Abstract
To study the clinical presentation and management outcomes in a series of patients with invasive rhino-orbital-cerebral mucormycosis presenting to a tertiary care center in central India. Medical records of eleven consecutive cases of invasive rhino-orbital–cerebral mucormycosis were reviewed. All clinically diagnosed cases, confirmed on microbiological examination were included. Their demographic data, clinical manifestations, underlying systemic conditions, microbiological and radiological reports, medical treatments, and surgical interventions were recorded and analyzed. There were nine male and two female patients with mean age of 46.8 years. Uncontrolled diabetes mellitus was noted in all patients. One patient had history of renal transplantation. The common presenting features were-ophthalmoplegia (73%), diminution of vision, (64%) proptosis (36%) and periorbital swelling (27%). CT scan/MRI revealed sino-orbital involvement in eight cases and rhino-orbital-cerebral involvement in three cases. Ethmoid sinus (100%) was the commonest paranasal sinus involved. KOH preparation and histopathology revealed broad aseptate filamentous fungi branching at right angles with tissue invasion. Culture on sabouraud’s dextrose agar showed growth of mucor species. All patients received intravenous amphotericin B and had undergone radical debridement of involved sinuses. The mean duration of follow up was 13 months. All survived except three, who developed cerebral mucormycosis. Rhino—orbital-cerebral mucormycosis is a fetal fungal infection requiring multidisciplinary approach. Uncontrolled diabetes mellitus is the main predisposing factor. Early diagnosis, reversal of predisposing co-morbidities, aggressive medical and surgical management are vital in managing this highly aggressive disease.
Keywords: Diabetes mellitus, Mucormycosis, Orbital cellulitis, Orbital apex syndrome, Ophthalmoplegia
Introduction
Mucormycosis is an acute, fulminating, often fetal fungal infection caused by fungi of the family Mucoraceae and is frequently seen in diabetic and immunocompromized patients [1, 2]. The fungal hyphae of mucoraceae family are angioinvasive, invades blood vessels, causes necrotizing vacuities and thrombosis resulting in extensive tissue infarcts and necrosis [3]. The disease usually starts in the nose and sinuses after inhalation of fungal spores. It proliferates and spreads to the paranasal sinuses (sino-nasal mucormycosis) and then to the orbit by direct extension or through hematogenous rout (sino-orbital mucormycosis). It can also spreads to the brain (sino-orbital–cerebral mucormycosis) which the commonest manifestation of mucormycosis [4–7]. Poorly controlled diabetes, especially with ketoacidosis and immunosuppression are the most important risk factors [4, 7–9]. Although the primary site of inoculation of fungus is nose and paranasal sinuses, the patients can initially present to the ophthalmologist with ocular signs and symptoms [5]. Therefore ophthalmologists should be familiar with the clinical spectrum of disease, as early diagnosis and aggressive management are crucial elements in the management of this invasive sino-orbital fungal infection [10].
Systemic Amphotericin B with surgical debridement of sinuses, orbital exenteration and control of systemic condition remains the main stay of treatment [11, 12]. However; there are reports of success with limited or no surgical intervention in the orbit [3, 13–15].
In this study we evaluated the clinical characteristics and treatment outcome of a series of 11 cases of invasive sino-orbital mucormycosis with diabetes mellitus.
Materials and Methods
Eleven microbiologically confirmed cases of invasive rhino-orbital-cerebral mucormycosis presenting between October 2012 and September 2016 were retrospectively reviewed from medical records. There clinical presentation, laboratory investigations and treatment outcomes were analyzed. Comprehensive workup at presentation included detailed history, comprehensive ocular examination, otorhinolaryngological and neurological examinations to assess the extent and severity of the disease.
Initial investigations included complete blood counts, blood sugar and serum creatinine. Diagnosis of mucormycosis was made on the basis of demonstration of broad aseptate hyphae with right angled branching on KOH preparation of specimens obtained from the nasal cavity and/or paranasal sinuses and culture reports. CT scan and/or MRI of paranasal sinuses, orbit and brain were obtained to assess the extent and severity of disease.
All patients received intravenous amphotericin B as soon as a diagnosis of mucormycosis was confirmed; it was given in dose of 1.0 mg/kg/day, dose was increased till a total dose of 2.5 to 3 g. Maximum duration of therapy was 6 weeks. Renal functions were monitored during therapy. Diabetes was controlled with insulin therapy.
