Abstract
We conducted a feasibility study to investigate the therapeutic effect of bevacizumab on vestibular schwannomas (VS) associated with neurofibromatosis type 2 (NF2) in a sample of Japanese patients. Ten NF2 patients were selected between 2013 and 2018: nine women and one man, with ages ranging from 12 to 45 years (mean: 29.4). Bevacizumab was administered intravenously in 5 mg/kg doses four times, with an inter-dose interval of 2 weeks. Seventeen tumors were followed for 3–72 months (mean: 39). A reduction from baseline tumor volume of at least 20% was considered a therapeutic radiologic response. Maximum reduction in tumor volume was identified in the 3rd month in 11 tumors, and in the 6th month in three tumors. Three tumors did not show any response to bevacizumab. A radiologic response was detected in seven tumors (41%). There was a significantly lower tumor volume mean in the 3rd month in comparison to the baseline for the entire sample. Tumors in patients aged 25 and above showed a significant reduction in volume in the 3rd month and significantly lower tumor-volume-to-baseline ratio than younger patients in both the 3rd and 6th months. The interaction between ‘time’ and ‘age group’ factors significantly affected the therapeutic outcome of bevacizumab on tumor volume. This study investigated the therapeutic effects of bevacizumab on NF2-associated vestibular schwannomas in Japanese patients. Bevacizumab appears to be a useful therapeutic choice in NF2 cases to control the growth of VS. Therefore, a randomised control trial to prove this assumption is necessary.
Keywords: bevacizumab, neurofibromatosis type 2, vestibular schwannoma
Introduction
Neurofibromatosis type 2 (NF2) is an autosomal dominant disease with a hallmark of bilateral vestibular schwannoma (VS). NF2 patients can suffer from multiple central or peripheral nervous system tumors, including schwannomas, meningiomas, and ependymomas. Among these tumors, VS has the greatest impact on the quality of life of NF2 patients. Bilateral lesions cause hearing disturbance and deafness, and later compress the brainstem and induce gait abnormality and ataxia.1)
Vestibular schwannoma is treated by either surgical resection or stereotactic radiosurgery. If the hearing function is still intact, the VS can be observed and managed conservatively. Thus, surgical intervention is usually reserved for large tumors. Total surgical resection is ideal but not easy. Surgical complications such as facial nerve palsy, hearing loss and ataxia are common.1)
Because NF2 patients typically suffer from multiple central nervous system tumors, the long-term outcome of NF2 is poor. Follow-up analysis of registered patients in a nationwide study in Japan between 1986 and 1987 revealed that the overall 10- and 20-year survival rates were 67% and 38%, respectively.2) In a retrospective study of the Japanese national NF2 registry between 2009 and 2013, the risk factors for progressive disability included younger age of onset, family history of NF2, and history of interventional therapy.3)
Bevacizumab is a humanized monoclonal antibody targeting vascular endothelial growth factor (VEGF), a critical angiogenic factor involved in both physiological and pathological conditions.4) A number of studies have previously reported expression of VEGF in a series of NF2-related schwannomas.5) In 2009, Plotkin et al.6) found that bevacizumab can reduce the volume of VS, with improvement in hearing function in some cases. Other articles demonstrated the therapeutic effects of bevacizumab in NF2 cases with VS.7–9) Since many NF2 patients requested to try bevacizumab therapy, we conducted a study to evaluate its efficacy in a sample of Japanese patients.
Patients and Methods
Patient selection
We included in this study NF2 patients who have poor candidacy for surgery and radiation therapy at the time of patients selection, and those who declined these treatments. From 2013 to 2018, 10 NF2 patients with bilateral VS were enrolled in this study in Fukushima Medical University Hospital: nine women and one man, with ages ranging from 12 to 45 years (mean: 29.4). Of the 20 lesions, three had been totally excised. Hence, a total of 17 lesions were included in the study. Among the 17 tumors, seven had previous treatment as follows: three with partial surgical excision and four with radiation therapy. The remaining 10 tumors had not received any treatment.
