporal and trophic axes3. Overlap in ecological niches causes competition for resources, which could lead to competitive exclusion4,5 and consequent niche shifts, whereby the position of a niche alters along the spatial, temporal, and/or trophic axis6,7. This niche partitioning commonly arises between sexes, but can also occur throughout ontogeny (hereby over an organism’s lifespan) and among individuals within a species8. The consequent reduction in intra-specific competition may lead to a greater carrying capacity for the population as a whole9–11. Understanding the causes and consequences of intra-specific niche partitioning is therefore a major goal of research into the ecology, evolution and conservation of species12,13.
Niche partitioning between sexes has been explained by several inter-connected hypotheses: (1) social roles: sexes segregating because they prefer to associate with the same class to benefit from social learning14,15; (2) activity budgets: sexes segregating to synchronise activities (e.g. sex-specific behaviours) to enable spatial coherence of the social group16,17; (3) life history strategies: including constraint of parental care; and (4) sexual size dimorphism (common in species with polgynous mating systems18). The sexual size dimorphism hypothesis has received considerable attention as body size is a key trait influencing fitness19. Indeed, males with larger body sizes could compete for mates more successfully20. Smaller animals can subsist on sparser resources than larger animals21,22, but may require higher quality food because of their higher mass-specific metabolic rates23. For example, adult female African elephants, Loxodonta africana, and their offspring feed in areas with greater plant diversity than larger adult males, which are less selective24. Size dimorphism also affects susceptibility to predation25,26 and physiological constraints (such as temperature and aerobic dive limits in diving predators27).
The above hypotheses relate to animals differently throughout ontogeny. As animals grow their life history priorities change, from maximising growth and survival as juveniles28 to reproduction as adults29,30. Ontogenetic niche shifts may occur as animals grow, become sexually mature and gain more experience with age. They could differ between the sexes, as a result of different life history constraints that affect growth patterns and resulting sexual-size dimorphism. Ontogenetic niche shifts may be particularly pronounced in the larger sex, as larger animals experience a greater diversity of body sizes (and therefore energetic requirements) throughout development3. For example, Northern death adders, Acanthopis praelongus, predate on frogs and lizards as juveniles, and frogs and mammals as adults, but adult females (the larger sex) consume a greater proportion of mammals than adult males31.
Niche differentiation can also occur among individuals, when individuals occupy only a subset of the population’s niche (individual specialisation)32. The optimum strategy for an individual depends on its particular priorities and restraints33. Individuals may rank resources differently according to their energy gain per unit time32 because of their size, age and experience, which affect diet preference, search efficiency and prey handling ability34,35. Different foraging strategies may therefore develop within the same sex. For example, female New Zealand sea lions, Phocarctos hookeri, have three distinct foraging strategies – a mechanism which could reduce intra-specific competition36.
Intra-specific niche partitioning may influence population carrying capacity. Theory on habitat selection predicts that as population density and competition increases, animals should distribute themselves relative to habitat profitability37. Selection favours behavioural and morphological traits that reduce aggressive encounters and competition for resources38,39. Individuals may specialise on particular resources40 and the population as a whole may exploit a wider range of resources37. For example, a population of feral horses, Equus ferus caballus, use a greater diversity of resources as population density increases37. It is therefore possible that generalist populations are composed of both generalist and specialist individuals40. These mechanisms reduce competition, which could increase individual reproductive success and consequently elevate population carrying capacity.
Antarctic fur seals, Arctocephalus gazella, are an ideal species to study intra-specific niche partitioning because of their large population size, breeding constraints and pronounced sexual size dimorphism (related to a highly polgygynous mating system). Intra-specific competition may be intense as populations have recovered from near extinction and are now in their millions with ~95% breeding at South Georgia, situated in the southern Atlantic Ocean41 (Fig. 1). The sexes have different breeding constraints, as females arrive at breeding beaches in late November/early December and are spatially restricted for four months while alternating foraging at sea with suckling their pups27. Males come ashore from October (peaking in numbers in December), to establish and defend harems (territorial males may fast at this time)41. After mating, males have no spatial or temporal constraints and observations suggest they migrate to higher latitudes in January27,41–43. Short-term tracking of individual females has shown that they migrate widely in winter, moving north towards Patagonia, south towards the Antarctic pack-ice and within waters around South Georgia44–46. However, it is unknown if these movement patterns are consistent across years and/or individuals.
Figure 1.
Map showing location of Bird Island, South Georgia, in relation to South America, Antarctica and the Polar Front. Map was created using R software (v3.6.1; https://www.R-project.org/).
The sexes have different growth trajectories that may facilitate ontogenetic niche shifts: females reach 90% of their maximum body length and become sexually mature by age four, while males grow to age seven (weighing up to four times more than females) and may not establish territories until age seven or eight41,47. Niche partitioning between sexes and throughout ontogeny has been determined in Antarctic fur seals breeding at Kerguelen48. However, Antarctic fur seals breeding at South Georgia may show different patterns in niche partitioning as a result of different environments, diets (i.e. Antarctic krill, Euphausia superba, predominates the diet at South Georgia41, while myctophids dominate at Kerguelen49), and the higher competitive pressure associated with a higher population density.
Stable isotope analysis can provide quantitative insights into intra-specific niche partitioning48,50 as stable isotope values are represented in delta-space as the ‘isotopic niche’51. Stable isotope values of a consumer’s tissues in part reflect diet, plus an added trophic discrimination factor (TDF) signifying the offset in stable isotope values between the tissue and the consumer’s food52. This offset occurs as a result of physical and biological processes involved in assimilating resources53. Nitrogen isotope ratios (15N/14N expressed as δ15N) are used as a proxy for trophic position, as the ratio increases stepwise with trophic level54,55. In marine systems carbon isotope ratios (13C/12C expressed as δ13C) indicate the geographic source of prey as they vary with offshore versus inshore regions, pelagic versus benthic regions, and notably latitude56,57. The δ13C values in particulate organic carbon in the oceans generally decline from the tropics to the poles58,59 and can be distinct between water masses separated by frontal zones: a pattern reflected by δ13C values of marine predators in sub-Antarctic regions60–64.
To investigate the existence and development of intra-specific niche partitioning and its role in reducing competition we analysed δ15N and δ13C values along the length of adult Antarctic fur seal whiskers. These are ideal tissues to study ontogeny and individual specialisation as they are metabolically inert once formed, grow continuously, and are retained for years so can reflect the animal’s foraging over long time periods. We hypothesise that for Antarctic fur seals within the world’s largest breeding colony: (1) Males will have lower δ13C values along their whiskers as they spend more time in maritime Antarctica than females; (2) Males will show greater ontogenetic changes in their isotopic niche as they exhibit greater growth than females and do not breed until they are older; (3) Females will show greater variation in δ15N values and δ13C values, reflecting a wider range of post-breeding migration strategies; (4) Consistent annual patterns in isotopic values will show that these migration strategies are consistent between years.
Results
Seal age
Average age of adult males, obtained from external growth ridges in canines, was 8.94 ± 0.89 years for all 34 males and 8.70 ± 0.73 for 20 randomly selected males. These age determinations were in close agreement between any two readers, with 92.1% of all readings showing a 0 or ±1 year difference. Age determinations were fairly consistent among all three readers, as the Index of Average Percentage Error (IAPE) was relatively low at 4.3%. Average minimum age of adult females at capture was 7.45 ± 2.17 years according to whisker growth rates.
Whisker growth rates
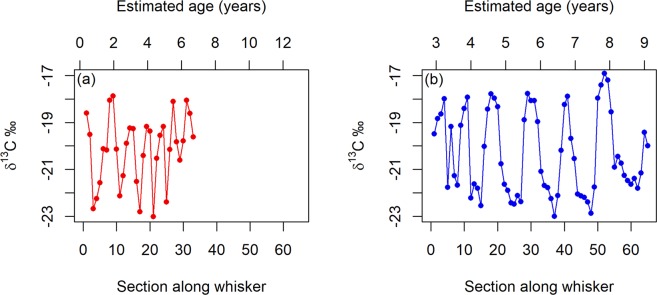
Whisker length significantly differed between males (mean = 25.75 cm, SD = 6.95) and females (mean = 16.29 cm, SD = 4.53, excluding the whisker root ~0.5 cm in length) (Mann-Whitney U test, U = 42, p < 0.001). Male whiskers also grew significantly faster (0.096 ± 0.026 mm/day) than female whiskers (0.063 ± 0.013 mm/day), assuming oscillations corresponded to annual migrations (Welch’s t-test, t = 5.29, p < 0.001) (e.g. Fig. 2; Supplementary Fig. S1). The calculated whisker growth rates suggest that male whiskers were grown over an average of 6.93 ± 2.03 years and females an average of 7.18 ± 1.20 years.
Figure 2.
Oscillations in δ13C values along the length of (a) a female Antarctic fur seal whisker (ID = w8859) and (b) a male Antarctic fur seal whisker (ID = w8821) from the distal to the facial end. Points are δ13C values of samples taken every 5 mm along the length of each whisker and lines join these points. Figure was created using R software (v3.6.1; https://www.R-project.org/).
δ13C value of polar front
When seals foraged at the Polar Front we estimated that δ13C values in their whiskers were about −18.92‰. This value was calculated from the average δ13C value of prey species (myctophids and krill) collected at the Polar Front in 2009 (−20.98‰) (Supplementary Fig. S2), added to the estimated TDF for Antarctic fur seal whiskers (2.06‰ ± 1.79 for δ13C). The variation in δ13C values along each whisker suggested all 20 females and only six males foraged north of the Polar Front at any point during their lives (as their maximum δ13C values exceeded −18.92‰) (Supplementary Tables S3 and S4). Stable isotope bi-plots (Fig. 3) revealed two isotopically distinct groups of females, separated by the estimated δ13C value of whiskers at the Polar Front: 14 individuals (female Group 1) had lower mean δ13C values (using all δ13C values along the whisker) than −18.92‰ and 6 individuals (female Group 2) had higher mean δ13C values than −18.92‰.
