Abstract
The incidences of diabetic mellitus and other metabolic diseases such as hypertension and hyperlipidemia are increasing worldwide; however, the current treatment is not able to control the rapidly increasing trend in diabetes mortality and morbidity. Studies related to the effectiveness of extracts and pure compounds obtained from plants have shown promising responses in preclinical and clinical studies related to these metabolic diseases. Plants belonging to the genus Berberis (Family: Berberidaceae) are widely distributed with nearly 550 species worldwide. Extracts and compounds obtained from Berberis species, especially Berberine alkaloid, showed effectiveness in the management of diabetes and other metabolic diseases. Various pharmacological experiments have been performed to evaluate the effects of Berberis extracts, berberine, and its natural and chemically synthesized derivatives against various cell and animal disease models with promising results. Various clinical trials conducted so far also showed preventive effects of Berberis extracts and berberine against metabolic diseases. The present review focuses on i) research updates on traditional uses, ii) phytopharmacology and clinical studies on Berberis species, and iii) active metabolites in the prevention and treatment of diabetes and other metabolic diseases with a detailed mechanism of action. Furthermore, the review critically analyzes current research gaps in the therapeutic use of Berberis species and berberine and provides future recommendations.
Keywords: Berberis, berberine, diabetes, metabolic diseases, pharmacology, clinical studies
Introduction
Diabetes mellitus (DM) is a metabolic disorder that is characterized by an abnormal long-term increase in plasma glucose levels. Diabetes is mainly classified into four types, i.e., type I diabetes (T1DM), type II diabetes (T2DM), gestational diabetes, and specific types of diabetes due to other causes (American Diabetes Association, 2019). Many factors, such as insulin deficiency or resistance as well as altered carbohydrate, protein, and fat metabolisms, are usually the reasons for high blood glucose levels leading to DM. Chronic hyperglycemia related to diabetes is often associated with many other complications, such as cardiovascular, dermatological, neurological, renal, retinal, and nerve diseases. Diabetes is one of the most common chronic disease, and it has shown an increasing rate of occurrence over the past decade (Bullard et al., 2018). According to the World Health Organization (WHO), the total number of people with diabetes worldwide substantially increased from 108 million in 1980 to 422 million in 2014 (World Health Organization, 2016). Along with diabetes, the incidence of other metabolic diseases, such as hyperlipidemia, is also increasing rapidly (Karr, 2017).
Metabolic syndrome (MS) is associated with a group of disease conditions that occur together, and it is composed of central adiposity, hyperglycemia, hypertriglyceridemia, low high-density lipoproteins (HDL)-cholesterol, and hypertension. This disease cluster of diabetes and cardiovascular diseases is also known as “The Deadly Quartet”, “Syndrome X”, and “The Insulin Resistance Syndrome” (Alberti, 2005). Various treatment options are available to mitigate MS, including the diabetic condition and related disorders (Deedwania and Volkova, 2005). As MS is manifested by the cluster of diseases, use of a single drug candidate might not be able to provide necessary therapeutic effects. Plant extracts and isolated compounds can be possible options as adjuvants in such cases. Traditionally, various medicinal plants and their products (extracts and isolated compounds) have been used in the treatment of diabetes and hypertension (Oyedemi et al., 2009; Tabassum and Ahmad, 2011; Rizvi and Mishra, 2013; Ezuruike and Prieto, 2014). Various research showed the protective/curative effect of plant extracts as a whole and/or an individual bioactive compound against diabetes and other metabolic diseases (Tabatabaei-Malazy et al., 2015; Waltenberger et al., 2016).
Plants belonging to the genus Berberis (Family: Berberidaceae) are widely distributed worldwide with nearly 550 species. A decoction prepared from the roots of Berberis plants is one of the common traditional recipes for the treatment of diabetes (Neag et al., 2018). Various studies have reported the traditional uses Berberis plants for the treatment of metabolic diseases (e.g., diabetes and hyperlipidemia) in many countries, including India, Pakistan, China, and Iran (Hamayun et al., 2006; Uniyal et al., 2006; Rahimi Madiseh et al., 2014; Rana et al., 2019). Various bioactive compounds, such as alkaloids, polyphenols, flavonoids, anthocyanins, etc., have been found in Berberis species along with various vitamins and mineral components (Andola et al., 2010; Srivastava et al., 2015; Belwal et al., 2016; Belwal et al., 2017). Berberine (BBR), a quaternary ammonium salt belonging to a group of benzylisoquinoline alkaloids, is the most active compound reported from Berberis species, and it is considered to be highly effective against diabetes and other metabolic diseases (Dong et al., 2012; Lan et al., 2015; Wang H. et al., 2018). BBR is also distributed in various plant species of other genera such as Coptis, Hydrastis, Mahonia, Tinospora, Xanthorhiza, and many others (Neag et al., 2018). In the genus Berberis, the distribution of BBR and other alkaloids is mostly in its root part, followed by the stem bark and the stem itself (Andola et al., 2010). In addition, its presence in trace amounts has been reported from leaves and berries. Various studies have been conducted to evaluate the effectiveness of Berberis extract or bioactive alkaloidal compounds against diabetes and other MS with promising results (Gulfraz et al., 2008; Meliani et al., 2011; Imenshahidi and Hosseinzadeh, 2016; Mirhadi et al., 2018). Moreover, various clinical trials were also conducted on testing their effectiveness against diabetes and other metabolic diseases and showed variable effects (Zhang et al., 2010; Pérez-Rubio et al., 2013).
Considering the Berberis species and their active alkaloidal components, the present review specifically focuses on their effectiveness against diabetes and other metabolic diseases. This review discusses various traditional uses of Berberis against metabolic diseases, along with its cell- and animal-model studies. The pharmacological effects of Berberis extracts and alkaloids against diabetes and other metabolic diseases are also discussed along with the molecular mechanism of action. Furthermore, based on the present studies of Berberis species against diabetes and metabolic diseases, research gaps were highlighted, and future recommendations were made.
Methodology
The scattered scientific information on Berberis species and isolated compounds used to counteract metabolic diseases was collected and documented. The synonyms of the various species were crosschecked with the plant name database The Plant List (www.theplantlist.org, Retrieved on November 22, 2019). Afterwards, the available articles on respective species were retrieved using popular search engines and various databases, such as SciFinder, ScienceDirect, PubMed, Scopus, Mendeley, JOAP, Microsoft academic, and Google Scholar. The keywords used were Berberis, berberine, diabetes, metabolic diseases, metabolic syndrome, ethnopharmacology, ethnobotany, chemical constituents, alkaloids, in vitro, in vivo, clinical study, and clinical trials. The data were congregated through the Boolean information retrieval method by using a plant name along with an “AND” operator followed by diabetes and metabolic syndrome. No prerequisite limitations on publications, i.e., language, year, and publication type (original contribution, review article, or key editorial note), were taken into consideration.
Taxonomy and Ecology of Genus Berberis
According to The Plant List database (www.theplantlist.org, retrieved on September 20, 2019), the family Berberidaceae consists of a total of 19 genera. The members of the genus Berberis are reported to be difficult to identify taxonomically due to their extreme morphological variation in relation to the environmental factors and natural hybridization (Ahrendt, 1961; Rao et al., 1998). Various overlapping morphological characters, such as flowers, leaves, stems, and berries—which also depend upon the season—and plant age also make it difficult to identify during field tasks (Rao and Hajra, 1993; Rao et al., 1998; Tiwari and Singh Adhikari, 2011). Berberis species are widely cultivated around the world due to their high medicinal and ornamental value. Most members of the genus Berberis are reported to be tolerant to shade, resistant to drought, and widely distributed in open and wooded habitats and wetlands. These plants are also studied as indicators of habitat degradation in the temperate region due traditionally to their thorny stem and unpalatable shoots (Champion and Seth, 1968). Representative photographs of some Berberis species from the Indian Himalayan Region (IHR) are shown in Figure 1, and their major plant parts used to extract berberine and other bioactive alkaloids are shown in Figure 2.
Figure 1.
Some Berberis species of Indian Himalayan Region (IHR). (A) B. aristata DC., (B) B. asiatica Roxb. ex DC., (C) B. jaeschkeana C.K.Schneid., (D) B. lycium Royle, (E) B. pseudumbellata R. Parker, (F) B. thomsoniana Schneider.
Figure 2.
Various plant parts of (A) Berberis asiatica collected from Indian Himalayan Region (IHR), includes, (B) roots, (C) stems and (D) stem barks. These parts are the major sources to extract Berberine (yellow color) from Berberis species.
Ethnopharmacology of Berberis spp. Against Diabetes and Other Metabolic Diseases
A literature review revealed that the ethnopharmacological uses of Berberis species have been documented from different parts of the world for the treatment of diabetes, hypertension, and obesity, and some of them also revealed the formulation methods. A majority of Berberis species were found to be used in the Himalayan region of India and Pakistan.
B. lycium Royle has been used traditionally for the treatment of diabetes mellitus and other diseases, particularly by the local inhabitants of the Himalayan region (Hamayun et al., 2006). Apart from diabetes, B. lycium is also used to treat bone fractures, diarrhoea, fever, intestinal colic, internal wounds, jaundice, menorrhagia, ophthalmic disorders, piles, rheumatism, sun blindness, and throat pain (Jabeen et al., 2015; Adhikari et al., 2019). Fruits and leaves of B. lycium are also reported to be used for the treatment of diabetes mellitus in south-west of Iran (Rahimi Madiseh et al., 2014) and Pakistan (Zain-Ul-Abidin et al., 2018). The water extract obtained by soaking the root bark in water is used for the treatment of diabetes (Ahmed et al., 2004). The whole plant is used to treat diabetes in Chamba district of Himachal Pradesh, West Himalaya, India (Rana et al., 2019). The Bhotiya tribal community of the Central Himalayan region of India used B. lycium roots with water for the treatment of diabetes (Phondani et al., 2010).
The stem of B. aristata DC. is widely used in Indian traditional medicine for the treatment of diabetes (Upwar et al., 2011), which is also reported in Ayurvedic Pharmacopoeia. The decoction (5–10 mL) of roots or stems of this species prepared with water was taken twice a day for 1–2 weeks to treat diabetes in Uttarakhand region (Kumar et al., 2019). It is also used by Uttarakhand people for the treatment of hypertension (Singh et al., 2019). The root, stem, and fruit also have been used to treat obesity (Chandrasekaran et al., 2018). B. asiatica is also used for the treatment of diabetes by the tribal communities of Chhota Bhangal, Western Himalaya, India. The decoction prepared from the roots is concentrated and dried in shade and then used with the sap of bitter guard for the treatment of diabetes (Uniyal et al., 2006).
In Iranian traditional medicine, B. vulgaris L. is extensively used to treat diabetes and hypertension (Rahimi-Madiseh et al., 2017). Local people use a decoction from the fruits and roots of B. vulgaris to treat hypertension (Baharvand-Ahmadi et al., 2016). The fruits are most frequently used in traditional and modern medicine (Rahimi Madiseh et al., 2014). Dried roots of B. crateagina DC. were recorded to be used as anti-diabetic agents locally in Turkey, and the decoction or infusion prepared from dried roots was taken orally one to two times a day for the treatment of diabetes (Durmuskahya and Öztürk, 2013). The anti-diabetic activity has also been reported for B. brevissima Jafri and B. parkeriana C.K.Schneid. (Alemardan et al., 2013). Bahmani et al. (2016) reported that the inhabitant of Urmia, Iran, use boiled and steamed B. integerrima Bunge extract for the treatment of diabetes.
Alkaloids From Berberis Species: Potential Compounds Against Metabolic Diseases
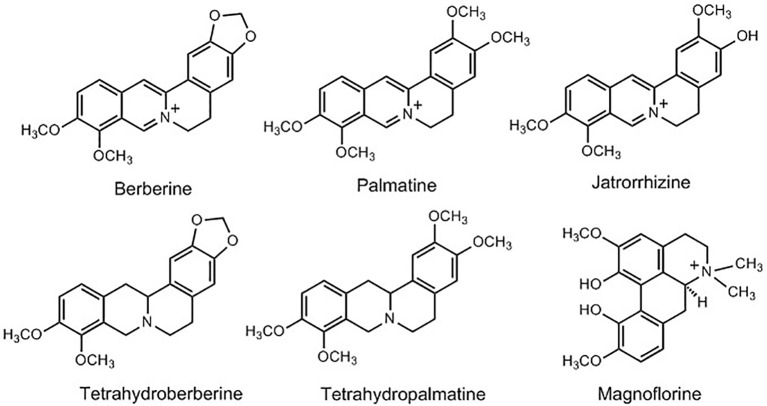
A large number of studies have been conducted on the isolation and quantification of bioactive compounds from Berberis species. The phytochemical investigations of the genus Berberis have shown the presence of more than 105 compounds with varying structural confirmations. Most of the studies on Berberis species are focused on phytochemical screening; for the presence and estimation of different secondary metabolites, such as alkaloids, flavonoids, steroids, sugars, triterpenoids, tannins, and other preliminary assays such as total ash content, acid soluble ash content, and moisture content (Belwal et al., 2016; Belwal et al., 2017; Andola et al., 2018; Srivastava et al., 2006; Shahid et al., 2009). However, the isolation and characterization of alkaloids from genus Berberis is well documented. Alkaloids are one of the major bioactive chemical constituents of the Berberis species, and they are responsible for various pharmacological activities of either whole extract or isolated individual compounds. Berberine (BBR) is one of the most commonly reported alkaloids from various Berberis species along with palmatine, magnoflorine, and jatrorrhizine, etc. (Figure 3) (Bhardwaj and Kaushik, 2012; Feng et al., 2018). Simple isoquinolone alkaloids are mainly reported from these species; however, studies have also reported their dimmers or dimeric benzylisoquinoline alkaloids (Leet et al., 1983). The detailed list of different alkaloids isolated from various Berberis species are given in Table 1. Among other compounds, BBR and its various natural and synthetic derivatives have also been evaluated and found effective in prevention and treatment of MS (Pérez-Rubio et al., 2013; Li et al., 2015; Zhao et al., 2017).
Figure 3.
Structures of some of the main bioactive alkaloids from Berberis species.
Table 1.
List of alkaloids isolated from various Berberis species.
| Plant source | Plant parts | Alkaloids | References |
|---|---|---|---|
| B. acanthifolium Mart. ex Schult. & Schult.f. | Stem bark | Berberine, tetrahydropalmatine | (Tiwari and Masood, 1977) |
| B. aetnensis C. Presl. | Root | Berberine | (Alamzeb et al., 2015) |
| B. amurensis Rupr. | Stem | Berberine, palmatine, berberine | (Wu et al., 2015) |
| B. amurensis Rupr. | Young shoot | Berberubine, oxyacanthine, pseudopalmatine, amurenine | (Yusupov et al., 1993b) |
| B. aristata DC. | Stem bark | Berberine phenoxide, ketoberberine benzoate A, ketoberberine benzoate B | (Ahamad et al., 2014) |
| Root and stem bark | Berberine, palmatine, berberrubine, jatrorrhizine, ketoberberine, dihydropalmatine, berbamine, pakistanamine |
(Bajpai et al., 2015) | |
| B. asiatica Roxb. ex DC. | Root | Berberine, oxyacanthine, berbamine, palmitine, jatrorrhizine, oxyberberine, tetrahydropalmatine, columbamine | (Bhakuni et al., 1968) |
| B. baluchistanica Ahrendt | Root | Pakistanine, pakistanamine, baluchistanamine, gandharamine | (Shamma et al., 1973; Shamma et al., 1974; Miana et al., 1979; Abu Zarga et al., 1982) |
| B. buxifolia Lam. | – | Chillanamine, (-)-osornine, (-)-curacutine, (-)-talcamine | (Leet et al., 1983) |
| B. calliobotrys Bien. ex Koehne | Root | Khyberine, pakistanamine, 1-O-methylpakistanine, pakistanine, chitraline, kalashine | (Fazal Hussain et al., 1980) |
| B. chitria Buch.-Ham. ex Lindl. | – | Berberine, palmatine, jatrorrhizine, oxyacanthine, O-methylcorydine-N-oxide | (Hussaini and Shoeb, 1985) |
| Root bark | Palmatine, | (Choudhary et al., 2010) | |
| B. coletioides Lechl. | - | Pronuciferine N-oxide, pronuciferine | (Fajardo et al., 2009) |
| B. concinna Hook.f. | Stem bark | Berberine, tetrahydropalmatine | (Tiwari and Masood, 1977) |
| B. crataegina DC. | Stem and root | Berberine, palmitine | (Petcu, 1968) |
| Seed | Berbaine, oxyacanthine | ||
| B. darwinii Hook. | – | magallanesine | (Valencia et al., 1985) |
| B. densiflora Boiss. & Buhse | Leaf | Berberine, β-allocryptopine, densinine, densiberine, glaucine, oxyacanthine, thalicmidine, isocorydine, O-methylcorypalline | (Khamidov et al., 1997c) |
| B. diaphana Maxim. | Bark | Berberine, palmatine, magnoflorine, jatrorrhizine | (Feng et al., 2018) |
| B. dictyophylla Franch. | Bark | Berberine, palmatine, magnoflorine, jatrorrhizine | (Feng et al., 2018) |
| B. glaucocarpa Stapf | Root | Oxyacanthine, tetrandrine | (Alamzeb et al., 2018) |
| B. heterobotrys E.L.Wolf | – | Berberine, palmatine, yatrorizine, oxyacanthine, berbamine, reticuline, obaberine, isocorydine, talikmidine, berberal. | (Karimov et al., 1993b) |
| B. heteropoda Schrenk | Young shoot and leaf | N-Methyldihydroberberine, 8-oxoberberrubine, berbamunine, aromoline, glaucine, talikmidine, isocorydine, reticuline, Pseudopalmatine, laudanosine, berpodine, isotetrandrine | (Karimov et al., 1992; Karimov et al., 1993a; Yusupov et al., 1993a) |
| B. hispanica Boiss. & Reut. | Root bark | Berberine tannate | (Aribi et al., 2017) |
| B. ilicifolia L.f. | – | Ilicifoline | (Fajardo et al., 1996) |
| B. iliensis Popov | Young shoot | (+)-β-N-Methylcorypalmine, berberrubine, berberine, magnoflorine | (Karimov and Shakirov, 1993) |
| B. integerrima Bunge | – | Berberine, berbamunine, oxyacanthine, magnoflorin, intebrine, intebrinine, intebrimine | (Karimov et al., 1977; Karimov et al., 1993g; Karimov et al., 1993h) |
| B. jaeschkeana C.K. Schneid | Root and bark | Berberine | (Andola et al., 2018) |
| B. jaeschkeana Schneid var. jaeschkeana | – | Berberine, palmatine, jatrorrhizine, chondrofoline, berberidione | (Alamzeb et al., 2015) |
| B. julianae C.K. Schneid. | Aerial part | Berberine, magnoflorine, glaucine, tetrahydrojatrorrhizine | (Brazdovicova et al., 1975) |
| B. kansuensis Schneid. | Bark | Berberine, palmatine, magnoflorine, jatrorrhizine | (Feng et al., 2018) |
| B. laurina Thunb. | Leaf | Berberine, (-)-tetrahydropalmatine, protopine | (Falco et al., 1968) |
| Trunk bark and root | Berberine, obaberine (O-methyloxyacanthine), O-methylisothalicaberine, lauberine | (Falco et al., 1968) | |
| B. libanotica Ehrenb. | Root, fruit | Oxycanthine, berbamine, jatrorrhizine, palmatine, berberine | (Alamzeb et al., 2015; Hosry et al., 2016) |
| B. lycium Royle | Fruit | Berberine, magnoflorine | (Sharma et al., 2018) |
| – | Berberine, berbericine | (Sehdev et al., 1971) | |
| B. nummularia Bunge | Leaf | Bernumine bernumidine and bernumicine, nummularine | (Karimov et al., 1993d; Faskhutdinov et al., 1997) |
| B. oblonga Scheid | Leaf | Glaucine, hydroxyacanthin, berbamine, berberin, isocoridin | (Khamidov et al., 2003) |
| – | Berberine, berbamunine, oxyacanthine, magnoflorine, palmitine, oblongamine | (Karimov et al., 1977) | |
| Root | Berberine iodide, magnoflorine iodide, columbamine iodide, oxyacanthine, berbamine, 2'-N-methylisotetrandrine iodide | (Karimov and Lutfullin, 1986) | |
| Leaves and shoots | Thalicmidine and in the shoots, berberin. Other alkaloids isolated included glaucine, hydroxyacanthine, berbamine, isocoridine | (Khamidov et al., 2003) | |
| B. pachycantha Koehne | Whole plant | Pachycanthine | (Ahmed et al., 2008) |
| B. petiolaris Wall. ex G. Don | Fruits, leaf, root and stem | Berberine, palmatine, magnoflorine, jatrorrhizine, tetrahydropalmatine, tetrahydroberberine, thalifendine/berberrubine, demethyleneberberine, reticuline, 8-oxoberberine, N-methyltetrahydroberberine, | (Singh et al., 2015) |
| Root | Berbamine, berberine chloride, palimitine | (Miana and Ikram, 1970) | |
| B. sibirica Pall. | Aerial part | (-)-Tetrahydropseudocoptisine, pseudoprotopine, (+)-chelidonine, (+)-glaziovine, berberine, palmatine, columbamine, berberubine, oxyacanthine, berbamine, 8-oxoberberine, 8-oxoberberubine, pakistanine, pronuciferine, N-acetylhomoveratrylamine | (Karimov et al., 1993e; Istatkova et al., 2007) |
| B. tabiensis L.A. Camargo | Stem | Tabienine | (Quevedo et al., 2008) |
| B. thunbergii DC | Stem | Berberine, berbamine, glaucine, isocorydine, oxycanthine, palmatine, thalicmidine | (Khamidov et al., 1997a) |
| Leaf | Thalicmidine, oxycanthine, isocorydine, heliamine, berberine | (Khamidov et al., 1997a) | |
| Fruit | Oxyxanthine, isotetrandrine, thalicmidine | (Khamidov et al., 1997a) | |
| – | Berberine, columbamine | (Och et al., 2017) | |
| – | Oxyacanthine, palmatine, thalicmidine, isotetrandrine, berberine, berbamine, glaucine, isocorydine,heliamine | (Khamidov et al., 1997b) | |
|
B. turcomanica Kar. ex Ledeb. |
Young shoot | Turconidine | (Karimov et al., 1993f) |
| – | Turcberine | (Karimov et al., 1993c) | |
| Young shoot | Berberine, isocorydine, glaucine, thalicmidine, aromoline, oxyacanthine, turcomanine, berberine, papaverine, cyclotriveratrilene | (Khamidov et al., 1996d; Khamidov et al., 1996a) | |
| Leaf | Turcomanidine, Turcamine, | (Khamidov et al., 1996b; Khamidov et al., 1996c) | |
| B. vernae Schneid. | Bark | Berberine, palmatine, magnoflorine, jatrorrhizine | (Feng et al., 2018) |
| B. virgetorum C.K. Schneid. | Whole plant | (-)-Berbervirine, berberine, jatrorrhizine, noroxyhydrastinine | (Liu et al., 1995) |
| B. vulgaris L. | Root bark | Berberine, palmatibne, bersavine, muraricine, berbostrejdine, berbamine, aromoline, obamegine, 8-oxoberberine, berbidine, bargustanine, Berberine, oxyacanthine, talikmidine, yatrorizine, berbamine, berbamunine, isocorydine | (Karimov et al., 1993i; Khamidov et al., 1995; Hošt'álková et al., 2013; Hostalkova et al., 2019) |
| B. vulgaris subsp. australis (Boiss.) | Root bark | Berbamine, sotetrandrine, oxyacanthine, obaberine, aromoline, obamegine, thaligrisine, thalifoline, 8-oxyberberine, chilenine, (-)-tejedine | (Suau et al., 1998) |
The effect of different habitat conditions (altitudinal variations and edaphic factors) of Berberis species has been investigated. Chandra and Purohit (1980) investigated eight Berberis species from different altitudinal range for determining the BBR concentration in different parts. Among these, B. asiatica was found to contain higher content of BBR than other species. Lower altitudinal range was found to contain higher BBR content within a species as compared to high altitude habitat. Among plant parts, roots contained a higher concentration of BBR (Chandra and Purohit, 1980). Similarly, variations in the BBR content of five Berberis species (i.e., B. aristata, B. asiatica, B. jaeschkeana, B. lycium, and B. pseudumbellata) depending upon the habitat have also studied. The presence of higher BBR content was recorded from rocky habitats in B. jaeschkeana (Andola et al., 2018). Both altitude and edaphic conditions were found to be responsible for the variation in BBR content in root and stem bark. Lower altitude populations showed significantly higher BBR content and positively correlated with moisture and potassium availability in soil species. Among these, B. asiatica contain significantly higher BBR content as compared to other species (Andola et al., 2010) Seasonal variations in the BBR content revealed higher percentage in summer and lower in rainy season (Andola et al., 2018). Low moisture and high soil potassium level is reported to be well correlated with high BBR content (Andola et al., 2011).
