Abstract
Masseteric-facial anastomosis has gained popularity in recent days compared to the facial–hypoglossal anastomosis. Masseteric nerve has numerous advantages like its proximity to the facial nerve, stronger motor impulse, its reliability, low morbidity in harvesting and sacrificing the nerve and faster re-innervation that is achievable in most patients. The present case series demonstrate the surgical technique and the effectiveness of the masseteric nerve as donor for early facial reanimation. Between January 2017 and February 2019, 6 patients (2 male, 4 female) with iatrogenic unilateral complete facial paralysis (grade VI, House Brackmann scale) who underwent masseteric-facial nerve anastomosis were included in the study. The time interval between the onset of paralysis and surgery ranged from 4 to 18 months (mean 8.5 months). In all patients pre-operative electromyography had facial mimetic muscle fibrillation potentials. All patients underwent end to end anastomosis except for one patient where greater auricular interposition graft was used. In all cases, the facial muscles showed earliest sign of recovery at 2–5 months. These movements were first noticed on the cheek musculature when the patients activated their masseter muscle. Eye movements started appearing at 6–9 months (in 3 cases) and forehead movements at 18 months (in 1 case). According to the modified House-Brackmann grading scale, one patient had Grade I function, two patients had Grade II function, and three had Grade V function. There was no morbidity except one patient who underwent interposition graft had numbness in the ear lobule. None of the patients could feel the loss of masseteric nerve function. Masseteric facial nerve anastomosis is a versatile, powerful early facial dynamic reanimation tool with almost negligible morbidity compared to other neurotization procedures for patients with complete facial nerve paralysis.
Electronic supplementary material
The online version of this article (10.1007/s12070-019-01758-z) contains supplementary material, which is available to authorized users.
Keywords: Masseteric anastomosis, Facial nerve palsy, Masseteric-facial anastomosis
Introduction
Permanent facial nerve palsy is a well-known pathological condition that has significant functional, aesthetic, and psychological consequences with a lot of impact on the quality of life. Functional consequences like lack of corneal protection due to lagophthalmos, impaired peripheral vision due to brow ptosis, nasal obstruction due to decreased nasal valve support and impaired oral competence and speech due to ptosis of oral commissure affects patient’s day to day life. Worsening facial asymmetry during facial expression makes patients reluctant to smile and to cover their mouths while speaking, laughing, and so on. All these affect the ability to communicate non-verbally and cause an overall sense of disfigurement leading to a state of reactive depression. Thus, permanent facial paralysis is a devastating disease and considered a feared complication presenting a formidable challenge to treatment for many otolaryngologists [1, 2].
Surgical techniques aimed at rehabilitating the paralyzed face are still imperfect. Restoration of facial symmetry and dynamic facial expression depends much more on the time elapsed since the onset of paralysis than on the etiology of the facial palsy [3]. Early facial reanimation with a new motor nerve can be attempted when the time is less than 18 months, where fibrillations of the facial mimetic musculature can be still seen on EMG.
Hypoglossal-facial anastomosis has been the gold standard first-line treatment for many years [4]. However, the significant morbidities in terms of chewing, speech impairment, and deglutition are not negligible when end to end anastomosis is performed [5]. When patients do achieve voluntary smile and eye closure, synkinesis almost always occurs [1, 6]. Though the morbidity associated with the use of this nerve is reduced in an end to side neuroraphy, the efficacy of such technique also decreases considerably.
Masseteric-facial nerve anastomosis is an alternative to hypoglossal nerve transfer because of its close proximity to the facial nerve, stronger motor impulse with powerful reinnervation, its reliability, lower morbidity in harvesting and sacrificing the nerve, greater ease of activation for the patient and faster clinical recovery that is achievable in most patients [1–3, 7]. The masseteric nerve is a motor branch of the anterior division of the trigeminal nerve. The patients should be selected for nerve anastomosis only if no further surgery that may impair its function is planned in near future.
