Abstract
Background
Helicobacter pylori frequently causes gastritis and peptic ulcers, and affected children are at risk of developing gastric carcinoma later in adulthood.
Methods
This was a Hospital based cross sectional study. A total of 200 children aged 6 months to 14 years were enrolled. Study subjects were tested for H. pylori using a standard serology rapid test measuring immunoglobulin G for H. pylori. For risk factors, Chi-square tests were used to test for association and then, odds ratios and their corresponding 95% confidence intervals and p-values were computed using logistic regression.
Results
The overall seroprevalence of H. pylori was 11.5%. The following factors were associated with H. pylori infection: Age group above 10 years, keeping a dog and household size. The independent predictors of H. pylori were: Fathers' occupation, keeping a dog, indoor tap water, age group, household size and diabetes mellitus type 1..
Conclusion
The seroprevalence of H. pylori antibodies was lower compared to most developing countries. Keeping a dog, household size, indoor tap water, fathers' occupation and diabetes mellitus type 1 were found to be independent predictors of presence of H. pylori antibodies.
Keywords: Helicobacter Pylori
Introduction
Helicobacter pylori (H. pylori) is a spiral-shaped gram negative bacterium found on the human gastric mucosa. It was first isolated by Warren and Marshall in 1982. Initially, this bacterium was classified as Campylobacter pyloridis but in 1989 was included in a new genus, Helicobacter, and renamed H. pylori. In 1994, the international agency for cancer classified H. pylori as the number one carcinogen or cancer causing agent and now it is the most common cause of gastric cancer which is the third cause of mortality amongst the cancers worldwide.
Globally, about half of the population, has H. pylori and the prevalence and its effects are more pronounced in the developing countries1,2. The estimated prevalence among children in developing countries is 30.9%3,4. H. pylori can cause iron and vitamin B12 depletion in the affected individual, as a result causing iron and vitamin B12 deficiency. Furthermore, H. pylori frequently causes gastritis and peptic ulcers, and affected children are at risk of developing gastric carcinoma5,6.
The risk factors for H. pylori include poverty related conditions such as overcrowding, poor hygiene and sanitation, low education level of mothers, and higher number of siblings7; all of which are common in Tanzania. In some cases, H. pylori among children presents with recurrent abdominal pain due to peptic ulcer disease; in a review article by Eren et al, 2011, H. pylori was found to be the most common cause of duodenal ulcers in children. Thus in thistudy the frequency of peptic ulcer disease was 9.4% amongst children and 76% were H. pylori positive.8
As a matter of fact, the aim of this study was to provide robust evidence on seroprevalence of H. pylori and its co-morbidities amongst children. In this case, it implies that clinicians will have a higher index of suspicion on presence of H. pylori infection and will be able to screen the population at risk and treat before development of any complications.
Methodology
Study population and study design
This was a cross-sectional hospital based study, which was done from June 2015 to May 2016 at the Kilimanjaro christian medical college (KCMC) pediatric department. The hospital is a regional referral health facility that has a pediatric bed capacity of 450 and it serves people within and outside Kilimanjaro region including both in-patients and out-patients. On average100 children are admitted to the ward every month. The study subjects were children admitted to the pediatric ward and those attending pediatrics out-patient clinics who are between 6 months and 14 years. In addition, those whose guardians did not give consent to participate in the study were excluded.
Sampling technique
All children fitting the criterion and coming for health care within the study period were enrolled if consent was granted by guardians until the desired sample size of 200 was achieved. Furthermore, the type 1 diabetes mellitus (T1DM) and Human immunodeficiency virus (HIV) units were purposively selected by the researcher to answer some of the objectives of the study.
Data collection tools
Structured questionnaires were used to collect information on social demographic and hygiene factors while laboratory data sheets were used to collect data on H. pylori and co-morbidities such as (T1DM) and (HIV).
