Abstract
Background
Although post-menopausal obesity is an important public national health problem in Saudi Arabia, to date no study has evaluated the effects of weight reduction on biochemical & clinical parameters and quality of Life for obese Saudi post-menopausal women.
Objective
The aim of this study was examine the effects of aerobic versus resisted exercise training effects upon systemic inflammation biomarkers and quality of life for obese post-menopausal Saudi women.
Material and Methods
One hundred Saudi post-menopausal obese women participated in this study, their age ranged from 50–58 years and their body mass index (BMI) ranged from 30–35 kg/m2. All participants were divided into two equal groups: The first group received aerobic exercise training on treadmill where, the second group received resisted exercise training. Health-related quality of life (SF-36 HRQL), tumor necrosis factor- alpha(TNF-α), Interleukin-2(IL-2), Interleukin-4 (IL-4), Interleukin-6 (IL-6) and C-reactive protein (CRP) were measured before and after 3 months at the end of the study.
Results
The mean values of SF-36 HRQL subscale scores were significantly increased, while the mean value of TNF-α, Il-2, IL-4, IL-6, CRP and BMI were significantly decreased in both groups after treatments. There were significant differences between mean levels of the investigated parameters in group (A) and group (B) after treatment with more changes in patients received aerobic exercise training.
Conclusion
The current study provides evidence that aerobic exercise is more appropriate than resisted exercise training in modulating inflammatory cytokines and quality of life among obese post-menopausal women.
Keywords: Aerobic exercise, resisted exercises, inflammatory cytokine, quality of life, obesity, menopause
Introduction
Post-menopausal women, especially those with body mass index (BMI) greater than 30 kg/m2, have lower health-related quality of life (HRQOL) in physical functioning, energy, and vitality compared with normal-weight women1. Nearly two-thirds of American adults are overweight or obese2. The current epidemic of overweight and obesity is one of the major public health concerns worldwide3. The prevalence of overweight and obesity has increased significantly over the past 30 years, with overweight and obesity now affecting 1.5 billion adults globally4. Worldwide, 2.8 million people die each year of obesity-related diseases, including type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), and metabolic syndrome5.
Obesity may significantly reduce HRQOL. A review of 8 studies examining HROQL among women aged over 55 years old concluded that post-menopausal women, especially those with BMI greater than 30 kg/m2, have lower HRQOL in physical functioning, energy, and vitality compared with normal-weight women1. Obesity impacts a variety of dimensions of quality of life, including vitality, bodily pain, and even social functioning. Obese individuals report significantly lower HRQL than normal-weight individuals6. General health problems, mobility/functional disability, depression and low self-esteem are commonly reported among obese subjects7.
Quality of life (QoL) in post-menopausal women may be compromised as a consequence of climacteric symptoms associated to psychosocial and cultural determinants8. There is a growing demand for health care proposals aimed at menopausal women and looking to promote a healthier female aging process and with a better QoL9. Hormone replacement therapy (HRT) has been indicated to minimize menopausal symptoms; however there are controversies as to whether its effects improve QoL. There are still some questions regarding the risks and benefits of HRT in post-menopausal women10,11.
Aerobic exercise training is an effective and cost-efficient alternative therapy for disorders of anxiety and mood disorders12. Also, exercise training slow down the progression of cognitive decline13, improves performance on tests of cognition and psychological wellbeing14 and enhances sleep quality15. However, the available previous studies involving the impact of exercise training upon the quality of life, psychological wellbeing along with systemic inflammation in post-menopausal women is limited in number16.
As the ideal exercise intensity that efficiently modulates the elevated inflammatory cytokines and abnormal HRQOL among obese post-menopausal women is inconclusive, this study was designed to compare the impact of aerobic versus resisted exercises on inflammatory cytokines and HRQOL in obese post-menopausal women.