Methods
Surgical Procedure (Video)
Under general anesthesia, a modified Blairs incision is marked and epinephrine (1:200,000 dilution) is infiltrated subcutaneously along the skin-incision line. A point 3 cm anterior to the tragus and 1 cm inferior to the zygomatic arch (surface landmark for masseteric nerve) is also marked. Skin flaps are elevated anteriorly to this point and the greater auricular nerve is delineated in its entire course till its entry into the parotid gland. If the need arises this can be used as an interposition nerve graft. Then the entire facial nerve trunk from its exit from stylomastoid foramen and the first 2 cm of the main branches after bifurcation are exposed and the nerve is divided close to exit from stylomastoid foramen (to gain additional length of nerve for anastomosis).
The masseteric nerve is then identified with the zygomatic arch and the posterior border of the masseter muscle as standard landmarks. It is advisable to enter masseter approximately 1 cm anterior to the posterior border. As the nerve lies deep at the level of the lower border of the zygomatic arch detachment of masseter from the zygomatic arch is not necessary. Care must be taken in preserving the branches of facial nerve over masseter. The masseteric nerve lies 1.5- to 2.0-cm deep from the muscle surface within its belly close to its internal surface than its external surface, between the middle and deep layers of the masseter. Gentle dissection of the muscle fibres usually reveals the nerve. The nerve courses from its origin deep to the zygomatic arch diagonally pointing towards the oral commissure. For anastomosis the proximal branches can be spared, preventing total denervation of the masseter muscle.
Case Series
Between January 2017 and February 2019, 6 patients (2 male, 4 female) with iatrogenic unilateral complete facial paralysis (grade VI, House Brackmann scale) who underwent masseteric-facial nerve anastomosis were included in the study (Table 1). The time interval between the onset of paralysis and surgery ranged from 4 to 18 months (mean 8.5 months). Patients were between 19 and 45 years of age (mean 29.6 years). In all patients, pre-operative needle electromyography (EMG) revealed complete facial nerve injury and had facial mimetic muscle fibrillation potentials.
Table 1.
Clinical characteristics of patients with masseteric facial anastomosis
| Case | Age | Duration of palsy (months) | Etiology | Preop HB grade | First facial mimetic activation (months) | Postop modified HB grade | Follow up [months] | Spontaneous smile | Cosmetic outcomesa |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 23/M | 12 | Petrous apex Cholesteatoma removal | VI | 4 | I | 30 | Yes | Excellent |
| 2 | 30/F | 5 | Glomus Jugulotympanicum removal | VI | 4 | V | 5 [lost to follow up] | No | Poor |
| 3 | 39/F | 4 | Vestibular Schwannoma removal | VI | 4 | II | 15 | No | Good |
| 4 | 22/F | 6 | Vestibular Schwannoma removal | VI | 2 | II | 11 | No | Good |
| 5 | 45/M | 6 | Facial nerve Schwannoma removal | VI | 5 | V | 7 | No | Fair |
| 6 | 19/F | 18 | Glomus jugulotympanicum removal | VI | 4 | V | 5 | No | Fair |
aFunctional and aesthetic grading system described by Terzis and Noah [9] poor = deformity, no contraction; fair = no symmetry, minimal contraction; moderate = moderate symmetry and contraction; good = symmetry, nearly full contraction; and excellent = symmetrical smile with full contraction
All patients were counseled preoperatively about the expected outcomes of surgery, the need for regular follow up and intensive postoperative physiotherapy. All patients underwent end to end anastomosis except for case 5, where interposition nerve graft using greater auricular nerve was performed. None of the patients underwent other ancillary procedures or Botulinum toxin injection for facial palsy. All patients had regular follow up (duration: 5–31 months) except case 2 who was lost to follow up after 5 months. The patients were also given specific instructions to consult us when they recognized the first signs of mimetic muscle contraction.
Postoperative physiotherapy commenced 15 days after surgery. The patients were encouraged to do mirror exercises actively emphasizing the importance of exercising the affected part of the face first. The patients were asked to express their emotions, to grimace, and to smile initially with clenching teeth (to activate the masseteric nerve and muscle) and later without clenching while trying to feel the amplitude of the movement. When they started having facial mimetic muscle activity, they were also taught to gradually reduce the strength of the bite necessary to achieve mimetic muscle activation during the rehabilitative period.
At the last follow up (July 2019) the patients were evaluated and graded for improvement according to the modified House-Brackmann scale [8] (Table 2) and cosmetic outcomes were evaluated using the Functional and Aesthetic Grading System described by Terzis and Noah [9] (poor = deformity, no contraction; fair = no symmetry, minimal contraction; moderate = moderate symmetry and contraction; good = symmetry, nearly full contraction; and excellent = symmetrical smile with full contraction).