Transnasal endoscopic radical debridement of involved sinuses was done in all patients and specimen obtained was sent for histopathology and culture.
The outcome for treatment was evaluated in terms of treatment success and treatment failure. Treatment success was defined as controlled metabolic status, stable and disease free patient. Treatment failure was defined as worsening of general condition and mortality due to intracranial spread of the infection.
Results
There were nine male and two female patients, with mean age of 46.8 years (range 15–65 years). Nine patients had type II diabetes and two had type I diabetes. The duration of diabetes ranged from 11 months to 7 years (mean 3.7 years). The lag time between onset of symptoms and presentation ranged from 2 days to 11 days (mean-4.7 days). The common presenting symptoms and signs were-Ophthalmoplegia (73%), diminution of vision, (64%) proptosis (36%) and periorbital swelling (27%). Two patients developed partial optic atrophy and two had central retinal artery occlusion. Palatal perforation was seen in one patient (Table 1).
Table 1.
Clinical presentation and treatment outcome of patients with rhino-orbital-cerebral mucormycosis
| S. No. | Age/gender | Presenting symptoms/signs | Orbit/CNS involvement | Paranasal sinuses involved | Treatment given | Outcome |
|---|---|---|---|---|---|---|
| 1. | 65/M | DOV ophthalmoplegia | Orbital apex syndrome, optic atrophy | Ethmoid and sphenoid | Sx debridement of sinuses + systemic antifungal | Success |
| 2. | 38/F | ophthalmoplegia | Orbital apex syndrome | Ethmoid sinusitis | Sx debridement of sinus + systemic antifungal | Success |
| 3. | 16/M | Proptosis with ophthalmoplegia | Orbital apex syndrome | Ethmoid and maxillary | Sx debridement of sinus + systemic antifungal | Success |
| 4. | 60 M | DOV,ophthalmoplegia, periorbital swelling | Orbital apex syndrome,optic atrophy | Ethmoid and maxillary | Sx debridement of sinuses + systemic antifungal | Success |
| 5. | 36/F | DOV, proptosis, globe displacement | Orbital cellulitis | Frontal, Ethmoid, maxillary | Sx debridement of sinus + systemic antifungal | Success |
| 6. | 48/M | DOV, ophthalmoplegia | Orbital apex syndrome | Ethmoid and maxillary | Sx debridement of sinus + systemic antifungal | Success |
| 7. | 52/M | DOV, proptosis, ophthalmoplegia | Orbital cellulitis with cerebral infarcts | Ethmoid and Maxillary | Sx debridement of sinus + systemic antifungal | Failure |
| 8. | 15/M | Periorbital swelling | Orbital cellulitis | Ethmoid | Sx debridement of sinus + systemic antifungal | Success |
| 9. | 62/M | DOV, ophthalmoplegia proptosis. Palatal perforation | Orbital apex syndrome CRAO, cerebral infarcts | Ethmoid, maxillary and sphenoid | Sx debridement of sinuses + systemic antifungal | Failure |
| 10 | 65/M | Periorbital swelling | Orbital cellulitis | Ethamoid | Sx debridement of sinuses + systemic antifungal | Success |
| 11 | 58/M | DOV, ophthalmoplegia | Orbital apex syndrome, CRA O,cerebral involvement | Ethmoid and sphenoid | Sx debridement of sinuses + systemic antifungal | Failure |
CRAO central retinal artery occlusion, DOV diminution of vision, Sx surgical
Uncontrolled diabetes was the commonest underlying disease in all patients; one was on immunosuppressive therapy for renal transplantation. KOH wet mount preparation demonstrated broad aseptate fungal hyphae with right angled branching (Fig. 1). Culture on sabouraud’s dextrose agar was positive for rhizopus in all cases. Lacto phenol cotton blue mount revealed broad, aseptate hyphae with rhizoids and sporangia (Figs. 2, 3, 4).