In Vitro Activities Against Diabetes and Other Metabolic Diseases
It has been suggested that physical exercise and a proper diet can act as controllers of the cause of T2DM and metabolic diseases. Currently available pharmacological interventions can control many aspects of diabetes and metabolic diseases, like microvascular and macrovascular complications, hypertension, dyslipidemia, and obesity. However, there is also a need for novel therapeutic agents that work alone or in combination with currently available drugs. Within the pharmacological options, phytochemicals have a great potential to act against T2DM, MS, and associated complications (Davì et al., 2010). Extracts of Berberis species and their components, especially alkaloids, have been documented for their potential activity against T2DM and MS in various in vitro studies (Table 2) (Potdar et al., 2012)
Table 2.
In vitro activity of extracts and/or isolated compounds from Berberis species against diabetes and metabolic diseases.
| Extracts from Berberis spp./isolated compounds | Model | Outcomes | References |
|---|---|---|---|
| Berberine | |||
| Berberine (BBR) | Mouse 3T3-L1 cells | Downregulated transcription factors (CCAAT/enhancer binding protein β, CCAAT/enhancer binding protein α) and PPARγ, suppress PPARs, A-FABP and FASN and inhibit 3T3-L1 fibroblast differentiation to adipocytes | (Kishimoto et al., 2015) |
| Berberine (BBR) | Mitochondria isolated from the liver of high-fat-fed rats | ↓capacity to accumulate calcium and OXPHOS capacity (MMP, oxygen consumption, and cellular ATP levels). ↑ mitochondrial SirT3 activity, normalizing mitochondrial function, and preventing a state of energetic deficit caused by impaired OXPHOS | (Teodoro et al., 2013) |
| Berberine (BBR) | C2C12 cell line | Reverted mitochondrial dysfunction induced by HFD and hyperglycemia in skeletal muscle, in part due to an ↑ in mitochondrial biogenesis. The prevention of mitochondrial dysfunction, ↑ in mitochondrial biogenesis, and BBR-induced AMPK activation, are blocked in cells in which SIRT1 has been knocked down. | (Gomes et al., 2012) |
| Berberine (BBR) | Cultured human liver and L6 rat skeletal muscle cells |
↑ InsR mRNA and ↑ protein expression in dose- and time-dependent results. InsR expression in the L6 rat skeletal muscle cells. BBR-enhanced InsR expression improved cellular glucose consumption only in the presence of insulin. Silencing InsR gene with small interfering RNA or blocking the pi3k ↓ this effect. BBR-induced InsR gene expression through a PKC-dependent activation of its promoter. Inhibition of PKC abolished BBR-caused InsR promoter activation and InsR mRNA transcription. | (Kong et al., 2009) |
| Berberine (BBR) | 3T3-L1 preadipocytes | Inhibitor of PPARγ and α | (Huang et al., 2006) |
| Berberine (BBR) | Human platelet | Inhibited platelet aggregation, superoxide production via modulating AR, NOX, and glutathione reductase activities in HG | (Paul et al., 2019) |
| Berberine (BBR) | Primary hepatocytes | Promotion of glucose uptake and prevention of gluconeogenesis by inhibition of SIRT3, and by regulation of mitochondria-related pathways. | (Zhang et al., 2018) |
| Berberine (BBR) | HepG2 and mouse primary hepatocytes | Prolonged activation of AMPK BBR-induced ↑CD36 expression in hepatocytes, evoking in FA uptake via processes associated to hepatocellular lipid accumulation and fatty liver. | (Choi et al., 2017) |
| Berberine (BBR) | H9c2 cardiomyocytes | Attenuation of palmitate-induced reduction in glucose uptake and consumption by ↓cellular DAG levels and accumulation of TAG. | (Chang et al., 2016) |
| Berberine (BBR) | Rat MCs | Inhibition of mesangial cell proliferation and hypertrophy by modulating cell cycle progress. Suppression of high glucose-induced TGF-β1 and FN expression through blocking NF-κB/AP-1 pathways. | (Lan et al., 2014) |
| Berberine (BBR) | human hepatoma cells |
Upregulated LDLR expression independent of sterol regulatory element-binding proteins, but dependent on ERK activation. Also ↑ LDLR expression through a post-transcriptional mechanism that stabilizes the mRNA. | (Kong et al., 2004) |
| Berberine (BBR) | Omental adipose tissue biopsies | Inhibition of human preadipocyte differentiation and leptin and adiponectin secretion accompanied by downregulation of PPARγ2, C/EBPα, adiponectin, and leptin mRNA expression | (Yang et al., 2012) |
| Berberine (BBR) | 3T3-L1 adipocytes, L6 myotubes, and L6 cells | ↑AMPK in 3T3-L1 adipocytes and L6 myotubes, ↑GLUT4 translocation in L6 cells in a pi3k -independent manner, and ↓ lipid accumulation in 3T3-L1 adipocytes | (Lee et al., 2006) |
| Berberine (BBR) | CEM, HCT-116, HepG2.2.15, SW1990, HT1080 and 293T cell lines |
↑gene expression of the insulin receptor | (Zhang et al., 2010) |
| Berberine (BBR) | L929 cells | Activation of GLUT 1 transporter | (Cok et al., 2011) |
| Berberine (BBR | 3T3-L1 and L6 cells | Inhibition of PTP1B, and ↑IR and ↑IRS1 phosphorylation | (Chen et al., 2010) |
| Berberine (BBR) | 3T3-L1 cells | ↓TG accumulation by ↑pIRS1-PI3KpAkt, ↑GLUT4 translocation and ↑insulin tropic action by pCREB-pIRS2-pAkt | (Ko et al., 2005) |
| Berberine (BBR) | L6 cells | ↑AMPK and ↑p38 MAPK phosphorylation | (Cheng et al., 2006) |
| Berberine (BBR) | 3T3-L1 cells | Regulation of PPARs and positive transcription elongation of factor b expression | (Zhou and Zhou, 2010) |
| Berberine (BBR) | HepG2 and C2C12 cells | ↑glucose metabolism by glycolysis stimulation and mitochondrial respiratory chain inhibition | (Xu et al., 2014) |
| Berberine (BBR) | HL-7702, normal human liver cell lines |
LDLR up-regulation by AMPK-dependent Raf-1 activation | (Li et al., 2014) |
| Combination of berberine and/or derivatives | |||
| Berberine (BBR) and dihydroberberine | L6 and LKB1−/− cells | AMPK activation, by complex I inhibition of the mitochondrial transport chain | (Turner et al., 2008) |
| 9-O-lipophilic group substituted) berberine (9-O-BBR) | HepG2 cells | ↑ hypoglycemic activity | (Zhang et al., 2016) |
| 13-Methylberberine (13-Me-BBR) | Mouse 3T3-L1 cells | Downregulated the expression of adipocyte differentiation transcription factors (PPARγ and C/EBPα). ↓PPARγ, ↓C/EBPα, and ↓SREBP-1 protein levels. Effect require AMPK signaling pathway | (Chow et al., 2016) |
| Berberine (BBR) and metformin | HepG2 hepatocytes and C2C12 myotubes | Promotion of glucose metabolism via stimulation of glycolysis, not be related to AMPK activity. | (Xiao et al., 2018) |
| BBR derivatives: thalifendine | Human HepG2 liver cells | ↑LDLR or InsR protein expression. | (Wang et al., 2009) |
| BBR amide derivatives | HL-7702 cells | ↑ glucose-lowering efficacies | (Ren et al., 2017) |
| Mannose modified berberine (m-BBR) | HepG2 cells | ↑ antidiabetic activity | (Han et al., 2019) |
| Pseudoberberine (pBBR) |
HepG2 cells | AMPK activation and LDR up-regulation. | (Wang et al., 2012) |
| Palmatine | Differentiated myocytes, L6 cells | anti-diabetic activity may be mediated through insulin dependent pathway by the activation of IRTK and PI3K | (Sangeetha et al., 2013) |
| Berberis extracts | |||
| B aristata bark methanolic extract | Dipeptidyl peptidase IV | Inhibition of dipeptidyl peptidase IV activity | (Chakrabarti et al., 2011) |
| B. mycrophylla roots ethanolic extract | non-resistant and insulin-resistant HepG2 cells | hypoglycemic effects and ↑ glucose uptake by activating AMPK protein. | (Furrianca et al., 2017) |
| B. vulgaris roots (ethanolic extract) and berberine (BBR) | α-Glucosidase | ↑ α-glucosidase activity, extract > BBR | (Abd El-Wahab et al., 2013) |
| B. vulgaris roots (methanolic extract) | α-Amylase | ↑ α-amylase activity | (Boudjelthia et al., 2017) |
| Jinqi Jiangtang tablet (berberine-contain) | α-Glucosidase, lipase and aldose | ↑α-glucosidase, ↑lipase, and ↑aldose reductase activities, | (Chang et al., 2015) |
The ↑ and ↓ signs shows significant increase and significant decrease of evaluated factors during mentioned studies.
Studies in mouse 3T3-L1 cells suggested that BBR has an pivotal role in regulating adipose tissues (Kishimoto et al., 2015). Experiments in mitochondria isolated from the liver of high-fat-fed rats have shown that BBR exhibited protective effects against MS that was associated with the increased mitochondrial sirtuin-3 (SIRT3) activity, normalizing mitochondrial function, and preventing a state of impaired oxidative phosphorylation (OXPHOS) that caused energetic deficit (Teodoro et al., 2013). In the same way, the preventive effects of BBR on diet-induced insulin resistance (InsR) was suggested to be linked to sirtuin-1 (SIRT1) and mitochondrial biogenesis (Gomes et al., 2012). It has been suggested that BBR is a unique natural medicine against insulin resistance in T2DM and MS (Kong et al., 2009). Different investigations have concluded that BBR as a new hypolipidemic drug works by a different mechanism of action to that of statin drugs (Kong et al., 2004). BBR works on multiple molecular targets as an inhibitor of peroxisome proliferator-activated receptor (PPAR) γ and α and is a potential weight reducing, hypolipidemic, and hypoglycemic agent (Huang et al., 2006). Prolonged activation of AMP-activated protein kinase (AMPK) by BBR improved CD36 expression in hepatocytes and was evoked in fatty acid uptake via processes associated with hepatocellular lipid accumulation (Choi et al., 2017). Also, BBR improved insulin sensitivity (InsS) by inhibiting fat storage and adjusting the adipokine profile in human preadipocytes (Yang et al., 2012). The hypoglycemic effects of BBR have also been attributed to its acute activation of the transport activity of glucose transporter 1 (GLUT1) (Cok et al., 2011).
Numerous studies of BBR in in vitro models have shed light on its positive effect on T2DM. BBR promoted glucose uptake and inhibited gluconeogenesis by inhibiting SIRT3, and regulating the mitochondria-related pathways (Zhang et al., 2018). BBR treatment attenuated a palmitate-induced reduction in glucose uptake and consumption through a reduction in cellular diacylglycerol (DAG) levels and the accumulation of triacylglycerol (TAG) in H9c2 cells (Chang et al., 2016). In addition, BBR displayed beneficial effects in the treatment of diabetes and obesity via stimulation of AMPK activity (Lee et al., 2006). The mechanisms of action of BBR in treatment of T2DM are suggested to be different than that of metformin and rosiglitazone (Zhang et al., 2010). BBR, as an insulin signal activator, had shown insulin-mimicry effects through the inhibition of protein tyrosine phosphatase 1B (PTP1B) activity on both adipocytes and myocytes (Chen et al., 2010) and acted as an effective insulin sensitizing and insulinotropic agent (Ko et al., 2005). Moreover, BBR and metformin promoted glucose metabolism by stimulating glycolysis through the inhibition of mitochondrial respiratory chain complex I and independent of AMPK activation (Xu et al., 2014). Besides, BBR circumvented the insulin signaling pathways and stimulated the glucose uptake through the AMP-AMPK-p38 MAPK pathway (Cheng et al., 2006). BBR modulated metabolism-related PPARs expression and differentiation-related positive transcription elongation factor b (P-TEFb) expression in adipocytes, which are associated with its hypoglycemic and hypolipidemic effects (Zhou and Zhou, 2010). In addition, BBR upregulated LDL receptor expression through Ras-independent (but AMPK-dependent) Raf-1 activation in liver cells (Li et al., 2014). BBR and metformin induced glycolysis and glucose consumption but are not related to the AMPK status (Xiao et al., 2018).
Different natural and synthetic derivatives of berberine are also evaluated for their in vitro activities. A BBR derivative, thalifendine, showed upregulatory activities for both LDLR and InsR, proving to be a potential treatment of both hyperlipidemia and hyperglycemia (Wang et al., 2009). Similarly, BBR amide derivatives improved the glucose-lowering effects (Ren et al., 2017). Mannose-modified BBR derivative exhibited high anti-diabetic activity at both high and low drug concentrations (Han et al., 2019). Palmatine showed anti-diabetic activity mediated through an insulin-dependent pathway by the activation of IRTK and PI3K (Sangeetha et al., 2013). Pseudoberberine (pBBR) has exhibited a potential effect on AMPK activation and LDLR upregulation as compared with BBR (Wang et al., 2012).
In the same way, the effects of extracts of species of the genus Berberis have been studied in several in vitro models and found effective. For instance, B. mycrophylla root extracts showed hypoglycemic effects and stimulated glucose uptake in HepG2 cells with and without resistance by activating AMPK protein (Furrianca et al., 2017). B. aristata bark methanolic extracts also inhibited the dipeptidyl peptidase–IV (DPP-IV) enzyme activity (Chakrabarti et al., 2011). B. vulgaris roots (ethanolic extract) and BBR showed α-glucosidase inhibition, where the inhibition caused by the extract was found to be higher than that of the BBR alone (Abd El-Wahab et al., 2013), and the extract also showed α-amylase inhibition activity (Boudjelthia et al., 2017).
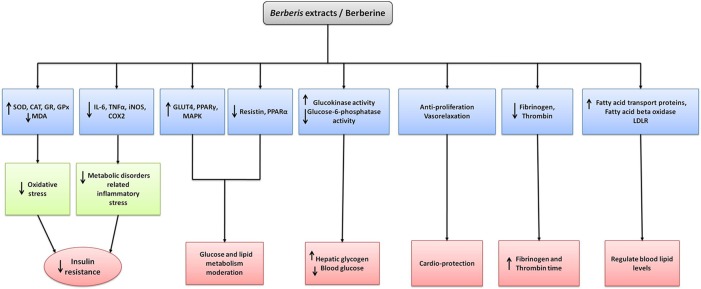
Some of the mechanisms of Berberis species and BBR against diabetes and metabolic diseases are depicted in Figure 4.
Figure 4.
The mechanism of action of extracts and its major isolated alkaloid of Berberis species in the treatment of diabetes and metabolic syndrome. Berberis spp. and berberine upregulate the anti-oxidant enzymes while decreasing reactive oxygen species and inflammatory mediators which in turn decreases oxidative and inflammatory stresses and thus decreasing insulin resistance. Upstream regulating expression of GLUT4, PPARγ, MAPK and downstream regulation of resistin, PPARα results glucose and lipid metabolism moderation. Increase in AMPK and glucokinase activities while decrease in glucose-6-phosphate activity results in decreasing gluconeogenesis, restoring hepatic glycogen and blood glucose. Upregulating AMPK and p38 MAPK activities also cause increasing insulin action and decreasing lipid synthesis. Antiproliferative action and vasorelaxation results in cardioprotection whereas decrease in fibrinogen and thrombin results in increasing fibrinogen and thrombin time respectively. Increasing expression of fatty acid transport proteins, fatty acid beta oxidase and LDLR aids in regulating blood lipid levels.
In Vivo Activities Against Diabetes and Metabolic Diseases
Extracts of Berberis species and their components, especially alkaloids, have been documented for their potential activity against T2DM and MS in in vivo models (Table 3). In the MS condition, BBR improved vascular inflammation and remodeling that was found to be correlated with the ability to inhibit p38 MAPK activation, ATF-2 phosphorylation, and MMP-2 expression (Li et al., 2015). Long-term treatment with BBR diminished the adipose tissue weight and decreased the renal injury (MS related diseases) in spontaneously hypertensive rats (Kishimoto et al., 2015). In normal diet-fed mice treated with BBR, hepatic CD36 expression and TG levels were increased; however, these effects were prevented when hepatic CD36 was silenced with an adenovirus containing CD36-specific short hairpin RNAs (shRNA) (Choi et al., 2017). BBR also improved the insulin-mediated vasodilatation of mesenteric arteries in diabetic rats through upregulation of insulin receptor-mediated signaling and increasing vascular InsS (Geng et al., 2016). Similarly, BBR increased both InsR and the low-density lipoprotein receptor (LDLR) expression, which resulted in a cellular response against InsR (Kong et al., 2009). In hyperlipidemic hamsters, the cholesterol-lowering effect of BBR was found to be due to its activity on upregulation of hepatic LDLR (Kong et al., 2004). Administration of BBR in hyperlipidemic and InsR rats decreased blood free fatty acid levels and increased the activity of lipoprotein lipase, leading to the amelioration of blood lipid and glucose metabolism (He et al., 2004). BBR administration resulted in the decrease of fasting blood glucose (FBL) level and ameliorated glycogen structural fragility (Li et al., 2019). Furthermore, BBR displayed beneficial effects in the treatment of obesity, and this was in part via improvement of adipose tissue fibrosis (Wang L. et al., 2018). BBR was reported to act in the liver to regulate lipid utilization and to maintain whole-body energy metabolism by mediating autophagy and FGF21 activation (Sun Y. et al., 2018). Additionally, BBR is also reported to reduce the systemic low-grade inflammation of T2DM mice to alleviate disease, and this effect may be achieved through regulating the gut microbes or inhibiting the TLR4 signaling pathway (Cao et al., 2017). Other in vivo investigations also showed the hypoglycemic effects of BBR through the improvement in gut-derived hormones and the attenuation of both intestinal mucosal mechanic and immune barrier damages (Gong et al., 2017). In the same way, the gut microbiota modulation was also suggested to be an effective mechanism of the antidiabetic effect of BBR (Han et al., 2011). The lipid-lowering effect of BBR chloride treatment in hyperlipidemic rats was found to be associated with a global change in the metabolism of lipids, carbohydrates, and amino acids as well as the structure of microbiota (Li et al., 2016).
Table 3.