Figure 3.
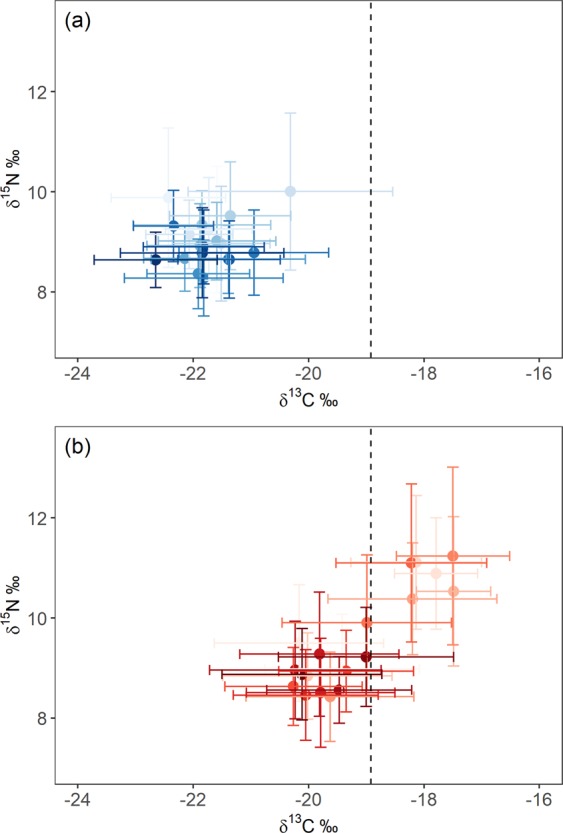
Bi-plots showing the means (points) and standard deviations (lines) of δ13C and δ15N values in whiskers of (a) 20 male and (b) 20 female Antarctic fur seals breeding at South Georgia. Dashed line indicates estimated δ13C value of whiskers when seals foraged at the Polar Front. Figure was created using R software (v3.6.1; https://www.R-project.org/).
Sex-specific niche partitioning
Sexual segregation occurred primarily in foraging distribution (along the carbon axis), but not in trophic position (along the nitrogen axis). Mean δ13C values were substantially lower in males than females (−21.68‰ ± 1.20 and −19.22‰ ± 1.58 respectively), while mean δ15N values were similar in males and females (8.98‰ ± 1.04 and 9.47‰ ± 1.45 respectively). Females occupied a larger isotopic niche than males as Standard Ellipse Areas (SEAs) were 5.39 for females (Bayesian Standard Ellipse Area (SEAB) mode: 5.49 with 95% credibility interval 5.00–5.82), and 3.72 for males (SEAB mode: 3.83 with 95% credibility interval 3.50–3.96) (Fig. 4a). Isotopic niches were distinct between the sexes, as male and female SEAs only overlapped by 1.2% (1.1% using Bayesian inference with 95% credibility interval 0.089–2.37).
Figure 4.
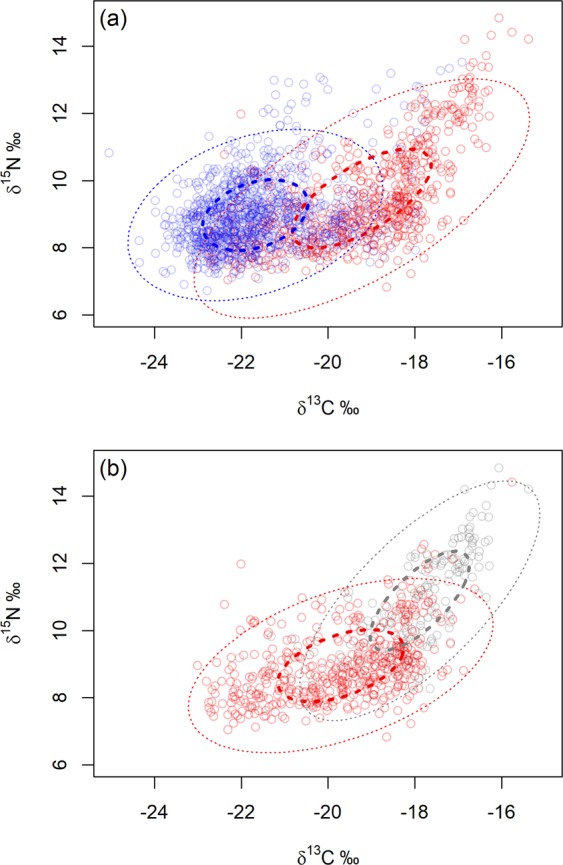
Standard Ellipse Areas (SEAs) representing the isotopic niches of (a) 20 male (blue) and 20 female (red) Antarctic fur seals by δ13C and δ15N values in their whiskers and (b) isotopic niches of females according to the estimated δ13C value of whiskers at the Polar Front (−18.92‰): female Group 1 (red) consists of 14 individuals with mean δ13C values below −18.92‰; female Group 2 (grey) consists of 6 individuals with mean δ13C values above −18.92‰. Points are isotopic values of each whisker sample, bold dashed ellipses use 40% of data points and dotted ellipses use 95% of data points. Figure was created using R software (v3.6.1; https://www.R-project.org/).
Isotopic differences within females
Female Group 1 mainly foraged at higher latitudes on potentially lower trophic level prey than female Group 2, as both mean δ15N and δ13C values were lower in female Group 1 than female Group 2 (means of each group: 8.96‰ ± 1.06 and 10.89‰ ± 1.46 respectively for δ15N; −19.71‰ ± 1.44 and −17.89‰ ± 1.13 respectively for δ13C). Female Group 1 occupied a slightly larger isotopic niche than female Group 2 as SEAs were 4.21 for female Group 1 (SEAB mode: 4.20 with 95% credibility interval 3.85–4.58) and 3.61 for female Group 2 (SEAB mode: 3.62 with 95% credibility interval 3.11–4.21). These female groups were largely distinct as SEAs overlapped by only 4.5% (3.1% using Bayesian inference with 95% credibility interval 1.01–9.17) (Fig. 4b). Males likely competed more with female Group 1 (SEAs overlapped by 5.1%; 4.8% using Bayesian inference with 95% credibility interval 3.26–6.77) than female Group 2 (SEAs did not overlap).
Body size differences within females
Females in Group 1 were significantly smaller than females in Group 2, as indicated by principal components analysis (PCA). Specifically, loadings for principal component 1(PC1) were highest for mass (−0.57), span (−0.52), length (−0.50) then girth (−0.40), while loadings for principal component 2 (PC2) were highest for girth (0.84), length (−0.47), span (−0.25) then mass (0.04). PC1 and PC2 explained 18.2% and 73.9% of variability in morphology data respectively. The mean scores between the two female groups differed by 1.97 for PC1 (Welch’s t-test: t = −2.70, p = 0.02) and 0.98 for PC2 (Welch’s t-test: t = 3.11, p = 0.01) (Supplementary Fig. S5).
Ontogeny of sexual segregation in isotopic niche
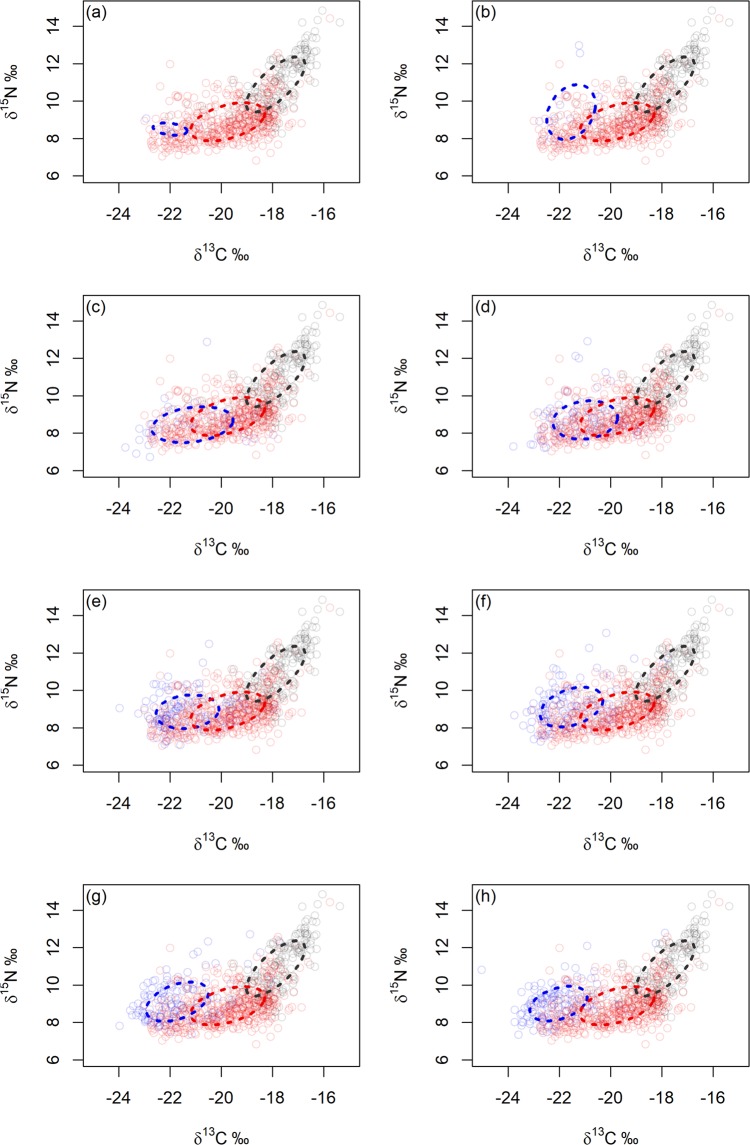
Ontogenetic niche shifts were present in males on an annual scale. SEA increased between ages 0.5–2 years (although there were only 6 samples for males aged 0.5–1), then generally declined with age thereafter (Fig. 5; Supplementary Table S6). Overlap in isotopic niche between males of different age classes and SEA of female Group 1 increased from males aged 0.5–3 years, then declined with increasing male age until only 0.0015% of overlap occurred when males aged 6–7 years and no overlap in SEA occurred thereafter (Fig. 6; Supplementary Table S7). No overlap in SEA occurred between any male age group and female Group 2.
