Abstract
Asthma, a disease classified as a chronic inflammatory disorder induced by airway inflammation, is triggered by a genetic predisposition or antigen sensitization. Drugs currently used as therapies present disadvantages such as high cost and side effects, which compromise the treatment compliance. Alternatively, traditional medicine has reported the use of natural products as alternative or complementary treatment. The aim of this review was to summarize the knowledge reported in the literature about the use of natural products for asthma treatment. The search strategy included scientific studies published between January 2006 and December 2017, using the keywords “asthma,” “treatment,” and “natural products.” The inclusion criteria were as follows: (i) studies that aimed at elucidating the antiasthmatic activity of natural-based compounds or extracts using laboratory experiments (in vitro and/or in vivo); and (ii) studies that suggested the use of natural products in asthma treatment by elucidation of its chemical composition. Studies that (i) did not report experimental data and (ii) manuscripts in languages other than English were excluded. Based on the findings from the literature search, aspects related to asthma physiopathology, epidemiology, and conventional treatment were discussed. Then, several studies reporting the effectiveness of natural products in the asthma treatment were presented, highlighting plants as the main source. Moreover, natural products from animals and microorganisms were also discussed and their high potential in the antiasthmatic therapy was emphasized. This review highlighted the importance of natural products as an alternative and/or complementary treatment source for asthma treatment, since they present reduced side effects and comparable effectiveness as the drugs currently used on treatment protocols.
1. Introduction
1.1. Physiopathology of Asthma
Asthma can be defined as a chronic inflammatory disorder that affects the lower airways, promoting an increase of bronchial reactivity, hypersensitivity, and a decrease in the airflow [1]. Furthermore, due to a complex interaction between the genetic predisposition and environmental factors, besides multiple related phenotypes, this disease may be considered as a heterogeneous disorder [2].
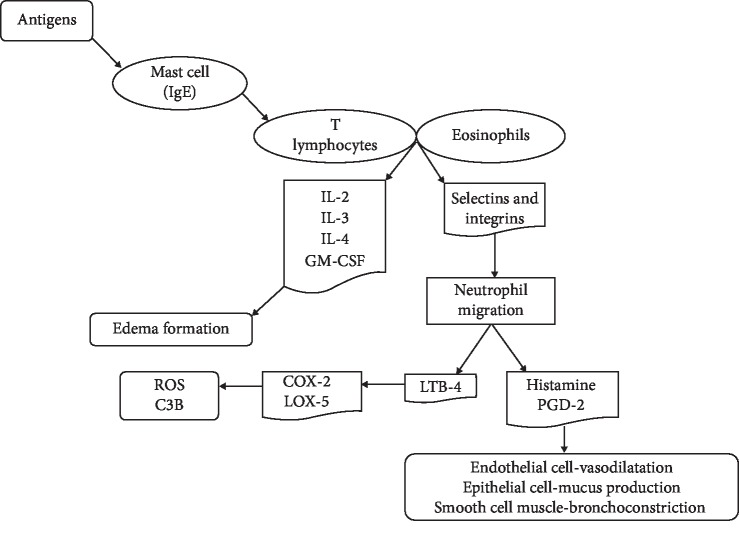
Sensitization by dust, pollen, and food represents the main environmental factors involved in the asthma physiopathology [1]. These antigens are recognized by the mast cells coated by IgE antibodies (Figure 1) and induce the release of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukins IL-2, IL-3, IL-4, IL-5, GM-CSF, prostaglandins, histamine, and leukotrienes [3, 4], by T lymphocytes and eosinophils. This degranulation process promotes an increase in the vascular permeability, leading to exudate and edema formation. This process is followed by leukocyte migration to the tissue affected by the inflammatory process through chemotaxis mediated by selectins and integrins [3, 6]. Subsequently, the neutrophil migration to the inflammatory site and the release of leukotrienes LTB4 induce the activation of type 2 cyclooxygenase (COX-2) and type 5 lipoxygenase (LOX-5), enhancing the expression of the C3b opsonin that produces reactive oxygen species (ROS) and thus promoting cell oxidative stress and pulmonary tissue injury [3, 7].
Figure 1.
Scheme of the immune response induced by allergen or antigen stimulation or the early stages of asthma. GM-CSF: granulocyte-macrophages colony-stimulating factor; IL: interleukin; C3b: opsonin; LOX-5: lipoxygenase type 5; ROS: reactive oxygen species; COX-2: cyclooxygenase type 2; LTB4: leukotriene type B; PGD2: prostaglandin type D (adapted from Bradding et al. [6].
Other mechanisms involved in asthma physiopathology are the inhalation of drugs, as well as respiratory viruses [8], which promote an immune response mediated by IgG antibodies. This process promotes an increase of the inflammatory cells influx, releasing inflammatory mediators responsible for the damage process [9].
Based on the factors and mechanisms presented above, asthma symptoms can be observed at different levels according to etiology and severity of clinical aspects, which define their classification [10]. The asthma severity is subdivided into (i) mild/low, also defined as intermittent/persistent, when the symptoms appear more than twice a week and their exacerbations can affect the daily activities of the patient; (ii) moderate, in which the daily symptom occurrence and their exacerbations affect the patient activities, requiring the use of short-acting β2-adrenergic drugs; or (iii) severe asthma, in which the patient presents persistent symptoms, physical activity limitations, and frequent exacerbations [10]. Based on this classification, it is estimated that 60% of the asthma cases are intermittent or persistent, 25% to 30% are moderate, and the severe cases account for only 10% of the total. However, it is important to highlight that although the proportion of severe asthmatics represents the minority of the cases, they are responsible for high mortality and high hospitalization costs [11], evidencing the high need of efficient treatments for this disease.
1.2. Asthma Epidemiology
According to the World Health Organization, asthma affects about 300 million of individuals across the world, regardless of the country development degrees [12]. In the United Kingdom, asthma affects approximately 5.2 million of individuals and is responsible for 60.000 hospital admissions per year [13], while in Brazil the annual incidence of hospital admissions due to asthma is around 173.442 patients, representing 12% of the total admissions for respiratory diseases in 2012 [14].
Furthermore, studies have demonstrated that asthma incidence and prevalence rates in different countries are not age related. In the United States of America, Albania, and Indonesia, the asthma prevalence is lower for children (around 8.4%, 2.1%, and 4.1%, respectively) when compared to adults [15]. On the other hand, in countries such as the United Kingdom and Costa Rica, children aged between 6 and 7 years represent approximately 32% of the asthma prevalence [16]. Additionally, the incidence or prevalence can be directly influenced by the socioeconomic characteristics of specific areas, as demonstrated by the analyses of the annual variation in the prevalence of asthma in which it was possible to observe, in Spain, that the asthma prevalence had an annual increase of 0.44% regardless of the age range studied. However, when these same data analyses were observed individually in Spain regions, the annual variation presented a different scenario, showing an increase or decrease according to the developmental degree of each region [17, 18]. Similar data were observed by Pearce et al. [19] and Schaneberg et al. [20], who demonstrated the influence of socioeconomic aspects on asthma. The studies showed a prevalent increase of asthma cases in metropolitan areas, fact attributed to the population growth with consequent exposure to the environmental factors and shortened access to asthma therapy, due to the high cost of the available medicines [19, 20]. Such phenomena directly interfere on the treatment compliance [21–23], evidencing the importance and the need of strategies that facilitate the access to the medicines for asthma therapy.
Studies that evaluate the importance of inclusion of antiasthmatic therapy on public health policy programs have demonstrated that asthma control can be achieved through a variety of approaches, promoting a decrease in hospital admissions of 90%. Indeed, this was demonstrated by two studies performed in Brazilian cities, in which public health programs offered free medicines and psychological and pharmaceutical care to treat chronic diseases [24, 25]. Furthermore, Ponte et al. [24] and Holanda [25] also showed that the hospital admissions of children decreased from 44.7% to 6.4% one year after the inclusion of these patients in the same project. Thus, these data corroborate the importance of public health policies that contribute to the reduction of hospital outlay, increasing the population's life quality.
1.3. Asthma Treatment
The asthma treatment recommended by the Global Initiative for Asthma (GINA) consists, especially, on the reduction of symptoms in order to decrease the inflammatory process [26, 27]. However, since asthma presents a complex physiopathology associated with variable manifestations, the treatment can lead to different response levels. Thus, the evaluation of the clinical aspects associated with the treatment response is defined as the most adequate approach to achieve treatment success [28]. Asthma therapy strategies are based on pulmonary (the main administration route on asthma therapy), oral or intravenous administration of class β2 agonist drugs (salbutamol, levalbuterol, terbutaline, and epinephrine), anticholinergics (ipratropium), corticosteroids (beclomethasone di- or monopropionate, ciclesonide, flunisolide, fluticasone propionate, mometasone furoate, triamcinolone acetonide, hydrocortisone, dexamethasone, budesonide, prednisone, prednisolone, and methylprednisolone), and xanthine drugs. Among these, the β2 agonists are often the drugs of first choice [13, 27].
To optimize the treatment for each patient, the drug dosage is determined by the patient's respiratory characteristics, mainly his/her respiratory rate. Patients with increased respiratory rate, due to the airways narrowing, present a low dispersion of the inhaled drug through the respiratory tract [29]. In these cases or when there is an absence of response on the first two hours after treatment, hospitalization should be performed, and adrenaline could be used, subcutaneously or intravenously, since this is an indicative of mucosal edema formation, which can be decreased by the adrenaline bronchodilator effect [30].
Overall, patients that present asthma exacerbation should be initially treated with the association of different dosage of corticosteroids and short-acting β2 agonists by intranasal oxygen administration, allowing the stimulation of β2 receptors that result in bronchodilation due to the inhibition of cholinergic neurotransmission and, thus, inhibition of mast cells degranulation [10]. Additionally, corticosteroids by oral or inhaled route are used on uncontrolled persistent asthma patients due to their direct effect on the inflammation site [31]. Accordingly, they improve the pulmonary function and decrease the asthma episodes [32], reducing hospitalizations and mortality of asthmatic patients [31]. Furthermore, because their systemic use can induce side effects, corticosteroids, mainly prednisone and prednisolone, are more commonly used in patients with severe persistent asthma who are not stabilized by other drugs [31].
In addition, xanthine drugs such as theophylline can be also used on asthma treatment, since they are able to promote the suppression of monocyte activation with consequent inhibition on the TNF-α release. Further, they promote the inhibition of neutrophil activation and its degranulation, inhibiting the catalytic activity of phosphodiesterase 4 (PDE4), allowing a reduction in the inflammatory process [33].
Regardless of the wide variety and associations of antiasthmatic medicines and their ability to promote the asthma symptoms control and to reduce the asthma episodes and hospital admissions, the antiasthmatic drugs present several side effects, including nausea, headaches, and convulsions (xanthine class) [3, 30], cardiovascular effects (β-adrenergic receptors antagonists) [20], vomiting (PDE4 inhibitors drugs) [34–36], osteoporosis, myopathies, adrenal suppression, and metabolic disturbs, compromising the patients' growth (corticosteroids) [30, 35, 37, 38]. These side effects compromise the life quality of the patients and reduce significantly the treatment compliance.
Another important drawback from the conventional asthma treatment is its cost. In fact, the required amount of money for asthma treatments represents a significant expenditure for health public organizations. Such situation has become a financial issue even for developed countries. In Sweden, for example, the cost of medicines for asthma treatment has increased since the 1990s and, in 2006, and it was responsible for 11.6% of the total healthcare expenditure. Furthermore, according to projections, an annual increase of 4% on the costs of asthma management is expected [22].
Additionally, studies revealed that in Europe and in the United States of America, the sum of the direct and indirect estimated annual costs with asthma management is approximately €18 billion and US$13 billion, respectively. This high expenditure was associated with the high incidence of uncontrolled asthma patients, since they represent an expense up to 5-fold higher than the controlled asthma ones [39] or than patients with other chronic diseases, as demonstrated in the study performed by O'Neil and colleagues [40]. These authors revealed that asthma costs up to £4,217 per person, while type II diabetes, chronic obstructive pulmonary disease, and chronic kidney disease represented, together, a cost of £3,630 [40].
Therefore, considering the therapies currently available, their side effects, and their high cost, the development of new therapeutic approaches or complementary treatments to the current asthma therapy become an important and essential strategy. In this context, the use of natural products allows easy access to treatment to all socioeconomic classes [41, 42] and shows advantages such as low cost, biocompatibility, and reduced side effects, besides their wide biodiversity and renewability [43, 44]. In addition, natural products, supported by the literature findings on their complex matrix as a source of bioactive compounds, represent one of the main access forms to the basic healthcare in the traditional medicine [45]. Thus, the present review aimed at summarizing the main natural products reported in the literature that show antiasthma activity.
2. Natural Products as Alternative for Asthma Treatment
The use of natural products for the treatment of physiologic disorders, especially in association with other drugs, has been widely reported through ethnopharmacological studies as an important scientific tool for bioprospection exploration and discovery of new bioactive compounds from natural sources [46]. Despite the wide scientific progress regarding chemical and pharmaceutical technology on synthesizing new molecules, drugs from natural sources still contribute tremendously to the discovery and development of new medicines [47]. These studies are based, initially, on the traditional use of the natural products, which draws the attention of pharmaceutical companies due to their easy and economical use, allowing the companies to perform many studies that evaluate their therapeutic activities, their toxicity, and their safety [48].
Moreover, the use of natural products as complementary therapy represents an important alternative for the treatment of several diseases [49]. In the United States of America, the use of natural products, vitamins, and other dietary supplements as auxiliary treatments represent about 40% of the conventional therapies [50]. Among the diseases that natural products are used for, those of allergic and inflammatory character can be highlighted. In fact, according to the literature, the alternative medicine associates the use of these products with biochemical mechanisms involved in immunomodulation, which could contribute to the management of these diseases [51].
The use of plant-based products for asthma treatment has been reported by the traditional medicine for over 5000 years, since the use by the Chinese culture of the infusion of Ephedra sinica, which is as an immune system stimulator able to decrease asthma crises [20]. More recently, a study performed by Costa and colleagues [49] described the main natural sources for the treatment of asthma used by the Brazilian families from the Northeast Region of the country [49]. The study included beet, honey, onion, lemon, garlic, yarrow, and mint, demonstrating the wide variety of natural products used on asthma treatment in children [49]. Additionally, other natural-derived products have been widely cited in asthma treatment, such as natural oils from plants and animals, which can be obtained by different extraction process [52, 53].
Plant-derived natural oils represent the main natural products used on the complementary asthma therapy due to the presence of compounds such as phenylpropanoids and mono- and sesquiterpenes as the major bioactive compounds, which provide their anti-inflammatory, antifungal, antibacterial, and anesthetic properties [54–56]. Similarly, oils obtained from animal sources have been used. They are rich in a mixture of different saturated, mono and polyunsaturated fatty acids, as well as compounds from animal organs and secretions, which are responsible for the immune-modulatory action and regulation of the tissue oxidative capacity [57, 58]. The activity credited to the oils derived from plants and animals is related to the presence of those bioactive compounds, which can inhibit COX-2 and COX-5. Additionally, these compounds are able to modulate the immune cells function by reducing levels of IL-4, IL-5, and IL-13 cytokines, decreasing the activity and proliferation of NK cells and leading to an increase in the level of endogenous corticosteroids, contributing to the regulation of NF-κB pathway, and reducing the mucus production and the inflammation in the lung tissues [59–61].
In this regard, Table 1 shows all products found in the studies included in this review after the inclusion criteria evaluation. Due to the wide variety of plant-derived products, only those with 3 or more citations were described in detail in this review. On the other hand, due to limited scientific investigations about the antiasthmatic activity of the natural products from animal and microorganism sources, all studies that fit the inclusion criteria were described in the next sections.
Table 1.
