Abstract
We analyzed the cell characteristics, neuroprotective, and transplantation effects of human cranial bone-derived mesenchymal stem cells (hcMSCs) in ischemic stroke model rats compared with human iliac bone-derived mesenchymal stem cells (hiMSCs). The expressions of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) as neurotrophic factors were analyzed in both MSCs. hiMSCs or hcMSCs were intravenously administered into ischemic stroke model rats at 3 or 24 h after middle cerebral artery occlusion (MCAO) and neurological function was evaluated. The survival rate of neuroblastoma × glioma hybrid cells (NG108-15) after 3 or 24 h oxidative or inflammatory stress and the neuroprotective effects of hiMSCs or hcMSCs-conditioned medium (CM) on 3 or 24 h oxidative or inflammatory stress-exposed NG108-15 cells were analyzed. The expressions of BDNF and VEGF were higher in hcMSCs than in hiMSCs. hcMSCs transplantation at 3 h after MCAO resulted in significant functional recovery compared with that in the hiMSCs or control group. The survival rate of stress-exposed NG108-15 was lower after 24 h stress than after 3 h stress. The survival rates of NG108-15 cells cultured with hcMSCs-CM after 3 h oxidative or inflammatory stress were significantly higher than in the control group. Our results suggest that hcMSCs transplantation in the early stage of ischemic stroke suppresses the damage of residual nerve cells and leads to functional recovery through the strong expressions of neurotrophic factors. This is the first report demonstrating a functional recovery effect after ischemic stroke following hcMSCs transplantation.
Keywords: mesenchymal stem cell, cranial bone, transplantation, stroke, vascular disorders
Introduction
Brain injury followed by an ischemic stroke may eventually lead to death in severe cases or considerable functional impairment, which might hamper the performance of regular activities in later stages of life. In recent years, due to advances in medication therapy and surgical treatment, including endovascular treatment, the number of treatment options available for ischemic stroke has increased. However, satisfactory functional recovery is achieved only in a limited number of cases. Therefore, stem cell therapy could be an effective and novel treatment option for central nervous system (CNS) injuries, including damage caused by ischemic stroke.
Several studies have demonstrated that transplantation of human mesenchymal stem cells (hMSCs) could improve functional recovery after ischemic stroke in rodents,1–8) a non-human primate model,9) and humans.10,11) Several neurotrophic factors released from the MSCs result in neuroprotection, nerve regeneration, and angiogenesis, which are considered to be the primary therapeutic effects of MSC transplantation in patients with CNS disorders.1,3–5,8,12–15)
hMSCs can be derived from various tissues, such as bone marrow,1,3–5,7,8) skin,16) muscle,16) adipose tissue,2,17) placenta,18) umbilical cord,19) and peripheral blood.6) However, hMSCs transplanted into ischemic stroke models or patients were generally harvested from iliac bone marrow [human iliac bone-derived mesenchymal stem cells (hiMSCs)], originating from the mesodermal layer.1,3–5,7,8) According to some studies, the transplantation of dental pulp-derived stem cells (DPSCs) originating from the neural crest in ischemic stroke model rats and spinal cord injury model rats enhances functional recovery,20–22) and it has been confirmed that DPSCs exhibit higher neurotrophic factor expression levels than bone marrow-derived MSCs.20) We previously established MSCs from human cranial bone [human cranial bone-derived mesenchymal stem cells (hcMSCs)] and reported that hcMSCs expressed neural crest markers at significantly higher levels than hiMSCs and exhibited a greater tendency to undergo neuronal differentiation, in an in vitro study.23) In addition, it has been demonstrated that MSCs established from rat cranial bone abundantly secrete neurotrophic factors.24) To the best of our knowledge, no report has explored the effects of transplanting hcMSCs on cerebral ischemic injury in an in vivo study.
The source of MSCs and the timing of MSCs transplantation both seem to affect the therapeutic outcome after transplantation. In fact, Ishizaka et al.25) have demonstrated timing-dependent cell distribution and functional recovery using a combination of neuroprotection, reactive astrocyte enhancement, and angiogenesis activities. In addition, Kawabori et al.26) have suggested the importance of optimal timing of MSC transplantation for functional recovery and engraftment of transplanted MSCs in ischemic stroke model rats. Toyoshima et al.27) have also examined the acute therapeutic time window of MSC transplantation and reported that extremely early transplantation of MSCs does not lead to functional recovery because the blood–brain–barrier may still be intact immediately after cerebral infarction. However, the optimal timing of hcMSCs transplantation in ischemic stroke model rats is still unclear. Therefore, the aim of the present study is to analyze the cytological features and neuroprotective effects of hcMSCs further and to clarify the effects of early transplantation of hcMSCs in ischemic stroke model rats.
