Abstract
The aim of the study was to establish a reliable system of transgenic hairy roots in Codonopsis pilosula through Agrobacterium-mediated genetic transformation. For this, we optimized several steps in the process of A. rhizogenes strain C58C1 mediated hairy root induction, including the most appropriate medium, explant type, time for infection and co-cultivation. We achieved an induction rate of up to 100% when the roots of C. pilosula seedlings were used as explants, infected with A. rhizogenes C58C1 harboring pCAMBIA1305 for 5 min, followed by induction on 1/2MS supplemented with 0.2 mg/L naphthylacetic acid and 200 mg/L cefotaxime sodium. The co-transformed hairy roots were confirmed by PCR amplification of hygromycin phosphotransferase II gene and histochemical GUS assay, and the efficiency of transformation was 70% and 68.3%, respectively, when no hygromycin selection pressure was exerted. To increase biomass production, we excised and self-propagated the transformed hairy roots, which produce saponins. Our successful establishment of an in vitro culture system of transgenic hairy root for this species lays the foundation not only for assessing gene expression and function but also for obtaining high levels of secondary metabolites through genetic engineering technology.
Keywords: Agrobacterium rhizogenes, Codonopsis pilosula, Gene transformation, Hairy root, Saponins
Introduction
Agrobacterium rhizogenes is a Gram-negative soil bacterium that induces plants to produce hairy roots upon infection at the wounding sites. Its root-inducing (Ri) plasmid, containing transfer DNA encoding root locus (rol) gene loci (rolA, rolB, and rolC), is responsible for the stable introduction of genetic material into host cells (Chilton et al. 1982). The hairy roots have some common features, such as a high rate of proliferation in phytohormone-free media, ageotropic growth, an elevated rate of lateral branching, and genetic stability, the latter being a feature that renders this system appropriate for producing valuable secondary metabolites through in vitro culture (Hosseini et al. 2017; Kochan et al. 2018; Contreras et al. 2019). Furthermore, researchers have exploited the capacity of A. rhizogenes to transfer the disarmed T-DNA region from the A. tumefaciens-based binary vector along with T-DNA from the Ri plasmid to develop co-transformed transgenic hairy root cultures (Hamill et al. 1987). These co-transformed hairy roots are an extremely powerful tool for elucidating gene functions (Limpens et al. 2004; DeBoer et al. 2009; Kajikawa et al. 2009; Ron et al. 2014), analyzing promoter activity (Preiszner et al. 2001), rapidly and efficiently visualizing gene expression (Wiśniewska et al. 2013; Ron et al. 2014), enhancing heterogenous protein expression (Menzel et al. 2003), producing antibodies (Sharp and Doran 2001), modifying plant metabolic pathways (Sommer et al. 1999; Mitra et al. 2002), and studying functional genomics (Seki et al. 2008).
The root of Codonopsis pilosula, known as “Dangshen”, is a famous traditional Chinese medicine that has been widely used to replenish qi (vital energy), strengthen the immune system, improve appetite, and cure gastric ulcers. Dangshen is also regarded as a health-care food in China, especially as a dietary supplement when brewing wine, making tea, cooking porridge, or making soup. Chemical and pharmacological studies have shown that its active ingredients are mainly composed of alkaloids, polysaccharides, and terpenoids, which participate in anti-gastric ulcer, anti-tumor, anti-aging, and anti-inflammatory activities; as well as aiding in improved respiratory function and memory; and treatments to cure diabetes, increase hemoglobin, and regulate immunity (Sun 2009; Xin et al. 2012; Barnes 2014; Chu et al. 2016; Li et al. 2017a). Because of its extensive pharmacological effects, the demand for Dangshen has greatly increased in domestic and foreign markets. This has stimulated the large-scale cultivation of C. pilosula, especially in Gansu Province of China, a region that accounts for 90% of the entire area planted with this species in that country (Deng et al. 2011).
Although some genes have been identified from C. pilosula (Gao et al. 2015; Ji et al. 2019), none has been verified by experiments. To test the functions of these candidate genes, a transformation system is necessary. Although hairy roots have been induced (Li et al. 2017b), no reports have been made about a highly effective transformation system for that species. The advantage associated with Agrobacterium-mediated transformation is that any foreign genes of interest placed in a binary vector can be simultaneously transferred to the transformed hairy root clones (Hamill et al. 1987). Here, we present a reproducible protocol for developing a transgenic hairy root system in C. pilosula through A. rhizogenes-mediated co-transformation of a recombinant binary vector with the beta-glucuronidase (GUS) reporter gene. To the best of our knowledge, this is the first description of a transgenic hairy root system for C. pilosula that facilitates the expression of a gene of interest, i.e., GUS. The adoption of our transformation techniques will greatly advance functional genomics studies in this species.
Materials and methods
Plant materials
Seeds of Codonopsis pilosula were collected from Gansu Province, China. They were disinfected with 15% NaClO for 13 min and rinsed with sterile water four or five times. Afterwards, the sterile seeds were placed on an MS medium (with 3% sucrose and 0.6% agar; pH 5.8 ± 0.2) and incubated under a 12-h photoperiod in a 24 ± 2 °C incubator. Aseptic seedlings were obtained after 4 weeks of such treatment.