Data collection methods
Data on risk factors and co-morbidities was collected using a precoded structured questionnaire which included the following information: Age, gender, residence, source of drinking thus whether it's from a spring, stream, well or tap, total number of people living in the house, guardians' educational level, father's and mother's occupation, contact with a community (a child who has been staying long time in community care services such as school, day care center), breast-feeding, recent antibiotic use, exposure to cigarette smoking, and hand washing before and after use of pit latrine, whether they eat from the same plate as a family or they use separate plate.
Laboratory analysis
A standard serology rapid test (FlexSure® HP) measuring immunoglobulin G for H. pylori in the serum was used to confirm the presence of H. pylori antibodies amongst the subjects. Thus 1 to 2 mls of whole blood was drawn from a vein by using a 2 mls syringe after observing sterile procedures, thus vein site was cleaned with alcohol swabs. The blood was then left to stand for 10 to 15 minutes in an Ethylenediaminetetraacetic acid tube and later the serum was tested for H. pylori antibodies.
Data analysis
The data collected was entered into EPIINFO 7 using double entry validity and exported to STATA 13 for analysis. The main study outcome was presence of H. pylori. The exposure variables include socio-demographic, recent antibiotic use, living in a crowded house, hygiene conditions and drinking spring water among others.
The analysis also explored the relationship between H. pylori and co-morbidities studied; these included diabetes and HIV.
Descriptive statistics were computed for the variables; frequencies and percentages were obtained for categorical variables while for continuous variables, means and respective standard deviations were presented.
For factors associated with H. pylori, at first, chi-square tests were used to test for association and then, odds ratios and their corresponding 95% confidence intervals and p-values were computed using logistic regression. Independent risk factors for H. pylori were assessed using the likelihood ratio test.
Ethical clearance
Ethical clearance was obtained from the KCMC ethical committee, college research ethical commitee form number 869 and signed informed consent were obtained from the parents/ legal guardians of the children before they were enrolled in the study. The signed consents were kept in a secure place and accessed only by the principal investigator.
Results
Descriptive analysis
Demographic characteristics
