Abstract
The aim of our study was to obtain wideband tympanometry (WBT) findings in Meniere’s disease (MD). It also aimed to evaluate whether the data obtained have diagnostic significance. 21 patients who were followed-up for unilateral Meniere’s Disease were evaluated. The ears with Meniere disease were grouped as the MD group and the opposite ears were grouped as the control group. WBT results were recorded as resonance frequency (RF) and frequency-specific absorbance values at 10 different frequencies in the 0.25–8.0 kHz range. Statistical analysis was performed with t test and receiver-operating characteristic analysis. Considering the WBT results, RF was significantly lower in the MD group compared to the control group (p < 0.001). Frequency-specific absorbance values at 0.25, 0.5, 0.75 and 1 kHz were significantly lower in the MD group compared to the control group (p < 0.05). No significant difference was found at 1.5 kHz and above (p > 0.05). For the MD, the RF below 598 Hz was 85.7% sensitive and 76.2% specific, the absorbance at 0.25 kHz below 8% was 66.7% sensitive and 61.9% specific, the absorbance below 17% at 0.5 kHz was 71.4% sensitive and 62.1% specific, the absorbance below 36% at 0.75 kHz was 81% sensitive and 57.8% specific, and the absorbance below 46% at 1 kHz was 71.5% sensitive and 66.7% specific. When MD was compared with intact ears, it was observed that RF was lower, and absorbance decreased in low frequencies. These data is statistically significant, but the sensitivity level is not enough for diagnostic use. Therefore, it is considered as an complementary test for the diagnosis.
Keywords: Meniere’s disease, Wideband tympanometry, Absorbance, Resonance frequency
Introduction
Meniere’s disease (MD) is an endolymphatic hydroplasty that develops in the inner ear as a result of overproduction or inadequate reabsorption of endolymph [1]. This hydrops is clinically characterized by vertigo in the form of attacks on the affected side, sensorineural hearing loss showing fluctuations at low to medium frequencies, tinnitus and fullness. The duration of the attack is between 20 min and 24 h [2, 3].
The disease is diagnosed by documenting the hearing loss with an audiogram in addition to these typical symptoms of the patient [4]. Several clinical and radiological tests have been tried and developed in time to assist the audiogram to support the diagnosis of endolymphatic hydrops. Electrocochleography (ECoG), vestibular-evoked myogenic potentials (VEMP), otoacoustic emissions (OAE) and Magnetic Resonance Imaging (MRI) tests with Gadolinium (Gd) have been reported to have benefits for the diagnosis. However, they either do not have sufficient sensitivity and specificity or it is difficult to reach and apply [2, 5–9]. In addition, it has been evaluated in recent years whether multifrequency tympanometry (MFT) device can be used as a new diagnostic test for MD. Although some significant results were obtained, the diagnostic accuracy was found to be limited [6, 10, 11]. Wideband tympanometry (WBT) is a relatively new measurement device that can provide a wider frequency range and more detailed frequency information than the MFT [12].
The wideband tympanometer provides immitansmetric measurement in the 226 Hz–8 kHz range. The measurement basically yields acoustic absorbance, i.e. absorbed sound energy and resonance frequency (RF) values. These data allow us to obtain important information related to both middle and inner ear [12]. As far as we know, there is no data about MD and WBT. Therefore, in our study, we aimed to obtain WBT results in MD patients. In addition, we also determined specificity and sensitivity levels to evaluate whether meaningful results could be used as a diagnostic tool for MD.
Materials and Method
The study was approved by the local ethics committee (2018/160).
Participants
Patients who were followed-up for unilateral Meniere’s Disease at the our Otorhinolaryngology Clinic in tertiary center October 2017 and February 2019 were evaluated. The patients were diagnosed according to the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) (Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease, 1995) criteria [4]. Patients with bilateral disease, patients who had undergone ear surgery, and patients with any pathology in their autoendoscopic examination were not included in the study. All the patients included in the study had normal autoendoscopic examinations and pure tone audiometry tests on the intact ears. In the pure tone audiometry test on the ear with MD, air–bone gap was below < 10 dB HL; that is, there was no conductive-type hearing loss. The ears with Meniere disease were grouped as the MD group and the opposite ears were grouped as the control group.