Materials and Methods
All experiments involving the use and care of animals were performed in compliance with the National Institutes of Health Guidelines. All studies were designed in accordance with the Animal Testing Committee Guidelines at Hiroshima University. In accordance with the Hiroshima University Hospital’s guidelines, cranial bone marrow waste was collected from the temporal and sphenoid bones during craniotomy after obtaining informed consent from the patients.
Isolation and culture of hiMSCs and hcMSCs
hiMSCs were purchased from Lonza Japan Ltd., (Tokyo, Japan). We have previously reported the isolation and establishment of hcMSCs from human cranial bone and confirmed that they had features consistent with that of MSCs using flow cytometric analysis and a multi-lineage differentiation assay.23) In the present study, hcMSCs were established using a method previously described.23) The cranial bone marrow samples were seeded onto 90-mm culture dishes (Sumitomo Bakelite Co., Ltd., Tokyo Japan) containing Dulbecco’s modified Eagle’s medium (DMEM) with low glucose (Sigma-Aldrich Co., St. Louis, MO, USA) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA), penicillin (100 units/mL), and streptomycin (100 μg/mL: both from Sigma-Aldrich Co.). The culture dishes were incubated at 37°C in a humidified atmosphere with 5% CO2. The medium was changed every 3 days. Both hMSCs were passaged several times upon reaching 80% confluence. All hiMSCs and hcMSCs were derived from different individuals.
Reverse-transcription polymerase chain reaction
Cells that reached confluence in the growth medium were collected with phosphate buffered saline (PBS). Total RNA was extracted using Nucleospin RNA (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) (hiMSCs: n = 4, hcMSCs: n = 3). We used the NanoDrop spectrophotometer (Thermo Fisher Scientific) to measure RNA concentration. In accordance with the manufacturer’s instructions, we used the ReverTra Ace-α (Toyobo Co., Ltd., Osaka, Osaka, Japan) to generate and amplify cDNA from 1 μg of total RNA. The 7500 Real-time PCR system (Applied Biosystems, Carlsbad, CA, USA) was used to perform real-time polymerase chain reaction (PCR). We used brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) as neurotrophic factors. Hypoxanthine phosphoribosyltransferase (HPRT) was examined as a housekeeping gene.
Establishment of transient middle cerebral artery occlusion model and cell transplantation
Adult male Sprague–Dawley rats (body weight, 250–300 g) were housed at a room temperature of 23 ± 1°C, humidity of 50 ± 10%, and a light/dark cycle of 12 h, with free access to food and water. The transient middle cerebral artery occlusion (MCAO) procedure was performed by an intraluminal thread occlusion method, as described previously.28,29) The rats were anesthetized with isoflurane, and the right common carotid, external carotid, internal carotid, and pterygopalatine arteries were exposed via midline cervical incision. A 4-0 nylon thread coated with silicone (403745PK10; Doccol Co., Sharon, MA, USA) was inserted from the cut stump of the external carotid artery toward the internal carotid artery until it blocked the origin of the right MCA. Following 2 h of occlusion, the thread was retracted and reperfusion was performed. During the procedure, rectal temperature was maintained at 37 ± 0.5°C using a thermostat-controlled heating pad (Bio Research Center Co., Ltd., Nagoya, Aichi, Japan). Cerebral infarction area was confirmed by magnetic resonance imaging (MRI) at 24 h after MCAO, 2,3,5-triphenyltetrazolium chloride (TTC) staining