All study aspects, including treatment methods, anticipated side effects, other treatment choices and medical expenses not covered by health insurance, were explained to the patients or their guardians when informed consent was obtained.
Bevacizumab administration
Four doses of bevacizumab at 5 mg/kg were administered intravenously, with an inter-dose interval of 2 weeks. Since the drug is not covered by medical insurance, we chose four doses to limit patients’ expenses. In one patient, a second trial with similar doses was given in the 18th month, due to the aggressive growth of the tumor. All patients were monitored carefully to detect potential drug side effects according to the Common Terminology Criteria for Adverse Events (CTCAE). Serious drug side effects are confirmed if they are CTCAE grade 3 or above.10)
MRI images and tumor volume measurements
Magnetic resonance imaging (MRI) images were taken before and after starting the drug administration (baseline, 3rd, 6th, 9th, 12th month and every 6 months thereafter if possible). Tumor volume was estimated by the equation (a*b*c)/2. The three measures are: the longest diameter of the cisternal portion of the tumor parallel to the petrous bone (a), and the width of the cisternal portion (b) perpendicular to (a), both using axial MRI images, and the height in a coronal MRI image (c). A reduction in tumor volume of at least 20% was considered a radiologic response. We also calculated the tumor volume change ratios (from the baseline volume).
Statistical analysis
We conducted an independent-samples t-test (or Welch t-test if the assumption of homogeneity was violated) to determine whether there were differences between the means of the tumor volume change ratios in the ‘age group’ categories and the ‘previous treatment group’ categories in each period of follow-up (see below).
Because the data showed skewed distributions, the tumor volume measurements were normalised by logarithmic transformation (log10) to achieve normality and homoscedasticity for the sake of the subsequent analyses. A two-way mixed ANOVA test was run to detect significant changes in tumor volumes between baseline and post-bevacizumab treatment in the whole sample, and whether an interaction effect exists between a within-subjects factor (‘time’ short for time of the observation) and a one of two between-subjects factor on tumor volume. Between-subjects factors were defined as follows: (1) age groups divided into either 25 years and above (‘above 25’) or below 25 years (‘below 25’); and (2) history of previous treatment divided into either present (‘yes’) or not present (‘no’). Post hoc analysis of pairwise comparisons was performed on factors with significant results using the Bonferroni method. If a significant interaction was found, an analysis to detect the simple main effect was conducted using a one-way ANOVA test for each factor. In cases of no interaction, only the main effect result was interpreted.
Descriptive data are expressed as mean ± standard deviation (SD), and results of the repeated-measures ANOVA and Bonferroni post hoc tests and the t-test are expressed as mean ± standard error (SE). Significance in all tests was set at P <0.05. All analyses were done using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and JASP version 0.10.0. This study was reviewed and approved by the Fukushima Medical University Institutional Review Board (#1139).