A total of 200 participants were enrolled for this study. Of these, 80 (40%) were below 5 years, 56 (26%) were between 5 to 10 years and 68 (34%) were older than 10 years; a majority of the study participants were male 109 (54.5%) while 91 (45.5%) were female; 109 (54.5%) came from rural residences while the rest came from urban residences; Furthermore, the average number of siblings per study participant was 2.1(SD=1.6). (Table 2)
Table 2.
Regression analysis of the risk factors for H. pylori
| Variable | Unadjusted OR (95%CI) | p-value | Adjusted OR (95%CI) | p-value |
| Sex | ||||
| Female | 1 | |||
| Male | 1.07 (0.4–2.6) | 0.836 | ||
| No of siblings | 0.91 (0.7–1.2) | 0.542 | ||
| Share plate | ||||
| No | 1 | |||
| Yes | 1.50 (0.6–3.9) | 0.407 | ||
| Day care | ||||
| Yes | 0.53 (0.1–1.9) | 0.326 | ||
| Antibiotics | ||||
| No | 1 | |||
| Yes | 0.72 (0.3–1.9) | 0.520 | ||
| Age group | ||||
| < 5 years | 1 | 1 | ||
| 5–10 years | 0.76 (0.2–3.2) | 0.701 | 0.89 (0.16–5.03) | 0.891 |
| > 10 years | 3.20 (1.2–8.9) | 0.025* | 7.13 (1.64–31.00) | 0.009* |
| Caretaker | ||||
| Education | ||||
| None | 1 | 1 | ||
| Primary | 0.35 (0.09–1.30) | 0.119 | 0.25 (0.04–1.63) | 0.148 |
| Secondary | 0.17 (0.03–0.79) | 0.024* | 0.15 (0.01–1.58) | 0.115 |
| Tertiary | 0.33 (0.61–1.73) | 0.189 | 1.32 (0.09–20.18) | 0.840 |
| Father Occupation | ||||
| Farmer | 1 | 1 | ||
| Business | 0.14(0.03–0.65) | 0.012* | 0.15 (0.01–1.95) | 0.148 |
| Professional | 0.33 (0.1–1.1) | 0.067 | 0.50 (0.04–5.89) | 0.584 |
| Other | 0.08 (0.01–0.55) | 0.018* | 0.01 (0.00–0.60) | 0.025* |
| Mother Occupation | ||||
| Housewife | 1 | 1 | ||
| Farmer | 3.05 (0.9–10.0) | 0.067 | 0.39 (0.04–4.29) | 0.441 |
| Business | 0.75 (0.2–3.5) | 0.717 | 0.34 (0.04–2.55) | 0.293 |
| Professional | 1.25 (0.3–6.0) | 0.781 | 0.58 (0.05–6.44) | 0.659 |
| Household size | 0.87 (0.7–1.1) | 0.273 | 0.64 (0.45–0.94) | 0.025* |
| No pit latrine use | ||||
| Yes | 0.40 (0.15–1.01) | 0.052 | 1.13 (0.24–5.40) | 0.881 |
|
Lack tap water indoor |
||||
| Yes | 2.87(1.15–7.11) | 0.023* | 4.41 (0.69–27.9) | 0.114 |
| Cigarette | ||||
| Yes | 0.28(0.03–2.14) | 0.218 | 0.28 (0.03–2.83) | 0.281 |
| Keeping dog | ||||
| Yes | 3.28 (1.3–8.3) | 0.012* | 4.80 (1.23–18.84) | 0.024* |
Statistically significant at p.0.05
With regards to caretaker education, majority attended primary school 97 (48.5%) and only 7% had no education. The predominant occupation for mothers and fathers was farming with 61 (30.8%) and 60 (30.9%) respectively falling in these categories.
In addition, for comorbidities, 42(21%) of the study participants had T1DM whereas 40(20%) had HIV. (Table 1)
