Abstract
Introduction
Gram-negative bacteria are the major cause of urinary tract infections (UTI) in children. There is limited data on UTI systemic response as measured using C-reactive protein (CRP). Here, we report the association of CRP and UTI among children attending the Bugando Medical Centre, Mwanza, Tanzania.
Methods
A cross-sectional study was conducted between May and July 2017. Urine and blood were collected and processed within an hour of collection. Data were analyzed using STATA version 13.
Results
Of 250 enrolled children, 76(30.4%) had significant bacteriuria with 56(22.4%, 95%CI; 11.5–33.3) having gram-negative bacteria infection. There was dual growth of gram-negative bacteria in 3 patients. Escherichia coli (32.2%, 19/59) was the most frequently pathogen detected. A total of 88/250(35.2%) children had positive CRP on qualitative assay. By multinomial logistic regression, positive CRP (RRR=4.02, 95%CI: 2.1–7.7, P<0.001) and age ≤ 2years (RRR=2.4, 95%CI: 1.23–4.73, P<0.01) significantly predicted the presence of significant bacteriuria due to gram-negative enteric bacteria.
Conclusion
C-reactive protein was significantly positive among children with UTI due to gram-negative bacteria and those with fever. In children with age ≤ 2 years, positive CRP indicates UTI due to gram-negative enteric bacteria.
Keywords: C - reactive protein, urinary tract infection, Gram-negative bacteria, Mwanza, Tanzania
Background
Urinary tract infection (UTI) have been noted as the commonest cause of bacterial infection among children under five years of age in low-income countries1–3. It is documented that 91% of bacteria causing UTI in children are gram negative bacteria2–4. This observation is of public health concern due to the increased antimicrobial resistance associated with gram negative bacteria. In the city of Mwanza, Tanzania, it was reported that more than 37% of gram negative bacteria causing UTI in children were producing extended spectrum beta lactamases (ESBL)1–3. In addition, ESBL producing gram negative bacteria had co-resistance to trimethoprim/sulfamethoxazole, fluoroquinolones and aminoglycosides5–8, which further complicates the management of infection caused by these pathogens. In children, if UTI is not properly and adequately treated can lead to complications like renal scaring9,10, the leading cause of end-stage renal disease in this population1. Therefore, a rapid test to indicate the presence of pathogens is highly needed especially in the place where there are no culture services.
The recommended diagnosis of UTI is quantitative urine culture, nevertheless this technique is expensive, take long time and not commonly available in many health facilities in resource limited settings. The C-reactive protein(CRP) often becomes elevated within few hours after tissue injury or the start of an infection11,12. In healthy individuals, CRP is normally present in a very low concentration of less than 6 mg/l11,13. Elevated CRP has been used as the predictor of inflammations among patients with infections including neonatal sepsis14, fungal infections15 and pelvic inflammatory diseases16.
Previous studies done in Europe and India reported a significant high CRP among patients with UTI than those without UTI17–19. Increase in CRP has commonly being detected in UTI caused by Escherichia coli, Proteus spp., Klebsiella pneumoniae, Staphylococcus aureus and others17. In addition, CRP had been suggested as the marker for the treatment progress20,21. However, the association of the CRP and UTI caused by gram negative bacteria among children with signs and symptoms of UTI has never being documented in many low-income settings. Therefore, this study was done to determine the association of C-reactive protein and UTI caused by gram negative bacteria among children attending the Bugando Medical Centre. The qualitative CRP assay is inexpensive and can be adopted in many settings to guide appropriate antibiotic treatment in patients with suspected bacterial infections including UTI.
Methodology
This cross-sectional hospital based study was conducted from May to July 2017 at pediatric out patients' clinic and pediatric wards of the Bugando Medical Centre (BMC). BMC is a tertiary and teaching hospital with a bed capacity of 1000. BMC serve about 13 million people from six regions. This study included children with presumptive diagnosis of UTI admitted or attending pediatric clinic at BMC. In this study children with either fever, painful micturition or pus in urine were presumptively diagnosed with UTI. To reduce CRP false positive results as indicated in the manufacturer guidelines; children with hepatitis and those who were HIV positive were excluded.
