Abstract
Endosaccular coiling is recognized as a feasible method for treating unruptured intracranial aneurysms (UIAs). We retrospectively reviewed cases of UIAs treated by coiling in the Japanese Registry of Neuroendovascular Therapy (JR-NET) 3, a nationwide survey of NET between 2010 and 2014, the beginning period of intracranial stents in Japan. Data were extracted for 6844 UIAs (6619 procedures) from 40,169 registered records of all NETs in the JR-NET 3 databases. The features of the aneurysms and procedures, immediate radiographic findings, procedure-related complications, and clinical outcomes at 30 days after the procedures were assessed. Of 6844 UIAs, 81.8% were located in the anterior circulation. The mean patient age was 61.3 years (72.4% females). Compared with the preceding JR-NET 1 and 2, there were significant increases (P <0.05) in the rates of the following in JR-NET 3: wide-necked and small UIAs measuring <10 mm (from 56.4% to 58.8%), adjunctive techniques (54.8% to 71.8%), and stent usage (1.1% to 22.1%). Both pre- (85.6% to 96.7%) and post-procedural (84.0% to 94.6%) antiplatelet therapy were more frequently administered in JR-NET 3. Although procedure-related complication rates did not differ between the two groups, ischemic complication rates increased from 4.6% to 5.9%, leading to an increase in the 30-day morbidity (modified Rankin Scale >2) from 2.1% to 2.8%. In conclusion, introduction of neck-bridge stent was associated with an increase in cases of wide-necked aneurysms. However, the ischemic complication rate increased despite the greater use of periprocedural antiplatelet therapy.
Keywords: unruptured intracranial aneurysms, coil embolization, Japanese Registry of Neuroendovascular Therapy
Introduction
The management of asymptomatic, unruptured intracranial aneurysms (UIAs) has been controversial. Several reports regarding the natural history of UIAs showed that factors such as size, location, can be the predictors of their rupture including those among Japanese people who are believed to be at higher risk.1–3)
Nowadays, endosaccular coiling of intracranial aneurysms is prevailing worldwide with its less invasiveness, lower incidence of complications compared with clipping4,5) and long-term durability.6,7) However, the complication rate and the possibility of retreatment due to recanalization have each been reported at 5–10%.6,8–10) Therefore, the assessment of current status of coiling is important for the management of patients with UIAs to compare the benefits and risks of this modality with surgical clipping or conservative management.
The Japanese Registry of Neuroendovascular Therapy (JR-NET) study group endorsed by Japanese Society for Neuroendovascular Therapy (JSNET) have conducted retrospective studies (JR-NET 1&2) to clarify the general status of neuroendovascular therapy delivered by JSNET-certified physicians, and to standardize endovascular procedures and to assist with education planning for Japanese neurointerventionists based on outcomes. This report details the results of JR-NET 3, in which clinical and procedural data were collected retrospectively from January 2010 through December 2014 for all endovascular procedures to further assess and guarantee the quality of NET in Japan. The primary end point was the 30-day clinical outcome [modified Rankin Scale (mRS)] and secondary end points comprised technical success, adverse events arising within 30 days.11)
Here, we collected a considerable amount of clinical data on NET for UIAs through the JR-NET 3 investigations and evaluated the outcomes of NET, especially endosaccular coiling for patients with asymptomatic UIAs in Japan.
Materials and Methods
JR-NET 3 protocols
The JR-NET 3 was conducted between 2010 and 2014 as the third nationwide survey of neuroendovascular treatment in Japan, targeting all endovascular procedures during the study period by JSNET board-certified physicians, who were instructed to register their endovascular procedures in the JR-NET 3 database. Patient-identifying information was anonymized, retrospectively registered via website (https://jr-net.tri-kobe.net/jr-net, and collected at the Translational Research Informatics Center (http://www.tri-kobe.org/). The Institutional Review Board (IRB) at the participating centers approved the use of the patient data for research (local IRB approval number: M25-024-2). A total of 40 169 endovascular procedures were registered for the JR-NET 3.11) For the current report, we extracted data for all cases of elective endosaccular coiling of UIAs. Cases with incomplete data were excluded.
Primary and secondary endpoints
The primary endpoint was activities of daily living, as indicated by the mRS scores. The secondary endpoints were the technical success of procedures and major adverse events within 30 days of the procedures. Adverse events were classified as minor and major when mRS scores deteriorated by 1 and ≥2 points, respectively.
Statistical methods
Variables are presented as mean ± standard deviation (SD), count, or percentage, as appropriate. The χ2-test was used to compare the categorical variables. The Wilcoxon signed-rank test was used to compare the ordinal variables while Student’s t-test was used to compare continuous variables. P-values <0.05 were considered statistically significant. Highly significant data with P-values <0.001 are indicated in the text. We used the JMP 10 software (SAS Institute Inc., Cary, NC, USA) to perform all the analyses.