Table 1.
Study participants' demographic characteristics
| Variable | Frequency (N=200) | Percentage (100) |
| Age group | ||
| < 5 years | 80 | 40 |
| 5–10 years | 52 | 26 |
| > 10 years | 68 | 34 |
| Sex | ||
| Female | 91 | 45.5 |
| Male | 109 | 54.5 |
| Residence | ||
| Urban | 93 | 46.7 |
| Rural | 106 | 53.3 |
| Caretaker Education | ||
| None | 14 | 07.0 |
| Primary | 97 | 48.5 |
| Secondary | 63 | 31.5 |
| Tertiary | 26 | 13.0 |
| Mother Occupation | ||
| Housewife | 49 | 24.8 |
| Farmer | 61 | 30.8 |
| Business | 48 | 24.2 |
| Professional | 30 | 15.2 |
| Other | 10 | 5.0 |
| Father Occupation | ||
| Farmer | 60 | 30.9 |
| Business | 49 | 25.3 |
| Professional | 44 | 22.7 |
| Other | 41 | 21.1 |
| Household size | ||
| Mean (SD) | 5.2 (1.8) | |
| No. of siblings | ||
| Mean (SD) | 2.1 (I.6) | |
| T1DM | ||
| No | 158 | 79 |
| Yes | 42 | 21 |
| HIV Status | ||
| Negative | 159 | 80 |
| Positive | 41 | 20 |
Hygiene characteristics
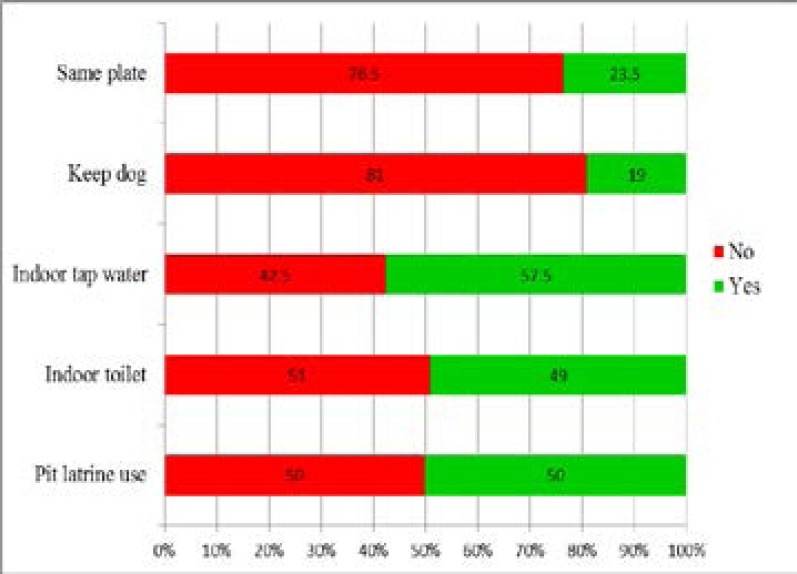
In general, 191 (95.5%) children had the tap as their drinking water source while 9 (4.5%) used wells as their drinking water source; 50% of the study participants came from homes with pit latrines and up to 98 (49%) of the study participants came from homes with indoor toilets, 115 (57.5%) had indoor tap water, 47 (23.5%) of the children shared a plate while 38 (19%) kept a dog. (Figure 2)
Figure 2.
Hygiene factors amongst study participants receiving care at KCMC (N=200)
Seroprevalence of H. pylori and cormobidity conditions
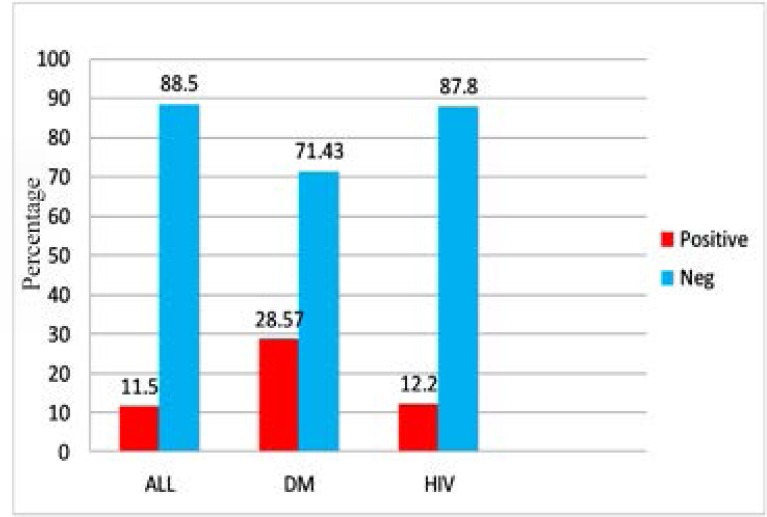
The overall seroprevalence of H. pylori amongst the study participants was 23(11.5%) while in those with T1DM and HIV it was 12(28.57%) and 5(12.5%), respectively. In this case a higher seroprevalence of H. pylori amongst those with T1DM was noted. (Figure 1)
Figure 1.
Seroprevalence of H. pylori amongst study subjects receiving care at KCMC (N=200)
Risk factors and H. pylori amongst children aged 6months to 14 years
Amongst the social demographic factors, age group above 10 years (χ2=8.5, p=0.015) and occupation of father (χ2=8.9, p=0.002) showed a significant relationship with H. pylori. In addition, the following hygiene factors were found to have association with H. pylori; having indoor tap water (χ2=5.5, p=0.019), using a pit latrine with hand washing (χ2=4.6, p=0.032), pit latrine use (χ2=4.0, p=0.046) and keeping a dog (χ2=6.8, p=0.02). Other factors such as daycare attendance (χ2=0.1, p=0.421), antibiotic use (χ2=0.4, p=0.518) and cigarette smoking (χ2=1.7, p=0.3) were not associated with H. pylori among children in this population.