Table 2.
Modified House Brackmann scale
| Facial zone | Movement (%) | Movement score | Synkinesis (quantity) | Synkinesis score | Total score | Grade |
|---|---|---|---|---|---|---|
| Eyebrow | 100 | 1 | None | 0 | 4 | I |
| Eye | > 75 | 2 | Slight | 1 | 5–9 | II |
| Nasolabial fold | > 50 | 3 | Obvious | 2 | 10–14 | III |
| Oral | < 50 | 4 | Disfiguring | 3 | 15–19 | IV |
| Poor | 5 | 20–23 | V | |||
| Whole face | None | 6 | 24 | VI |
Results
All patients (100%) showed visible activation of the facial mimetic musculature at 2–5 months. These movements were first noticed on the cheek musculature while the patients activated their chewing musculature. The activation was seen as early as 2 months in case 4 (Fig. 1). In case 5 where interposition graft was used the facial mimetic activation was seen at 5 months. Rest all cases had their activation at 4 months. Eye movements started appearing at 6–9 months in 3 cases and forehead movements appeared at 18 months in only case 1 (Fig. 2). Three patients also learnt automatic smile at 9 months duration. However, the spontaneity of smiling (laughing at a joke or while watching a funny movie) was observed only in case 1 at 20 months.
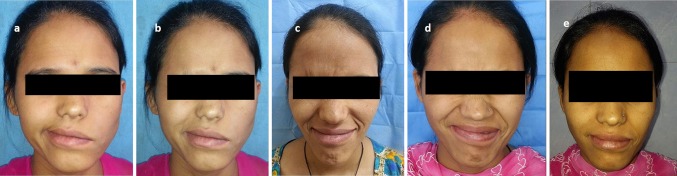
Fig. 1.
Preoperative images a note the lip sagging and loss of left nasolabial fold during smile; b complete facial palsy; c at 2 months post-op; note the activation of facial mimetic musculature; d at 8 months post-op; note eye contraction; e 15 months post-op; note lip sagging improvement during active smile
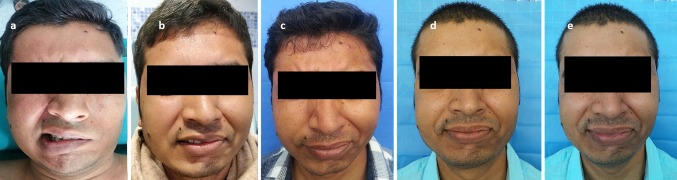
Fig. 2.
Preoperative image a complete facial palsy; b at 4 months post-op; note the first mimetic activation; c at 18 months post-op, note the forehead muscle contraction and almost complete eye closure; d, e at 30 months post-op, note the symmetrical smile and complete eye closure
According to the modified House-Brackmann grading scale at the last follow up visit (July 2019), case 1 had Grade I function, case 3 and 4 had Grade II function, and all others had Grade V function. Synkinesis was present only in case 3. The cosmetic results were fair in 3 patients, good in 2 and excellent in 1 patient.
The average surgical duration was 2 h and there were no intraoperative or postoperative complications. Patient 5 who underwent interposition graft using greater auricular nerve had numbness in the ear postoperatively. Though there was an initial weakness in bite strength on the anastomosis side, none of the patients complained regarding the same in subsequent follow-ups.
Discussion
Facial reanimation always proved to be a challenging topic. Though options for nerve transfer include cross facial, spinal accessory, motor roots of cervical plexus, hypoglossal, and masseteric nerve, no single nerve transfer can replicate the nuanced function of the native facial nerve. Massetric nerve has gained recent popularity compared to other nerves since its use in a series of 7 patients showed excellent results and minimal morbidity [7].
In a larger series of 34 patients who underwent facial masseteric anastomosis, 91.2% patient’s experienced facial nerve function reactivation as early as 2 months (range: 2–12 months) with the restoration of facial symmetry at rest [3]. In a systematic review by murphy et al., it was seen that the overall time for nerve recovery was around 5 months (range 2–7 months), and varied based on the facial nerve branch targeted. Selective anastomosis to the zygomatic/buccal branch recovered much faster than anastomosis to the main trunk (3.76 vs 5.76 months). Also, interposition nerve graft delayed recovery time, 6.24 versus 4.06 months [10]. In case 5 there was a delay of 1 month compared to other cases with respect to the first activation of mimetic musculature.