Results
Patients characteristics and multiplicity of the procedure
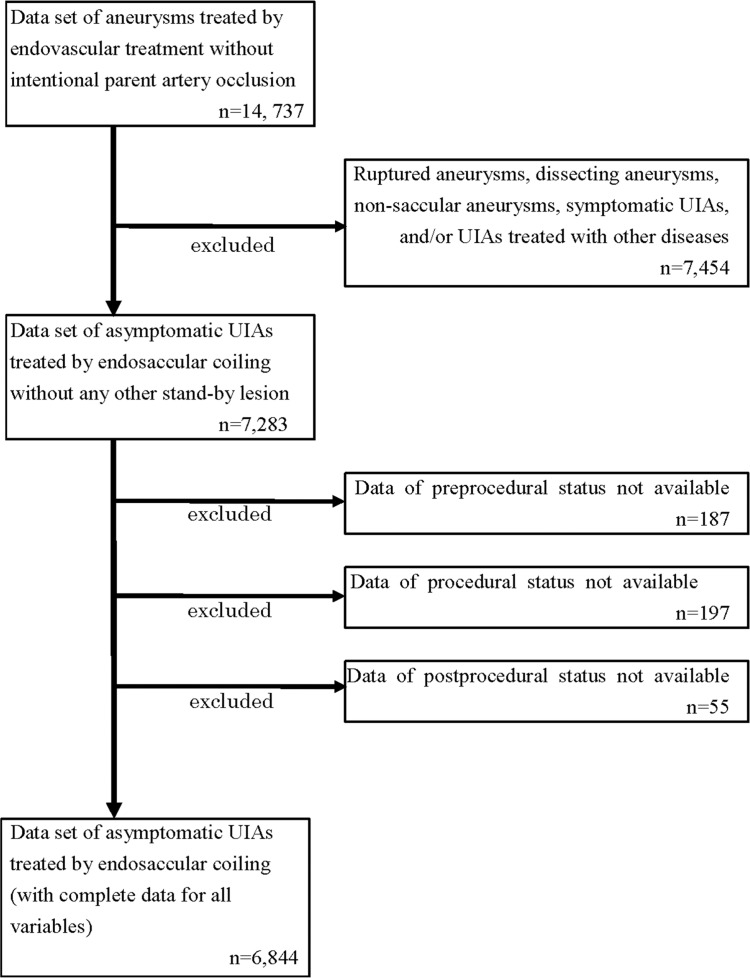
In total, 6844 cases were selected for analysis, among which 6619 procedures were performed. Of these, 1811 (27.4%) were for men and 4808 (72.6%) were for women. The flowchart for data selection is shown in Fig. 1. The mean patient age was 61.3 ± 11.6 years (range 17–89 years), and 98.9% were independent (mRS 0–2) at the time of the procedure. The numbers of treated UIAs were one (96.8%) of 6404, two of 205, and three of 10 procedures.
Fig. 1.
Figure shows the flowchart of data extraction from the JR-NET 3. The pre-procedural status included sex, age, date of treatment, pre-procedural modified Rankin Scale (mRS), antithrombotic therapy, and aneurysm characteristics. The intra-procedural status included the techniques and antithrombotic therapy. The post-procedural status included the radiographic outcome, procedure-related adverse events and mRS 30 days after the procedures. JR-NET: Japanese Registry of Neuroendovascular Therapy, UIA: unruptured intracranial aneurysm.
Aneurysm characteristics
The features of the aneurysms are summarized in Table 1. Most UIAs were located in the anterior circulation (n = 5599; 81.8%), while the remainder were located in the posterior circulation (n = 1245; 18.2%). Specifically, 33.8% were in the paraclinoid region, followed by the internal carotid artery–posterior communicating artery (14.7%), anterior communicating artery (13.9%), bifurcation of the basilar artery (9.0%), and middle cerebral artery (5.2%). As for the maximal diameters, 2318 (33.9%) were 5–6 mm and 1436 (21.0%) were 7–9 mm. Regarding the minimal diameters, 2125 (31.0%) were 3–4 mm and 124 (1.8%) were <3 mm. Notably, 3548 (58.8%) UIAs with diameters <10 mm had wide necks (neck diameter >4 mm or dome-to-neck ratio <1.5).12)
Table 1.
Characteristics of unruptured intracranial aneurysms treated by endosaccular coiling, based on data in JR-NET 3
| n (%) | |
|---|---|
| Location | |
| Anterior circulation | 5599 (81.8) |
| ICA-cav | 295 (4.3) |
| Paraclinoid | 2316 (33.8) |
| ICA-PCom | 1006 (14.7) |
| ICA-AChA | 233 (3.4) |
| ICA-bif | 191 (2.8) |
| MCA | 354 (5.2) |
| A1 | 76 (1.1) |
| ACoA | 952 (13.9) |
| DACA | 130 (1.9) |
| Other (AC) | 46 (0.7) |
| Posterior circulation | 1245 (18.2) |
| VA | 154 (2.3) |
| BA-trunk | 64 (0.9) |
| BA-SCA | 208 (3.0) |
| BA-bif | 617 (9.0) |
| PCA | 56 (0.8) |
| VA-PICA | 131 (1.9) |
| Other (PC) | 15 (0.2) |
| Size | (r, mm)* |
| <3 | 124 (1.8) |
| 3 to <5 | 2125 (31.0) |
| 5 to <7 | 2318 (33.9) |
| 7 to <10 | 1436 (21.0) |
| 10 to <25 | 827 (12.1) |
| ≥25 | 14 (0.2) |
| Appearance (% of UIA <10 mm) | (n = 6003) |
| Narrow neck† | 2475 (41.2) |
| Wide neck‡ | 3528 (58.8) |
r: maximal diameter,
Neck: ≤4 mm and dome-to-neck (D/N) ratio ≥1.5,
Neck: >4 mm or D/N ratio <1.5. ACoA: anterior communicating artery, A1: anterior cerebral artery proximal to anterior communicating artery, BA: basilar artery, BA-bif: basilar bifurcation, BA-SCA: junction of basilar artery and superior cerebellar artery, BA-trunk: trunk of basilar artery, DACA: anterior cerebral artery distal to anterior communicating artery, IC-AChA: anterior choroidal artery, ICA-bif: bifurcation of internal carotid artery, ICA-cav: cavernous segment of ICA, ICA-Pcom: posterior communicating artery, JR-NET 3: Japanese Registry of Neuroendovascular Therapy 3, MCA: middle cerebral artery, Other (AC): other locations in anterior circulation, Other (PC): other locations in posterior circulation, PCA: posterior cerebral artery, UIA: unruptured intracranial aneurysm, VA: vertebral artery.