After adjusting for potential confounders, age above 10 years had 7.13 times higher odds of having H. pylori, thus a 3.93-fold increase compared to unadjusted odds ratio. In addition, those with tap water indoor had 90% lower odds for H. pylori compared to those without indoor tap water. Furthermore, with regards to household size, there was a 36% reduction in odds for H. pylori for each 1 person increase in the household and for those keeping a dog, they had 4.8 times higher odds of having H. pylori compared to those without a dog. Age above 10 years (95% CI=1.64–31.0), household size (95% CI=0.45–0.94), and keeping a dog (95% CI=1.23–18.84) were still associated with H. pylori in the multivariate analysis. (Table 2)
Independent predictors of H. pylori
All variables in the multivariate model were tested to see if they were independent predictors of H. pylori. Thus, from the Likelihood ratio test, age group (χ2=10.63, p-value= 0.005), father's occupation (χ2=9.94, p-value=0.019), water indoor (χ2=4.35, p-value=0.037), keeping a dog (χ2=5.21, p-value=0.022), and household size (χ2=5.96, p-value=0.015) were found to be independent predictors of H. pylori among children aged 6months–14 years.
Relationship between H. pylori and T1DM (multivariate analysis)
After running the univariate analysis it was noticed that there was a significant relationship between H. pylori and T1DM (p=0.001). However, there was no significant association between H. pylori and HIV (p=0.791).
Furthermore, in the multivariate analysis, after adjusting T1DM for the independent risk factors of H. pylori such as; fathers occupation, indoor tap water, age group above 10, keeping a dog and household size, it was found out that T1DM still remains significantly associated with H. pylori (OR: 8.19, 95% CI: 2.01–33.28) and there was 2.85 fold increase in the odds. In this model, apart from the adjusting T1DM for the independent risk factors of H pylori, we also included HIV in order to assess its effect on T1DM if there was any.(Table 3). In addition, we run a likelihood ratio test to test for independence and we found out that T1DM was independently associated with H. pylori (χ2=9.3, p=0.002).
Table 3.
Relationship between H. pylori and T1DM
| Variable | Unadjusted OR (95%CI) | p-value | Adjusted OR (95%CI) | p-value |
| Father Occupation | ||||
| Farmer | 1 | 1 | ||
| Business | 0.14(0.03–0.65) | 0.012* | 0.17 (0.03–1.02) | 0.052 |
| Professional | 0.33 (0.1–1.1) | 0.067 | 0.68 (0.15–3.01) | 0.608 |
| Other | 0.08 (0.01–0.55) | 0.018* | 0.05 (0.00–0.50) | 0.011 |
| No Water indoor | ||||
| Yes | 2.87 (1.15–7.11) | 0.023* | 3.58 (0.96–13.36) | 0.058 |
| Age group | ||||
| < 5 years | 1 | 1 | ||
| 5–10 years | 0.76 (0.2–3.2) | 0.701 | 0.59 (0.11–3.03) | 0.524 |
| > 10 years | 3.20 (1.2–8.9) | 0.025* | 1.58 (0.37–6.67) | 0.537 |
| Keeping dog | ||||
| Yes | 3.28 (1.3–8.3) | 0.012* | 3.47 (1.04–11.50) | 0.042* |
| Household size | 0.91 (0.7–1.2) | 0.542 | 0.69 (0.49–0.99) | 0.045* |
| HIV status | ||||
| Negative | 1 | 1 | ||
| Positive | 1.09 (0.38–3.13) | 0.876 | 2.54 (0.63–10.28) | 0.190 |
| T1DM | ||||
| No | 1 | 1 | ||
| Yes | 5.34 (2.16–13.25) | <0.0001* | 8.19 (2.01–33.28) | 0.003* |
Statistically significant at p.0.05
Discussion
In this study, the overall seroprevalence of H. pylori antibodies was 11.5% and the seroprevalence was higher in children with T1DM. Furthermore, among the variables we studied, in the multivariate analysis the following risk factors had a significant relationship with H. pylori infection: Age group above 10 years, keeping a dog, household size and fathers' occupation. In addition, T1DM was independently associated with presence of H. pylori antibodies.