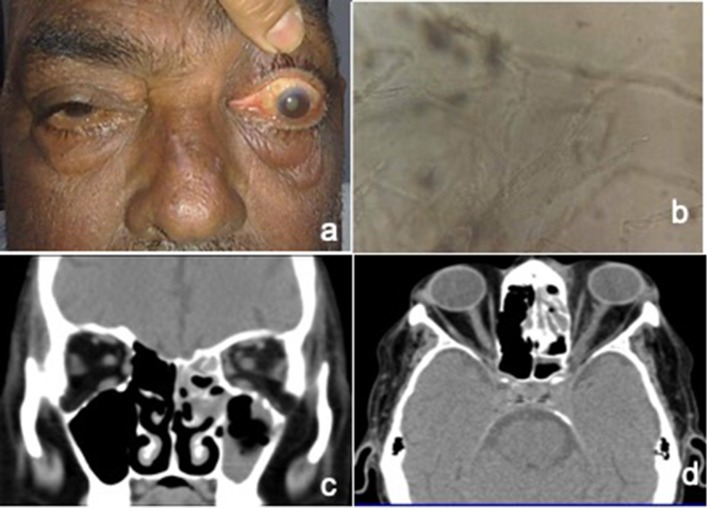
Fig. 1.
a Photograph of patient with left eye proptosis and ophthalmoplegia. b KOH mount showing broad aseptate fungal hyphae with right angled branching. c CT Scan coronal image showing opacification of ethmoid and maxillary sinus on left side. d CT axial image showing proptosis left eye with orbital fat stranding and opacification of ethmoid sinus. There is evidence of ill defined soft tissue density at the orbital apex with bulky left cavernous sinus
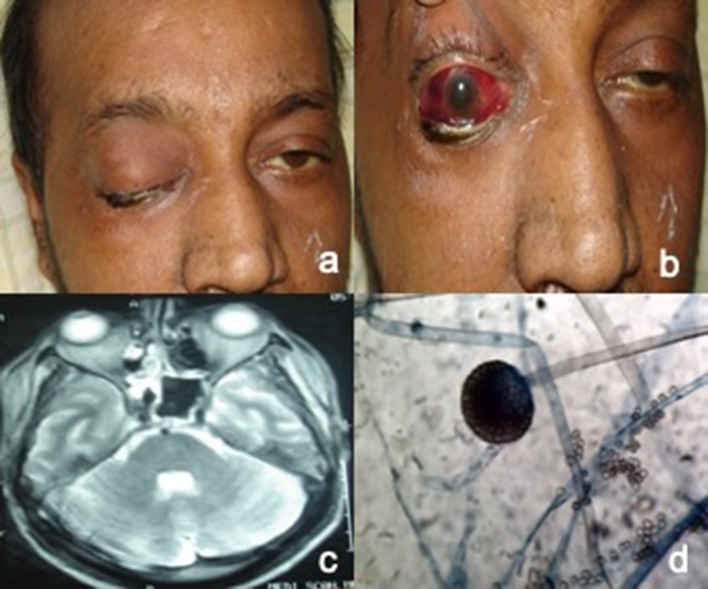
Fig. 2.
a, b Photograph of patient with right eye total ophthalmoplegia with subconjunctival hemorrhage and ecchymosis. c Axial MRI T2W image showing signs of ethmoid and sphenoid sinusitis with involvement of orbital apex. d Lactophenol cotton blue mount with rhizoids and sporangia
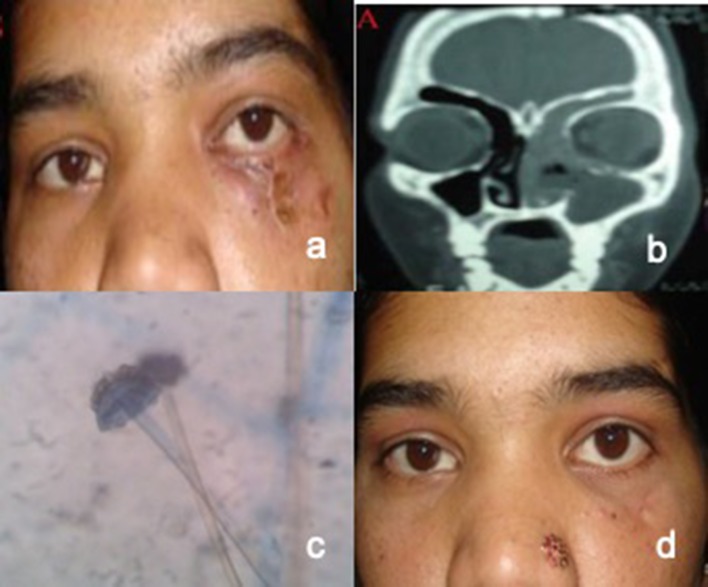
Fig. 3.