Endovascular modalities
The techniques used for endosaccular coiling are shown in Table 2. Simple technique by which a single catheter and coils were used were performed in 1933 cases (28.2%), while adjunctive techniques were used in the remaining 4911 (71.8%) cases. Among them, balloon assist, double catheter, stent-assisted, stent monotherapy techniques were used in 2806 (41.0%), 439 (6.4%), 1253 (18.3%), 23 (0.3%), respectively. A combination of techniques was used in 391 cases (5.7%). Specifically, neck bridge stents were used in 1512 cases (22.1%).
Table 2.
The techniques and periprocedural antithrombotic therapy used according to data in JR-NET 3
| n (%) | |
|---|---|
| Technique (pa, 6844 cases) | |
| Simple | 1933 (28.2) |
| Adjunctive | 4911 (71.8) |
| DCT | 439 (6.4) |
| BAT | 2806 (41.0) |
| SAC | 1253 (18.3) |
| DCT + BAT | 129 (1.9) |
| DCT + SAC | 84 (1.2) |
| BAT + SAC | 146 (2.1) |
| DCT + BAT + SAC | 15 (0.2) |
| Stenting only | 23 (0.3) |
| Other | 17 (0.3) |
| Antithrombotic therapy (pp, 6619 cases) | |
| Anticoagulation | |
| Intraprocedural systemic heparinization | 6457 (97.6) |
| Continuous anticoagulation | 3995 (60.4) |
| heparin | 949 (14.3) |
| argatroban | 2730 (41.2) |
| heparin + argatroban | 107 (1.6) |
| Antiplatelet therapy | |
| Preprocedural | 6398 (96.7) |
| SAPT | 1647 (24.9) |
| DAPT | 4405 (66.6) |
| TAPT or more | 346 (5.2) |
| Postprocedural | 6262 (94.6) |
| SAPT | 1738 (26.3) |
| DAPT | 3872 (58.5) |
| TAPT or more | 652 (9.9) |
With mRS deterioration. BAT: balloon assist technique, DAPT: dual antiplatelet therapy, DCT: doble catheter technique, EVT: endovascular therapy, mRS: modified Rankin scale, pa: per aneurysm, pp: per procedure, RA: residual aneurysm, RN: residual neck, SAC: stent-assisted coiling, SAPT: single antiplatelet therapy, TAPT: triple antiplatelet therapy, uPAO: unpredicted parent artery occlusion.
Periprocedural antithrombotic management
Table 2 also shows the antithrombotic therapy. Systemic heparinization was used in 6458 (97.6%) procedures. Continuous anticoagulation therapy was applied in 3786 procedures (55.3%). Argatroban, a direct thrombin inhibitor, was preferentially used (41.2%).
Notably, pre- and post-procedural antiplatelet therapy (APT) was executed in 6398 (96.6%) and 6263 (94.6%), respectively.
Mode of the APT in the pre-procedural period were single in 1647 (24.9%), dual in 4405 (66.5%), and triple or more in 346 (5.2%); the corresponding figures in the 30-day post-procedural period were 1739 (26.3%), 3872 (58.5%), and 652 (9.8%), respectively.
Feasibility and immediate angiographic outcome
Failure of endosaccular coiling was noted in for 78 (1.1%) aneurysms (Table 3) at rates of 5.4%, 1.2%, 0.8%, 0.7%, 1.4%, and 0% for aneurysms with diameters of <3, 3–4, 5–7, 7–9, 10–25, and >25 mm, respectively (Table 4).
Table 3.
Outcomes of coiling for unruptured intracranial aneurysms
| n | (%) | |
|---|---|---|
| Feasibility (pa, 6844 cases) | ||
| Success | 6766 | 98.9 |
| Failure | 78 | 1.1 |
| Anatomic outcome (per successfully treated UIA, 6766 cases) | ||
| Complete occlusion | 3147 | 46.5 |
| Residual neck | 2324 | 34.3 |
| Residual aneurysm | 1288 | 19.0 |
| Unpredicted parent artery occlusion | 7 | 0.1 |
| Adverse events (pp, 6619 cases) | ||
| Procedure-related complications | 664 (154)* | 10.0 (2.3)* |
| Hemorrhagic | 143 (46)* | 2.2 (0.7)* |
| Intraprocedural aneurysmal rupture | 86 (20)* | 1.3 (0.3)* |
| Aneurysmal rupture in post-treatment period | 5 (3)* | 0.1 (0.05)* |
| Ischemic | 389 (103)* | 5.9 (1.6)* |
| Puncture site | 89 | 1.3 |
| Other | 67 | 1.0 |
| mRS 30 days after EVT (pp, 6619 cases) | ||
| 0 | 5988 | 90.5 |
| 1 | 337 | 5.1 |
| 2 | 153 | 2.3 |
| 3 | 58 | 0.9 |
| 4 | 45 | 0.7 |
| 5 | 23 | 0.3 |
| 6 | 15 | 0.2 |
| Clinical outcome (pp = 6619) | ||
| 30-day morbidity | 186 | 2.8 |
| 30-day mortality | 15 | 0.2 |
With mRS deterioration. EVT: endovascular therapy, mRS: modified Rankin scale, pa: per aneurysm, pp: per procedure.
Table 4.