Patients and methods
Subjects
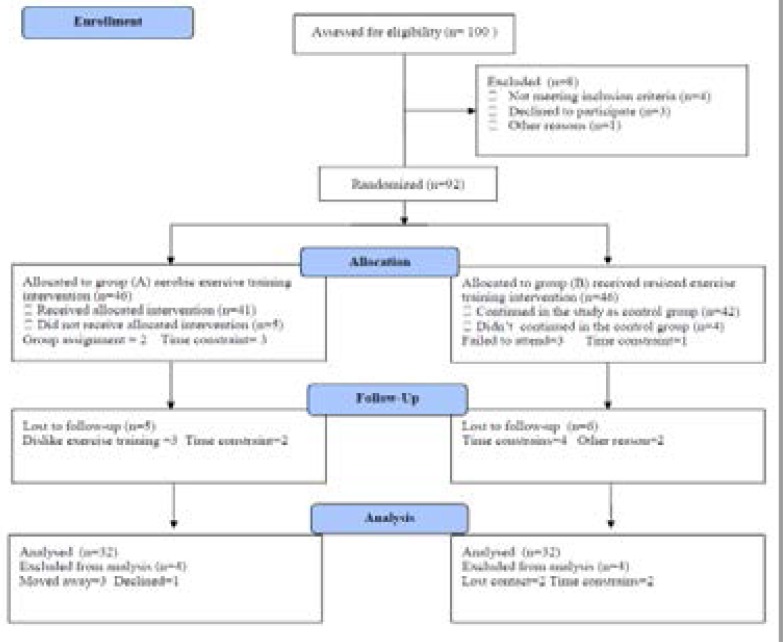
One-hundred Saudi post-menopausal obese women participated in this study, their age ranged from 50–58 years and their BMI ranged from 30–35 kg/m2. All volunteers were asked to read and sign an informed consent document prior to participation. Women who were smokers, having any endocrine, musculoskeletal, renal, liver, cardiac disorders, diabetes and chest diseases were excluded. Participants were assigned into 2 groups; group (A) received aerobic exercise training for 3 months and group (B) received resisted exercise training. The CONSORT diagram outlining the details of the screening, run-in and randomization phases of the study and reasons for participant exclusion can be found in figure (1). Informed consent was obtained from all participants. This study was approved by the Scientific Research Ethical Committee, Faculty of Applied Medical Sciences at King University.
Figure (1).
Subjects screening and recruitment CONSORT diagram.
Measurements
1. Health-related quality of life (SF-36 HRQL): The SF-36 included eight subscales: Vitality, Bodily Pain, General Health, Physical Functioning, Social Functioning, Physical Role Functioning, Emotional Role Functioning, and Mental Health. In addition to these subscales, the SF-36 also included a question asking participants to report the amount of change in their general health over the past year referred to as health transition17.
2. Biochemical analyses: Blood samples were drained from the antecubital vein after a 12-h fasting, the blood samples were centrifuged at + 4 °C (1000 = g for 10 min). Interleukin-2 (IL-2) Interleukin-4 (IL-4), Interleukin-6 (IL-6) levels were analyzed by “Immulite 2000” immunassay analyzer (Siemens Healthcare Diagnostics, Deerfield, USA). However, tumor necrosis factor- alpha (TNF-α) and C-reactive protein (CRP) levels were measured by ELISA kits (ELX 50) in addition to ELISA microplate reader (ELX 808; BioTek Instruments, USA). Measurements of TNF-α, Il-2, IL-4, IL-6 and CRP were taken before starting of the study (pre-test) and after 12 weeks (post-test).
All measurements were taken before the starting of the study (pre-test) and after three months at the end of the study (post-test).
Procedures
Following the previous evaluation, all participants were assigned into two groups:
1. Group (A) participated in a treadmill aerobic exercise (Enraf Nonium, Model display panel Standard, NR 1475.801, Holand) which was conducted according to recommendation of aerobic exercise application approved by the American College of Sports Medicine18. Training program was included five minutes for warming-up in the form of range motion and stretching exercises, thirty minutes of aerobic exercise training (60–70% of maximum heart rate) and five minutes of cooling down (on treadmill with low speed and without inclination). Participants had three sessions /week for 3 months with close supervision of physical therapist.
2. Group (B) participated in the resistance exercises on some resistance gym machines (Nautilus Sports/Medical Industries, Independence, VA), three sessions of resistance exercise training /week for 3 months. Each session of resistance training program included five minutes for warming -up in the form of range motion and stretching exercises, 30 minutes of resistance exercise training and 5 minutes of cooling down19.
Statistical analysis
The mean values of the investigated parameters obtained before and after three months in both groups were compared using paired “t” test. Independent “t” test was used for the comparison between the two groups (P<0.05).
Results
Table 1 shows the baseline characteristics of the participants who entered the trial. There was no significant differences in baseline characteristics between the two groups were found.
Table 1.
Baseline characteristics of study participants
| Characteristic | Group (A) | Group (B) | Significance |
| Age (years) | 56.42 ± 4.81 | 54.97 ± 5.63 | P>0.05 |
| BMI (kg/m2) | 34.28 ± 3.74 | 33.52 ± 3.68 | P>0.05 |
| SBP (mmHg) | 136.51 ± 11.46 | 135.14 ±13.12 | P>0.05 |
| DBP (mmHg) | 82.33 ± 6.27 | 80.81 ± 7.52 | P>0.05 |
| Glucose (mmol/L) | 4.51 ± 0.64 | 4.35 ± 0.53 | P>0.05 |
| ALT (U/L) | 40.16 ± 7.23 | 38.62 ± 6.11 | P>0.05 |
| AST (U/L) | 28.45 ± 6.18 | 27.71 ± 5.39 | P>0.05 |
BMI: Body mass index; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
The mean values of SF-36 HRQL subscale scores and 6MWT were significantly increased, while the mean value of TNF-α, Il-2, IL-4, IL-6, CRP and BMI were significantly decreased in both groups after treatments (Tables 2–5). There were significant differences between mean levels of the investigated parameters of group (A) and group (B) after treatment with more changes in patients received aerobic exercise training (Tables 6 and 7). These results confirm that aerobic exercise is more appropriate than resisted exercise training in modulating inflammatory cytokines and quality of life among obese post-menopausal women.