Figure 5.
The ontogeny in isotopic niche of male Antarctic fur seals (blue) as they age compared to Standard Ellipse Areas (SEAs) of female Group 1 (red) and female Group 2 (grey). Males are aged (a) 0.5–1 year; (b) 1–2 years; (c) 2–3 years; (d) 3–4 years; (e) 4–5 years; (f) 5–6years; (g) 6–7 years; (h) 7–8 years. Points are isotopic values of each whisker sample and bold dashed ellipses represent SEAs using 40% of data points for each group: blue represents the isotopic niche of males; red SEA represents the overall isotopic niche of female Group 1 (females with lower mean δ13C values than estimated δ13C value of whiskers at the Polar Front) and grey SEA represents the overall isotopic niche of female Group 2 (females with lower mean δ13C values than estimated δ13C value of whiskers at the Polar Front). Figure was created using R software (v3.6.1; https://www.R-project.org/).
Figure 6.
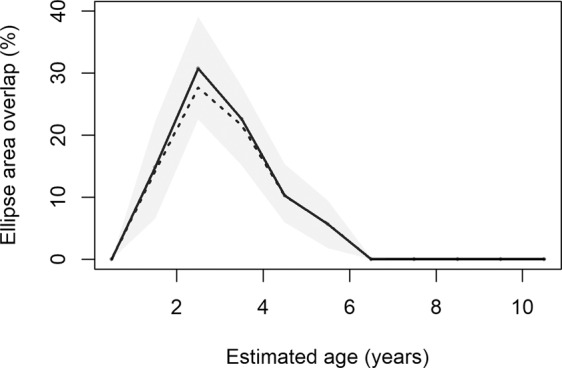
Percentage overlap in Standard Ellipse Area (SEA) of male Antarctic fur seals as they age with female Group 1 (females with lower mean δ13C values than estimated δ13C value of whiskers at the Polar Front). Bold line shows overlap in SEA using maximum likelihood, dashed line shows mode overlap using Bayesian inference, and grey shaded region shows 95% credibility interval around this mode. Figure was created using R software (v3.6.1; https://www.R-project.org/).
Contributions of sex, age and individual to isotopic niche differentiation
The δ13C values along the length of whiskers, indicating changes in foraging distribution throughout ontogeny, were best explained by group (males, female Group 1 and female Group 2) and age (linear mixed model; likelihood ratio test LR = 76.4, p < 0.001; conditional R-squared = 49.3%). The δ13C values significantly differed among all three groups and values declined as seals aged (Table 1; Fig. 7a). Foraging distribution was highly generalised within the sample population, as the individual specialisation value was 0.89.
Table 1.
Results of best-fit linear mixed models explaining the change in δ13C and δ15N values along the length of Antarctic fur seal whiskers: males, female Group 1 (females with lower mean δ13C values than estimated δ13C value of whiskers at the Polar Front) and female Group 2 (females with higher mean δ13C values than estimated δ13C value of whiskers at the Polar Front).
| Fixed effects | Intercept Value | Degrees of freedom | p-value |
|---|---|---|---|
| δ13C | |||
| Male (Intercept) | −21.61 | 1596 | <0.001 |
| Female Group 1 | 1.66 | 37 | <0.001 |
| Female Group 2 | 3.56 | 37 | <0.001 |
| Age | −0.30 | 1596 | <0.001 |
| δ15N | |||
| Male (Intercept) | 9.00 | 1596 | <0.001 |
| Female Group 1 | −0.07 | 37 | 0.69 |
| Female Group 2 | 1.93 | 37 | <0.001 |
| Age | 0.15 | 1596 | 0.0038 |
Figure 7.
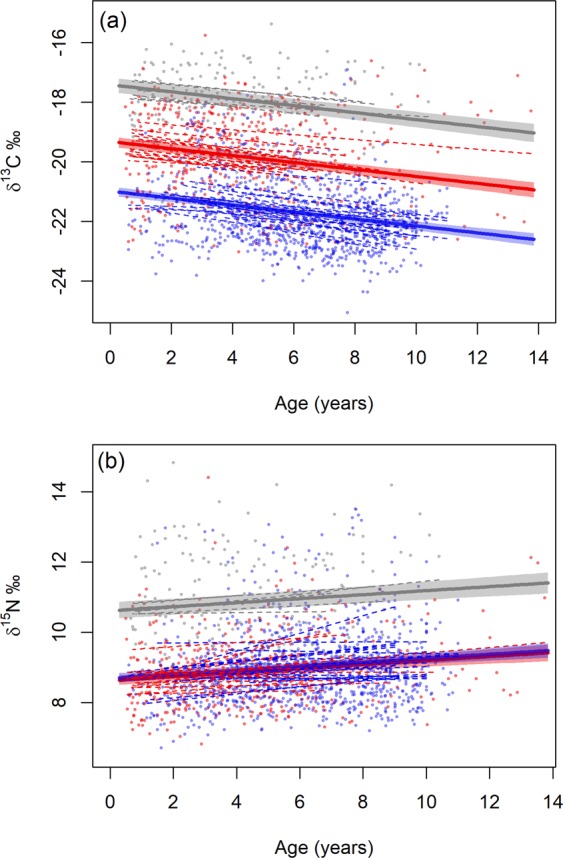
Best-fit linear mixed models explaining the change in (a) δ13C and (b) δ15N values along Antarctic fur seal whiskers with estimated age of males (blue) and minimum estimated age of females: female Group 1 (red; females with lower mean δ13C values than estimated δ13C value at the Polar Front) and female Group 2 (grey; females with higher mean δ13C values than estimated δ13C value of whiskers at the Polar Front). Points are isotope values of each whisker sample, dashed lines are fitted slopes explaining trend for each individual seal, bold lines are fitted trends for each group and shaded areas indicate standard error. Figure was created using R software (v3.6.1; https://www.R-project.org/).
The estimated portion of time that seals spent north of the Polar Front (based on δ13C values in sampled whisker segments and estimated δ13C value of whiskers when seals foraged at the Polar Front) was best described by group alone (Generalised linear mixed model; likelihood ratio test LR = 90.4, df = 2, p < 0.001). Female Group 2 spent the highest percentage of time north of the Polar Front (mean = 85.8% ± 8.7), followed by female Group 1 (mean = 32.6% ± 14.4) and males (mean = 2.6% ± 6.6).
The δ15N values along the length of whiskers, indicating changes in trophic position throughout ontogeny, were best explained by group and age (linear mixed model; likelihood ratio test LR = 7.72, p = 0.006; conditional R2 = 43.4%). Trends in δ15N values significantly increased with age for all groups and δ15N values significantly differed between males and female Group 2 but not between males and female Group 1 (Table 1; Fig. 7b). Individuals were slightly less generalised in trophic position than foraging distribution within the sample population, as the individual specialisation value was 0.76.
Discussion
Niche partitioning plays a fundamental role in ecology by reducing competition for resources. This study revealed that the behaviour of Antarctic fur seals in the world’s largest breeding colony show distinct niche partitioning between sexes, throughout ontogeny and among individuals, which may help facilitate the high population density on South Georgia and the recovery of this population post-sealing. By analysing stable isotopes along whiskers we found strong support that (1) Males spend more time foraging in maritime Antarctica than females; (2) Males progressively spend more time foraging in maritime Antarctica during each annual cycle as they age; (3) Females demonstrate two main foraging strategies with 70% of females (female Group 1) mainly foraging south of the Polar Front and the remainder (female Group 2) mainly foraging to the north of it; (4) Migration strategies remained consistent between years. We discuss the potential underlying drivers of these findings and propose their key ecological consequences.
Methodological considerations
Prior to interpreting results there are several caveats of our study to consider. The estimated whisker δ13C value when Antarctic fur seals foraged at the Polar Front was based on only one season of prey data and the proportion of each species’ contribution to the diet was unknown. Our estimated value was very close to −19‰: estimated by Cherel et al.62 and used by Kernaléguen et al.63 for Antarctic fur seals breeding at the Crozet Islands. It also closely aligned with isotope values in blood of seabirds foraging at the Polar Front from South Georgia61. However, the location and width of the Polar Front is not constant as a result of high variability in mesoscale meanderings, eddies and ring formations61,65. The value can therefore vary spatially and temporally and should only be considered as a broad indicator of foraging distribution. Baseline isotope ratios also change in time and space with sources of organic matter66. We could not account for these changes due to uncertainties inferring fine-scale foraging locations at set points in time from the isotope data, coupled with the lack of comprehensive isoscapes available for the geographical area (which vary seasonally and annually). The available isoscapes for the Antarctic Peninsula region revealed that δ13C values in particulate organic matter showed high annual variation (standard deviation of 2.9‰) in February each year between 2013 and 2016, but no general trend over time67. It is unlikely that trends in our results reflect changes in baselines, as there is currently no evidence of simultaneous trends in baseline isotope values in the Southern Ocean68.