List of natural compounds described in the literature reviewed.
| Product | Product form | Product source | Active compound | Compound class/type | Mechanism of action | Reference |
|---|---|---|---|---|---|---|
| 1,8-Cineol | Isolated compound | Essential oil of Eucalyptus globulus leaves | 1,8-Cineol | Monoterpene | Reduces the expression of NF-κB target gene MUC2 | Greiner et al. [62] |
|
| ||||||
| 3-Methoxy-catalposide | Isolated compound | P. rotundum var. subintegrum extract | 3-Methoxy-catalposide | Iridoid glycoside | Inhibits the expression of cyclooxygenase (COX)-2, nitric oxide synthase (iNOS), and proinflammatory genes (IL-6, IL-1β, and TNF-α) | Ryu et al. [63] |
|
| ||||||
| Achyranthes aspera L | Ethanolic extract | Roots | Not reported | Not reported | Bronchoprotective activity | Dey [64] |
|
| ||||||
| Ailanthus excelsa Roxb | Aqueous extract | Barks | Not reported | Not reported | Bronchodilator and mast cell stabilizing activities | Kumar [65] |
|
| ||||||
| Allium Cepa L. and quercetin | Extract and isolated compound | Methanolic extract and vegetable | Quercetin [2-(3, 4-dihydroxyphenyl)-3, 5, 7-trihydroxy-4H-1-benzopyran-4-one, 3, 3′, 4′, 5, 6-entahydroxyflavone] | Flavonoid | Reduce the production of proinflammatory cytokines (IL-4, IL-5, IL-13) and promote the relaxation of tracheal rings | Oliveira et al. [66] |
|
| ||||||
| Alstonia scholaris (L.) R. Br. | Extract | Leaves of Alstonia scholaris (L.) R. Br. | Scholaricine, 19-epi-scholaricine, vallesamine, picrinine | Alkaloid | Reduce the eosinophilia, the production of proinflammatory cytokine (IL-4) and the expression of serum IgE and eotaxin | Zhao et al. [67] |
|
| ||||||
| Amorphophallus konjac (konjac) | Gel extract | Not reported | Not reported | Plant | Not elucidated | Chua et al. [68] |
|
| ||||||
| Andropogon muricatus | Crude extract | Aerial parts | Vetivenes. vetivenol, vetivenic acid, and vetivenyl acetate | Sesquiterpenic compounds | Inhibit the Ca2+ channels and phosphodiesterase activity | Shah and Gilani [69] |
|
| ||||||
| Anoectochilus formosanus Hayata | Aqueous extract | Whole plant | Kinsenoside | Plant | Reduce the IL-4 production by Tregs and enhance the production of IL-12 and IFN-γ by Th1 differentiation | Hsieh et al. [70] |
|
| ||||||
| Artemisia maritima | Essential oil | Leaves | 1,8-Cineol, camphor, camphene, and β-caryophyllene | Terpenoid | Inhibit the Ca2+ channels and phosphodiesterase activity | Shah et al. [71] |
|
| ||||||
| Aster tataricus L. f. | Extract | Rhizomes | Kaempferol, aurantiamide, and astin C | Flavonoid | Inhibit the expression of NF-κB and promote the activation of beta-2 adrenergic receptor | Chen and Zheng [72] |
| Aster yomena (Kitam.) Honda | Ethanolic extract | Leaves | Phenolic compounds not specified | Phenolic compounds | Attenuate the production of NO and IL-1β, and suppress the expression of NF-κB. In addition, suppress the activation of TLR4 and promote a reduction of intracellular ROS production | Kang et al. [73] |
|
| ||||||
| Baicalin | Isolated compound | Leaves and branch | 7-Glucuronic acid-5,6-dihydroxyflavone | Flavonoid | Suppress the lipopolysaccharide-induced TNF-α expression and inhibit the cyclic adenosine monophosphate-specific phosphodiesterase 4 (PDE4) | Park et al. [74] |
|
| ||||||
| Baliospermum montanum Müll. Arg. (Euphorbiaceae) | Chloroformic and ethanolic extracts | Leaves | Alkaloids, triterpenoids, diterpenoids, and glycosides | Alkaloids, triterpenoids, diterpenoids, and glycosides | Stabilize the mast cell degranulation and decrease the histamine release | Venkatesh et al. [75] |
|
| ||||||
| Berry fruit | Polyphenolic extract | Not reported | Phenolic compounds not specified | Phenolic compounds not specified | Not reported | Power et al. [76] |
|
| ||||||
| Boswellia serrata, Boswellia carterii, and frankincense | Essential oil | Resinous part | Fl-boswellic acid, acetyl-fl-boswellic acid, 11-keto-fl-boswellic acid, and acetyl-11-keto-fl-boswellic acid | Boswellic acids | Inhibition of leukotriene biosynthesis | Hamidpour et al. [77] and Al-Yasiry and Kiczorowska [78] |
|
| ||||||
| Boswellia serrata, Glycyrrhiza glabra, and Curcuma longa | Essential oil extract and extract | Resinous part, licorice root and turmeric root, respectively | Curcumin and fl-boswellic acid | Polyphenol | Reduce the plasma level of the leukotriene C4, nitric oxide and malondialdehyde | Houssen et al. [79] |
|
| ||||||
| Buffalo spleen lipid and a bacterial polypeptide | Extract | Animal-derived and microorganism-derived, respectively | Not reported | Not reported | Reduce the tracheal responsiveness and the amount of white blood cells | Neamati et al. [80] |
|
| ||||||
| Bullfrog oil (Rana catesbiana shaw) | Oil | Bullfrog adipose tissue | Oleic, linolenic, stearic, palmitic, and myristic acids. Eicosapentaenoic acids and decosahexaenoic acid | Fatty acids | Not elucidated | Amaral-Machado et al. [81] |
|
| ||||||
| Bu-zhong-yi-qi-tang | Aqueous extract | Root of Astragalus mongholicus Bunge, Panax ginseng C.A.Mey, Angelica dah-rica Fisch. Ex Hoffm and Bupleurum chinense DC. Rhizome of Zingiber officinale Rosc, Atractylodes macrocephala Koidz, and Cimicifuga foetida L. Fruit of Ziziphus jujuba Mill. var. inermis Rehd. Pericarp of Citrus reticulata Blanco. Root and rhizome of Glycyrrhiza uralensis Fisch | Not reported | Not reported | Reduce the level of eotaxin, Th2-related cytokines (IL-4, IL-5, IL-13), IgE, and eosinophilia | Yang et al. [82] |
|
| ||||||
| Caenorhabditis elegans | Crude extract | Microorganism | Not reported | Not reported | Modulate the immunologic Th1/Th2 response | Huang et al. [83] |
|
| ||||||
| Camellia sinensis L. | Aqueous extract | Not reported | Polyphenois and flavonoids | Polyphenois and flavonoids | Not elucidated | Sharangi [84] |
|
| ||||||
| Carica papaya | Extract | Leaves | Tanins, alkaloids, steroids, and quinones | Tanins, alkaloids, steroids, and quinones | Reduce the expression of IL-4, IL-5, eotaxin, TNF-α, NF-κB, and iNOS | Elgadir et al. [85] |
|
| ||||||
| Carum roxburghianum | Crude extract | Seeds | Hydrocarbons, wax esters, sterol esters, triacylglycerols, free fatty acids, diacylglycerols, lysophosphatidylethanolamines, and phosphatidylinositols | Hydrocarbons, wax esters, sterol esters, triacylglycerols, free fatty acids, diacylglycerols, lysophosphatidylethanolamines, and phosphatidylinositols | Bronchodilator activity | Khan et al. [86] |
|
| ||||||
| Chitin | Isolated compound | Shrimp | Chitin | Polysaccharide | Not elucidated | Ozdemir et al. [87] |
|
| ||||||
| Chrysin | Isolated compound | Marketable synthetic compound | 5,7-Dihydroxy-2-phenyl-1-4H-chromen-4-one | Flavonoid | Reduces the histamine release and decreases the gene expression of proinflammatory cytokines (IL-1β, IL-4, IL-6, TNF-α, NF-κB) | Yao et al. [88]; Yao et al. [89]; Bae et al. [90] |
| Cissampelos sympodialis Eichl | Extract | Leaves | Warifteine | Alkaloid | Reduce the expression of IL-3 and IL-5, increase the IL-10 level, and decrease the density of inflammatory cells | Cerqueira-lima et al. [91] |
|
| ||||||
| Citrus tachibana | Ethanolic extract | Leaves | Coumarins, carotenoids, and flavonoids | Coumarins, carotenoids, and flavonoids | Modulate the Th1/Th2 imbalance by inhibition of NF-κB signaling and histamine secretion | Bui et al. [92] |
|
| ||||||
| Conjugated linoleic acid | Conjugated compound | Fatty tissue from ruminants | Cis, cis-9,12-octadecadienoic acid | Polyunsaturated fatty acid | Modulate the PPARγ-dependent and PPARγ-independent inflammation signaling, the eicosanoid production, and humoral immune response | Macredmond and Dorscheid [93] |
|
| ||||||
| Coumarins | Isolated compound | Synthetic compounds | 6,7-Dihydroxycoumarin, 7-hydroxycoumarin and 4-methyl-7-hydroxycoumarin | Coumarin | Not elucidated | Sanchez-Recillas et al. [94] |
|
| ||||||
| Crocetin | Isolated compound | Marketable synthetic compound | Crocetin | Carotenoid | Activates the FOXP3 signaling through TIPE2 | Ding et al. [95] |
|
| ||||||
| Curcumin | Isolated compound | Curcuma longa | (1E, 6E)-1,7-Bis (4-hydroxy- 3-methoxyphenyl)-1,6- heptadiene-3,5-dione | Polyphenol | Inhibits the Notch1-GATA3 signaling pathway | Zheng et al. [96]; Chong et al. [97] |
|
| ||||||
| Cyclotheonamides | Isolated compound | Marine | Not reported | Cyclic pentapeptides | Inhibit the human ß-tryptase | Schaschke and Sommerhoff [98] |
|
| ||||||
| Diallyl-disulfide | Isolated compound | Garlic oil | Diallyl-disulfide | Organosulfur | Activates the NrF-2/HO-1 pathway and suppresses the NF-κB | Shin et al. [99] |
|
| ||||||
| Dietary plant stanol esters | Not reported | Fatty acid | Not reported | Stanol ester | Reduce the total plasma IgE, IL-1β, IL-13, and TNF-α | Brull et al. [100] |
|
| ||||||
| Dioscorea nipponica | Isolated compound | Not reported | Diosgenin | Steroidal saponin | Suppress the secretion of TNF-α, IL-1β, and IL-6 | Junchao et al. [101] |
|
| ||||||
| D-α-tocopheryl acetate | Isolated compound | Natural source | D-α-tocopheryl acetate | Vitamin | Inhibits the oxidative stress. Modulates the allergic inflammation and the airway hyperresponsiveness | Hoskins et al. [102] |
|
| ||||||
| Echinodorus scaber | Hydroethanolic extract | Leaves | Vitexin, rutin, and gallic acid | Phenolic compounds | Decrease the migration of inflammatory cells and reduce the Th2 cytokines and IgE levels | Rosa et al. [103] |
|
| ||||||
| Eclipta prostrata (L.)L. | Methanolic extract | Whole plant | Wedelolactone and demethylwedelolactone | Coumestan | Reduce the bronchial hyperresponsiveness and the production of Th2 cytokines | De Freitas Morel et al. [104] |
|
| ||||||
| Ecklonia cava | Marine alga | Brown macroalgae | Fucodiphloroethol and phlorofucofuroeckol A | Phlorotannins | Downregulate the FcεRI expression and block the IgE-FcεRI binding | Vo et al. [105] |
|
| ||||||
| Ephedra intermedia | Crude extract | Aerial parts | Ephedrine and pseudoephedrine | Alkaloids | Not elucidated | Gul et al. [106] |
|
| ||||||
| Ellagic acid | Isolated compound | Marketable synthetic compound | Ellagic acid | Polyphenol | Inhibits the activation of the NF-κB | Zhou et al. [107] |
|
| ||||||
| Emodin | Isolated compound | Roots and barks of Rheum palmatum and Polygonum multiflorum | 1,3,8-Trihydroxy-6-methylanthraquinone | Anthraquinone | Suppresses the characteristics of airway inflammation, mucin components, and chitinase protein expression. Inhibits the NF-κB signaling pathway | Shrimali et al. [108] |
|
| ||||||
| Euphorbia hirta | Aqueous extract | Not reported | Galloylquinic acid, phorbol acid, leucocyanidol, quercitol, camphol, quercetin, chlorophenolic acid, shikimic acid | Tanins, leucoanthocyanidins, flavonoids, and phenolic compounds | Not elucidated | Kunwar et al. [109] |
|
| ||||||
| Sesame | Fixed oil | Seeds | 5,5′-(1S, 3aR, 4S, 6aR)-Tetrahydro-1H, 3H-furo [3,4-c]furan-1,4-diylbis-1,3-benzodioxole | Polyphenol | Decreases the levels of IL-4, Il-5, IL-13, and serum IgE. Reduces the amount of inflammatory cells and the eosinophil infiltration | Lin et al. [41] |
|
| ||||||
| Farnesol | Isolated compound | Fruits, leaves, flowers | 3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol | Sesquiterpene | Increases the level of IgG2a/IgE and reduces the total IgE, IgA, IgM, IgG | Ku and Lin [110] |
|
| ||||||
| Feverfew (Tanacetum parthenium L.) | Extract | Leaves and parts above the ground | Parthenolide | Sesquiterpene | Inhibit the IκB kinase complex and the histamine release | Pareek et al. [111] |
|
| ||||||
| Flavonoids | Isolated compound | Vegetables (capers, tomatoes, fennel, sweet potato leaves, etc.), fruits (apple, apricots, grapes, plums, and berries), cereals (green/yellow beans and buckwheat) | Not reported | Polyphenol | Prevent the IgE synthesis and the mast cell degranulation. Reduce the airway hyperresponsiveness and inhibit the human phospholipase A2 | Castell et al. [112]; Lattig et al. [113] |
|
| ||||||
| Fumaria parviflora Linn | Aqueous methanolic extract | Aerial parts | Fumarophycine, cryptopine, sanactine, stylopine, bicuculline, adlumine, perfumidine, and dihydrosanguirine | Alkaloids | Block the muscarinic receptors and the Ca2+ channels | Najeeb ur et al. [114] |
|
| ||||||
| Galangin | Synthetic compound | Alpinia officinarum | 3,5,7-Trihydroxy-2-phenylchromen-4-one | Flavonol | Inhibits the TGF-β1 signaling by ROS generation and MAPK/Akt phosphorylation | Liu et al. [115] |
|
| ||||||
| Geastrum saccatum | Solid extract | Fruiting bodies of Geastrum saccatum | β-Glucose | Polysaccharide | Inhibit the NOS and COX | Guerra dore et al. [116] |
|
| ||||||
| Ginsenosides | Synthetic compound | Root of ginseng | Ginsenosides | Glycoside | Suppress the IL-4 level, increase the production of IFN-γ, and inhibit the mucus overproduction and recruitment of eosinophils | Chen et al. [117] |
|
| ||||||
| Grape seed | Extract | Seeds | Not reported | Not reported | Not elucidated. | Mahmoud [118] |
|
| ||||||
| Gymnema sylvestre R. Br. | Extract | Leaves | Not reported | Tanins and saponins | Not elucidated. | Tiwari et al. [119]; Di Fabio et al. [120] |
|
| ||||||
| Herba epimedii | Extract | Leaves | Icariin | Flavonoids, iridoid glycosides, and alkaloids | Inhibit the mRNA expression of TGF-β1 and TGF-β2. Modulate the TGF-β signaling | Tang et al. [121] |
|
| ||||||
| Higenamine | Isolated compound | Tinospora crispa, Nandina domestica THUNBERG, Gnetum parvifolium C.Y. Cheng, Asarum heterotropoides | 1-[(4-Hydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol | Alkaloid | Not elucidated | Zhang et al. [122] |
|
| ||||||
| Homoegonol | Isolated compound | Styrax japonica | 3-[2-(3,4-Dimethoxyphenyl)-7-methoxy-1-benzofuran-5-yl]propan-1-ol | Lignan | Reduces the inflammatory cells count and Th2 cytokines | Shin et al. [123] |
|
| ||||||
| Hypericum sampsonii | Isolated compound | Aerial parts | Not reported | Polycyclic polyprenylated acylphloroglucinols | Not elucidated | Zhang et al. [124] |
|
| ||||||
| Justicia pectoralis | Extract | Aerial parts | 7-Hydroxycoumarin | Coumarin | Decrease the tracheal hyperresponsiveness and the IL-1β and TNF-α levels | Moura et al. [125] |
|
| ||||||
| Juniperus excelsa | Crude extract | Aerial parts | (+)-Cedrol, (+)-Sabinene, (+)-limonene, terpinolene, endo-fenchol, cis-pinene hydrate, α-campholena, camphor, borneol, triene cycloheptane 1,3,5-trimethylene, β-myrcene, o-allyl toluene | Anthraquinones, flavonoids, saponins, sterol, terpenoids, and tanins | Inhibit the Ca2+ influx and the phosphodiesterase activity | Khan et al. [126] |
|
| ||||||
| Kaempferol | Isolated compound | Biotransformation of synthetic kaempferol by genetically engineered E. coli | Kaempferol-3-O-rhamnoside | Flavonoid | Reduces the inflammatory cells number, suppresses the production of Th2 cytokines and TNF-α | Chung et al. [127] |
|
| ||||||
| Kefir | Isolated compound | Kefir grains | Kefiran | Microorganism derived | Reduces the inflammatory cell number and decreases the level of IL-4, IL-13, IL-5, and IgE | Kwon et al. [128]; Lee et al. [129] |
|
| ||||||
| Laurus nobilis L. | Isolated compound | Leaves of Laurus nobilis L | Magnolialide | Sesquiterpene | Inhibit the mast cell degranulation and reduce the IL-4 and IL-5 production | Lee et al. [130] |
|
| ||||||
| Lepidium sativum | Crude extract | Seeds | Ascorbic acid, linoleic acid, oleic acid, palmitic acid, stearic acid | Vitamin and fatty acids | Promote a anticholinergic effect, inhibit the Ca2+ influx, and inhibit the phosphodiesterase activity | Rehman et al. [131] |
|
| ||||||
| L-Theanine | Isolated compound | Green tea of Camellia sinensis | L-Theanine (N-ethyl-L-glutamine) | Amino acid | Reduces the ROS production and decreases the levels of NF-κB and MMP-9 | Hwang et al. [132] |
|
| ||||||
| Luteolin | Isolated compound | Perilla frutescens | (2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-chromenone) | Flavonoid | Inhibits the mucus overproduction and the GABAergic system | Shen et al. [133] |
|
| ||||||
| Lysate bacterial (OM-85 Broncho-Vaxom) | Extract | H. influenzae, S. pneumoniae, Klebsiella pneumoniae, smelly nose Klebsiella, S. aureus, Streptococcus pyogenes, Streptococcus viridans, Neisseria catarrhalis | Not reported | Not reported | Increase the level of IL-4, IL-10, and IFN-γ | Lu et al. [134] |
|
| ||||||