Results
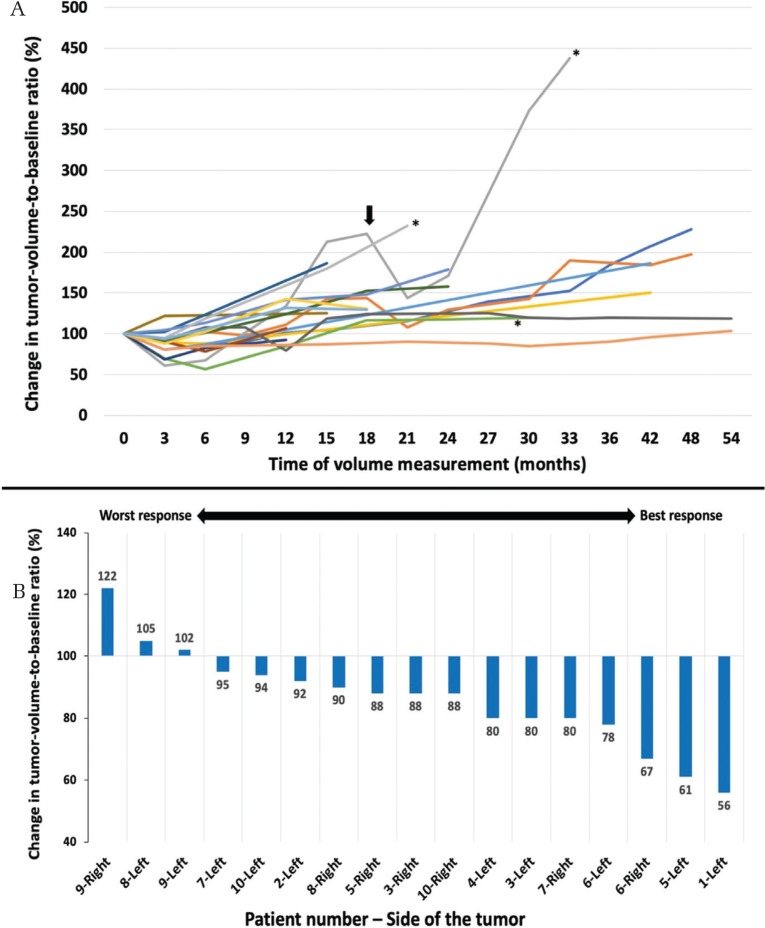
Patient characteristics, tumor volume measurements and change of tumor volume from baseline ratios are shown in Table 1. Before bevacizumab therapy, facial palsy was observed bilaterally in two patients, and useful hearing was detected on one side in four patients; two of them had a normal hearing [pure tone average (PTA) <10 dB] and did not change through the course of this study. The remaining two patients, one had the PTA changed from 20 to 22.5 dB, and the other from 13.8 to 7.5 dB, both in 3 months. All patients received four doses of bevacizumab and were followed for 3–72 months (mean: 39 months) (Fig. 1A). Side effects of CTCAE grade 1 were observed in five patients: delay of menstruation in four; nasal bleeding in two; and bilateral eye redness, delayed wound healing, nausea, headache and diarrhoea each in one patient. No patient developed CTCAE grade 3 or above complications or any degree of hypertension or proteinuria. Neither general condition nor Karnofsky Performance Score changed during bevacizumab therapy in all patients. In three patients, enlarged tumors were removed in the 25th, 30th, and 32nd months of follow-up, respectively (Fig. 1A, asterisks). One patient died after 60 months due to the aggressive growth of intracranial schwannomas and meningiomas.
Table 1.
Patients characteristics and tumor volume measurement
| Patient no. | Age | Sex | Tumor side | Previous treatment | Age group (years) | Baseline volume (cm3) | 3rd month volume (cm3) | 6th month volume (cm3) | 3rd month ratio (%)* | 6th month ratio (%)* | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Time before bevacizumab (months) | ||||||||||
| 1 | 38 | F | Left | No | - | ≥25 | 1.15 | 0.8 | 0.64 | 70 | 56† |
| 2 | 28 | F | Left | SRS | 113 | ≥25 | 1.67 | 1.54 | 1.8 | 92† | 108 |
| 3 | 39 | F | Right | Surgical excision | 127 | ≥25 | 0.92 | 0.82 | 0.81 | 89 | 88† |
| 39 | F | Left | Surgical excision | 161 | ≥25 | 3.17 | 2.53 | 2.75 | 80† | 87 | |
| 4 | 45 | F | Left | No | - | ≥25 | 1.54 | 1.23 | 1.32 | 80† | 86 |
| 5 | 41 | F | Right | Surgical excision | 13 | ≥25 | 0.49 | 0.43 | 0.5 | 88† | 102 |
| 41 | F | Left | No | - | ≥25 | 0.94 | 0.57 | 0.63 | 61† | 67 | |
| 6 | 33 | F | Right | No | - | ≥25 | 0.24 | 0.16 | 0.2 | 67† | 83 |
| 33 | F | Left | SRS | 62 | ≥25 | 1.53 | 1.37 | 1.2 | 90 | 78† | |
| 7 | 18 | F | Right | SRS | 11 | <25 | 4.7 | 3.77 | 4 | 80† | 85 |
| 18 | F | Left | No | - | <25 | 2.75 | 2.6 | 3.28 | 95† | 119 | |
| 8 | 18 | M | Right | No | - | <25 | 0.81 | 0.73 | 0.82 | 90† | 101 |
| 18 | M | Left | No | - | <25 | 2.25 | 2.36 | 2.54 | 105‡ | 113 | |