Table 2.
Mean value and significance of TNF-α, IL-2, IL-4, IL-6 and CRP of group (A) before and at the end of the study
| Mean +SD | T-value | Significance | ||
| Before | After | |||
| TNF-α (pg/ml) | 6.36 ± 1.74* | 4.38 ± 1.86 | 7.11 | P<0.05 |
| IL-2 (pg/ml) | 8.23 ± 2.31* | 4.75 ± 1.97 | 6.58 | P<0.05 |
| IL-4 (pg/ml) | 5.72 ± 1.44* | 3.46 ± 1.23 | 6.25 | P<0.05 |
| IL-6 (pg/ml) | 7.85 ± 2.13* | 4.55 ± 1.81 | 6.22 | P<0.05 |
| CRP (mg/dl) | 14.93 ± 3.82* | 8.31 ± 2.75 | 8.43 | P<0.05 |
TNF-α: tumor necrosis factor – alpha; IL-2: Interleukin-2; IL-4: Interleukin-4; IL-6: Interleukin-6; CRP: C-reactive protein
indicates a significant difference between the two groups, P < 0.05.
Table 5.
Mean value and significance of SF-36 subscale scores of group (B) before and at the end of the study
| SF-36 subscale variables |
Mean +SD | T-value | Significance | |
| Before | After | |||
| SF-36: Health transition |
2.61 ± 0.92* | 2.11 ± 0.84 | 3.19 | P<0.05 |
| SF-36: Physica l functioning |
73.27 ± 8.51* | 77.68 ± 8.96 | 3.23 | P<0.05 |
| SF-36: Role functioning: Physical |
80.21 ± 10.39* | 84.34 ± 10.75 | 3.42 | P<0.05 |
| SF-36: Bodily pain | 73.92 ± 7.48* | 70.74 ± 7.23 | 3.22 | P<0.05 |
| SF-36: General health |
72.15 ± 9.87* | 75.22 ± 10.12 | 3.15 | P<0.05 |
| SF-36: Vitality | 57.81 ± 6.42* | 62.13 ± 6.76 | 3.41 | P<0.05 |
| SF-36: Social functioning |
86.24 ± 11.26* | 89.57 ± 11.87 | 3.27 | P<0.05 |
| SF-36: Role functioning: Emotional |
90.83 ± 14.91* | 87.14 ± 13.22 | 3.31 | P<0.05 |
| SF-36: Mental health |
84.16 ± 10.38* | 81.25± 10.18 | 3.45 | P<0.05 |
indicates a significant difference between the two groups; P < 0.05.
Table 6.
Mean value and significance of TNF- α, IL-6, IL-8 and CRP of group (A) and group (B) at the end of the study
| Mean +SD | T-value | Significance | ||
| Group (A) | Group (B) | |||
| TNF-α (pg/ml) | 4.38 ± 1.86* | 5.13 ± 1.78 | 3.41 | P<0.05 |
| IL-2 (pg/ml) | 4.75 ± 1.97* | 6.74 ± 2.21 | 3.53 | P<0.05 |
| IL-4 (pg/ml) | 3.46 ± 1.23* | 4.37 ± 1.54 | 3.68 | P<0.05 |
| IL-6 (pg/ml) | 4.55 ± 1.81* | 6.14 ± 2.25 | 3.47 | P<0.05 |
| CRP (mg/dl) | 8.31 ± 2.75* | 11.82 ± 3.27 | 4.15 | P<0.05 |
TNF- α: tumor necrosis factor – alpha; IL-2: Interleukin-2; IL-4: Interleukin-4; IL-6: Interleukin-6; CRP: C-reactive protein
indicates a significant difference between the two groups, P < 0.05.
Table 7.