Hearing Evaluation
Pure sound audiometry test was performed with AC40 (Interacoustics; Assens, Denmark) device in a soundproof room. Air conduction thresholds were measured with the Telephonics TDH39 headset and bone conduction thresholds were measured between 0.25 and 6.0 kHz using a B71 bone vibrator. The audiometric thresholds were determined by the modified Hughson–Westlake method [13]. Pure sound averages were calculated from the average of 0.5, 1 and 2 kHz.
Wideband Tympanometric Evaluation
WBT was measured using a Titan Suite (Interacoustics; Assens, Denmark) device. Wideband absorbance and resonance frequency values were measured at ambient pressure and in the frequency range of 0.25–8.0 kHz by using 90 ± 3 decibel sound stimulus. Absorbance rates in ambient pressure (APARs) range from 0 to 1. The higher the amount absorbed by the middle ear, the closer the value is to 1. These values between 0 and 1, i.e. the absorbance rates can be evaluated as %. WBT results were recorded as frequency-specific absorbance values at 10 different frequencies at the 0.25–8.0 kHz range (0.25, 0.50, 0.75, 1, 1.5, 2, 3, 4, 6, 8 kHz). To calculate the data, OtoAccess 1.2.1 (Interacoustics; Assens, Denmark) software was used. The first two digits after 0 gives the percentage value of the sound energy absorbed by the middle ear.
Statistical Analysis
Statistical analysis was performed using SPSS software (ver. 20.0; SPSS Inc., Chicago, IL, USA). RF and frequency-specific absorbance rates of MD and control groups were compared using t-test. Statistical significance was taken as p < 0.05. Receiver operating characteristic (ROC) analysis was performed to determine the cut-off value at the diagnosis of MD at significant values.
Results
There were a total of 21 patients included in the study. The mean age was 43.47 ± 9.6 (24–56); 8 (38%) of the patients were female and 13 (62%) were male. MD was in 7 (33%) right ears and in 14 (67%) left ears. In pure tone audiometry, 8 (38%) patients had mild and 13 (62%) patients had moderate sensorineural hearing loss.
For the WBT results, mean RF was 414.7 ± 343 (124–1559) in MD group and 860.2 ± 266.9 (239–2743) in the control group. There was a statistically significant difference (p < 0.001) in RF values of both groups. While there were significant differences in the frequency-specific absorbance values of 0.25, 0.5, 0.75 and 1 kHz evaluated at 10 different frequencies at ambient pressure, there was no significant difference at 1.5 kHz and above (Table 1).
Table 1.