| 9 | 24 | F | Right | No | - | <25 | 1.08 | 1.32 | NA | 122‡ | NA |
| 24 | F | Left | No | - | <25 | 1.67 | 1.71 | NA | 102‡ | NA | |
| 10 | 12 | F | Right | SRT | 9 | <25 | 16.16 | 14.21 | 16.54 | 88† | 102 |
| 12 | F | Left | No | - | <25 | 9.68 | 9.11 | 10.23 | 94† | 106 | |
Cases with radiologic response (20% and above reduction in volume) are shown in bold.
Ratio of tumor volume from the baseline volume in percentage,
Maximum volume reduction achieved,
Tumors did not show any reduction of volume, SRS: Stereotactic radiosurgery, SRT: Stereotactic radiation therapy.
Fig. 1.
(A) Change of the tumor-volume-to-baseline ratio (%) of 17 tumors after giving bevacizumab. Arrow indicates the second trial of bevacizumab in one patient. Asterisks indicate that three lesions were surgically excised. (B) Maximal change in tumor-volume-to-baseline ratio (%) of 17 tumors in descending order in the first 6 months after giving bevacizumab. The lower the ratio, the better the radiological response, and vice versa.
The maximum reduction in tumor volume was identified in the 3rd month in 11 tumors and in the 6th month in three tumors, while three tumors did not show any reduction in tumor volume (Fig. 1B). Radiologic response with a volume reduction of 20% or more was achieved in seven tumors (41%). The effect of volume reduction faded in and beyond 12 months period (Fig. 1A). When analysing the effects of bevacizumab, we included the measurements in the baseline, 3rd, and 6th month from 15 out of 17 tumors (data of patient no. 9 at 6th month are missing).
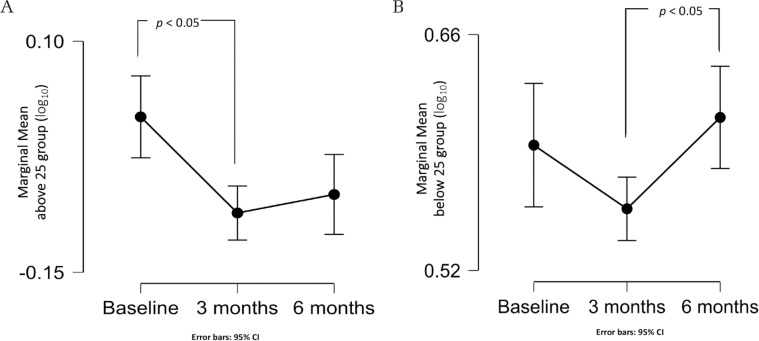
There was a statistically significant main effect of the ‘time’ factor, F(2, 28) = 9.2, P <0.001, partial η2 = 0.40, with tumor volume in the 3rd month of observation significantly smaller than baseline (P = 0.001). A statistically significant interaction between the ‘age group’ and the ‘time’ factor was found, F(2, 26) = 4.85, P = 0.016, partial η2 = 0.27. Accordingly, an analysis of the simple main effect of the factors was conducted. For the ‘above 25’ age group, tumor volume (log10) was significantly reduced in the 3rd month compared to the baseline measurement (P = 0.004) (Fig. 2A). For the ‘below 25’ age group, tumor volume (log10) was statistically significantly increased in the 6th month compared to the 3rd month (P = 0.01) (Fig. 2B). No significant interaction was found between the ‘time’ and ‘previous treatment’ factors (P = 0.68). As no interaction was detected, an analysis of the main effect of the ‘previous treatment’ factor was performed, and it did not show a statistically significant difference in mean tumor volume (log10) between groups (P = 0.45).