Mean value and significance of SF-36 subscale scores of group (A) and group (B) at the end of the study
| SF-36 subscale variables |
Mean +SD | T-value | Significance | |
| Group (A) | Group (B) | |||
| SF-36: Health transition |
1.69 ± 0.61* | 2.11 ± 0.84 | 3.27 | P<0.05 |
| SF-36: Physical functioning |
80.32 ± 10.12* | 77.68 ± 8.96 | 3.46 | P<0.05 |
| SF-36: Role functioning: Physical |
87.13 ± 12.37* | 84.34 ± 10.75 | 3.49 | P<0.05 |
| SF-36: Bodily pain |
69.85 ± 6.25* | 70.74 ± 7.23 | 3.35 | P<0.05 |
| SF-36: General health |
77.38 ± 10.19* | 75.22 ± 10.12 | 3.18 | P<0.05 |
| SF-36: Vitality | 66.97 ± 7.81* | 62.13 ± 6.76 | 3.52 | P<0.05 |
| SF-36: Social functioning |
92.26 ± 13.31* | 89.57 ± 11.87 | 3.32 | P<0.05 |
| SF-36: Role functioning: Emotional |
84.51 ± 14.13* | 87.14 ± 13.22 | 3.61 | P<0.05 |
| SF-36: Mental health |
78.24+ 8.97* | 81.25± 10.18 | 3.54 | P<0.05 |
indicates a significant difference between the two groups; P < 0.05.
Table 3.
Mean value and significance of SF-36 subscale scores of group (A) before and at the end of the study
| SF-36 subscale variables |
Mean ± SD | T-value | Significance | |
| Before | After | |||
| SF-36: Health transition |
2.75 ± 0.83* | 1.69 ± 0.61 | 5.34 | P<0.05 |
| SF-36: Physical functioning |
72.18 ± 9.43* | 80.32 ± 10.12 | 6.76 | P<0.05 |
| SF-36: Role functioning: Physical |
78.96 ± 10.15* | 87.13 ± 12.37 | 6.81 | P<0.05 |
| SF-36: Bodily pain | 75.11 ± 7.63* | 69.85 ± 6.25 | 6.52 | P<0.05 |
| SF-36: General health |
71.16± 9.44* | 77.38 ± 10.19 | 7.35 | P<0.05 |
| SF-36: Vitality | 57.27 ± 6.32* | 66.97 ± 7.81 | 6.41 | P<0.05 |
| SF-36: Social functioning |
85.18 ± 11.17* | 92.26 ± 13.31 | 8.15 | P<0.05 |
| SF-36: Role functioning: Emotional |
92.42 ± 15.26* | 84.51 ± 14.13 | 7.86 | P<0.05 |
| SF-36: Mental health |
84.71 ± 10.14* | 78.24+ 8.97 | 6.91 | P<0.05 |
indicates a significant difference between the two groups; P < 0.05.
Table 4.
Mean value and significance of TNF- α, IL-6, IL-8 and CRP of group (B) before and after treatment
| Mean +SD | T-value | Significance | ||
| Before | After | |||
| TNF-α (pg/ml) | 5.93 ± 1.82* | 5.13 ± 1.78 | 3.61 | P<0.05 |
| IL-2 (pg/ml) | 8.15 ± 2.49* | 6.74 ± 2.21 | 3.87 | P<0.05 |
| IL-4 (pg/ml) | 5.63 ± 1.61* | 4.37 ± 1.54 | 3.52 | P<0.05 |
| IL-6 (pg/ml) | 7.65 ± 2.38* | 6.14 ± 2.25 | 3.68 | P<0.05 |
| CRP (mg/dl) | 14.67 ± 3.91* | 11.82 ± 3.27 | 4.36 | P<0.05 |
TNF- α: tumor necrosis factor – alpha; IL-2: Interleukin-2; IL-4: Interleukin-4; IL-6: Interleukin-6; CRP: C-reactive protein
indicates a significant difference between the two groups, P < 0.05.
Discussion
Regular physical activity may attenuate post-menopausal symptoms related to physical and behavioral changes, positively influencing QOL9,11,20. However, studies on the effects of aerobic exercises alone are found in literature11,21. The fact that resisted exercise enhances muscle strength22 and body composition23 is well established, but the association of resisted exercise to QOL improvement has not been demonstrated. Therefore, the aim of this study was to examine the effects of aerobic versus resisted exercise training effects upon systemic inflammation biomarkers and quality of life among sedentary obese post-menopausal women.
The results of our study regarding the QOL proved that both aerobic and resisted exercise training group improved mean values of SF-36 subscale scores in obese post-menopausal women. In addition, there was a significant difference between both groups at the end of the study with more reduction of group (A) received aerobic exercise training. Our findings were consistent with several studies have shown that exercise intervention might enhance health-related quality of life and psychological well-being24–31 as Teomana et al. enrolled 81 volunteer post-menopausal women who have been taking hormone replacement treatment (HRT) were divided randomly into two groups: exercise group (which was omposed of sub-maximal aerobic exercises for a 6-week period 3 times a week) and control group (received no training intervention). At the end of 6 weeks exercise period, they found statistically significant differences in quality of life, strength, endurance, flexibility and balance parameters in the exercise group24. However, Kanemaru et al. randomly assigned sixty-three osteoporotic women over 60 years to 12 months of muscle exercise or to no intervention, they proved that QOL, grip strength and maximum walking speed increased significantly in the intervention group, while there were no improvements in any of the parameters in the control group25.