Data and sample collection
A clean catch method for obtaining a midstream urine was used to collect urine sample from children above 2 years of age22,23. For children below two years of age and in all children who have not been pre-trained on toileting supra-pubic aspiration was aseptically done3,24. Urine samples were collected in clean sterile container (HiMedia Laboratories. Pvt. Ltd, India) and transported to the microbiology laboratory for processing within an hour of sample collection. Standard quantitative urine culture was done on Cysteine lactose electrolyte deficient (CLED), MacConkey and blood agar plates (Oxoid, UK)3. Plates were aerobically incubated at 37°c for 18–24 hours.
The presence of at least 105 CFU/ml for midstream urine and any growth for supra-pubic urine was defined as significant bacteriuria. Bacterial specie identifications were done using in house biochemical test22,25. Antibiotic susceptibility test was performed using Kirby-Bauer disc diffusion method following the guidelines laid down by the Clinical Laboratory Standard Institute(CLSI)26. E. coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used for quality control.
Furthermore, 2 ML of venous blood was collected and placed in to the plain vacutainer tube (BD Vacutainer, Nairobi Kenya). Qualitative C-reactive protein assay was performed following manufacturer instructions (Diagnostics Euromedi Equip UK). Presence of agglutinations similar with a positive control was considered as positive C-reactive protein (i.e greater than 6mg/dl).
Data management and analysis
Data entry was done using Microsoft excel 2007 cleaned and transferred to STATA version 13 (College station, Texas) for analysis. Categorical variables were presented as proportions while continuous variables were summarized using median (Inter quartile range). Chi square or Fisher's exact were used to establish statistical differences in proportions while logistic regression analysis was used to establish strength of association between CRP and the age, sex, gram-negative culture results, diarrhea and fever. Multivariate logistic was performed for all factors with P less than 0.2 controlled by age and sex to establish independent predictors of elevated CRP levels. Furthermore, multinomial logistic regression analysis was done using gram reaction (no significant bacteriuria, gram positive significant bacteriuria and gram negative significant bacteriuria) as the outcome. Statistical significance was set at 95% confidence interval with a p value of less than 0.05 as significant.
Results
Demographic Characteristics of Study population
A total of 250 children were enrolled in the study, their median age (IQR) was 3(1–4.5) years. The slightly majority of the study participants were male 142(56.8%) and 128(51.2%) had fever (Table 1). Failure to gain weight was observed in 6(2.4%) children while 29 (11.6%) had diarrhoea.
Table 1.
Demographic features and clinical data representing 250 pediatric patients at BMC
| Children characteristics | Frequency | Percentage (%) |
| Median age* | 3(IQR;1–4.5) | |
| Sex | ||
| Male | 142 | 56.8 |
| Female | 108 | 43.2 |
| Diarrhea | ||
| Yes | 29 | 11.6 |
| No | 221 | 88.4 |
| Fever | ||
| Yes | 128 | 51.2 |
| No | 122 | 48.8 |
| Cough | ||
| Yes | 58 | 23.20 |
| No | 192 | 76.80 |
| Body swelling | ||
| Yes | 46 | 18.40 |
| No | 204 | 81.60 |
| Failure to gain weight | ||
| Yes | 6 | 2.40 |
| No | 244 | 97.60 |
| Weight loss | ||
| Yes | 38 | 15.26 |
| No | 211 | 84.74 |
| Oral thrush | ||
| Yes | 3 | 1.2 |
| No | 247 | 98.8 |
Median age and interquatile range are presented
Culture results and antibiotic susceptibility pattern
Of 250 enrolled children, 76(30.4%, 95%CI 24.6–36.1) had significant bacteriuria with a total of 56(22.4%, 95%CI; 11.5–33.3) children having significant bacteriuria due to gram negative bacteria; making 73.6% of UTI cases being due to gram negative enteric bacteria. Three children had significant bacteriuria of dual gram negative pathogens. Escherichia coli, Klebsiella oxytoca and K.pneumoniae accounted for 32.2%(19/59), 18.6%(11/59) and 15.3%(9/59) of isolates, respectively. Two gram negative bacteria(3.4%) could not be identified (Table 2). Other uropathogens detected include: Candida spp: 13.2%(10/76), Staphylococcus aureus: 9.2%(7/76) and Streptococcus pyogenes: 3.9%(3/76).
