Abstract
Improving artificial oocyte activation is essential for assisted reproduction or animal biotechnology that can obtain healthy offspring with a high success rate. Here, we examined whether intracytoplasmic injection of equine sperm-specific phospholipase C zeta (ePLCζ) mRNA, the PLCζ with the strongest oocyte activation potential in mammals, could improve the mouse oocyte activation rate and subsequent embryonic development using inactivated spermatozoa. mRNA of mouse PLCζ (mPLCζ) or ePLCζ were injected into mouse oocytes to determine the optimal mRNA concentration to maximize the oocyte activation rate and developmental rate of parthenogenetic embryos in vitro. Full-term development was examined using NaOH-treated inactive spermatozoa using the optimal activation method. We found that the most optimal ePLCζ mRNA concentration was 0.1 ng/µl for mouse oocyte activation, which was ten times stronger than mPLCζ mRNA. The concentration did not affect parthenogenetic embryo development in vitro. Relatively normal blastocysts were obtained with the same developmental rate (52–53% or 48–51%, respectively) when inactive spermatozoa were injected into activated oocytes using ePLCζ or mPLCζ mRNA injection. However, the birth rate after embryo transfer was slightly but significantly decreased in oocytes activated by ePLCζ mRNA (24%) compared to mPLCζ mRNA (37%) or strontium treatment (40%) activation. These results suggest that the higher activation rate does not always correlate the higher birth rate, and some mechanisms might exist in the oocyte activation process that could affect the later developmental stages like full-term development.
Keywords: Horse, Inactivated spermatozoa, Mouse, Oocytes activation, Sperm-specific phospholipase C zeta (PLCζ)
In the fields of assisted reproductive technology or animal reproductive biotechnology, oocytes are often activated artificially before or after injection of defective spermatozoa [1], round spermatids [2], or somatic cell nuclei [3]. However, artificially activated oocytes show poor embryonic development and a lower birth rate compared to natural fertilization with mature spermatozoa. The poor birth rate is thought to be due to defective sperm cell nuclei or the incomplete reprogramming of donor cell nuclei. However, it is also possible that poor development is caused by artificial activation of oocytes rather than a defect in sperm nuclei or somatic cells. If this is the case, then improving the activation method would improve the offspring birth success rate.
Suitable oocyte activation methods differ between species. While strontium chloride (SrCl2) is one of the most efficient activation methods for mouse oocytes [4, 5], electric pulses or calcium (Ca2+) ionophores are more efficient for porcine, bovine, or human oocytes [6,7,8]. However, the activated oocytes have a physiological reaction that differs from oocytes naturally activated by mature spermatozoa even when using an appropriate activation method. When normal spermatozoa penetrate the oocyte, the oocyte initiates repetitive increases in intracellular Ca2+ concentration (Ca2+ oscillations) and resumes its meiotic cell cycle to exit metaphase II (MII) [9, 10]. This process is triggered by a sperm factor known as sperm-specific phospholipase C zeta (PLCζ) [11]. On the other hand, the pattern of Ca2+ oscillation differs when oocytes are activated by electrostimulation or EtOH stimulation [12, 13]. Some oocytes undergo lysis during this treatment [3] when oocytes are activated by Sr, but a relatively similar Ca2+ oscillation occurs in them [9].
Purified PLCζ or PLCζ mRNA, which are closer to the natural activation factors, were used to activate oocytes. The activated oocytes showed Ca2+ oscillations similar to naturally fertilized oocytes [11, 14,15,16,17]. Healthy offspring was obtained when the activated oocytes were fertilized with mouse round spermatids; however, the birth rate was lower than that with the electro-stimulus or Sr activation methods [18, 19]. Thus, although PLCζ allows for a more natural activation process, the injection treatment remains very artificial. A high concentration of PLCζ mRNA injection can cause embryonic developmental arrest [14]. However, the blastocyst development and offspring success rate were increased when excessive PLCζ mRNA was degraded using the Auxin-inducible degron technology [20].
The Ca2+ oscillation-inducing activity of PLCζ is conserved among many species [16, 21], and spermatozoa from one species can activate the oocyte of a different species when artificially fertilized [22]. However, the spermatozoa activation efficiency varies between species. While sea urchin spermatozoa can activate mouse oocytes, more than five spermatozoa are required to activate one oocyte [23]. Importantly, oocytes activated by the sperm factor from a different species can support full-term development after fertilization with their own species’ spermatozoa or spermatids [19]. Recently, Miura et al. reported that mouse oocyte activation by human PLCζ (hPLCζ) mRNA injection using their optimized method followed by spermatid injection yielded live offspring at a higher success rate compared to mouse PLCζ (mPLCζ) mRNA injection [20]. This result suggests that using a different species’ PLCζ might increase the birth rate if it has a stronger potential for oocyte activation. Furthermore, if the strongest PLCζ mRNA is used at an appropriate mRNA concentration, the birth rate from a deficient sperm cell or somatic cell nuclei will be increased.