While, de Souza Santos et al. conducted a longitudinal study that was quasi-experimental and correlational on 323 post-menopausal elderly women who practiced dancing or walking over a 10-month period, their results demonstrated significant differences for the post-test assessment of functional capacity (5.63%) and general QoL (9.19%)26. In addition, Berin et al. conducted a randomized controlled study on sixty symptomatic and sedentary post-menopausal women with a mean of at least four moderate to severe hot flushes per day or 28 per week will be randomized to an exercise intervention or unchanged physical activity (control group). The intervention consists of 15 weeks of standardized resistance training performed three times a week under supervision of a physiotherapist. They proved that exercise training is an effective treatment for hot flushes among post-menopausal women27. Moreover, Küçükçakır et al. enrolled seventy women with the diagnosis of post-menopausal osteoporosis in a supervised Pilates exercise program twice a week for one year, they concluded that Pilates exercises may be a safe and an effective treatment alternative for the quality of life in patients with post-menopausal osteoporosis28. In the other hand, Sattar et al. investigated the impact of aquatic-resistance training on Quality of life in twenty four post-menopausal women. The aquatic-resistance training was administered for a period of 8 weeks, 3 days a week; a session each day. Their results revealed aquatic-resistance training produced positive impacts on the quality of life in post-menopausal women29. Also, Bonganha et al. assessed the body composition, muscle strength and quality of life (QoL) changes following 16 weeks of resistance training in 32 healthy, non-active women who were not under hormone replacement therapy (HRT), classified as control group (CG, n=16) and training group (TG, n=16), they concluded that the 16-week program of RT was not enough to influence the perception of QoL in post-menopausal women30. While, Imayama et al. conducted a study one Middle-aged women (n=100) and men (n=102) were randomly assigned to either exercise (360 min/week of moderate-to-vigorous aerobic exercise) or control and HRQOL (SF-36) were assessed at baseline and 12 months and proved that this level of exercise may increase HRQOL among overweight women31.
Although the exact mechanism for the effect of exercises on mental health is still unknown, several physiological and psychological mechanisms have been proposed, including increased feelings of self-efficacy, self-perceptions of control, reduced emotional strain and physiological responses to stress, and beneficial effects on neurotransmitters32. In addition, physical activity appears to stimulate neurogenesis33, enhance neuronal survival34 and increase synaptic plasticity35. Exercise promotes brain vascularization36,37. Social contact may be an important mechanism, and subjects who take regular exercise may, as a result, get positive feedback from other people and an increased sense of self-worth38.
In our study, the mean values of TNF-α, IL-2, IL-4, IL-6 and CRP were significantly decreased in both groups. In addition, there was a significant difference between both groups at the end of the study with more reduction of group (A) received aerobic exercise training. This means that in obese post-menopausal women aerobic exercise is more appropriate for modulating inflammatory cytokine levels than is resisted exercise training. These results are in line with many previous studies39–50 as Dekker et al. stated that a 12-week exercise intervention resulted in a significant decrease in circulating IL-6 in subjects with type 2 diabetes mellitus who underwent an exercise program without weight loss39. In addition, there is evidence of lowered IL-6 and TNF-α after prolonged exercise in obese women40 and decreased TNF-α after 12 weeks of aerobic exercise in patients with heart disease41. Moreover, in obese post-menopausal women with type 2 diabetes, 14 weeks of aerobic exercise decreased CRP by 15% and marginally decreased IL-6 (p=0.07)42. Likewise, 12 week of exercise reduced IL-18 levels by 17.5% in patients with metabolic syndrome43. On the other side, Rosety-Rodriguez and colleagues in their study on 24 young male adults with Down syndrome who were assigned to perform resistance circuit training with 6 stations, 3 days per week for 12 weeks. They proved that Resistance circuit training improved low-grade systemic inflammation44. In addition, Brooks et al. stated that a 16-week resistance training intervention reduced CRP and increased adiponectin levels in older adults with type 2 diabetes45. While, Neto et al. proved that high-intensity exercise training program induced an anti-inflammatory effect (a reduction of IL-6, TNF-α & leptin and an increase of IL-4 & IL-10)46. In contrast to the previous studies Rall et al. reported that 12 week of high-intensity progressive resistance strength training does not affect TNFα, IL-6, or IL-247.