Bacterial strain and recombinant DNA
Agrobacterium rhizogenes strain C58C1 was purchased from Huayueyang Biotechnology Co. Ltd. (Beijing, China). The Agrobacterium binary vector pCAMBIA1305, with hygromycin- and kanamycin- resistance and GUSPlus genes driven by the Cauliflower Mosaic Virus 35S (CaMV35S) promoter, was also purchased from Huayueyang. It was transformed into A. rhizogenes C58C1 by the freeze–thaw method (Weigel and Glazebrook 2006). The A. rhizogenes C58C1 harboring pCAMBIA1305 was conserved in our laboratory.
Bacterial culture
The native C58C1 Agrobacterium strain (negative control) and recombinant C58C1 strain carrying pCAMBIA1305 were streaked on a solid TY medium containing either 50 mg/L rifamycin (Rif) or 50 mg/L Rif plus 50 mg/L kanamycin (Kan). They were then incubated at 27 °C for 2–3 days. A single colony was inoculated into the TY liquid medium containing the specified antibiotics and incubated overnight at 27 °C (shaking at 180 rpm) until the OD600 value of the bacterial solution was 1.2–1.3. This solution was centrifuged for 8 min (5000 rpm). Afterward, the precipitated cells were suspended in a 1/2 MS liquid medium to achieve an OD600 value of 0.6–0.8. This then served as our infection solution.
Optimization of protocol for inducing hairy roots
The leaves of 4-week-old aseptic seedlings were cut into small pieces (approx. 0.5 cm2), and the stems and roots were trimmed to an average length of 0.5 cm. The surface of each explant type was gently scratched with the back of a scalpel, then placed on the MS medium. The tissues (stem, leaf, or root) were pre-cultured at 24 ± 2 °C for 2 days in the dark. Afterwards, they were immersed in the infection solution for 5 min and placed on a 1/2 MS solid medium for 2 days of co-cultivation. Following this inoculation period, the explants were transferred to a 1/2 MS solid medium supplemented with 200 mg/L cefotaxime sodium (Cef) plus different concentrations of naphthylacetic acid (NAA) to induce hairy roots. The effects on induction efficiency were investigated according to explant type and NAA concentration. Then, we optimized infection time and co-cultivation period with roots explants on the 1/2 MS solid medium supplemented with 0.2 mg/L NAA and 200 mg/L Cef. The single root about 2–3 cm in length was excised and sub-cultured every 14 days on the 1/2MS solid medium containing 0.2 mg/L NAA and 200 mg/L Cef. The induction rate was calculated after the explants had been cultured for 7 days on hygromycin-free induction medium.
Ten explants were placed on each plate (two to three plates per replicate) and all experiments were conducted with three replicates. All data were expressed as means ± standard errors and compared using Duncan’s multiple range tests at a 5% level of significance.
PCR identification of transgenic hairy roots
Genomic DNA was extracted from the proliferated transgenic hairy roots with a DNA Extraction Kit according to the manufacturer’s instructions (Huayueyang). Genomic DNA from the roots of wild-type (WT) seedlings was used as the negative control. Each PCR-amplification was conducted in a 50-µL volume containing 40 ng of genomic DNA, 0.4 μM of each primer, and 25 μL of 2 × EasyTaq PCR SuperMix (TransGen, Beijing, China). The forward and reverse gene-specific primers for the Ri plasmid-derived RolA were RolA-F (5′-CATGTTTCAGAATGGAATTA-3′) and RolA-R (5′-AGCCACGTGCGTAT TAATCC-3′), while those for the pCAMBIA1305 derived hygromycin phosphotransferase II gene (hptII) were hptII-F (5′-CTATTTCTTTGCCCTCGGAC-3′) and hptII-R (5′-CACTGGCAAACTGTGATGGA-3′). The PCR reactions were performed as follows: initial denaturation at 95 °C for 3 min; then 34 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 45 s; with a final extension at 72 °C for 5 min. After this amplification, we performed a 1% agarose gel electrophoresis analysis.
Histochemical GUS assay
Histochemical GUS assays were conducted as described previously (Chattopadhyay et al. 2011).
Growth of transgenic hairy roots in liquid culture
Fresh samples of our proliferated transgenic hairy roots (0.5 g each) were transferred to 250-mL flasks (total of 15 flasks), each containing 50 mL of a liquid medium (1/2MS + 200 mg/L Cef) and cultured at 24 °C (120 rpm). The fresh weights of these transgenic hairy root cultures were recorded at 10-day intervals (three flasks per event) to create a growth curve for the liquid culture. To do so, the sampled roots were oven-dried for 24 h at 50 °C to a constant weight.