Failure rate, anatomic outcome, and complication rate by the aneurysm location and maximal radius (6404 cases)
| Location of the aneurysms (n, %) | P-value | Maximal radius (n, %) | P-value | Overall (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior Circ. | Posterior Circ. | <3 | 3 to <5 | 5 to <7 | 7 to <10 | 10 to <25 | ≥25 | ||||
| Number | 5216 | 1188 | 92 | 1943 | 2174 | 1379 | 802 | 14 | 6404 | ||
| Failure rate | 59 (1.1) | 8 (0.7) | 0.20 | 5 (5.4) | 24 (1.2) | 18 (0.8) | 9 (0.7) | 11 (1.4) | 0 | 0.008 | 67 (1.0) |
| CO | 2452 (47.0) | 498 (41.9) | 0.002 | 54 (58.7) | 1030 (53.0) | 1018 (46.8) | 573 (41.6) | 273 (34.0) | 2 (14.3) | <.001 | 2950 (46.1) |
| RA | 977 (18.7) | 232 (19.5) | 0.54 | 10 (10.9) | 291 (15.0) | 399 (18.4) | 286 (20.7) | 215 (26.8) | 8 (57.1) | <.001 | 1209 (18.9) |
| Procedure-related complications | 465 (8.9) | 163 (13.7) | <.001 | 10 (10.9) | 151 (7.8) | 182 (8.4) | 155 (11.2) | 129 (16.1) | 1 (7.1) | <.001 | 628 (9.8) |
| Hemorrhagic complications | 101 (2.9) | 31 (2.6) | 0.142 | 5 (5.4) | 45 (2.3) | 42 (1.9) | 21 (1.5) | 19 (2.4) | 0 (0) | 0.146 | 132 (2.1) |
| Ischemic complications | 264 (5.1) | 105 (8.8) | <.001 | 4 (4.3) | 75 (3.9) | 105 (4.8) | 98 (7.1) | 86 (10.7) | 1 (7.1) | <.001 | 369 (5.8) |
circ.: circulation, CO: complete occlusion, RA: residual aneurysm.
The failure rate decreased significantly with increasing aneurysmal size. There was no significant difference in failure rate between aneurysms in the anterior (1.1%) and posterior (0.7%) circulations. The immediate radiographic outcomes of 6766 successfully treated aneurysms demonstrated that 3147 (46.5%) were completely occluded, 2324 (34.3%) had residual necks, 1288 (19.0%) had residual aneurysmal domes, and 7 (0.1%) resulted in unpredicted parent artery occlusion (Table 3).
Adverse events related to endosaccular coiling
Procedure-related adverse events occurred in 664 patients (10.0%), of whom 23.2% (n = 154; 2.3% of total) had increases in their 30-day mRS scores (Table 3). Intracranial hemorrhage and ischemia were noted in 143 (2.2%) and 389 (5.9%), respectively; there were 89 intra-procedural aneurysmal ruptures, or 1.3% per procedure. The anterior communicating artery was the most frequent site of these ruptures (30 cases or 3.0% of aneurysms in this region). Delayed aneurysmal rupture occurred in five cases within 30 days (Table 3).
Ischemic complication rates occurred at a lower rate in smaller aneurysms (3–7 mm) and at a higher rate in larger aneurysms (7–25 mm); however, there was no statically significant correlation between hemorrhagic complications and aneurysmal size (Table 4).
Concerning the location, aneurysms in the posterior circulation (8.8%) were more prone to ischemic complications than those in the anterior circulation (5.1%), which resulted in a higher incidence of procedure-related complications (13.7% versus 8.9%, respectively; Table 4).
30-Day clinical outcomes
The 30-day morbidity and mortality rates were 2.8% and 0.2% among 6404 procedures, respectively (Table 3). Of the 15 deaths within 30 days of treatment, 12 were associated with hemorrhagic complications (11 aneurysmal ruptures and one vessel rupture). A total of 6478 patients (97.9%) remained independent (mRS score 0–2).
Impact of stent-assisted coiling in JR-NET 3
Table 5 shows the comparison between stented and non-stented cases in this study.
Table 5.
Comparison of stented and non-stented coiling procedure based on data in JR-NET 3 (pa, 6404 cases)
| Stents used (n = 1462) | Stents not used (n = 4942) | P-value | |
|---|---|---|---|
| Age | 61.5 ± 11.6 | 61.3 ± 11.6 | 0.500 |
| Anterior circulation* | 1058 (72.4) | 4158 (84.1) | <0.001 |
| Posterior circulation* | 404 (27.6) | 784 (15.9) | <0.001 |
| Maximum radius >7 mm | 906 (62.0) | 1289 (26.1) | <0.001 |
| Unfavorable anatomy† | 1373 (93.9) | 2772 (56.1) | <0.001 |
| DAPT or more | 1368 (93.6) | 3211 (65.0) | <0.001 |
| Complete obliteration | 581 (39.7) | 2369 (47.9) | <0.001 |
| Residual aneurysm | 391 (26.7) | 818 (16.6) | <0.001 |
| Procedure-related complications | 204 (14.0) | 424 (8.6) | <0.001 |
| Hemorrhagic complications | 31 (2.1) | 101 (2.0) | 0.834 |
| Ischemic complications | 130 (8.9) | 239 (4.8) | <0.001 |
| 30-day morbidity | 88 (6.0) | 94 (1.9) | <0.001 |
| 30-day mortality | 4 (0.3) | 9 (0.2) | 0.510 |
Significantly different between anterior circulation and posterior circulation (P <0.001),
Aneurysms with wide neck and/or maximal diameter >10 mm. DAPT: dual antiplatelet therapy, pa: per aneurysm.
Neck bridge stents were used in 1462 cases, but were used more frequently in the posterior circulation (404 of 1188 cases; 34.0%) than in the anterior circulation (1058 of 5116 cases; 20.3%) (P <0.001). Also, the proportion of aneurysms >7 mm and aneurysms with unfavorable anatomy (i.e., either wide-necked or >10 mm) in terms of initial occlusion status and recanalization for non-stented coiling13) were larger in the stented cases than those in non-stented cases (62% versus 26.1%; P <0.001, 93.9% versus 56.0%; P <0.001, respectively).