Table 2.
Distribution of the gram-negative bacteria isolates causing UTI
| Gram negative bacteria Isolate | Frequency | Percentage (%) |
| Escherichia coli | 19 | 32.2 |
| Klebsiella oxytoca | 11 | 18.6 |
| Klebsiella pneumonia | 9 | 15.3 |
| Enterobacter aerogenes | 8 | 13.6 |
| Acinetobacter spp. | 4 | 6.8 |
| Citrobacter freundii | 3 | 5.1 |
| Unidentified gram negative | 2 | 3.4 |
| Proteus mirabilis | 1 | 1.7 |
| Pseudomonas aureginosa | 1 | 1.7 |
| Morganella morganii | 1 | 1.7 |
| TOTAL | 59 | 100 |
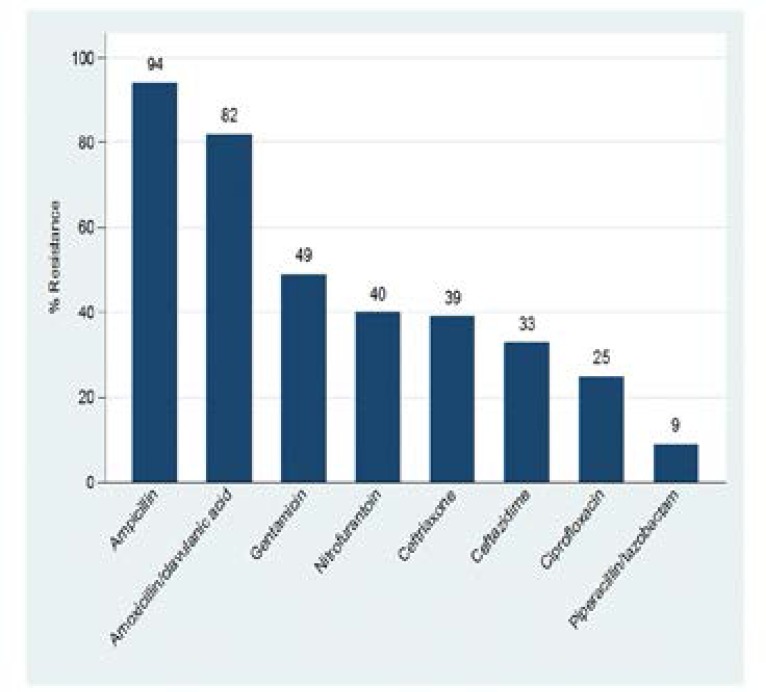
The isolated gram negative bacteria were highly resistant to ampicillin (94%) and amoxicillin/clavulanic acid (82%). Regarding the resistance to third generation cephalosporins, 39% and 33% of enteric gram negative isolates were resistant to ceftriaxone and ceftazidime, respectively (Figure 1). All gram negative bacteria detected were 100% sensitive to meropenem.
Figure 1.
Antibiotic resistant patterns of gram negative bacteria isolates causing UTI among children at BMC.
Qualitative C-reactive protein assay
A total of 88(35.2%) children had positive results on qualitative CRP assay. Out of 128 children with fever, 55(55.5%) had positive results on qualitative CRP assay compared to 17/122(13.9%) of children with no fever (P<0.001). Positive CRP was significantly more in children with UTI due to gram negative bacteria than in children with gram positive UTI (30%) and those with no significant bacteriuria (28.2%), P<0.001, Table 3.
Table 3.