In vivo activity extracts and/or isolated compounds from Berberis species against diabetes and metabolic diseases.
| Extracts from Berberis spp./isolated compounds | Model | Outcomes | References |
|---|---|---|---|
| Berberine | |||
| Berbamine (BBA) | STZ-induced diabetic Sprague-Dawley rats | ↑metabolic enzymes activities and preserved the glucose homeostasis | (Chandrasekaran et al., 2018) |
| Berberine (BBR) | Specific-pathogen-free male C57BL/6 mice | prolonged activation of AMPK BBR-induced ↑CD36 expression and fatty acid uptake | (Choi et al., 2017) |
| Berberine (BBR) | male Sprague–Dawley diabetic rats | ↑DVIS and ↑mesenteric vasodilatation by insulin receptor-mediated signaling upregulation. | (Geng et al., 2016) |
| Berberine (BBR) | male Wistar rats | ↓secretion of inflammatory factors and ↑vascular remodeling. Inhibition of p38 MAPK activation, ATF-2 phosphorylation, and MMP-2 expression. | (Li et al., 2015) |
| Berberine (BBR) | Male spontaneously hypertensive rats |
↓BWG, ↓retroperitoneal adipose tissues, ↓mesenteric adipose tissues, and ↓urinary albumin excretion. | (Kishimoto et al., 2015) |
| Berberine (BBR) | T2DM STZ-induced Wistar rats | ↓FBGL, ↓FSIL, ↑InsS, ↑InsR-mRNA, and ↑PKC activity in the liver. | (Kong et al., 2009) |
| Berberine (BBR) | hyperlipidemic hamsters | ↓TC, ↓LDL-C, ↑hepatic LDLR mRNA, and ↑hepatic LDLR protein | (Kong et al., 2004) |
| Berberine (BBR) | Hyperlipidemic and IR rats | ↓TC, ↓TG, ↓ApoB, ↓LDL-C, ↓FFA, ↑HDL-C, ↑ISI, ↑ApoAI, and ↑lipoprotein lipase activity |
(He et al., 2004) |
| Berberine (BBR) | T2DM db/db mice | ↓FBGL and ameliorated glycogen structural fragility | (Li et al., 2019) |
| Berberine (BBR) | HFD Obese rats | ↓BWG, ↑glucose tolerance, ↓collagen deposition and reversed the upregulation of fibrosis related genes in the adipose tissue of HFD. | (Wang L, et al., 2018) |
| Berberine (BBR) | Liver-specific SIRT1 knockout mice | Regulation of lipid usage and preserved whole-body energy metabolism via autophagy and FGF21 activation. | (Sun Y, et al., 2018) |
| Berberine (BBR) | Rat islets | Inhibition of glucose-stimulated insulin secretion with AMPK activation, ↓OCR and ↓ATP production induced by high glucose, and attenuation of glucose-stimulated expression of fatty acid synthase |
(Bai et al., 2018) |
| Berberine (BBR) | T2DM mice | ↓systemic low-grade inflammation to alleviate disease, by regulating the gut microbes and/or inhibiting TLR4 signaling pathways. | (Cao et al., 2017) |
| Berberine (BBR) | Diabetic rats | hypoglycemic effects associated to ↑ gut-derived hormones. | (Gong et al., 2017) |
| Berberine (BBR) | T2DM rats | ↓MALA, ↑InsR and ↑liver enzymes by | (Almani et al., 2017) |
| Berberine (BBR) | Diabetic rats | Attenuation of hyperglycemia, oxidative stress and inflammation by potentiation of the antioxidant defenses and up-regulation of PPARγ expression | (Mahmoud et al., 2017) |
| Berberine (BBR) | SD rats | ↓2h-PPG level by local inhibition of intestinal DPP-IV. | (Wang J, et al., 2016) |
| Berberine (BBR) | Diabetic rat model | ↓ expressions of Nrf2 and HO-1 | (Tao et al., 2017) |
| Berberine (BBR) | Diabetic rats | Inhibition of hepatic gluconeogenesis via the regulation of the LKB1-AMPK-TORC2 signaling pathway. | (Jiang et al., 2015) |
| Berberine (BBR) | Diabetic hamsters | ↓BGL, ↓TC, ↓TG, ↓FFA, ↓LDL-C, ↓Glucose, ↓insulin levels, ↓malondialdehyde, ↓thiobarbituric acid-reactive substance, and ↓8-isoprostane levels, ↑expression of skeletal muscle glucose transporter 4 mRNA and ↓liver LDL receptor mRNA expression. |
(Liu et al., 2015) |
| Berberine (BBR) | Zucker Diabetic Fatty Rats |
↓HbA1c, ↓TC, ↓TG, ↑insulin secretion, regulation of glucose and lipid metabolism and activation of pAMPK. |
(Dong et al., 2016) |
| Berberine (BBR) | db/db mice and high-fat–fed Wistar rats | ↓BWG, ↑glucose tolerance, ↓TG, and ↑ insulin action | (Lee et al., 2006) |
| Berberine (BBR) | Diabetic rats | Direct inhibition of liver gluconeogenesis | (Xia et al., 2011) |
| Berberine (BBR) | Diabetic rats | Intestinal microbiome modulation | (Han et al., 2011) |
| Berberine (BBR) | Diabetic rats | Lipid metabolism regulation and ↑ elimination of free radicals | (Tang et al., 2006) |
| Berberine (BBR) | Diabetic rats | PPAR α/δ up-regulation and PPARδ repression in liver | (Zhou et al., 2008) |
| Berberine (BBR) | Non-obese Diabetic rats | Regulation of MAPK activity to control the differentiation of Th17 and Th1 | (Cui et al., 2009) |
| Berberine (BBR) | Diabetic rats | Promotes secretion of glucagon-like peptide type I | (Lu et al., 2009) |
| Berberine (BBR) | Diabetic rats | Tyrosine phosphatase 1B activity inhibition and insulin-like effect | (Chen et al., 2010) |
| Berberine (BBR) | Diabetic hamster | Up-regulation of LXRα, PPARα, and down-regulation of SREBPs | (Liu et al., 2010) |
| Berberine (BBR) | Diabetic rats | ↓ intestinal disaccharidases and β-glucuronidases activities | (Liu et al., 2008) |
| Berberine (BBR) | Diabetic rats | Glucose metabolism modulation by GnRH-GLP-1 and MAPK pathway in the gut | (Zhang et al., 2014) |
| Berberine chloride (BC) | Diabetic rats | ↓FBG, ↓WBC, ↓HbAlc ↑plasma insulin, ↑hemoglobin, ↑RBC, ↑Ht, ↑MCH and ↑MCHC. | (Chandirasegaran et al., 2017) |
| Berberine chloride (BC) | Diabetic rats | ↓TC, ↓TG, ↓phospholipids, ↓LDL-C, ↓VLDL, ↓LOOH, ↓TBARS. ↑SOD, ↑CAT, ↑GPx, non-enzymatic antioxidant (↑GSH, ↑vitamin C, ↑vitamin E) and ↑IRS-1, ↑PKB, ↑Akt and ↑GLUT-4) |
(Chandirasegaran et al., 2019) |
| Berberine fumarate (BF) | T2DM rats | ↑metabolic disorder and ↓ inflammation by ↓over-expression of TLR4 and p-JNK and ↑PI3K and VGLUT2 expression. | (Cui et al., 2018) |
| Combination of berberine and other compounds/extracts | |||
| Berberine chloride (BC), oryzanol and vitamin B2 | Male Wistar hyperlipidemic rats | ↓lipid effect without apparent adverse side effects. | (Li et al., 2016) |
| Berberine (BBR), Ortosiphon staminensis, policosanol, red yeast rice extract, folic acid and coenzyme Q10 |
Rats | ↓TC, ↓LDL-C, ↓DBP, ↓TG, and ↑HDL-C. antihypertensive effect, which allows an effective control of blood pressure | (Rozza et al., 2009) |
| Berberine - Metformin Hybrid (BMH473) | T2DM obese rats | ↑maintaining glucose and ↑ lipid homeostasis, ↑antihyperlipidemic activity. | (Jia et al., 2019) |
| berberine (BBR) and Timosaponin B2 (TB-2) | Goto-Kakizaki rats | ↑anti-diabetic efficacy. | (Huang et al., 2019) |
| berberine (BBR) and Glycyrrhizic acid | Rats | ↓FBG, and ↑Insulin level | (Qiao et al., 2018) |
| Berberine (BBR) with resveratrol | High fat diet-induced mice | ↓TC, ↓TG, and ↓LDL-C | (Zhu et al., 2018) |
| Berberine (BBR) and Gelucire44/14 | diabetic mice | Gelucire44/14 showed potential ↑oral absorption of BBR thus ↑ anti-diabetic efficacy. | (Sun J, et al., 2018) |
| Berberine organic acid salts (BOAs), including berberine citrate, berberine fumarate, berberine malate, and berberine succinate | T2DM rats | ↑ hypoglycemic effects | (Li et al., 2017) |
| Berberine (BBR) and Coptis chinensis extract (CCE) | T2DM rats | ↑pancreatic insulin secretion via ↑ islet β-cell proliferation and ↑ protein expression of PARP-1. | (Jiang et al., 2017) |
| Berberine (BBR) combined with Canagliflozin | Diabetic mice | ↓FBG and ↓insulin. Antidiabetic effect associated with ↑ pAMPK and ↓ TNFα in kidneys. | (Cai-Ming et al., 2016) |
| Berberine (BBR) and Ginsenoside Rb1 (Rb1) | Diabetic mice | Improved abnormal metabolism of glucose and lipid. | (Shang et al., 2015) |
| Berberin glycyrrhizinate complex salt (BGC) | GK rats | ↓PBG, ↓insulin level, ↓GSP, ↓LDL-C and ↓MDA, and ↑ histopathological changes in kidney and pancreas. | (Wang et al., 2014) |
| Berberis extracts | |||
|
B. aristata roots (ethanolic extract) |
Diabetic rats | ↓dose-dependent in hyperglycemia, ↓TC, ↓TG, ↓AST, and ↓ALT levels of serum, ↓serum creatinine and ↓blood urea. | (Mittal et al., 2012) |
|
B. aristata stem (ethanolic extract) |
T1DM and T2DM albino rats | ↑Liver glycogen and ↓FBS | (Rameshwar et al., 2009) |
|
B. aristata roots (ethanolic extract) |
STZ-induced diabetic rats | ↓PBG | (Pareek and Suthar, 2010) |
|
B. aristata stem bark (aqueous extract) |
STZ-induced diabetic rats | ↓TC and ↑HDL-C | (Ahamad et al., 2012) |
| B. aristata bark (ethanolic extract) | alloxan-induced diabetic rats | ↓PBG | (Semwal et al., 2008) |
| B. aristata stem bark (methanolic extract) | Alloxan-Induced DiabeticRats | ↓PBG | (Gupta et al., 2010) |
| B. aristata roots (methanolic-water extract | Diabetic rabbits | ↓PBG | (Akhtar et al., 2008) |
|
B. aristata roots (water-ethanolic extract) |
Diabetic rats | Regulated glucose homeostasis via ↓ gluconeogenesis and ↓oxidative stress. | (Singh and Kakkar, 2009) |
| B asiatica roots (water-ethanolic extract) | Diabetic rats | ↓BW | (Singh and Jain, 2010) |
| B. dictyophylla roots (extract) | Diabetic mice and normal mice | ↓FBG, ↓ICAM-1, ↓ANGII, and ↓SOD in serum expression | (Yue et al., 2013) |
|
B. holstii roots (aqueous extract) |
Alloxan-induced diabetic male mice |
↓FBGL | (Kimani et al., 2017) |
|
B. integerrima roots (aqueous extract) |
Diabetic male Wistar rats | ↑renal by control of blood glucose and renal protective effects. | (Ashraf et al., 2013) |
| B. integerrima fruits (anthocyanin fraction) | Diabetic Male Sprague Dawley rats | ↓FBG, ↑ liver glycogen level, and ↑ body weight. | (Sabahi et al., 2016) |
| B. julianae roots (methanolic extract) | T2DM mice | ↑ GLUT4 translocation, ↑ oral glucose tolerance, ↑LDL-C, ↓BWG, ↓blood glucose and ↓other related blood-lipid contents. | (Yang et al., 2014) |
|
B. lycium roots (aqueous extract) |
Diabetic rabbits | ↓ FBG. | (Ahmad and Alamgeer, 2009) |
| B. lycium extract (BLE) | Diabetic rabbits | ↓TG, ↓TC, ↓LDL-C, and ↑HDL-C | (Ahmad et al., 2008) |
|
B. lycium leaves (methanolic extract) |
Female diabetic rabbits | ↓FBG | (Hussain et al., 2017) |
|
B. lycium roots (ethanolic extract) |
Alloxan treated rats | ↓FBG | (Gulfraz et al., 2007) |
| B. lycium roots (powder) | Broilers chickens | ↓TG, ↓TC, ↓LDL-C, and ↑HDL-C | (Chand et al., 2007) |
| B. lycium roots (aqueous extract) | Diabetic rats | ↓FBG, ↓TC, ↓TG, ↓LDL-C, ↓VLDL, ↓SGOT, ↓SGPT, and ↓ALP | (Mustafa et al., 2011) |
|
B. lycium fruits (aqueous extract) |
Diabetic rats | ↓TC, ↓TG, ↓LDL-C, ↓VLDL, and ↓MDA | (Rahimi Madiseh et al., 2014) |
| B. lycium root (methanolic extract) and berberine (BBR) | Diabetic rats | ↓FBG, ↑glucose tolerance, positive serum lipid profiles, glycosylated hemoglobin and body weight. | (Gulfraz et al., 2008) |
| B. vulgaris roots (aqueous extract) | Diabetic rats | ↓TC and ↓TG. | (Meliani et al., 2011) |
|
B. vulgaris fruits (aqueous and hydro-ethanolic extract) |
T1DM Rats | ↑ serum glucose levels, ↑ serum alanine aminotransferase activities, and ↓ HbA1c. | (Karami et al., 2016) |
|
B. vulgaris fruits (ethanolic extract) |
Diabetic rats | ↑total antioxidant levels, ↓MDA and ↓FBG, and ↑mRNA level of GK | (Hemmati et al., 2016) |
|
B. vulgaris fruits (Hydro-ethanolic extract) |
Diabetic rats | ↓ liver damage by influencing hepatic histopathological and biochemical markers |
(Rahimi-Madiseh et al., 2017) |
| Jatrorrihizine | Hyperglycemic mice | ↓FBG and ↑aerobic glycolysis | (Yan et al., 2005) |
| Jatrorrihizine and berberine | Diabetic rats | ↓FBG. Berberine > Jatrorrihizine | (Fu et al., 2005) |
| Palmatine | Normal rats | ↓FBG. | (Patel and Mishra, 2011) |
The ↑ and ↓ signs show significant increase and significant decrease, respectively, of evaluated factors during mentioned studies.
On the other hand, BBR protects against metformin-associated lactic acidosis (MALA) in streptozotocin (STZ)-induced T2DM (Almani et al., 2017). BBR attenuated hyperglycemia and its associated oxidative stress and inflammation through, possibly, the potentiation of the antioxidant defenses and upregulation of PPARγ expression (Mahmoud et al., 2017). BBR decreased 2-hour postprandial plasma glucose (2h-PPG) level in STZ-induced diabetic rats by locally inhibiting intestinal DPP-IV (Wang J. et al., 2016). Moreover, BBR also reduced the blood glucose level in diabetic rats, improving the blood lipid and decreasing the retinal vascular injury, suggesting its association with the reduced expressions of Nrf2/HO-1 (Tao et al., 2017). BBR also upregulated protein expressions of LKB1, AMPK, p-AMPK, and p-TORC2 and also inhibited the translocation of TOCR2 into the cell nucleus (Jiang et al., 2015). Moreover, BBR was also found to be effective in lowering blood glucose and lipid levels, reducing the body weight, and alleviating the oxidative stress in diabetic hamsters (Liu et al., 2015).
The anti-diabetic effect of BBR was suggested to be mainly due to its activity in the regulation of glycometabolism and lipometabolism and the activation of AMPK (Lee et al., 2006; Dong et al., 2016). BBR improved glucose metabolism through an insulin-independent pathway (Xia et al., 2011). BBR also significantly inhibited the progression of diabetes induced by alloxan, and the effect of BBR on diabetes was suggested to be associated with its hypoglycemic effect, modulating lipids metabolic effects and its ability to scavenge free radicals (Tang et al., 2006). BBR improved glucolipid metabolism in diabetic rats both in the blood and liver, possibly through modulating the metabolic related PPARα/δ/γ protein expression in liver (Zhou et al., 2008). BBR targeted MAPK to suppress Th17 and Th1 differentiation in T1DM NOD mice and showed a novel role of ERK in Th17 differentiation through downregulation of STAT3 phosphorylation and RORt expression (Cui et al., 2009). Altered hepatic SREBPs, LXRα, and PPARα transcriptional programs were suggested to be involved in the therapeutic mechanisms of BBR on fat-induced hepatic insulin resistance (FIHIR) in T2DM hamsters (Liu et al., 2010). The inhibitory effect on intestinal disaccharidases and β-glucuronidase of BBR might be one of the mechanisms for BBR as an antihyperglycaemic agent (Liu et al., 2008). BBR caused the glucose metabolism modulation by the GnRH-GLP-1 and MAPK pathway in the gut (Zhang et al., 2014). The treatment of BBR chloride notably protected the blood components (Chandirasegaran et al., 2017) and significantly reversed the abnormal levels of lipids, oxidant status, and insulin signaling molecules in the diabetic rat model (Chandirasegaran et al., 2019). BBR also reduced the release of lipopolysaccharides and ameliorated inflammation by reducing the level of lipolysaccharide binding protein (LBP), thus alleviating intestinal injury and improving InsR (Cui et al., 2018).
The combination of Ortosiphon staminensis, policosanol, red yeast rice extract, BBR, folic acid, and coenzyme Q10 provided an antihypertensive effect, which allowed for an effective control of blood pressure in patients with MS (Rozza et al., 2009). The berberine-metformin hybrid compound BMH473 was found to be beneficial for maintaining glucose and lipid homeostasis in T2DM rats, and it exhibited better anthyperlipidaemic effects compared to metformin and BBR alone (Jia et al., 2019).
Combining timosaponin B2 (TB-2) and BBR in a single formulation enhanced the anti-diabetic efficacy by improving the intestinal absorption (Huang et al., 2019). Glycyrrhizic acid was also reported to improve the oral absorption of BBR by inhibiting P-gp, and it thus increased the anti-diabetic effects of BBR in db/db mice (Qiao et al., 2018). Lipid-lowering effects of BBR were also reported to be increased with resveratrol, which may be associated with upregulation of a low-density-lipoprotein (LDL) receptor (Zhu et al., 2018). Similarly, gelucire44/14 was found to enhance the oral absorption of BBR and thus improve the antidiabetic efficacy of BBR (Sun J. et al., 2018). Berberine organic acids (BOAs) were found to be comparable to berberine hydrochloride (BH) in terms of hypoglycaemic effects, they were but superior with regard to safety from hyperchloraemia in T2DM rats (Li et al., 2017). Coptis chinensis (containing berberine) and BBR exerted similar effects when used for the treatment of T2MD rats, mainly via the stimulation of the pancreatic secretion of insulin (Jiang et al., 2017). Berberine chloride was a stronger antidiabetic agent than BBR or canagliflozin alone with fewer side effects on kidneys in the diabetic mice (Cai-Ming et al., 2016). BBR and ginsenoside Rb1 (Rb1) improve abnormal metabolism of glucose and lipid (Shang et al., 2015).
Extracts of Berberis plants have shown interesting results in in vivo models. The ethanolic extract of B. aristata showed antidiabetic activity due to its significant dose-dependent reduction effect on the blood glucose levels (Semwal et al., 2008; Mittal et al., 2012), which were also reported to be better than glibenclamide (Rameshwar et al., 2009) and comparable to metformin in diabetic rats (Pareek and Suthar, 2010). In addition, the aqueous extract of B. aristata showed significant antidiabetic activity, decreased total cholesterol, increased HDL-C levels, and prevented the body weight loss in diabetic rats (Ahamad et al., 2012).
The aqueous extract of B. lycium roots showed an antihyperlipidemic effect (Ahmad et al., 2008). B. lycium leaf extracts alleviated lipid profile levels and might be used efficiently in hyperglycemic and diabetic patients (Hussain et al., 2017). Also, the root extract of B. lycium reduced the serum glucose levels in normal and diabetic rats (Gulfraz et al., 2007). In chicken Broilers, the powder of B. lycium reduced the serum cholesterol (Chand et al., 2007). The oral administration of extracts of B. lycium showed hypoglycemic activity (Mustafa et al., 2011) and alleviated lipid profile levels (Rahimi Madiseh et al., 2014). Similarly, the methanolic extract of the B. lycium root and its main alkaloid BBR showed hypoglycemic activity (Gulfraz et al., 2008) and showed antiglycation activity (Khan et al., 2014).
On the other hand, in diabetic rats, the beneficial effects of B. vulgaris extracts showed positive effects in attenuating the side effects of T2DM (Karami et al., 2016), ameliorating oxidative stress (Hemmati et al., 2016), decreasing the liver damage by influencing hepatic histopathological and biochemical markers (Rahimi-Madiseh et al., 2017), and showed that the serum cholesterol and serum triglycerides levels were decreased (Meliani et al., 2011).
Other species of Berberis have also been studied. For instance, B. asiatica hydro-ethanolic root extracts have shown to be a potent orally effective antidiabetic extract (Singh and Jain, 2010). Likewise, the B. dictyophylla cortex could significantly reduce the level of fasting blood glucose, ICAM-1, and ANG II expression (Yue et al., 2013). The B. holstii extract showed the reduction of blood glucose levels (Kimani et al., 2017). Furthermore, the aqueous extract of B. integerrima roots improved renal dysfunction in STZ-induced diabetic rats through controlling blood glucose, and it also showed renal protective effects (Ashraf et al., 2013). The anthocyanin fraction of the fruits of B. integerrima also showed hypoglycemic effects (Sabahi et al., 2016). Moreover, the methanolic extract of B. julianae roots was also reported to possess promising beneficial effects for the treatment of T2DM with the possible mechanism via stimulating AMPK activity (Yang et al., 2014).