The rate of complete obliteration was lower in the stented cases (39.7%) than in the non-stented cases (47.9%) (P <0.001). Compared with patients who treated without stents, those who treated with stents had higher rates of procedure-related (14.0% versus 8.6%, P <0.001) and ischemic (8.9% versus 4.8%, P <0.001) complications. Although the overall rates of hemorrhagic complications were similar between the two groups (2.1% versus 2.0%, P = 0.834), there was a significant difference in 30-day morbidity between the stented (6.0%) and non-stented (1.9%) cases (P <0.001).
Comparison between JR-NET 3 and JR-NET 1&2
Table 6 shows the comparison of data from JR-NET 3 with that from JR-NET 1&2,12) which covered the data of UIAs treated by endosaccular coiling between 2005 and 2009.
Table 6.
Comparison of the data from JR-NET 1&2 and JR-NET 3
| JR-NET 1, 2 (2005–2009) | JR-NET 3 (2010–2014) | P-value | |
|---|---|---|---|
| UIAs (n) | 4767 | 6844 | |
| Procedures (n) | 4573 | 6619 | |
| Age (y, mean ± SD, range) | 60.6 ± 11.1 (6–93) | 61.3 ± 11.6 (17–89) | 0.001 |
| Female (n, %) | 3311 (72.4) | 4808 (72.6) | 0.796 |
| Anterior circulation | 3814 (80.0) | 5614 (82.0) | 0.006 |
| Size | (n = 4767) | (n = 6844) | |
| <3 | 119 (2.5) | 124 (1.8) | 0.012 |
| 3 to <5 | 1569 (32.9) | 2125 (31.0) | 0.035 |
| 5 to <10 | 2476 (51.9) | 3754 (54.9) | 0.002 |
| ≥10 | 603 (12.6) | 841 (12.3) | 0.568 |
| Appearance (% of UIA <10 mm) | n = 4164 | n = 6003 | |
| Narrow neck | 1816 (43.6) | 2475 (41.2) | 0.017 |
| Wide neck | 2348 (56.4) | 3528 (58.8) | |
| Technique (pa) | n = 4767 | n = 6844 | |
| Adjunctive | 2612 (54.8) | 4911 (71.8) | <0.001 |
| Use of stent | 51 (1.1) | 1512 (22.1) | <0.001 |
| Antithrombotic regimen (pp) | n = 4573 | n = 6619 | |
| PRE antiplatelet therapy | 3914 (85.6) | 6398 (96.7) | <0.001 |
| INTRA systemic heparinization | 4488 (98.1) | 6457 (97.6) | 0.043 |
| CONT anticoagulation | 3108 (68.0) | 3995 (60.4) | <0.001 |
| POST antiplatelet therapy | 3841 (84.0) | 6262 (94.6) | <0.001 |
| Feasibility (pa) | n = 4767 | n = 6844 | |
| Technical success | 4665 (97.9) | 6766 (98.9) | <0.001 |
| Anatomic outcome (st) | n = 4665 | n = 6766 | |
| CO | 2690 (57.7) | 3147 (46.5) | <0.001 |
| RN | 1490 (31.9) | 2324 (34.3) | 0.007 |
| RA | 468 (10.0) | 1288 (19.0) | <0.001 |
| uPAO | 17 (0.4) | 7 (0.1) | 0.003 |
| Adverse events (pp) | n = 4573 | n = 6619 | |
| Procedure-related complications | 417 (9.1) | 664 (10.0) | 0.111 |
| Hemorrhagic complications | 90 (2.0) | 143 (2.2) | 0.500 |
| Ischemic complications | 210 (4.6) | 389 (5.9) | 0.003 |
| mRS 30 days after EVT (pp) | n = 4573 | n = 6619 | |
| 0 to 2 | 4481 (98.0) | 6478 (97.9) | 0.686 |
| 3 to 6 | 92 (2.0) | 141 (2.1) | |
| 30-day morbidity (pp) | 97 (2.1) | 186 (2.8) | 0.024 |
| 30-day mortality (pp) | 14 (0.3) | 13 (0.2) | 0.247 |
CO: complete occlusion, CONT: continuous, EVT: endovascular therapy, INTRA: intraprocedural, JR-NET: Japanese Registry of Neuroendovascular Therapy, mRS: modified Rankin scale, pa: per aneurysm, POST: postprocedural, pp: per procedure, PRE: preprocedural, RA: residual aneurysm, RN: residual neck, UIA: unruptured intracranial aneurysms, uPAO: unpredicted parent artery occlusion.
The number of patients with UIAs in JR-NET 3 (n = 6844) was higher than in JR-NET 1&2 (n = 4767). The mean age was also older (P <0.001), and the ratio of anterior circulation aneurysms was higher (P = 0.006), in JR-NET 3. The rate of involvement of anterior communicating arteries increased from 12.3% in JR-NET 1&2 to 13.9% in JR-NET 3. As to the size and appearance of the treated aneurysms, there was a decrease in aneurysms smaller than 5 mm from JR-NET 1&2 to JR-NET 3 (35.4–32.8%, P = 0.004) while the rate of wide-necked, small aneurysms (<10 mm) were higher in JR-NET 3 than that in JR-NET 1&2 (58.8% versus 56.4%, P = 0.017). Angiographically, the rate of complete obliteration was lower in JR-NET 3 (46.5%) compared with JR-NET 1&2 (57.7%) (P <0.001).
The use of adjunctive technique was more frequent in JR-NET 3 than in the previous two cohorts (71.8% versus 54.8%, P <0.001), and the use of stent markedly increased from JR-NET 1&2 to JR-NET 3 (1.1–22.1%, P <0.001).
Regarding antithrombotic therapy, the rates of both pre- and post-procedural antiplatelet therapy increased from JR-NET 1&2 (85.6% and 84.0%, respectively) to JR-NET 3 (96.7% and 94.6%, respectively), whereas the rate of continuous heparinization decreased (68.0% in JR-NET 1&2 to 60.4% in JR-NET 3).