| Mangifera indica L. extract (Vimang®) | Extract | Stem bark | Mangiferin (1,3,6,7-tetrahydroxyxanthone-c2-b-D-glucoside) | Xanthone | Inhibit the IgE production, the histamine release, and mast cell degranulation. Decrease the MMP-9 activity | Rivera et al. [135] |
| Aqueous extract | Barks | Mangiferin (1,3,6,7-tetrahydroxyxanthone-c2-b-D-glucoside) | Xanthone | Reduce the inflammatory cells recruitment and the airway hyperresponsiveness. Increase the Th2 cytokines and attenuated the increase of the PIK3 activity | Alvarez et al. [136] | |
|
| ||||||
| Mangosteen | Isolated compound | Garcinia mangostana Linn. | α- and γ-mangostin | Xanthone | Inhibits the histamine release and modulates the cytokine production | Jang et al. [137] |
|
| ||||||
| Marine bioactives | Isolated compound | Marine sponges Petrosia contignata and Xestospongia bergquisita | Contignasterol and xestobergsterol | Steroids | Upregulation of TNF-β and IL-10 expression | D'Orazio et al. [138] |
|
| ||||||
| Marshallagia marshalli | Isolated compound | Marshallagia marshalli | Secretory/excretory antigen | Microorganism derived | Prevent the release of TNF-α and IL-1β. Suppress the neutrophil migration | Jabbari et al. [139] |
|
| ||||||
| Mikania laevigata and M. glomerata | Extract | Leaves | Dihydrocoumarin, coumarin, spathulenol, hexadecanoic acid, 9, 12-octadecadienoic acid, 9,12,15-octadecatrineoic acid, cupressenic acid, kaurenol, kaurenoic acid, isopropyloxigrandifloric acid, isobutyloxy-grandifloric acid | Coumarins, terpenoids, steroids, and flavonoids | Not elucidated | Napimoga and Yatsuda [140] |
|
| ||||||
| Milk and colostrum | Conjugated compound | Bovine milk | Conjugated linoleic acid | Fatty acid | Modulate the cytokine and antibodies (IgE, IgM) production, interferon NO synthesis and iNOS activity. Modulate the mast cell degranulation | Kanwar et al. [141] |
|
| ||||||
| Monoterpenes | Isolated compound | Essential oil of several medicinal plants (Matricaria recutita, Boswellia carterii, Pelargonium graveolens, Lavandula angustifolia, Citrus limon, Melaleuca alternifolia, Melaleuca viridiflora, Santalum spicatum, Cedrus atlantica, and Thymus vulgaris) | Hydroxydihydrocarvone, fenchone, α-pinene, (S)-cis-verbenol, piperitenone oxide, α-terpinene, α-terpineol, terpinen-4-ol, α-carveol, menthone, pulegone, geraniol, citral, citronellol, perillyl alcohol, perillic acid, β-myrcene, carvone, limonene, thymol, carvacrol, linalool, linalyl acetate, borneol, l-borneol, bornyl acetate, terpineol, thymoquinone, thymohydroquinone, 1,8-cineol, l-menthol, menthone, and neomenthol | Terpenoids | Reduce the expression of NF-κB target gene MUC2 | Cassia et al. [142] |
|
| ||||||
| Mandevilla longiflora | Hydroethanolic extract | Plant xylopodium | Ellagic acid, hesperidin, luteolin, naringin, naringenin, and rutin | Polyphenol and flavonoids | Decrease the eosinophils, neutrophils, and mononuclear cell migration in BALF and by histopathological analysis. Decrease the IL-4, IL-5, IL-13, IgE, and LTB4 levels | Almeida et al. [143] |
|
| ||||||
| Morus alba L. | Isolated compound | Root bark | Moracin M. (5-(6-hydroxy-1-benzofuran-2-yl)benzene-1,3-diol) | Not reported | Inhibit the PDE4 | Chen et al. [144] |
|
| ||||||
| Haemanthus coccineus | Extract | Dried bulbs | Narciclasine | Alkaloid | Inhibit the edema formation, the leucocyte infiltration, and cytokine synthesis in vivo. Block the interaction between the leucocyte and endothelial cells, the activation of isolated leucocytes (cytokine synthesis and proliferation) and of primary endothelial cells (adhesion molecule expression) in vitro. Suppress the NF-κB-dependent gene transcription | Fuchs et al. [145] |
| Naringin | Isolated compound | Common grapefruit | Naringin | Flavone | Attenuates the bronchoconstriction by reduction of calcium influx | Wang et al. [146] |
|
| ||||||
| Nielumbo nucifera | Extract | Leaves | Nuiciferine and aporphine | Alkaloids | Attenuate the bronchoconstriction by reduction of calcium influx | Yang et al. [147] |
|
| ||||||
| Nigella sativa | Oil | Seeds | Thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone) | Quinone | Decrease the NO and IgE levels. Increase the IFN-γ | Salem et al. [148]; Koshak et al. [149] |
|
| ||||||
| NujiangexanthoneA | Isolated compound | Leaves of Garcinia nujiangensis | 1,2,5,6-Tetrahydroxy-3-methoxy-4,7,8-tri(3-meth-ylbut-2-enyl)-xanthone | Xanthone | Suppresses the IgE/Ag activation and degranulation of mast cell. Suppresses the production of cytokines and eicosanoids, through inhibiting Src kinase activity and Syk-dependent pathways. Inhibits the release of histamine, PGD2 and leukotriene C4 generation. Inhibits the increase of IL-4, IL-5, IL-13, and IgE levels. Inhibits the cell infiltration and increases mucus production | Lu et al. [150] |
|
| ||||||
| Oleanolic acid | Synthetic compound | Forsythia viridissima | Oleanolic acid | Triterpenoid | Modulates the transcription factors T-bet, GATA-3, RORγt, and Foxp3 | Kim et al. [151] |
|
| ||||||
| Omega 3 | Isolated compound | Fish oil | n – 3 Polyunsaturated fatty acid | Fatty acid | Decreases the IL-17 and TNF-α levels | Hansen et al. [152]; Farjadian et al. [153] |
|
| ||||||
| Organic acids | Isolated compound | Berberis integerrima and B. vulgaris fruits | Malic, citric, tartaric, oxalic, and fumaric acids | Organic acids | Inhibits the Th2 cytokines | Ardestani et al. [154]; Shaik et al. [155] |
|
| ||||||
| Oroxylin A | Isolated compound | Scutellariae radix | 5′7-Dihydroxy-6-methoxy-2phenyl-4H-1-benzopyran-4-one | Flavonoid | Reduces the airway hyperactivity. Decreases the levels of IL-4, IL-5, IL-13 and IgE in BALF | Lu et al. [156]; Zhou et al. [157] |
|
| ||||||
| Oxymatrine | Isolated compound | Root of the Sophora flavescens Aiton (Fabaceae) | Oxymatrine | Alkaloid | Inhibits the eosinophil migration, IL-4, IL-5, IgE, and IL-13 levels. Inhibits the expression of CD40 protein | Zhang et al. [158] |
|
| ||||||
| P. integerrima Gall and Pistacia integerrima stew. Ex brand | Methanolic and crude extract | Galls and whole plant | Not reported | Carotenoids, terpenoids, catechins, and flavonoids | Attenuate the TNF-α, IL-4, and IL-5 expression levels, and pulmonary edema by elevation of AQP1 and AQP5 expression levels | Rana et al. [159]; Bibi et al. [160] |
|
| ||||||
| Paeonia emodi royle | Extract | Rhizomes | 1β, 3β, 5α, 23, 24-Pentahydroxy-30-nor-olean-12, 20(29)-dien-28-oic acid; 6α, 7α-epoxy-1α, 3β, 4β, 13β-tetrahydroxy-24, 30-dinor-olean-20-ene-28, 13β-olide; paeonin B; paeonin C; methyl grevillate; 4-hydroxy benzoic acid, and gallic acid | Terpenoids and phenolic compounds | Inhibits the lipoxygenase activity | Zargar et al. [161] |
|
| ||||||
| Petasites japonicus | Extract | Leaves | Petatewalide B | Not reported | Inhibit the degranulation of β-hexosaminidase in mast cells, the iNOS induction, and the NO production. Inhibits the accumulation of eosinophils, macrophages, and lymphocytes in BALF | Choi et al. [162] |
|
| ||||||
| Peucedanum praeruptorum dunn | Extract | Roots | Dihydropyranocoumarin, linear furocoumaris, and simple coumarin | Coumarins | Attenuate the airway hyperreactivity and Th2 responses | Xiong et al. [163] |
|
| ||||||
| Peucedani Radix | Extract | Roots | Nodakenin, nodakenetin, pteryxin, praeruptorin A, and praeruptorin B | Not reported | Inhibit the Th2 cell activation | Lee et al. [164] |
|
| ||||||
| Eryngium | Extract | Leaves, fruits, and roots | A1-barrigenol, R1-barrigenol, tiliroside, kaempferol 3-O-β-D-glucosyde-7-O-α-L-rhamnoside, rutin, agasyllin, grandivittin, aegelinol benzoate, aegelinol, R-(+)-rosmarinic (61), and R-(+)-3′-O-β-D-glucopyranosyl rosmarinic acid | Phenol, flavonoids, tannins, and saponins | Not elucidated | Erdem et al. [165] |
|
| ||||||
| Pericampylus glaucus | Extract | Stems, leaves, roots, and fruits | Periglaucine A-D and mangiferonic acid | Alkaloids, terpenoids, isoflavones, and sterols | Inhibit the COX enzymes activity | Shipton et al. [166] |
|
| ||||||
| Aquilaria malaccensis | Ethanolic extract | Seeds | Aquimavitalin | Phorbol ester | Inhibit the mast cell degranulation | Korinek et al. [167] |
|
| ||||||
| Phytochemicals | Isolated compound | Several medicinal plants | Luteolin, kaempferol, quercetin, eudesmin, magnolin, woorenoside, zerumbone, aucubin, triptolide, nitocine, berberine, and piperine | Flavonoids, lignans, terpenoids, and alkaloids | Suppress the TNF-α expression | Iqbal et al. [168] |
|
| ||||||
| Picrasma quassioides (D.Don) Benn | Alcoholic extract | Not reported | 4-Methoxy-5- hydroxycanthin-6-one | Alkaloid | Decreases the inflammatory cell count in BALF. Reduces the IL-4, IL-5, IL-13, and IgE levels. Reduces the airway hyperresponsiveness. Attenuates the recruitment of inflammatory cells and the mucus production in the airways. Reduces the overexpression of inducible nitric oxide synthase (iNOS) | Shin et al. [169] |
|
| ||||||
| Pinus maritime (Pycnogenol®) | Extract | Barks | Procyanidin | Flavonoid | Decrease the NO production, the inflammatory cell count, and the levels of IL-4, IL-5, IL-13, and IgE in BALF or serum. Reduces the IL-1β and IL-6 levels, the expression of iNOS and MMP-9. Enhances the expression of heme oxygenase (HO)-1. Attenuates the airway inflammation and mucus hypersecretion | Shin et al. [170] |
|
| ||||||
| Ping chuan ke li | Not elucidated | Wang et al. [171] | ||||
|
| ||||||
| Piperine | Isolated compound | Piper nigrum (black pepper) and Piper longum (long pepper) | Piperine | Alkaloid | Inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production | Chinta et al. [172] |
|
| ||||||
| Piperlongumine | Isolated compound | Piper longum | Piperlongumine (5,6-dihydro-1-[(2E)-1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propenyl]-2(1H)-pyridinone) | Alkaloid | Inhibits the activity of inflammatory transcription factors, NF-κB, and signal transducer and activator of transcription (STAT)-3 as well as the expression of IL-6, IL-8, IL-17, IL-23, matrix metallopeptidase (MMP)-9, and intercellular adhesion molecule (ICAM)-1. Suppresses the permeability and leukocyte migration, the production of TNF-α, IL-6, and extracellular regulated kinases (ERK) 1/2 along with the activation of NF-κB | Prasad and Tyagi [173] |
|
| ||||||
| Piper nigrum | Ethanolic extract | Not reported | Piperine | Alkaloid | Inhibit the Th2/Th17 responses and mast cell activation | Bui et al. [174]; Khawas et al. [175] |
|
| ||||||
| Plectranthus amboinicus (Lour.) spreng. | Ethanol, methanol, and hexane extracts | Aerial parts | Rosmarinic acid, shimobashiric acid, salvianolic acid L, rutin, thymoquinone, and quercetin | Flavonoids | Not elucidated | Arumugam et al. [176] |
|
| ||||||
| Podocarpus sensu latissimo | Extract | Barks | 3-Methoxyflavones and 3-O-glycosides | Flavonoids | Provinol and flavin-7 | Abdillahi et al. [177] |
|
| ||||||
| Polyphenols and their compounds | Isolated compound | Provinol and flavin-7 | Quercetin and resveratrol | Polyphenol | Decrease IL-4 and IL-5 levels, the airway hyperresponsiveness, and mucus overproduction | Joskova et al. [178] |
|
| ||||||
| Propolis | Isolated compound | Honey bees from several plants | Pinocembrin and caffeic acid phenethyl ester | Polyphenol and terpenoids | Inhibits TGF-β1 | Kao et al. [179] |
|
| ||||||
| Psoralea corylifolia | Extract | Fruits | 7-O-Methylcorylifol A, 7-O-isoprenylcorylifol A, and 7-O-isoprenylneobavaisoflavone | Flavonoids | Inhibit the N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP)-induced O2– generation and/or elastase release | Chen et al. [180] |
|
| ||||||
| Quercetin | Isolated compound | Tea, fruits and vegetables | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one | Flavonoid | Inhibits LOX and PDE4. Reduce leukotrienes and histamine release with a decrease in the IL-4 level. Inhibit prostaglandins release and the human mast cell activation by Ca2+ influx | Townsend et al. [181]; Mlcek et al. [182] |
|
| ||||||
| Radix Rehmanniae Preparata | Extract | Not reported | Catalpol | Glycoside | Inhibit IgE secretion. Decrease IL-4 and IL-5. Inhibit eosinophil infiltration and suppress eotaxin and its receptor CCR3. Reduce IL-5Rα levels | Chen et al. [183] |
|
| ||||||
| Resveratrol | Isolated compound | Skin and barks of red fruits | Resveratrol (3,4,5-trihydroxystilbene) | Polyphenol | Decreases eosinophilia. Reduce neutrophil migration and inhibit PGD-2 release. Decrease IL-4 and IL-5 and also the hyperresponsiveness and mucus production | Lee et al. [184]; Hu et al. [185]; Chen et al. [186] |
|
| ||||||
| Schisandra chinensis | Extract | Dried fuits | α-Cubebenoate | Not reported | Suppress bronchiolar structural changes. Inhibit the accumulation of lymphocytes, eosinophils, and macrophages in BALF. Suppress IL-4, IL-13, and TGF-β1. Increase the intracellular Ca2+ | Lee et al. [187] |
|
| ||||||
| Sea cucumber (Holothurians) | Tonic | Marine animal (Sea cucumber) | Holothurin A3, pervicoside A, and fuscocinerosides A | Toxins | Reduce COX enzymatic activity | Guo et al. [188] |
| Selaginella uncinata (Desv.) | Extract | Dried herbs | Amentoflavone, hinokiflavone, and isocryptomerin | Flavonoids | Attenuate hyperresponsiveness and goblet cell hyperplasia. Decrease IL-4, IL-5, IL-13, and IgE levels in serum. Upregulation of T2R10 gene expression and downregulation of IP3R1 and Orai1 gene expression. Suppression of eotaxin, NFAT1, and c-Myc protein expression | Yu et al. [189] |
|
| ||||||
| Selaginella pulvinata | Isolated compound | Air-dried powder of the whole plant of S. pulvinata | Selaginpulvilin A, selaginpulvilin B, and selaginpulvilin C | Phenol | Inhibit the PDE4 | Liu et al. [190] |
|
| ||||||
| Sideritis scardica | Extract | Leaves | Echinacoside, verbascoside, luteolin, apigenin, caffeic acid, vanillic acid | Glycosides, flavonoids, and phenolic acids | Not elucidated | Todorova and Trendafilova [191] |
|
| ||||||
| Siegesbeckia glabrescens | Extract | Aerial roots | 3,40-O-Dimethylquercetin, 3,7-O-dimethylquercetin, 3-O-methylquercetin, and 3,7,40-O-trimethylquercetin | Flavonoids | Reduce inflammatory cell infiltration in BALF. Decrease IL-4, IL-5, IL-13, eotaxin, and IgE. Reduce airway inflammation and mucus overproduction. Decrease iNOS and COX-2 expression and reduce NO levels | Jeon et al. [192] |
|
| ||||||
| Sitostanol | Isolated compound | Marketable synthetic compound | Sitostanol | Steroid | Suppresses IL-4 and IL-13 release | Brüll et al. [193] |
|
| ||||||
| Soft coral | Isolated compound | Sarcophyton ehrenbergi | Not reported | Prostaglandins | Inhibits PDE4 | Cheng et al. [194] |
|
| ||||||
| Solanum paniculatum L | Extract | Fruits | Stigmasterol and β-sitosterol | Steroid | Reduce IL-4 and NO levels. Decrease IFN-γ without changes in IL-10 levels. Reduce NF-κB, TBET, and GATA3 gene expression | Rios et al. [195] |
|
| ||||||
| Squill (Drimia maritima (L.) stearn) oxymel | Crude extract | Not reported | Scillaren A, scillirubroside, scilliroside, scillarenin, and proscillaridin A | Glycosides | Not elucidated | Nejatbakhsh et al. [196] |
|
| ||||||
| Sorbus commixta Hedl. (Rosaceae) | Methanolic extract | Fruits | Neosakuranin | Glycosides | Not elucidated | Bhatt et al. [197] |
|
| ||||||
| Thuja orientalis | Extract | Fruits | Cupressuflavone, amentoflavone, robustaflavone, afzelin, (+)-catechin, quercetin, hypolaetin 7-O-β-xylopyranoside, isoquercitrin, and myricitrin | Flavonoids | Reduce nitric oxide production and reduce the relative mRNA expression levels of inducible nitric oxide synthase (iNOS), IL6, cyclooxygenase-2, MMP-9, TNF-α in vitro. Decrease the inflammatory cell counts in BALF. Reduce IL-4, IL-5, IL-13, eotaxin, and IgE levels and reduce the airway hyperresponsiveness, in vivo. Attenuate mucus hypersecretion | Shin et al. [198] |
|
| ||||||
| Tonggyu-tang | Extract | Ledebouriella divaricata Hiroe, Angelica koreanum Kitagawa, Angelica tenuissima Nakai, Cimicifuga heracleifolia Kom., Pueraria thunbergiana Benth., Ligusticum wallichii var. officinale Yook., Atractylodes lancea DC., Thuja orientalisl., Ephedra sinica Stapf., Zanthoxylum schinifolium S.Z., Asarum sieboldii var. seoulense Nakai, Glycyrrhiza glabra, Astragalus membranaceus var. mongholicus Bung, Xanthium strumarium L., Magnolia denudate Desr., Mentha arvensis var. piperascens Makinv | Not reported | Plant | Inhibit inflammatory cytokines (IL-4, IL-6, IL-8, and TNF-α). Suppress mitogen activated protein kinase (MAPK) and NF-κB in mast cells and keratinocytes | Kim et al. [199] |
|
| ||||||