The isotope data also presented additional sources of variation. In females, the exact point that whiskers were cut likely differed (within ~2 mm) among individuals, which may slightly affect the minimum ages of females. Since only minimum female age could be determined, we could not assess changes in isotope values with exact age. In males, we were unable to account for fasting during the breeding season. Fasting enriches δ15N values in organisms by 0.5‰ on average and has no signficiant effect on δ13C values69. However, male Antarctic fur seals will also forage during the breeding season27, so we were unable to determine the length of fasting by each male each year (as well as determine the enrichment in nitrogen, which may depend on seal age, size and health). Although this short period of fasting may have slightly increased the values of some data points, it is unlikely that this explains patterns in our results.
Niche partitioning between sexes
Sexual segregation occurred along the spatial and temporal axis of the niche: male Antarctic fur seals had lower δ13C values than females, indicating they spent more time foraging further south in maritime Antarctica during each annual cycle than females, supporting hypothesis (1). This sexual segregation might be partially driven by breeding constraints, as females are restricted in the distance they can travel from pupping beaches when foraging to provision their pups, while males have no temporal or spatial limitations post-mating so can forage further afield27,70. Indeed, three Antarctic fur seal adult males tracked with satellite transmitters migrated south post-mating44 and young males marked with flipper tags have been re-sighted further south at Signy Island43.
There may also be a link between sexual size dimorphism and foraging niche. Lower δ13C values (indicating more southerly foraging) in males than females have also been reported in sexually dimorphic albatrosses and giant petrels breeding at South Georgia, but not in monomorphic burrowing petrels61. According to optimal foraging theory71 larger animals should prefer spatially clustered resources to decrease foraging costs72, as they have higher energetic needs20,21. For example, Albrecht et al. (2018) found that larger birds (of over 80 species) sampled along Mount Kilimanjaro foraged on plants with higher resource density than smaller birds. Male Antarctic fur seals require an estimated 3.8 tonnes of krill per year – twice as much as females73 and may therefore exploit the most productive areas available48. Krill density tends to be higher near the Antarctic Peninsula than South Georgia (1996–201674) and there is large inter-annual variability in krill abundance and availability in the Scotia Sea75, which has been associated with sporadic declines in breeding success and population sizes of predators at South Georgia76–78. Males may exploit the greater density and predictability of krill near the Antarctic Peninsula to maintain a large body size. The Antarctic Peninsula and nearby islands appear to be less suitable for females to provision pups, as shown by the low numbers that breed there (e.g.77,79,80) relative to South Georgia.
Males and female Group 1 had similar δ15N values, indicating they competed for the same prey. They are likely opportunistic foragers, as the individual specialisation index for δ15N values showed greater generalisation than specialisation. Males potentially forage more successfully in the absence of females42,44,70, which reduces intra-specific competition. Spatial segregation between the sexes also occurs in grey seals, Halichoerus grypus, as males primarily use the continental shelf and females the mid-shelf, which the authors suggest acts to maximise fitness by reducing intersexual competition81. Shifting habitat, as opposed to diet, may be a more effective strategy to reduce competition6. By migrating south, male Antarctic fur seals could also reduce inter-specific competition with millions of breeding seabirds that congregate at South Georgia in summer. However, males likely increase spatial overlap with the krill fishery (largest fishery by tonnage in the Southern Ocean82), which operates at the Antarctic Peninsula in summer: a time when it is closed at South Georgia.
Niche ontogeny
Individual niches are not fixed and can differentiate throughout an animal’s life26,28,29. We found that δ15N values and therefore trophic level of prey in male and female Antarctic fur seal whiskers gradually increased with age. Similar patterns have been documented with increasing body size in striped dolphins, Stenella coeruleoalba50 and great white sharks, Carcharodon carcharias83. This pattern may result from development of a larger mouth gape84, greater physiological capabilities (e.g. travel speed and aerobic dive limits27) and foraging experience, enabling larger individuals to handle larger higher-trophic-level prey with greater nutritional value. Alternatively, the increasing δ15N values may relate to changes in prey availability over time, such as declines in krill abundance as a result of climate change74,85. Indeed, Tarroux et al.68 attributed increasing δ15N values in blood and plasma of Antarctic fur seals breeding at Bouvetøya (from 1997–2015) to a shift in diet towards greater consumption of higher-trophic-level prey (replacing krill). This best explains trends in δ15N values along Antarctic fur seal whiskers at South Georgia, as δ15N values did not level when adults reached maximum body size.
Distinct ontogenetic niche shifts were present in males, supporting hypothesis (2). The δ13C values declined as males aged, indicating they progressively spent more time south during each annual cycle. This trend in δ13C values was also apparent in growth layers of male Antarctic fur seal teeth86. As males age they gain experience of the best foraging areas and may exploit abundant resources in maritime Antarctica to meet their growing energetic needs. A larger body size and better body condition will improve a male’s ability to gain and retain high quality territories with greater mating opportunities42. Larger body sizes also facilitate better heat retention87, enabling larger males to withstand the higher thermoregulatory costs of foraging in colder environments. Thermal tolerance also influenced sexual segregation in the most sexually dimorphic bird species: great bustards, Otis tarda88, and could be an overlooked factor driving sexual segregation.
Trajectories in ontogenetic niche shifts may differ between the sexes, as females reach maximum body size and become sexually mature earlier than males. Kernaléguen et al.48 found that female Antarctic fur seals breeding at Kerguelen had a similar isotopic niche to adult females by age 2. We could not assess whether this pattern occurred in females breeding at South Georgia, as we could only determine minimum female age since body length was a poor indicator of age (varying substantially among individuals; see Forcada & Hoffman78). However, trends in δ13C values along female whiskers suggest a more continuous change in isotopic niche, which requires further investigation.
Ontogenetic niche shifts can reduce intra-specific competition11,89, as only a subset of individuals will compete with one another at a specific time29,90. Sample sizes for males aged under 2 years were small (as a result of whisker wear). However, throughout the remainder of male Antarctic fur seal development, the greatest isotopic niche overlap (indicating competition for resources) occurred between female Group 1 and males aged 2–3 years. This overlap may result from similarities in body size47 and energetic requirements. Isotopic niche overlap gradually declined between the sexes as males grew and aged, showing progressive development of sexual segregation as ontogenetic shifts in males freed up resources available to females. This mechanism (which may occur in other sexually dimorphic species) substantially reduces intra-specific competition, which potentially increases female survival, reproductive rates and ultimately elevates population carrying capacity.
Niche partitioning within females
Female Antarctic fur seals occupied a broader isotopic niche (SEA 1.5 × larger) than males, supporting hypothesis (3). This concurs with tracking studies whereby females migrated north to the continental shelf east of Patagonia45,46, south to the northern tip of Antarctic pack ice45, or remained within the vicinity of South Georgia44–46. However, stable isotope analysis allowed us to quantify foraging strategies into two main groups supporting hypothesis 4. The consistency of these foraging strategies within the two groups highlights the potential of familiarisation with a foraging area, allowing individuals to maximise net energy gain46.
Size dimorphism may be a cause or consequence of divergent foraging strategies, as female Group 1 and Group 2 differed in body size: a phenomenon also observed in female loggerhead turtles, Caretta caretta91. Size dimorphism could lead to distinct foraging strategies as larger animals are generally less susceptible to predation and have greater competitive abilities than smaller animals91. They tend to have lower stroke frequencies92 and lower mass-specific maintenance costs93, enabling them to migrate over greater distances than smaller animals using the same amount of energy. This size dimorphism could stem from early life e.g. size of tadpoles at metamorphosis affects size of adult frogs94. However, foraging strategies were consistent in female Antarctic fur seals and body mass and girth were the most important components in PC1 and PC2 respectively, suggesting body dimorphism was more likely a consequence of divergent foraging strategies. These strategies may initially develop when pups disperse after weaning and explore potential foraging sites95. Pups with bolder personalities could show greater exploration than shyer individuals, as documented in wandering albatrosses, Diomedea exulans96. Female Group 2 may discover better foraging opportunities north of the Polar Front, resulting in their larger body size. Indeed, loggerhead turtles that migrated further and foraged in more productive waters were significantly larger than other turtles – potentially investing more resources into growth91.
According to Schoener3, competition should result in overdispersion of niches. Marginal value theorem97 also predicts that an animal should leave a patch of resources and search for another when rate of resource gain falls under expected mean rate. Competitive interactions and low resource availability could have alternatively initiated the longer foraging trips by female Group 2. For example, at high population densities some three-spined stickleback, Gasterosteus aculeatus, become more opportunistic while others form novel dietary groups98 and when resource availability is limited some female parasitic wasps, Pachycrepoideus vindemmiae, immediately retreat from a resource patch, while others remain and are more involved in competitive interactions99. Female Group 2 may minimise intra-specific competition with males and Female Group 1, while gaining nutritional benefits that outweigh the energetic costs of locomotion100. Since Antarctic krill is almost exclusively distributed south of the Polar Front101, female Group 2 must predominantly feed on alternative species such as squid, myctophids and other fish – as found in the diet of Antarctic fur seals breeding at Kerguelen, Heard Island and Marion Island49,102,103. They are likely more susceptible to competition and interaction with an abundance of squid jiggers, longliners and benthic trawlers that operate in the South Atlantic. Prey consumption by female Group 2 (satisfying potentially 30% of the female population) could result in a greater impact on the South Atlantic marine ecosystem than previously realised.