a Photograph of patient showing facial cellulitis with discharging sinus along inferior orbital margin and globe displacement on left side. b CT Coronal image showing opacification of frontal, ethmoid and maxillary sinus with erosion of floor and medial orbital wall on left side. c Lactophenol cotton blue mount with broad aseptate hyphae and sporangium d Photograph of the patient after treatment
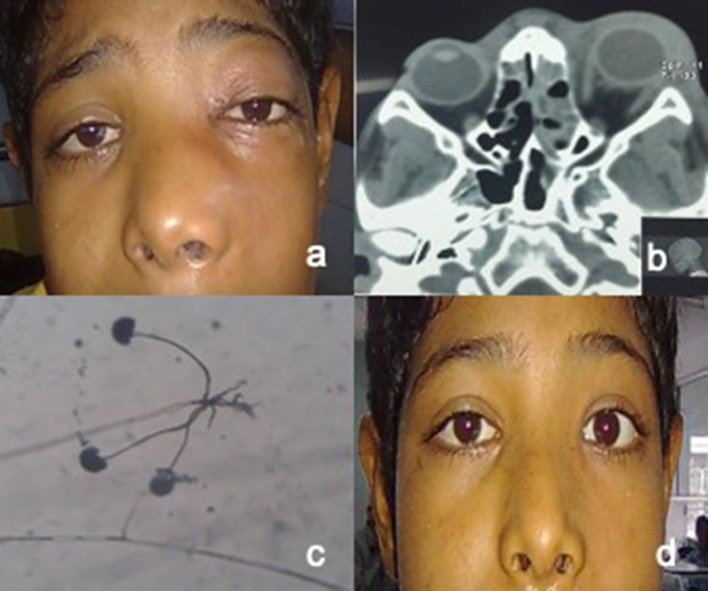
Fig. 4.
a Photograph showing proptosis with periorbital swelling in left eye. b Axial CT scan showing left sided periorbital and orbital cellulitis with opacification of ethmoid sinus. c Lactophenol cotton blue mount showing rhizoids and sporangia. d Photograph of the patient after recovery
CT scan/MRI, revealed signs of paranasal sinusitis with spread to orbital. The ethmoid (100%) and maxillary sinuses (55%) were most commonly involved followed by sphenoid (36%). One patient had frontal, ethmoid and maxillary sinusitis with osteomyelitis and discharging sinus along inferior orbital margin (Fig. 3a, b). Diffuse enhancement at orbital apex was seen in seven patients presenting with orbital apex syndrome (Figs. 1, 2).
Out of eleven, eight patients responded to systemic amphotericin B and sinus surgery. Vision did not improve in patients with central retinal artery occlusion and optic atrophy.
Three patients presented very late (8th, 11th and 13th day), had high blood sugar levels, developed ketoacidosis during treatment. One patient was on immunosuppressive therapy as well, for renal transplantation. These patients developed cerebral infarcts, and could not be saved.
The hospital stay of patients ranged from 12 to 47 days with average of 28.5 days. The mean duration of follow up was 13 months (range 6 months to 2 years).
Discussion
Mucormycosis is considered as an emergency owing to the rapidly aggressive and invasive nature of the fungus [16]. In India, the prevalence of mucormycosis is approximately 0.14 cases/1000 population [9]. It can present in various forms depending on the immunological status of the host. Rhino-orbital-cerebral mucormycosis (ROCM) is the most frequent (51.9%) presentation [7].
The infection begins with inhalation of the spores into the oral and nasal cavity. In persons with an intact immune system infection rarely develops because the fungal spores are phagocytized by macrophages. However, in individuals with uncontrolled diabetes mellitus and in immunocompromized patients with severe neutropenia, infection develops as their immune system is weak [17]. From here infection spreads to the paranasal sinuses and to orbit via ethamoid and maxillary sinuses or through nasolacrimal duct resulting in orbital cellutiltis [18]. The infection may extend posteriorly to the orbital apex, leading to orbital apex syndrome. The fungus may gain access to the cavernous sinus and to the brain parenchyma through cribriform plate, orbital apex or orbital vessels [18, 19].