Meniere disease and control groups data of APARs, and RFs
| Meniere disease group | Control group | p values | |
|---|---|---|---|
| Mean ± SD (min–max) | Mean ± SD (min–max) | ||
| APARs | |||
| 0.25 kHz |
9.4 ± 6.8 (0.01–0.29) |
15.2 ± 9.2 (0.01–0.39) |
0.026 |
| 0.5 kHz |
14.9 ± 9.2 (0.05–0.39) |
22.2 ± 9.4 (0.10–0.43) |
0.016 |
| 0.75 kHz |
30.6 ± 15.3 (0.07–0.62) |
42.4 ± 18.4 (0.11–0.85) |
0.03 |
| 1 kHz |
42.7 ± 18.6 (0.17–0.80) |
56.0 ± 18.7 (0.20–0.92) |
0.026 |
| 1.5 kHz |
54.4 ± 17.5 (0.30–0.96) |
61.5 ± 16.8 (0.29–0.96) |
0.192 |
| 2 kHz |
63 ± 17 (0.37–0.87) |
62.9 ± 18.8 (0.28–0.87) |
0.986 |
| 3 kHz |
69.9 ± 19.2 (0.35–0.99) |
72.6 ± 19.8 (0.35–0.99) |
0.661 |
| 4 kHz |
55.6 ± 20 (0.02–0.80) |
57.8 ± 18.1 (0.26–0.90) |
0.706 |
| 6 kHz |
32.2 ± 18.1 (0.01–0.60) |
31.2 ± 15.5 (0.08–0.60) |
0.856 |
| 8 kHz |
31.3 ± 19.6 (0.01–0.88) |
27.4 ± 21.6 (0.03–0.88) |
0.496 |
| RFs |
414.7 ± 343 (124–1559) |
860.2 ± 266.9 (239–2743) |
<0.001 |
Bold and italics expresses statistical significant
APARs ambient-pressure absorbance rations, RFs resonance frequencies, SD standard deviation, min minimum, max maximum)
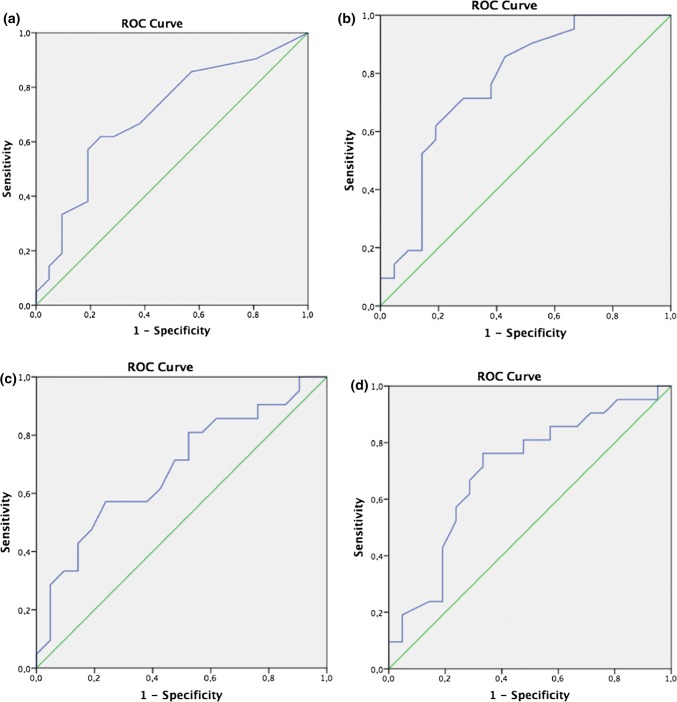
ROC analysis was performed to determine the cut-off values of the data with significant differences. For the MD, the RF being below 598 Hz was 85.7% sensitive and 76.2% specific (Fig. 1). Frequency-specific absorbance values of 0.25 kHz below 8% of the absorbance was 66.7% sensitive and 61.9% specific; absorbance below 17% at 0.5 kHz was 71.4% sensitive and 62.1% specific; absorbance below 36% at 0.75 kHz was 81% sensitive and 57.8% specific; and the absorbance below 46% at 1 kHz was 71.5% sensitive and 66.7% specific (Fig. 2). Since the area under the curve (AUC) in all of these values with the ROC analysis was in 0.5–1 range, the diagnostic use of the cut-off values given can be evaluated as reliable.
Fig. 1.