Determination of total saponins content
The previous method was used to extract saponin and determine the saponin content (Dou et al. 2015). Standard substance of ginsenoside Re was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The dried and powdered hairy roots (0.1 g) were extracted with methanol (3 mL) in an ultrasonic bath (Kunshan Instrument Co., Ltd., China) in a designed ultrasonic power (50–150 W) for 90 min. After filtration, the solvent was removed. Then it was dissolved in 1 ml water, followed by extraction with petroleum ether for three times and butyl alcohol extraction three times. The total saponins were obtained by removing butyl alcohol and dissolved in 1 mL methanol, which was used as samples for content determination. Samples of 0.1 mL were transferred to vials and dried at 80 °C, followed by 0.2 mL 5% (w/v) vanillin solution in acetic acid and 0.8 mL perchloric acid. A control sample using methanol was prepared. Samples were vortexed and heated at 60 °C for 30 min. Vials were cooled and added 5 mL acetic acid before absorbance was measured at 536 nm using a spectrophotometer (BioSpectrometer, Eppendorf, Germany) against the control sample containing methanol. The total saponins content was obtained from a standard curve of ginsenoside Re.
Results
Induction of hairy roots in Codonopsis pilosula
The results from our investigation of explant type and NAA concentration indicated that the induction rate for hairy roots was optimal when we selected an infection time of 5 min and a 2-day co-cultivation period. Specifically, hairy roots were observed from the C. pilosula samples at 7–9 days after exposure to A. rhizogenes C58C1 carrying pCAMBIA1305 (Fig. 1). The root explants proved to be much more susceptible when compared with the stem and leaf explants. The optimal medium for initiating hairy roots combined 1/2 MS + 0.2 mg/L NAA (Table 1). Based on the data shown in Table 1, induction rates on that particular medium were highest for root explants, i.e., 77.78 ± 8.31%, followed by only 3.33 ± 2.36% for the stems. No hairy roots were induced from leaf explants when tested on that medium.
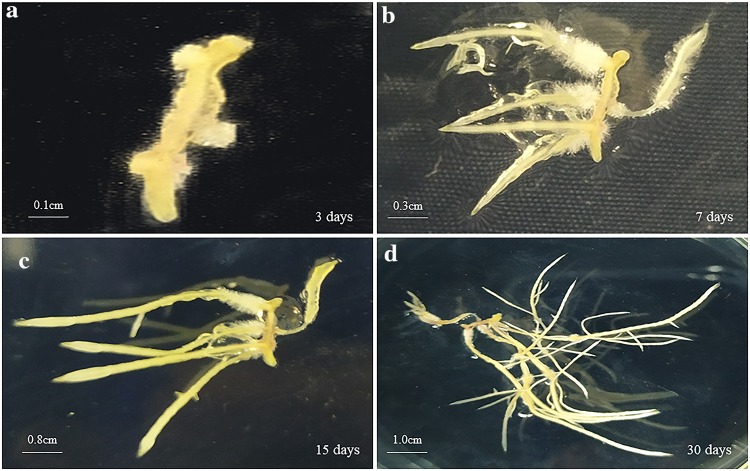
Fig. 1.
Hairy roots of Codonopsis pilosula induced from root explants at 3 days (a), 7 days (b), 15 days (c), and 30 days (d) after infection by Agrobacterium rhizogenes C58C1 harboring pCAMBIA1305
Table 1.
Effects of explant type and NAA concentration on the rate of hairy root induction
| NAA concentration (mg/L) | Explant type | Number of explants | Number of explants with hairy roots | Induction rate of hairy roots (%)* |
|---|---|---|---|---|
| 0.00 | Root | 30 | 16.00 ± 1.00 | 53.33 ± 2.72b |
| 0.05 | Root | 30 | 18.67 ± 0.58 | 62.22 ± 1.57bc |
| 0.10 | Root | 30 | 19.67 ± 4.73 | 65.56 ± 12.86c |
| 0.20 | Root | 30 | 23.33 ± 3.06 | 77.78 ± 8.31d |
| 0.00 | Stem | 20 | 0.00 ± 0.00 | 0.00 ± 0.00a |
| 0.05 | Stem | 20 | 0.33 ± 0.58 | 1.67 ± 2.36a |
| 0.10 | Stem | 20 | 0.67 ± 1.15 | 3.33 ± 4.71a |
| 0.20 | Stem | 20 | 0.67 ± 0.58 | 3.33 ± 2.36a |
| 0.00 | Leaf | 20 | 0.00 ± 0.00 | 0.00 ± 0.00a |
| 0.05 | Leaf | 20 | 0.00 ± 0.00 | 0.00 ± 0.00a |
| 0.10 | Leaf | 20 | 0.00 ± 0.00 | 0.00 ± 0.00a |
| 0.20 | Leaf | 20 | 0.00 ± 0.00 | 0.00 ± 0.00a |
*Rates followed by same letter are not significantly different (P = 0.05), based on Duncan’s multiple range tests
Further experiments were conducted to determine the optimum infection time and co-cultivation period for inducing hairy roots from root explants. During a co-cultivation period of 2 days, we found that the induction rate was 73.33 ± 4.71%, 48.33 ± 2.88%, 55.00 ± 13.29%, and 35.00 ± 5.00% when the infection time was set as 5 min, 10 min, 15 min, and 20 min, respectively (Table 2). We also tested the effects of different lengths of co-cultivation on the induction rate, based on an infection time of 5 min. That rate reached 100% when infected root explants did not undergo co-culturing. In contrast, induction rates were 88.33 ± 2.88%, 73.33 ± 4.71%, and 46.67 ± 2.89% when the infected root explants were co-cultured for 1, 2, and 3 days, respectively. This demonstrated that the success of induction was gradually reduced as co-culturing became prolonged. From these data we determined that the optimized protocol for inducing hairy roots, i.e., at a rate of up to 100%, involved the use of root explants that were first immersed in an Agrobacterium solution for 5 min and then transferred to an Agrobacterium-elimination medium.