Factors associated with positive C-reactive protein among 250 children with UTI at BMC
| Variables | Univariate analysis | Multivariate analysis | |||
| CRP (+ve) Median/n (%) | OR(95%; CI) | P value | OR(95%;CI) | P value | |
| Age | 3(IQR:1–4.5) | 0.98(0.89–1.08) | 0.788 | ||
| Sex | |||||
| Male (142) | 49(34.5) | 1 | |||
| Female (108) | 39(36.1) | 1.07(0.63–1.81) | 0.793 | ||
| Fever | |||||
| No (122) | 17(13.9) | 1 | |||
| Yes (128) | 71(55.5) | 7.69(4.1414.29) | <0.001 | 7.57(3.94–14.55) | <0.001 |
| Diarrhea | |||||
| No (221) | 74(33.5) | 1 | |||
| Yes (29) | 14(48.3) | 1.85(0.84–4.04) | 0.121 | 1.44(0.58–3.42) | 0.444 |
| Bacteriuria | |||||
| Culture negative (174) | 49(28.2) | 1 | |||
| Gram Positive (20) | 6(30.0) | 1.1(0.39–3.00) | 0.863 | 1.54(0.49–4.78) | 0.452 |
| Gram negative (56) | 33(58.9) | 3.66(1.96–6.84) | <0.001 | 3.54(1.73–7.22) | 0.001 |
By multivariate logistic regression analysis, children with fever (OR 7.57, 95% CI: 3.94–14.55, P<0.001) and those with UTI due to gram negative bacteria (OR 3.54, 95% CI: 1.73–7.22, P=0.001) were more likely to have positive CRP results (Table 3). By multinomial logistic regression analysis was done, no bacteria was used as base and other outcomes being gram positive significant bacteriuria and gram negative significant bacteriuria, factors found to independently predict gram negative significant bacteriuria were positive CRP (RRR=4.02, 95%CI: 2.1–7.7, P<0.001) and age ≤ 2years(RRR=2.4, 95%CI: 1.23–4.73, P<0.01). Fever was not subjected to multinomial logistic regression due to its strong collinearity with positive CRP.
Discussion
The prevalence of significant bacteriuria due to gram negative enteric bacteria among children with presumptive diagnosis of UTI in the current study was 22.4%. This is similar to the results from the previous study conducted 6 years ago in the same settings which observed the prevalence of enteric gram negative bacteria UTI to be 20.3%1,2. The current results indicate that the contribution of gram negative in causing UTI at BMC has remained relative the same. Nevertheless, the observed prevalence of gram negative enteric bacteria causing UTI in children is slightly lower than 29.3% and 39.7% reported from Kenya and Uganda27,28, respectively. The observed difference could be explained by the differences in study population, a significant proportion of children in studies from Kenya and Uganda had co-morbidities such as malnutrition and HIV. Co-morbidities have been found to pedispose to infections such as UTI1,27. In the current study out of six children who failed to gain weight only 1 had significant bacteriuria, the number is too low for any statistical test therefore no conclusion could be made.
Escherichia coli and Klebsiella spp., have been reported in previous studies29–31 to contribute more than three quarter of the bacteria causing UTI in children of all ages. This was confirmed in the present study whereby these were predominant pathogens causing UTI. This can partly be explained by the fact that Escherichia coli and Klebsiella spp. are among the normal flora in the gastrointestinal tract (GIT), hence can easily cause UTI due to the close proximity of GIT and urinary tract.
As it was reported previous by other studies in the same settings1–3,5, bacteria detected in the current study were highly resistant to ampicillin and amoxicillin/clavulanic acid. This could be explained by the fact that these antimicrobial agents are inappropriately used to treat respiratory infections among children in Tanzania, hence increase the chance of pathogens to develop resistance against them3. Similar findings were reported elsewhere32,33 which necessity the need of increasing effort in promoting appropriate antimicrobial use through antimicrobial stewardship programmes.
Early detection of bacterial infection is crucial for the proper management of patients to reduce the associated morbidity and mortality. In the current study about one third of the enrolled children had positive CRP (i.e elevated CRP). CRP has been found to be a more sensitive maker in predicting gram negative bacterial infections than gram positive bacterial infections in children14. This has been confirmed in the current study whereby patient with gram negative bacterial infections had 3.54 times higher odds of having positive CRP results with positive CRP being an independent predictor of presence of gram negative significant bacteriuria. These findings are similar to previous studies reported in Turkey, England, Iran and US among children with UTI20,29,34,35.