This is the first study looking at H. pylori in children done in the Kilimanjaro region and to our knowledge there are no publications seen on studies done in Tanzania on similar topics. This result is similar to those observed in other developed countries where by a prevalence of H. pylori of less than 15% was observed9,10.
In contrast this result is lower compared to most studies done in Nigeria and Kenya where they found the prevalence of H. pylori to be 63.3% and 73.3%11,12, respectively. This difference could be attributed to the fact that in these studies they included only symptomatic children while in our study we screened for H. pylori antibodies in all children receiving care within the given period of time at KCMC. In addition, it could also be as a result of differences in environment and hygiene practices in the places where studies were done.
In our study, we found out that the seroprevalence of H. pylori was quite higher with increase in age which was consistent with other studies 7. As a matter of fact, the seroprevalence of H. pylori in those above 10 years was 20.6% which was higher compared to those under 5 (6%) and 5 to 10 years (3%). (Table 1). In this case it means this is the age group with highest risk and a clinician should have a higher index of suspicion of H. pylori infection if the child in this age group presents with signs and symptoms of the H. pylori infection i.e. abdominal pain or dyspepsia.
Furthermore, occupation of father, household size and keeping a dog and indoor tap water were found to be independently associated with H. pylori which is similar to some studies7,13,14. The knowledge of these factors is important since they can guide the clinician to assess the risk of acquiring H. pylori infection especially if the patient is presenting with signs and symptoms of H. pylori such as abdominal pain or dyspepsia in a low resource setting. In this case the clinician can undertake a decision to treat the child empirically which is not only clinically sound but also cost effective in the developing countries, especially in hard to reach low resource areas where tools for H. pylori detection may not be available.
In our study we did not find any significant relationship between breast feeding, use of antibiotics and cigarrete smoking with H. pylori. In general, our results contrast with some studies where they found out that there was no role of environmental factors i.e. hygiene factors, rather advocates person to person transmission i.e. breast feeding. As a matter of fact, they only found association of H. pylori to social demographic factors such as age 14. The differences could be explained by sample size differences which probably limited strength of association between H. pylori and other risk factors. In addition, study settings could also contribute to the observed differences i.e. some studies were done exclusively in one setting such as Urban or rural, and were community based15 while in our study it was a mixture of both urban and rural population. Furthermore, it was a hospital based study.
Similar to other studies we found out that T1DM is significantly associated with H. pylori (Table 3). A study done in Egypt looking at the relationship between H. pylori and T1DM reported a significant association where by children with T1DM had a significant higher level of H. pylori16. As a matter of fact, Dai et al, 2015, stipulates that poor glycemic control amongst children with T1DM is the reason for increased seroprevalence of H. pylori amongst the children with T1DM which was also the case in our study since the average HbA1c was 11.5% which was high and an indication for poorly controlled T1DM17. In contrast Candelli et al, 2000, found out that there was no significant association between H. pylori and diabetes type 1, instead his explanation was that most previous association may have arisen from failure to consider social economic status and age, or a different prevalence of a specific strain of H. pylori known as Cag-A positive cytotoxic strain. Thus Cag A cytotoxic strains induce a higher production of proinflammatory cytokines which cause a remarkable impairment of metabolic control in T1DM patients than Cag-A negative strain18. However, in our study after adjusting H. pylori for independent risk factors such as age above 10 years, fathers' occupation, household size, keeping a dog and having tap water indoor, thus we run a likelihood ratio test and found out that T1DM has a true association with H. pylori. This information is clinically significant since it can help in decision making as far as management of children in diabetic clinic who are presenting with dyspepsia or abdominal pain are concerned. In this case it would be reasonable to start the patient on empirical treatment for H. pylori infection.