| Trigonella foenum-graecum | Extract | Seeds | Not reported | Flavonoids | Reduce IL-5, IL-6, IL-1β, and TNF-α. Reduce collagen deposition in goblet cells. Suppress inflammatory cells | Piao et al. [200] |
|
| ||||||
| Tropidurus hispidus | Oil | Fat of tropidurus hispidus | Croton oil, arachidonic acid, phenol, and capsaicin | Fatty acids and its derivated | Affect the arachidonic acid and their metabolites and reduce proinflammatory mediators | Santos et al. [201] |
|
| ||||||
| Urtica dioica L. | Extract | Leaves | Caffeic acid, gallic acid, quercetin, scopoletin, carotenoids, secoisolariciresinol, and anthocyanidins | Polyphenols, flavonoids, cumarin, and lignan | Reduce leucocytes and lymphocytes levels in serum. Inhibit the eosinophilia increase in BALF. Suppress inflammatory cells recruitment and attenuation of lipid peroxidation of lung tissues | Zemmouri et al. [202] |
|
| ||||||
| Verproside | Isolated compound | Pseudolysimachion | Verproside | Glycoside | Suppress the NF-κB and TNF-α expression | Lee et al. [203] |
|
| ||||||
| Vitamin D | Isolated compound | Not reported | Calcitriol | Vitamin | Inhibit lymphocytes (Th1 and Th2) and reduces cytokines production | Szekely and Pataki [204] |
|
| ||||||
| Vitamin E | Isolated compound | Plant lipids | α-, β-, γ-, and δ-Tocopherols and the α-, β-, γ-, and δ-tocotrienols | Vitamin | Reduce airway hyperresponsiveness, IL-4, IL-5, IL-13, OVA-specific IgE, eotaxin, TGF-β, 12/15-LOX, lipid peroxidation, and lung nitric oxide metabolites | Cook-Mills and McCary [205]; Abdala-Valencia et al. [206] |
|
| ||||||
| Vitex rotundifolia linn til (Verbenaceae) | Methanolic extract | Fruits | 1H, 8H-Pyrano [3, 4-c]pyran-1,8-dione | Not reported | Inhibit eotaxin, IL-8, IL-16, and VCAM-1 mRNA | Lee et al. [207] |
|
| ||||||
| Viticis fructus | Extract | Dried fruit | Pyranopyran-1,8-dione | Not reported | Inhibit eosinophils and lymphocytes cell infiltration into the BAL fluid. Reduce to normal levels of IL-4, IL-5, IL-13 and eotaxin. Suppress IgE levels | Park et al. [208] |
|
| ||||||
| Yu ping feng san | Extract | Radix Saposhnikoviae (Fangfeng), Radix Astragali (Huangqi), and Rhizoma Atractylodis macrocephalae (Baizhu) | Calycosin-7-O-β-d-glucoside, calycosin, formonetin, atractylenolide III, II, and I; 5-O-methylvisammioside, 8-methoxypsoralen and bergapten | Flavonoids, terpenoids, saponins, and furocoumarins | Inhibit TNF-α, IFN-γ, and IL-1β | Stefanie et al. [209] |
|
| ||||||
| Zygophyllum simplex L | Extract | Aerial parts | Isorhamnetin-3-O-β-D-rutinoside, myricitrin, luteolin-7- O-β-D-glucoside, isorhamnetin-3-O-β-D-glucoside, and isorhamnetin | Phenol | Inhibit NF-κB, TNF-α, IL-1β, and IL-6 | Abdallah and Esmat [210] |
|
| ||||||
| Ziziphus amole | Extract | Leaves, stems, barks, and roots | Alphitolic acid, sitosterol, ziziphus-lanostan-18-oico acid | Terpenoid and steroid | Inhibit myeloperoxidase activity | Romero-Castillo et al. [211] |
2.1. Natural Products from Plants
The use of natural products obtained from plants by the traditional medicine has been reported from centuries, especially in countries as China, Japan, and India [212]. Thus, the topics below concern these products or bioactive compounds originated from the most studied plants used on asthma therapy.
2.1.1. Flavonoids
Flavonoids are natural compounds from plants, nuts, and fruits that are chemically characterized by the presence of two benzene rings (A and B) linked through a heterocyclic pyrene ring (C). They represent a large group of polyphenolic secondary metabolites [213] with more than 8,000 different compounds already identified [214]. Considering their chemical structure, they can be classified as flavans, flavanones, isoflavanones, flavones, isoflavones, anthocyanidins, and flavonolignans [214]. Flavans or isoflavans possess a heterocyclic hydrocarbon skeleton, chromane, and a substitution on its C ring, in carbons 2 or 3, by a phenyl group (B ring). Flavanones and isoflavanones show an oxo-group in position 4. The presence of a double bond between C2 and C3 indicates flavones and isoflavones, and the addition of a C1 to C2 double bond represents anthocyanidins [214].
The diversity in their chemical structure contributes to their broad range of physiological and biological activities, from which it can be highlighted the antioxidant, anti-inflammatory, antiallergic, antiviral, hepatoprotective, antithrombotic, and anticarcinogenic activities [213]. In this review, 14 studies reported flavonoids as a group of compounds able to be used on asthma treatment. The following subsections show the main flavonoids with antiasthmatic activity reported in the literature and used by the traditional medicine. These studies attributed the antiasthmatic activity of plant extracts containing these compounds, in part, due to their presence in the phytocomplex.
(1) Flavone Compounds: Chrysin, Baicalin, Luteolin, and Oroxylin A. Defined as 5,7-dihydroxy-2-phenyl-1-4H-chromen-4-one, chrysin is classified as a flavone that can be found in Passiflora caerulea and Passiflora incarnate flowers, as well as in Matricaria chamomilla, popularly known as chamomile, besides being present in propolis and other plants [90, 100]. Chrysin is a compound able to suppress the proliferation of airway smooth muscle cells as well as to promote a reduction in the IL-4, IL-13, IgE, and interferon-γ levels that lead to an attenuation in the asthma inflammatory process [89]. Bae et al. [90] performed their studies through an in vitro cell culture model with the purpose to describe how the chrysin was able to promote the inhibitory effect in the proinflammatory cytokines. They suggested that this effect was caused by the intracellular calcium reduction in mast cells, since calcium is responsible for proinflammatory cytokine gene transcription [90]. In addition, a study performed by Yao and colleagues [88] investigated the activity of chrysin against asthma in mice sensitized with ovalbumin (OVA). Their results revealed that chrysin would be a promising compound able to be used for controlling airway remodeling and clinical manifestations of asthma [88].
Baicalin, a 7-glucuronic acid-5,6-dihydroxyflavone, is a natural metabolite easily found in leaves and barks from several species of the Scutellaria genus [215]. Studies performed by Park and colleagues [208] investigated the anti-inflammatory activity of baicalin using an asthma-induced animal model. The results showed that this compound decreased the inflammatory cell infiltration and the levels of TNF-α in the bronchoalveolar lavage fluids (BALF). The activity of the baicalin was attributed to the fact that this metabolite selectively inhibits the enzyme activity of PDE4 and suppresses the TNF-α expression induced by the lipopolysaccharides on macrophages, indicating a potential use of this metabolite in asthma treatment [74].
Additionally, luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromenone), another compound that had also demonstrated antiasthma activity, is widely found in aromatic flowering plants, such as Salvia tomentosa and Lamiaceae, as well as in broccoli, green pepper, parsley, and thyme [216]. Shen and colleagues [133] studied its pharmacological activity through inhibition of the GABAergic system, which is responsible for the overproduction of mucus during the asthmatic crisis by overstimulation of the epithelial cells. The study indicated that this compound was able to promote the attenuation of the goblet cell hyperplasia by the partial inhibition of GABA activities [133].
Another antiasthmatic flavonoid compound is oroxylin A, a flavone found in the extract of Scutellaria baicalensis Georgi and Oroxylum indicum tree [156]. According to Zhou [157], oroxylin A, or 5-7-dihydroxy-6-methoxy-2-phenylchromen-4-one, was able not only to reduce the airway hyperactivity in an OVA-induced asthma murine model, but also to decrease the levels of IL-4, IL-5, IL-13, and OVA-specific IgE in BALF [157]. This study also showed the ability of oroxylin A in inhibiting the alveolar wall thickening in addition to avoid the inflammatory cell infiltration in the perivascular and peribronchial areas assessed by histopathological evaluation [157].
(2) Flavonol Compounds: Quercetin, Galangin, and Kaempferol. Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one), a flavonol compound widely found in onions, apples, broccoli, cereals, grapes, tea, and wine, has been known as the main active compound of these plants and, therefore, responsible for their widespread use in traditional medicine for the treatment of inflammatory, allergic, and viral diseases [213]. The studies using this compound as antiasthma were performed in cell cultures and rats, as in vitro and in vivo models, respectively, showing its high capacity to reduce inflammatory processes. According to these studies, the anti-inflammatory mechanism of quercetin is attributed to the lipoxygenase and PDE4 inhibition and reduction on histamine and leukotriene release, which promote a decrease in the proinflammatory cytokine formation and production of IL-4, respectively. In addition, quercetin also promoted the inhibition of human mast cell activation by Ca2+ influx and prostaglandin release inhibition [182], favoring the therapeutic relief of the asthma symptoms and decreasing the short-acting β-agonist dependence [181, 182].
Galangin, a compound chemically defined as 3,5,7-trihydroxy-2-phenylchromen-4-one, easily found on Alpinia officinarum [217], had its pharmacological activity evaluated using a specific-pathogen-free mice model [115]. The study, performed by Liu [115], showed an effective response against the in vivo OVA-induced inflammation as well as a reduction on the ROS levels in vitro. Furthermore, galangin acted as an antiremodeling agent in asthma, since this compound inhibited the goblet cell hyperplasia, lowering the TGF-β1 levels and suppressing the expression of vascular endothelial grown factor (VEGF) and matrix metalloproteinase-9 (MMP-9) in BALF or lung tissue. This result highlighted its antiremodeling activity in the TGF-β1-ROS-MAPK pathway, proving its potential use on asthma treatment [115].
Another flavonol, kaempferol, chemically defined as 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, is widely found in citrus fruits, broccoli, apples, and other plant sources [213]. This compound has been studied due to its pharmacological potential, especially against inflammation. In the study performed by Chung et al. [127], an OVA-induced airway inflammation mouse model of asthma was performed, demonstrating that kaempferol can significantly reduce the inflammatory process due to the decrease of the inflammatory cell infiltration and the decrease of production of inflammatory cytokines and IgE antibodies. In addition, this compound was also able to reduce the intracellular ROS production in the airway inflammation reaction [127].
Furthermore, Mahat et al. [218] demonstrated that the anti-inflammatory activity of kaempferol occurs through the inhibition of nitric oxide and nitric oxide-induced COX-2 enzyme activation, further inhibiting the cytotoxic effects of nitric oxide, reducing the prostaglandin-E2 production [218]. To improve the possibility of the use of kaempferol as a bioactive on the development of new drugs or medicines, the previously mentioned study by Chung [127] also describes the antiasthma activity of a glycosylated derivative of kaempferol, the kaempferol-3-O-rhamnoside. The glycosylation of kaempferol improved its solubility and stability, besides reducing its toxicity [127], allowing the production of a compound with great potential to increase the asthma therapeutic arsenal. According to this rationale, this compound may be responsible for the anti-inflammatory properties of the plant extracts containing this substance and that have been used to asthma treatment.
2.1.2. Resveratrol
Resveratrol is a natural stilbenoid compound, a class of polyphenol obtained from the bark of red fruits, with known antioxidant and promising anti-inflammatory and antiasthma activities [186]. In studies using eosinophils obtained from asthmatic individuals, Hu et al. [185] demonstrated that resveratrol induces not only cell cycle arrest in the G1/S phase, but also apoptosis, allowing a decrease in the eosinophil number [185], thus reducing the neutrophil migration and, consequently, preventing the histamine and PGD-2 release, avoiding vasodilatation, mucus production, and bronchoconstriction (Figure 1). Additionally, Lee and colleagues [129] demonstrated that resveratrol was effective against the asthmatic mouse model once this polyphenol induced a significant decrease in the plasma level of T-helper-2-type cytokines, such as IL-4 and IL-5. It also decreased the airway hyperresponsiveness, eosinophilia, and mucus hypersecretion [184]. Although performed by different methods, the studies are in agreement regarding the scientific evidence that supports the use of resveratrol by oral route as an effective natural compound to treat asthma patients.
2.1.3. Boswellia
Boswellia is a tree genus that produces oil known as frankincense, which is obtained through incisions in the trunks of these trees. This oil is composed by 30–60% resin, 5–10% essential oils, and polysaccharides [219]. Studies performed using this product evaluated its pharmacological activities revealing that the Boswellia bioactives are boswellic acids and AKBA (3-O-acetyl-11-keto-β-boswellic acid), both responsible for preventing NF-κB activation and, consequently, inhibiting IL-1, IL-2, IL-4, IL-6, and IFN-gamma release [52]. They also inhibit LOX-5, thus preventing leukotriene release [78]. Thus, based on the physiopathology of asthma, it is possible to infer that these compounds may act as antiasthma molecules from the tree genus, once these enzymes and mediators are involved in the asthma-related inflammation. Moreover, another study that aimed at evaluating the antiasthma activity of these compounds showed that the association between Boswellia serrata, Curcuma longa, and Glycyrrhiza had a pronounced effect on the management of bronchial asthma [79], suggesting its potential on asthma therapy.
2.2. Natural Products from Animal Source
Animal-derived natural products still represent the minority of natural sources for products intended for asthma treatment. Nonetheless, many studies describe the use of animal-based products, such as oils, milk, and spleen as a complementary therapy for several diseases, including asthma. The traditional medicine reports the benefits of consuming some animal parts and animal products, once they can be rich in compounds such as lipids, prostaglandins, unsaturated fatty acids, enzymes, and polysaccharides, which are responsible for their pharmacological activities [220, 221]. In addition, animal sources are also widely cited as biocompatible and biodegradable sources, suggesting their safe use. The animal products and compounds cited in this session can be obtained from several sources, such as mammals, amphibians, and crustaceans, demonstrating its wide range of possibilities.
2.2.1. Animal Sea Source: Holothuroidea, Penaeus, and Sarcophyton ehrenbergi
Marine ecosystems represent an important source of natural compounds due to their wide biodiversity, which include animals and plants that are unique to this environment. Therefore, many studies have been performed to evaluate the antimicrobial, anti-inflammatory, antiviral, and antiasthmatic potential of algae and sea animals.
On this concern, the sea cucumber, a marine invertebrate animal that belongs to the class Holothuroidea, usually found in the benthic areas and deep seas, has been used by Asian and Middle Eastern communities in the traditional medicine as elixir, due to its pharmacological activity on the treatment of hypertension, asthma, rheumatism, cuts, burns, and constipation [188]. These pharmacological activities are attributed to the presence of saponins, cerebrosides, polysaccharides, and peptides on its composition [188, 220]. Bordbar et al. [220], in a literature review, mentioned an experimental study from Herencia et al. [222] in which sea cucumber extract showed a reduction of the enzymatic activity of cyclooxygenase in inflamed mice tissues, without promoting any modification on the cyclooxygenase enzyme, showing that the sea cucumber extract is a potent natural product able to be used against several inflammatory diseases [220].