Here, we used equine PLCζ (ePLCζ) mRNA to activate mouse oocytes. Although the pattern of oscillation by ePLCζ differed compared to mPLCζ, previously shown to yield the highest potency for oocyte activation among all mammalian species studied to date [15]. First, we tried to determine the optimal concentration of mRNA for mouse oocytes activation and in vitro development of parthenogenetic embryos. We used this method to obtain mouse offspring from inactivated spermatozoa, and we compared the birth rate between mPLCζ, ePLCζ, SrCl2, and intact spermatozoa.
Materials and Methods
Animals
ICR and BDF1 (C57BL/6N × DBA/2) mice (8–10 weeks of age) were obtained from SLC (Hamamatsu, Japan). The surrogate pseudopregnant ICR females, used as embryo recipients, were mated with vasectomized ICR males that had been shown to be sterile. Mice were euthanized using CO2 inhalation or cervical dislocation on the day of the experiment or after completion of all experiments. All animal experiments followed the Guide for the Care and Use of Laboratory Animals, and the study was approved by the Institutional Committee of Laboratory Animal Experimentation of the University of Yamanashi.
Oocyte preparation
Female mice were superovulated by injecting 5 IU of equine chorionic gonadotropin followed by 5 IU of human chorionic gonadotropin after 48 h. Cumulus–oocyte complexes (COCs) were collected from the oviducts 14–16 h later and moved to a Falcon dish containing HEPES–CZB medium [24]. To disperse the cumulus, the COCs were transferred to a 50-µl droplet of HEPES–CZB medium containing 0.1% bovine testicular hyaluronidase for 3 min. The cumulus-free oocytes were washed twice and before moving to a 20-µl droplet of CZB medium [25] for culturing.
Plasmid construction and in vitro transcription for mouse and horse PLCζ mRNA synthesis
cDNA sequences encoding mPLCζ and ePLCζ cloned into pCS2 [16] were used as mRNA synthesis templates. mRNA was synthesized from the linearized template plasmids using in vitro transcription with a mMESSAGE MACHINE sp6 kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The synthesized mRNAs were precipitated using lithium chloride and dissolved in nuclease-free water. The concentration was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and aliquots (500 ng/µl) were stored at −80°C until use.
mRNA microinjection
mPLCζ mRNA was diluted with nuclease-free water to 0.1, 1, and 10 ng/µl before use. ePLCζ mRNA was diluted to 0.01, 0.1, and 1 ng/µl before use. Microinjection of mRNA into oocytes was done using a Piezo-driven micropipette (Prime Tech, Ibaraki, Japan) [26]. Briefly, microinjection was performed in HEPES-buffered CZB [27] on an inverted microscope (Olympus, Tokyo, Japan) with a micromanipulator (Narishige, Tokyo, Japan). The zona pellucida and cytosolic membrane were penetrated with a piezo drive. Each oocyte received ~10 pl mRNA of various concentrations. After mRNA injection, the oocytes were kept in HEPES-buffered CZB at room temperature for 10 min and before culturing in CZB medium. Control oocytes were activated using 5 mM SrCl2 in Ca2+-free CZB medium for 20 min before injecting inactivated sperm.
Diploidization of parthenogenetically activated oocyte
The parthenogenetically activated oocytes were diploidized by adding 5 μg/ml cytochalasin B (Calbiochem) to the culture medium for 6 h after mRNA injection. After washing, the cells were further cultured in CZB medium for 90 h.
Spermatozoa collection and inactivation using NaOH
Epididymides were removed from male ICR mice. After both epididymal ducts were cut with sharp scissors, a few drops of the dense sperm mass was placed into an Eppendorf tube with 100 μl of HTF medium [28] and incubated for 30 min at 37°C. We used the method described previously to NaOH treat spermatozoa [29]. The sperm suspension (10 μl) was mixed 1:10 with 10 mM NaOH solution (Wako Pure Chemical, Osaka, Japan) in Eppendorf tubes and placed at room temperature for 30 min. The suspension was neutralized using the same HCl concentration (Wako) to a final pH value of 7.3. The treated spermatozoa were kept on ice until ICSI use.