As it was reported previously from other studies13,36; this study has confirmed that, fever strongly predicts positive CRP results. This may be due to presence of pro-inflammatory cytokines like interleukin 1 and interleukin 6 that signal the liver to produce high concentration of CRP37. Therefore, in the place with limited diagnostic facilities positive qualitative CRP assay in children with fever and other symptoms and signs of UTI can be used to predict the possible group of pathogens which is important for early appropriate antibiotic treatment. Early appropriate initiation of the right antibiotics has been found to reduce morbidity such as renal scarring and mortality associated with UTI.
Limitation
Failure to do serial dilution of CRP and possibility of having other undiagnosed conditions might affect the result of this study.
Conclusion and recommendations
Qualitative CRP assay is mostly likely to be positive in children with fever and can predict the significant bacteriuria of gram negative bacteria especially in young children. In place with limited culture services, clinicians should start appropriate treatment for gram negative bacteria in young children(≤ 2years) with presumptive diagnosis of UTI and positive qualitative CRP assay in order to reduce UTI associated morbidity and mortality. Further studies to evaluate the effectiveness of CRP in monitoring treatment of UTI and establish cutoff value of quantitative CRP in the diagnosis of gram negative enteric bacteria UTI are warranted.
Acknowledgement
Authors would like to acknowledge the support provided by Pediatric and Child health of the Bugando Medical Centre and Microbiology and Immunology department of Catholic University of Health and Allied Sciences. We thank all parents/guardians for allowing their children to participate in the study.
Declaration
Ethical approval and consent to participate
The protocol for conducting the study was approved by the Joint Catholic University of Health and Allied Sciences/Bugando Medical Centre (CUHAS/BMC) research ethics and review committee (CREC) with certificate no: CREC/274/2017. Consent to participate was obtained from guardians/parents.
Consent for publication
Not applicable.
Availability of data and material
The data is available upon request and the request should be made to the Director of research and Innovation Catholic University of Health and allied Sciences.
Competing interest
All authors have declared that they have no competing interest in publishing this work.
Funding
This study was supported by the research grant from Department of Microbiology and Immunology of Catholic University of Health and Allied Sciences to VGA.
Authors' contributions
MFM and SEM designed the work. VGA and MS participated in the collection of data and specimens. MFM, SEM, VGA and VS performed laboratory investigations. MFM and SEM analyzed and, interpreted the data. MFM wrote the first draft of the manuscript which was critically reviewed by SEM. All authors read and approved the final version of the manuscript.
References
- 1.Ahmed M, Moremi N, Mirambo MM, Hokororo A, Mushi MF, Seni J, Kamugisha E, Mshana SE. Multi-resistant gram negative enteric bacteria causing urinary tract infection among malnourished underfives admitted at a tertiary hospital, northwestern, Tanzania. Italian Journal of Ppediatrics. 2015;41(1):44. doi: 10.1186/s13052-015-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Msaki BP, Mshana SE, Hokororo A, Mazigo HD, Morona D. Prevalence and predictors of urinary tract infection and severe malaria among febrile children attending Makongoro health centre in Mwanza city, North-Western Tanzania. Archives of Public Health. 2012;70(1):4. doi: 10.1186/0778-7367-70-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Festo E, Kidenya BR, Hokororo A, Mshana SE. Predictors of urinary tract infection among febrile children attending at Bugando Medical Centre Northwestern, Tanzania. Archives of Clinical Microbiology. 2011 [Google Scholar]