This study found no association between H. pylori and HIV. Hestivic et al, 2011, from Uganda, found a significant relationship between H. pylori and HIV. In this cross-sectional hospital based study and exclusively done in Kampala urban, Hestivic found out that HIV positive children with advanced disease were less likely to be infected with H. pylori15. In our case absence of association between H. pylori and HIV, could be explained by limited sample size and additionally all our children were on anti-retroviral therapy and had had antibiotics or on antibiotics due to some serious bacterial infection or prophylaxis and the latter could possibly have eliminated H. pylori in those found H. pylori antibody negative.
In general, our study satisfied the objectives as far as a hospital based cross-sectional study is concerned. Thus from this study, we will be able to generate hypotheses for future studies and it will enable us to formulate a treatment protocol that will include the tools provided for by this study such as understanding of independent predictors of H. pylori infection which is cost effective to a resource limited setting. However, our study was a hospital based and that means the guardians of these patients have health seeking behavior thus there is a possibility that the study subjects had had antibiotic treatment at some point which eradicated H. pylori infection in some of those who tested H. pylori antibody negative. Furthermore, these results cannot be used to generalize presence of active infection since we screened for H. pylori using an immunoglobulin G antibody test which detects both exposure as well as chronic infection which means we may have missed the newly infected subjects with very low or no antibody titers.
Conclusion
Conclusively, in this study the seroprevalence of H. pylori was lower compared to most studies done in the developing countries. However, the mere presence of the H. pylori antibodies in the study subjects on its own has significant clinical implications and should be taken seriously. In addition, T1DM, socio-demographic factors such as age, fathers' occupation, household size as well as hygiene and environmental factors such as lack of indoor tap water and keeping a dog have been identified as independent predictors of H. pylori infection. Therefore, routine screening for H. pylori in higher risk groups such as those above age of 10 years with T1DM and exposed to the other risk factors such as owning a dog is highly recommended. In this case, it means that improved hygiene and sanitation can help eradicate H. pylori in our population.
Acknowledgements
This research was funded by Malawi government who is the sponsor for the authors education at KCMC. The author would also like to thank Florence Nakkagwa from Uganda International Health Sciences University who assisted with knowledge on statistical analysis and KCMC department of statistics and epidemiology who dedicated themselves to teach the author research methodology, management and statistics.
Abbreviations
- KCMC
Kilimanjaro Christian Medical Center
- T1DM
Type 1 diabetes mellitus
- HIV
Human Immunodeficiency virus
- HBA1c
Glycated hemoglobin
Declarations
Ethical approval and consent
The study was approved by KCMC ethics committee and the guardians of the children signed a consent form to grant permission for the researcher to proceed with data collection.
Authors' contribution
AM is the one who developed proposal, collected and analyzed data. RP, LV GK and BM: These were major contributors in development of proposal, they reviewed the topic, offered advice on how it could be modified to address the intended goal, they all looked at the feasibility of analytical methods and help intellectual understanding and editing of the manuscript.