Compared to hypoglossal nerve transfer, masseteric nerve anastomosis was shown to have a faster recovery rate and more impressive contraction in 2 studies; by Albathi et al. (5.6 vs 10.8 months) and Hontanilla et al. (62 vs 136 days) [11, 12].
Consistent with other literature all our patients demonstrated signs of facial nerve recovery at 2–5 months. We also noted the recovery was seen earliest in the cheek followed by eye and the frontal area at the last.
Although there are numerous scales which used to evaluate outcomes after facial nerve rehabilitation like Smile Recovery Score, Terzis Smile Function Score, Sunnybrook Facial Grading System, Facial Disability Index (FDI), postoperative facial reanimation scale, Sunnybrook Facial Grading System, Yanagihara Facial Nerve Grading System, House Brackmann and modified HB scale, till date no single scale exists as standardized reporting measure [8, 10, 13–15].
The original HB scale is an imperfect tool to be used for grading after facial reanimation. It was originally made to classify facial paralysis or its spontaneous healing only. The presence of synkinesis or isolated frontal branch paralysis will downgrade the results significantly after reanimation. Grading also becomes difficult when a patient has little symmetry at rest but has an optimal function of mimetic musculature during movement. Hence we used a modified HB scale in our study to address these issues.
The main advantage of masseteric nerve anastomosis is its low morbidity and ease of surgery compared with other neurotization procedures. There were only 12 reported minor complications in 183 patients [1, 10]. Synkinesis rate is also lower than hypoglossal nerve anastomosis. Yoshioka et al. [16] evaluated postoperative masseter atrophy secondary to masseteric nerve transfer in a series of 10 patients and found that there was a statistically significant reduction (p = 0.021) in the superficial masseter muscle area. However, there was no externally visible postoperative facial asymmetry appreciated in any of the patients. The primary drawback initially thought lies in its inability to produce an emotive smile in isolation. However, Hontanilla et al. found a spontaneous smile in 56% of their patients in a series of 30 cases with facial masseteric anastomosis [17]. In a systematic review, this was noted in 25 of the 108 reported patients [10]. The reason may be attributed to the fact that the masseter muscle was found to contract 40% of the time on EMG during a natural, spontaneous smile [18].
Limitation
The study is limited by its smaller sample size and all the patients have not completed a minimum follow-up duration of at least 1 year. However, the excellent promising results of the initial three cases shows this technique is promising and it will help many otorhinolaryngologists and reconstructive surgeons to adopt this easy technique for early facial reanimation.
Conclusion
Masseteric facial nerve anastomosis is a versatile powerful early facial dynamic reanimation tool with almost negligible morbidity compared to other neurotization procedures for patients with complete facial nerve paralysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [PS, GT, SR] and [CAS]. The first draft of the manuscript was written by [PS] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Nil.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no financial or non-financial conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the All India Institute of Medical Sciences, New Delhi, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pirabu Sakthivel, Email: pirabusakthivel@gmail.com.
Chirom Amit Singh, Email: amitchirom@gmail.com.
Alok Thakar, Email: drathakar@gmail.com.
Geeta Thirumeni, Email: drgeetha2010@gmail.com.
Sarath Raveendran, Email: sharu_2_u@yahoo.co.in.
Suresh Chandra Sharma, Email: suresh6sharma@yahoo.com.