Fig. 2.
(A) Line graph showing the marginal mean of the tumor volume (log10) measurement in the ‘above 25’ group, with a significant difference between the baseline and 3rd month values. (B) Line graph showing the marginal mean of the tumor volume (log10) measurement in the ‘below 25’ group, showing a significant difference between the 3rd and the 6th month values.
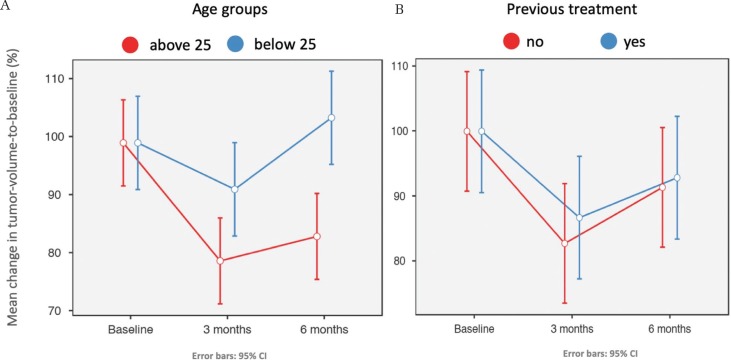
Results of analyses of tumor-volume-to-baseline change ratios are shown in Fig. 3. The means of volume change ratios in the 3rd month for the age groups ‘above 25’ and ‘below 25’ were 79.7 ± 11.3% and 92.0 ± 8.3%, respectively, with a significant mean difference of 12.3 ± 5.4% (P = 0.04). In the 6th month, they were 83.9 ± 16.0% and 104.3 ± 11.7%, respectively, with a significant mean difference of 20.4 ± 7.6% (P = 0.02). The means of volume change ratios in the 3rd month for the ‘previous treatment’ groups ‘no’ and ‘yes’ were 82.8 ± 15.7% and 86.7 ± 4.8%, respectively, with a mean difference of 4.0 ± 5.8 (P = 0.51). In the 6th month, they were 91.4 ± 22.3% and 92.9 ± 11.1%, respectively, with a mean difference of 1.5 ± 9.3 (P = 0.88).
Fig. 3.
(A) Line graph showing the relation between the mean change of tumor-volume-to-baseline ratio (%) in both age groups over 6 months period of observation. The ‘above 25’ group showed a significant favorable response and lower volume ratio in both the 3rd and the 6th months. (B) Line graph describing the relation between the mean change of tumor-volume-to-baseline ratio (%) in both previous treatment groups over 6 months period of observation. There was no significant difference between the “no” and “yes” groups.
An illustrative case
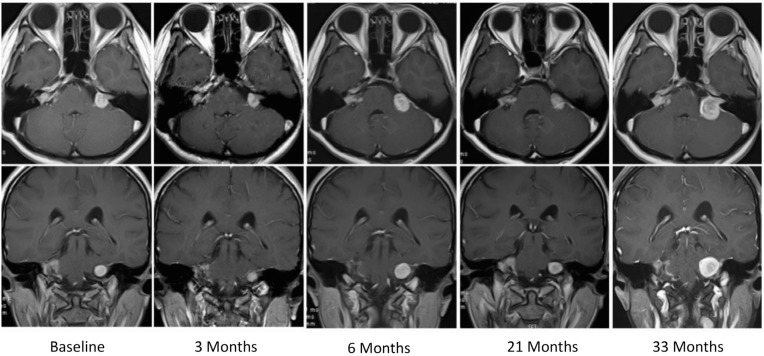
A 41-year-old woman (patient no. 5) was referred to us for a bevacizumab trial after partial removal of her right VS. She had lost her hearing on the right side. After receiving bevacizumab, her left VS shrank to 61% from the baseline volume in the 3rd month and relapsed to 101% in the 9th month (Fig. 4). Left PTA was 20 dB before the trial and 22.5 and 26.3 dB in the 3rd and 15th months, respectively. In the 18th month, she received a second trial of bevacizumab because the tumor had grown to 223% of the baseline volume (Fig. 1A, arrow). It shrank to 144% and 171% in the 21st and 24th months, respectively, and relapsed again to 438% in the 33rd month. Eventually, the tumor was surgically removed. Later, her left hearing was restored through an auditory brainstem implant.