Other alkaloids isolated from Berberis species have also shown promising activities against T2DM and MS. For example, berbamine increased the activity of metabolic enzymes and preserved the glucose homeostasis in HFD/STZ induced diabetic rats (Chandrasekaran et al., 2018). Jatrorrihizine (JAT) induced an important decrease in FBG in normal and hyperglycemic mice, attributed to improve in aerobic glycolysis (Yan et al., 2005). JAT, BBR, and a combination of BBR and JAT decreased the FBG of diabetic and normal mice at different degrees. JAT also possessed the function of decreasing FBG, which was found less than that of BBR at the same dose level (Fu et al., 2005). Palmatine was also found to decrease FBG and suppressed the increase of blood glucose level in normal rats (Patel and Mishra, 2011).
Studies in Humans
Several pilot studies as well as pre-clinical studies and clinical trials have evaluated the beneficial effects of Berberis extracts and isolated compounds on diabetes, metabolic syndrome, and other metabolic diseases (Table 4).
Table 4.
Studies in diabetic and/or metabolic syndrome patients using treatment with extract and/or isolated compounds of Berberis species.
| Berberis spp./isolated compound | Study design/Model | Results | References |
|---|---|---|---|
| Berberine | |||
| Berberine (BBR, 0.05g, 4 tablets/time, 3 times/day) |
MS patients (n=80) RCT, 1 month | ↓FBG, ↓PBG, ↓InsR, ↓TG, ↓TC, ↓hs-CRP, and ↓IL-6 and ↓TNF-α | (Cao and Su, 2019) |
| Berberine (BBR, 0.5 g, 2 times/day) | T2DM patients (n = 300), double-blind, RCT, 16 weeks | ↓FPG | (Ming et al., 2018) |
| Berberine (BBR, 0.5 g, 3 times/day) |
MS patients (n=24) double-blind, placebo-controlled, RCT, 3 months | ↓WC, ↓SBP, ↓TG, ↓AUC of glucose, ↓AUC of insulin, ↓insulinogenic index, and ↑Matsuda index | (Pérez-Rubio et al., 2013) |
| Berberine (BBR, 0.4 g, 3 times/day) |
T2DM patients (n=114), RCT, 6 months | ↓HbA1c, ↓BUN, ↓SP, ↓hs-CRP, ↓ESR, and ↓eGFR | (Li et al., 2018) |
| Berberine (BBR, 0.5 g, 2 times/day) |
Mild mixed hyperlipidemia (n=32), double-blind, RCT, 12 weeks |
↓TC, ↓LDL-C and ↓TG. | (Kong et al., 2004) |
| Berberine (BBR, 1 g, 1 time/day) |
T2DM and mixed hyperlipidemia patients (n=116), double-blind, RCT, 3 months | ↓FPG, ↓PPG, ↓HbA1c, ↓TG, ↓TC, ↓LDL-C, and ↑GDR | (Zhang et al., 2008) |
| Berberine (BBR, 0.5 g, 3 times/day) |
Newly diagnosed T2DM patients (n=36) double-blind, RCT, 3 months | ↓HbA1c, ↓FBG, ↓PBG, ↓TG, ↓TC ↓FPI, ↓IR, and ↓LDL-C. | (Yin et al., 2008) |
| Berberine (BBR, 0.5 g, 2 times/day) |
Hyperlipidemic patients (n =86), Open study, 3 months | ↓LDL-C, ↓TC and ↓TG. | (Zhao et al., 2008) |
| Berberine (BBR, 0.3g, 3 times/day) | MS patients (n=41) Double‐blind, RCT, 3 months | ↓BMI, and ↓leptin levels, ↓leptin/adiponectin ratio, ↓HOMA-IR, and ↑IS | (Yang et al., 2012) |
| Berberine (BBR, 0.5 g, 3 times/day) |
PCOS and IR patients (n=89) randomized, single center, placebo-controlled, 3 months | ↓WHR, ↓TC, ↓TG, ↓LDLC, ↓FPG, ↓HOMA-IR, ↓AUC of insulin, ↑HDLC, and ↑SHBG | (Wei et al., 2012) |
| Berberine (BBR, 1.0 g, 1 time/day) |
T2DM and dyslipidemic patients (n = 116) double-blind, placebo-controlled and multiple-center trial consisting of a screening visit, RCT, 2-week | ↓FFA | (Gu et al., 2010) |
| Berberine (BBR, 1.0 g, 1 time/day) | T2DM patients with fasting blood glucose (n = 96), 2 months | ↓FBG, ↓HA1c, ↓TG, and ↓insulin levels | (Zhang et al., 2010) |
| Berberine (BBR, 0.5 g, 2 times/day) | T2DM patients (n=228) double-blind randomized controlled placebo, 4 weeks |
↓FPG, ↓PMBG, and ↓FA. | (Rashidi et al., 2018) |
| Berberine (BBR, 0.5 g, 2 times/day) |
T2DM patients (n=30), open labelled, observational and single centre study, 12 weeks | ↓FBG, ↓PPBG, and ↓GHb | (Dange et al., 2016) |
| Berberine (BBR, 0.3 g, 3 times/day) |
T2DM patients (n=30), 8 weeks | ↓BMI, ↓FBG, ↓HbAlc, ↓fasting insulin, ↓TG, ↓TC, ↓HDL-C, ↓LDL-C, ↓CPR, ↓TNF-α, and ↓LPS |
(Chen et al., 2016) |
| Berberine (BBR, N.I., 2 times/day) | T2DM patients (n=41), open-label interventional RCT, 3 months | ↓HbA1C, ↓FBG, and ↓PPG | (Rao, 2017) |
| Berberine (BBR, 0.3 g, 3 times/day) | Mild hyperlipemic patients (n=97) Double‐blind, RCT, 3 months | ↓TG, ↓TC, and ↓LDL-C | (Wang L et al., 2016) |
| Berberine (BBR, 0.4 g, 1 time/day) |
Hypercholesterolemia in tolerance to more than one statin (n=91), 3 months | ↓ LDL-C and ↓TG. | (Cicero and Ertek, 2008) |
| Berberine combined with others compounds and extracts | |||
| Berberine (BBR, 1.0 g, 1 time/day.) and simvastatin (SIMVA) |
Hypercholesterolemic patients (n=63), double-blind, RCT, 2 months | ↓LDL-C, ↓TC, and ↓TG | (Kong et al., 2008) |
| (Berberine, BBR, 0.5 g; red yeast, 200 mg; and policosanol, 10 mg; 1 time/day) | Hypercholesterolemic patients (n=50), double-blind, single-centered, placebo-controlled, RCT, 6 weeks | ↓TC, ↓LDL-C, ↓TG, ↑FMD, and ↑InsS | (Affuso et al., 2010) |
| (Berberine, BBR, 0.5 g; policosanols, 10 mg; and red yeast rice, 200 mg; 1 time/day) | Hypercholesterolemic patients (n=135) randomized, double-blind, EZE-controlled, 6 months | ↓LDL-C, and ↓TG | (Pisciotta et al., 2012) |
| Armolipid Plus ™ composed by (Berberine, BBR, 0.5 g; red yeast rice, 200 mg; policosanol, 10 mg; folic acid, 0.2 mg; coenzyme Q10, 2.0 mg; and astaxanthin, 0.5 mg; 1 time/day) | Hypercholesterolemic patients (n=106), single-blind, single centered, placebo-controlled, RCT, 12 months | ↓TC, ↓LDL-C, and ↓InsR | (Marazzi et al., 2011) |
| Armolipid Plus ™ composed by (Berberine, BBR, 0.50g; red yeast rice, 200 mg; policosanol, 10 mg; folic acid, 0.2 mg; coenzyme Q10, 2.0 mg; and astaxanthin, 0.5 mg; 1 time/day) | Hyperlipidemic patients (n=102), double-blind, parallel, controlled, Multiple centered, placebo-controlled, RCT, 12 weeks | ↓LDL-C, ↓apo B-100, ↓TC/HDL-C, ↓ApoB/ApoA1 ratio, and ↑ApoA1 | (Sola et al., 2014) |
| Armolipid Plus ™ composed by (Berberine, BBR, 0.5g; red yeast rice, 200 mg; policosanol, 10 mg; folic acid, 0.2 mg; coenzyme Q10, 2.0 mg; and astaxanthin, 0.5 mg; 1 time/day) | Dyslipidemic patients (n = 1751) Double‐blind, RCT, 16 weeks | ↓TC and ↓LDL-C | (Trimarco et al., 2011) |
| Armolipid Plus ™ composed by (Berberine, BBR, 0.5g; red yeast rice, 200 mg; policosanol, 10 mg; folic acid, 0.2 mg; coenzyme Q10, 2.0 mg; and astaxanthin, 0.5 mg; 1 time/day) | Hypercholesterolemic patients (n=66), single-blind, placebo-controlled, RCT, 3 weeks | ↓TC, ↓LDL-C, and ↓TG | (Gonnelli et al., 2015) |
| Armolipid Plus ™ composed by (Berberine, BBR, 0.5 g; red yeast rice, 200 mg; policosanol, 10 mg; folic acid, 0.2 mg; coenzyme Q10, 2.0 mg; and astaxanthin, 0.5 mg; 1 time/day) | Moderate dyslipidemic and MS patients (n=30), double-blind, centered, placebo-controlled, RCT, | ↓TC, ↓LDL-C, ↓leptin-to-adiponectin ratio, and ↑HDL-C | (Ruscica et al., 2014) |
| Armolipid Plus ™ composed by (Berberine, BBR, 0.5 g; red yeast rice, 200 mg; policosanol, 10 mg; folic acid, 0.2 mg; coenzyme Q10, 2.0 mg; and astaxanthin, 0.5 mg; 1 time/day) | Dyslipidemic with ischemic heart disease treated patients (n=100), single-blind, EZE-controlled, RCT, 12 months | ↓LDL-C, ↓TC, ↓TG, and ↑HDL-C | (Marazzi et al., 2015) |
| Berberine (BBR, 500mg) and Armolipid Plus ™ Composed by (Berberine, BBR, 0.5 g; red yeast extract, 200 mg; policosanol, 10 mg; folic acid, 200 mg; coenzyme Q10, 2 mg; and astaxanthin, 0.5 mg; 1 time/day) |
Hyperlipidemic patients (n=40) single-blind, no placebo-controlled, 4 weeks | ↓TC, ↓LDL-C, ↓ApoB, ↓TG, and ↑HDL-C | (Cicero et al., 2007) |
| Body Lipid ™ composed by (Berberine, BBR, 0.5 g; red yeast rice, 10 mg; coenzyme Q10, 2 mg; and hydroxytyrosol, 5 mg; 1 time/day) |
Hypercholesterolemic patients (n = 158) Double‐blind, RCT, 4 weeks | ↓TC and ↓LDL-C | (D'Addato et al., 2017) |
| Berberine (BBR, 0.2g; monacolin K, 3 mg; chitosan, 10 mg; and coenzyme Q10, 10 mg; 1 time/day) | Hypercholesterolemic patients (n =36) Double-blind phase II placebo-controlled study, 12 weeks | ↓nHDL-C, ↓LDL-C and ↓apoB | (Spigoni et al., 2017) |
| Estromineral lipid ™ composed by (Berberine, BBR, 0.5 g; soy isoflavones, 60 mg; Lactobacillus sporogenes, 1x109 spores; calcium phosphate dehydrate, 137 mg; vitamin D3, 5 μg; and folic acid, 0.2 mg; 1 time/day) | Menopausal women (n=120) RCT, 12 weeks | ↓TC, ↓LDL-C, and ↓TG | (Cianci et al., 2012) |
| Berberine (BBR, 1.0 g; phytosterols, 4 g; antioxidants, 2 capsules; probiotics, 12 billion colony forming units; fish oil, 2g; and soy, pea, and whey proteins, 40 g, 2-3 times/day) | CMS patients (n=44) open-label, 2-arm, RCT, 13 weeks |
↓body mass, ↓fat mass, ↓TC, ↓LDL-C, ↓TG, ↓TC/HDL-C, ↓TG/HDL-C, ↓apoB/apoA1, and ↓hs-CRP. | (Dahlberg et al., 2017) |
| Berberine sulfate trihydrate (0.1 g, equiv. 69 mg berberine, BBR); Hop rho iso-alpha acids, 200 mg; vitamin D3, 500 IU; and vitamin K1 500 μg; 2 times/day) | MS postmenopausal women patients (n=51), randomized, single-blind, 2-arm placebo-controlled, RCT, 14 weeks | ↓serum OC, serum ↑25(OH)D, and ↑IGF-I | (Lamb et al., 2011) |
| Berberine (BBR, 0.5 g, 3 times/day) and methylglyoxal (0.5 g ×3 times/day) | T2DM patient (n=200), case–control study, 3 months | ↓HOMA-IR, and ↓MGO | (Memon et al., 2018) |
| Berberine (BBR, 0.5 g; orthosiphon, 300 mg; red yeast rice, 60 mg; monacolin, 3 mg; policosanol, 10 mg; folic acid, 0.2 mg; and coenzyme Q10, 15mg; 1 time/day) | MS patients (n=1161), Double-blind, Randomized, controlled, 1 year | ↓TC, ↓LDL-C, ↓HDL-C, ↓TG, ↓SBP, and ↓DBP | (Manzato and Benvenuti, 2014) |
| Berberis extracts | |||
|
B. aristata stem powder (1.5 and 3 g in two divided doses daily) |
T2DM with dyslipidemic patients (n=90) open parallel, RCT, 9 months | ↓FBS, ↑HDL, ↓TC, ↓TG, and ↓LDL. | (Sharma et al., 2017) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) and only B. aristata extract (Berberine, BBR, 1.0 g) 2 time/day | T2DM patients (n=69), single-blind, RCT, 120 days | ↓IFG, ↓HbA1c, ↓TC, ↓TG, ↓LDL (only Berberol ®), ↓AST, and ↓ALT |
(Di Pierro et al., 2013) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) 2 times/day | T1DM patients (n=85) double-blind, randomized, placebo-controlled, 6 months |
↓TIC, ↓HgbA1c, ↓FPG, ↓PPG, ↓TC, ↓TG, ↓LDL-C, and ↑HDL-C | (Derosa et al., 2016) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) 2 times/day |
Dyslipidemic patients (n=105), Double‐blind, RCT, 3 months | ↓TC, ↓LDL-C, ↓TG, ↑HDL-C, ↓FPI, and ↓HOMA-IR | (Derosa et al., 2013) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) 2 times/day |
T2DM and MS patients (n=50) double-blind placebo-controlled, 6 months | ↓BMI, ↓HOMA-R, ↓TC, ↓WC, ↓HbA1c, and ↓TF% | (Guarino et al., 2015) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) 2 times/day |
T2DM and MS patients (n=136), placebo RCT, 52 weeks | ↓TC, ↑HDL-C, ↓TG, ↓LDL-C, ↓HOMA-R, ↓WC, ↓TF(%), ↓VF(%), ↓UA, ↓HbA1c, ↓SBP, and ↓DBP | (Guarino et al., 2017) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) 2 times/day |
T2DM patients (n = 26), 6 months | ↓HbA1c, ↓basal insulin, ↓TC, ↓LDL-C, ↓TG, ↓HOMA-R, ↓ ALT, and ↓AST | (Di Pierro et al., 2012) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) 2 times/day |
Dyslipidemic patients (n =175), double blind, placebo-controlled, RCT, 6 months | ↓FPG, ↓IC, ↓HOMA, and ↓dosage of statin | (Derosa et al., 2015a) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) 2 times/day |
Euglycemic, dyslipidemic subjects (n=137) double-blind, RCT, placebo-controlled, 6-months | ↓FPG, ↓IC, and ↓HOMA-index | (Derosa et al., 2015b) |
| Berberol ® compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg), Berberol ® + statin, and Berberol ® +ezetimibe; 2 times/day |
T2DM and hypercholesterolemic patients (n=45), 6-months | ↓TC, ↓LDL-C, ↓HDL-C (only Berberol ®), ↓FPG, and ↓HbA1c. |
(Di Pierro et al., 2015) |
| Berberol ® K compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg) and Monakopure™-K20, 50 mg; 1 time/day | Dyslipidemic patients (n=226), non-blind non-randomized, 6 months |
↓TC, ↓LDL-C, ↓TG, and ↓CPK. | (Di Pierro et al., 2018) |
| Berberol ® K compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg), and Monakopure™-K20, 50 mg; 1 time/day | Low cardiovascular risk patients (n=73), double-blind, placebo-controlled, RCT, 3 months | ↑FPI, ↓HOMA, ↓TC, ↓TG, ↓LDL-C, and ↓hs-CRP | (Derosa et al., 2017) |
| Berberol ® K compose by B. aristata (Berberine, BBR, 1.0 g) and S. marianum (silymarin, 210 mg), and Monakopure™-K20, 50 mg; 1 time/day | Diabetic and dyslipidemic patients (n = 59), 6 months | ↓HbA1c, ↓TC, ↓LDL-C), and ↓TG | (Di Pierro et al., 2017) |
| B. aristata (83.3 mg), Cyperus rotundus (83.3 mg), Cedrus deodara (83.3 mg), Emblica officinalis (83.3 mg), Terminalia chebula (83.3 mg) and T. bellirica (83.3 mg) 1-6 timea/day | T2DM patients (n=93) Pilot RCT, 24 weeks | ↓PBG, ↓FBG, ↓TC, and ↓HbA1c. | (Awasthi et al., 2015) |
|
B. vulgaris fruit (aqueous extract, 3 g/day) |
T2DM patients (n=31) Double‐blind, RCT, 3 months |
↓TG, ↓TC, ↓LDL-C, ↓apoB, ↓glucose, ↓insulin, and ↑TAC. | (Shidfar et al., 2012) |
|
B. vulgaris fruit (600 mg/day) |
MS patients (n=106) Double-blind, RCT, 6 weeks | ↓PAB | (Mohammadi et al., 2014) |
|
B. vulgaris juice (10 c.c. of processed extract/day) |
MS patients (n=57) Double-blind, RCT, 8 weeks | ↓LDL-C, ↓TC/HDL-C ratio, ↑HDL, ↑IC, and ↑IR. | (Ebrahimi-Mamaghani et al., 2009) |
|
B. vulgaris fruit (ethanolic extract 1 mg, 3 times/day) |
T2DM patients (n=30) Double-blind, RCT, 8 weeks | ↓SGL, ↓FG, and ↓HbA1c | (Moazezi and Qujeq, 2014) |
|
B. vulgaris juice (480 mL/day) |
women diagnosed with BBD (n =85), 8 weeks | ↓IC, ↓C-peptide, ↓HOMA-IR, ↓glucose/insulin ratio, and ↑HOMA-B. | (Asemani et al., 2018) |
|
B. vulgaris fruit (600 mg/day) |
(n = 106) Double-blind, RCT, 6 weeks | ↓LDL-C, ↓TC, ↑HDL-C, ↓anti-HSPs 27, ↓anti-HSPs 60, and ↓hs-CRP |
(Zilaee et al., 2014) |
The ↑ and ↓ signs show significant increase and significant decrease, respectively, of evaluated factors during mentioned studies. N.I., not informed.
The administration of BBR in patients with MS was found to be effective in regulating the blood glucose and blood lipid levels, improving the InsR, and reducing the level of inflammatory responses in the body (Cao and Su, 2019). BBR also decreased the waist circumference, systolic blood pressure (SBP), triglycerides, and total insulin secretion along with an increase in InsS (Pérez-Rubio et al., 2013). BBR was suggested as a promising new hypolipidemic drug that acts through signaling pathways distinct from those of statins in the treatment of hyper mild mixed hyperlipidemia patients (Kong et al., 2004). Besides, BBR has been shown to have a good potential as a drug to control lipid metabolism alone or in combination with other drugs for hyperlipidemic hepatitis or liver cirrhosis patients (Zhao et al., 2008). Moreover, BBR improved the InsS by limiting fat storage and adjusting adipokine profile in human preadipocytes and MS patients (Yang et al., 2012), and attenuated some of the metabolic and hormonal derangements in women with polycystic ovary syndrome (PCOS (Wei et al., 2012). The administration of BBR was found to be effective in the regulation of blood glucose and blood lipid in T2DM patients (Ming et al., 2018) and in improving diabetic kidney disease by reducing UACR and serum Cys C (Li et al., 2018). On the other hand, BBR had also shown glucose-lowering activity with a mechanism different from metformin and rosiglitazone (Zhang et al., 2010). In pilot study, BBR demonstrated a potent oral hypoglycemic activity with positive effects on lipid metabolism (Yin et al., 2008). Also, the benefits of BBR in lowering blood glucose, lipids, body weight, and blood pressure have been confirmed in T2DM and MS patients (Zhang et al., 2008). BBR played an important role in the treatment T2DM through downregulating the higher levels of free fatty acids (Gu et al., 2010). In another study, BBR reduced the fasting plasma glucose, post-meal blood glucose, and fructosamine; however, no signification changes were found in lipid profiles, fasting insulin, HOMA-IR, and HOMA-β% in T2DM patients (Rashidi et al., 2018).
In addition, BBR improved the glycemic parameters comparable to metformin in T2DM patients (Dange et al., 2016). BBR significantly ameliorated T2DM via modulation of Bifidobacterium species, TNF-α, and LPS (Chen et al., 2016). BBR improved the blood lipid level in mild hyperlipidemia patients (Wang L, et al., 2016). Likewise, it reduced the plasma LDL-C and TG in mixed hyperlipidaemic subjects (Cicero and Ertek, 2008).