Ozdemir and colleagues [87] investigated the pharmacological activity of chitin, a polysaccharide formed by repeated units of N-acetylglucosamine to form long chain through β-(1-4) linkage [221], the major compound of the shrimp (Penaeus) exoskeleton. In this study, the authors performed the intranasal administration of chitin microparticles in the asthma-induced mice model, which promoted the reduction of serum IgE and peripheral blood eosinophilia, besides the decrease of the airway hypersensitivity [87]. Additionally, another study identified and isolated ten new prostaglandin derivatives from the Sarcophyton ehrenbergi extract, a soft coral species found in the Red sea [194], from which five of them showed inhibitory activity against PDE4 (44.3%) at 10 μg.mL–1, suggesting its utilization on asthma and chronic obstructive pulmonary disease treatment, once PDE4 is the drug target on the treatment of both diseases [194].
Finally, these studies demonstrated that marine source needs to be further investigated, since a wide variety of bioproducts and/or bioactives with potential anti-inflammatory activity and antiasthmatic proprieties can be found in this environment.
2.2.2. Bullfrog (Rana catesbeiana Shaw) Oil
The bullfrog oil is a natural oil extracted from the adipose tissue of the amphibian Rana catesbeiana Shaw, which is originated from North America and has its meat widely commercialized around the world [223]. This oil has been used by the traditional medicine to treat inflammatory disorders, especially asthma [223]. This oil is composed of a mixture of mono- and polyunsaturated fatty acids and bile-derived steroid compound (ethyl iso-allocholate) [81, 224], which are responsible for its therapeutic properties [81].
According to Yaqoob [57], the presence of oleic, linolenic, stearic, palmitic, and myristic fatty acids can promote the suppression of immune cell functions [58]. Based on such evidence, it is possible to infer that the bullfrog oil, due to its chemical composition, can be used on the treatment of inflammation-related disorders such as asthma. However, further studies are needed to confirm this hypothesis.
2.2.3. Other Products Derived from Animals
Although the majority of the currently used animal products by the traditional medicine for asthma treatment belong from animal tissues, there is evidence that mammal fluids, for example, buffalo spleen liquid, milk, and colostrum, can act on the immune system promoting the decrease of asthma symptoms [80].
The buffalo spleen liquid was investigated in a study performed by Neamati and colleagues [80], in which pigs were asthma sensitized using ovalbumin, followed by administration of the buffalo spleen liquid-based adjuvant. A decrease in the tracheal response as well as a reduction in the white blood cell number in lung lavage was observed on sensitized animals when compared to healthy animals [80], showing the potentiality of this fluid in promoting asthma control. In addition, another study was performed to evaluate the antiasthma activity using milk and colostrums, which contain linolenic acid and proteins like lactoferrin [141], as a natural product. This study showed a modulation in the plasma lipid concentration in human and animal models and a decrease in the allergic airway inflammation induced by ragweed pollen grain extract.
2.3. Bioactives Obtained from Microorganisms
The use of bacteria and fungi metabolites on the treatment of several diseases is widely reported since the penicillin discovery. However, more recent studies have further investigated the antiasthmatic potential of these metabolites [225]. On this concern, a study performed by Lu and colleagues [156] evaluated the antiasthma activity of the bacterial lysate OM-85 Broncho-Vaxom (BV), a patented pharmaceutical product [134]. The study observed that the bacterial lysate coupled with the conventional treatment was able to increase the rate of natural killer T cells on the peripheral blood, decreasing the cytokine level (cytokines type not described) and, then, promoting the reduction of asthma symptoms. Furthermore, kefir, a fermented milk drink produced by lactic and acetic acids from bacteria, which also presents the kefiran, an insoluble polysaccharide as main component [128, 129], had its in vivo anti-inflammatory activity evaluated. This compound was able to reduce at normal levels the release of IL-4, IL-6, and IL-10 along with the production of INF-γ and TNF-α [128]. In addition, the intragastric administration of kefiran promoted the reduction of OVA-induced cytokine production in a murine asthma model, decreasing the pulmonary eosinophilia and mucus hypersecretion [128, 129].
Therefore, based on these reports and historical facts regarding the use of microorganisms as source for isolation of new bioactives and the development of medicines, it is important to highlight that these new agents may contribute to the current asthma treatment.
3. Conclusion: Widely Used Active Pharmaceutical Ingredients from Natural Source
As previously demonstrated, natural products have been extensively used as a complementary treatment for asthma therapy. Studies concerning these products have aimed at investigating their activity as a matrix of compounds to complement or replace current asthma treatment, while others aim at isolating compounds to generate new medicines based on synthetic drugs of natural origin [226].
Historically, natural products have contributed tremendously to the development of marketable medicines to the treatment of several diseases [226]. The evaluation of their therapeutic activities and identification and isolation of their bioactive molecules allowed not only their clinical use, but also the discovery of the pharmacophore groups and the radicals responsible for their toxicity or their biopharmaceutics aspects. In fact, based on such studies, it is possible to perform structural or delivery changes on these compounds that would increase their safety or would be able to module their half-life allowing to target them to specific action sites [227].
This review shows the experimental studies that identified the antiasthma activity of different natural sources in the last decade, along with the molecules responsible for that. Altogether, these studies presented preliminary data that require further investigations about these compounds in order to, in a near future, be used on the production of designing medicines. Currently, a few natural-based active compounds are already available in the market, such as ipratropium bromide, theophylline, epinephrine, and sodium cromoglycate [226, 228–231].
Ipratropium bromide, an anticholinergic drug able to promote bronchodilation, has been widely used for the treatment of asthma. This compound was synthesized from atropine, a compound extracted for the first time in 1809 from Atropa belladonna L. However, it can be also found in other plants from the Solanaceae family [228, 229]. In spite of that, only in 1833, its chemical structure was elucidated, and in 1850, it was implemented for clinical use, allowing the proper understanding of its in vivo biopharmaceutics and therapeutic characteristics [232].
Theophylline is an antiasthmatic drug widely used in the management of severe persistent asthma, promoting the bronchodilation and attenuation of asthma inflammation. Also known as 1,3-dimethylxanthine, this molecule was extracted in 1888 from Theobroma cacao L. and Camellia sinensis L., plants presented in several countries. Later in 1922, this drug was introduced on asthma therapy [233]. Years after, epinephrine, also known as adrenaline, was extracted from the Ephedra sinica, a plant widely used in the Chinese traditional medicine, allowing the synthesis of beta-agonist antiasthmatic drugs, such as salbutamol and salmeterol, currently used in asthma treatment [226].
Furthermore, sodium cromoglycate, a drug obtained from the khellin bioactive extracted from Ammi visnaga (L) Lamk, has been used as a bronchodilator based on its ability of inhibiting mast cell degranulation, which enabled its use on the asthma treatment [226, 230].
Overall, these reports highlight the relevance of the investigation and isolation of new bioactive compounds that could present antiasthmatic potential. As the current asthma treatment involves drugs that have been extensively studied in the past decades, the experimental studies that evaluate the activity of compounds obtained from diverse natural sources might allow the development of new antiasthmatic drugs in the near future.
4. Final Considerations
The current asthma treatment is of high cost and has many side effects, which compromises the patient treatment compliance. Literature reports show that asthma treatment can be improved using natural products to complement the traditional drugs, since those products are of low cost and biocompatible and show reduced side effects. The literature search included the keywords asthma, natural products, and treatment, individually, resulted in 14,296,762 studies, including scientific articles, reviews, editorial reference works, and abstracts. Additionally, the keyword combination “Asthma + Natural Products” found 18,111 studies, “Asthma + Treatment,” 209,423 studies, “Natural Products + Treatment,” 459,685 studies, and “Asthma + Treatment + Natural Products,” 1,986 studies. Thus, after screening for duplicate studies, 1,934 abstracts were evaluated. Finally, based on the inclusion criteria, 172 studies reporting the use of natural products on asthma treatment were included in this review, summarizing a total of 160 studies that reported plants as natural source, 9 from animal source, and 3 studies describing bacteria and fungi as bioactive sources, totalizing 134 compounds which can be used as complementary or alternative medicine on asthma treatment. Plants were found to be the major source of products used by the folk medicine to treat asthma, since they are a renewable source of easy access. Also, due to their variety of secondary metabolites, plants are able to promote antiasthma activity mainly due to their anti-inflammatory and bronchodilator properties. This study revealed that flavonoids, phenolic acids, and terpenoids are the main elucidated compounds able to promote the attenuation of asthma symptoms. On the other hand, a lack of scientific reports regarding the pharmaceutical activity of natural products from animal and microorganism sources has limited their use. However, these products still represent an important source of bioactive compounds able to be used on asthma treatments. In addition, despite the relevant antiasthmatic activity, the literature search showed a lack of investigations concerning the pharmacokinetics properties as well as more accurate information regarding efficacy, safety, and the required dosage to induce in vivo antiasthma activity. In conclusion, due to the fact that current asthma treatment involves drugs obtained from natural products widely explored in the past, the current experimental studies reported in this review may lead to the development of new drugs in the future, able to improve the antiasthmatic treatment.
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brazil (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) for supporting the students.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Lambrecht B. N., Hammad H. The immunology of asthma. Nature Immunology. 2015;16(1):45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 2.Richards L. B., Neerincx A. H., van Bragt J. J. M. H., Sterk P. J., Bel E. H. D., Maitland-van der Zee A. H. Biomarkers and asthma management. Current Opinion in Allergy and Clinical Immunology. 2018;18(2):96–108. doi: 10.1097/aci.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 3.Rang H. P., Dalle M. M., Ritter J. M., Flower R. J., Henderson G. Farmacologia. 7th. Amsterdam, Netherlands: Elsevier; 2004. [Google Scholar]
- 4.Luster A. D., Tager A. M. T-cell trafficking in asthma: lipid mediators grease the way. Nature Reviews Immunology. 2004;4(9):711–724. doi: 10.1038/nri1438. [DOI] [PubMed] [Google Scholar]
- 5.Bradding P., Walls A. F., Holgate S. T. The role of the mast cell in the pathophysiology of asthma. Journal of Allergy and Clinical Immunology. 2006;117(6):1277–1284. doi: 10.1038/nri1438. [DOI] [PubMed] [Google Scholar]
- 6.Murdoch J. R., Lloyd C. M. Chronic inflammation and asthma. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2010;690(1-2):24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner J. G., Roth R. A. Neutrophil migration during endotoxemia. Journal of Leukocyte Biology. 1999;66(1):10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Singh A. M., Moore P. E., Gern J. E., Lemanske R. F., Hartert T. V. Bronchiolitis to asthma. American Journal of Respiratory and Critical Care Medicine. 2007;175(2):108–119. doi: 10.1164/rccm.200603-435pp. [DOI] [PubMed] [Google Scholar]
- 9.Maestrelli P., Boschetto P., Fabbri L. M., Mapp C. E. Mechanisms of occupational asthma. Journal of Allergy and Clinical Immunology. 2009;123(3):531–542. doi: 10.1016/j.jaci.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. Borås, Sweden: GINA; 2019. http://www.ginasthma.org. [Google Scholar]
- 11.Nunes C., Pereira A. M., Morais-Almeida M. Asthma costs and social impact. Asthma Research and Practice. 2017;3(1):p. 1. doi: 10.1186/s40733-016-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Global Health Observatory (GHO) Data 2017. Geneva, Switzerland: WHO; 2017. http://www.who.int/gho/en/ [Google Scholar]
- 13.Mohammed S., Goodacre S. Intravenous and nebulised magnesium sulphate for acute asthma: systematic review and meta-analysis. Emergency Medicine Journal. 2007;24(12):823–830. doi: 10.1136/emj.2007.052050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministério da Saúde. Morbidade Hospitalar do SUS—por Local de Internação—Brasil. Rio de Janeiro, Brazil: Ministério da Saúde—DataSUS; 2012. http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/niuf.def. [Google Scholar]
- 15. Asthma Surveillance Data. 2015.
- 16.Strachan D. P., Sihbald B., Weiland S. K., et al. Worldwide variations in the prevalence of asthma symptoms: the international study of asthma and allergies in childhood (ISAAC) European Respiratory Journal. 1998;12(2):315–335. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- 17.Asher M. I., Stewart A. W., Mallol J., et al. Which population level environmental factors are associated with asthma, rhinoconjunctivitis and eczema? Review of the ecological analyses of ISAAC Phase One. Respiratory Research. 2011;11(1):p. 8. doi: 10.1186/1465-9921-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai C. K. W., Beasley R., Crane J., et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the international study of asthma and allergies in childhood (ISAAC) Thorax. 2009;64(6):476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 19.Pearce N., Ait-Khaled N., Beasley R., et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the international study of asthma and allergies in childhood (ISAAC) Thorax. 2007;62(9):758–766. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaneberg B. T., Crockett S., Bedir E., Khan I. A. The role of chemical fingerprinting: application to Ephedra. Phytochemistry. 2003;62(6):911–918. doi: 10.1016/s0031-9422(02)00716-1. [DOI] [PubMed] [Google Scholar]
- 21.Chatkin J. M., Cavalet-Blanco D., Scaglia N. C., Tonietto R. G., Wagner M. B., Fritscher C. C. Adesão ao tratamento de manutenção em asma (estudo ADERE) Jornal Brasileiro de Pneumologia. 2006;32(4):277–283. doi: 10.1590/s1806-37132006000400004. [DOI] [PubMed] [Google Scholar]
- 22.Arnlind M. H., Wettermark B., Nokela M., Hjemdahl P., Rehnberg C., Jonsson E. W. Regional variation and adherence to guidelines for drug treatment of asthma. European Journal of Clinical Pharmacology. 2010;66(2):187–198. doi: 10.1007/s00228-009-0731-7. [DOI] [PubMed] [Google Scholar]
- 23.Drotar D., Bonner M. S. Influences on adherence to pediatric asthma treatment: a review of correlates and predictors. Journal of Developmental & Behavioral Pediatrics. 2009;30(6):574–582. doi: 10.1097/dbp.0b013e3181c3c3bb. [DOI] [PubMed] [Google Scholar]
- 24.Ponte E., Franco R. A., Souza-Machado A., Souza-Machado C., Cruz Á. A. Impacto de um programa para o controle da asma grave na utilização de recursos do Sistema Único de Saúde. Jornal Brasileiro de Pneumologia. 2007;33(1):15–19. doi: 10.1590/s1806-37132007000100006. [DOI] [PubMed] [Google Scholar]
- 25.Holanda M. A. Asmáticos brasileiros: o tratamento desejado. Jornal Brasileiro de Pneumologia. 2000;26(3):7–9. doi: 10.1590/s0102-35862000000300001. [DOI] [Google Scholar]
- 26.Kaufman G. Asthma: pathophysiology, diagnosis and management. Nursing Standard. 2011;26(5):48–57. doi: 10.7748/ns2011.10.26.5.48.c8744. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen S. E., Hurd S. S., Lemanske R. F., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatric Pulmonology. 2011;46(1):1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 28.Marc G., Anuradha R., Sally E. W. Evolving concepts of asthma. American Journal of Respiratory and Critical Care Medicine. 2015;192(6):660–668. doi: 10.1164/rccm.201504-0763PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolovich M., Ruffin R., Corr D., Newhouse M. T. Clinical evaluation of a simple demand inhalation MDI aerosol delivery device. Chest. 1983;84(1):36–41. doi: 10.1378/chest.84.1.36. [DOI] [PubMed] [Google Scholar]