In a review of 175 patients of sino–orbital mucormycosis, males were more commonly affected (68.5%) and mean age of 43 years. The common signs and symptoms in orbital involvement were periorbital swelling (27%), decreased vision (20%), ptosis (18%), and ophthalmoplegia (15%) [18]. In our study mean age was 46.8 years and males were more commonly affected (82%). The common presenting signs were ophthalmoplegia, proptosis, periorbital swelling and decreased vision. Four patients presented with orbital cellulitis and seven with orbital apex syndrome. Yohai et al. and Ferry et al. reported proptosis and ophthalmoplegia in the majority of their patients. Bhadada et al. reported six patients of ROCM with type I diabetes mellitus. Proptosis and ptosis were the most common symptoms [20].
Similar to our study, Jiang et al. reported a series of eleven patients with ROCM presenting with orbital apex syndrome [21].
In a comprehensive review of 929 cases of mucormycosis, diabetes (36%), hematological malignancy (17%) and solid organ or haemopoetic cell transplantation (12%) were the most common risk factors and ROCM was the most common clinical manifestation [17].
Mucormycosis is known to cause of central retinal occlusion (CRAO) [22]. Qingli et al. reported acute loss of vision due to CRAO and Bullock et al. in one of two cases reported acute obstruction of the retinal and choroidal circulations in orbital mucormycosis. Similarly in this study two patients had acute loss of vision due to central retinal artery occlusion.
Diagnosis of invasive rhino orbital mucormycosis is made by typical clinical presentation and by detection of broad aseptate hyphae with right-angled branching in KOH mount which is pathognomonic of mucormycosis [5]. In this study, broad aseptate fungal hyphae with right angled branching were demonstrated in all patients on KOH smear and culture and lacto phenol cotton blue stain. Histopathology of the biopsy will show narcotizing vacuities, with invasion of vessel wall with fungal hyphae. Veins are relatively spared [23]. Alternate techniques for tissue diagnosis include immunohistochemistry, polymerase chain reaction (PCR) for fungal DNA and in situ hybridization [10].
CT scan and MRI provides evidence for mucosal thickening, opacification of sinuses, orbital and intracranial involvement [24]. In this study, nine cases of rhino-orbital and three cases of rhino-orbital-cerebral mucormycosis were identified by these imaging modalities. In a study by Abdollahi et al. maxillary sinuses were the most frequently involved sinuses (66.7%) followed by the ethmoid [25]. In our study, ethmoid sinus was involved in all the cases (100%).
Early diagnosis, prompt initiation of systemic antifungal therapy, control of underlying systemic condition and aggressive radical debridement of sinuses is the key in improving outcomes and conservation of orbits, even in the presence of total ophthalmoplegia and CRAO [26]. Exenteration is however, not required in all cases of orbital mucormycosis [22]. Eight cases of sino-orbital mucormycosis were managed successfully without exenteration as reported by Kohn et al. All our patients received intravenous amphotericin B and transnasal endoscopic radical debridement of sinuses was performed early therefore none of the patient required exenteration.
Amphotericin B is the first-line drug for mucormycosis [8, 27]. It should be initiated as soon as the diagnosis is suspected. It is given in a dose of 1.0–1.5 mg/kg/day in dextrose 5% solution intravenously. The liposomal amphotericin B is more effective and less toxic facilitating prolonged administration without side effects [28, 29]. Due to financial constraints, our patients received the conventional form of amphotericin B.
Medical management alone with anti fungal is not effective because extensive vascular thrombosis and ischemic necrosis prevents entry of antifungal agents in adequate concentrations. Therefore radical debridement of infected and necrotic tissue, with drainage of infected paranasal sinuses should be performed early. It also minimizes the fungal load in the tissue [17].
Early commencement of treatment is related to better survival outcomes. Those presenting late with signs of intracranial involvement tend to have a poorer outcome [18]. Three patients presented very late (8th, 11th and 13th day) had high blood sugar levels and ketoacidosis. One patient was on immunosuppressive therapy for renal transplantation. These patients had intracranial spread, developed cerebral infarcts and could not be saved.