Also, Rodrigo and colleagues founded that weight-lifting exercises for 6 muscle groups in the upper and lower limbs (2 sets of 8 repetitions each), and the initial load was set at 80% of the 1-repetition maximum load for one month led to improvement in the six minute walking test, health related quality of life (HRQOL) and lower-limb muscle strength, without altering the levels of systemic inflammation48. Also, Nikseresht et al. conducted a 12 week study on middle-aged obese men and proved that aerobic interval training consisted of running on a treadmill (4 sets of 4 minutes at 80–90% of maximal heart rate, with 3-minute recovery intervals) had better anti-inflammatory effects (as indicated by the IL-10: TNF-α ratio) than nonlinear resistance training consisted of 40–65 minutes of weight training at different intensities with flexible periodization49. While, Kohut et al. proved that aerobic exercise intervention, but not flexibility/resistance exercise, reduces serum inflammatory cytokines including interleukin-18 (IL-18), CRP and IL-6 among older adults; this reduction would be mediated, in part, by improvements in psychosocial factors and/or by β-adrenergic receptor mechanisms50.
The exact mechanisms by which physical activity may reduce inflammation are not entirely understood, there are some data pointing to factors that may contribute to an effect of repeated bouts of muscle contraction leading to improvements in inflammatory status over time as exercise training-induced improvements in inflammatory status may also result from the modulation of intracellular signaling pathways and cellular function that are mediated by nitric oxide (NO) and ROS51. Moreover, the potential mechanisms for the anti-inflammatory effect of exercise may include reduced percentage of body fat and macrophage accumulation in adipose tissue, muscle-released IL-6 inhibition of TNF-α and the cholinergic anti-inflammatory pathway52.
The current study has important strengths and limitations. The major strength is the supervised nature of the study. However, all exercise sessions were supervised. Moreover, the study was randomized; hence, we can extrapolate adherence to the general population. In the other hand, the major limitations is only post-menopausal women enrolled in the study, so the value of this study only related to women in this age group, also small sample size in both groups may limit the possibility of generalization of the findings in the present study. Finally, within the limit of this study, aerobic exercise training is recommended for modulation of low grade systemic inflammation and quality of life among obese post-menopausal women. Further researches are needed to explore the impact of weight reduction on quality of life and other biochemical parameters among post-menopausal women.
Conclusion
The current study provides evidence that weight reduction program improves biochemical & clinical parameters and quality of life among obese post-menopausal women. The strengths of this trial include its large sample size and randomized controlled design. There is no doubt that further studies are necessary to understand cellular and molecular mechanisms involved in relationships among weight reduction and changes of overall subscales of SF-36.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G-4-142-1439). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Conflict of interest
None declared.
References
- 1.Jones G, Sutton A. Quality of life in obese post-menopausal women. Menopause Int. 2008;14(1):26–32. doi: 10.1258/mi.2007.007034. [DOI] [PubMed] [Google Scholar]
- 2.Flegal K, Carroll M, Ogden C, Curtin L. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Fuller N, Lau N, Denyer G, Simpson A, Gerofi J, Wu M, Holmes A, Markovic T, Kang J, Caterson I. A 12-week, randomised, controlled trial to examine the acceptability of the Korean diet and its effectiveness on weight and metabolic parameters in an Australian overweight and obese population. Obesity Research & Clinical Practice. 2012;6(1):e71–e83. doi: 10.1016/j.orcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation, author. Obesity and overweight. Geneva, Switzerland: World Health Organisation; 2011. [Google Scholar]
- 5.World Health Organisation, author. Global strategy on diet, physical activity and health. Geneva, Switzerland: World Health Organisation; 2011. [Google Scholar]
- 6.Kruger J, Bowles HR, Jones DA, Ainsworth BE, Kohl HW. Health related quality of life, BMI and physical activity among US adults (N = 18 years): National physical activity and weight loss survey, 2002. International Journal of Obesity. 2003;31:321–327. doi: 10.1038/sj.ijo.0803386. [DOI] [PubMed] [Google Scholar]
- 7.McInnes R, Gray C. Obese Women and Quality of Life. Obesity. 2013;15:585–595. [Google Scholar]
- 8.Dennerstein L, Lehert P, Guthrie J. The effects of the menopausal transition and biopsychossocial factors on well-being. Arch Women Ment Health. 2002;5:15–22. doi: 10.1007/s007370200018. [DOI] [PubMed] [Google Scholar]