Conclusion
Stable isotope analysis (complimented with findings from short-term tracking studies) enabled us to reveal niche partitioning in the world’s largest Antarctic fur seal colony. Analysing stable isotopes along progressively growing tissues may be more practical, more cost-effective and less invasive than using short-term tracking methods alone36. We propose that the Antarctic fur seal colony breeding at South Georgia is generalist as a whole (indicated by individual specialisation indices), as seals could inhabit a range of environments, from warmer South Atlantic waters to colder Antarctic waters. However, the population is composed of more specialised strategies that may develop as a function of body size (with males experiencing a large range of body sizes and energetic requirements throughout ontogeny and female size differing according to foraging strategy). These strategies relax intra-specific competition, which may benefit population stability and carrying capacity, as well as the behavioural plasticity of the colony to adapt to changing environmental conditions. Intra-specific niche partitioning therefore has implications for ecology, evolution and conservation and is important to study in other species.
Methods
Ethics statement
The animal handling procedures in this study were reviewed and approved by the British Antarctic Survey Animal Ethics and Welfare Review Body (AWERB). The procedures adhered to the ASAB guidelines, ARRIVE guidelines and legal requirements of the South Georgia Government. The behavioural responses of adult females during restraint were predictable (given previous research conducted within the colony) and all efforts were made to minimise stress to individuals.
Sample collection
Whiskers were collected from 30 freshly dead adult male and 25 live adult female Antarctic fur seals from September 2016 – February 2017 during the breeding season at Bird Island, South Georgia (54.010°S, 38.059°W). Dead males are regularly found ashore during the mating season: a reflection of the intense competition among males to gain access to females104. For males the two longest whiskers were pulled from both sides of the face, body length and girth measurements were recorded (fresh dead males only), an ID tag was applied to the skull, and after decomposition an upper canine was extracted from the jaw. An upper canine was extracted from an additional four dead males (34 teeth total), in which whiskers could not be obtained because of prolonged decomposition. Females rarely die ashore and no dead females were observed during this period. For each live female, the single longest whisker was cut from the right side of the face (as close to the skin as possible) during restraint (enabling whiskers to regrow). Females were weighed and body length, body span (nose to tail) and girth measurements recorded. The longest whisker (representing the longest period of growth) on the right side of the face was chosen from 20 randomly selected males and 20 randomly selected females for sample preparation.
Sample preparation
Whiskers were washed with a sponge and Ecover detergent, transferred to a water bath for five minutes to remove contaminants (i.e. blood and dirt), then dried in an oven at 70 °C. Adhesive measuring tape was placed along each whisker and clear thin plastic positioned on the alternate side to ensure samples could be cut with accuracy and remained enclosed (to secure samples during cutting). Samples weighing a target weight of 0.7 mg were cut at the start of every 5 mm segment along the length of each whisker (most samples were 1–2 mm in length). Each 5 mm segment represented approximately 1.5–2.8 months of whisker growth based on growth rates calculated by Kernaléguen et al.48. Samples were removed from the tape using tweezers and placed in glass vials with a 2:1 chloroform:methanol solvent to remove any lipids and tape residue stuck to the whisker to leave clean keratin105. Samples were dried in a fume hood overnight then weighed into 3 × 5 mm tin capsules for mass spectrometry. Total sample sizes were 1011 for males and 642 for females.
Mass spectrometry
Tin capsules were loaded into the autosampler of an Elementar (Hanau, Germany) Pyrocube Elemental Analyser, which converted carbon and nitrogen in the samples to CO2 and N2 gases. The ratios of carbon and nitrogen isotopes in these gases were measured on a Thermo-Fisher-Scientific (Bremen, Germany) Delta XP Plus Isotope-Ratio Mass Spectrometer. The internal reference materials (mean ± SD) were GEL (gelatin solution, δ13C= −20.09 ± 0.19‰, δ15N= 5.59 ± 0.12‰), ALAGEL (alanine-gelatine solution spiked with 13C-alanine, δ13C = −8.69 ± 0.17, δ15N = 2.22 ± 0.08‰), and GLYGEL (glycine-gelatine solution spiked with 15N-alanine, δ13C = −38.35 ± 0.13‰, δ15N = 23.19 ± 0.22‰), each dried for two hours at 70 °C. Four USGS 40 glutamic acid standards106,107 were used as independent checks of accuracy. Delta values were corrected for instrument drift (changes in isotopic composition of gases through the mass spectrometer) and linearity (variability in sample masses). Stable isotope ratios were expressed in parts per thousand (‰) deviation from the international standards (Vienna Pee Dee Belemnite for carbon and AIR for nitrogen), according to the equation:
where X is 15N or 13C and R is the corresponding ratio (15N/14N) or (13C/12C). Stable isotope ratios were reported as δ13C values for carbon and δ15N values for nitrogen.
Age determination
Each male seal was aged by three readers by counting external growth ridges on the extracted upper canine. These ridges, formed from annual deposition of dentin, are prominent in male Antarctic fur seals108. The modal ages were assumed for each individual. Precision in age determination was estimated using the IAPE, as described by Beamish and Fournier109 according to equation:
N is the total number of individuals aged, R is the number of times each individual is aged, and Xij is the ith age determination of the jth individual. A smaller IAPE indicates more precise age determinations. Females were first aged according to their measured body length by extrapolating age from a modelled body length-to-age curve (Fig. 1d in Forcada & Hoffman78). Age was not estimated for five females, as body lengths exceeded modelled lengths in the growth curve.
Whisker growth rates were calculated using wavelet analysis, which can assess the degree of periodicity in stable isotope values along the length of whiskers (as described by Kernaléguen et al.63). For each seal whisker the wavelet transform was applied and a power spectrum produced using the ‘WaveletComp’ package110 in R111. The power spectrum specified significant periodicity in δ13C values, which were used to reconstruct the original time series by ‘denoising’ the series and retaining the smooth components. These reconstructed time series were used to calculate the growth rate of each whisker, assuming oscillations corresponded to annual migrations. Since whiskers of four females and four males demonstrated no clear periodicity in δ13C values, the average growth rates of all female and male whiskers were applied respectively for these individuals. For each of the 20 male seals, age was estimated along the length of the whisker using whisker growth rate and seal age (obtained from external growth ridges in canine) by back-tracking along the whisker (facial end to whisker tip). This method was repeated for the 20 females using the estimated ages obtained from body length. However, age estimates are highly variable with body length78 and body length substantially underestimated female age at capture (by 3.5 years on average) according to female age determined by whisker growth rates. Whisker growth rates were considered more reliable (since oscillations in δ13C values likely correspond to annual migration patterns) and were used alone to determine minimum female age along the length of each whisker for following analyses. Exact female ages could not be determined as a result of whisker wear/breakage at the tips and because whisker growth rates were not definite. Characteristic peaks in δ15N values at the tips of six female whiskers likely corresponded to suckling patterns, suggesting these whiskers had not broken. In these cases, δ15N peaks were lined up and age was estimated along the whisker (whisker tip to facial end) using the calculated whisker growth rates.
Data analysis
To broadly determine Antarctic fur seal foraging distribution using stable isotope values we approximated the δ13C value for whiskers when seals foraged at the Polar Front (convergence between cold Antarctic waters and warmer sub-Antarctic waters). We first determined the TDF for Antarctic fur seal whiskers using the SIDER package112 in R111. SIDER estimates the TDF for a particular consumer and tissue (in which controlled feeding studies are impractical) using a phylogenetic regression model, fitted using Bayesian inference to a compiled dataset of TDF values of phylogenetically and ecologically related species112. We secondly added this TDF to the average δ13C value of prey items (myctophids and krill) collected at two locations at the Polar Front (50.0632°S, 34.0287°W and 49.9357°S, 34.2078°W) during research cruise JR200 (British Antarctic Survey) in Autumn 2009. The resulting δ13C value for Antarctic fur seals whiskers when seals foraged at the Polar Front was then overlayed on stable isotope bi-plots to assess differences in foraging distributions between and within the sexes. Since bi-plots revealed two isotopically distinct groups of females, with average δ13C value of each individual falling lower or higher than the estimated δ13C value of whiskers when seals foraged at the Polar Front, females were split into two groups (female Group 1 and female Group 2 respectively) for subsequent analyses. To test whether body morphology significantly differed between female Group 1 and Group 2, we ran a PCA on body mass, length, span and girth measurements, and used the output from PC1 and PC2 as separate response variable in Welch’s t-tests.
To compare male and female isotopic niche areas we used the SIBER package in R43,113 to calculate SEAs (encompassing 40% of data points) according to maximum-likelihood estimation, as well as SEABs according to Bayesian inference to account for uncertainty in ellipse areas. The Bayesian Standard Ellipse Areas were calculated using 100,000 posterior draws and the mode and 95% credibility intervals were reported. The proportions of overlap between male and female prediction ellipse areas, and between female Group 1 and Group 2 ellipse areas, were calculated to quantify isotopic niche differentiation between these groups – first using maximum-likelihood estimation, then using Bayesian inference with 100,000 posterior draws. This method was repeated to quantify ontogeny of isotopic niche differentiation on an annual scale in males (from 0 to 11 years of age) and to assess overlap among these niches and overall SEAs of female Group 1 and 2.