As to the adverse events and 30-day clinical outcomes, ischemic complication rates were higher in JR-NET 3 (5.9%) than in JR-NET 1&2 (4.6%) (P <0.001), but there was no significant difference in the procedure-related complication rate (10.0% and 9.1%, respectively). The 30-day morbidity rate was also higher in JR-NET 3 (2.8%) than in JR-NET 1&2 (2.1%) (P = 0.024), but there was no significant difference in the independence rates (mRS 0–2) and 30-day mortality rates between JR-NET 3 and JR-NET 1&2.
Discussion
We have reported the current status of endosaccular coiling for UIAs in Japan between 2010 and 2014. The most noteworthy event in this study period was that reimbursement was introduced for two neck bridge stents—the Enterprise VRD (Cerenovus, Johnson and Johnson, New Brunswick, NJ, USA) in 2010 and the Neuroform EZ (Stryker Neurovascular, Fremont, CA, USA) in 2012—for treating intracranial aneurysms. Flow diverters were used in some cases, but these were not reimbursed during the study period. Thus, we can consider the main theme of this study as being to verify the feasibility and safety of endovascular treatment for UIAs after the two neck bridge stents were introduced.
The increase in the rate of treated aneurysms in the anterior circulation was considered to be multifactorial, with stents potentially of benefit for aneurysms of both the anterior and posterior circulations.14) Other factors, such as the increased number of neuroendovascular physicians and the increased awareness of coil embolization in Japan, probably contributed to this result. The increases in the number of wide-necked aneurysms and in the use of adjunctive techniques likely resulted from the introduction of stents, which were used in 22.1% of all procedures in the JR-NET 3 period.
The size of the treated aneurysms significantly changed, with the proportion of aneurysms measuring <3 and 3–5 mm decreasing from JR-NET 1&2 to JR-NET 3 (2.5–1.8% and 32.9–31.0%, respectively). This followed the publication of the UCAS Japan in 2012,2) which reported on the natural history of UIAs in Japan. That study reported that the annual rupture rate of UIAs was 0.95%, with aneurysms measuring >7 mm having the greatest risk of rupture. At the same time, the Japanese Guidelines for the Management of Stroke recommended continuing to treat UIAs measuring 5–7 mm.15) Thus, fewer UIAs measuring <5 mm and more UIAs measuring 5–7 mm were treated.
Concerning the antithrombotic therapy, periprocedural APT became more frequent in JR-NET 3 period. Several reports about the efficacy of antiplatelet therapy16–18) including its use with stents,19,20) likely accelerated this increased usage. However, it should be noted that the use of heparinization decreased both intra- and post-procedurally. Although the reduction in post-procedural anticoagulation can be explained by the lack of verification in the literature, no clear explanation can be found to account for the reduction in intra-procedural anticoagulation.
The technical success rate increased marginally from 97.9% in JR-NET 1&2 to 98.9% in JR-NET 3. This may be explained by greater technical skill among Japanese physicians, even though the use of stents will have been associated with a learning curve.21)
That said, the percentage of complete occlusion decreased from 57.7% to 46.5%, with several plausible causes. First, physicians likely avoided the risk of thromboembolic complications by meticulous coiling because progressive thrombosis could be expected, even if complete occlusion was not achieved immediately,22) and because retreatment is not associated with excessive risk.23) Second, stent-assisted coiling tends to show progressive thrombosis of the aneurysms at follow-up.24,25) The fact that stented cases had a significantly lower incidence of complete occlusion compared with non-stented cases probably reflected such reports.
Procedure-related complications were noted in 10.0% in JR-NET 3, representing a non-significant increase from 9.1% in JR-NET 1&2. These rates are consistent with previously reported data, although comparisons between studies are difficult because of the different criteria use for complications.9,10,26) Unfortunately, the prevalence of ischemic complications increased to 5.9% in JR-NET 3 from a level of 4.6% in JR-NET 1&2. When stents were used, this rate was as high as 8.9% and the 30-day morbidity rate was 6.0% despite dual or triple antiplatelet therapy being given in 93.6% of the cases that received stent-assisted coiling. Coupled with this, the rates of ischemic events associated with non-stented cases were comparable between JR-NET 3 (4.8%) and JR-NET 1&2 (4.6%). We concluded that stent introduction led to an expanded indication of endosaccular coiling, especially for wide-necked aneurysms, but that this was at the expense of an increased incidence of ischemia. By contrast, the rate of hemorrhagic complications, including intra-procedural, was comparable to that in previous studies.12,27)
Although there were no statistically significant differences in patient independence between JR-NET 3 and the preceding JR-NET 1&2 studies, the 30-day morbidity rate was significantly higher in the former (2.8% versus 2.1%, respectively). Given that the ischemic complication rate contributed heavily to this, we propose using a tailored antiplatelet therapy regimen based on pre-procedural platelet function,28,29) data of which we could not obtain in this study.
Limitations
There are several limitations in this study. First, we relied on retrospectively collected registry data that covered only about 35% of all cases receiving endovascular therapy in Japan. Second, our results could be biased because the radiographic findings, clinical outcomes, and procedure-related complications were only assessed by the treating physicians. Decisions on therapeutic indications might also have introduced inclusion bias. Third, clinical outcomes were recorded at the 30-day follow-up, and there was a lack of information on long-term outcomes after endosaccular coiling, after which recurrence rates are more important than after surgical clipping. In the future, a more complete and prospective registry would help to assess the therapeutic standards in the endovascular treatment of UIAs. The impact of using new devices, such as flow diverters and intrasaccular flow disruptors, should also be assessed.30,31)
Conclusion
The recent status of endosaccular coiling for UIAs in Japan is reported. The introduction of neck bridge stents has led to an increase in the number of cases of wide-necked aneurysms being treated and has been associated with a higher technical success rate compared with previous cohorts. However, the rate of ischemic complications has increased despite the increased use of periprocedural APT.