- 9.De Lorenzi DR, Baracat EC, Saciloto B, Padilha JI. Fatores associados àqualidade de vida após menopausa. Rev Asso Med Bras. 2006;52:312–317. doi: 10.1590/s0104-42302006000500017. [DOI] [PubMed] [Google Scholar]
- 10.Hess R, Colvin A, Avis NE, Bromberger JT, Schocken M, Johnston JM, Matthews KA. The impact of hormone therapy on health-related quality of life: longitudinal results from the Study of Women's Health Across the Nation. Menopause. 2007;15:422–428. doi: 10.1097/gme.0b013e31814faf2b. [DOI] [PubMed] [Google Scholar]
- 11.Moriyama CK, Oneda B, Bernardo FR, Cardoso CG, Junior, Forjaz CL, Abrahao SB, Mion D, Fonseca AM, Tinucci T. Arandomized, placebo-controlled trial of the effects of physical exercises and estrogen therapy on health-related quality of life in post-menopausal women. Menopause. 2008;15:613–618. doi: 10.1097/gme.0b013e3181605494. [DOI] [PubMed] [Google Scholar]
- 12.Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychology Review. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Larson EB. Prospects for delaying the rising tide of worldwide, late-life dementias. International Psychogeriatrics. 2010;22:1196–1202. doi: 10.1017/S1041610210001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt T, Schneider S, Bruumer V, Struder H K. Frontal EEG asymmetry: The effects of sustained walking in the elderly. Neuroscience Letters. 2010;485:134–137. doi: 10.1016/j.neulet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Benloucif S, Orbeta L, Ortiz R, Janssen I, Finkel SI, Bleiberg J. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;15:1542–1551. doi: 10.1093/sleep/27.8.1542. PubMed. [DOI] [PubMed] [Google Scholar]
- 16.van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: The FINE study. Neurology. 2004;28:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg S, Boll D, Nicolaije K, Vos M, Pijnenborg J, Coebergh J, Beijer S, van de Poll-Franse L, Ezendam N. The relationship of body mass index with quality of life among endometrial cancer survivors: A study from the population-based PROFILES registry. Gynecologic Oncology. 2013;129:216–221. doi: 10.1016/j.ygyno.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Deibert P, Konig D, Vitolins MZ. Effect of a weight loss intervention on anthropometric measures and metabolic risk factors in pre- versus post-menopausal women. J Nutr. 2007;6:31. doi: 10.1186/1475-2891-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagey AR, Warren MP. Role of exercise and nutrition in menopause. Clin Obstet Gynecol. 2008;51:627–641. doi: 10.1097/GRF.0b013e318180ba84. [DOI] [PubMed] [Google Scholar]
- 20.Woodward MJ, Lu CW, Levandowski R, Kostis J, Bachmann G. The exercise prescription for enhancing overall health of midlife and older women. Maturitas. 2015 Sep;82(1):65–71. doi: 10.1016/j.maturitas.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Ji W, Lv X, Zhu Y, Zhao J, Miao L. Impact of physical activity on health-related quality of life in osteoporotic and osteopenic post-menopausal women: A systematic review. International Journal of Nursing Sciences. 2015;2:204–217. [Google Scholar]
- 22.Orsatti FL, Nahas EAP, Maesta N, Nahas-Neto J, Burini RC. Plasma hormones, muscle mass and strength in resistance-trained post-menopausal women. Maturitas. 2008;59:394–404. doi: 10.1016/j.maturitas.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Maestá N, Nahas EAP, Nahas-Neto J, Orsatti FL, Fernandes CE, Traiman P, Burini RC. Effects of soy protein and resistance exercise on body composition and blood lipids in post-menopausal women. Maturitas. 2007;56:350–358. doi: 10.1016/j.maturitas.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Teomana N, Özcan A, Acar B. The effect of exercise on physical fitness and quality of life in post-menopausal women. Maturitas. 2004;47:71–77. doi: 10.1016/s0378-5122(03)00241-x. [DOI] [PubMed] [Google Scholar]
- 25.Kanemaru A, Arahata K, Ohta T, Katoh T, Tobimatsu H, Horiuchi T. The efficacy of home-based muscle training for the elderly osteoporotic women: the effects of daily muscle training on quality of life (QoL) Arch Gerontol Geriatr. 2010;51(2):169–172. doi: 10.1016/j.archger.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 26.de Souza Santos CA, Dantas EE, Moreira MH. Correlation of physical aptitude; functional capacity, corporal balance and quality of life (QoL) among elderly women submitted to a post-menopausal physical activities program. Arch Gerontol Geriatr. 2011;53(3):344–349. doi: 10.1016/j.archger.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Berin E, Hammar ML, Lindblom H, Lindh-Åstrand L, Spetz Holm AC. Resistance training for hot flushes in post-menopausal women: Randomized controlled trial protocol. Maturitas. 2016 Mar;85:96–103. doi: 10.1016/j.maturitas.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Küçükçakır N, Altan L, Korkmaz N. Effects of Pilates exercises on pain, functional status and quality of life in women with post-menopausal osteoporosis. J Bodyw Mov Ther. 2013 Apr;17(2):204–211. doi: 10.1016/j.jbmt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Sattar M, Esfarjani F, Nezakatalhosseini M. The effect of aquatic-resistance training on quality of life in post-menopausal women. Procedia - Social and Behavioral Sciences. 2013;70:1732–1739. [Google Scholar]