Since sexual segregation can also occur along the δ13C and δ15N axes separately, δ13C and δ15N values were used as separate response variables in linear mixed models36. We tested whether δ13C and δ15N values significantly differed among males, female Group 1 and female Group 2 using a global model, refined by backward-stepwise deletion and likelihood ratio tests using the ‘nmle’ package114 in R. Each global model included group (males, female Group 1 and female Group 2), age, and the interaction between group and age as fixed effects. Age was used as a random intercept and slope to account for variability in δ13C and δ15N values among individuals as they aged and a corARMA structure (p = 2, q = 0) was used to account for temporal autocorrelation in residuals. We additionally tested whether males, female Group 1 and female Group 2 differed in time spent north of the Polar Front as they aged by calculating the proportion of time spent north of the Polar Front (based on whether δ13C values exceeded the estimated δ13C value of whiskers when seals foraged at the Polar Front), which was used as the response variable in a generalised linear mixed model with a Beta error family, refined as above. Individual specialisation indices were determined, corresponding to the average similarity among individuals and the population115. The variance components were partitioned from each best-fit model and the within individual component (WIC) was divided by trophic niche width (TNW). An individual specialisation index of 0 indicates individuals are complete specialists, while a value of 1 indicates individuals use the whole range of the sample population’s niche116,117. All results were reported as means plus standard deviations unless stated.
Supplementary information
Acknowledgements
We sincerely thank Dr Emily Humble and James Robbins for their help collecting Antarctic fur seal whiskers at Bird Island, South Georgia, during the 2016–2017 breeding season. We also thank Oliver Thomas for his contribution aging male Antarctic fur seal teeth and Dr Rona McGill for her support while running whisker samples through the Isotope Ratio Mass Spectrometer at the Scottish Universities Environmental Research Centre. This project was led by the British Antarctic Survey and supported by the Natural Environment Research Council (NERC) Capability Fund, the NERC Life Sciences Mass Spectrometry Facility, and the NERC Great Western Four+ Doctoral Training Partnership (GW4+ DTP, NE/L002434/1).
Author contributions
Study design: I.J.S. Fieldwork: J.F. and J.D. Laboratory work: K.A.J., J.N. and G.S. Contributed data: G.S. Advised data analysis: I.J.S., N.R. and S.V. Analysed data: K.A.J. Drafted manuscript: K.A.J. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59992-3.
References
- 1.Svanbäck R, Bolnick DI. Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society B: Biological Sciences. 2006;274(1611):839–844. doi: 10.1098/rspb.2006.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchinson GE. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology. 1957;22:415–427. doi: 10.1101/SQB.1957.022.01.039. [DOI] [Google Scholar]
- 3.Schoener TW. Resource partitioning in ecological communities. Science. 1974;185:27–38. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 4.Hardin G. The competitive exclusion principle. Science. 1960;131(3409):1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- 5.Staniewicz A, Behler N, Dharmasyah S, Jones G. Niche partitioning between juvenile sympatric crocodilians in Mesangat Lake, East Kalimantan, Indonesia. Raffles Bulletin of Zoology. 2018;66:528–537. [Google Scholar]
- 6.MacArthur, R. & Wilson, E. The Theory of Island Biogeography (Princeton University Press, 1967).
- 7.Root R. The niche exploitation pattern of the blue-gray gnatcatcher. Ecological Monographs. 1967;37:317–350. doi: 10.2307/1942327. [DOI] [Google Scholar]
- 8.Ratcliffe N, et al. The roles of sex, mass and individual specialisation in partitioning foraging-depth niches of a pursuit-diving predator. PloS One. 2013;8(10):e79107. doi: 10.1371/journal.pone.0079107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tschumy WO. Competition between juveniles and adults in age-structured populations. Theoretical Population Biology. 1982;21(2):255–268. doi: 10.1016/0040-5809(82)90017-X. [DOI] [Google Scholar]
- 10.Johst K, Berryman A, Lima M. From individual interactions to population dynamics: individual resource partitioning simulation exposes the causes of nonlinear intra‐specific competition. Population Ecology. 2008;50(1):79–90. doi: 10.1007/s10144-007-0061-5. [DOI] [Google Scholar]
- 11.Zhao T, Villéger S, Lek S, Cucherousset J. High intraspecific variability in the functional niche of a predator is associated with ontogenetic shift and individual specialization. Ecology and Evolution. 2014;4:4649–4657. doi: 10.1002/ece3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiemann GW, Iverson SJ, Stirling I, Obbard ME. Individual patterns of prey selection and dietary specialization in an Arctic marine carnivore. Oikos. 2011;120(10):1469–1478. doi: 10.1111/j.1600-0706.2011.19277.x. [DOI] [Google Scholar]
- 13.Carneiro AP, Bonnet-Lebrun AS, Manica A, Staniland IJ, Phillips RA. Methods for detecting and quantifying individual specialisation in movement and foraging strategies of marine predators. Marine Ecology Progress Series. 2017;578:151–166. doi: 10.3354/meps12215. [DOI] [Google Scholar]
- 14.Bon R, Campan R. Unexplained sexual segregation in polygamous ungulates: a defense of an ontogenetic approach. Behavioural Processes. 1996;38(2):131–154. doi: 10.1016/S0376-6357(96)00029-0. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrini AD. Sexual segregation in childhood: A review of evidence for two hypotheses. Animal Behaviour. 2004;68(3):435–443. doi: 10.1016/j.anbehav.2003.07.023. [DOI] [Google Scholar]
- 16.Ruckstuhl KE. Foraging behaviour and sexual segregation in bighorn sheep. Animal Behaviour. 1998;56(1):99–106. doi: 10.1006/anbe.1998.0745. [DOI] [PubMed] [Google Scholar]
- 17.Conradt L, Roper TJ. Activity synchrony and social cohesion: a fission-fusion model. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2000;267(1458):2213–2218. doi: 10.1098/rspb.2000.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gittleman JL, Valkenburgh BV. Sexual dimorphism in the canines and skulls of carnivores: effects of size, phylogency, and behavioural ecology. Journal of Zoology. 1997;242(1):97–117. doi: 10.1111/j.1469-7998.1997.tb02932.x. [DOI] [Google Scholar]
- 19.Mayer M, Shine R, Brown G. Bigger babies are bolder: effects of body size on personality of hatchling snakes. Behaviour. 2016;153:313–323. doi: 10.1163/1568539X-00003343. [DOI] [Google Scholar]
- 20.Isaac JL. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mammal Review. 2005;35:101–115. doi: 10.1111/j.1365-2907.2005.00045.x. [DOI] [Google Scholar]
- 21.Clutton-Brock TH, Harvey PH. The functional significance of variation in body size among mammals. Special Publication of the American Society of Mammalogists. 1983;7:632–663. [Google Scholar]
- 22.Prins, H. H. T. & Olff, H. Species richness of African grazer assemblages: towards a functional explanation in Dynamics of Tropical Communities (Eds Newbery, D. M., Prin, H. H. T. & Brown, N. D.) 449–490 (Blackwell, 1998).
- 23.Ruckstuhl, K. E., Clutton-Brock, T. & Neuhaus, P. Sexual segregation and the ecology of the two sexes (Cambridge University Press, 2005).
- 24.Stokke S, du Toit JT. Sex and size related differences in the dry season feeding patterns of elephants in Chobe National Park, Botswana. Ecography. 2000;23(1):70–80. doi: 10.1111/j.1600-0587.2000.tb00262.x. [DOI] [Google Scholar]
- 25.Jakimchuk RD, Ferguson SH, Sopuck LG. Differential habitat use and sexual segregation in the Central Arctic caribou herd. Canadian Journal of Zoology. 1987;65(3):534–541. doi: 10.1139/z87-083. [DOI] [Google Scholar]
- 26.Lingle S. Coyote predation and habitat segregation of white‐tailed deer and mule deer. Ecology. 2002;83(7):2037–2048. doi: 10.1890/0012-9658(2002)083[2037:CPAHSO]2.0.CO;2. [DOI] [Google Scholar]
- 27.Staniland IJ, Robinson SL. Segregation between the sexes: Antarctic fur seals, Arctocephalus gazella, foraging at South Georgia. Animal Behaviour. 2008;75(4):1581–1590. doi: 10.1016/j.anbehav.2007.10.012. [DOI] [Google Scholar]
- 28.Heupel MR, Carlson JK, Simpfendorfer CA. Shark nursery areas: concepts, definition, characterization and assumptions. Marine Ecology Progress Series. 2007;337:287–297. doi: 10.3354/meps337287. [DOI] [Google Scholar]
- 29.Werner EE, Gilliam JF. The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology, Evolution, and Systematics. 1984;15:393–425. doi: 10.1146/annurev.es.15.110184.002141. [DOI] [Google Scholar]
- 30.Grubbs, R. D. Ontogenetic shifts in movements and habitat use in Sharks and their Relatives II: Biodiversity, Physiology, and Conservation (Eds Carrier, J. C., Musick, J. A. & Heithaus, M. R.) 319–350 (CRC Press, 2010).
- 31.Webb JK, Shine R, Christian KA. Does intraspecific niche partitioning in a native predator influence its response to an invasion by a toxic prey species? Austral Ecology. 2005;30(2):201–209. doi: 10.1111/j.1442-9993.2005.01437.x. [DOI] [Google Scholar]
- 32.Bolnick DI, et al. The ecology of individuals: incidence and implications of individual specialization. The American Naturalist. 2002;161(1):1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- 33.Durell SELVD. Individual feeding specialisation in shorebirds: population consequences and conservation implications. Biological Reviews. 2000;75(4):503–518. doi: 10.1111/j.1469-185X.2000.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 34.Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE. Individual variation in prey selection by sea otters: patterns, causes and implications. Journal of Animal Ecology. 2003;72:144–155. doi: 10.1046/j.1365-2656.2003.00690.x. [DOI] [Google Scholar]
- 35.Jeglinski J, Werner C, Robinson P, Costa D, Trillmich F. Age, body mass and environmental variation shape the foraging ontogeny of Galapagos sea lions. Marine Ecology Progress Series. 2012;453:279–296. doi: 10.3354/meps09649. [DOI] [Google Scholar]
- 36.Chilvers BL. Whisker stable isotope values indicate long-term foraging strategies for female New Zealand sea lions. Endangered Species Research. 2019;38:55–66. doi: 10.3354/esr00934. [DOI] [Google Scholar]
- 37.Beest FM, et al. Increasing density leads to generalization in both coarse‐grained habitat selection and fine‐grained resource selection in a large mammal. Journal of Animal Ecology. 2014;83(1):147–156. doi: 10.1111/1365-2656.12115. [DOI] [PubMed] [Google Scholar]
- 38.Dayan, T. & Simberloff, D. Patterns of size separation in carnivore communities in Carnivore Behavior, Ecology, and Evolution, vol 2. (Ed. Gittleman, J. L.) 243–266 (Cornell University Press, 1996).