Table 2.
Effects of infection time and co-cultivation period on induction of hairy roots from root explants
| Infection time (min) | Co-cultivation time (d) | Number of explants | Number of explants with hairy roots | Induction rate of hairy roots (%)* |
|---|---|---|---|---|
| 5 | 2 | 20 | 14.67 ± 0.67 | 73.33 ± 4.71c |
| 10 | 2 | 20 | 9.67 ± 0.57 | 48.33 ± 2.88b |
| 15 | 2 | 20 | 11.00 ± 2.64 | 55.00 ± 13.29b |
| 20 | 2 | 20 | 7.00 ± 1.00 | 35.00 ± 5.00a |
| 5 | 0 | 20 | 20.00 ± 0.00 | 100.00 ± 0.00e |
| 5 | 1 | 20 | 17.67 ± 0.57 | 88.33 ± 2.88d |
| 5 | 2 | 20 | 14.67 ± 0.67 | 73.33 ± 4.71c |
| 5 | 3 | 20 | 9.33 ± 0.57 | 46.67 ± 2.89b |
*Rates followed by same letter are not significantly different (P = 0.05), based on Duncan’s multiple range tests
Detection of co-transformed transgenic hairy roots by PCR
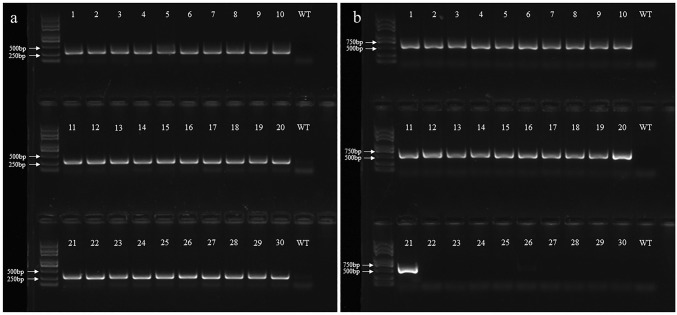
The Rol genes are important Ri plasmid genes of A. rhizogenes and play an important role in hairy root induction (Lima et al. 2009). Using PCR-amplification with RolA-F/R and hptII-F/R gene-specific primers, we detected the Ri-derived rolA as well as the pCAMBIA1305-derived hptII in our co-transformed hairy root lines. An expected band of 360 bp was amplified in all 30 co-transformed hairy root lines, whereas no detectable band was observed in the WT control (Fig. 2a), indicating the successful presence of the RolA in all tested hairy root lines genome. Among those 30 lines, a desired 546-bp amplicon was found in 21 of them (Fig. 2b). This demonstrated that 70% of the co-transformed hairy roots contained pCAMBIA1305-derived T-DNA in the plant genome.
Fig. 2.
PCR-amplification of 360-bp rolA DNA fragment (a) and 546-bp hygromycin phosphotransferase II gene (b) in different co-transformed hairy root lines of Codonopsis pilosula and wild-type (WT) control
Detection of transgenic hairy roots by GUS-staining
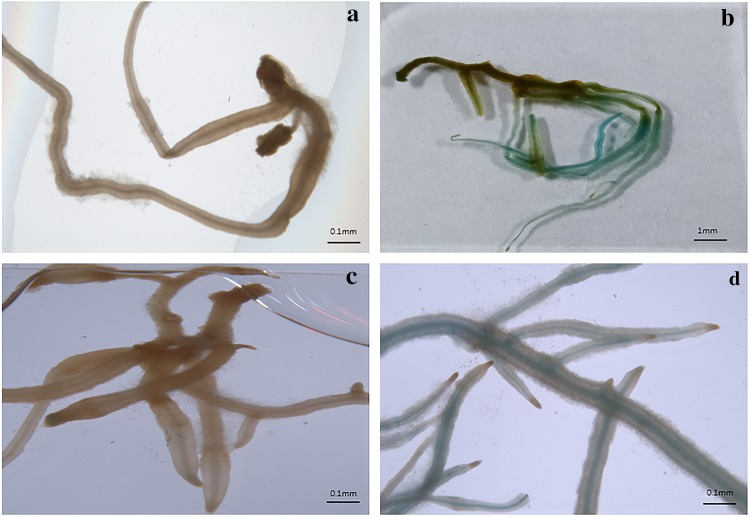
Because the vector pCAMBIA1305 carries the gusA reporter gene, we further examined the protein expression level of the pCAMBIA1305-derived gusA in transgenic hairy roots. Results from our histochemical GUS assays revealed that 28 out of the 41 transgenic lines tested here showed the characteristic intense blue color, i.e., 68.3% co-transformation efficiency, while no blue coloration was observed in the control hairy roots (Fig. 3a, b).