- 30.Adams B. K., Cydulka R. K. Asthma evaluation and management. Emergency Medicine Clinics of North America. 2003;21(2):315–330. doi: 10.1016/s0733-8627(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 31.Stirbulov R., Bernd L. A. G., Sole D. IV diretizes brasileiras para o manejo da Asma. Jornal Brasileiro de Pneumologia. 2006;32:447–474. [PubMed] [Google Scholar]
- 32.Chen Y. Z., Busse W. W., Pedersen S., et al. Early intervention of recent onset mild persistent asthma in children aged under 11 yrs: the steroid treatment as regular therapy in early asthma (START) trial. Pediatric Allergy and Immunology. 2006;17(17):7–13. doi: 10.1111/j.1600-5562.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 33.Lal D., Manocha S., Ray A., Vijayan V., Kumar R. Comparative study of the efficacy and safety of theophylline and doxofylline in patients with bronchial asthma and chronic obstructive pulmonary disease. Journal of Basic and Clinical Physiology and Pharmacology. 2015;26(5):443–451. doi: 10.1515/jbcpp-2015-0006. [DOI] [PubMed] [Google Scholar]
- 34.Banner K. H., Trevethick M. A. PDE4 inhibition: a novel approach for the treatment of inflammatory bowel disease. Trends in Pharmacological Sciences. 2004;25(8):430–436. doi: 10.1016/j.tips.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Barnes P. J. Drugs for asthma. British Journal of Pharmacology. 2006;147(1):297–303. doi: 10.1038/sj.bjp.0706437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacology & Therapeutics. 2006;109(3):366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.DiMartino S. J. Idiopathic inflammatory myopathy: treatment options. Current Rheumatology Reports. 2008;10(4):321–327. doi: 10.1007/s11926-008-0051-4. [DOI] [PubMed] [Google Scholar]
- 38.Kesler S. M., Sprenkle M. D., David W. S., Leatherman J. W. Severe weakness complicating status asthmaticus despite minimal duration of neuromuscular paralysis. Intensive Care Medicine. 2009;35(1):157–160. doi: 10.1007/s00134-008-1267-5. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan S. D., Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008;63(6):670–684. doi: 10.1111/j.1398-9995.2008.01723.x. [DOI] [PubMed] [Google Scholar]
- 40.O’Neill S., Sweeney J., Patterson C. C., et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British thoracic society difficult asthma registry. Thorax. 2015;70(4):376–378. doi: 10.1136/thoraxjnl-2013-204114. [DOI] [PubMed] [Google Scholar]
- 41.Lin C. H., Shen M. L., Zhou N., et al. Protective effects of the polyphenol sesamin on allergen-induced TH2 responses and airway inflammation in mice. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0096091.e96091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bielory L. Complementary and alternative interventions in asthma, allergy, and immunology. Annals of Allergy, Asthma & Immunology. 2004;93(2):45–54. doi: 10.1016/s1081-1206(10)61486-x. [DOI] [PubMed] [Google Scholar]
- 43.Biavatti M. W., Marensi V., Leite S. N., Reis A. Ethnopharmacognostic survey on botanical compendia for potential cosmeceutic species from Atlantic Forest. Revista Brasileira de Farmacognosia. 2007;17(1):640–653. doi: 10.1590/s0102-695x2007000400025. [DOI] [Google Scholar]
- 44.Huntley A., Ernst E. Herbal medicines for asthma: a systematic review. Thorax. 2000;55(11):925–929. doi: 10.1136/thorax.55.11.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agra M. D. F., Freitas P. F. D., Barbosa-Filho J. M. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Revista Brasileira de Farmacognosia. 2007;17(1):114–140. doi: 10.1590/s0102-695x2007000100021. [DOI] [Google Scholar]
- 46.Albuquerque U. P. D., Hanazaki N. As pesquisas etnodirigidas na descoberta de novos fármacos de interesse médico e farmacêutico: fragilidades e pespectivas. Revista Brasileira de Farmacognosia. 2006;16(1):678–689. doi: 10.1590/s0102-695x2006000500015. [DOI] [Google Scholar]
- 47.Yauan G., Wahlqvist M., He G., Yang M., Li D. Natural products and anti-inflammatory activity. Asia Pacific Journal of Clinical Nutrition. 2006;15(2):143–52. [PubMed] [Google Scholar]
- 48.Lam K. S. New aspects of natural products in drug discovery. Trends in Microbiology. 2007;15(6):279–289. doi: 10.1016/j.tim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Costa R. D. S., Brasil T. C., Santos C. D. J., et al. Produtos naturais utilizados para tratamento de asma em crianças residentes na cidade de Salvador-BA, Brasil. Revista Brasileira de Farmacognosia. 2010;20(4):594–599. doi: 10.1590/s0102-695x2010000400020. [DOI] [Google Scholar]
- 50.Ventola C. L. Current issues regarding complementary and alternative medicine (CAM) in the United States: part 1: the widespread use of CAM and the need for better-informed health care professionals to provide patient counseling. Pharmacology & Therapeutics. 2010;35(8):461–468. [PMC free article] [PubMed] [Google Scholar]
- 51.Mainardi T., Kapoor S., Bielory L. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. Journal of Allergy and Clinical Immunology. 2009;123(2):283–294. doi: 10.1016/j.jaci.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Ammon H. P. T. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine. 2010;17(11):862–867. doi: 10.1016/j.phymed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Silva L. P., Miyasaka C. K., Martins E. F., et al. Effect of bullfrog (Rana catesbeiana) oil administered by gavage on the fatty acid composition and oxidative stress of mouse liver. Brazilian Journal of Medical and Biological Research. 2004;37(10):1491–1496. doi: 10.1590/s0100-879x2004001000007. [DOI] [PubMed] [Google Scholar]
- 54.Leandro L. M., de Sousa Vargas F., Barbosa P. C. S., Neves J. K. O., da Silva J. A., da Veiga-Junior V. F. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules. 2012;17(4):3866–3889. doi: 10.3390/molecules17043866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos A. O. d., Ueda-Nakamura T., Dias Filho B. P., Veiga Junior V. F., Pinto A. C., Nakamura C. V. Antimicrobial activity of Brazilian copaiba oils obtained from different species of the Copaifera genus. Memórias Do Instituto Oswaldo Cruz. 2008;103(3):277–281. doi: 10.1590/s0074-02762008005000015. [DOI] [PubMed] [Google Scholar]
- 56.Veiga V. F., Rosas E. C., Carvalho M. V., Henriques M. G. M. O., Pinto A. C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne-a comparative study. Journal of Ethnopharmacology. 2007;112(2):248–254. doi: 10.1016/j.jep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Yaqoob P., Knapper J. M. E., Webb D. H., Williams C. M., Newsholme E. A., Calder P. C. The effect of chronic consumption of monounsaturated fat on immune function in middle-aged men. Biochemical Society Transactions. 1997;25(2):p. 350S. doi: 10.1042/bst025350s. [DOI] [PubMed] [Google Scholar]
- 58.Yaqoob P. Monounsaturated fats and immune function. Proceedings of the Nutrition Society. 1998;57(4):511–520. doi: 10.1079/pns19980075. [DOI] [PubMed] [Google Scholar]
- 59.Ahn K. S., Noh E. J., Zhao H. L., Jung S. H., Kang S. S., Kim Y. S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7 cells. Life Sciences. 2005;76(20):2315–2328. doi: 10.1016/j.lfs.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 60.Leung C. Y., Liu L., Wong R. N. S., Zeng Y. Y., Li M., Zhou H. Saikosaponin-d inhibits T cell activation through the modulation of PKCθ, JNK, and NF-κB transcription factor. Biochemical and Biophysical Research Communications. 2005;338(4):1920–1927. doi: 10.1016/j.bbrc.2005.10.175. [DOI] [PubMed] [Google Scholar]
- 61.Vasconcelos J. F., Teixeira M. M., Barbosa-Filho J. M., et al. The triterpenoid lupeol attenuates allergic airway inflammation in a murine model. International Immunopharmacology. 2008;8(9):1216–1221. doi: 10.1016/j.intimp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Greiner J. F.-W., Müller J., Zeuner M.-T., et al. 1,8-cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2013;1833(12):2866–2878. doi: 10.1016/j.bbamcr.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Ryu H. W., Lee S. U., Lee S., et al. 3-methoxy-catalposide inhibits inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Cytokine. 2017;91(1):57–64. doi: 10.1016/j.cyto.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Dey A. Achyranthes aspera L: phytochemical and pharmacological aspects. International Journal of Life Sciences Biotechnology and Pharma Research. 2011;9(2):72–82. [Google Scholar]
- 65.Kumar D., Bhujbal S., Deoda R., Mudgade S. Bronchodilator activity of aqueous extract of stem bark of ailanthus excelsaroxb. Pharmacognosy Research. 2010;2(2):102–106. doi: 10.4103/0974-8490.62955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oliveira T. T., Campos K. M., Cerqueira-Lima A. T., et al. Potential therapeutic effect of Allium cepa L. and quercetin in a murine model of Blomia tropicalis induced asthma. DARU Journal of Pharmaceutical Sciences. 2015;23(1):p. 18. doi: 10.1186/s40199-015-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Y.-L., Cao J., Shang J.-H., et al. Airways antiallergic effect and pharmacokinetics of alkaloids from Alstonia scholaris. Phytomedicine. 2017;27(1):63–72. doi: 10.1016/j.phymed.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Chua M., Baldwin T. C., Hocking T. J., Chan K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. Journal of Ethnopharmacology. 2010;128(2):268–278. doi: 10.1016/j.jep.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 69.Shah A. J., Gilani A. H. The calcium channel blocking and phosphodiesterase inhibitory activities of the extract of Andropogon muricatus explains its medicinal use in airways disorders. Phytotherapy Research. 2012;26(8):1256–1258. doi: 10.1002/ptr.3687. [DOI] [PubMed] [Google Scholar]
- 70.Hsieh C. C., Hsiao H. B., Lin W. C. A standardized aqueous extract of Anoectochilus formosanus modulated airway hyperresponsiveness in an OVA-inhaled murine model. Phytomedicine. 2010;17(8-9):557–562. doi: 10.1016/j.phymed.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Shah A. J., Gilani A.-H., Abbas K., Rasheed M., Ahmed A., Ahmad V. U. Studies on the chemical composition and possible mechanisms underlying the antispasmodic and bronchodilatory activities of the essential oil of Artemisia maritima L. Archives of Pharmacal Research. 2011;34(8):1227–1238. doi: 10.1007/s12272-011-0801-0. [DOI] [PubMed] [Google Scholar]
- 72.Chen L. S., Zheng D. S. Bioactive constituents from the rhizomes of aster tataricus L. F. Afford the treatment of asthma through activation of beta(2)AR and inhibition of NF-kappa b. Latin American Journal of Pharmacy. 2015;34(2):291–295. [Google Scholar]
- 73.Kang H.-J., Jeong J.-S., Park N.-J., et al. An ethanol extract of Aster yomena (Kitam.) Honda inhibits lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 macrophages. BioScience Trends. 2017;11(1):85–94. doi: 10.5582/bst.2016.01217. [DOI] [PubMed] [Google Scholar]
- 74.Park K., Lee J. S., Choi J. S., et al. Identification and characterization of baicalin as a phosphodiesterase 4 inhibitor. Phytotherapy Research. 2016;30(1):144–151. doi: 10.1002/ptr.5515. [DOI] [PubMed] [Google Scholar]
- 75.Venkatesh P., Mukherjee P. K., Mukherjee D., Bandyopadhyay A., Fukui H., Mizuguchi H. Potential of Baliospermum montanum against compound 48/80-induced systemic anaphylaxis. Pharmaceutical Biology. 2010;48(11):1213–1217. doi: 10.3109/13880201003677827. [DOI] [PubMed] [Google Scholar]
- 76.Power S., Williams M., Semprini A., et al. RCT of the effect of berryfruit polyphenolic cultivar extract in mild steroid-naive asthma: a cross-over, placebo-controlled study. BMJ Open. 2017;7(3) doi: 10.1136/bmjopen-2016-013850.e013850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamidpour R., Hamidpour S., Hamidpour M., Shahlari M. Frankincense (乳香 Rǔ Xiāng; Boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. Journal of Traditional and Complementary Medicine. 2013;3(4):221–226. doi: 10.4103/2225-4110.119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Yasiry A. R. M., Kiczorowska B. Frankincense—therapeutic properties. Postępy Higieny i Medycyny Doświadczalnej. 2016;70:380–391. doi: 10.5604/17322693.1200553. [DOI] [PubMed] [Google Scholar]
- 79.Houssen M. E., Ragab A., Mesbah A., et al. Natural anti-inflammatory products and leukotriene inhibitors as complementary therapy for bronchial asthma. Clinical Biochemistry. 2010;43(10-11):887–890. doi: 10.1016/j.clinbiochem.2010.04.061. [DOI] [PubMed] [Google Scholar]
- 80.Neamati A., Boskabady M. H., Afshari J. T., Hazrati S. M., Rohani A. H. The effect of natural adjuvants on tracheal responsiveness and cell count in lung lavage of sensitized guinea pigs. Respirology. 2009;14(6):877–884. doi: 10.1111/j.1440-1843.2009.01566.x. [DOI] [PubMed] [Google Scholar]
- 81.Amaral-Machado L., Xavier-Júnior F., Rutckeviski R., et al. New trends on antineoplastic therapy research: bullfrog (Rana catesbeiana Shaw) oil nanostructured systems. Molecules. 2016;21(5):p. 585. doi: 10.3390/molecules21050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang S. H., Kao T. I., Chiang B. L., et al. Immune-modulatory effects of bu-zhong-Yi-Qi-tang in ovalbumin-induced murine model of allergic asthma. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0127636.e0127636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Y., Zhang Y., Li C., et al. Immunoregulation effect of crude extract of C. elegans on allergic asthma. International Journal of Clinical and Experimental Medicine. 2014;7(4):886–892. [PMC free article] [PubMed] [Google Scholar]
- 84.Sharangi A. B. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—a review. Food Research International. 2009;42(5-6):529–535. doi: 10.1016/j.foodres.2009.01.007. [DOI] [Google Scholar]
- 85.Inam A., Shahzad M., Shabbir A., Shahid H., Shahid K., Javeed A. Carica papaya ameliorates allergic asthma via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-κB, and iNOS levels. Phytomedicine. 2017;32(1):1–7. doi: 10.1016/j.phymed.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Khan M., Khan A.-U., Najeeb-ur-Rehman R., Gilani A.-H. Gut and airways relaxant effects of Carum roxburghianum. Journal of Ethnopharmacology. 2012;141(3):938–946. doi: 10.1016/j.jep.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 87.Ozdemir C., Yazi D., Aydogan M., et al. Treatment with chitin microparticles is protective against lung histopathology in a murine asthma model. Clinical Experimental Allergy. 2006;36(7):960–968. doi: 10.1111/j.1365-2222.2006.02515.x. [DOI] [PubMed] [Google Scholar]
- 88.Yao J., Jiang M., Zhang Y., et al. Chrysin alleviates allergic inflammation and airway remodeling in a murine model of chronic asthma. International Immunopharmacology. 2016;32(1):24–31. doi: 10.1016/j.intimp.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Yao J., Zhang Y. S., Feng G. Z., Du Q. Chrysin inhibits human airway smooth muscle cells proliferation through the extracellular signal-regulated kinase 1/2 signaling pathway. Molecular Medicine Reports. 2015;12(5):7693–7698. doi: 10.3892/mmr.2015.4401. [DOI] [PubMed] [Google Scholar]
- 90.Bae Y., Lee S., Kim S. H. Chrysin suppresses mast cell-mediated allergic inflammation: involvement of calcium, caspase-1 and nuclear factor-κB. Toxicology and Applied Pharmacology. 2011;254(1):56–64. doi: 10.1016/j.taap.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 91.Cerqueira-Lima A. T., Alcantara-Neves N. M., de Carvalho L. C., et al. Effects of Cissampelos sympodialis Eichl. and its alkaloid, warifteine, in an experimental model of respiratory allergy to Blomia tropicalis. Current Cancer Drug Targets. 2010;11(11):1458–1467. doi: 10.2174/1389450111009011458. [DOI] [PubMed] [Google Scholar]
- 92.Bui T. T., Piao C. H., Kim S. M., et al. Citrus tachibana leaves ethanol extract alleviates airway inflammation by the modulation of Th1/Th2 imbalance via inhibiting NF-κB signaling and histamine secretion in a mouse model of allergic asthma. Journal of Medicinal Food. 2017;20(7):676–684. doi: 10.1089/jmf.2016.3853. [DOI] [PubMed] [Google Scholar]
- 93.Macredmond R., Dorscheid D. R. Conjugated linoleic acid (CLA): is it time to supplement asthma therapy? Pulmonary Pharmacology & Therapeutics. 2011;24(5):540–548. doi: 10.1016/j.pupt.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 94.Sanchez-Recillas A., Navarrete-Vazquez G., Hidalgo-Figueroa S., et al. Semisynthesis, ex vivo evaluation, and SAR studies of coumarin derivatives as potential antiasthmatic drugs. European Journal of Medicinal Chemistry. 2014;77(1):400–408. doi: 10.1016/j.ejmech.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 95.Ding J., Su J., Zhang L., Ma J. Crocetin activates foxp3 through tipe2 in asthma-associated treg cells”. Cellular Physiology and Biochemistry. 2015;37(6):2425–2433. doi: 10.1159/000438595. [DOI] [PubMed] [Google Scholar]
- 96.Zheng M., Zhang Q., Joe Y., et al. Curcumin induces apoptotic cell death of activated human CD4+ T cells via increasing endoplasmic reticulum stress and mitochondrial dysfunction. International Immunopharmacology. 2013;15(3):517–523. doi: 10.1016/j.intimp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 97.Chong L., Zhang W., Nie Y., et al. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation. 2014;37(5):1476–1485. doi: 10.1007/s10753-014-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaschke N., Sommerhoff C. P. Upgrading a natural product: inhibition of human beta-tryptase by cyclotheonamide analogues. Chem Med Chem. 2010;5(3):367–370. doi: 10.1002/cmdc.200900484. [DOI] [PubMed] [Google Scholar]