Intracytoplasmic spermatozoa injection, in vitro culture, and embryo transfer
Approximately 1 μl of sperm suspension was mixed with 5–10 μl drop of HEPES–CZB containing 10% (w/v) polyvinylpyrrolidone (Irvine Scientific, Santa Ana, CA, USA) to inject NaOH treated inactive spermatozoa. The sperm head was separated from the tail using several Piezo pulses, and the head was injected into the oocyte according to the method described by Kimura and Yanagimachi [24]. The oocytes that survived ICSI were incubated in CZB medium at 37°C in 5% CO2. Pronucleus formation was checked 6 h after ICSI. Embryos at the two-cell stage were transferred to a day 0.5 pseudopregnant mouse that had been mated with a vasectomized male the night before transfer. Six to ten embryos were transferred into each oviduct. The offspring was delivered by cesarean section at day 18.5 of gestation. The remaining unused embryos were cultured for up to four days to evaluate their potential development to the blastocyst stage.
Blastocyst immunostaining
Blastocyste immunofluorescence staining was performed as described to evaluate the quality of blastocysts derived from mPLCζ or ePLCζ mRNA injected embryos [30]. The primary antibodies used were an anti-CDX2 rabbit monoclonal antibody (1:500; BioGenex, California, CA, USA) to detect the TE cells and an anti-NANOG mouse polyclonal antibody (1:500; Abcam, Cambridge, UK) to detect the ICM cells. The secondary antibodies used were Alexa Fluor 568-labeled goat anti-rabbit IgG (1:500; Thermo Fisher Scientific, Massachusetts, USA) and Alexa Fluor 488-labeled goat anti-mouse IgG (1:500; Thermo). DNA was stained with DAPI (2 µg/ml; Molecular Probes, Oregon, USA).
Statistical analysis
The blastocyst formation rate and offspring development rate were evaluated using Chi-squared tests. P < 0.05 was considered to represent a statistically significant difference.
Results
Optimal concentration of mPLCζ or ePLCζ mRNA for mouse oocyte activation and parthenogenetic embryo development
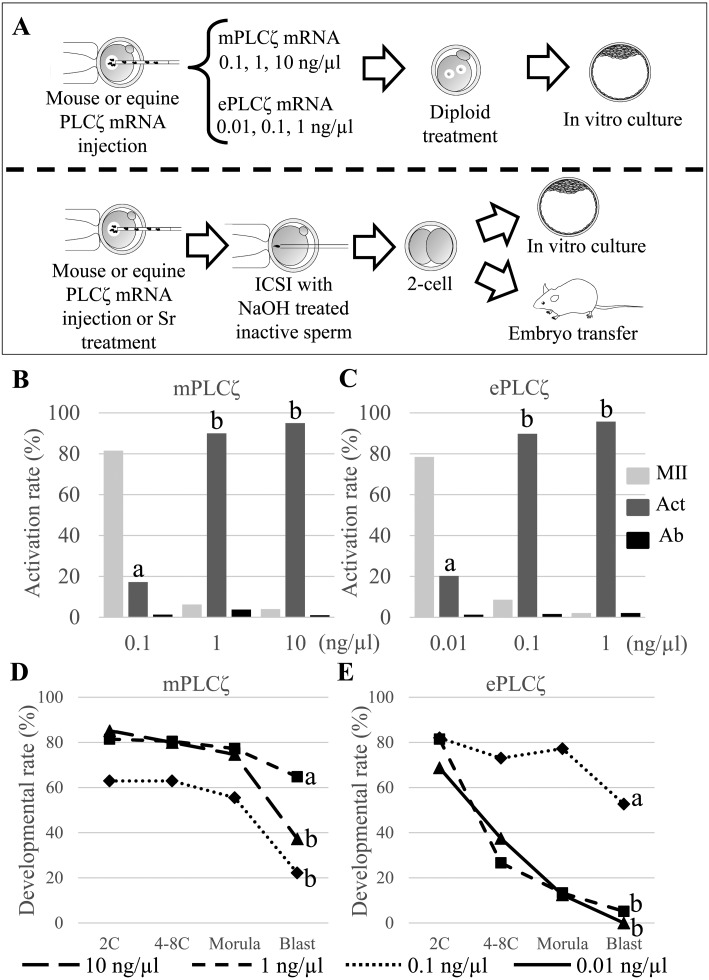
First, 0.1, 1, and 10 ng/µl of mPLCζ mRNA or 0.01, 0.1, and 1 ng/µl of ePLCζ mRNA were injected into mouse oocytes to examine the optimal concentration of mPLCζ or ePLCζ mRNA for mouse oocyte activation (Fig. 1A). The oocytes were moved into CB containing CZB medium approximately 20 min after mRNA injection to inhibit the second polar body extrusion. The oocyte activation rate was determined based on pronuclear formation 6 h after mRNA injection. Activated oocytes were cultured for 4 days to examine parthenogenetic blastocyst development. Only 18.5% of oocytes were activated when 0.1 ng/µl of mPLCζ mRNA was injected, and 93.8% or 96.0% of oocytes were activated with two pronuclei when 1 or 10 ng/µl of mRNA were injected, respectively (Fig. 1B). On the other hand, only 21.5% of oocytes were activated when 0.01 ng/µl of ePLCζ mRNA was injected into mouse oocytes. 88.5% or 97.9% of oocytes were activated with two pronuclei when 0.1 or 1 ng/µl of mRNA were injected, respectively (Fig. 1C).