Fig. 3.
Histochemical GUS assay of Codonopsis pilosula transgenic hairy roots and untransformed control. a GUS activity in emerging hairy roots after 15 days of infection by Agrobacterium rhizogenes C58C1. b GUS activity in co-transformed transgenic hairy roots after 15 days of infection by strain C58C1 harboring pCAMBIA1305. c GUS activity in hairy roots after 28 days in liquid culture. d GUS activity in co-transformed transgenic hairy roots after 28 days in liquid culture
We further monitored GUS activity in the proliferated transgenic hairy roots, which were confirmed by PCR to contain pCAMBIA1305-derived T-DNA and cultured in the 1/2MS liquid medium for 4 weeks. GUS activity was observed in all the transgenic hairy root lines tested, whereas no such activity was detected in control lines (Fig. 3c, d).
Growth of transgenic hairy roots
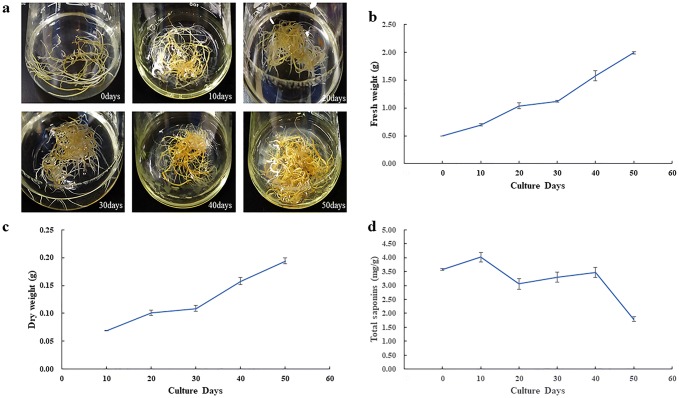
Hairy root growth was observed in the 1/2MS liquid medium. As culture time became prolonged, the hairy roots gradually changed from white to pale-yellow and dark-yellow (Fig. 4a). Their fresh weights increased to 0.70, 1.04, 1.12, 1.58, and 1.99 g when the hairy roots were cultured in the liquid medium for 10, 20, 30, 40, and 50 days, respectively (Fig. 4b). The fresh weight on Day 50 was 3.98 times greater than that recorded at the start of the inoculation period. Hairy root dry weights were 0.07, 0.10, 0.11, 0.16, and 0.19 g after being cultured in the liquid medium for 10, 20, 30, 40, and 50 days, respectively (Fig. 4c).
Fig. 4.
Growth of Codonopsis pilosula hairy roots in phytohormone-free 1/2 strength liquid MS medium. a Growth of transgenic hairy roots (initial fresh weight of 0.5 g per sample) after 10, 20, 30, 40, and 50 days. b Growth curve for fresh weights of hairy root. c Growth curve for dry weights of hairy root biomass. d Total saponins content in transgenic hairy roots
Content of total saponins in transgenic hairy roots
Dangshen extract saponins clearly demonstrated the protective effects on kidney ischemia–reperfusion injury after kidney transplantation (He et al. 2011). We determined the content of total saponins in transgenic hairy roots. Our result showed that the content of total saponins was 3.58, 4.01, 3.05, 3.29, 3.47 and 1.79 mg/g when the hair roots were cultured in the 1/2MS liquid medium for 0, 10, 20, 30, 40 and 50 days, respectively (Fig. 4d).
Discussion
Hairy roots can be utilized as a biological reactor for the production of valuable compounds from medicinal plants (Guillon et al. 2006). Some of those species can quickly produce hairy roots, including Salvia miltiorrhiza (Huang et al. 2012), Panax ginseng (Sathiyamoorthy et al. 2010), P. quinquefolius (Kochan et al. 2013), Tetrastigma hemsleyanum (Du et al. 2015), Stevia rebaudiana (Fu et al. 2015), and Anisodus luridus (Qin et al. 2014). Here, we established a rapid in vitro system for the culturing of C. pilosula transgenic hairy roots. This initial effort provides a promising alternative for the accelerated production of bioactive components in that species.