The combination of BBR and simvastatin (SIMVA) in hypercholesterolemic patients significantly improved LDL-receptor upregulation and LDL-cholesterol downregulation compared to monotherapies, and the combined effect also reduce the statins dosage (Kong et al., 2008). The administration of BBR along with red yeast and policosanol on a daily basis was found to be effective in reducing cholesterol levels and was associated with the enhancement of endothelial function and InsS (Affuso et al., 2010). The administration of this supplementation in patients with familial hypercholesterolemia heterozygotes on stable treatment with LDL-C-lowering validated that the supplement reduced the LDL-C superior to that obtained by doubling the dose of statins (Pisciotta et al., 2012).
Also, the dietary supplement Armolipid Plus™ composed of BBR, red yeast rice, policosanol, folic acid, coenzyme Q10, and astaxanthin showed significant reduction of cholesterolemia and positive plasma LDL-C levels in elderly (statin-intolerant) hypercholesterolemic patients (Marazzi et al., 2011). Moreover, it reduced LDL-C levels as well as total cholesterol/HDLc and ApoB/ApoA1 ratios, and it increased the Apo A1; tjos demonstrated the improvements in CVD risk indicators in patients with hypercholesterolemia (Sola et al., 2014) and amelioration of blood lipids and significant reduction of global CVD risk in dyslipidemic patients (Trimarco et al., 2011). In patients with low- to moderate-risk hypercholesterolemia, Armolipid Plus ™ in association with a hypolipidic diet significantly reduced the total cholesterol and LDL-C levels (Gonnelli et al., 2015). In addition, Armolipid Plus ™ improved the lipid profile similar to a low dose of a standard statin and also increased the HDL-C levels and improved the leptin-to-adiponectin ratio in patients with moderate dyslipidemia and MS (Ruscica et al., 2014). Armolipid Plus™ alone or in combination with ezetimibe enhanced the lipid profile in statin-intolerant patients with coronary heart disease (Marazzi et al., 2015). BBR and Armolipid Plus™ could be a useful alternative to correct dyslipidemias and to reduce CVD risk in subjects with moderate mixed dyslipidemias (Cicero et al., 2007).
Other food supplements containing BBR, including Body Lipid™, were suggested as an alternative to pharmacological treatment for patients with mild-to-moderate hypercholesterolemia (D'Addato et al., 2017). A new nutraceutical formulation containing BBR, monacolin K, chitosan, and coenzyme Q10 has proven effective in reducing non-HDL/LDL-C levels, representing an emergent therapeutic strategy in dyslipidemic patients (Spigoni et al., 2017). On the other hand, the combination of BBR and isoflavones was found to be effective in lowering CVD risk factors in menopausal women with moderate dyslipidaemia (Cianci et al., 2012).
Treatment with BBR and rho iso-alpha acids, vitamin D3, and vitamin K1 produced a more favorable bone biomarker profile, indicative of healthy bone metabolism in postmenopausal women with MS (Lamb et al., 2011). In a case–control study, BBR is more effective in decreasing the serum MGO levels and InsR through increasing the glycemic control in newly diagnosed T2DM patients (Memon et al., 2018). The intake of the natural formulation (containing BBR, orthosiphon, red yeast rice equivalent to monacolin, policosanol, folic acid, and coenzyme Q10) has evidenced the effective control of plasma lipids and keeps borderline high blood pressure within normal values compared with diet alone (Manzato and Benvenuti, 2014).
Stem powder of B. aristata was found to be effective in improving glycemic control and lipid profiles with no major adverse effects on T2DM patients (Sharma et al., 2017). The effect of B. vulgaris extract on T2DM and MS patients has been widely studied in humans. The intake of 3 g/d of B. vulgaris fruits aqueous extract for 3 months may have beneficial effects on lipoproteins, apoproteins, glycemic control, and TAC in T2DM patients (Shidfar et al., 2012). B. vulgaris juice reduced oxidative burden in patients with MS (Mohammadi et al., 2014). Other study showed the beneficial effects of processed B. vulgaris on certain atherosclerosis risk factors in T2DM patients (Ebrahimi-Mamaghani et al., 2009). B. vulgaris fruit extract showed beneficial metabolic effects in T2DM patients, improving the glucose catabolism via the glycolysis pathway, stimulating the insulin secretion or improving the insulin function, and later decreasing the glucose uptake (Moazezi and Qujeq, 2014). Another study demonstrated that the B. vulgaris juice evoked regulatory roles on HOMA-IR and improved HOMA-B with the metabolic controlling insulin-related indices in benign breast disease (Asemani et al., 2018). Also, B. vulgaris supplementation in patients with MS significantly diminished anti-HSPs 27 and 60 and hs-CRP levels and improved lipid profiles (Zilaee et al., 2014). It is reported that the Hsp60 protein is able to induce the production of anti-Hsp60 antibodies, which leads to the destruction of β-islet cells. In the same way, Hsp60 acts as a proinflammatory signaling molecule, which plays a role in the non-resolved vascular inflammation, and this is recognized as one of the characteristic of T2DM (Juwono and Martinus, 2016). Others natural formulations containing Berberis have also been tested in humans. A clinical trial demonstrated that daily intake of polyherbal capsule composed by B. aristata and Cyperus rotundus, Cedrus deodara, Emblica officinalis, Terminalia chebula, and T. bellirica decreased the glucose level, enhanced lipid homeostasis, and maintained other serum biochemical levels to the normal in patients with T2DM (Awasthi et al., 2015).
The nutraceutical product Berberol®, containing a B. aristata extract (titrated in 85% BBR) plus a Silybum marianum extract (titrated in 60% silymarin), has been evaluated for its antidiabetic potential in humans. Berberol® was demonstrated to be more effective than BBR alone (administered at the same dose), reducing HbA1c in T2DM patients (Di Pierro et al., 2013). The incorporation of Berberol® into insulin therapy in patients with T1DM has the effect of a diminution of the insulin dose necessary for adequate glycemic control (Derosa et al., 2016). In dyslipidemic patients, Berberol® has proven to be safe and effective in improving lipid profile, InsR, and adipocytokines levels (Derosa et al., 2013). Berberol® also improved the cholesterol-lowering properties of statins and showed the positive effects on liver enzymes and glycemic control in patients with T2DM (Guarino et al., 2015). In addition, Berberol® significantly lowered abdominal adiposity and decreased the circulating uric acid level in overweight/obese patients with T2DM (Guarino et al., 2017). Berberol® was suggested as a good candidate for an adjunctive treatment option in diabetes, especially in patients with suboptimal glycemic control (Di Pierro et al., 2012). Berberol® administered as a single or add-on therapy in statin-intolerant subjects is an effective treatment to improve the lipidic and glycemic profiles in T2DM and hypercholesterolemia patients (Di Pierro et al., 2015). The combination of Berberol® and a reduced dosage of statin is found effective for the treatment of hyperlipidemia in patients intolerant to statins at high dosage (Derosa et al., 2015a) and in dyslipidemic euglycemic patients (Derosa et al., 2015b)
Berberol K®, was found to be a potentially good alternative in primary intervention in low cardiovascular-risk subjects with dyslipidemia, as an add-on therapy in mildly statin-intolerant patients, and as an alternative for dyslipidemic patients with a negative perception of statins (Di Pierro et al., 2017). Berberol K® reduced lipid profile effectively and improved the inflammatory parameters under a safe dose (Derosa et al., 2017). It was also found to be effective in diabetic subjects with dyslipidemia statin intolerant or with diarrhea caused by IBS or metformin (Di Pierro et al., 2018).
Few studies have also reported the effectiveness of BBR in non-alcoholic fatty liver disease (NAFLD). NAFLD is a result of abnormal fat accumulation in the liver due to the reasons other than alcohol, and it is considered to be a hepatic manifestation of MS. NAFLD results in the overproduction of sugars and triglycerides and plays a central role in the development of InsR and various other glucose- and lipid metabolism-related diseases (Yki-Järvinen, 2014). Recently, Yan et al. (2015) conducted a randomized, parallel controlled, open-label clinical trial in 188 NAFLD patients. Patients received lifestyle intervention (LSI) or LSI and 15 mg of pioglitazone qd or LSI and of BBR for 16 weeks. Parameters, including hepatic fat content, serum glucose level, serum lipid profiles, liver enzymes, and serum and urine BBR concentrations, were measured before and after treatment. LSI and BBR showed a reduction in hepatic fat content as compared to LSI and were better than pioglitazone in reducing body weight and resulted in better lipid profiles (Yan et al., 2015). Furthermore, a mechanism-based study revealed that BBR reduced hepatic TG accumulation and decreased the expressions of hepatic stearyl-coenzyme A desaturase 1 (SCD1) and other TG synthesis-related genes (Zhu et al., 2019). Berberine administration was also reported to recruit and activate BAT in both humans and mice (Wu et al., 2019).
Conclusion
Although there are many effective therapeutic drugs for the treatment of metabolic diseases, the current treatment did not control the rapid increasing trend in diabetes mortality and morbidity. Various therapeutic agents from both natural and synthetic sources are being investigated in patients with clinical signs of diabetic and other metabolic diseases. Formulations prepared from the various plant parts of Berberis species were found to be used traditionally in the treatment of diabetes and other metabolic diseases and related complications. A review of the scientific literature revealed that the extracts, isolated alkaloids from Berberis species including BBR and their derivatives, have shown promising effects in the studies related to diabetes and other metabolic diseases. The relatively low cost of BBR or supplements or extracts containing BBR, compared to other synthetic medications, will be of an advantage to the patients living in developing countries with poor socioeconomic circumstances. However, currently available scientific evidence is still not fully sufficient to prove their efficacy clinically. Further randomized double-blind clinical trials with a large number of patients and standardized clinical assessments are required to prove the effectiveness of the Berberis extracts and isolated compounds on metabolic diseases alone or in combinations. Novel pharmacological assessment techniques and analytical techniques will further provide additional opportunities for these agents. Moreover, the development of novel formulations of berberine could be an effective strategy for increasing its effectiveness against diabetes and other metabolic diseases.
Author Contributions
TB, IB and JE conceptualized the manuscript. TB, AB, HD, HU, HK, IB and JE wrote the initial manuscript. TB, HD, HU, AP, IB and JE revised the manuscript. All authors agreed on the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
TB and IB are thankful to the Director of GBPNIHESD for providing necessary facilities and to the State Biotech Department, Dehradun, Uttarakhand, for partial financial support under the Grant No. SBP/R&D-02/11/432. JE is grateful for support from CONICYT PAI/ACADEMIA project N° 79160109.
Abbreviations
2h-PPG, 2-hour postprandial plasma glucose; A-FABP, Adipocyte fatty acid–binding protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AMPK, AMP-activated protein kinase; BBA, berbamine; BBD, benign breast disease; BBR, berberine; BFFAL, blood free fatty acids level; BF, berberine fumarate; BGL, blood glucose levels; BJ, Berberis juice; b.i.d., twice daily; BMI, body mass index; BP, blood pressure; BW, body weight; BWG, body weight gain; CAT, catalase; C/EBPα, CCAAT enhancer binding protein alpha; CMS, cardio metabolic syndrome; COX2, Cyclooxygenase-2; CPK, serum creatine phosphokinase; DAG, diacylglycerol; DBP, diastolic blood pressure; DVIS, diabetic vascular insulin sensitivity; DPP-IV, dipeptidyl-peptidase IV; DN, Diabetic nephropathy; eNOS, endothelial nitric oxide synthase; EZE, ezetimibe; FA, fructosamine; FASN, fatty acid synthase; FBS, Fasting Blood Sugar; FBGL, fasting blood glucose levels; FOP, fibrodysplasia ossificans progressive; FPG, fasting plasma glucose; FPI, fasting plasma Insulin; FSIL, fasting serum insulin level; GHb, glycosylated hemoglobin; GLP-1, glucagon-like peptide-1; GLUT4, Glucose transporter type 4; GPx, glutathione peroxidase; GR, glutathione reductase; HbA1c, glycated haemoglobin; HDL-C, high density lipoprotein HDL-cholesterol; HFD, High Fat Diet; HOMA-R, Homeostatic Model Assessment; HOMA-IR, and HOMA-β%; IC, insulin concentration; IFG, Impaired fasting glycemia; IL-6, Interleukin-6; iNOS, Inducible nitric oxide synthase; INSR-mRNA, insulin receptor gene messenger RNA; InsR, Insulin resistance; InsS, insulin sensitivity; LDL-C, low density lipoprotein cholesterol; LDLR, Low density lipoprotein receptor; LEL, liver enzyme levels; Lp, lipid profile; MALA, metformin-associated lactic acidosis; MAPK, Mitogen activated protein kinase; MDA, Malondialdehyde; MMP, mitochondrial membrane potential; MS, metabolic syndrome; RCT: randomized, controlled trial; OXPHOS, impaired oxidative phosphorylation; PAB, prooxidant-antioxidant balance; pBBR, pseudoberberine; PG, plasma glucose; PGs, prostaglandins; P-gp, P-glycoprotein; pi3k, phosphoinositol 3 kinase; PKC, protein kinase C; PMBG, post-meal blood glucose; PON1, Paraoxonase-1; PPARα, peroxisome proliferator activated receptor alpha; PPARγ, peroxisome proliferator activated receptor gamma; PPBG, postprandial blood glucose; SBP, systolic blood pressure; SOD, superoxide dismutase; SREBP-1, sterol regulatory element-binding protein 1; STZ, streptozotocin; T1DM: type-1 diabetes mellitus; T2DM, type-2 diabetes mellitus; TAG, triacylglycerol; TC, total cholesterol; TG, triglycerides; TIC, total insulin consumption; TIS, total insulin secretion; TLR4, Toll-like receptor 4; TNFα, tumor necrosis factor alpha; ULK1, Unc-51-like autophagy-activating kinase 1.
References
- Abd El-Wahab A. E., Ghareeb D. A., Sarhan E. E. M., Abu-Serie M. M., El Demellawy M. A. (2013). In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 13, 218. 10.1186/1472-6882-13-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Zarga M. H., Miana G. A., Shamma M. (1982). Gandharamine: a new benzylisoquinoline alkaloid from Berberis baluchistanica. Heterocycles 18, 63–65. 10.3987/S(B)-1982-01-0063 [DOI] [Google Scholar]
- Adhikari M., Thapa R., Kunwar R. M., Devkota H. P., Poudel P. (2019). Ethnomedicinal uses of plant resources in the machhapuchchhre rural municipality of kaski district, nepal. Medicines 6, 69. 10.3390/medicines6020069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affuso F., Ruvolo A., Micillo F., Saccà L., Fazio S. (2010). Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 20, 656–661. 10.1016/j.numecd.2009.05.017 [DOI] [PubMed] [Google Scholar]
- Ahamad J., Mir S. R., Naquivi K. J. (2012). Hypoglycemic activity of aqueous extract of Berberis aristata stems bark in STZ-induced rats. Int. J. Pharm. Pharm. Sci. 4, 473–474. [Google Scholar]
- Ahamad J., Naquvi K. J., Ali M., Mir S. R. (2014). New isoquinoline alkaloids from the stem bark of Berberis aristata. Indian J. Chem. - Sect. B. Org. Med. Chem. 53B, 1237–1241. [Google Scholar]
- Ahmad M., Alamgeer S. T. (2009). A potential adjunct to insulin: Berberis lycium Royle. Diabetol Croat 38, 13–18. [Google Scholar]
- Ahmad M., Alamgeer C. M. Z., Nadeem M., Sharif T., Ahmad B. (2008). Hepatoprotective effect of Berberis lycium (Royle) in hepatotoxic rabbits. Gomal. Uni J. Res. 24, 1–9. [Google Scholar]
- Ahmed E., Arshad M., Ahmad M., Saeed M., Ishaque M. (2004). Ethnopharmacological survey of some medicinally important plants of galliyat areas of NWFP, Pakistan. Asian J. Plant Sci. 3, 410–415. 10.3923/ajps.2004.410.415 [DOI] [Google Scholar]
- Ahmed B., Masoodi M. H., Khan S. (2008). Pachycanthine: A new isoquinoline alkaloid and its antihepatotoxic activity from Berberis pachycantha Koehne. Indian J. Chem. - Sect. B. Org. Med. Chem. 47, 945–951. 10.1002/chin.200841201 [DOI] [Google Scholar]
- Ahrendt L. W. A. (1961). Berberis and Mahonia A taxonomic revision. J. Linn. Soc. London Bot. 57, 1–410. 10.1111/j.1095-8339.1961.tb00889.x [DOI] [Google Scholar]
- Akhtar M. S., Sajid S. M., Akhtar M. S., Ahmad M. (2008). Hypoglycaemic effect of Berberis aristata roots, aqueous and methanolic extracts in normal and alloxan-diabetic rabbits. Pharmacologyonline 2, 845–856. [Google Scholar]
- Alamzeb M., Khan M. R., Mamoon Ur R. U. R., Ali S., Khan A. A. (2015). A new isoquinoline alkaloid with anti-microbial properties from Berberis jaeschkeana Schneid. var. jaeschkeana. Nat. Prod. Res. 29, 692–697. 10.1080/14786419.2014.981187 [DOI] [PubMed] [Google Scholar]
- Alamzeb M., Omer M., Ur-Rashid M., Raza M., Ali S., Khan B., et al. (2018). NMR, novel pharmacological and in silico docking studies of oxyacanthine and tetrandrine: bisbenzylisoquinoline alkaloids isolated from Berberis glaucocarpa roots. J. Anal. Methods Chem. 2018, 7692913. 10.1155/2018/7692913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti G. (2005). Introduction to the metabolic syndrome. Eur. Hear. J. Suppl. 7, D3–D5. 10.1093/eurheartj/sui021 [DOI] [Google Scholar]
- Alemardan A., Asadi W., Rezaei M., Tabrizi L., Mohammadi S. (2013). Cultivation of iranian seedless barberry (Berberis integerrima ‘bidaneh’): a medicinal shrub. Ind. Crops Prod. 50, 276–287. 10.1016/j.indcrop.2013.07.061 [DOI] [Google Scholar]
- Almani S. A., Memon I. A., Shaikh T. Z., Khoharo H. K., Ujjan I. (2017). Berberine protects against metformin-associated lactic acidosis in induced diabetes mellitus. Iran. J. Basic Med. Sci. 20, 511–515. 10.22038/IJBMS.2017.8675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (2019). 2. Classification and diagnosis of diabetes: standards of medical care in diabetes. Diabetes Care 42, S13–S28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- Andola H. C., Gaira K. S., Rawal R. S., Rawat M. S. M., Bhatt I. D. (2010). Habitat-dependent variations in berberine content of Berberis asiatica Roxb. ex. DC. in Kumaon, Western Himalaya. Chem. Biodivers. 7, 415–420. 10.1002/cbdv.200900041 [DOI] [PubMed] [Google Scholar]
- Andola H. C., Rawal R. S., Bhatt I. D. (2011). Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis species of West Himalaya, India. Food Res. Int. 44, 2352–2356. 10.1016/j.foodres.2010.07.017 [DOI] [Google Scholar]
- Andola H. C., Gaira K. S., Pandey A., Bhatt I. D., Rawal R. S. (2018). Influence of habitat characteristics and altitude on berberine content in Berberis jaeschkeana C.K. Schneid. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 89, 967–972. 10.1007/s40011-018-1014-9 [DOI] [Google Scholar]
- Aribi I., Chemat S., Hamdi-Pacha Y., Luyten W. (2017). Isolation of berberine tannate using a chromatography activity-guided fractionation from root bark of Berberis hispanica Boiss. & Reut. J. Liq. Chromatogr. Relat. Technol. 40, 894–899. 10.1080/10826076.2017.1381850 [DOI] [Google Scholar]
- Asemani S., Montazeri V., Baradaran B., Tabatabiefar M. A., Pirouzpanah S. (2018). The effects of Berberis vulgaris juice on insulin indices in women with benign breast disease: a randomized controlled clinical trial. Iran. J. Pharm. Res. IJPR 17, 110–121. [PMC free article] [PubMed] [Google Scholar]
- Ashraf H., Heidari R., Nejati V., Ilkhanipoor M. (2013). Aqueous extract of Berberis integerrima root improves renal dysfunction in streptozotocin induced diabetic rats. Avicenna J. phytomedicine 3, 82–90. [PMC free article] [PubMed] [Google Scholar]