Fig. 1.
Activation and in vitro development of mouse oocytes injected with mouse PLCζ (mPLCζ) or equine PLCζ (ePLCζ) mRNA. (A) Upper; Schematic representation of injection of different concentrations of mPLCζ or ePLCζ mRNA into mouse oocytes. The oocytes were treated with cytochalasin B for 6 h to induce diploid parthenogenesis. Lower: NaOH-treated inactive spermatozoa were injected into oocytes activated by mPLCζ or ePLCζ mRNA injection before culturing in vitro for four days or transferring into a recipient female at the 2-cell stage. (B, C) Rates of activated oocytes after injection of different concentration of mPLCζ or ePLCζ mRNA. The oocytes were injected with 0.1, 1, and 10 ng/µl of mPLCζ mRNA (B), or 0.01, 0.1, and 1 ng/µl of ePLCζ mRNA (C). Experiments were repeated more than six times for each concentration. (D, E) Rates of parthenogenetic embryo development in vitro at different concentrations of mPLCζ or ePLCζ mRNA. Embryos were observed at 24 h (2-cell stage), 48 h (4–8-cell stage), 72 h (morula stage), and 96 h (blastocyst stage). The “a” vs. “b” denotes significant differences between experiments (P < 0.01).
Parthenogenetically activated oocytes were cultured for four days to examine the developmental potential in vitro. In the mPLCζ mRNA experiment, oocytes activated by 0.1 ng/µl mPLCζ mRNA injection showed lower developmental potential in all stages, and only 22.2% of embryo developed to blastocyst. When oocytes were activated by 1 or 10 ng/µl of mPLCζ mRNA, the zygotes showed a higher developmental rate until the morula stage. However, the blastocyst rate was reduced when 10 ng/µl of mRNA was used for activation (Fig. 1D). In the ePLCζ mRNA experiment, the oocytes injected with the lowest (0.01 ng/µl) or the highest (1 ng/µl) mRNA concentration showed lower developmental potential at each stage, and only a few or no embryos reached the blastocyst stage (5% and 0%, respectively). However, 77.2% of embryos developed to the morula stage when 0.1 ng/µl mRNA was injected, and 52.7% reached the blastocyst stage (Fig. 1E).
Based on these results, 1 ng/µl of mPLCζ mRNA and 0.1 ng/µl of ePLCζ mRNA are the optimal concentrations for both oocyte activation and embryo development in vitro.
Optimal timing for inactive sperm injection after mPLCζ or ePLCζ mRNA injection into oocytes
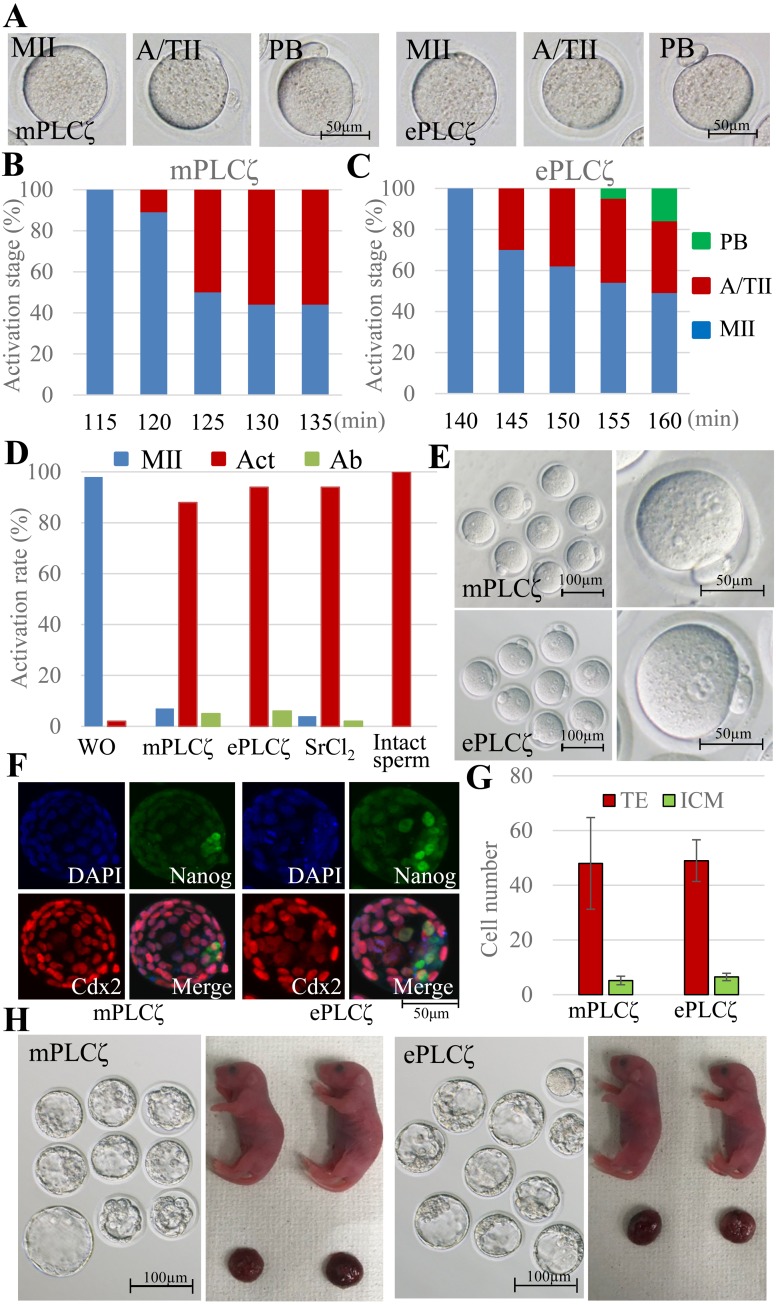
Next, we found the optimal timing for inactive sperm injection into the activated oocytes. The windows for fertilization between pre-activated oocytes and spermatozoa are very narrow and strict [31]. The mPLCζ mRNA or ePLCζ mRNA injected oocytes were observed from 115 to 135 min or 140 to 160 min after injection, respectively. Those oocytes were classified into MII (not activated), anaphase/telophase II (A/TII: activation started), or polar body extrusion (PB) (Fig. 2A). A/TII is an obvious sign of oocyte activation, and it is easy to observe without staining. As shown in Fig. 2B and 2C, A/TII was observed starting at 120 or 145 min after mPLCζ or ePLCζ mRNA injection, respectively. Therefore, we injected the inactive spermatozoa at these times after PLCζ mRNA injection into mouse oocytes.