- 30.Bonganha V, Modeneze DM, Madruga VA, Vilarta R. Effects of resistance training (RT) on body composition, muscle strength and quality of life (QoL) in post-menopausal life. Arch Gerontol Geriatr. 2012 Mar-Apr;54(2):361–365. doi: 10.1016/j.archger.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Imayama I, Alfano CM, Cadmus Bertram LA, Wang C, Xiao L, Duggan C, Campbell KL, Foster-Schubert KE, McTiernan A. Effects of 12-month exercise on health-related quality of life: a randomized controlled trial. Prev Med. 2011;52(5):344–351. doi: 10.1016/j.ypmed.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paluska SA, Schwenk TL. Physical activity and mental health: current concepts. Sports Med. 2000;29:167–180. doi: 10.2165/00007256-200029030-00003. PubMed. [DOI] [PubMed] [Google Scholar]
- 33.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and longterm potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. PubMed. [PubMed] [Google Scholar]
- 35.Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58:76–87. PubMed. [PubMed] [Google Scholar]
- 36.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci, USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 38.Greist JH, Klein MH, Eischens RJ, Faris J, Gurman AS, Morgan WE. Running as treatment for depression. Compr Psychiatry. 1979;20:41–54. doi: 10.1016/0010-440x(79)90058-0. PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Dekker M, Lee S, Hudson R, Kilpatrick K, Graham T, Ross R. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56(3):332–338. doi: 10.1016/j.metabol.2006.10.015. PubMed. [DOI] [PubMed] [Google Scholar]
- 40.You T, Berman D, Ryan A, Nicklas B. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese post-menopausal women. J Clin Endocrinol Metab. 2004;89(4):1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 41.Larsen A, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am J Cardiol. 2001;88(7):805–808. doi: 10.1016/s0002-9149(01)01859-8. PubMed. [DOI] [PubMed] [Google Scholar]
- 42.Giannopoulou I, Fernhall B, Carhart R, Weinstock R, Baynard T, Figueroa A, Kanaley J. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of post-menopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. PubMed. [DOI] [PubMed] [Google Scholar]
- 43.Troseid M, Lappegard KT, Mollnes T, Arnesen H, Seljeflot I. The effect of exercise on serum levels of interleukin-18 and components of the metabolic syndrome. Metab Syndr Relat Disord. 2009;7(6):579–584. doi: 10.1089/met.2009.0003. [DOI] [PubMed] [Google Scholar]
- 44.Rosety-Rodriguez M, Camacho A, Rosety I, Fornieles G, Rosety MA, Diaz AJ, Rosety M, Ordonez FJ. Resistance circuit training reduced inflammatory cytokines in a cohort of male adults with Down syndrome. Med Sci Monit. 2013;19:949–953. doi: 10.12659/MSM.889362. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks N, Layne J, Gordon P, Roubenoff R, Nelson M, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4:19–27. doi: 10.7150/ijms.4.19. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosa N, Lira F, Oyama L, Zanchi N, Yamashita A, Batista M, Oller do Nascimento C, Seelaender M. Exhaustive exercise causes an anti-inflammatory effect in skeletal muscle and a pro-inflammatory effect in adipose tissue in rats. Eur J Appl Physiol. 2009;106:697–704. doi: 10.1007/s00421-009-1070-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 47.Rall L, Roubenoff R, Cannon J, Abad L, Dinarello C, Meydani S. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med Sci Sports Exerc. 1996;28:1356–1365. doi: 10.1097/00005768-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigo C, Celso R, Carvalho C. Impact of Resistance Training in Chronic Obstructive Pulmonary Disease Patients during Periods of Acute Exacerbation. Archives of Physical Medicine and Rehabilitation. 2014;95(9):1638–1645. doi: 10.1016/j.apmr.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Nikseresht M, Agha-Alinejad H, Azarbayjani MA, Ebrahim K. Effects of nonlinear resistance and aerobic interval training on cytokines and insulin resistance in sedentary men who are obese. J Strength Cond Res. 2014;28(9):2560–2568. doi: 10.1519/JSC.0000000000000441. PubMed. [DOI] [PubMed] [Google Scholar]
- 50.Kohut M, McCann D, Russell D, Konopka D, Cunnick J, Franke W, Castillo M, Reighard A, Vanderah E. Aerobic exercise, but not flexibility/ resistance exercise, reduces serum IL -18, CRP and IL-6 independent of beta-blockers, BMI and psychosocial factors in older adults. Brain Behav Immun. 2006;20(3):201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Scheele C, Nielsen S, Pedersen B. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab. 2009;20:95–99. doi: 10.1016/j.tem.2008.12.002. PubMed. [DOI] [PubMed] [Google Scholar]
- 52.Woods J, Vieira V, Keylock K. Exercise, inflammation, and innate immunity. Neurol Clin. 2006;24(3):585–599. doi: 10.1016/j.ncl.2006.03.008. PubMed. [DOI] [PubMed] [Google Scholar]