- Awasthi H., Nath R., Usman K., Mani D., Khattri S., Nischal A., et al. (2015). Effects of a standardized Ayurvedic formulation on diabetes control in newly diagnosed type-2 diabetics; a randomized active controlled clinical study. Complement. Ther. Med. 23, 555–561. 10.1016/j.ctim.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Baharvand-Ahmadi B., Bahmani M., Eftekhari Z., Jelodari M., Mirhoseini M. (2016). Overview of medicinal plants used for cardiovascular system disorders and diseases in ethnobotany of different areas in Iran. J. HerbMed. Pharmacol. 5, 39–44. 10.15171/jnp.2016.08 [DOI] [Google Scholar]
- Bahmani M., Zargaran A., Rafieian-Kopaei M., Saki K. (2014). Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Biomed. 7, S348–S354. 10.1016/S1995-7645(14)60257-1 [DOI] [PubMed] [Google Scholar]
- Bai M., Liu Y., Zhou F., Zhang Y., Zhu Q., Zhang L., et al. (2018). Berberine inhibits glucose oxidation and insulin secretion in rat islets. Endocr. J. 65, 469–477. 10.1507/endocrj.EJ17-0543 [DOI] [PubMed] [Google Scholar]
- Bajpai V., Singh A., Arya K. R., Srivastava M., Kumar B. (2015). Rapid screening for the adulterants of Berberis aristata using direct analysis in real-time mass spectrometry and principal component analysis for discrimination. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 32, 799–807. 10.1080/19440049.2015.1022885 [DOI] [PubMed] [Google Scholar]
- Belwal T., Dhyani P., Bhatt I. D., Rawal R. S., Pande V. (2016). Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 207, 115–124. 10.1016/j.foodchem.2016.03.081 [DOI] [PubMed] [Google Scholar]
- Belwal T., Giri L., Bhatt I. D., Rawal R. S., Pande V. (2017). An improved method for extraction of nutraceutically important polyphenolics from Berberis jaeschkeana C.K. Schneid. fruits. Food Chem. 230, 657–666. 10.1016/j.foodchem.2017.03.086 [DOI] [PubMed] [Google Scholar]
- Bhakuni D. S., Shoeb A., Popli S. P. (1968). Medicinal plants. I. Chemical constituents of Berberis asiatica. Indian J. Chem. 6, 123–127. [Google Scholar]
- Bhardwaj D., Kaushik N. (2012). Phytochemical and pharmacological studies in genus Berberis. Phytochem. Rev. 11, 523–542. 10.1007/s11101-013-9272-x [DOI] [Google Scholar]
- Boudjelthia K., Hammadi K., Kouidri M., Djebli N. (2017). Evaluation of antidiabetic activity of two plants Berberis vulgaris and Zygophyllum geslini. J. Phys. Chem. Biophys. 7, 398–2161. 10.4172/2161-0398.1000236 [DOI] [Google Scholar]
- Brazdovicova B., Kostalova D., Slavik J., Tomko J. (1975). Alkaloids of Berberis julianae. Chem. Zvesti 29, 265–268. [Google Scholar]
- Bullard K. M., Cowie C. C., Lessem S. E., Saydah S. H., Menke A., Geiss L. S., et al. (2018). Prevalence of diagnosed diabetes in adults by diabetes type — United States 2016. Morb. Mortal. Wkly. Rep. 67, 359–361. 10.15585/mmwr.mm6712a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai-Ming T., Jiang X., Ouyang X.-X., Zhang Y.-O., Wei-Dong X. I. E. (2016). Berberine enhances antidiabetic effects and attenuates untoward effects of canagliflozin in streptozotocin-induced diabetic mice. Chin. J. Nat. Med. 14, 518–526. 10.1016/S1875-5364(16)30061-9 [DOI] [PubMed] [Google Scholar]
- Cao C., Su M. (2019). Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. Exp. Ther. Med. 17, 3009–3014. 10.3892/etm.2019.7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Hu L., Chen H., Gao Y., Liang Y., Wu Y., et al. (2017). Berberine alleviates chronic inflammation of mouse model of type 2 diabetes by adjusting intestinal microbes and inhibiting TLR4 signaling pathway. Int. J. Clin. Exp. Med. 10, 10267–10276. [Google Scholar]
- Chakrabarti R., Bhavtaran S., Narendra P., Varghese N., Vanchhawng L., Mohamed Sham Shihabudeen H., et al. (2011). Dipeptidyl peptidase-IV inhibitory activity of Berberis aristata. J. Nat. Prod. 4, 158–163. [Google Scholar]
- Champion S. H., Seth S. K. (1968). A revised survey of the forest types of India (Delhi: Publisher-Manager of Publications; ). [Google Scholar]
- Chand N., Durrani F. R., Qureshi M. S., Durrani Z. (2007). Role of Berberis lycium in reducing serum cholesterol in broilers. Asian-australasian J. Anim. Sci. 20, 563–568. 10.5713/ajas.2007.563 [DOI] [Google Scholar]
- Chandirasegaran G., Elanchezhiyan C., Kavisa G., Hemalatha S. (2017). Protective role of berberine chloride on blood components in streptozotocin induced diabetic rats. Chem. Pharm. Res. 9, 69–73. 10.7897/2230-8407.079107 [DOI] [Google Scholar]
- Chandirasegaran G., Elanchezhiyan C., Ghosh K. (2019). Modulatory effects of berberine chloride on lipid profile, oxidant status and insulin signaling molecules in Streptozotocin induced diabetic rats. Indian J. Clin. Biochem. 34, 254–262. 10.1007/s12291-018-0754-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chandra P., Purohit A. N. (1980). Berberine contents and alkaloid profile of Berberis species from different altitudes. Biochem. Syst. Ecol. 8, 379–380. 10.1016/0305-1978(80)90040-X [DOI] [Google Scholar]
- Chandrasekaran S., Ramajayam N., Pachaiappan P. (2018). Ameliorating effect of berbamine on hepatic key enzymes of carbohydrate metabolism in high-fat diet and streptozotocin induced type 2 diabetic rats. Biomed. Pharmacother. 103, 539–545. 10.1016/j.biopha.2018.04.066 [DOI] [PubMed] [Google Scholar]
- Chang Y., Ge A., Donnapee S., Li J., Bai Y., Liu J., et al. (2015). The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine Jinqi Jiangtang Tablet. J. Ethnopharmacol. 164, 210–222. 10.1016/j.jep.2015.02.018 [DOI] [PubMed] [Google Scholar]
- Chang W., Chen L., Hatch G. M. (2016). Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in H9c2 cardiomyocytes. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1861, 352–362. 10.1016/j.bbalip.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Chen C., Zhang Y., Huang C. (2010). Berberine inhibits PTP1B activity and mimics insulin action. Biochem. Biophys. Res. Commun. 397, 543–547. 10.1016/j.bbrc.2010.05.153 [DOI] [PubMed] [Google Scholar]
- Chen L., Lu W., Li Y. (2016). Berberine ameliorates type 2 diabetes via modulation of Bifidobacterium species, tumor necrosis factor-alpha, and lipopolysaccharide. Int. J. Clin. Exp. Med. 9, 9365–9372. [Google Scholar]
- Cheng Z., Pang T., Gu M., Gao A.-H., Xie C.-M., Li J.-Y., et al. (2006). Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta - Gen. Subj. 1760, 1682–1689. 10.1016/j.bbagen.2006.09.007 [DOI] [PubMed] [Google Scholar]
- Choi Y.-J., Lee K.-Y., Jung S.-H., Kim H. S., Shim G., Kim M.-G., et al. (2017). Activation of AMPK by berberine induces hepatic lipid accumulation by upregulation of fatty acid translocase CD36 in mice. Toxicol. Appl. Pharmacol. 316, 74–82. 10.1016/j.taap.2016.12.019 [DOI] [PubMed] [Google Scholar]
- Choudhary A. S., Sharma A., Sharma P., Joshi Y. C., Sharma M. C., Dobhal M. P. (2010). Isolation and characterization of isoquinoline alkaloids from methanolic extract of Berberis chitria Lindl. J. Indian Chem. Soc 87, 635–636. [Google Scholar]
- Chow Y.-L., Sogame M., Sato F. (2016). 13-Methylberberine, a berberine analogue with stronger anti-adipogenic effects on mouse 3T3-L1 cells. Sci. Rep. 6, 38129. 10.1038/srep38129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci A., Cicero A. F. G., Colacurci N., Matarazzo M. G., De Leo V. (2012). Activity of isoflavones and berberine on vasomotor symptoms and lipid profile in menopausal women. Gynecol. Endocrinol. 28, 699–702. 10.3109/09513590.2011.652250 [DOI] [PubMed] [Google Scholar]
- Cicero A. F. G., Ertek S. (2008). Natural sources of antidyslipidaemic agents: is there an evidence-based approach for their prescription? Med. J. Nutr. Metab. 1, 85–93. 10.3233/s12349-008-0011-6 [DOI] [Google Scholar]
- Cicero A. F. G., Rovati L. C., Setnikar I. (2007). Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. Arzneimittelforschung 57, 26–30. 10.1055/s-0031-1296582 [DOI] [PubMed] [Google Scholar]
- Cok A., Plaisier C., Salie M. J., Oram D. S., Chenge J., Louters L. L. (2011). Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 93, 1187–1192. 10.1016/j.biochi.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G., Qin X., Zhang Y., Gong Z., Ge B., Zang Y. Q. (2009). Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J. Biol. Chem. 284, 28420–28429. 10.1074/jbc.M109.012674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H.-X., Hu Y.-N., Li J.-W., Yuan K. (2018). Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid. Med. Cell. Longev. 2018, 8930374. 10.1155/2018/8930374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addato S., Scandiani L., Mombelli G., Focanti F., Pelacchi F., Salvatori E., et al. (2017). Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q(10) on lipid levels: a randomized, double-blind, placebo controlled study. Drug Des. Devel. Ther. 11, 1585–1592. 10.2147/DDDT.S128623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg C. J., Ou J. J., Babish J. G., Lamb J. J., Eliason S., Brabazon H., et al. (2017). A 13-week low glycemic load diet and lifestyle modification program combining low glycemic load protein shakes and targeted nutraceuticals improved weight loss and cardio-metabolic risk factors. Can. J. Physiol. Pharmacol. 95, 1414–1425. 10.1139/cjpp-2016-0704 [DOI] [PubMed] [Google Scholar]
- Dange S. V., Shende S. S., Rane B. T., Tilak A. V., Vaidya M. U., Limaye M. V. (2016). An observational study of the antidiabetic activity of berberine in newly diagnosed type 2 diabetes mellitus patients. J. Pharm. Biomed. Sci. 6, 230–233. [Google Scholar]
- Davì G., Santilli F., Patrono C. (2010). Nutraceuticals in diabetes and metabolic syndrome. Cardiovasc. Ther. 28, 216–226. 10.1111/j.1755-5922.2010.00179.x [DOI] [PubMed] [Google Scholar]
- Deedwania P. C., Volkova N. (2005). Current treatment options for the metabolic syndrome. Curr. Treat. Options Cardiovasc. Med. 7, 61–74. 10.1007/s11936-005-0007-1 [DOI] [PubMed] [Google Scholar]
- Derosa G., Bonaventura A., Bianchi L., Romano D., D'Angelo A., Fogari E., et al. (2013). Effects of Berberis aristata/Silybum marianum association on metabolic parameters and adipocytokines in overweight dyslipidemic patients. J. Biol. Regul. Homeost. Agents 27, 717–728. [PubMed] [Google Scholar]
- Derosa G., Romano D., D'Angelo A., Maffioli P. (2015. a). Berberis aristata/Silybum marianum fixed combination (Berberol®) effects on lipid profile in dyslipidemic patients intolerant to statins at high dosages: a randomized, placebo-controlled, clinical trial. Phytomedicine 22, 231–237. 10.1016/j.phymed.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Derosa G., Romano D., D'Angelo A., Maffioli P. (2015. b). Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis 239, 87–92. 10.1016/j.atherosclerosis.2014.12.043 [DOI] [PubMed] [Google Scholar]
- Derosa G., D'Angelo A., Maffioli P. (2016). The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 35, 1091–1095. 10.1016/j.clnu.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Derosa G., D'Angelo A., Romano D., Maffioli P. (2017). Effects of a combination of Berberis aristata, silybum marianum and monacolin on lipid profile in subjects at low cardiovascular risk; a double-blind, randomized, placebo-controlled trial. Int. J. Mol. Sci. 18, 343. 10.3390/ijms18020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F., Villanova N., Agostini F., Marzocchi R., Soverini V., Marchesini G. (2012). Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes. Metab. Syndr. Obes. 5, 213–217. 10.2147/DMSO.S33718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F., Putignano P., Villanova N., Montesi L., Moscatiello S., Marchesini G. (2013). Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin. Pharmacol. Adv. Appl. 5, 167–174. 10.2147/CPAA.S54308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F., Bellone I., Rapacioli G., Putignano P. (2015). Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins. Diabetes Metab. Syndr. Obes. Targets Ther. 8, 89–96. 10.2147/DMSO.S78877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F., Putignano P., Ferrara T., Raiola C., Rapacioli G., Villanova N. (2017). Retrospective analysis of the effects of a highly standardized mixture of Berberis aristata, Silybum marianum, and monacolins K and KA in patients with dyslipidemia. Clin. Pharmacol. Adv. Appl. 9, 1–9. 10.2147/CPAA.S120032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F., Putignano P., Villanova N. (2018). Retrospective analysis of the effects of a highly standardized mixture of Berberis aristata, Silybum marianum, and monacolins K and KA in diabetic patients with dyslipidemia. Acta Biomed. 88, 462–469. 10.23750/abm.v88i4.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Wang N., Zhao L., Lu F. (2012). Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evidence-Based Complement. Altern. Med. 2012, 591654. 10.1155/2012/591654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Chen Y.-T., Yang Y.-X., Zhou X.-J., Dai S.-J., Tong J.-F., et al. (2016). Metabolomics study of type 2 diabetes mellitus and the antidiabetic effect of Berberine in Zucker Diabetic fatty rats using Uplc-ESI-Hdms. Phyther. Res. 30, 823–828. 10.1002/ptr.5587 [DOI] [PubMed] [Google Scholar]
- Durmuskahya C., Öztürk M. (2013). Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malaysiana. 42, 1431–1438. 10.2174/2210289201304010288 [DOI] [Google Scholar]
- Ebrahimi-Mamaghani M., Arefhosseini S. R., Golzarand M., Aliasgarzadeh A., Vahed-Jabbary M. (2009). Long-term effects of processed Berberis vulgaris on some metabolic syndrome components. Iran. J. Endocrinol. Metab. 39, 41–47. [Google Scholar]
- Ezuruike U. F., Prieto J. M. (2014). The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J. Ethnopharmacol. 155, 857–924. 10.1016/j.jep.2014.05.055 [DOI] [PubMed] [Google Scholar]
- Fajardo V., Cárcamo C., Moreno B. (1996). Ilicifoline: new berbine dimer alkaloid from Berberis ilicifolia. Heterocycles 43, 949–951. 10.3987/COM-94-6909 [DOI] [Google Scholar]
- Fajardo V., Araya M., Cuadra P., Oyarzun A., Gallardo A., Cueto M., et al. (2009). Pronuciferine N-oxide, a proaporphine N-oxide alkaloid from Berberis coletioides. J. Nat. Prod. 72, 1355–1356. 10.1021/np9000976 [DOI] [PubMed] [Google Scholar]
- Falco M. R., de Vries J. X., de Brovetto A. G., Macció Z., Rebuffo S., Bick I. R. C. (1968). Two new alkaloids from Berberis laurina billb. Tetrahedron Lett. 9, 1953–1959. 10.1016/S0040-4039(01)99064-1 [DOI] [PubMed] [Google Scholar]
- Faskhutdinov M. F., Karimov A., Levkovich M. G., Abdullaev N. D., Shakirov R. (1997). Berberis alkaloids XXXV. the structure of nummularine. Chem. Nat. Compd. 33, 70–72. 10.1007/BF02273928 [DOI] [Google Scholar]
- Fazal Hussain S., Tariq Siddiqui M., Shamm M. (1980). Khyberine and the biogenesis of dimeric aporphine-benzylisqoquinoline alkaloids. Tetrahedron Lett. 21, 4573–4576. 10.1016/0040-4039(80)80076-1 [DOI] [Google Scholar]
- Feng T., Du H., Chen H., Xiao Q., He Y., Fan G. (2018). Comparative analysis of genetic and chemical differences between four Berberis Herbs based on molecular phylogenetic and HPLC methods. Biol. Pharm. Bull. 41, 1870–1873. 10.1248/bpb.b18-00327 [DOI] [PubMed] [Google Scholar]
- Fu Y., Hu B. R., Tang Q., Fu Q., Zhang Q., Xiang J. Z. (2005). Effect of jatrorrhizine, berberine, Huanglian Decoction and compound-mimic prescription on blood glucose in mice. Chin. Tradit. Herb. Drugs 36, 548–551. [Google Scholar]
- Furrianca M. C., Alvear M., Zambrano T., Fajardo V., Salazar L. A. (2017). Hypoglycemic effect of Berberis microphylla G Forst root extract. Trop. J. Pharm. Res. 16, 2179–2184. 10.4314/tjpr.v16i9.19 [DOI] [Google Scholar]
- Geng F., Li G., Zhang X., Zhang P., Dong M., Zhao Z., et al. (2016). Berberine improves mesenteric artery insulin sensitivity through up-regulating insulin receptor-mediated signalling in diabetic rats. Br. J. Pharmacol. 173, 1569–1579. 10.1111/bph.13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A. P., Duarte F. V., Nunes P., Hubbard B. P., Teodoro J. S., Varela A. T., et al. (2012). Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1822, 185–195. 10.1016/j.bbadis.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Hu M., Huang Z., Fang K., Wang D., Chen Q., et al. (2017). Berberine attenuates intestinal mucosal barrier dysfunction in type 2 diabetic rats. Front. Pharmacol. 8, 42. 10.3389/fphar.2017.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnelli S., Caffarelli C., Stolakis K., Cuda C., Giordano N., Nuti R. (2015). Efficacy and tolerability of a nutraceutical combination (red yeast rice, policosanols, and berberine) in patients with low-moderate risk hypercholesterolemia: a double-blind, placebo-controlled study. Curr. Ther. Res. 77, 1–6. 10.1016/j.curtheres.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Zhang Y., Shi X., Li X., Hong J., Chen J., et al. (2010). Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta 81, 766–772. 10.1016/j.talanta.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Guarino G., Della Corte T., Sofia M., Carbone L., Marino G., Martedì E., et al. (2015). Metabolic effects of the association Berberis aristata/Silybum marianum: a preliminary double-blind, placebo-controlled study in obese patients with type 2 diabetes. Nutrafoods 14, 181–188. 10.1007/s13749-015-0052-7 [DOI] [Google Scholar]
- Guarino G., Strollo F., Carbone L., Della Corte T., Letizia M., Marino G., et al. (2017). Bioimpedance analysis, metabolic effects and safety of the association Berberis aristata/Silybum marianum: a 52-week double-blind, placebo-controlled study in obese patients with type 2 diabetes. J. Biol. Regul. Homeost. Agents 31, 495–502. [PubMed] [Google Scholar]
- Gulfraz M., Qadir G., Nosheen F., Parveen Z. (2007). Antihyperglycemic effects of Berberis lyceum Royle in alloxan induced diabetic rats. Diabetol. Croat. 36, 49–54. [Google Scholar]
- Gulfraz M., Mehmood S., Ahmad A., Fatima N., Praveen Z., Williamson E. M. (2008). Comparison of the antidiabetic activity of Berberis lyceum root extract and berberine in alloxan-induced diabetic rats. Phyther. Res. 22, 1208–1212. 10.1002/ptr.2438 [DOI] [PubMed] [Google Scholar]
- Gupta J. K., Mishra P., Rani A., Mazumder P. M. (2010). Blood glucose lowering potential of stem bark of Berberis aristata Dc in alloxan-induced diabetic rats. Iran. J. Pharmacol. Ther. 9, 20–21. [Google Scholar]
- Hamayun M., Khan S. A., Sohn E. Y., Lee I.-J. (2006). Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia 11, 101–113. 10.1300/J044v12n04_02 [DOI] [Google Scholar]
- Han J., Lin H., Huang W. (2011). Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 17, RA164–RA167. 10.12659/MSM.881842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Sheng W., Li X., Sik A., Lin H., Liu K., et al. (2019). Novel carbohydrate modified berberine derivatives: synthesis and in vitro anti-diabetic investigation. MedChemComm 10, 598–605. 10.1039/C9MD00036D [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M.-K., Lu F.-E., Wang K.-F., Leng S. H., Xu L. J., Zhou X. (2004). Effect and mechanisms of berberine on hyperlipidemic and insulin resistant rats. Chin. J. Hosp. Pharm. 24, 389–390. [Google Scholar]
- Hemmati M., Serki E., Gholami M., Hoshyar R. (2016). Effects of an ethanolic extract of Berberis vulgaris fruits on hyperglycemia and related gene expression in streptozotocin-induced diabetic rats. Clin. Phytosci. 2, 1–7. 10.1186/s40816-016-0017-4 [DOI] [Google Scholar]
- Hošt'álková A., Novák Z., Pour M., Jirošová A., Opletal L., Kuneš J., et al. (2013). Berbanine: a new isoquinoline-isoquinolone alkaloid from Berberis vulgaris (Berberidaceae). Nat. Prod. Commun. 8, 441–442. 10.1177/1934578X1300800407 [DOI] [PubMed] [Google Scholar]
- Hosry L. E., Boyer L., Garayev E. E., Mabrouki F., Bun S.-S., Debrauwer L., et al. (2016). Chemical composition, antioxidant and cytotoxic activities of roots and fruits of Berberis libanotica. Nat. Prod. Commun. 11, 645–648. 10.1177/1934578X1601100523 [DOI] [PubMed] [Google Scholar]