Fig. 2.
Activation and full term development of mouse oocytes injected with mouse PLCζ (mPLCζ) or equine PLCζ (ePLCζ) mRNA. (A–C) Morphology and resumption of meiosis in mPLCζ or ePLCζ mRNA-injected oocytes. Oocytes were observed every 5 min from 115 min to 135 min or 140 to 160 min, respectively. (A) Morphology of oocytes. (B, C) Rates of each stage of meiosis II in mPLCζ or ePLCζ mRNA-injected oocytes, respectively. MII, metaphase II; A/TII, anaphase /telophase II; PB, extrusion of second polar body. (D, E) Activation and pronuclear formation of zygote fertilized with inactivated spermatozoa. The oocytes were activated by mPLCζ or ePLCζ mRNA injection or SrCl2 treatment. In the control, intact oocytes were injected with intact or inactivated spermatozoa. (F, G) Immunostaining of blastocysts activated with mPLCζ or ePLCζ mRNA injection. (F) CDX2+ cells (trophectoderm; TE) are shown in red, and Nanog+ cells (inner cell mass; ICM) are in green. (G) The average number of TE and ICM in blastocysts. (H) Development of embryo to blastocyst and full-term. The embryos were activated by mPLCζ or ePLCζ mRNA injection and fertilized with inactivated spermatozoa.
In vitro and in vivo development of embryos generated by inactive sperm injection into oocytes activated by mouse and horse PLCζ mRNA injection
We obtained offspring using NaOH-treated inactive spermatozoa. The mouse oocytes were activated using mPLCζ or ePLCζ mRNA injection or SrCl2 treatment. As controls, some oocytes were not activated and injected with intact or inactivated spermatozoa. In the positive control experiment, 95% of oocytes were activated 6 h after intact spermatozoa were injected into intact oocytes. Most oocytes remained at the MII stage with two metaphase spindles when inactivated sperm were injected into intact oocytes (data not shown). When oocytes injected with mPLCζ or ePLCζ mRNA were examined, 94% and 88% of oocytes showed two pronuclei, respectively (Fig. 2D, E).
Next, we performed the same experiment after activated embryos were cultured for 96 h in vitro. As shown in Table 1, 51% and 53% of mPLCζ or ePLCζ mRNA injected ICR mouse oocytes developed to blastocyst, respectively. Although there was no significant difference, those proportions were slightly higher than oocytes activated by Sr treatment (40%) and lower than oocytes activated with intact sperm injection (65%). Similar results were obtained when BDF1 oocytes were used. We examined the quality of those blastocysts derived from mPLCζ or ePLCζ activated oocytes using immunostaining of Nanog or Cdx2-expressing blastomeres (Fig. 2F). As shown in Fig. 2G, the cell ratio of ICM and TE of blastocyst were similar irrespective of activation methods.