Mucorales have an affinity for acidic environments and possess ketone reductase enzyme that allows them to thrive in states of diabetic ketoacidosis [30]. Furthermore, these organisms survive through acquisition of iron from their hosts [16]. Therefore patients with diabetic ketoacidosis have poor survival rate.
Yohai et al. in a systematic review of 145 cases reported survival rate of 60%, following factors were associated with a lower survival rate: delayed diagnosis and treatment, hemiparesis or haemiplegia, bilateral sinus involvement, leukemia, renal disease and treatment with deferoxamine. In another review, Roden et al. reported survival rate 70% in those treated with both systemic amphotericin B and radical debridement.
This study showed a higher survival rate of 72.7%, as patients were diagnose early and treated very aggressively with intravenous amphotericin B and radical surgical debridement of sinuses was done. Similar survival rate (73.3%) was reported by Abdullah et al.
Conclusions
Invasive rhino-orbital-cerebral mucormycosis is a severe, fatal infection requiring multidisciplinary approach. Mucormycosis should be kept in mind when dealing with a case of orbital cellulitis or orbital apex syndrome with uncontrolled diabetes and immunodeficiency disorders.
Early diagnosis, urgent systemic antifungal therapy and sinus debridement surgery are of extreme importance for successful eradication of infection and patient survival.
Compliance with Ethical Standards
Conflicts of interest
All authors have declare that they have no conflict of interest.
Ethical Approval
The manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work.
Footnotes
The study was done at: People’s College of Medical Sciences and Research Centre, Bhopal, Madhya Pradesh, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Saroj Gupta, Email: gsaroj40@gmail.com.
Rashmi Goyal, Email: drrashmi03@gmail.com.
Navinchandra M. Kaore, Email: navinnmk@gmail.com
References
- 1.Yohai RA, Bullock JD, Aziz AA, Markert RJ. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol. 1994;39:3–22. doi: 10.1016/S0039-6257(05)80041-4. [DOI] [PubMed] [Google Scholar]
- 2.Uğurlu KS, Selim S, Kopar A, Songu M. Rhino-orbital mucormycosis: clinical findings and treatment outcomes of four cases. TJO. 2015;45(4):169–174. doi: 10.4274/tjo.82474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohn R, Hepler R. Management of limited rhino-orbital mucormycosis without exenteration. Ophthalmology. 1985;92:1440–1443. doi: 10.1016/S0161-6420(85)33844-7. [DOI] [PubMed] [Google Scholar]
- 4.Bala K, Chander J, Handa U, Singh R, Kumar A. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. 2015;53:248–257. doi: 10.1093/mmy/myu086. [DOI] [PubMed] [Google Scholar]
- 5.Ferry AP, Abedi S. Diagnosis and management of rhino-orbito-cerebral mucormycosis (phycomycosis): A report of 16 personally observed cases. Ophthalmology. 1983;90:1096–1104. doi: 10.1016/S0161-6420(83)80052-9. [DOI] [PubMed] [Google Scholar]
- 6.Grewal RK, Grewal SS, Zachariah RM. Orbital mucormycosis (phycomycosis) Indian J Ophthalmol. 1985;33:239–241. [PubMed] [Google Scholar]
- 7.Patel AK, Patel KK, Patel K, Gohel S, Chakrabarti A. Mucormycosis at a tertiary care centre in Gujarat. India Mycoses. 2017;60(6):407–411. doi: 10.1111/myc.12610. [DOI] [PubMed] [Google Scholar]
- 8.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, et al. Presentation and outcome of rhino orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80(949):670–674. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chander J, Kaur M, Singla N, Punia RPS, Singhal SK, Attri AK, et al. Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi. 2018;4(2):46. doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee B, Raichura ND, Alam MS. Fungal infections of the orbit. Indian J Ophthalmol. 2016;64:337–345. doi: 10.4103/0301-4738.185588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochi JW, Harris JP, Feldman JI, Press GA. Rhino cerebral mucormycosis: results of aggressive surgical debridement and amphotericin B. Laryngoscope. 