- 99.Shin I. S., Hong J., Jeon C. M., et al. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food and Chemical Toxicology. 2013;62(1):506–513. doi: 10.1016/j.fct.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 100.Brull F., De Smet E., Mensink R. P., et al. Dietary plant stanol ester consumption improves immune function in asthma patients: results of a randomized, double-blind clinical trial. The American Journal of Clinical Nutrition. 2016;103(2):444–453. doi: 10.3945/ajcn.115.117531. [DOI] [PubMed] [Google Scholar]
- 101.Junchao Y., Zhen W., Yuan W., et al. Anti-trachea inflammatory effects of diosgenin from Dioscorea nipponica through interactions with glucocorticoid receptor α. Journal of International Medical Research. 2017;45(1):101–113. doi: 10.1177/0300060516676724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoskins A., Roberts J. L., Milne G., Choi L., Dworski R. Natural source d-α-tocopheryl acetate inhibits oxidant stress and modulates atopic asthma in humans in vivo. Allergy. 2012;67(5):676–682. doi: 10.1111/j.1398-9995.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosa S. I. G., Rios-Santos F., Balogun S. O., et al. Hydroethanolic extract from Echinodorus scaber rataj leaves inhibits inflammation in ovalbumin-induced allergic asthma. Journal of Ethnopharmacology. 2017;203(1):191–199. doi: 10.1016/j.jep.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 104.Freitas-Morel L. J., Azevedo B. C., Carmona F., et al. A standardized methanol extract of Eclipta prostrata (L.) L.(Asteraceae) reduces bronchial hyperresponsiveness and production of Th2 cytokines in a murine model of asthma. Journal of Ethnopharmacology. 2017;198(1):226–234. doi: 10.1016/j.jep.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Vo T. S., Ngo D. H., Kim S. K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochemistry. 2012;47(3):386–394. doi: 10.1016/j.procbio.2011.12.014. [DOI] [Google Scholar]
- 106.Gul R., Jan S. U., Faridullah S., Sherani S., Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from ephedra intermedia indigenous to balochistan. The Scientific World Journal. 2017;2017:7. doi: 10.1155/2017/5873648.5873648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou E., Fu Y., Wei Z., Yang Z. Inhibition of allergic airway inflammation through the blockage of NF-kappaB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food & Function. 2014;5(9):2106–2112. doi: 10.1039/c4fo00384e. [DOI] [PubMed] [Google Scholar]
- 108.Shrimali D., Shanmugam M. K., Kumar A. P., et al. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Letters. 2013;341(2):139–149. doi: 10.1016/j.canlet.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 109.Kunwar R. M., Shrestha K. P., Bussmann R. W. Traditional herbal medicine in far-west Nepal: a pharmacological appraisal. Journal of Ethnobiology and Ethnomedicine. 2010;6(1):p. 35. doi: 10.1186/1746-4269-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ku C. M., Lin J. Y. Farnesol, a sesquiterpene alcohol in essential oils, ameliorates serum allergic antibody titres and lipid profiles in ovalbumin-challenged mice. Allergol Immunopathol (Madr) 2016;44(2):149–159. doi: 10.1016/j.aller.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 111.Pareek A., Suthar M., Rathore G. S., Bansal V. Feverfew (Tanacetum parthenium L.): a systematic review. Pharmacognosy Reviews. 2011;5(9):103–110. doi: 10.4103/0973-7847.79105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Castell M., Perez-Cano F. J., Abril-Gil M., Franch A. Flavonoids on allergy. Current Pharmaceutical Design. 2014;20(6):973–987. doi: 10.2174/13816128113199990041. [DOI] [PubMed] [Google Scholar]
- 113.Lattig J., Bohl M., Fischer P., et al. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: rationale for lead design. Journal of Computer-Aided Molecular Design. 2007;21(8):473–483. doi: 10.1007/s10822-007-9129-8. [DOI] [PubMed] [Google Scholar]
- 114.Najeeb ur R., Bashir S., Al-Rehaily A. J., Gilani A. H. Mechanisms underlying the antidiarrheal, antispasmodic and bronchodilator activities of Fumaria parviflora and involvement of tissue and species specificity. Journal of Ethnopharmacology. 2012;144(1):128–137. doi: 10.1016/j.jep.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y. N., Zha W. J., Ma Y., et al. Galangin attenuates airway remodelling by inhibiting TGF-β1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Scientific Reports. 2015;5(1):11758–11761. doi: 10.1038/srep11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guerra Dore C. M., Azevedo T. C., de Souza M. C., et al. Antiinflammatory, antioxidant and cytotoxic actions of beta-glucan-rich extract from Geastrum saccatum mushroom. International Immunopharmacology. 2007;7(9):1160–1169. doi: 10.1016/j.intimp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 117.Chen T., Xiao L., Zhu L., et al. Anti-asthmatic effects of ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation. 2015;38(5):1814–1822. doi: 10.1007/s10753-015-0159-4. [DOI] [PubMed] [Google Scholar]
- 118.Mahmoud Y. I. Grape seed extract attenuates lung parenchyma pathology in ovalbumin-induced mouse asthma model: an ultrastructural study. Micron Technology. 2012;43(10):1050–1059. doi: 10.1016/j.micron.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 119.Tiwari P., Mishra B. N., Sangwan N. S. Phytochemical and pharmacological properties of gymnema sylvestre: an important medicinal plant. BioMed Research International. 2014;2014:18. doi: 10.1155/2014/830285.830285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di Fabio G., Romanucci V., Zarrelli M., Giordano M., Zarrelli A. C-4 gem-dimethylated oleanes of Gymnema sylvestre and their pharmacological activities. Molecules. 2013;18(12):14892–14919. doi: 10.3390/molecules181214892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang X., Nian H., Li X., et al. Effects of the combined extracts of herba epimedii and fructus ligustrilucidi on airway remodeling in the asthmatic rats with the treatment of budesonide. BMC Complementary and Alternative Medicine. 2017;17(1):380–392. doi: 10.1186/s12906-017-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang N., Lian Z., Peng X., Li Z., Zhu H. Applications of higenamine in pharmacology and medicine. Journal of Ethnopharmacology. 2017;196(1):242–252. doi: 10.1016/j.jep.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 123.Shin I. S., Ahn K. S., Shin N. R., et al. Homoegonol attenuates the asthmatic responses induced by ovalbumin challenge. Archives of Pharmacal Research. 2014;37(9):1201–1210. doi: 10.1007/s12272-013-0327-8. [DOI] [PubMed] [Google Scholar]
- 124.Zhang J. S., Zou Y. H., Guo Y. Q., et al. Polycyclic polyprenylated acylphloroglucinols: natural phosphodiesterase-4 inhibitors from Hypericum sampsonii. RSC Advances. 2016;6(58):53469–53476. doi: 10.1039/c6ra08805h. [DOI] [Google Scholar]
- 125.Moura C., Batista-Lima F. J., Brito T. S., et al. Inhibitory effects of a standardized extract of Justicia pectoralis in an experimental rat model of airway hyper-responsiveness. Journal of Pharmacy and Pharmacology. 2017;69(6):722–732. doi: 10.1111/jphp.12689. [DOI] [PubMed] [Google Scholar]
- 126.Khan M., Khan A. U., Najeeb ur R., Gilani A. H. Pharmacological explanation for the medicinal use of Juniperus excelsa in hyperactive gastrointestinal and respiratory disorders. Journal of Natural Medicines. 2012;66(2):292–301. doi: 10.1007/s11418-011-0605-z. [DOI] [PubMed] [Google Scholar]
- 127.Chung M. J., Pandey R. P., Choi J. W., et al. Inhibitory effects of kaempferol-3-O-rhamnoside on ovalbumin-induced lung inflammation in a mouse model of allergic asthma. International Immunopharmacology. 2015;25(2):302–310. doi: 10.1016/j.intimp.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 128.Kwon O. K., Ahn K. S., Lee M. Y., et al. Inhibitory effect of kefiran on ovalbumin-induced lung inflammation in a murine model of asthma. Archives of Pharmacal Research. 2008;31(12):1590–1596. doi: 10.1007/s12272-001-2156-4. [DOI] [PubMed] [Google Scholar]
- 129.Lee M. Y., Ahn K. S., Kwon O. K., et al. Anti-inflammatory and anti-allergic effects of kefir in a mouse asthma model. Immunobiology. 2007;212(8):647–654. doi: 10.1016/j.imbio.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 130.Lee T., Lee S., Ho Kim K., et al. Effects of magnolialide isolated from the leaves of Laurus nobilis L. (Lauraceae) on immunoglobulin E-mediated type I hypersensitivity in vitro. Journal of Ethnopharmacolog. 2013;149(2):550–556. doi: 10.1016/j.jep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 131.Rehman N., Khan A., Alkharfy K. M., Gilani A. H. Pharmacological basis for the medicinal use of Lepidium sativum in airways disorders. Evidence-Based Complementary and Alternative Medicine. 2012;2012(1):8. doi: 10.1155/2012/596524.596524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hwang Y. P., Jin S. W., Choi J. H., et al. Inhibitory effects of l-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food and Chemical Toxicology. 2017;99(1):162–169. doi: 10.1016/j.fct.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 133.Shen M. L., Wang C. H., Lin C. H., Zhou N., Kao S.-T., Wu D. C. Luteolin attenuates airway mucus overproduction via inhibition of the GABAergic system. Scientific Reports. 2016;6(1):32756–68. doi: 10.1038/srep32756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu Y., Li Y., Xu L., Xia M., Cao L. Bacterial lysate increases the percentage of natural killer T cells in peripheral blood and alleviates asthma in children. Pharmacology. 2015;95(3-4):139–144. doi: 10.1159/000377683. [DOI] [PubMed] [Google Scholar]
- 135.Rivera D. G., Balmaseda I. H., Leon A. A., et al. Anti-allergic properties of Mangifera indica L. extract (vimang) and contribution of its glucosylxanthone mangiferin. Journal of Pharmacy and Pharmacology. 2006;58(3):385–392. doi: 10.1211/jpp.58.3.0014. [DOI] [PubMed] [Google Scholar]
- 136.Alvarez A., Sanchez C., Garcia D., Rodríguez J., Lemus Y. Treatment of bronchial asthma with an aqueous extract of Mangifera indica L. (vimang®): two cases report. Blacpma. 2009;8(2):63–66. [Google Scholar]
- 137.Jang H. Y., Kwon O. K., Oh S. R., et al. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food and Chemical Toxicology. 2012;50(11):4042–4050. doi: 10.1016/j.fct.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 138.D’Orazio N., Gammone M. A., Gemello E., et al. Marine bioactives: pharmacological properties and potential applications against inflammatory diseases. Marine Drugs. 2012;10(4):812–833. doi: 10.3390/md10040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jabbari Azad F., Kiaee F., Rezaei A., et al. Downregulation of immune responses in asthmatic humans by ES products of Marshallagia marshalli. The Clinical Respiratory Journal. 2017;11(1):83–89. doi: 10.1111/crj.12309. [DOI] [PubMed] [Google Scholar]
- 140.Napimoga M. H., Yatsuda R. Scientific evidence for mikania laevigata and Mikania glomerata as a pharmacological tool. Journal of Pharmacy and Pharmacology. 2010;62(7):809–820. doi: 10.1211/jpp.62.07.0001. [DOI] [PubMed] [Google Scholar]
- 141.Kanwar J. R., Kanwar R. K., Sun X., et al. Molecular and biotechnological advances in milk proteins in relation to human health. Current Protein & Peptide Science. 2009;10(4):308–338. doi: 10.2174/138920309788922234. [DOI] [PubMed] [Google Scholar]
- 142.Cassia S. S. R., Andrade L. N., Sousa D. P. A review on anti-inflammatory activity of monoterpenes. Molecules. 2013;18(1):1227–1254. doi: 10.3390/molecules18011227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Almeida D. A. T., Rosa S. I. G., Cruz T. C. D., et al. Mandevilla longiflora (Desf.) Pichon improves airway inflammation in a murine model of allergic asthma. Journal of Ethnopharmacology. 2017;200(1):51–59. doi: 10.1016/j.jep.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 144.Chen S. K., Zhao P., Shao Y. X., et al. Moracin M from Morus alba L. is a natural phosphodiesterase-4 inhibitor. Bioorganic & Medicinal Chemistry Letters. 2012;22(9):3261–3264. doi: 10.1016/j.bmcl.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 145.Fuchs S., Hsieh L. T., Saarberg W., et al. Haemanthus coccineus extract and its main bioactive component narciclasine display profound anti-inflammatory activities in vitro and in vivo. Journal of Cellular and Molecular Medicine. 2015;19(5):1021–1032. doi: 10.1111/jcmm.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Y., Lu Y., Luo M., et al. Evaluation of pharmacological relaxation effect of the natural product naringin on in vitro cultured airway smooth muscle cells and in vivo ovalbumin-induced asthma balb/c mice. Biomedical Reports. 2016;5(6):715–722. doi: 10.3892/br.2016.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang X., Xue L., Zhao Q., et al. Nelumbo nucifera leaves extracts inhibit mouse airway smooth muscle contraction. BMC Complementary and Alternative Medicine. 2017;17(1):159–167. doi: 10.1186/s12906-017-1674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salem A. M., Bamosa A. O., Qutub H. O., et al. Effect of Nigella sativa supplementation on lung function and inflammatory mediators in partly controlled asthma: a randomized controlled trial. Annals of Saudi Medicine. 2017;37(1):64–71. doi: 10.5144/0256-4947.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koshak A., Wei L., Koshak E., et al. Nigella sativa supplementation improves asthma control and biomarkers: a randomized, double-blind, placebo-controlled trial. Phytotherapy Research. 2017;31(3):403–409. doi: 10.1002/ptr.5761. [DOI] [PubMed] [Google Scholar]
- 150.Lu Y., Cai S., Nie J., et al. The natural compound nujiangexanthone A suppresses mast cell activation and allergic asthma. Biochemical Pharmacology. 2016;100(1):61–72. doi: 10.1016/j.bcp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 151.Kim S. H., Hong J. H., Lee Y. C. Oleanolic acid suppresses ovalbumin-induced airway inflammation and Th2-mediated allergic asthma by modulating the transcription factors T-bet, GATA-3, RORγt and Foxp3 in asthmatic mice. International Immunopharmacology. 2014;18(2):311–324. doi: 10.1016/j.intimp.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 152.Hansen S., Strom M., Maslova E., et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. Journal of Allergy and Clinical Immunology. 2017;139(1):104–111. doi: 10.1016/j.jaci.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 153.Farjadian S., Moghtaderi M., Kalani M., Gholami T., Hosseini Teshnizi S. Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine. 2016;85:61–66. doi: 10.1016/j.cyto.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 154.Ardestani S. B., Sahari M. A., Barzegar M. Effect of extraction and processing conditions on organic acids of barberry fruits. Journal of Food Biochemistry. 2015;39(5):554–565. doi: 10.1111/jfbc.12158. [DOI] [Google Scholar]
- 155.Shaik F. B., Panati K., Narasimha V. R., Narala V. R. Chenodeoxycholic acid attenuates ovalbumin-induced airway inflammation in murine model of asthma by inhibiting the T(H)2 cytokines. Biochemical and Biophysical Research Communications. 2015;463(4):600–605. doi: 10.1016/j.bbrc.2015.05.104. [DOI] [PubMed] [Google Scholar]
- 156.Lu L., Guo Q., Zhao L. Overview of oroxylin A: a promising flavonoid compound. Phytotherapy Research. 2016;30(11):1765–1774. doi: 10.1002/ptr.5694. [DOI] [PubMed] [Google Scholar]
- 157.Zhou D. G., Diao B. Z., Zhou W., Feng J. L. Oroxylin A inhibits allergic airway inflammation in ovalbumin (OVA)-Induced asthma murine model. Inflammation. 2016;39(2):867–872. doi: 10.1007/s10753-016-0317-3. [DOI] [PubMed] [Google Scholar]
- 158.Zhang T. Z., Fu Q., Chen T., Ma S. P. Anti-asthmatic effects of oxymatrine in a mouse model of allergic asthma through regulating CD40 signaling. Chinese Journal of Natural Medicines. 2015;13(5):368–374. doi: 10.1016/s1875-5364(15)30028-5. [DOI] [PubMed] [Google Scholar]
- 159.Rana S., Shahzad M., Shabbir A. Pistacia integerrima ameliorates airway inflammation by attenuation of TNF-alpha, IL-4, and IL-5 expression levels, and pulmonary edema by elevation of AQP1 and AQP5 expression levels in mouse model of ovalbumin-induced allergic asthma. Phytomedicine. 2016;23(8):838–845. doi: 10.1016/j.phymed.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 160.Bibi Y., Qayyum A., Nisa S., Waheed A., Chaudhary M. F. Isolation studies from stem extract of Pistacia integerrima stew. ex brand. Journal of the Chilean Chemical Society. 2016;61(2):2916–2920. doi: 10.4067/s0717-97072016000200014. [DOI] [Google Scholar]
- 161.Zargar B. l. A., Masoodi M. H., Khan B. A., Akbar S. Paeonia emodi royle: ethnomedicinal uses, phytochemistry and pharmacology. Phytochem Letters. 2013;6(2):261–266. doi: 10.1016/j.phytol.2013.03.003. [DOI] [Google Scholar]
- 162.Choi Y. W., Lee K. P., Kim J. M., et al. Petatewalide B, a novel compound from Petasites japonicus with anti-allergic activity. Journal of Ethnopharmacology. 2016;178(1):17–24. doi: 10.1016/j.jep.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 163.Xiong Y. Y., Wu F. H., Wang J. S., Li J., Kong L. Y. Attenuation of airway hyperreactivity and T helper cell type 2 responses by coumarins from Peucedanum praeruptorum Dunn in a murine model of allergic airway inflammation. Journal of Ethnopharmacology. 2012;141(1):314–321. doi: 10.1016/j.jep.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 164.Lee A. R., Chun J. M., Lee A. Y., et al. Reduced allergic lung inflammation by root extracts from two species of Peucedanum through inhibition of Th2 cell activation. Journal of Ethnopharmacology. 2017;196(1):75–83. doi: 10.1016/j.jep.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 165.Erdem S. A., Nabavi S. F., Orhan I. E., et al. Blessings in disguise: a review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU Journal of Pharmaceutical Sciences. 2015;23(1):p. 53. doi: 10.1186/s40199-015-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shipton F. N., Khoo T. J., Hossan M. S., Wiart C. Activity of pericampylus glaucus and periglaucine A in vitro against nasopharangeal carcinoma and anti-inflammatory activity. Journal of Ethnopharmacology. 2017;198(1):91–97. doi: 10.1016/j.jep.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 167.Korinek M., Wagh V. D., Lo I. W., et al. Antiallergic phorbol ester from the seeds of aquilaria malaccensis. International Journal of Molecular Sciences. 2016;17(3):398–411. doi: 10.3390/ijms17030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iqbal M., Verpoorte R., Korthout H. A. A. J., Mustafa N. R. Phytochemicals as a potential source for TNF-α inhibitors. Phytochemistry Reviews. 2013;12(1):65–93. doi: 10.1007/s11101-012-9251-7. [DOI] [Google Scholar]