Table 1. Preimplantation development of embryos fertilized with NaOH-treated inactive spermatozoa and activated by mouse PLCζ (mPLCζ) or equine PLCζ (ePLCζ) mRNA injection, or SrCl2 treatment.
| Mouse strain | Oocyte activation method | Sperm treatment | No. of fertilized embryos |
No. of 2-cell embryos at 24 h (%) |
No. of 4–8-cell embryos at 48 h (%) |
No. of morula/ blastocysts at 72 h (%) |
No. of expanded blastocysts at 96 h (%) * |
|---|---|---|---|---|---|---|---|
| ICR | – | intact | 57 | 56 (98) | 48 (84) | 47 (82) | 37 (65) a |
| mPLCζ | NaOH | 84 | 73 (87) | 68 (81) | 66 (79) | 43 (51) | |
| ePLCζ | NaOH | 60 | 58 (97) | 55 (92) | 53 (88) | 32 (53) | |
| SrCl2 | NaOH | 85 | 81 (95) | 65 (76) | 61 (72) | 34 (40) b | |
| BDF1 | – | intact | 26 | 25 (96) | 24 (92) | 24 (92) | 19 (73) a |
| mPLCζ | NaOH | 52 | 45 (87) | 37 (71) | 36 (69) | 25 (48) b | |
| ePLCζ | NaOH | 48 | 43 (90) | 38 (79) | 36 (75) | 25 (52) b | |
| SrCl2 | NaOH | 32 | 25 (78) | 24 (75) | 24 (75) | 14 (44) b | |
* a. vs. b; P < 0.05.
Finally, some of the embryos that developed to the two-cell stage were transferred into recipient females. As shown in Table 2, although healthy offspring were obtained from activated oocytes using either mPLCζ or ePLCζ mRNA injection (Fig. 2H), the success rate derived from the ePLCζ activated oocyte was 24%. This was significantly lower than the rate derived from any other group (intact sperm: 46%, mPLCζ activated: 37%, SrCl2 activated: 40%).
Table 2. Full term development of embryos fertilized with NaOH treated inactive spermatozoa activated by mouse PLCζ (mPLCζ) or equine PLCζ (ePLCζ) mRNA injection, or SrCl2 treatment.
| Oocyte activation method | Sperm treatment | No. of fertilized embryos | No. (%) of 2-cell embryos at 24 h | No. of transferred embryos | No. (%) of offspring * |
|---|---|---|---|---|---|
| – | Intact | 184 | 176 (96) | 165 | 76 (46) a |
| mPLCζ | NaOH | 217 | 156 (72) | 116 | 43 (37) a |
| ePLCζ | NaOH | 204 | 187 (92) | 173 | 41 (24) b |
| SrCl2 | NaOH | 145 | 119 (82) | 114 | 46 (40) a |
* a. vs. b; P < 0.05.
Discussion
Here, we obtained mouse offspring from oocytes activated by injecting a different species’ sperm factor, ePLCζ mRNA, along with NaOH-treated inactive spermatozoa.
Previously, live offspring were obtained from spermatids with oocytes activated by either mPLCζ or hPLCζ mRNA injection [20]. Those results demonstrated that the birth rate increases when using PLCζ from a different species compared to PLCζ from the same species when an appropriate PLCζ mRNA injection method is established, with electro-stimulus, or with Sr stimulation methods [4, 17]. However, the birth success rate is still lower than zygotes fertilized with normal spermatozoa even with the appropriate method for oocyte activation with PLCζ. The lower birth rate seems inevitable when fertilization is attempted with round spermatids due to unknown or epigenetic reasons. This makes it difficult to evaluate the real potential of PLCζ for oocyte activation. To overcome these confounding parameters, we used mature spermatozoa treated with NaOH to remove the activation factors from the cell surface but preserve development potential after fertilization [29].