- 39.Van Valkenburgh B. Major patterns in the history of carnivorous mammals. Annual Review of Earth and Planetary Sciences. 1999;27:463–493. doi: 10.1146/annurev.earth.27.1.463. [DOI] [Google Scholar]
- 40.Costa A, et al. Generalisation within specialization: inter-individual diet variation in the only specialized salamander in the world. Scientific Reports. 2015;5:13260. doi: 10.1038/srep13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forcada, J. & Staniland, I. J. Antarctic fur seal Arctocephalus gazella in Encyclopedia of Marine Mammals, 2nd edition (Eds Perrin, W. F., Würsig, B. & Thewissen, J. G. M.) 36–42 (Academic Press, 2009).
- 42.Staniland, I. Sexual segregation in seals in Sexual segregation in Vertebrates: Ecology of the Two Sexes (Eds Ruckstuhl, K. E. & Clutton-Brock, T. H.) 53–73 (Cambridge University Press, 2005).
- 43.Waluda CM, Gregory S, Dunn MJ. Long-term variability in the abundance of Antarctic fur seals Arctocephalus gazella at Signy Island, South Orkneys. Polar Biology. 2010;33(3):305–312. doi: 10.1007/s00300-009-0706-2. [DOI] [Google Scholar]
- 44.Boyd IL, McCafferty DJ, Reid K, Taylor R, Walker TR. Dispersal of male and female Antarctic fur seals (Arctocephalus gazella) Canadian Journal of Fisheries and Aquatic Sciences. 1998;55(4):845–852. doi: 10.1139/f97-314. [DOI] [Google Scholar]
- 45.Staniland IJ, Robinson SL, Silk JRD, Warren N, Trathan PN. Winter distribution and haul-out behaviour of female Antarctic fur seals from South Georgia. Marine Biology. 2012;159(2):291–301. doi: 10.1007/s00227-011-1807-3. [DOI] [Google Scholar]
- 46.Arthur B, et al. Return customers: Foraging site fidelity and the effect of environmental variability in wide-ranging Antarctic fur seals. PloS One. 2015;10(3):e0120888. doi: 10.1371/journal.pone.0120888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne MR. Growth in the Antarctic fur seal Arctocephalus gazella. Journal of Zoology. 1979;187(1):1–20. doi: 10.1111/j.1469-7998.1979.tb07709.x. [DOI] [Google Scholar]
- 48.Kernaléguen L, et al. Early-life sexual segregation: ontogeny of isotopic niche differentiation in the Antarctic fur seal. Scientific Reports. 2016;6:33211. doi: 10.1038/srep33211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lea MA, et al. Colony-based foraging segregation by Antarctic fur seals at the Kerguelen Archipelago. Marine Ecology Progress Series. 2008;358:273–287. doi: 10.3354/meps07305. [DOI] [Google Scholar]
- 50.Giménez J, et al. Intra-and interspecific niche partitioning in striped and common dolphins inhabiting the southwestern Mediterranean Sea. Marine Ecology Progress Series. 2017;567:199–210. doi: 10.3354/meps12046. [DOI] [Google Scholar]
- 51.Newsome SD, Martınez del Rio C, Bearhop S, Phillips DL. A niche for isotopic ecology. Frontiers in Ecology and the Environment. 2007;5:429–436. doi: 10.1890/1540-9295(2007)5[429:ANFIE]2.0.CO;2. [DOI] [Google Scholar]
- 52.DeNiro MJ, Epstein S. You are what you eat (plus a few ‰): the carbon isotope cycle in food chains. Geological Society of America. 1976;6:834–835. [Google Scholar]
- 53.Ben-David M, Flaherty EA. Stable isotopes in mammalian research: a beginner’s guide. Journal of Mammalogy. 2012;93(2):312–328. doi: 10.1644/11-MAMM-S-166.1. [DOI] [Google Scholar]
- 54.Minagawa M, Wada E. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta. 1984;48(5):1135–1140. doi: 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- 55.Fry B. Food web structure on Georges Bank from stable C, N, and S isotopic compositions. Limnology and Oceanography. 1988;33(5):1182–1190. doi: 10.4319/lo.1988.33.5.1182. [DOI] [Google Scholar]
- 56.Hobson KA, Piatt JF, Pitocchelli J. Using stable isotopes to determine seabird trophic relationships. Journal of Animal Ecology. 1994;63:786–798. doi: 10.2307/5256. [DOI] [Google Scholar]
- 57.Kelly JF. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Canadian Journal of Zoology. 2000;78(1):1–27. doi: 10.1139/z99-165. [DOI] [Google Scholar]
- 58.Goericke R, Fry B. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochemical Cycles. 1994;8(1):85–90. doi: 10.1029/93GB03272. [DOI] [Google Scholar]
- 59.Rau GH, Takahashi T, Des Marais DJ. Latitudinal variations in plankton δ13C: implications for CO2 and productivity in past oceans. Nature. 1989;341(6242):516. doi: 10.1038/341516a0. [DOI] [PubMed] [Google Scholar]
- 60.Cherel Y, Hobson KA, Guinet C, Vanpe C. Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. Journal of Animal Ecology. 2007;76(4):826–836. doi: 10.1111/j.1365-2656.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 61.Phillips RA, Bearhop S, Mcgill RA, Dawson DA. Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia. 2009;160(4):795–806. doi: 10.1007/s00442-009-1342-9. [DOI] [PubMed] [Google Scholar]
- 62.Cherel Y, Kernaléguen L, Richard P, Guinet C. Whisker isotopic signature depicts migration patterns and multi-year intra-and inter-individual foraging strategies in fur seals. Biology Letters. 2009;5(6):830–832. doi: 10.1098/rsbl.2009.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kernaléguen L, et al. Long-term species, sexual and individual variations in foraging strategies of fur seals revealed by stable isotopes in whiskers. PloS One. 2012;7(3):e32916. doi: 10.1371/journal.pone.0032916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stowasser G, et al. Food web dynamics in the Scotia Sea in summer: a stable isotope study. Deep Sea Research Part II: Topical Studies in Oceanography. 2012;59:208–221. doi: 10.1016/j.dsr2.2011.08.004. [DOI] [Google Scholar]
- 65.Moore JK, Abbott MR, Richman JG. Variability in the location of the Antarctic Polar Front (90–20 W) from satellite sea surface temperature data. Journal of Geophysical Research: Oceans. 1997;102(C13):27825–27833. doi: 10.1029/97JC01705. [DOI] [Google Scholar]
- 66.Wakefield ED, et al. Long‐term individual foraging site fidelity – why some gannets don’t change their spots. Ecology. 2015;96(11):3058–3074. doi: 10.1890/14-1300.1. [DOI] [PubMed] [Google Scholar]
- 67.Seyboth E, et al. Isotopic evidence of the effect of warming on the northern Antarctic Peninsula ecosystem. Deep Sea Research Part II: Topical Studies in Oceanography. 2018;149:218–228. doi: 10.1016/j.dsr2.2017.12.020. [DOI] [Google Scholar]
- 68.Tarroux A, Lowther AD, Lydersen C, Kovacs KM. Temporal shift in the isotopic niche of female Antarctic fur seals from Bouvetøya. Polar Research. 2016;35(1):31335. doi: 10.3402/polar.v35.31335. [DOI] [Google Scholar]
- 69.Hertz E, Trudel M, Cox MK, Mazumder A. Effects of fasting and nutritional restriction on the isotopic ratios of nitrogen and carbon: a meta‐analysis. Ecology and evolution. 2015;5(21):4829–4839. doi: 10.1002/ece3.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salton M, Kirkwood R, Slip D, Harcourt R. Mechanisms for sex-based segregation in foraging behaviour by a polygynous marine carnivore. Marine Ecology Progress Series. 2019;624:213–226. doi: 10.3354/meps13036. [DOI] [Google Scholar]
- 71.Pyke GH, Pulliam HR, Charnov EL. Optimal foraging: a selective review of theory and tests. The Quarterly Review of Biology. 1977;52(2):137–154. doi: 10.1086/409852. [DOI] [Google Scholar]
- 72.Haskell JP, Ritchie ME, Olff H. Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature. 2002;418:527–530. doi: 10.1038/nature00840. [DOI] [PubMed] [Google Scholar]
- 73.Boyd IL. Estimating food consumption of marine predators: Antarctic fur seals and macaroni penguins. Journal of Applied Ecology. 2002;39(1):103–119. doi: 10.1046/j.1365-2664.2002.00697.x. [DOI] [Google Scholar]
- 74.Atkinson A, et al. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nature Climate Change. 2019;9:142–147. doi: 10.1038/s41558-018-0370-z. [DOI] [Google Scholar]
- 75.Murphy EJ, et al. Interannual variability of the South Georgia marine ecosystem: biological and physical sources of variation in the abundance of krill. Fisheries Oceanography. 1998;7(3–4)):381–390. doi: 10.1046/j.1365-2419.1998.00081.x. [DOI] [Google Scholar]
- 76.Reid K, Croxall JP. Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2001;268(1465):377–384. doi: 10.1098/rspb.2000.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waluda CM, Hill SL, Peat HJ, Trathan PN. Long-term variability in the diet and reproductive performance of penguins at Bird Island, South Georgia. Marine Biology. 2017;164(3):39. doi: 10.1007/s00227-016-3067-8. [DOI] [Google Scholar]
- 78.Forcada J, Hoffman JI. Climate change selects for heterozygosity in a declining fur seal population. Nature. 2014;511:462–465. doi: 10.1038/nature13542. [DOI] [PubMed] [Google Scholar]
- 79.Bengtson J. L., Ferm L. M., Härkönen T. J. & Stewart B. S. Abundance of Antarctic Fur Seals in the South Shetland Islands, Antarctica, During the 1986/87 Austral Summer in Antarctic Ecosystems (Eds Kerry, K. R. & Hempel, G.) 265–270 (Springer, 1990).