Fig. 4.
Series of MRI scans with contrast enhancement of a 41-year-old woman (case no. 5). After receiving bevacizumab, the volume of her left VS shrank to 61% from baseline volume in the 3rd month, then, it regrown to reach 223% in 18th month. A second trial of bevacizumab was restarted, and it shrank again to reach 144% in the 21st month and again relapsed to reach 438% in the 33rd month.
Discussion
The Food and Drug Administration has approved the VEGF-neutralizing antibody bevacizumab for use in the treatment of cancers, but no randomized clinical trials have been conducted in patients with NF2-associated VS. However, there are a few reports in the literature on the effect of bevacizumab in a limited number of cases. In a retrospective analysis of 31 patients by Plotkin et al., therapeutic radiologic response to bevacizumab, defined as a decrease in tumor volume of 20% or more, occurred in 55% of the sample, and a hearing response, defined as a statistically significant increase in word recognition score, occurred in 57% of the sample. The median time to radiologic response was 3 months. About 88% of patients had stable or decreased tumor size after 1 year and 54% after 3 years.9) There was no correlation between hearing response and radiologic response.9,11) Blakeley et al.11) and Sverak et al.12) also studied the effect of bevacizumab in NF2-associated VS and reported a hearing response in 36% and 56% of cases, respectively, and a radiologic response in 43% and 47% of cases, respectively. Goutagny et al. reviewed the literature and reported that bevacizumab induced hearing improvement and tumor shrinkage in more than 50% of progressive lesions. They pointed out that the need for intravenous injections and long-term adverse effects (hypertension, proteinuria, haemorrhage) were the drawbacks of such clinical studies.13)
Although prolonged treatment with bevacizumab may contribute to long-term control of tumor growth and hearing preservation, it can induce adverse effects. In the study by Plotkin et al.,9) 25 patients received treatment for a median duration of 14 months (range: 6–41), but six patients stopped therapy due to the clinical progression of their tumors. There were 17 drug interruptions in 12 patients due to adverse events or other reasons. Eventually, discontinuation of bevacizumab was associated with an increase in tumor volume.
Slusarz et al. described long-term toxicity in 33 NF2 patients receiving bevacizumab. With a median treatment time of 34.1 months, 58% became hypertensive (systolic blood pressure ≥140 or diastolic ≥90) and 62% developed proteinuria. The median time to develop hypertension was 12.8 months, and the median time to develop 1+ and 2+ proteinuria was 23.7 and 31.9 months, respectively.14) A study from the UK showed that prolonged treatment of NF2 patients with bevacizumab doses of 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks for at least 6 months resulted in either grade 2 or grade 3 hypertension in 24% of patients, and proteinuria in 17.5% of patients.15) A trial of dose escalation to 10 mg/kg for the progressive tumors was unsuccessful.16) Alanin et al. administered a 10 mg/kg dose every 2 weeks for 6 months, followed by a 15 mg/kg dose every 3 weeks, in 12 patients. They observed a radiologic response in 39% of patients and neurological benefit in 42%, but one patient died from cerebral haemorrhage.17) Farschtschi et al. reported that a dose reduction from 5 to 2.5 mg bi- or tri-weekly was an option to avoid side effects.18)
We concluded that a 5 mg/kg dose every 2 weeks was an adequate choice for bevacizumab administration to NF2 patients. Ideal doses and duration of bevacizumab administration have not been determined and remain a matter of debate, so our conclusion was based only on the limited data available. Continued administration for several months, followed by interruption periods or dose reduction and restart before the tumor volume returns to the baseline volume might be an option, although no scientific evidence exists to support this assumption. Due to the limited (four times) doses, no patient in the current study experienced serious side effects such as hypertension or proteinuria.