In the first series of experiments, we detected the ePLCζ mRNA concentration appropriate for both oocyte activation and parthenogenetic embryo development. ePLCζ is approximately ten times more potent at activating oocytes compared to mPLCζ, and it retains the in vitro developmental potential. However, we found that excessive ePLCζ mRNA is harmful to the embryonic development during the blastocyst stage; this is consistent with previous studies [15, 20]. The appropriate timing for ICSI after mRNA injection was studied, because the spermatozoa injection timing into pre-activated oocytes is crucial for fertility [31]. In addition, the injected mRNA first needed to be translated into PLCζ to start the oocyte activation process. This caused a delay compared to the normal fertilization process. We clarified the delay, finding that ePLCζ activation of injected oocytes was delayed by approximately 20 min compared to mPLCζ injected oocytes. The delay was likely caused by the ten times lower concentration of ePLCζ mRNA compared to mPLCζ mRNA. This ten times lower concentration of ePLCζ mRNA injected could still efficiently activate mouse oocytes with only a slight delay. This also suggests that ePLCζ mRNA more efficiently activates mouse oocytes compared to mPLCζ mRNA .
Finally, we used the above settings to examine the effect of PLCζ on embryo development in vitro and in vivo. Previously, recombinant human PLCζ or porcine PLCζ mRNAs were injected into mouse oocytes before injecting heat inactivated spermatozoa or round spermatids, respectively [17, 32]. Although both reports did not examine the full-term development, blastocysts were obtained without a reduction developmental potential in vitro. In contrast, when mouse oocytes were activated by hamster sperm factors or mouse PLCζ mRNA injection and fertilized with round spermatid, the embryo developed to the blastocyst stage normally and healthy offspring were obtained; however, the birth rate was decreased compared to electro-stimulus or Sr activation methods [18, 19]. Similarly, we show here that the developmental rate to blastocysts derived from ePLCζ mRNA injected oocytes was comparable to the control when the optimal ePLCζ mRNA concentration was used. The quality of blastocysts was also similar between oocytes activated by ePLCζ and mPLCζ mRNA injection. However, the full-term development after inactive sperm injection was significantly decreased in ePLCζ mRNA injected oocytes compared to all other groups. This suggests that the development to the blastocyst stage is not an accurate indicator of developmental competence. For example, parthenogenetic embryos or cloned embryos can easily develop to blastocysts; however, they never or rarely develop to full-term, respectively [3, 33, 34]. Ozil et al. reported that a change in Ca2+ signaling events following fertilization (an excess or reduction) has effects on gene expression and offspring development [35]. Cox et al. reported that a high concentration of PLCζ mRNA injection could cause embryonic developmental arrest [14]. Excessive PLCζ mRNA is likely degraded to yield the high success rate of embryo development like using Auxin-inducible degron technology [20]. Thus, while ePLCζ has a strong potential to initiate mouse oocyte activation, the higher activation rate does not always correlate to higher birth rate. Some unknown mechanisms might exist in the oocyte activation process that affect the later stages of development, like full-term development.
Acknowledgments
This work was partially funded by the Japan Society for the Promotion of Science to SK (26450458), JI (15H04584), MO (17K15394), and TW (16H02593); the Naito Foundation to SW; Asada Science Foundation to TW; the Takeda Science Foundation to TW, and the international scientific partnership program (ISPP) at King Saud university through ISPP- 135.
References
- 1.Chao SB, Guo L, Ou XH, Luo SM, Wang ZB, Schatten H, Gao GL, Sun QY. Heated spermatozoa: effects on embryonic development and epigenetics. Hum Reprod 2012; 27: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 2.Ogura A, Matsuda J, Yanagimachi R. Birth of normal young after electrofusion of mouse oocytes with round spermatids. Proc Natl Acad Sci USA 1994; 91: 7460–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394: 369–374. [DOI] [PubMed] [Google Scholar]
- 4.Ogura A, Matsuda J, Asano T, Suzuki O, Yanagimachi R. Mouse oocytes injected with cryopreserved round spermatids can develop into normal offspring. J Assist Reprod Genet 1996; 13: 431–434. [DOI] [PubMed] [Google Scholar]
- 5.Sasagawa I, Yanagimachi R. Comparison of methods for activating mouse oocytes for spermatid nucleus transfer. Zygote 1996; 4: 269–274. [DOI] [PubMed] [Google Scholar]
- 6.Yanagida K, Fujikura Y, Katayose H. The present status of artificial oocyte activation in assisted reproductive technology. Reprod Med Biol 2008; 7: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de León FA, Robl JM. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science 1998; 280: 1256–1258. [DOI] [PubMed] [Google Scholar]