Acknowledgments
Dr. Satow contributed to the conception and design of the work, data acquisition, data interpretation, and manuscript drafting. Dr. Ikeda contributed to the design of the work, data acquisition, data interpretation. Dr. Satow and Dr. Ikeda performed the statistical analyses. Dr. Iihara and Dr. Sakai contributed to data acquisition, data interpretation, and critical revision of the manuscript for important intellectual content. Dr. Takahashi contributed to critical revision of the manuscript for important intellectual content. All authors approved the final version to be published.
Investigators of the Japanese Registry of Neuroendovascular Therapy (JR-NET) study group are listed below:
Co-principal investigators; Nobuyuki Sakai, Kobe City Medical Center General Hospital, Kobe, Japan; Koji Iihara, Kyushu University, Fukuoka, Japan; and Tetsu Satow, National Cerebral and Cardiovascular Center, Suita, Japan.
Investigators; Masayuki Ezura, Sendai Medical Center, Sendai, Japan; Akio Hyodo, Dokkyo Medical University Saitama Medical Center, Koshigaya, Japan; Shigeru Miyachi, Aichi Medical University, Aichi, Japan; Susumu Miyamoto, Kyoto University, Kyoto, Japan; Yoji Nagai, Kobe University, Kobe, Japan; Kunihiro Nishimura, National Cerebral and Cardiovascular Center, Suita, Japan; and Kazunori Toyoda, National Cerebral and Cardiovascular Center, Suita, Japan.
Co-investigators; Toshiyuki Fujinaka, Osaka Medical Center, Osaka, Japan; Toshio Higashi, Fukuoka University, Fukuoka, Japan; Masaru Hirohata, Kurume University, Kurume, Japan; Akira Ishii, Kyoto University, Kyoto, Japan; Hirotoshi Imamura, Kobe City Medical Center General Hospital, Kobe, Japan; Yasushi Ito, Shinrakuen Hospital, Niigata, Japan; Naoya Kuwayama, Toyama University, Toyama, Japan; Hidenori Oishi, Juntendo University, Tokyo, Japan; Yuji Matsumaru, Tsukuba University, Tsukuba, Japan; Yasushi Matsumoto, Konan Hospital, Sendai, Japan; Ichiro Nakahara, Fujita Medical University, Aichi, Japan; Chiaki Sakai, Hyogo College of Medicine, Nishinomiya, Japan; Kenji Sugiu, Okayama University, Okayama, Japan; Tomoaki Terada, Showa University Fujigaoka Hospital, Kanagawa, Japan; Shinichi Yoshimura, Hyogo College of Medicine, Nishinomiya, Japan; and board-certified physicians of Japanese Society of Neuroendovascular Therapy.
The authors express heartfelt thanks to the neuroendovascular physicians who devoted their time to this investigation.
Footnotes
Sources of Funding
This study was supported in part by a Grant-in-Aid (Junkanki-Kaihatsu H24-4-3) from the National Cerebral and Cardiovascular Center, Japan and by Hatazaki Foundation, Kobe, Japan.
Conflicts of Interest Disclosure
Dr. Sakai received Speakers’ Bureau/Honoraria from Otsuka Pharmaceutical Co., Stryker Co., Medtronic Co, Medico’s Hirata Co., and Biomedical Solutions Co., and research funding from Otsuka Pharmaceutical Co., Terumo Co., and Daiichi Sankyo Co. Dr. Iihara received Speakers’ Bureau/Honoraria from Otsuka Pharmaceutical Co., and research funding from Otsuka Pharmaceutical Co, Mitsubishi Tanabe Pharma Co., Kaneka Medix Co., Chugai Pharmaceutical Co., and Eisai Co. Dr. Satow, Dr. Ikeda and Dr. Takahashi have no conflicts of interest to declare. All authors who are members and non-members of The Japan Neurosurgical Society (JNS) have registered self-reported COI disclosure statements through the website for JNS. This manuscript has not been published or presented elsewhere in part or in entirety, and is not under consideration by another journal.