In the current study, 14 out of 17 tumors showed a drop in tumor volume after a limited number of doses and seven (41%) reached a radiologic response level, a value that is comparable to results in similar published reports. Bevacizumab showed a significant favorable effect on patients aged above 25. The drop in tumor-volume-to-baseline ratios was significantly higher than in the ‘below 25’ group in both the 3rd and 6th months of observation (P = 0.04 and 0.02, respectively). In the ‘above 25’ group, the tumor-volume-to-baseline ratio dropped significantly from the baseline level, reaching a radiologic response level in the third month (mean ± SD = 79.7 ± 11.3%) (Fig. 3A). This finding, supported by mixed ANOVA analysis results, suggests an existing significant ‘age group’ and ‘time’ factors interaction, causing an effect on bevacizumab therapy outcome [F(2, 26) = 4.85, P = 0.02, partial η2 = 0.27] (Fig. 2). In contrast, the ‘below 25’ age group showed a significant relapse and progressive tumor enlargement in the 6th month in comparison with the 3rd month (P = 0.01) (Fig. 2B). The tumor-volume-to-baseline ratio in this group had relapsed and grown even further beyond the baseline level (mean ± SD = 104.3 ± 11.7%, P = 0.02) (Fig. 3A). These findings agree with Morris et al.,16) who found that age was one of the predictors of therapeutic response in 61 patients in the UK: younger patients tended to have faster-growing tumors and weaker responses to bevacizumab. The rationale behind bevacizumab been effective in older patients is not clear to us, and still a matter of research. We found that the ‘previous treatment’ factor did not play a significant role in bevacizumab therapeutic effect, a finding which is similar to other reports.6,8) Predicting factors and biological mechanisms for positive bevacizumab responses need to be evaluated further.
In one patient who was offered a second trial of bevacizumab in the 18th month of observation with a tumor volume ratio of 223% from the baseline (Fig. 1A, arrows), the treatment showed a strong effect, and the tumor declined to 144% from the baseline volume in the 21st month of observation. This observation suggests that repeated or interrupted doses of bevacizumab can also impair tumor progression. Prescribing bevacizumab to NF2 patients is not covered by the Japanese health insurance system. Accordingly, we chose to give only four doses of 5 mg/kg bevacizumab every 2 weeks, so as to limit patients’ expenses.
The current study has some limitations. We could not assess the hearing response since useful hearing was observed before the beginning of bevacizumab therapy on one side of four patients only. Moreover, we did not use volumetric analysis for tumor size measurement due to the heterogeneous thicknesses and low resolution of the MRI scans. Also, we used only four doses of bevacizumab in a limited number of patients, despite witnessing and confirming the positive effects of bevacizumab in NF2 patients.
A major clinical trial that investigate the efficacy of bevacizumab in NF2-associated VS will help to uncover the significance of bevacizumab efficacy in NF2 patients. If such a trial does indeed demonstrate a promising result, then it should be possible to extend insurance cover for this expensive drug in Japan.
Conclusion
The current study was conducted to investigate the effects of bevacizumab in a limited sample of Japanese NF2 patients. It is a feasibility study for a larger clinical trial. By administering four doses of bevacizumab, the study achieved a radiologic response with a reduction of 20% or more from tumor baseline volume in 41% of patients. This finding agrees with most published data. We found a significant interaction between time of observation and age on the effect of bevacizumab in VS. A major clinical trial to examine the efficacy of bevacizumab in NF2 has become a necessity, as this drug has the potential to become the first-line treatment of choice for NF2-related VS.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflict of interest to disclose.