Several factors influence the induction of hairy roots, including explant type, choice of Agrobacterium strain, infection time, and period of co-cultivation. The part of the plant that is most susceptible to A. rhizogenes infection for hairy root formation differs among species. For example, leaf explants from Salvia miltiorrhiza show the highest induction rate for hairy roots (Huang et al. 2012) while the bud is the most suitable explant to use when inducing hairy roots in Semecarpus anacardium (Panda et al. 2017). For Chenopodium murale, the best target explant is the root (Mitic et al. 2012). For C. pilosula hairy root, the stem is better than either the leaf or petiole, and the induction rate is maximized, at 48%, when those stem explants are infected with A. rhizogenes A4 rather than with strain C58C1 or A1476 (Li et al. 2017b). In the present study, we found that root explants were highly susceptible to A. rhizogenes C58C1. Furthermore, our optimized protocol led to an induction rate of up to 100%.
The hptII is widely used as a selectable marker in plant transformation systems. Its expression allows transformed hairy roots to grow on media supplemented with that antibiotic. When selecting for highly sensitive transgenic hairy roots, the induction medium is often supplemented with 5 mg/L hygromycin (Chattopadhyay et al. 2011; Rizvi et al. 2015). However, we noted in the experiments described here that hairy roots of C. pilosula could be induced on a hygromycin-free medium, at a transformation efficiency of 68.3%, when we used the T-DNA from pCAMBIA1305. This indicated that co-transformation was very successful for C. pilosula even in the absence of antibiotic selective pressure. In fact, we found C. pilosula were highly sensitive to hygromycin and the rate of induction for co-transformed hairy roots was only 28.5% on the selection medium with 2 mg/l hygromycin (data not shown).
As a well-known traditional Chinese medicine, Dangshen has the effect of strengthening spleen and tonifying lung, nourishing blood and engendering liquid. Modern research showed that saponins extracted from Dangshen had protective effects on kidney ischemia–reperfusion injury after kidney transplantation (He et al. 2011). We observed accumulation of saponins in the co-transformed hairy roots, which indicated that transgenic hairy roots of C. pilosula could be used to produce secondary metabolites.
Conclusion
We have developed an efficient A. rhizogenes-mediated transformation approach for Codonopsis pilosula transgenic hairy roots. The rate of induction for hairy roots reached 100% when root explants were used. This method was most successful when the samples were infected for 5 min with A. rhizogenes C58C1 harboring pCAMBIA1305, followed by induction on 1/2MS + 0.2 mg/L NAA + 200 mg/L Cef. Overall, 70% of the co-transformed hairy roots contained pCAMBIA1305-derived hptII in the plant genome and 68.3% of those hairy roots showed GUS activity, even when no selection pressure was exerted. These findings indicated that T-DNA from the exogenous plasmid pCAMBIA1305 could be efficiently integrated into the C. pilosula genome and then expressed stably. Our protocol helps lay the foundation for genetic engineering of that species.
Acknowledgements
This work was supported financially by the Major Project of Shaanxi Province, China (Grant No. 2017ZDXM-SF-005) and Fundamental Research Funds for the Central Universities (Grant No. GK201706004).
Author contributions
XC designed the study and wrote the manuscript. JY performed experiments and wrote the manuscript. XY analyzed the data. BL, XL and JK performed experiments.
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflicts of interest.
Ethical statements
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay T, Roy S, Mitra A, Maiti KM. Development of a transgenic hairy root system in jute (Corchorus capsularis L.) with gusA reporter gene through Agrobacterium rhizogenes mediated co-transformation. Plant Cell Rep. 2011;30:485–493. doi: 10.1007/s00299-010-0957-y. [DOI] [PubMed] [Google Scholar]
- Chilton MD, Tepfer DA, Petit A, David C, Casse-Delbart F, Tempé J. Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature. 1982;295:432–434. doi: 10.1038/295432a0. [DOI] [Google Scholar]