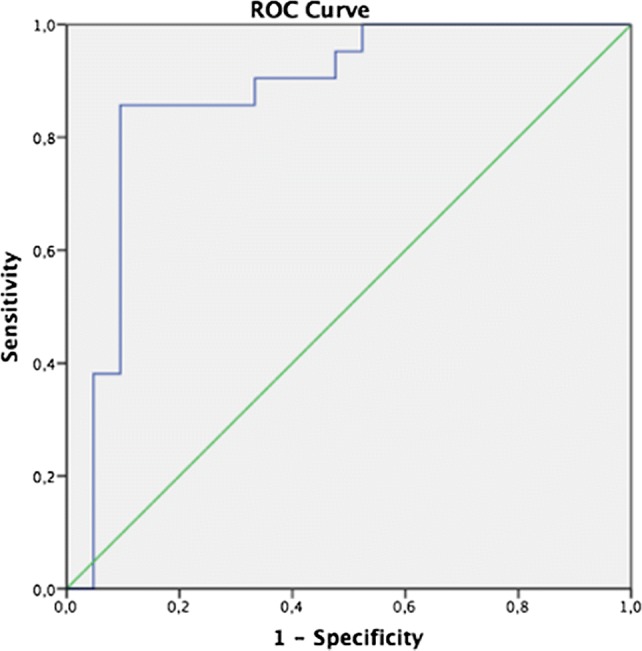
Receiver operating characteristic curves that resonance frequency (RF) below 598 Hz afforded 85.7% sensitivity and 76.2% specificity
Fig. 2.
Receiver operating characteristic curves that a an APARs on 0.25 kHz below 8% afforded 77.7% sensitivity and 61.9% specificity, b an APARs on 0.5 kHz below 17% afforded 71.4% sensitivity and 62.1% specificity, c an APARs on 0.75 kHz below 36% afforded 81% sensitivity and 57.8% specificity, d an APARs on 1 kHz below 46% afforded 71.5% sensitivity and 66.7% specificity
Discussion
It is compulsory to perform audiological evaluation after anamnesis is taken for the diagnosis of MD. The most important finding is the detection of sensorineural type hearing loss at low and medium frequencies. Pure tone audiometry test is the basic test used in the diagnosis and follow-up of MD. It also plays an important role in the staging and treatment of the disease. However, new laboratory techniques have been used to evaluate MD with radiological and audiovestibular equipment enriched in time [2, 5, 14, 15].
Along with the advances in technology, 3-Dimensional Fluid-Attenuated Inversion Recovery (3D-FLAIR) Magnetic Resonance Imaging (MRI) with Gadolinium (Gd) imaging of the inner ear is used for the diagnosis of MD. In routine use, images are taken using intravenous Gd for the diagnosis of EH [5]. In this imaging, the second dose should be administered 4 h after the first Gd injection for a clear perilymph and endolymph image distinction. However, high-dose Gd has a risk of systemic toxicity [16]. For this reason, Nakshima et al. [17] performed intratympanic Gd and after 24 h, they showed EH in the inner ear with 3D-FLAIR MRI and stated that they differentiated perilymphatic and endolymphatic compartments better. However, the intratympanic application is more invasive; the transition characteristics of Gd to the inner ear may vary due to oval window permeability; has a waiting period; and may lead to various complications such as perforation in the tympanic membrane due to application [5, 14]. The main limitations include necessity of serious equipment in radiological diagnostic methods, application of contrast agent to the patient, cost and transportation difficulties. In addition, there is no routine procedure in the diagnosis of MD. Therefore, these conditions lead us to clinical tests.
The vestibular-evoked myogenic potentials (VEMP) test allows us to evaluate the vestibular system through the saccular and inferior vestibular nerve with a sound stimulus. The diagnostic value of the VEMP test for MD was investigated, but the measurements of intact ears and the ears with MD were found quite similar. Therefore, no significant diagnostic features could be obtained [7, 8]. OAE is a measurement that evaluates the undulant hairy cells of the inner ear. In OAE, different results were obtained in patients with MD compared to the ears with normal hearing. However, there was no difference between patients with hearing loss due to a different cause and those with MD [9].
Electrocochleography (ECOG) is the most specific clinical test used for a long time. But the ideal electrode placement is on the promontorium. This situation has lost its popularity since it is not practical and cannot be done in routine office conditions [18]. Nowadays, MFT is a new test for MD with a moderate level, specificity and sensitivity similar to ECoG. There are a limited number of recent studies evaluating whether MFT can be used for the diagnosis of MD [6, 10, 11]. Although these studies show some changes in MFT during MD attack, it is known that the diagnostic accuracy is not enough yet [10]. However, the non-invasiveness of MFT and its ease of application are advantages compared to ECoG [6]. WBT is a more comprehensive and more recent measurement of the MFT. Therefore, WBT measurements may provide valuable information for MD. When we examine the literature, we have found that WBT is mostly evaluated according to age, gender and ear side normative data. Polat et al. [19] showed in their study on the Turkish population that gender and ear side did not affect outcomes in adult patients. In our study, since all patients were adults, we did not feel the need to separate gender and the ear sides affected.