- 169.Shin N. R., Shin I. S., Jeon C. M., et al. Inhibitory effects of Picrasma quassioides (D.Don) Benn. on airway inflammation in a murine model of allergic asthma. Molecular Medicine Reports. 2014;10(3):1495–1500. doi: 10.3892/mmr.2014.2322. [DOI] [PubMed] [Google Scholar]
- 170.Shin I. S., Shin N. R., Jeon C. M., et al. Inhibitory effects of Pycnogenol(R) (French maritime pine bark extract) on airway inflammation in ovalbumin-induced allergic asthma. Food and Chemical Toxicology. 2013;62:681–686. doi: 10.1016/j.fct.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 171.Wang X., Tian Z., Gao F., et al. Traditional Chinese medicine as an adjunctive therapy to oral montelukast for treating patients with chronic asthma. Medicine. 2017;96(51):p. e9291. doi: 10.1097/md.0000000000009291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chinta G., Syed S. B., Coumar M. S., Periyasamy L. Piperine: a comprehensive review of pre-clinical and clinical investigations. Current Bioactive Compounds. 2015;11(3):156–169. doi: 10.2174/1573407211666150915214425. [DOI] [Google Scholar]
- 173.Prasad S., Tyagi A. K. Historical spice as a future drug: therapeutic potential of piperlongumine. Current Pharmaceutical Design. 2016;22(27):4151–4159. doi: 10.2174/1381612822666160601103027. [DOI] [PubMed] [Google Scholar]
- 174.Bui T. T., Piao C. H., Ong C. H., et al. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cellular Immunology. 2017;322(1):64–73. doi: 10.1016/j.cellimm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 175.Khawas S., Nosáľová G., Majee S. K., et al. In vivo cough suppressive activity of pectic polysaccharide with arabinogalactan type II side chains of Piper nigrum fruits and its synergistic effect with piperine. International Journal of Biological Macromolecules. 2017;99:335–342. doi: 10.1016/j.ijbiomac.2017.02.093. [DOI] [PubMed] [Google Scholar]
- 176.Arumugam G., Swamy M. K., Sinniah U. R. Plectranthus amboinicus (lour.) spreng: botanical, phytochemical, pharmacological and nutritional significance. Molecules. 2016;21(4):p. 369. doi: 10.3390/molecules21040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abdillahi H. S., Stafford G. I., Finnie J. F., Van Staden J. Ethnobotany, phytochemistry and pharmacology of Podocarpus sensu latissimo (s.l.) South African Journal of Botany. 2010;76(1):1–24. doi: 10.1016/j.sajb.2009.09.002. [DOI] [Google Scholar]
- 178.Joskova M., Sadlonova V., Nosalova G., Novakova E., Franova S. Advances in Experimental Medicine and Biology. 1. Vol. 756. Berlin, Germany: Springer; 2013. Polyphenols and their components in experimental allergic asthma; pp. 91–98. [DOI] [PubMed] [Google Scholar]
- 179.Kao H. F., Chang-Chien P. W., Chang W. T., Yeh T. M., Wang J. Y. Propolis inhibits TGF-β1-induced epithelial-mesenchymal transition in human alveolar epithelial cells via PPARγ activation. International Immunopharmacology. 2013;15(3):565–574. doi: 10.1016/j.intimp.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 180.Chen C. H., Hwang T. L., Chen L. C., Chang T.-H., Wei C.-S., Chen J.-J. Isoflavones and anti-inflammatory constituents from the fruits of Psoralea corylifolia. Phytochemistry. 2017;143(1):186–193. doi: 10.1016/j.phytochem.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 181.Townsend E. A., Emala C. W. Quercetin acutely relaxes airway smooth muscle and potentiates beta-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2013;305(5) doi: 10.1152/ajplung.00125.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mlcek J., Jurikova T., Skrovankova S., Sochor J. Quercetin and its anti-allergic immune response. Molecules. 2016;21(5):623–638. doi: 10.3390/molecules21050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen Y., Zhang Y., Xu M., et al. Catalpol alleviates ovalbumin-induced asthma in mice: reduced eosinophil infiltration in the lung. International Immunopharmacology. 2017;43(1):140–146. doi: 10.1016/j.intimp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 184.Lee M., Kim S., Kwon O. K., Oh S.-R., Lee H.-K., Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. International Immunopharmacology. 2009;9(4):418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 185.Hu X., Wang J., Xia Y., et al. Resveratrol induces cell cycle arrest and apoptosis in human eosinophils from asthmatic individuals. Molecular Medicine Reports. 2016;14(6):5231–5236. doi: 10.3892/mmr.2016.5884. [DOI] [PubMed] [Google Scholar]
- 186.Chen G., Tang J., Ni Z., et al. Antiasthmatic effects of resveratrol in ovalbumin-induced asthma model mice involved in the upregulation of PTEN. Biological & Pharmaceutical Bulletin. 2015;38(4):507–513. doi: 10.1248/bpb.b14-00610. [DOI] [PubMed] [Google Scholar]
- 187.Lee K. P., Kang S., Park S. J., et al. Anti-allergic effect of alpha-cubebenoate isolated from Schisandra chinensis using in vivo and in vitro experiments. Journal of Ethnopharmacology. 2015;173(1):361–369. doi: 10.1016/j.jep.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 188.Guo Y., Ding Y., Xu F., et al. Systems pharmacology-based drug discovery for marine resources: an example using sea cucumber (Holothurians) Journal of Ethnopharmacology. 2015;165(1):61–72. doi: 10.1016/j.jep.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 189.Yu B., Cai W., Zhang H. H., et al. Selaginella uncinata flavonoids ameliorated ovalbumin-induced airway inflammation in a rat model of asthma. Journal of Ethnopharmacology. 2017;195(1):71–80. doi: 10.1016/j.jep.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 190.Liu X., Luo H. B., Huang Y. Y., et al. Selaginpulvilins A–D, new phosphodiesterase-4 inhibitors with an unprecedented skeleton from Selaginella pulvinata. Organic Letters. 2014;16(1):282–285. doi: 10.1021/ol403282f. [DOI] [PubMed] [Google Scholar]
- 191.Todorova M., Trendafilova A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: traditional uses, cultivation, chemical composition, biological activity. Journal of Ethnopharmacology. 2014;152(2):256–265. doi: 10.1016/j.jep.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 192.Jeon C. M., Shin I. S., Shin N. R., et al. Siegesbeckia glabrescens attenuates allergic airway inflammation in LPS-stimulated RAW 264.7 cells and OVA induced asthma murine model. International Immunopharmacology. 2014;22(2):414–419. doi: 10.1016/j.intimp.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 193.Brüll F., Mensink R. P., Steinbusch M. F., et al. Beneficial effects of sitostanol on the attenuated immune function in asthma patients: results of an in vitro approach. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046895.e46895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheng Z. B., Deng Y. L., Fan C. Q., et al. Prostaglandin derivatives: nonaromatic phosphodiesterase-4 inhibitors from the soft coral Sarcophyton ehrenbergi. Journal of Natural Products. 2014;77(8):1928–1936. doi: 10.1021/np500394d. [DOI] [PubMed] [Google Scholar]
- 195.Rios R., da Silva H. B. F., Carneiro N. V. Q., et al. Solanum paniculatum L. decreases levels of inflammatory cytokines by reducing NF-κB, TBET and GATA3 gene expression in vitro. Journal of Ethnopharmacology. 2017;209(1):32–40. doi: 10.1016/j.jep.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 196.Nejatbakhsh F., Karegar-Borzi H., Amin G., et al. Squill oxymel, a traditional formulation from Drimia maritima (L.) Stearn, as an add-on treatment in patients with moderate to severe persistent asthma: a pilot, triple-blind, randomized clinical trial. Journal of Ethnopharmacology. 2017;196(1):186–192. doi: 10.1016/j.jep.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 197.Bhatt L. R., Bae M. S., Kim B. M., Oh G. S., Chai K. Y. A chalcone glycoside from the fruits of Sorbus commixta Hedl. Molecules. 2009;14(12):5323–5327. doi: 10.3390/molecules14125323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shin I. S., Shin N. R., Jeon C. M., et al. Thuja orientalis reduces airway inflammation in ovalbumin-induced allergic asthma. Molecular Medicine Reports. 2015;12(3):4640–4646. doi: 10.3892/mmr.2015.3910. [DOI] [PubMed] [Google Scholar]
- 199.Kim H. I., Hong S. H., Ku J. M., et al. Tonggyu-tang, a traditional Korean medicine, suppresses pro-inflammatory cytokine production through inhibition of MAPK and NF-κB activation in human mast cells and keratinocytes. BMC Complementary and Alternative Medicine. 2017;17(1):178–86. doi: 10.1186/s12906-017-1704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Piao C. H., Bui T. T., Song C. H., et al. Trigonella foenum-graecum alleviates airway inflammation of allergic asthma in ovalbumin-induced mouse model. Biochemical and Biophysical Research Communications. 2017;482(4):1284–1288. doi: 10.1016/j.bbrc.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 201.Santos I. J., Leite G. O., Costa J. G., et al. Topical anti-inflammatory activity of oil from Tropidurus hispidus (spix, 1825) Evidence-Based Complementary and Alternative Medicine. 2015;2015(1) doi: 10.1155/2015/140247.140247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zemmouri H., Sekiou O., Ammar S., et al. Urtica dioica attenuates ovalbumin-induced inflammation and lipid peroxidation of lung tissues in rat asthma model. Pharmaceutical Biology. 2017;55(1):1561–1568. doi: 10.1080/13880209.2017.1310905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee S. U., Sung M. H., Ryu H. W., et al. Verproside inhibits TNF-α-induced MUC5AC expression through suppression of the TNF-α/NF-κB pathway in human airway epithelial cells. Cytokine. 2016;77(1):168–175. doi: 10.1016/j.cyto.2015.08.262. [DOI] [PubMed] [Google Scholar]
- 204.Székely J. I., Pataki Á. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: a short review. Expert Review of Respiratory Medicine. 2012;6(6):683–704. doi: 10.1586/ers.12.57. [DOI] [PubMed] [Google Scholar]
- 205.Cook-Mills J. M., McCary C. A. Isoforms of vitamin E differentially regulate inflammation. Endocrine‚ Metabolic & Immune Disorders-Drug Targets. 2010;10(4):348–366. doi: 10.2174/1871530311006040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Abdala-Valencia H., Berdnikovs S., Cook-Mills J. M. Vitamin E isoforms as modulators of lung inflammation. Nutrients. 2013;5(11):4347–4363. doi: 10.3390/nu5114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lee H., Han A. R., Kim Y., et al. A new compound, 1H,8H-pyrano[3,4-C]pyran-1,8-dione, suppresses airway epithelial cell inflammatory responses in a murine model of asthma. International Journal of Immunopathology and Pharmacology. 2009;22(3):591–603. doi: 10.1177/039463200902200305. [DOI] [PubMed] [Google Scholar]
- 208.Park S., Park M. S., Jung K. H., et al. Treatment with pyranopyran-1, 8-dione attenuates airway responses in cockroach allergen sensitized asthma in mice. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087558.e87558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nikles S., Monschein M., Zou H., et al. Metabolic profiling of the traditional Chinese medicine formulation Yu Ping Feng San for the identification of constituents relevant for effects on expression of Tnf-α, Ifn-γ, Il-1β and Il-4 in U937 cells. Journal of Pharmaceutical and Biomedical Analysis. 2017;145(1):219–229. doi: 10.1016/j.jpba.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 210.Abdallah H. M., Esmat A. Antioxidant and anti-inflammatory activities of the major phenolics from Zygophyllum simplex L. Journal of Ethnopharmacology. 2017;205:51–56. doi: 10.1016/j.jep.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 211.Romero-Castillo P. A., Amador Barron M. C. P., Guevara Fefer P., et al. Anti-inflammatory activity of Ziziphus amole. Phyton (B Aires) 2013;82(1):75–80. [Google Scholar]
- 212.Khan H. Medicinal plants in light of history: recognized therapeutic modality. Journal of Evidence-Based Complementary & Alternative Medicine. 2014;19(3):216–219. doi: 10.1177/2156587214533346. [DOI] [PubMed] [Google Scholar]
- 213.Kumar S., Pandey A. K. Chemistry and biological activities of flavonoids: an overview. The Scientific World Journal. 2013;2013:p. 16. doi: 10.1155/2013/162750.162750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kawai M., Hirano T., Higa S., et al. Flavonoids and related compounds as anti-allergic substances. Allergology International. 2007;56(2):113–123. doi: 10.2332/allergolint.r-06-135. [DOI] [PubMed] [Google Scholar]
- 215.Baylor N. W., Fu T., Yan Y. D., Ruscetti F. W. Inhibition of human T cell leukemia virus by the plant flavonoid baicalin (7-glucuronic acid, 5,6-dihydroxyflavone) Journal of Infectious Diseases. 1992;165(3):433–437. doi: 10.1093/infdis/165.3.433. [DOI] [PubMed] [Google Scholar]
- 216.Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini-Reviews in Medicinal Chemistry. 2009;9(1):31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 217.Zhang H. T., Wu J., Wen M., Su L. J., Luo H. Galangin induces apoptosis in hepatocellular carcinoma cells through the caspase 8/t-Bid mitochondrial pathway. Journal of Asian Natural Products Research. 2012;14(7):626–633. doi: 10.1080/10286020.2012.682152. [DOI] [PubMed] [Google Scholar]
- 218.Mahat M. Y., Kulkarni N. M., Vishwakarma S. L., et al. Modulation of the cyclooxygenase pathway via inhibition of nitric oxide production contributes to the anti-inflammatory activity of kaempferol. European Journal of Pharmacology. 2010;642(1–3):169–176. doi: 10.1016/j.ejphar.2010.05.062. [DOI] [PubMed] [Google Scholar]
- 219.Siddiqui M. Z. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian Journal of Pharmaceutical Sciences. 2011;73(3):255–261. doi: 10.4103/0250-474X.93507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bordbar S., Anwar F., Saari N. High-value components and bioactives from sea cucumbers for functional foods—a review. Marine Drugs. 2011;9(10):1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hao C., Wang W., Wang S. K., Zhang L., Guo Y. An overview of the protective effects of chitosan and acetylated chitosan oligosaccharides against neuronal disorders. Marine Drugs. 2017;15(4):89–104. doi: 10.3390/md15040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Herencia F., Ubeda A., Ferrándiz M. L., et al. Anti-inflammatory activity in mice of extracts from Mediterranean marine invertebrates. Life Sciences. 1998;62(9):115–120. doi: 10.1016/s0024-3205(97)01188-0. [DOI] [PubMed] [Google Scholar]
- 223.Lopes V. S., Dantas T. N. C., Cunha A. F., Moura E. F., Maciel M. A. M. Obtençao de um tensoativo aniônico a partir de óleo de Rana catesbeiana Shaw. Revista Universidade Rural. Série Ciências Exatas e da Terra (UFRRJ) 2010;30(2):85–97. [Google Scholar]
- 224.Rutckeviski R., Xavier-Júnior F. H., Morais A. R., et al. Thermo-oxidative stability evaluation of bullfrog (Rana catesbeiana Shaw) Oil. Molecules. 2017;22(4):p. 606. doi: 10.3390/molecules22040606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Abraham W. R. Bioactive sesquiterpenes produced by fungi: are they useful for humans as well? Current Medical Chemistry. 2001;8(6):583–606. doi: 10.2174/0929867013373147. [DOI] [PubMed] [Google Scholar]
- 226.Cragg G. M., Newman D. J. J. Natural products: a continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA)—General Subjects. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shen B. A new golden age of natural products drug discovery. Cell. 2015;163(6):1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Balunas M. J., Kinghorn A. D. Drug discovery from medicinal plants. Life Sciences. 2005;78(5):431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 229.Barnes P. J. The role of anticholinergics in chronic obstructive pulmonary disease. The American Journal of Medicine Supplements. 2004;117(12):24–32. doi: 10.1016/j.amjmed.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 230.Bizumukama L., Ferster A., Gulbis B., Kumps A., Cotton F. Effects of disodium cromoglycate on cationic exchange of deoxygenated sickle cells. European Journal of Pharmacology. 2011;665(1–3):13–18. doi: 10.1016/j.ejphar.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 231.Cragg G. M., Kingston D. G. I., Newman D. J. Anticancer Agents from Natural Products. Boca Raton, FL, USA: CRC Press; 2011. [Google Scholar]
- 232.Shutt L. E., Bowes J. B. Atropine and hyoscine. Anaesthesia. 1979;34(5):476–490. doi: 10.1111/j.1365-2044.1979.tb06327.x. [DOI] [PubMed] [Google Scholar]
- 233.Schultze-Werninghaus G., Meier-Sydow J. The clinical and pharmacological history of theophylline: first report on the bronchospasmolytic action in man by SR Hirsch in Frankfurt (main) 1922. Clinical Experimental Allergy. 1982;12(2):211–215. doi: 10.1111/j.1365-2222.1982.tb01641.x. [DOI] [PubMed] [Google Scholar]