- Chu X, Liu XJ, Qiu JM, Zeng XL, Bao HR, Shu J. Effects of astragalus and Codonopsis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to PM2.5. Environ Toxicol Pharmacol. 2016;48:76–84. doi: 10.1016/j.etap.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Contreras A, Leroy B, Mariage PA, Wattiez R. Proteomic analysis reveals novel insights into tanshinones biosynthesis in Salvia miltiorrhiza hairy roots. Sci Rep. 2019;9:5768. doi: 10.1038/s41598-019-42164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer KD, Lye JC, Aitken CD, Su AK, Hamill JD. The A622 gene in Nicotiana glauca (tree tobacco): evidence for a functional role in pyridine alkaloid synthesis. Plant Mol Biol. 2009;69:299–312. doi: 10.1007/s11103-008-9425-2. [DOI] [PubMed] [Google Scholar]
- Deng ML, Wang J, Deng CQ, Shi L. Progress of modern research on Codonopsis taxa. Guide China Med. 2011;9:279–281. [Google Scholar]
- Dou WY, Cui ZJ, Hou J, Wang MW, Yang FD. Determination of total saponins in Codonopsis pilosula in different producing areas of Gansu. J Tradit Chin Vet Med. 2015;34:59–62. [Google Scholar]
- Du SR, Xiang TH, Song YL, Huang LX, Sun Y, Han YX. Transgenic hairy roots of Tetrastigma hemsleyanum: induction, propagation, genetic characteristics and medicinal components. Plant Cell Tiss Organ Cult. 2015;122:373–382. doi: 10.1007/s11240-015-0775-6. [DOI] [Google Scholar]
- Fu X, Yin ZP, Chen JG, Shang GX, Wang X, Zhang Q, Peng D. Production of chlorogenic acid and its derivatives in hairy root cultures of Stevia rebaudiana. J Agric Food Chem. 2015;63:262–268. doi: 10.1021/jf504176r. [DOI] [PubMed] [Google Scholar]
- Gao JP, Wang D, Cao LY, Sun HF. Transcriptome sequencing of Codonopsis pilosula and identification of candidate genes involved in polysaccharide biosynthesis. PLoS ONE. 2015;10:e117342. doi: 10.1371/journal.pone.0117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon S, Tremouillaux-Guiller J, Pati PK, Rideau M, Gantet P. Hairy root research: recent scenario and exciting prospects. Curr Opin Plant Biol. 2006;9:341–346. doi: 10.1016/j.pbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Hamill JD, Prescott A, Martin C. Assessment of the efficiency of cotransformation of the T-DNA of disarmed binary vectors derived from Agrobacterium tumefaciens and the T-DNA of A rhizogenes. Plant Mol Biol. 1987;9:573–584. doi: 10.1007/BF00020534. [DOI] [PubMed] [Google Scholar]
- He B, Zhang YT, Yuan XG, Sun JS, Wei GH, Lin T. Protective effects of Radix Codonopsis on ischemia–reperfusion injury in rats after kidney transplantation. Pediatr Surg Int. 2011;27:1203–1212. doi: 10.1007/s00383-011-2935-z. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Bahramnejad B, Douleti Baneh H, Emamifar A, Goodwin PH. Hairy root culture optimization and resveratrol production from Vitis vinifera subsp. sylvesteris. World J Microbiol Biotechnol. 2017;33:67. doi: 10.1007/s11274-017-2235-4. [DOI] [PubMed] [Google Scholar]
- Huang LQ, Zhang XN, Cui GH, Jiang X. Establishment and analysis of in vitro culture system for transgenic Salvia miltiorrhiza hairy roots. China J Chin Materia Med. 2012;37:2257–2261. [PubMed] [Google Scholar]
- Ji JJ, Feng Q, Sun HF, Zhang XJ, Li XX, Li JK, Gao JP. Response of bioactive metabolite and biosynthesis related genes to methyl jasmonate elicitation in Codonopsis pilosula. Molecules. 2019;24(3):533. doi: 10.3390/molecules24030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa M, Hirai N, Hashimoto T. A PIP-family protein is required for biosynthesis of tobacco alkaloids. Plant Mol Biol. 2009;69:287–298. doi: 10.1007/s11103-008-9424-3. [DOI] [PubMed] [Google Scholar]
- Kochan E, Wasiela M, Sienkiewicz M. The production of ginsenosides in hairy root cultures of American Ginseng, Panax quinquefolium L. and their antimicrobial activity. Vitro Cell Dev Biol Plant. 2013;49:24–29. doi: 10.1007/s11627-012-9469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan E, Szymczyk P, Kuźma Ł, Szymańska G, Wajs-Bonikowska A, Bonikowski R, Sienkiewicz M. The increase of triterpene saponin production induced by trans-anethole in hairy root cultures of Panax quinquefolium. Molecules. 2018;23(10):2674. doi: 10.3390/molecules23102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JK, Wang T, Zhu ZC, Yang FR, Cao LY, Gao JP. Structure features and anti-gastric ulcer effects of inulin-type fructan CP-A from the roots of Codonopsis pilosula (Franch.) Nannf. Molecules. 2017;22(12):2258. doi: 10.3390/molecules22122258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XX, Xue WX, Liu LL, Yuan JY, Liu Q, Wei TS, Zheng YJ. Study on the induction condition of hairy roots of Codonopsis pilosula. J Anhui Agric Sci. 2017;45:121–123. [Google Scholar]
- Lima JE, Benedito VA, Figueira A, Peres LE. Callus, shoot and hairy root formation in vitro as affected by the sensitivity to auxin and ethylene in tomato mutants. Plant Cell Rep. 2009;28:1169–1177. doi: 10.1007/s00299-009-0718-y. [DOI] [PubMed] [Google Scholar]