Franco-Vidal et al. [10] and Yasuhi et al. [11] showed that admittance decreased at low frequencies in multifrequency tympanometry in Meniere’s disease patients. Similarly, in our study, the absorbance at low frequencies in the ears with MD decreased significantly compared to the intact ears. The concepts of absorbance and admittance indicate the ease of transition of sound energy into a system. These findings indicate that hydrops formed in the Meniere constitute a resistance to the passage of sound energy.
Sugasawa et al. [6] reported that RF was significantly lower in the ear affected from MD than in the control ear. Similarly, in our study, RF was significantly lower in the ears with MD than in the intact ears. Previous studies have shown a decrease in RF in the large vestibular aqueduct and superior semicircular canal dehiscence in which the inner ear is affected [12, 20]. When this condition is evaluated together with the data obtained in our study, it suggests that it may be related to abnormal inner ear pressure.
In our study, WBT data were obtained for MD. RF below 598 in the ear with Meniere’s disease had a sensitivity of 85.7% and a specificity of 76.2%. When the frequency-specific absorbance values were evaluated, a difference was found only at the low frequencies (0.25–1 kHz) and the absorbance was significantly lower in the ear with MD. The absorbance below 8% at 0.25 kHz was 66.7% sensitive and 61.9% specific; the absorbance below 17% at 0.5 kHz was 71.4% sensitive and 62.1% specific; the absorbance below 36% at 0.75 kHz was 81% sensitive and 57.8% specific; and the absorbance below 46% at 1 kHz was 71.5% sensitive and 66.7% specific.
The main limitation of our study was that the test was not re-applied to the patients after the attacks. In future studies with larger patient series, a comparison can be made by taking measurements both during an attack and after an attack. Thus, changes in data may help us obtain further helpful diagnostic information.
Conclusion
In our study, it was seen that, in MD, the absorbance decreased in low frequencies and RF was lower than in the intact ear. These findings suggest that WBT can be used as an adjuvant test for the diagnosis of MD. Being an easily-applicable, fast and non-invasive test can be reasons for preference. Although its diagnostic value is statistically reliable, its moderate sensitivity suggests that it can be used as a supportive test for the diagnosis.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paparella MM. The cause (multifactorial inheritance) and pathogenesis (endolymphatic mal absorption) of Meniere’s disease and its symptoms (mechanical and chemical) Acta Otolaryngol. 1985;99:445–451. doi: 10.3109/00016488509108936. [DOI] [PubMed] [Google Scholar]
- 2.Magnan J, Özgirgin ON, Trabalzini F, et al. European position statement on diagnosis, and treatment of Meniere’s disease. J Int Adv Otol. 2018;14(2):317–321. doi: 10.5152/iao.2018.140818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvakumar P, Balraj A, Kurien R, Krishnan T. Clinical and audio vestibular profile of Meniere’s disease in a tertiary care Centrein India. Indian J Otolaryngol Head Neck Surg. 2012;64(4):351–355. doi: 10.1007/s12070-011-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease American Academy of Otolaryngology-Head and Neck Foundation Inc. Otolaryngol Head Neck Surg. 1995;113(3):181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 5.Loureiro RM, Sumi DV, Lemos MD, et al. The role of magnetic resonance imaging in Ménière disease: the current state of endolymphatic hydrops evaluation. Einstein (Sao Paulo) 2019;17(1):eMD4743. doi: 10.31744/einstein_journal/2019MD4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugasawa K, Iwasaki S, Fujimoto C, et al. Diagnostic usefulness of multifrequency tympanomatry for Ménière’s disease. Audiol Neurotol. 2013;18:152–160. doi: 10.1159/000346343. [DOI] [PubMed] [Google Scholar]