References
- 1).Sonobe M, Yamazaki T, Yonekura M, Kikuchi H: Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke 41: 1969–1977, 2010 [DOI] [PubMed] [Google Scholar]
- 2).UCAS Japan Investigators. Morita A, Kirino T, et al. : The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366: 2474–2482, 2012 [DOI] [PubMed] [Google Scholar]
- 3).Greving JP, Wermer MJ, Brown RD, et al. : Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13: 59–66, 2014 [DOI] [PubMed] [Google Scholar]
- 4).Hwang JS, Hyun MK, Lee HJ, et al. : Endovascular coiling versus neurosurgical clipping in patients with unruptured intracranial aneurysm: a systematic review. BMC Neurol 12: 99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Lin N, Cahill KS, Frerichs KU, Friedlander RM, Claus EB: Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J Neurointerv Surg 4: 182–189, 2012 [DOI] [PubMed] [Google Scholar]
- 6).Pierot L, Wakhloo AK: Endovascular treatment of intracranial aneurysms: current status. Stroke 44: 2046–2054, 2013 [DOI] [PubMed] [Google Scholar]
- 7).Koyanagi M, Ishii A, Imamura H, et al. : Long-term outcomes of coil embolization of unruptured intracranial aneurysms. J Neurosurg 129: 1492–1498, 2018 [DOI] [PubMed] [Google Scholar]
- 8).Ferns SP, Sprengers ME, van Rooij WJ, et al. : Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 40: e523–e529, 2009 [DOI] [PubMed] [Google Scholar]
- 9).Ji W, Liu A, Lv X, et al. : Risk score for neurological complications after endovascular treatment of unruptured intracranial aneurysms. Stroke 47: 971–978, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Kwon SC, Kwon OK, Korean Unruptured Cerebral Aneurysm Coiling (KUCAC) Investigators : Endovascular coil embolization of unruptured intracranial aneurysms: a Korean multicenter study. Acta Neurochir (Wien) 156: 847–854, 2014 [DOI] [PubMed] [Google Scholar]
- 11).Sakai N, Uchida K, Iihara K, et al. : Japanese surveillance of neuroendovascular therapy in JR-NET - Part II. Japanese registry of neuroendovascular treatment 3. Main report. Neurol Med Chir (Tokyo) 59: 106–115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Shigematsu T, Fujinaka T, Yoshimine T, et al. : Endovascular therapy for asymptomatic unruptured intracranial aneurysms: JR-NET and JR-NET2 findings. Stroke 44: 2735–2742, 2013 [DOI] [PubMed] [Google Scholar]
- 13).Murayama Y, Nien YL, Duckwiler G, et al. : Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 98: 959–966, 2003 [DOI] [PubMed] [Google Scholar]
- 14).Chalouhi N, Jabbour P, Gonzalez LF, et al. : Safety and efficacy of endovascular treatment of basilar tip aneurysms by coiling with and without stent assistance: a review of 235 cases. Neurosurgery 71: 785–794, 2012 [DOI] [PubMed] [Google Scholar]
- 15).Shinohara Y, Yanagihara T, Abe K, et al. : V. Asymptomatic cerebrovascular diseases. J Stroke Cerebrovasc Dis 20: S116–S128, 2011 [DOI] [PubMed] [Google Scholar]
- 16).Hwang G, Jung C, Park SQ, et al. : Thromboembolic complications of elective coil embolization of unruptured aneurysms: the effect of oral antiplatelet preparation on periprocedural thromboembolic complication. Neurosurgery 67: 743–748; discussion 748, 2010 [DOI] [PubMed] [Google Scholar]
- 17).Nishikawa Y, Satow T, Takagi T, Murao K, Miyamoto S, Iihara K: Efficacy and safety of single versus dual antiplatelet therapy for coiling of unruptured aneurysms. J Stroke Cerebrovasc Dis 22: 650–655, 2013 [DOI] [PubMed] [Google Scholar]
- 18).Yamada NK, Cross DT, Pilgram TK, Moran CJ, Derdeyn CP, Dacey RG: Effect of antiplatelet therapy on thromboembolic complications of elective coil embolization of cerebral aneurysms. AJNR Am J Neuroradiol 28: 1778–1782, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Kanaan H, Jankowitz B, Aleu A, et al. : In-stent thrombosis and stenosis after neck-remodeling device-assisted coil embolization of intracranial aneurysms. Neurosurgery 67: 1523–1532; discussion 1532–1533, 2010 [DOI] [PubMed] [Google Scholar]
- 20).King B, Vaziri S, Singla A, Fargen KM, Mocco J: Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with enterprise and neuroform stents: a comparative analysis of the literature. J Neurointerv Surg 7: 905–909, 2015 [DOI] [PubMed] [Google Scholar]
- 21).Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK: Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol 33: 159–163, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Funakoshi Y, Imamura H, Tani S, et al. : Progressive thrombosis of unruptured aneurysms after coil embolization: analysis of 255 consecutive aneurysms. J Neurointerv Surg 11: 1113–1117, 2019 [DOI] [PubMed] [Google Scholar]
- 23).Renowden SA, Koumellis P, Benes V, Mukonoweshuro W, Molyneux AJ, McConachie NS: Retreatment of previously embolized cerebral aneurysms: the risk of further coil embolization does not negate the advantage of the initial embolization. AJNR Am J Neuroradiol 29: 1401–1404, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Geyik S, Yavuz K, Yurttutan N, Saatci I, Cekirge HS: Stent-assisted coiling in endovascular treatment of 500 consecutive cerebral aneurysms with long-term follow-up. AJNR Am J Neuroradiol 34: 2157–2162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Phan K, Huo YR, Jia F, et al. : Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracranial aneurysms. J Clin Neurosci 31: 15–22, 2016 [DOI] [PubMed] [Google Scholar]
- 26).Pierot L, Spelle L, Vitry F, ATENA Investigators : Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 39: 2497–2504, 2008 [DOI] [PubMed] [Google Scholar]
- 27).Park YK, Yi HJ, Choi KS, Lee YJ, Chun HJ: Intraprocedural rupture during endovascular treatment of intracranial aneurysm: clinical results and literature review. World Neurosurg 114: e605–e615, 2018 [DOI] [PubMed] [Google Scholar]
- 28).Hwang G, Huh W, Lee JS, et al. : Standard vs modified antiplatelet preparation for preventing thromboembolic events in patients with high on-treatment platelet reactivity undergoing coil embolization for an unruptured intracranial aneurysm: a randomized clinical trial. JAMA Neurol 72: 764–772, 2015 [DOI] [PubMed] [Google Scholar]
- 29).Shim EJ, Ryu CW, Park S, Lee HN, Shin HS, Kim SB: Relationship between adverse events and antiplatelet drug resistance in neurovascular intervention: a meta-analysis. J Neurointerv Surg 10: 942–948, 2018 [DOI] [PubMed] [Google Scholar]
- 30).Becske T, Brinjikji W, Potts MB, et al. : Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 80: 40–48, 2017 [DOI] [PubMed] [Google Scholar]
- 31).Pierot L, Moret J, Barreau X, et al. : Safety and efficacy of aneurysm treatment with WEB in the cumulative population of three prospective, multicenter series. J Neurointerv Surg 10: 553–559, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]