References
- 1.Bayrak SB, Kriet JD, Humphrey CD. Masseteric to buccal branch nerve transfer. Curr Opin Otolaryngol Head Neck Surg. 2017;25:280–285. doi: 10.1097/MOO.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi B, Ferri A, Ferrari S, Copelli C, Salvagni L, Sesenna E. The masseteric nerve: a versatile power source in facial animation techniques. Br J Oral Maxillofac Surg. 2014;52:264–269. doi: 10.1016/j.bjoms.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Biglioli F, Colombo V, Rabbiosi D, Tarabbia F, Giovanditto F, Lozza A, Cupello S, Mortini P. Masseteric-facial nerve neurorrhaphy: results of a case series. J Neurosurg. 2017;126:312–318. doi: 10.3171/2015.12.JNS14601. [DOI] [PubMed] [Google Scholar]
- 4.Pitty LF, Tator CH. Hypoglossal-facial nerve anastomosis for facial nerve palsy following surgery for cerebellopontine angle tumors. J Neurosurg. 1992;77:724–731. doi: 10.3171/jns.1992.77.5.0724. [DOI] [PubMed] [Google Scholar]
- 5.Viterbo F, Amr AH, Stipp EJ, Reis FJ. End-to-side neurorrhaphy: past, present, and future. Plast Reconstr Surg. 2009;124:e351–e358. doi: 10.1097/PRS.0b013e3181bf8471. [DOI] [PubMed] [Google Scholar]
- 6.Venail F, Sabatier P, Mondain M, Segniarbieux F, Leipp C, Uziel A. Outcomes and complications of direct end-to-side facial–hypoglossal nerve anastomosis according to the modified May technique. J Neurosurg. 2009;110:786–791. doi: 10.3171/2008.9.JNS08769. [DOI] [PubMed] [Google Scholar]
- 7.Biglioli F, Frigerio A, Colombo V, Colletti G, Rabbiosi D, Mortini P, Dalla Toffola E, Lozza A, Brusati R. Masseteric-facial nerve anastomosis for early facial reanimation. J Craniomaxillofac Surg. 2012;40:149–155. doi: 10.1016/j.jcms.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Henstrom DK, Skilbeck CJ, Weinberg J, Knox C, Cheney ML, Hadlock TA. Good correlation between original and modified House Brackmann facial grading systems. Laryngoscope. 2011;121:47–50. doi: 10.1002/lary.21163. [DOI] [PubMed] [Google Scholar]
- 9.Terzis JK, Noah ME. Analysis of 100 cases of free-muscle transplantation for facial paralysis. Plast Reconstr Surg. 1997;99:1905–1921. doi: 10.1097/00006534-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Murphey AW, Clinkscales WB, Oyer SL. Masseteric nerve transfer for facial nerve paralysis: a systematic review and meta-analysis. JAMA Facial Plast Surg. 2018;20:104–110. doi: 10.1001/jamafacial.2017.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albathi M, Oyer S, Ishii LE, Byrne P, Ishii M, Boahene KO. Early nerve grafting for facial paralysis after cerebellopontine angle tumor resection with preserved facial nerve continuity. JAMA Facial Plast Surg. 2016;18:54–60. doi: 10.1001/jamafacial.2015.1558. [DOI] [PubMed] [Google Scholar]
- 12.Hontanilla B, Marré D. Comparison of hemihypoglossal nerve versus masseteric nerve transpositions in the rehabilitation of short-term facial paralysis using the Facial Clima evaluating system. Plast Reconstr. 2012;130:662e–672e. doi: 10.1097/PRS.0b013e318267d5e8. [DOI] [PubMed] [Google Scholar]
- 13.Hu WL, Ross B, Nedzelski J. Reliability of the Sunnybrook facial grading system by novice users. J Otolaryngol. 2001;30:208–211. doi: 10.2310/7070.2001.20148. [DOI] [PubMed] [Google Scholar]
- 14.Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001;111:387–398. doi: 10.1097/00005537-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RP, WernickRobinson M, Hadlock TA. Validation of the synkinesis assessment questionnaire. Laryngoscope. 2007;117:923–926. doi: 10.1097/MLG.0b013e3180412460. [DOI] [PubMed] [Google Scholar]
- 16.Yoshioka N. Masseter atrophication after masseteric nerve transfer. Is it negligible? Plast Reconstr Surg Glob Open. 2016;4:e692. doi: 10.1097/GOX.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hontanilla B, Cabello A. Spontaneity of smile after facial paralysis rehabilitation when using a non-facial donor nerve. J Craniomaxillofac Surg. 2016;44:1305–1309. doi: 10.1016/j.jcms.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Schaverien M, Moran G, Stewart K, Addison P. Activation of the masseter muscle during normal smile production and the implications for dynamic reanimation surgery for facial paralysis. J Plast Reconstr Aesthet Surg. 2011;64:1585–1588. doi: 10.1016/j.bjps.2011.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.