- Limpens E, Ramos J, Franken C, Raz V, Compaan B, Franssen H, Bisseling T, Geurts R. RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J Exp Bot. 2004;55:983–992. doi: 10.1093/jxb/erh122. [DOI] [PubMed] [Google Scholar]
- Menzel G, Harloff HJ, Jung C. Expression of bacterial poly (3-hydroxybutyrate) synthesis genes in hairy roots of sugar beet (Beta vulgaris L.) Appl Microbiol Biotechnol. 2003;60:571–576. doi: 10.1007/s00253-002-1152-z. [DOI] [PubMed] [Google Scholar]
- Mitić N, Dmitrović S, Djordjević M, Zdravković-Korać S, Nikolić R, Raspor M, Djordjević T, Maksimović V, Zivković S, Krstić-Milošević D, Stanišić M, Ninković S. Use of Chenopodium murale L. transgenic hairy root in vitro culture system as a new tool for allelopathic assays. J Plant Physiol. 2012;169:1203–1211. doi: 10.1016/j.jplph.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Mitra A, Mayer MJ, Mellon FA, Michael AJ, Narbad A, Parr AJ, Waldron KW, Walton NJ. 4-hydroxycinnamoyl-CoA hydratase/lyase, an enzyme of phenylpropanoid cleavage from Pseudomonas, causes formation of C(6)–C(1) acid and alcohol glucose conjugate when expressed in hairy root of Datura stramonium L. Planta. 2002;215:79–89. doi: 10.1007/s00425-001-0712-2. [DOI] [PubMed] [Google Scholar]
- Panda BM, Mehta UJ, Hazra S. Optimizing culture conditions for establishment of hairy root culture of Semecarpus anacardium L. 3 Biotech. 2017;7:21. doi: 10.1007/s13205-017-0608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiszner J, Van Toai TT, Huynh L, Bolla R, Yen H. Structure and activity of a soybean Adh promoter in transgenic hairy roots. Plant Cell Rep. 2001;20:763–769. doi: 10.1007/s002990100385. [DOI] [Google Scholar]
- Qin BF, Ma LL, Wang Y, Chen M, Lan XZ, Wu NB, Liao ZH. Effects of acetylsalicylic acid and UV-B on gene expression and tropane alkaloid biosynthesis in hairy root cultures of Anisodus luridus. Plant Cell Tissue Organ Cult. 2014;117:483–490. doi: 10.1007/s11240-014-0454-z. [DOI] [Google Scholar]
- Rizvi NF, Cornejo M, Stein K, Weaver J, Cram EJ, Lee-Parsons CW. An efficient transformation method for estrogen-inducible transgene expression in Catharanthus roseus hairy roots. Plant Cell Tiss Organ Cult. 2015;120:475–487. doi: 10.1007/s11240-014-0614-1. [DOI] [Google Scholar]
- Ron M, Kajala K, Pauluzzi G, Wang D, Reynoso MA, Zumstein K, Garcha J, Winte S, Masson H, Inagaki S, Federici F, Sinha N, Deal RB, Bailey-Serres J, Brady SM. Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol. 2014;166(2):455–469. doi: 10.1104/pp.114.239392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyamoorthy S, In JG, Gayathri S, Kim YJ, Yang DC. Gene ontology study of methyl jasmonate-treated and nontreated hairy roots of Panax ginseng to identify genes involved in secondary metabolic pathway. Genetika. 2010;46:932–939. [PubMed] [Google Scholar]
- Seki H, Ohyama K, Nishizawa T, Yoshida S, Muranaka T. The ‘‘all-in-one’’ rol-type binary vectors as a tool for functional genomics studies using hairy roots. Plant Biotechnol. 2008;25:347–355. doi: 10.5511/plantbiotechnology.25.347. [DOI] [Google Scholar]
- Sharp JM, Doran PM. Strategies for enhancing monoclonal antibody accumulation in plant cell and organ cultures. Biotechnol Prog. 2001;17:979–992. doi: 10.1021/bp010104t. [DOI] [PubMed] [Google Scholar]
- Sommer S, Köhle A, Yazaki K, Shimomura K, Bechthold A, Heide L. Genetic engineering of shikonin biosynthesis hairy root cultures of Lithospermum erythrorhizon transformed with the bacterial ubiC gene. Plant Mol Biol. 1999;39:683–693. doi: 10.1023/A:1006185806390. [DOI] [PubMed] [Google Scholar]
- Sun YX. Immunological adjuvant effect of a water-soluble polysaccharide, CPP, from the roots of Codonopsis pilosula on the immune responses to ovalbumin in mice. Chem Biodivers. 2009;6:890–896. doi: 10.1002/cbdv.200800154. [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J (2006) Transformation of Agrobacterium using the freeze-thaw method. CSH Protoc 2006: pdb.prot4666 [DOI] [PubMed]
- Wiśniewska A, Dąbrowska-Bronk J, Szafrański K, Fudali S, Święcicka M, Czarny M, Wilkowska A, Morgiewicz K, Matusiak J, Sobczak M, Filipecki M. Analysis of tomato gene promoters activated in syncytia induced in tomato and potato hairy roots by Globodera rostochiensis. Transgenic Res. 2013;22:557–569. doi: 10.1007/s11248-012-9665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin T, Zhang F, Jiang Q, Chen C, Huang D, Li Y, Shen W, Jin Y, Sui G. The inhibitory effect of a polysaccharide from Codonopsis pilosula on tumor growth and metastasis in vitro. Int J Biol Macromol. 2012;51:788–793. doi: 10.1016/j.ijbiomac.2012.07.019. [DOI] [PubMed] [Google Scholar]