- 8.Miyagawa S, Matsunari H, Watanabe M, Nakano K, Umeyama K, Sakai R, Takayanagi S, Takeishi T, Fukuda T, Yashima S, Maeda A, Eguchi H, Okuyama H, Nagaya M, Nagashima H. Generation of α1,3-galactosyltransferase and cytidine monophospho-N-acetylneuraminic acid hydroxylase gene double-knockout pigs. J Reprod Dev 2015; 61: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol 1992; 149: 80–89. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol 1993; 158: 62–78. [DOI] [PubMed] [Google Scholar]
- 11.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 2002; 129: 3533–3544. [DOI] [PubMed] [Google Scholar]
- 12.Cuthbertson KS, Whittingham DG, Cobbold PH. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature 1981; 294: 754–757. [DOI] [PubMed] [Google Scholar]
- 13.Swann K, Ozil JP. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol 1994; 152: 183–222. [DOI] [PubMed] [Google Scholar]
- 14.Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 2002; 124: 611–623. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Wakai T, Seita Y, Takizawa A, Fissore RA, Ito J, Kashiwazaki N. Molecular characteristics of horse phospholipase C zeta (PLCζ). Anim Sci J 2013; 84: 359–368. [DOI] [PubMed] [Google Scholar]
- 16.Ito J, Parrington J, Fissore RA. PLCζ and its role as a trigger of development in vertebrates. Mol Reprod Dev 2011; 78: 846–853. [DOI] [PubMed] [Google Scholar]
- 17.Yoneda A, Watanabe T. Involvement of mouse and porcine PLCζ-induced calcium oscillations in preimplantation development of mouse embryos. Biochem Biophys Res Commun 2015; 460: 476–481. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi T, Ishibashi N, Kubota H, Inoue K, Ogonuki N, Ogura A, Kashiwabara S, Baba T. Birth of normal offspring from mouse eggs activated by a phospholipase Czeta protein lacking three EF-hand domains. J Reprod Dev 2008; 54: 244–249. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai A, Oda S, Kuwabara Y, Miyazaki S. Fertilization, embryonic development, and offspring from mouse eggs injected with round spermatids combined with Ca2+ oscillation-inducing sperm factor. Mol Hum Reprod 1999; 5: 132–138. [DOI] [PubMed] [Google Scholar]
- 20.Miura K, Matoba S, Ogonuki N, Namiki T, Ito J, Kashiwazaki N, Ogura A. Application of auxin-inducible degron technology to mouse oocyte activation with PLCζ. J Reprod Dev 2018; 64: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod 2008; 78: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 22.Maleszewski M, Kline D, Yanagimachi R. Activation of hamster zona-free oocytes by homologous and heterologous spermatozoa. J Reprod Fertil 1995; 105: 99–107. [DOI] [PubMed] [Google Scholar]
- 23.Wakayama T, Uehara T, Hayashi Y, Yanagimachi R. The response of mouse oocytes injected with sea urchin spermatozoa. Zygote 1997; 5: 229–234. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod 1995; 52: 709–720. [DOI] [PubMed] [Google Scholar]
- 25.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679–688. [DOI] [PubMed] [Google Scholar]
- 26.Yamagata K, Suetsugu R, Wakayama T. Long-term, six-dimensional live-cell imaging for the mouse preimplantation embryo that does not affect full-term development. J Reprod Dev 2009; 55: 343–350. [DOI] [PubMed] [Google Scholar]
- 27.Chatot CL, Lewis JL, Torres I, Ziomek CA. Development of 1-cell embryos from different strains of mice in CZB medium. Biol Reprod 1990; 42: 432–440. [DOI] [PubMed] [Google Scholar]
- 28.Quinn P. Enhanced results in mouse and human embryo culture using a modified human tubal fluid medium lacking glucose and phosphate. J Assist Reprod Genet 1995; 12: 97–105. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Mizutani E, Ono T, Wakayama T. Production of normal mice from spermatozoa denatured with high alkali treatment before ICSI. Reproduction 2009; 137: 779–792. [DOI] [PubMed] [Google Scholar]
- 30.Wakayama S, Kamada Y, Yamanaka K, Kohda T, Suzuki H, Shimazu T, Tada MN, Osada I, Nagamatsu A, Kamimura S, Nagatomo H, Mizutani E, Ishino F, Yano S, Wakayama T. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc Natl Acad Sci USA 2017; 114: 5988–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishigami S, Wakayama S, Nguyen VT, Wakayama T. Similar time restriction for intracytoplasmic sperm injection and round spermatid injection into activated oocytes for efficient offspring production. Biol Reprod 2004; 70: 1863–1869. [DOI] [PubMed] [Google Scholar]
- 32.Sanusi R, Yu Y, Nomikos M, Lai FA, Swann K. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ. Mol Hum Reprod 2015; 21: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hikichi T, Ohta H, Wakayama S, Wakayama T. Functional full-term placentas formed from parthenogenetic embryos using serial nuclear transfer. Development 2010; 137: 2841–2847. [DOI] [PubMed] [Google Scholar]
- 34.Barton SC, Surani MA, Norris ML. Role of paternal and maternal genomes in mouse development. Nature 1984; 311: 374–376. [DOI] [PubMed] [Google Scholar]
- 35.Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol 2006; 300: 534–544. [DOI] [PubMed] [Google Scholar]