- 4.Weinstein RA, Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clinical Infectious Diseases. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 5.Christopher A, Mshana SE, Kidenya BR, Hokororo A, Morona D. Bacteremia and resistant gram-negative pathogens among under-fives in Tanzania. Italian Journal of Pediatrics. 2013;39(1):27. doi: 10.1186/1824-7288-39-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomberg B, Manji KP, Urassa WK, Tamim BS, Mwakagile DS, Jureen R, Msangi V, Tellevik MG, Holberg-Petersen M, Harthug S. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infectious Diseases. 2007;7(1):43. doi: 10.1186/1471-2334-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clinical Infectious Diseases. 2006;42(Supplement_2):S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 8.Ramphal R. > PGA: Extended-spectrum β-lactamases and clinical outcomes: current data. Clinical Infectious Diseases. 2006;42(Supplement_4):S164–S172. doi: 10.1086/500663. [DOI] [PubMed] [Google Scholar]
- 9.Holland NH, Jackson EC, Kazee M, Conrad GR, Ryo UY. Relation of urinary tract infection and vesicoureteral reflux to scars: follow-up of thirty-eight patients. The Journal of Pediatrics. 1990;116(5):S65–S71. doi: 10.1016/s0022-3476(05)82705-2. [DOI] [PubMed] [Google Scholar]
- 10.Rushton HG, Majd M, Jantausch B, Wiedermann BL, Belman AB. Renal scarring following reflux and nonreflux pyelonephritis in children: evaluation with 99m technetium-dimercaptosuccinic acid scintigraphy. The Journal of Urology. 1992;147(5):1327–1332. doi: 10.1016/s0022-5347(17)37555-9. [DOI] [PubMed] [Google Scholar]
- 11.Young B, Gleeson M, Cripps AW. C-reactive protein: a critical review. Pathology. 1991;23(2):118–124. doi: 10.3109/00313029109060809. [DOI] [PubMed] [Google Scholar]
- 12.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clinical Infectious Diseases. 2004;39(2):206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 13.Chan T, Gu F. Early diagnosis of sepsis using serum biomarkers. Expert Review of Molecular Diagnostics. 2011;11(5):487–496. doi: 10.1586/erm.11.26. [DOI] [PubMed] [Google Scholar]
- 14.Chacha F, Mirambo MM, Mushi MF, Kayange N, Zuechner A, Kidenya BR, Mshana SE. Utility of qualitative C-reactive protein assay and white blood cells counts in the diagnosis of neonatal septicaemia at Bugando Medical Centre, Tanzania. BMC Pediatrics. 2014;14(1):248. doi: 10.1186/1471-2431-14-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marková M, Brodská H, Malíčková K, Válková V, Cetkovský P, Kolář M, Haluzík M. Substantially elevated C-reactive protein (CRP), together with low levels of procalcitonin (PCT), contributes to diagnosis of fungal infection in immunocompromised patients. Supportive Care in Cancer. 2013;21(10):2733–2742. doi: 10.1007/s00520-013-1844-1. [DOI] [PubMed] [Google Scholar]
- 16.Hemilä M, Henriksson L, Ylikorkala O. Serum CRP in the diagnosis and treatment of pelvic inflammatory disease. Archives of Gynecology and Oobstetrics. 1987;241(3):177–182. doi: 10.1007/BF00931315. [DOI] [PubMed] [Google Scholar]
- 17.Khan F, Malik M, Afzal K, Malik A, Khalid M. Renal biometry and serum c-reactive protein levels in the evaluation of urinary tract infections. Indian J Nephrol. 2004;14:10–14. [Google Scholar]
- 18.Honkinen O, Jahnukainen T, Mertsola J, Eskola J, Ruuskanen O. Bacteremic urinary tract infection in children. The Pediatric Infectious Disease Journal. 2000;19(7):630–634. doi: 10.1097/00006454-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Galloway A, Green H, Windsor J, Menon K, Gardner B, Krishnan K. Serial concentrations of C-reactive protein as an indicator of urinary tract infection in patients with spinal injury. Journal of Clinical Pathology. 1986;39(8):851–855. doi: 10.1136/jcp.39.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadi HM, Shalan YA, Al-Qatan HY, Alotaibi S. Urinary tract infection in boys less than five years of age: a general pediatric perspective. The Kuwait Medical Journal: KMJ: the official Journal of the Kuwait Medical Association. 2006;38(3):220. [PMC free article] [PubMed] [Google Scholar]