- 7.Lin MY, Timmer FC, Oriel BS, et al. Vestibular evoked myogenic potentials (VEMP) can detect asymptomatic saccular hydrops. Laryngoscope. 2006;116:987–992. doi: 10.1097/01.mlg.0000216815.75512.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmer FC, Zhou G, Guinan JJ, Kujawa SG, Herrmann BS, Rauch SD. Vestibular evoked myogenic potential (VEMP) in patients with Ménière’s disease with drop attacks. Laryngoscope. 2006;116(5):776–779. doi: 10.1097/01.mlg.0000205129.78600.27. [DOI] [PubMed] [Google Scholar]
- 9.de Kleine E, Mateijsen DJ, Wit HP, Albers FW. Evoked otoacoustic emissions in patients with Ménière’s disease. Otol Neurotol. 2002;23(4):510–516. doi: 10.1097/00129492-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Franco-Vidal V, Legarlantezec C, Blanchet H, Convert C, Torti F, Darrouzet V. Multifrequency admittancemetry in Ménière’s disease: a preliminary study for a new diagnostic test. Otol Neurotol. 2005;26:723–727. doi: 10.1097/01.mao.0000178136.81729.7c. [DOI] [PubMed] [Google Scholar]
- 11.Yasui T, Iwasaki S, Sugasawa K, et al. Admittance tympanometry with 2-kHz probe tones in patients with low-frequency hearing loss. Laryngoscope. 2012;122(10):2252–2255. doi: 10.1002/lary.23439. [DOI] [PubMed] [Google Scholar]
- 12.Demir E, Afacan NN, Celiker M, et al. Can wideband tympanometry be used as a screening test for superior semicircular canal dehiscence? Clin Exp Otorhinolaryngol. 2018 doi: 10.21053/ceo.2018.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carhart R, Jerger JF. Pregerred method for clinical determination of pure-tone thresholds. J Speech Hear Dis. 1959;24(4):330–345. doi: 10.1044/jshd.2404.330. [DOI] [Google Scholar]
- 14.Lingam RK, Connor SE, Casselman JW, Beale T. MRI in otology: applications in cholesteatoma and Ménière’s disease. Clin Radiol. 2018;73(1):35–44. doi: 10.1016/j.crad.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Guneri EA, Çakır A, Mutlu B. Validity and reliability of the diagnostic tests for Ménière’s disease. Turk Arch Otorhinolaryngol. 2016;54:124–130. doi: 10.5152/tao.2016.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai T, Uno A, Kitahara T, et al. Evaluation of endolymphatic hydrops using 3-T MRI after intravenous gadolinium injection. Eur Arch Otorhinolaryngol. 2017;274:4103–4111. doi: 10.1007/s00405-017-4739-9. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima T, Naganawa S, Sugiura M, et al. Visualization of endolymphatic hydrops in patients with Meniere’s disease. Laryngoscope. 2007;117(3):415–420. doi: 10.1097/MLG.0b013e31802c300c. [DOI] [PubMed] [Google Scholar]
- 18.Ng M, Srireddy S, Horlbeck DM, Niparko JK. Safety and patient experience with transtympanic electrocochleography. Laryngoscope. 2001;111:792–795. doi: 10.1097/00005537-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Polat Z, Bas B, Hayır D, Bulut E, Atas A. Wideband tympanometry normative data for Turkish young adult population. J Int Adv Otol. 2015;11:157–162. doi: 10.5152/iao.2015.809. [DOI] [PubMed] [Google Scholar]
- 20.Bilgen C, Kirkim G, Kirazli T. Middle ear impedance measurements in large vestibular aqueduct syndrome. Auris Nasus Larynx. 2009;36:263–268. doi: 10.1016/j.anl.2008.07.002. [DOI] [PubMed] [Google Scholar]