References
- 1).Evans DG: Neurofibromatosis 2 [Bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II]. Genet Med 11: 599–610, 2009 [DOI] [PubMed] [Google Scholar]
- 2).Otsuka G, Saito K, Nagatani T, Yoshida J: Age at symptom onset and long-term survival in patients with neurofibromatosis type 2. J Neurosurg 99: 480–483, 2003 [DOI] [PubMed] [Google Scholar]
- 3).Iwatate K, Yokoo T, Iwatate E, et al. : Population characteristics and progressive disability in neurofibromatosis type 2. World Neurosurg 106: 653–660, 2017 [DOI] [PubMed] [Google Scholar]
- 4).Wang Y, Fei D, Vanderlaan M, Song A: Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 7: 335–345, 2004 [DOI] [PubMed] [Google Scholar]
- 5).Wong HK, Lahdenranta J, Kamoun WS, et al. : Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res 70: 3483–3493, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Plotkin SR, Stemmer-Rachamimov AO, Barker FG, et al. : Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 361: 358–367, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Mautner VF, Nguyen R, Kutta H, et al. : Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol 12: 14–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Eminowicz GK, Raman R, Conibear J, Plowman PN: Bevacizumab treatment for vestibular schwannomas in neurofibromatosis type two: report of two cases, including responses after prior gamma knife and vascular endothelial growth factor inhibition therapy. J Laryngol Otol 126: 79–82, 2012 [DOI] [PubMed] [Google Scholar]
- 9).Plotkin SR, Merker VL, Halpin C, et al. : Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol 33: 1046–1052, 2012 [DOI] [PubMed] [Google Scholar]
- 10).Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017 [PubMed] [Google Scholar]
- 11).Blakeley JO, Ye X, Duda DG, et al. : Efficacy and biomarker study of bevacizumab for hearing loss resulting from neurofibromatosis type 2-associated vestibular schwannomas. J Clin Oncol 34: 1669–1675, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Sverak P, Adams ME, Haines SJ, et al. : Bevacizumab for hearing preservation in neurofibromatosis type 2: emphasis on patient-reported outcomes and toxicities. Otolaryngol Head Neck Surg 160: 526–532, 2019 [DOI] [PubMed] [Google Scholar]
- 13).Goutagny S, Kalamarides M: Medical treatment in neurofibromatosis type 2. Review of the literature and presentation of clinical reports. Neurochirurgie 64: 370–374, 2018 [DOI] [PubMed] [Google Scholar]
- 14).Slusarz KM, Merker VL, Muzikansky A, Francis SA, Plotkin SR: Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol 73: 1197–1204, 2014 [DOI] [PubMed] [Google Scholar]
- 15).Morris KA, Golding JF, Blesing C, et al. : Toxicity profile of bevacizumab in the UK neurofibromatosis type 2 cohort. J Neurooncol 131: 117–124, 2017 [DOI] [PubMed] [Google Scholar]
- 16).Morris KA, Golding JF, Axon PR, et al. : Bevacizumab in neurofibromatosis type 2 (NF2) related vestibular schwannomas: a nationally coordinated approach to delivery and prospective evaluation. Neurooncol Pract 3: 281–289, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Alanin MC, Klausen C, Caye-Thomasen P, et al. : The effect of bevacizumab on vestibular schwannoma tumour size and hearing in patients with neurofibromatosis type 2. Eur Arch Otorhinolaryngol 272: 3627–3633, 2015 [DOI] [PubMed] [Google Scholar]
- 18).Farschtschi S, Kollmann P, Dalchow C, Stein A, Mautner VF: Reduced dosage of bevacizumab in treatment of vestibular schwannomas in patients with neurofibromatosis type 2. Eur Arch Otorhinolaryngol 272: 3857–3860, 2015 [DOI] [PubMed] [Google Scholar]