- 80.Hucke-Gaete R, Osman LP, Moreno CA, Torres D. Examining natural population growth from near extinction: the case of the Antarctic fur seal at the South Shetlands, Antarctica. Polar Biology. 2004;27(5):304–311. doi: 10.1007/s00300-003-0587-8. [DOI] [Google Scholar]
- 81.Breed GA, Bowen WD, McMillan JI, Leonard ML. Sexual segregation of seasonal foraging habitats in a non-migratory marine mammal. Proceedings of the Royal Society B: Biological Sciences. 2006;273(1599):2319–2326. doi: 10.1098/rspb.2006.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicol S, Foster J, Kawaguchi S. The fishery for Antarctic krill – recent developments. Fish and Fisheries. 2012;13(1):30–40. doi: 10.1111/j.1467-2979.2011.00406.x. [DOI] [Google Scholar]
- 83.Estrada JA, Rice AN, Natanson LJ, Skomal GB. Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology. 2006;87(4):829–834. doi: 10.1890/0012-9658(2006)87[829:UOIAOV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 84.Drago M, et al. Isotopic niche partitioning between two apex predators over time. Journal of Animal Ecology. 2017;86(4):766–780. doi: 10.1111/1365-2656.12666. [DOI] [PubMed] [Google Scholar]
- 85.Hill SL, Atkinson A, Pakhomov EA, Siegel V. Evidence for a decline in the population density of Antarctic krill Euphausia superba still stands. A comment on Cox et al. Journal of Crustacean Biology. 2019;39(3):316–322. doi: 10.1093/jcbiol/ruz004. [DOI] [Google Scholar]
- 86.Hanson NN, Wurster CM, Bird MI, Reid K, Boyd IL. Intrinsic and extrinsic forcing in life histories: patterns of growth and stable isotopes in male Antarctic fur seal teeth. Marine Ecology Progress Series. 2009;388:263–272. doi: 10.3354/meps08158. [DOI] [Google Scholar]
- 87.Bergmann KGLC. Über die Verhältnisse der wärmeokönomie der Thiere zu ihrer Grösse. Göttinger Studien. 1847;3:595–708. [Google Scholar]
- 88.Alonso JC, Salgado I, Palacín C. Thermal tolerance may cause sexual segregation in sexually dimorphic species living in hot environments. Behavioral Ecology. 2015;27(3):717–724. doi: 10.1093/beheco/arv211. [DOI] [Google Scholar]
- 89.Agashe D, Bolnick DI. Intraspecific genetic variation and competition interact to influence niche width. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2915–2924. doi: 10.1098/rspb.2010.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cloyed CS, Eason PK. Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. Royal Society open science. 2017;4(3):170060. doi: 10.1098/rsos.170060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hawkes LA, et al. Phenotypically linked dichotomy in sea turtle foraging requires multiple conservation approaches. Current Biology. 2006;16(10):990–995. doi: 10.1016/j.cub.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 92.Sato K, et al. Stroke frequency, but not swimming speed, is related to body size in free-ranging seabirds, pinnipeds and cetaceans. Proceedings of the Royal Society B: Biological Sciences. 2006;274(1609):471–477. doi: 10.1098/rspb.2006.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams TM. The evolution of cost efficient swimming in marine mammals: limits to energetic optimization. Philosophical Transactions of the Royal Society B. 1999;354:193–201. doi: 10.1098/rstb.1999.0371. [DOI] [Google Scholar]
- 94.Ficetola GF, De Bernardi F. Trade-off between larval development rate and post-metamorphic traits in the frog Rana latastei. Evolutionary Ecology. 2006;20(2):143–158. doi: 10.1007/s10682-005-5508-6. [DOI] [Google Scholar]
- 95.Warren NL, Trathan PN, Forcada J, Fleming A, Jessopp MJ. Distribution of post-weaning Antarctic fur seal Arctocephalus gazella pups at South Georgia. Polar Biology. 2006;29(3):179–188. doi: 10.1007/s00300-005-0037-x. [DOI] [Google Scholar]
- 96.Patrick SC, Pinaud D, Weimerskirch H. Boldness predicts an individual’s position along an exploration–exploitation foraging trade‐off. Journal of Animal Ecology. 2017;86(5):1257–1268. doi: 10.1111/1365-2656.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Charnov EL. Optimal foraging, the marginal value theorem. Theoretical Population Biology. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-X. [DOI] [PubMed] [Google Scholar]
- 98.Araújo MS, et al. Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology. 2008;89(7):1981–1993. doi: 10.1890/07-0630.1. [DOI] [PubMed] [Google Scholar]
- 99.Goubault M, Outreman Y, Poinsot D, Cortesero AM. Patch exploitation strategies of parasitic wasps under intraspecific competition. Behavioral Ecology. 2005;16(4):693–701. doi: 10.1093/beheco/ari043. [DOI] [Google Scholar]
- 100.Casey TM. Energetics of locomotion. Advances in Comparative and Environmental Physiology. 1992;11:251–275. doi: 10.1007/978-3-642-76693-0_8. [DOI] [Google Scholar]
- 101.Atkinson A, et al. Oceanic circumpolar habitats of Antarctic krill. Marine Ecology Progress Series. 2008;362:1–23. doi: 10.3354/meps07498. [DOI] [Google Scholar]
- 102.Green K, Burton HR, Williams R. The diet of Antarctic fur seals Arctocephalus gazella (Peters) during the breeding season at Heard Island. Antarctic Science. 1989;1(4):317–324. doi: 10.1017/S0954102089000489. [DOI] [Google Scholar]
- 103.Klages NTW, Bester MN. Fish prey of fur seals Arctocephalus spp. at subantarctic Marion Island. Marine Biology. 1998;131(3):559–566. doi: 10.1007/s002270050348. [DOI] [Google Scholar]
- 104.Baker JR, McCann TS. Pathology and bacteriology of adult male Antarctic fur seals, Arctocephalus gazella, dying at Bird Island, South Georgia. British Veterinary Journal. 1989;145(3):263–275. doi: 10.1016/0007-1935(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 105.Paritte JM, Kelly JF. Effect of cleaning regime on stable-isotope ratios of feathers in Japanese Quail (Coturnix japonica) The Auk. 2009;126(1):165–174. doi: 10.1525/auk.2009.07187. [DOI] [Google Scholar]
- 106.Qi H, Coplen TB, Geilmann H, Brand WA, Böhlke JK. Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Communications in Mass Spectrometry. 2003;17(22):2483–2487. doi: 10.1002/rcm.1219. [DOI] [PubMed] [Google Scholar]
- 107.Coplen TB, et al. New guidelines for δ 13C measurements. Analytical Chemistry. 2006;78(7):2439–2441. doi: 10.1021/ac052027c. [DOI] [PubMed] [Google Scholar]
- 108.Boyd IL, Roberts JP. Tooth growth in male Antarctic fur seals (Arctocephalus gazella) from South Georgia: an indicator of long‐term growth history. Journal of Zoology. 1993;229(2):177–190. doi: 10.1111/j.1469-7998.1993.tb02630.x. [DOI] [Google Scholar]
- 109.Beamish RJ, Fournier DA. A method for comparing the precision of a set of age determinations. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38(8):982–983. doi: 10.1139/f81-132. [DOI] [Google Scholar]
- 110.Rösch, A. & Schmidbauer, H. WaveletComp 1.1. WaveletComp: Computational Wavelet Analysis. R package version 1.1. At https://CRAN.R-project.org/package=WaveletComp (Date accessed: 15-11-2019) (2018).
- 111.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. At http://www.r-project.org/ (Date accessed: 15-11-2019) (2019).
- 112.Healy K, et al. SIDER: An R package for predicting trophic discrimination factors of consumers based on their ecology and phylogenetic relatedness. Ecography. 2018;41(8):1393–1400. doi: 10.1111/ecog.03371. [DOI] [Google Scholar]
- 113.Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER – Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology. 2011;80(3):595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- 114.Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. At https://CRAN.R-project.org/package=nlme (Date accessed: 15-11-2019) (2019).
- 115.Araújo MS, Bolnick DI, Layman CA. The ecological causes of individual specialisation. Ecology Letters. 2011;14(9):948–958. doi: 10.1111/j.1461-0248.2011.01662.x. [DOI] [PubMed] [Google Scholar]
- 116.Roughgarden J. Evolution of niche width. American Naturalist. 1972;106:683–718. doi: 10.1086/282807. [DOI] [Google Scholar]
- 117.Bolnick DI, Yang LH, Fordyce JA, Davis JM, Svanbäck R. Measuring individual‐level resource specialization. Ecology. 2002;83(10):2936–2941. doi: 10.1890/0012-9658(2002)083[2936:MILRS]2.0.CO;2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.