- 21.Putto A, Meurman O, Ruuskanen O. C-reactive Protein In Viral And Bacterial Infections. Pediatric Research. 1985;19(10):1103–1103. [Google Scholar]
- 22.PR M, WL D, GS K, J TJ. Medical microbiology: Tavistock square. London: Wolfe Medical Publications Ltd; 1990. [Google Scholar]
- 23.Downs SM. Technical report: urinary tract infections in febrile infants and young children. Pediatrics. 1999;103(4):e54–e54. doi: 10.1542/peds.103.4.e54. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JD, Peters PC. Suprapubic aspiration of urine in premature and term infants. Pediatrics. 1965;36(1):132–134. [PubMed] [Google Scholar]
- 25.Mshana SE, Kamugisha E, Mirambo M, Chakraborty T, Lyamuya EF. Prevalence of multiresistant gram-negative organisms in a tertiary hospital in Mwanza, Tanzania. BMC Research Notes. 2009;2(1):49. doi: 10.1186/1756-0500-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen JH, Turnidge JD. Manual of Clinical Microbiology. Eleventh Edition. American Society of Microbiology; 2015. Susceptibility test methods: dilution and disk diffusion methods; pp. 1253–1273. [Google Scholar]
- 27.Okwara F, Obimbo E, Wafula E, Murila F. Bacteraemia, urinary tract infection and malaria in hospitalised febrile children in Nairobi: is there an association? East African Medical Journal. 2004;81(1):47–51. doi: 10.4314/eamj.v81i1.8795. [DOI] [PubMed] [Google Scholar]
- 28.Batwala V, Magnussen P, Nuwaha F. Antibiotic use among patients with febrile illness in a low malaria endemicity setting in Uganda. Malaria Journal. 2011;10(1):377. doi: 10.1186/1475-2875-10-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildiz B, Poyraz H, Cetin N, Kural N, Colak O. High sensitive C-reactive protein: a new marker for urinary tract infection, VUR and renal scar. Eur Rev Med Pharmacol Sci. 2013;17(19):2598–2604. [PubMed] [Google Scholar]
- 30.Hooton TM, Besser R, Foxman B, Fritsche TR, Nicolle LE. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clinical Infectious Diseases. 2004;39(1):75–80. doi: 10.1086/422145. [DOI] [PubMed] [Google Scholar]
- 31.Ayazi P, Mahyar A, Jahani HH, Khabiri S. Urinary tract infections in children. Iran J Pediatr Soc. 2010;2(1):9–14. [Google Scholar]
- 32.Picozzi SC, Casellato S, Mattia Rossini GP, Tejada M, Costa E, Carmignani L. Extended-spectrum beta-lactamase-positive Escherichia coli causing complicated upper urinary tract infection: Urologist should act in time. Urology Annals. 2014;6(2):107. doi: 10.4103/0974-7796.130536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladhani S, Gransden W. Increasing antibiotic resistance among urinary tract isolates. Archives of disease in childhood. 2003;88(5):444–445. doi: 10.1136/adc.88.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yen C, Chen D. Urinary tract infection in children. Journal of microbiology, immunology, and infection= Wei mian yu gan ran za zhi. 1999;32(3):199–205. [PubMed] [Google Scholar]
- 35.Naseri M. Alterations of peripheral leukocyte count, erythrocyte sedimentation rate, and C-reactive protein in febrile urinary tract infection. Iran J Kidney Dis. 2008;2(3):137–142. [PubMed] [Google Scholar]
- 36.Chan Y-L, Liao H-C, Tsay P-K, Chang S-S, Chen J-C, Liaw S-J. C-reactive protein as an indicator of bacterial infection of adult patients in the emergency department. Chang Gung Medical Journal. 2002;25(7):437–445. [PubMed] [Google Scholar]
- 37.Lacour AG, Gervaix A, Zamora SA, Vadas L, Lombard PR, Dayer J-M, Suter S. Procalcitonin, IL-6, IL-8, IL-1 receptor antagonist and C-reactive protein as identificators of serious bacterial infections in children with fever without localising signs. European Journal of Pediatrics. 2001;160(2):95–100. doi: 10.1007/s004310000681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available upon request and the request should be made to the Director of research and Innovation Catholic University of Health and allied Sciences.