- Hostalkova A., Marikova J., Opletal L., Korabecny J., Hulcova D., Kunes J., et al. (2019). Isoquinoline alkaloids from Berberis vulgaris as potential lead compounds for the treatment of alzheimer's disease. J. Nat. Prod. 82, 239–248. 10.1021/acs.jnatprod.8b00592 [DOI] [PubMed] [Google Scholar]
- Huang C., Zhang Y., Gong Z., Sheng X., Li Z., Zhang W., et al. (2006). Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARγ pathway. Biochem. Biophys. Res. Commun. 348, 571–578. 10.1016/j.bbrc.2006.07.095 [DOI] [PubMed] [Google Scholar]
- Huang C., Tian X., Liu F., Li Z., Lin Y., Liu H., et al. (2019). Enhanced anti-diabetic effect of berberine combined with timosaponin B2 in Goto-Kakizaki rats, associated with increased variety and exposure of effective substances through intestinal absorption. Front. Pharmacol. 10, 19. 10.3389/fphar.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Q., Mushtaq W., Ishtiaq M., Anjum M., Faisal M., Mazhar M. (2017). Comparative In vivo antidiabetic evaluation of leaves and bark of Berberis lyceum Royle in alloxan induced diabetic rabbits. Int. J. Biosci. 11, 91–98. 10.12692/ijb/11.2.91-98 [DOI] [Google Scholar]
- Hussaini F. A., Shoeb A. (1985). Isoquinoline derived alkaloids from Berberis chitria. Phytochemistry 24, 633. 10.1016/S0031-9422(00)80794-3 [DOI] [Google Scholar]
- Imenshahidi M., Hosseinzadeh H. (2016). Berberis vulgaris and Berberine: an update review. Phyther. Res. 30, 1745–1764. 10.1002/ptr.5693 [DOI] [PubMed] [Google Scholar]
- Istatkova R., Philipov S., Tuleva P., Amgalan S., Samdan J., Dangaa S. (2007). Alkaloids from Mongolian species Berberis sibirica Pall. Comptes Rendus L'Academie Bulg. Des. Sci. 60, 1177–1182. [Google Scholar]
- Jabeen N., Saleem A., Anwaar S., Hussain Z. (2015). Berberis lycium Royle (Royle 1837): a threatened medicinal plant and its biological activities. EC Agric. 1, 100–108. [Google Scholar]
- Jia D., Li Z. W., Zhou X., Gao Y., Feng Y., Ma M., et al. (2019). A novel berberine-metformin hybrid compound exerts therapeutic effects on obese type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 46, 533–544. 10.1111/1440-1681.13085 [DOI] [PubMed] [Google Scholar]
- Jiang S.-J., Dong H., Li J.-B., Xu L.-J., Zou X., Wang K.-F., et al. (2015). Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol. WJG 21, 7777. 10.3748/wjg.v21.i25.7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Cui H., Wang J., Liu H., Dang M., Zhang Q., et al. (2017). Protective role of berberine and Coptis chinensis extract on T2MD rats and associated islet Rin−5f cells. Mol. Med. Rep. 16, 6981–6991. 10.3892/mmr.2017.7467 [DOI] [PubMed] [Google Scholar]
- Juwono J., Martinus R. D. (2016). Does Hsp60 Provide a link between mitochondrial stress and inflammation in diabetes Mellitus? J. Diabetes Res. 2016, 8017571. 10.1155/2016/8017571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami M., Sepehrimanesh M., Koohi-Hosseinabadi O., Fattahi M., Jahromi I. R., Mokhtari M., et al. (2016). Therapeutic effects of hydroalcoholic and aqueous extracts of Berberis vulgaris fruits in streptozotocin induced type 1 diabetes mellitus rats. Rom. J. Diabetes Nutr. Metab. Dis. 23, 239–245. 10.1515/rjdnmd-2016-0028 [DOI] [Google Scholar]
- Karimov A., Lutfullin K. L. (1986). Berberis alkaloids. 2'-N-methylisotetrandrine from Berberis oblonga. Khimiya Prir. Soedin. 2, 249–251. [Google Scholar]
- Karimov A., Shakirov R. (1993). Berberis alkaloids. XX. investigation of the alkaloids of Berberis iliensis. Chem. Nat. Compd. 29, 69–70. 10.1007/BF00631020 [DOI] [Google Scholar]
- Karimov A., Telezhenetskaya M. V., Lutfullin K. L., Yunusov S. Y. (1977). Berberis alkaloids. the new alkaloid oblongamine. Chem. Nat. Compd. 13, 68–70. 10.1007/BF00565503 [DOI] [Google Scholar]
- Karimov A., Butayarov A. B., Yusupov M. M., Mirzamatov R. T., Shakirov R. S. (1992). Berberis alkaloids XIII. an investigation of the alkaloids of Berberis heteropoda. Chem. Nat. Compd. 28, 523–524. 10.1007/BF00630680 [DOI] [Google Scholar]
- Karimov A., Abdullaev N. D., Shakirov R. (1993. a). Berberis alkaloids. XVI. Structure of berpodine. Chem. Nat. Compd. 29, 219–221. 10.1007/BF00630120 [DOI] [Google Scholar]
- Karimov A., Faskhutdinov M. F., Abdullaev N. D., Levkovich M. G., Mil'grom E. G., Rashkes Y. V., et al. (1993. b). Berberis alkaloids XXXII. Berberal—A new alkaloid from Berberis heterobotrys. Chem. Nat. Compd. 29, 774–777. 10.1007/BF00629649 [DOI] [Google Scholar]
- Karimov A., Levkovich M. G., Abdullaev N. D., Shakirov R. (1993. c). Berberis alkaloids. XXIII. structure of turcberine. Chem. Nat. Compd. 29, 63–67. 10.1007/BF00631018 [DOI] [Google Scholar]
- Karimov A., Levkovich M. G., Abdullaev N. D., Shakirov R. (1993. d). Berberis alkaloids. XXIV. structure of bernumine. Chem. Nat. Compd. 29, 331–334. 10.1007/BF00630532 [DOI] [Google Scholar]
- Karimov A., Levkovich M. G., Abdullaev N. D., Shakirov R. (1993. e). Berberis alkaloids. XXIX. an investigation of the alkaloids of Berberis sibirica. Chem. Nat. Compd. 29, 361–364. 10.1007/BF00630540 [DOI] [Google Scholar]
- Karimov A., Levkovich M. G., Abdullaev N. D., Shakirov R. (1993. f). Berberis alkaloids XXXI. the structure of turconidine. Chem. Nat. Compd. 29, 771–773. 10.1007/BF00629648 [DOI] [Google Scholar]
- Karimov A., Tashkhodzhaev B., Rashkes Y. V., Makhmudov M. K., Mil'grom E. G. (1993. g). Berberis alkaloids. XXI. intebrine—a new N-benzylisoquinoline alkaloid from Berberis integerrima. Chem. Nat. Compd 29, 53–57. 10.1007/BF00631015 [DOI] [Google Scholar]
- Karimov A., Vinogradova V. I., Shakirov R. (1993. h). Berberis alkaloids. XXII. interbrinine and intebrimine—New alkaloids from Berberis integerrima. Chem. Nat. Compd. 29, 57–60. 10.1007/BF00631016 [DOI] [Google Scholar]
- Karimov A., Yusupov M. M., Shakirov R. (1993. i). Berberis alkaloids. XV. Structure of bargustanine. Chem. Nat. Compd. 29, 35–38. 10.1007/BF00631010 [DOI] [Google Scholar]
- Karr S. (2017). Epidemiology and management of hyperlipidemia. Am. J. Manage. Care 23, S139–S148. [PubMed] [Google Scholar]
- Khamidov I., Telezhenetskaya M. V., Karimov A., Shakirov R. (1995). Berberis alkaloids. XXXIII. Investigations of the alkaloids of Berberis vulgaris. Chem. Nat. Compd. 31, 417–418. 10.1007/BF01165220 [DOI] [Google Scholar]
- Khamidov I., Faskhutdinov M., Telezhenetskaya M. V., Karimov A., Levkovich M. G., Abdullaev N. D., et al. (1996. a). Berberis alkaloids. XXXIV. Turcomanine, a new alkaloid from Berberis turcomanica. Khimiya Prir. Soedin. 1, 74–76. 10.1007/BF01373793 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Telezhenetskaya M. V., Faskhutdinov M. F., Karimov A. K., Dzhepberov I. (1996. b). Berberis alkaloids XXXVI. turcomanidine—a new alkaloid from Berberis turcomanica. Chem. Nat. Compd. 32, 873–875. 10.1007/BF01374018 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Telezhenetskaya M. V., Karimov A. K. (1996. c). Berberis Alkaloids XXXVIII. turcamine—a new isoquinoline alkaloid from Berberis turcomanica. Chem. Nat. Compd. 32, 880–881. 10.1007/BF01374020 [DOI] [Google Scholar]
- Khamidov I., Karimo A. K., Telezhenetskaya M. V., Tashkhodzhaev B. (1996. d). Berberis alkaloids XXXV. An Investigation of Berberis turcomanica. Chem. Nat. Compd. 32, 89–90. 10.1007/BF01373805 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Karimov A., Yusupov M. M. (1997. a). Berberis alkaloids. XL. an investigation of the alkaloids of Berberis thunbergii. Chem. Nat. Compd. 33, 599. 10.1007/BF02254817 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Karimov A., Yusupov M. M. (1997. b). Berberis alkaloids. XL. an investigation of the alkaloids of Berberis thunbergii. Chem. Nat. Compd. 33, 599–599. 10.1007/BF02254817 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Telezhenetskaya M. V., Karimov A., Dzhenberov I. (1997. c). Berberis alkaloids XXXIX. new alkaloids from B. densiflora. Chem. Nat. Compd. 33, 323–325. 10.1007/BF02234886 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Karimov A. K. (2003). Berberis alkaloids. XLI. Alkaloids from leaves of cultivated Berberis oblonga. Chem. Nat. Compd. 39, 407. 10.1023/B:CONC.0000003429.41497.b6 [DOI] [Google Scholar]
- Khan I., Ahmad H., Ahmad B., Azam S. (2014). Antiglycation and antioxidation properties of Berberis lyceum and Terminalia chebula: possible role in curing diabetes and slowing aging. Pak J. Bot. 46, 1469–1471. [Google Scholar]
- Kimani N. L., Njangiru I. K., Njagi E. N. M., Orinda G. O. (2017). Antidiabetic activity of administration of aqueous extract of Berberis holstii. J. Diabetes Metab. 8, 11. 10.4172/2155-6156.1000774 [DOI] [Google Scholar]
- Kishimoto A., Dong S., Negishi H., Yasui N., Sun J., Ikeda K. (2015). Effects of berberine on adipose tissues and kidney function in 3T3-L1 cells and spontaneously hypertensive rats. Nat. Prod. Commun. 10, 1543–1546. 10.1177/1934578X1501000914 [DOI] [PubMed] [Google Scholar]
- Ko B.-S., Choi S. B., Park S. K., Jang J. S., Kim Y. E., Park S. (2005). Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol. Pharm. Bull. 28, 1431–1437. 10.1248/bpb.28.1431 [DOI] [PubMed] [Google Scholar]
- Kong W., Wei J., Abidi P., Lin M., Inaba S., Li C., et al. (2004). Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10, 1344–1351. 10.1038/nm1135 [DOI] [PubMed] [Google Scholar]
- Kong W. J., Wei J., Zuo Z. Y., Wang Y. M., Song D. Q., You X. F., et al. (2008). Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 57, 1029–1037. 10.1016/j.metabol.2008.01.037 [DOI] [PubMed] [Google Scholar]
- Kong W.-J., Zhang H., Song D.-Q., Xue R., Zhao W., Wei J., et al. (2009). Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism 58, 109–119. 10.1016/j.metabol.2008.08.013 [DOI] [PubMed] [Google Scholar]
- Kumar A., Aswal S., Chauhan A., Semwal R. B., Kumar A., Semwal D. K. (2019). Ethnomedicinal Investigation of Medicinal Plants of Chakrata Region (Uttarakhand) Used in the Traditional Medicine for Diabetes by Jaunsari Tribe. Nat. Prod. Bioprospect. 9, 175–200. 10.1007/s13659-019-0202-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. J., Holick M. F., Lerman R. H., Konda V. R., Minich D. M., Desai A., et al. (2011). Nutritional supplementation of hop rho iso-alpha acids, berberine, vitamin D3, and vitamin K1 produces a favorable bone biomarker profile supporting healthy bone metabolism in postmenopausal women with metabolic syndrome. Nutr. Res. 31, 347–355. 10.1016/j.nutres.2011.03.016 [DOI] [PubMed] [Google Scholar]
- Lan T., Wu T., Chen C., Chen X., Hao J., Huang J., et al. (2014). Berberine attenuates high glucose-induced proliferation and extracellular matrix accumulation in mesangial cells: involvement of suppression of cell cycle progression and NF-κB/AP-1 pathways. Mol. Cell. Endocrinol. 384, 109–116. 10.1016/j.mce.2014.01.022 [DOI] [PubMed] [Google Scholar]
- Lan J., Zhao Y., Dong F., Yan Z., Zheng W., Fan J., et al. (2015). Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 161, 69–81. 10.1016/j.jep.2014.09.049 [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Kim W. S., Kim K. H., Yoon M. J., Cho H. J., Shen Y., et al. (2006). Berberine, a natural plant product, activates AMP-Activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55, 2256 LP–2264. 10.2337/db06-0006 [DOI] [PubMed] [Google Scholar]
- Leet J. E., Freyer A. J., Shamma M., Fajardo V. (1983). Some dimeric benzylisoquinoline alkaloids with an unusual oxygenation pattern. J. Nat. Prod. 46, 908–912. 10.1021/np50030a013 [DOI] [Google Scholar]
- Li Z., Jiang J.-D., Kong W.-J. (2014). Berberine upregulates hepatic low-density lipoprotein receptor through ras-independent but amp-activated protein kinase-dependent raf-1 activation. Biol. Pharm. Bull. 37, 1766–1775. 10.1248/bpb.b14-00412 [DOI] [PubMed] [Google Scholar]
- Li X.-X., Li C.-B., Xiao J., Gao H.-Q., Wang H.-W., Zhang X.-Y., et al. (2015). Berberine attenuates vascular remodeling and inflammation in a rat model of metabolic syndrome. Biol. Pharm. Bull. Pharm. Bull. 38, 862–868. 10.1248/bpb.b14-00828 [DOI] [PubMed] [Google Scholar]
- Li M., Shu X., Xu H., Zhang C., Yang L., Zhang L., et al. (2016). Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds. J. Transl. Med. 14, 237–250. 10.1186/s12967-016-0987-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yuan K., Shang S., Guo Y. (2017). A safer hypoglycemic agent for type 2 diabetes—Berberine organic acid salt. J. Funct. Foods 38, 399–408. 10.1016/j.jff.2017.09.031 [DOI] [Google Scholar]
- Li Z. Y., Liu B., Zhuang X. J., Shen Y. D., Tian H. R., Ji Y., et al. (2018). Effects of berberine on the serum cystatin C levels and urine albumin/creatine ratio in patients with type 2 diabetes mellitus. Zhonghua Yi Xue Za Zhi 98, 3756–3761. 10.3760/cma.j.issn.0376-2491.2018.46.007 [DOI] [PubMed] [Google Scholar]
- Li C., Gan H., Tan X., Hu Z., Deng B., Sullivan M. A., et al. (2019). Effects of active ingredients from traditional Chinese medicines on glycogen molecular structure in diabetic mice. Eur. Polym. J. 112, 67–72. 10.1016/j.eurpolymj.2018.12.039 [DOI] [Google Scholar]
- Liu C., Beecher C. W. W., Zhao S. (1995). A benzylisoquinoline alkaloid from Berberis virgetorum. J. Nat. Prod. 58, 1100–1102. 10.1021/np50121a021 [DOI] [Google Scholar]
- Liu L., Deng Y., Yu S., Lu S., Xie L., Liu X. (2008). Berberine attenuates intestinal disaccharidases in streptozotocin-induced diabetic rats. Die Pharm. Int. J. Pharm. Sci. 63, 384–388. 10.1691/ph.2008.7778 [DOI] [PubMed] [Google Scholar]
- Liu X., Li G., Zhu H., Huang L., Liu Y., Ma C., et al. (2010). Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPs, LXRα and PPARα transcriptional programs. Endocr. J. 57, 881–893. 10.1507/endocrj.K10E-043 [DOI] [PubMed] [Google Scholar]
- Liu C., Wang Z., Song Y., Wu D., Zheng X., Li P., et al. (2015). Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. BioMed. Res. Int. 2015, 313808. 10.1155/2015/313808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. S., Yu Y. L., Zhu H. J., Liu X. D., Liu L., Liu Y. W., et al. (2009). Berberine promotes glucagon-like peptide-1 (7-36) amide secretion in streptozotocin-induced diabetic rats. J. Endocrinol. 200, 159–165. 10.1677/JOE-08-0419 [DOI] [PubMed] [Google Scholar]
- Mahmoud A. M., Abdel-Rahman M. M., Bastawy N. A., Eissa H. M. (2017). Modulatory effect of berberine on adipose tissue PPARγ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 7, 1–10. 10.7324/JAPS.2017.70401 [DOI] [Google Scholar]
- Manzato E., Benvenuti C. (2014). Controlled clinical study on the effect of a patented combination of berberine, red yeast rice and orthosiphon on lipids and borderline high blood pressure versus diet alone in metabolic syndrome. Eur. J. Prev. Cardiol. 21. [Google Scholar]
- Marazzi G., Cacciotti L., Pelliccia F., Iaia L., Volterrani M., Caminiti G., et al. (2011). Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv. Ther. 28, 1105–1113, S116. 10.1007/s12325-011-0082-5 [DOI] [PubMed] [Google Scholar]
- Marazzi G., Pelliccia F., Campolongo G., Quattrino S., Cacciotti L., Volterrani M., et al. (2015). Usefulness of nutraceuticals (Armolipid Plus) versus ezetimibe and combination in statin-intolerant patients with dyslipidemia with coronary heart disease. Am. J. Cardiol. 116, 1798–1801. 10.1016/j.amjcard.2015.09.023 [DOI] [PubMed] [Google Scholar]
- Meliani N., Dib M. E. A., Allali H., Tabti B. (2011). Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 1, 468–471. 10.1016/S2221-1691(11)60102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon M. A., Khan R. N., Riaz S., Ain Q. U., Ahmed M., Kumar N. (2018). Methylglyoxal and insulin resistance in berberine-treated type 2 diabetic patients. J. Res. Med. Sci. 23, 110. 10.4103/jrms.JRMS_1078_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miana G. A., Ikram M. (1970). Alkaloids of Berberis petiolaris Wall. Pakistan J. Sci. Indus Res. 13, 49–51. [Google Scholar]
- Miana G. A., Foy J. E., Minard R. D., Shamma M. (1979). Baluchistine, a new bisbenzylisoquinoline alkaloid. Experientia 35, 1137–1138. 10.1007/BF01963244 [DOI] [Google Scholar]
- Ming J., Xu S., Liu C., Liu X., Jia A., Ji Q. (2018). Effectiveness and safety of bifidobacteria and berberine in people with hyperglycemia: study protocol for a randomized controlled trial. Trials 19, 72. 10.1186/s13063-018-2438-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhadi E., Rezaee M., Malaekeh-Nikouei B. (2018). Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 104, 465–473. 10.1016/j.biopha.2018.05.067 [DOI] [PubMed] [Google Scholar]
- Mittal M., Juyal V., Singh A. (2012). Phytochemical, antidiabetic, and cytoprotective properties of Berberis aristata DC. root extracts. Pharm. Crop 3, 64–68. 10.2174/2210290601203010064 [DOI] [Google Scholar]
- Moazezi Z., Qujeq D. (2014). Berberis fruit extract and biochemical parameters in patients with type II diabetes. Jundishapur J. Nat. Pharm. Prod. 9, e13490. 10.17795/jjnpp-13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi A., Sahebkar A., Kermani T., Zhilaee M., Tavallaie S., Mobarhan M. G. (2014). Barberry administration and pro-oxidant–antioxidant balance in patients with metabolic syndrome. Iran. Red Crescent Med. J. 16, e16786. 10.5812/ircmj.16786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa K., Ganai B., Akbar S., Dar M., Tantry M., Masood A. (2011). The extracts of Berberis lycium and diabetes mellitus in alloxan monohydrate induced diabetic rats. J. Pharm. Res. 4, 2570–2573. [Google Scholar]
- Neag M. A., Mocan A., Echeverría J., Pop R. M., Bocsan C. I., Crisan G., et al. (2018). Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 9, 557. 10.3389/fphar.2018.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Och A., Szewczyk K., Pecio Ł., Stochmal A., Załuski D., Bogucka-Kocka A. (2017). UPLC-MS/MS Profile of alkaloids with cytotoxic properties of selected medicinal plants of the Berberidaceae and Papaveraceae families. Oxid. Med. Cell. Longev. 2017, 9369872. 10.1155/2017/9369872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyedemi S. O., Bradley G., Afolayan A. J. (2009). Ethnobotanical survey of medicinal plants used for the management of diabetes mellitus in the Nkonkobe municipality of South Africa. J. Med. Plants Res. 3, 1040–1044. [Google Scholar]
- Pérez-Rubio K. G., González-Ortiz M., Martínez-Abundis E., Robles-Cervantes J. A., Espinel-Bermúdez M. C. (2013). Effect of Berberine Administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 11, 366–369. 10.1089/met.2012.0183 [DOI] [PubMed] [Google Scholar]
- Pareek A., Suthar M. (2010). Antidiabetic activity of extract of Berberis aristata root in streptozotocin induced diabetic rats. Pharmacologyonline 2, 179–185. [Google Scholar]
- Patel M. B., Mishra S. (2011). Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine 18, 1045–1052. 10.1016/j.phymed.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Paul M., Hemshekhar M., Kemparaju K., Girish K. S. (2019). Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free Radic. Biol. Med. 130, 196–205. 10.1016/j.freeradbiomed.2018.10.453 [DOI] [PubMed] [Google Scholar]
- Petcu P. (1968). Berberis crataegina DC plants acclimated to Romania. Arch. Pharm. (Weinheim). 301, 680. 10.1002/ardp.19683010906 [DOI] [PubMed] [Google Scholar]
- Phondani P. C., Maikhuri R. K., Rawat L. S., Farooquee N. A., Kala C. P., Vishvakarma S. C. R., et al. (2010). Ethnobotanical uses of plants among the Bhotiya tribal communities of niti valley in central Himalaya, India. Ethnobot. Res. Appl. 8, 233–244. 10.17348/era.8.0.233-244 [DOI] [Google Scholar]
- Pisciotta L., Bellocchio A., Bertolini S. (2012). Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 11, 123. 10.1186/1476-511X-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potdar D., Hirwani R. R., Dhulap S. (2012). Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 83, 817–830. 10.1016/j.fitote.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Qiao X., Wang Q., Wang S., Kuang Y., Li K., Song W., et al. (2018). A 42-markers pharmacokinetic study reveals interactions of berberine and glycyrrhizic acid in the anti-diabetic Chinese medicine formula gegen-qinlian decoction. Front. Pharmacol. 9, 622. 10.3389/fphar.2018.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo R., Valderrama K., Moreno-Murillo B., Laverde M., Fajardo V. (2008). A new bisbenzyltetrahydroisoquinoline alkaloid from Berberis tabiensis (Berberidaceae). Biochem. Syst. Ecol. 36, 812–814. 10.1016/j.bse.2008.07.007 [DOI] [Google Scholar]