1988;98:1339–1342. doi: 10.1288/00005537-198812000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Abedi E, Sismanis A, Choi K, Pastore P. Twenty-five years experience treating cerebro-rhino-orbital mucormycosis. Laryngoscope. 1984;94:1060–1062. doi: 10.1288/00005537-198408000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Pelton RW, Peterson EA, Patel BC, Davis K. Successful treatment of rhino-orbital mucormycosis without exenteration: The use of multiple treatment modalities. Ophthal Plast Reconstr Surg. 2001;17:62–66. doi: 10.1097/00002341-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Bullock JD, Jampol LM, Fezza AJ. Two cases of orbital phycomycosis with recovery. Am J Ophthalmol. 1974;78:811–815. doi: 10.1016/0002-9394(74)90305-5. [DOI] [PubMed] [Google Scholar]
- 15.Ferry AP. Discussion on limited rhino-orbital mucormycosis. Ophthalmology. 1985;92:1443–1444. doi: 10.1016/s0161-6420(85)33844-7. [DOI] [PubMed] [Google Scholar]
- 16.Binder U, Maurer E, Lass-Florl C. Mucormycosis- from the pathogens to the disease. Clin Microbiol Infect. 2014;20(Suppl. 6):60–66. doi: 10.1111/1469-0691.12566. [DOI] [PubMed] [Google Scholar]
- 17.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;5(41):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan C, Bartolo A, Vallabh N, Leong SC. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis-has anything changed in the past 20 years? Clin Otolaryngol. 2018;43(6):1454–1464. doi: 10.1111/coa.13175. [DOI] [PubMed] [Google Scholar]
- 19.Snaith J, Burns K, Kok J, Chen S, Cheung NW. A case of rhino-orbital mucormycosis in diabetes with haematogenous cerebral spread. Med Mycol Case Rep. 2016;13:22–24. doi: 10.1016/j.mmcr.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhadada S, Bhansali A, Reddy KS, Bhat RV, Khandelwal N, Gupta AK. Rhino-orbito-cerebral mucormycosis in type 1 diabetes mellitus. Indian J Pediatr. 2005;72(8):671–674. doi: 10.1007/BF02724075. [DOI] [PubMed] [Google Scholar]
- 21.Jiang N, Zhao G, Yang S, Lin J, Hu L, Che C, et al. A retrospective analysis of eleven cases of invasive rhino-orbito-cerebral mucormycosis presented with orbital apex syndrome initially. BMC Ophthalmol. 2016;16:10. doi: 10.1186/s12886-016-0189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qingli L, Orcutt JC, Seifter LS. Orbital mucormycosis with retinal and ciliary artery occlusions. Br J Ophthalmol. 1989;73:680–683. doi: 10.1136/bjo.73.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim AS, Kontoyiannis DP. Update on mucormycosis pathogenesis. Curr Opin Infect Dis. 2013;26:508–515. doi: 10.1097/QCO.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant P, Skilbeck CJ. Rhinocerebral mucormycosis: a devastating rhinological condition. Pract Diabetes. 2014;31(1):37–39. doi: 10.1002/pdi.1826. [DOI] [Google Scholar]
- 25.Abdollahi A, Shokohi T, Amirrajab N, Poormosa R, Kasiri AM, Motahari SJ, et al. Clinical features, diagnosis, and outcomes of rhino-orbito-cerebral mucormycosis—a retrospective analysis. Curr Med Mycol. 2016;2(4):15–23. doi: 10.18869/acadpub.cmm.2.4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson KL, Wang M, Canalis FR, Abemayor E. Rhinocerebral mucormycosis: evolution of the disease and treatment options. Laryngoscope. 1997;107:855–862. doi: 10.1097/00005537-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D’Souza O. Rhino cerebral mucormycosis. A retrospective analysis and treatment options. Indian J Ophthalmol. 2003;51:231–236. [PubMed] [Google Scholar]
- 28.Fisher EW, Toma A, Fisher PH, et al. Rhinocerebral mucormycosis: use of liposomal amphotericin B. J Laryngol Otol. 1991;105:575–577. doi: 10.1017/S0022215100116652. [DOI] [PubMed] [Google Scholar]
- 29.Ericsson M, Anniko M, Gustafsson H, Hjalt CA, Stenling R, Tarnvik A. Case of chronic progressive rhinocerebral mucormycosis treated with liposomal amphotericin B and surgery [letter] Clin Infect Dis. 1993;16:585–586. doi: 10.1093/clind/16.4.585. [DOI] [PubMed] [Google Scholar]
- 30.Spellberg B, Edwards J, John A Ibrahim. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]