References
- 1.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori Infection. Helicobacter. 2004;9:1–6. doi: 10.1111/j.1083-4389.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 3.Yücel O, Sayan A, Yildiz M. The factors associated with asymptomatic carriage of Helicobacter pylori in children and their mothers living in three socio-economic settings. Jpn J Infect Dis. 2009;62:120–124. [PubMed] [Google Scholar]
- 4.Yucel O, Prevention of. Helicobacter pylori infection in childhood. World J Gastroenterol. 2014:10348–10354. doi: 10.3748/wjg.v20.i30.10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol Helicobacter pylori. 2007;21:205–214. doi: 10.1016/j.bpg.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Salih BA. Helicobacter pylori Infection in Developing Countries: The Burden for How Long? Saudi J Gastroenterol Off J Saudi Gastroenterol Assoc. 2009;15:201–207. doi: 10.4103/1319-3767.54743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasosah M, Satti M, Shehzad A, Alsahafi A, Sukkar G, Alzaben A, et al. Prevalence and risk factors of Helicobacter pylori infection in Saudi children: a three-year prospective controlled study. Helicobacter. 2015:56–63. doi: 10.1111/hel.12172. [DOI] [PubMed] [Google Scholar]
- 8.Eterm D. Helicobacter pylori infection in children. Journal of Pediatric Sciences. 2011;3(4):e102. [Google Scholar]
- 9.Hoffmann A, Krumbiegel P, Richter T, Richter M, Röder S, Rolle-Kampczyk U, Herbarth O. Helicobacter pylori prevalence in children influenced by non-specific antibiotic treatments. Cent Eur Journal of Public Health. 2014;22(1):48–53. doi: 10.21101/cejph.a3890. [DOI] [PubMed] [Google Scholar]
- 10.Segal I, Otley A, Jacobson K. Low prevalence of H. pylori infection in Canadian children: a cross-sectional analysis. Canadian Journal Gastroenterology. 2008;22(5):585–489. doi: 10.1155/2008/410176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senbanjo IO, Oshikoya KA, Njokanma OF. Helicobacter pylori associated with breastfeeding, nutritional status and recurrent abdominal pain in healthy Nigerian children. J Infect Dev Ctries. 2014;8:448–453. doi: 10.3855/jidc.3196. [DOI] [PubMed] [Google Scholar]
- 12.Kimang'a AN, Revathi G, Kariuki S, Sayed S, Devani SS. H. pylori prevalence and antibiotic susceptibility among Kenyans. Afr Med J. 2010;100(1):53–57. [PubMed] [Google Scholar]
- 13.Hestvik E, Tylleskar T, Kaddu-Mulindwa DH, Ndeezi G, Grahnquist L, Olafsdottir E, Tumwine JK. Helicobacter pylori in apparently healthy children aged 0–12years in Kampala, Uganda. BMC Gastroenterol. 2010;16(10):62. doi: 10.1186/1471-230X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen BV, Nguyen KG, Phung CD, Kremp O, Kalach N, Dupont C, Raymond J, Vidal-Trecan G. Prevalence and factors associated with Helicobacter pylori infection in children in the north of Vietnam. Am J Trop Med Hyg. 2006;74:536–539. [PubMed] [Google Scholar]
- 15.Hestvik E, Tylleskar T, Kaddu-Mulindwa DH, Ndeezi G, Grahnquist L, Olafsdottir E, et al. Prevalence of Helicobacter pylori in HIV-infected, HAART-naïve Ugandan children. Journal of International Aids Society. 2011;14:34. doi: 10.1186/1758-2652-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zekry OA, Abd E. The association between Helicobacter pylori infection, type 1 DM, and autoimmune thyroiditis. Journal of Egypt Public Health Association. 2013;(3):143–147. doi: 10.1097/01.EPX.0000437621.23560.de. [DOI] [PubMed] [Google Scholar]
- 17.Dai YN, Yu WL, Ding JX, Yu CH, Li YM. Is Helicobacter pylori infection associated with glycemic control in diabetes? World Journal of Gastroenterology. 2015;21(17):5407–5418. doi: 10.3748/wjg.v21.i17.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candelli C, Rigante D, Marietti G, Nista EC, Gasbarrini A. Helicobacter pylori, Gastrointestinal symptoms, and metabolic control in young type 1 DM patients. Official Journal of the American Pediatrics. 2003;111:800. doi: 10.1542/peds.111.4.800. [DOI] [PubMed] [Google Scholar]