- Rahimi Madiseh M., Heidarian E., Rafieian-Kopaei M. (2014). Biochemical components of Berberis lycium fruit and its effects on lipid profile in diabetic rats. J. HerbMed. Pharmacol. 3, 15–19. [Google Scholar]
- Rahimi-Madiseh M., Karimian P., Kafeshani M., Rafieian-Kopaei M. (2017). The effects of ethanol extract of Berberis vulgaris fruit on histopathological changes and biochemical markers of the liver damage in diabetic rats. Iran. J. Basic Med. Sci. 20, 552–556. 10.22038/IJBMS.2017.8681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar N. K., Shenoy R. R., Theerthahalli S., Arun R. C. M. (2009). Effect of Berberis aristata on type I and II diabetes mellitus models in albino rats. Pharmacologyonline 1, 89–96. [Google Scholar]
- Rana D., Bhatt A., Lal B. (2019). Ethnobotanical knowledge among the semi-pastoral Gujjar tribe in the high altitude (Adhwari's) of Churah subdivision, district Chamba, Western Himalaya. J. Ethnobiol. Ethnomed. 15, 10. 10.1186/s13002-019-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. R., Hajra P. K. (1993). “Berberis“ in Flora of India, ed. Sharma B. D. et al. Vol. 1 (New Delhi: Botanical Survey of India; ), 352–402. [Google Scholar]
- Rao R. R., Husain T., Datt B., Garg A. (1998). Revision of the family Berberidaceae of the Indian region: 2. Rheedia 8, 109–143. [Google Scholar]
- Rao A. (2017). Efficacy of berberine hydrochloride on biochemical parameters in Indian type 2 diabetic patients. Endocr. Pract. 23, 18A. [Google Scholar]
- Rashidi H., Namjoyan F., Mehraban Z., Zakerkish M., Ghaderian S. B., Latifi S. M. (2018). The effects of active ingredients of barberry root (Berberine) on Glycemic control and insulin resistance in type 2 diabetic patients. Jundishapur J. Nat. Pharm. Prod. 13, e64180. 10.5812/jjnpp.64180 [DOI] [Google Scholar]
- Ren G., Wang Y.-X., Li Y.-H., Song D.-Q., Kong W.-J., Jiang J.-D. (2017). Structure-activity relationship of berberine derivatives for their glucose-lowering activities. Int. J. Clin. Exp. Med. 10, 5054–5060. [Google Scholar]
- Rizvi S. I., Mishra N. (2013). Traditional Indian medicines used for the management of diabetes mellitus. J. Diabetes Res. 2013, 712092. 10.1155/2013/712092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozza F., de Simone G., Izzo R., De Luca N., Trimarco B. (2009). Nutraceuticals for treatment of high blood pressure values in patients with metabolic syndrome. High Blood Press Cardiovasc. Prev. 16, 177–182. 10.2165/11530420-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Ruscica M., Gomaraschi M., Mombelli G., Macchi C., Bosisio R., Pazzucconi F., et al. (2014). Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 8, 61–68. 10.1016/j.jacl.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Sabahi Z., Khoshnood-Mansoorkhani M. J., Namadi S. R., Moein M. (2016). Antidiabetic and synergistic effects of anthocyanin fraction from Berberis integerrima fruit on streptozotocin-induced diabetic rats model. Trends Phram. Sci. 2, 43–50. [Google Scholar]
- Sangeetha M. K., Priya C. D. M., Vasanthi H. R. (2013). Anti-diabetic property of Tinospora cordifolia and its active compound is mediated through the expression of Glut-4 in L6 myotubes. Phytomedicine 20, 246–248. 10.1016/j.phymed.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Sehdev R. K., Handa K. L., Rao P. R. (1971). Note on the alkaloids of Berberis lycium Royle. Indian J. Chem. 9, 503. [Google Scholar]
- Semwal B. C., Shah K., Chauhan N. S., Badhe R., Divakar K. (2008). Anti-diabetic activity of stem bark of Berberis aristata DC in alloxan induced diabetic rats. Internet J. Pharmacol. 6, 1531–1576. 10.5580/90 [DOI] [Google Scholar]
- Shahid M., Rahim T., Shahzad A., Latif T. A., Fatma T., Rashid M., et al. (2009). Ethnobotanical studies on Berberis aristata DC. root extracts. Afr. J. Bio-technol. 8, 556–563. [Google Scholar]
- Shamma M., Moniot J. L., Yao S. Y., Miana G. A., Ikram M. (1973). Pakistanine and Pakistanamine, two new dimeric isoquinoline alkaloids. J. Am. Chem. Soc 95, 5742–5747. 10.1021/ja00798a050 [DOI] [PubMed] [Google Scholar]
- Shamma M., Foy J. E., Miana G. A. (1974). Baluchistanamine. novel type dimeric isoquinoline alkaloid. J. Am. Chem. Soc 96, 7809–7811. 10.1021/ja00832a033 [DOI] [Google Scholar]
- Shang W., Guo C., Yu X., Zhao J., Z. H. (2015). Effects of combination of ginsenoside Rb1 and berberine on glucose and lipid metabolism in db/db obese diabetic mice. Lishizhen Med. Mater. Med. Res. 3, 518–521. [Google Scholar]
- Sharma R. K., Sharma B., Jindal M., Gupta A. K., Kunwar R., Lata S., et al. (2017). Evaluation of hypolipidemic effect of stem part of Berberis aristata in Type 2 diabetes mellitus patients as add on therapy. Natl. J. Physiol. Pharm. Pharmacol. 7, 1A–11A. 10.5455/njppp.2017.7.0517510062017 [DOI] [Google Scholar]
- Sharma A., Sharma R., Kumar D., Padwad Y. (2018). Berberis lycium Royle fruit extract mitigates oxi-inflammatory stress by suppressing NF-κB/MAPK signalling cascade in activated macrophages and Treg proliferation in splenic lymphocytes. Inflammopharmacology. 1–20. 10.1007/s10787-018-0548-z [DOI] [PubMed]
- Shidfar F., Ebrahimi S. S., Hosseini S., Heydari I., Shidfar S., Hajhassani G. (2012). The effects of Berberis vulgaris fruit extract on serum lipoproteins, apoB, apoA-I, homocysteine, glycemic control and total antioxidant capacity in type 2 diabetic patients. Iran. J. Pharm. Res. IJPR 11, 643. [PMC free article] [PubMed] [Google Scholar]
- Singh P., Jain S. (2010). Antidiabetic activity of Berberis asiatica (DC) roots. Int. J. Pharm. Sci. Res. 1, 109–112. 10.13040/IJPSR.0975-8232.1(6).109-12 [DOI] [Google Scholar]
- Singh J., Kakkar P. (2009). Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J. Ethnopharmacol. 123, 22–26. 10.1016/j.jep.2009.02.038 [DOI] [PubMed] [Google Scholar]
- Singh A., Bajpai V., Srivastava M., Arya K. R., Kumar B. (2015). Rapid screening and distribution of bioactive compounds in different parts of Berberis petiolaris using direct analysis in real time mass spectrometry. J. Pharm. Anal. 5, 332–335. 10.1016/j.jpha.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Hart R., Chandra S., Nautiyal M. C., Sayok A. K. (2019). Traditional Herbal Knowledge among the Inhabitants: A Case Study in Urgam Valley of Chamoli Garhwal, Uttarakhand, India. Evid. Based. Complement. Alternat. Med. 2019, 5656925. 10.1155/2019/5656925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola R., Valls R.-M., Puzo J., Calabuig J.-R., Brea A., Pedret A., et al. (2014). Effects of poly-bioactive compounds on lipid profile and body weight in a moderately hypercholesterolemic population with low cardiovascular disease risk: a multicenter randomized trial. PloS One 9, e101978. 10.1371/journal.pone.0101978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigoni V., Aldigeri R., Antonini M., Micheli M. M., Fantuzzi F., Fratter A., et al. (2017). Effects of a new nutraceutical formulation (berberine, red yeast rice and chitosan) on non-HDL cholesterol levels in individuals with dyslipidemia: results from a randomized, double blind, placebo-controlled study. Int. J. Mol. Sci. 18, 1498. 10.3390/ijms18071498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Rawat A. K. S., Srivastava M., Mehrotra S. (2006). Pharmacognostic evaluation of the roots of Berberis chitria Lindl. Nat. Prod. Sci. 12, 19–23. [Google Scholar]
- Srivastava S., Srivastava M., Misra A., Pandey G., Rawat A. K. S. (2015). A review on biological and chemical diversity in Berberis (Berberidaceae). EXCLI J. 14, 247–267. 10.17179/excli2014-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suau R., Rico R., López-Romero J. M., Nájera F., Cuevas A. (1998). Isoquinoline alkaloids from Berberis vulgaris subsp. australis. Phytochemistry 49, 2545–2549. 10.1016/S0031-9422(98)00121-6 [DOI] [Google Scholar]
- Sun J., Bao H., Peng Y., Zhang H., Sun Y., Qi J., et al. (2018). Improvement of intestinal transport, absorption and anti-diabetic efficacy of berberine by using Gelucire44/14: in vitro, in situ and in vivo studies. Int. J. Pharm. 544, 46–54. 10.1016/j.ijpharm.2018.04.014 [DOI] [PubMed] [Google Scholar]
- Sun Y., Xia M., Yan H., Han Y., Zhang F., Hu Z., et al. (2018). Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br. J. Pharmacol. 175, 374–387. 10.1111/bph.14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum N., Ahmad F. (2011). Role of natural herbs in the treatment of hypertension. Pharmacogn. Rev. 5, 30–40. 10.4103/0973-7847.79097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei-Malazy O., Larijani B., Abdollahi M. (2015). Targeting metabolic disorders by natural products. J. Diabetes Metab. Disord. 14, 57. 10.1186/s40200-015-0184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L.-Q., Wei W., Chen L.-M., Liu S. (2006). Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 108, 109–115. 10.1016/j.jep.2006.04.019 [DOI] [PubMed] [Google Scholar]
- Tao K., Chen J., Wang L. (2017). Effects of berberine on the expressions of NRF2 and HO-1 in endothelial cells of diabetic rat. BioMed. Res. 28, 3860–3864. 10.1155/2017/6352858 [DOI] [Google Scholar]
- Teodoro J. S., Duarte F. V., Gomes A. P., Varela A. T., Peixoto F. M., Rolo A. P., et al. (2013). Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: a possible role for SirT3 activation. Mitochondrion 13, 637–646. 10.1016/j.mito.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Tiwari K. P., Masood M. (1977). Alkaloidal constituents of Berberis concina and Berberis acanthifolium. Proc. Natl. Acad. Sci. India Sect. A 47, 93–94. [Google Scholar]
- Tiwari U. L., Singh Adhikari B. (2011). Berberis rawatii sp. nov. (Berberidaceae) from India. Nord. J. Bot. 29, 184–188. 10.1111/j.1756-1051.2011.00940.x [DOI] [Google Scholar]
- Trimarco B., Benvenuti C., Rozza F., Cimmino C. S., Giudice R., Crispo S. (2011). Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet. Med. J. Nutr. Metab. 4, 133–139. 10.1007/s12349-010-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N., Li J.-Y., Gosby A., To S. W. C., Cheng Z., Miyoshi H., et al. (2008). Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 57, 1414–1418. 10.2337/db07-1552 [DOI] [PubMed] [Google Scholar]
- Uniyal S. K., Singh K. N., Jamwal P., Lal B. (2006). Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomed. 2, 14. 10.1186/1746-4269-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upwar N., Patel R., Waseem N., Mahobia N. K. (2011). Hypoglycemic effect of methanolic extract of Berberis aristata DC stem on normal and streptozotocin induced diabetic rats. Int. J. Pharm. Pharm. Sci. 3, 222–224. [Google Scholar]
- Valencia E., Fajardo V., Freyer A. J., Shamma M. (1985). Magallanesine: an isoindolobenzazocine alkaloid. Tetrahedron Lett. 26, 993–996. 10.1016/S0040-4039(00)98494-6 [DOI] [Google Scholar]
- Waltenberger B., Mocan A., Smejkal K., Heiss E. H., Atanasov A. G. (2016). Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 21, 807. 10.3390/molecules21060807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-X., Wang Y.-P., Zhang H., Kong W.-J., Li Y.-H., Liu F., et al. (2009). Synthesis and biological evaluation of berberine analogues as novel up-regulators for both low-density-lipoprotein receptor and insulin receptor. Bioorg. Med. Chem. Lett. 19, 6004–6008. 10.1016/j.bmcl.2009.09.059 [DOI] [PubMed] [Google Scholar]
- Wang Y.-X., Kong W.-J., Li Y.-H., Tang S., Li Z., Li Y.-B., et al. (2012). Synthesis and structure–activity relationship of berberine analogues in LDLR up-regulation and AMPK activation. Bioorg. Med. Chem. 20, 6552–6558. 10.1016/j.bmc.2012.09.029 [DOI] [PubMed] [Google Scholar]
- Wang P., Liu X., Hong Y., Reng X., Wu X. (2014). Anti-diabetic effects of Berberin Glycyrrhizinate complex salt on GK rat. Chin. Arch. Tradit. Chin. Med. 12, 2995–2997. [Google Scholar]
- Wang J., Dai G., Li W. (2016). Berberine regulates glycemia via local inhibition of intestinal dipeptidyl peptidase-IV. Zhejiang Da Xue Xue Bao Yi Xue Ban 45, 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Peng L., Wei G., Ge H. (2016). Therapeutic effects of berberine capsule on patients with mild hyperlipidemia. Zhongguo Zhong Xi Yi Jie He Za Zhi 36, 681–684. [PubMed] [Google Scholar]
- Wang H., Zhu C., Ying Y., Luo L., Huang D., Luo Z. (2018). Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget 9, 10135–10146. 10.18632/oncotarget.20807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ye X., Hua Y., Song Y. (2018). Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed. Pharmacother. 105, 121–129. 10.1016/j.biopha.2018.05.110 [DOI] [PubMed] [Google Scholar]
- Wei W., Zhao H., Wang A., Sui M., Liang K., Deng H., et al. (2012). A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur. J. Endocrinol. 166, 99–105. 10.1530/EJE-11-0616 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016). Global report on diabetes. ISBN ISBN 978 92 4 156525 7.
- Wu J., Yu D., Sun H., Zhang Y., Zhang W., Meng F., et al. (2015). Optimizing the extraction of anti-tumor alkaloids from the stem of Berberis amurensis by response surface methodology. Ind. Crops Prod. 69, 68–75. 10.1016/j.indcrop.2015.01.053 [DOI] [Google Scholar]
- Wu L., Xia M., Duan Y., Zhang L., Jiang H., Hu X., et al. (2019). Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 10, 1–18. 10.1038/s41419-019-1706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Yan J., Shen Y., Tang K., Yin J., Zhang Y., et al. (2011). Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PloS One 6, 1–10. 10.1371/journal.pone.0016556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Xu M., Alimujiang M., Bao Y., Wei L., Yin J. (2018). Bidirectional regulation of adenosine 5′-monophosphate–activated protein kinase activity by berberine and metformin in response to changes in ambient glucose concentration. J. Cell. Biochem. 119, 9910–9920. 10.1002/jcb.27312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Xiao Y., Yin J., Hou W., Yu X., Shen L., et al. (2014). Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PloS One 9, e103702. 10.1371/journal.pone.0103702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Benrong H., Qiang T., Qin F., Jizhou X. (2005). Hypoglycemic activity of jatrorrhizine. J. Huazhong Univ. Sci. Technol. [Med. Sci. 25, 491–493. 10.1007/BF02895996 [DOI] [PubMed] [Google Scholar]
- Yan H. M., Xia M. F., Wang Y., Chang X. X., Yao X. Z., Rao S. X., et al. (2015). Efficacy of berberine in patients with non-alcoholic fatty liver disease. PloS One 10, e0134172. 10.1371/journal.pone.0134172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yin J., Gao H., Xu L., Wang Y., Xu L., et al. (2012). Berberine improves insulin sensitivity by inhibiting fat store and adjusting adipokines profile in human preadipocytes and metabolic syndrome patients. Evidence-Based Complement. Altern. Med. 2012, 363845. 10.1155/2012/363845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhao P., Wan D., Zhou Q., Wang C., Shu G., et al. (2014). Antidiabetic effect of methanolic extract from Berberis julianae Schneid. via activation of AMP-activated protein kinase in type 2 diabetic mice. Evidence-Based Complement. Altern. Med. 2014, 106206. 10.1155/2014/106206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Xing H., Ye J. (2008). Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 57, 712–717. 10.1016/j.metabol.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H. (2014). Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910. 10.1016/S2213-8587(14)70032-4 [DOI] [PubMed] [Google Scholar]
- Yue L., Zhang Y., Xiang L., Lai X., Meng X. (2013). Study on the effect of Berberis dictyophlla cortex on diabetic retinopathy and the mechanism. Chin. J. Exp. Tradit. Med. Formulae 2013, 43. [Google Scholar]
- Yusupov M. M., Karimov A., Levkovich M. G., Abdullaev N. D., Shakirov R. (1993. a). Berberis alkaloids. XVII. investigation of the alkaloids of Berberis heteropoda. Chem. Nat. Compd. 29, 43–48. 10.1007/BF00631012 [DOI] [Google Scholar]
- Yusupov M. M., Karimov A., Shakirov R., Gorovoi P. G., Faskhutdinov M. F., Levkovich M. G., et al. (1993. b). Berberis alkaloids. XXVI. an investigation of the alkaloids of Berberis amurensis. Chem. Nat. Compd. 29, 338–340. 10.1007/BF00630534 [DOI] [Google Scholar]
- Zain-Ul-Abidin S., Khan R., Ahmad M., Bhatti M. Z., Zafar M., Saeed A., et al. (2018). Ethnobotanical survey of highly effective medicinal plants and phytotherapies to treat diabetes mellitus ii in South-West Pakistan. Indian J. Tradit. Knowl. 17, 682–690. [Google Scholar]
- Zhang Y., Li X., Zou D., Liu W., Yang J., Zhu N., et al. (2008). Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 93, 2559–2565. 10.1210/jc.2007-2404 [DOI] [PubMed] [Google Scholar]
- Zhang H., Wei J., Xue R., Wu J.-D., Zhao W., Wang Z.-Z., et al. (2010). Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 59, 285–292. 10.1016/j.metabol.2009.07.029 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Xiao X., Li M., Li W., Yu M., Zhang H., et al. (2014). Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine. BMC Complement. Altern. Med. 14, 188. 10.1186/1472-6882-14-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang X., Yin W., Liu Z., Zhou M., Xiao D., et al. (2016). Synthesis and hypoglycemic activity of 9-O-(lipophilic group substituted) berberine derivatives. Bioorg. Med. Chem. Lett. 26, 4799–4803. 10.1016/j.bmcl.2016.08.027 [DOI] [PubMed] [Google Scholar]
- Zhang B., Pan Y., Xu L., Tang D., Dorfman R. G., Zhou Q., et al. (2018). Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3. Endocrine 62, 576–587. 10.1007/s12020-018-1689-y [DOI] [PubMed] [Google Scholar]
- Zhao X., Zhang J.-J., Wang X., Bu X.-Y., Lou Y.-Q., Zhang G.-L. (2008). Effect of berberine on hepatocyte proliferation, inducible nitric oxide synthase expression, cytochrome P450 2E1 and 1A2 activities in diethylnitrosamine- and phenobarbital-treated rats. Biomed. Pharmacother. 62, 567–572. 10.1016/j.biopha.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Zhao W., Ge H., Liu K., Chen X., Zhang J., Liu B. (2017). Nandinine, a derivative of berberine, inhibits inflammation and reduces insulin resistance in adipocytes via regulation of AMP-Kinase activity. Plant. Med. 83, 203–209. 10.1055/s-0042-110576 [DOI] [PubMed] [Google Scholar]
- Zhou J., Zhou S. (2010). Berberine regulates peroxisome proliferator-activated receptors and positive transcription elongation factor b expression in diabetic adipocytes. Eur. J. Pharmacol. 649, 390–397. 10.1016/j.ejphar.2010.09.030 [DOI] [PubMed] [Google Scholar]
- Zhou J. Y., Zhou S. W., Zhang ,. K. B., Tang J. L., Guang L. X., Ying Y., et al. (2008). Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol. Pharm. Bull. 31, 1169–1176. 10.1248/bpb.31.1169 [DOI] [PubMed] [Google Scholar]
- Zhu X., Yang J., Zhu W., Yin X., Yang B., Wei Y., et al. (2018). Combination of berberine with resveratrol improves the lipid-lowering efficacy. Int. J. Mol. Sci. 19, 3903. 10.3390/ijms19123903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Bian H., Wang L., Sun X., Xu X., Yan H., et al. (2019). Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 141, 192–204. 10.1016/j.freeradbiomed.2019.06.019 [DOI] [PubMed] [Google Scholar]
- Zilaee M., Kermany T., Tavalaee S., Salehi M., Ghayour-Mobarhan M., Ferns G. A. A. (2014). Barberry treatment reduces serum anti-heat shock protein 27 and 60 antibody titres and high-sensitivity C-reactive protein in patients with metabolic syndrome: a double-blind, randomized placebo-controlled trial. Phyther. Res. 28, 1211–1215. 10.1002/ptr.5117 [DOI] [PubMed] [Google Scholar]