at 24 h after MCAO, and hematoxylin–eosin (H&E) staining on day 35 after MCAO (Figs. 1A–1C). The rats were divided according to the time of treatment administration after MCAO (3 or 24 h). At 3 or 24 h after MCAO, the rats were divided into the following three groups according to the treatment received: administration of only 300 μL of PBS after MCAO [control (Ctrl) group, n = 7 and 7, respectively], hiMSCs (1 × 106 cells/300 μL of PBS) (hiMSCs group, n = 8 and 5, respectively), and hcMSCs (1 × 106 cells/300 μL of PBS) (hcMSCs group, n = 9 and 6, respectively). The cells were administered intravenously through the tail vein. Human iMSCs and hcMSCs from different individuals were passaged three times and used for transplantation. All the rats were immunosuppressed with cyclosporine-A (10 mg/kg, intraperitoneally) daily from the day before MCAO.6,7)
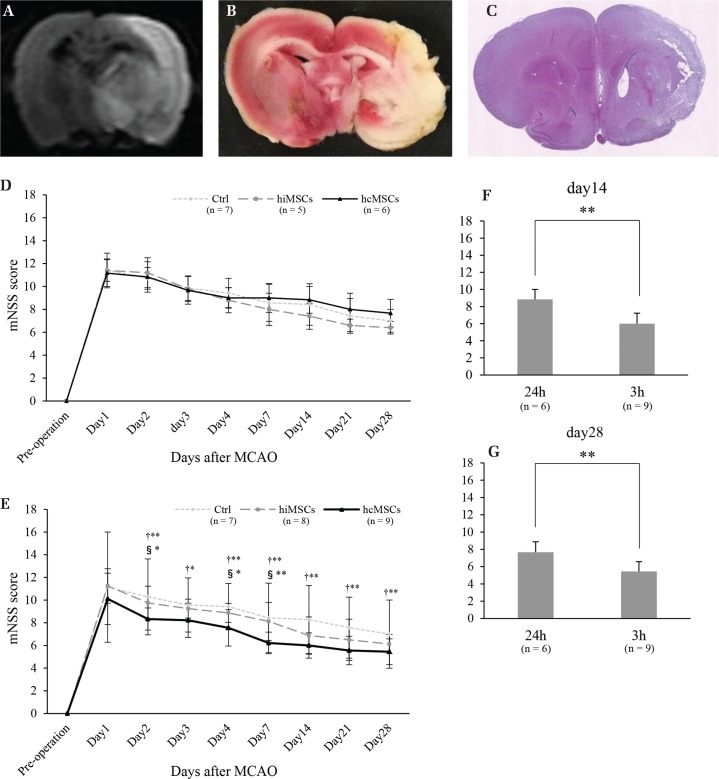
Fig. 1.
Various images of brain infarction and neurological functional recovery. Brain infarction was confirmed by magnetic resonance imaging (MRI) (A), 2,3,5-triphenyltetrazolium chloride (TTC) staining (B), and hematoxylin–eosin (H&E) staining (C). Results of neurological functional recovery in the rats transplanted at 24 h after middle cerebral artery occlusion (MCAO) (D) [Control (Ctrl) group: n = 7, human iliac bone-derived mesenchymal stem cells (hiMSCs) group: n = 5, human cranial bone-derived mesenchymal stem cells (hcMSCs) group: n = 6] or 3 h after MCAO (Ctrl group: n = 7, hiMSCs group: n = 8, hcMSCs group: n = 9) (E). †Ctrl group vs. hcMSCs group; and §hiMSCs group vs. hcMSCs group. Neurological function in the rats transplanted with hcMSCs [24 h (n = 6) or 3 h (n = 9)] at day 14 (F) or day 28 (G). *P <0.05, **P <0.01.
MRI experiment
The MRI was performed using a 4.7-T superconducting magnet system (BioSpec47/40USR; Bruker BioSpin, Ettlingen, Germany) with a combination of transmit quadrature volume coil (154-mm inner diameter) and a 30-mm receiver surface coil. The rats were anesthetized with isoflurane, placed in a prone position inside the imaging coil, and monitored by breathing excursions of the thoracic wall. The diffusion-weighted images (DWIs) of coronal forebrain sections were obtained at the level of the caudate–putamen complex. The DWIs were obtained with the multi-shot echo-planar imaging technique with the following scanning parameters: TR: 2848 ms, TE: 48 ms, matrix: 128 × 128, segments: 3, b-values: 0, 1000, and 2000 s/mm2, and number of excitations (NEX): 11, and incorporated chemical shift selective radiofrequency (RF) pulses to provide effective fat suppression. The other parameters were identical between the two sequences: 17 coronal slices, slice thickness: 1 mm, inter-slice gap: 0.25 mm, and field of view: 35 × 35 mm. The scan time of DWIs was 4 min 41 s. A region with a high-intensity signal in the DWIs was diagnosed as a cerebral infarction lesion.
Neurological functional evaluation
Neurological function was evaluated using the previously reported modified neurological severity score (mNSS), which is a complex of motor, sensory, balance, and reflexes.12) mNSS is graded on a scale from 0, reflecting a normal status, to 18, reflecting a large deficit. mNSS tests were performed before MCAO and 1–4, 7, 14, 21, and 28 days after MCAO. In the present study, rats with mNSS of 9–13 points before transplantation were used.
Oxidative or inflammatory stress exposure and cell death assay on NG108-15 cells
To analyze the neural cell viability after ischemic damage in vitro, neuroblastoma × glioma hybrid cells (NG108-15; ECACC, Porton Down, UK) were exposed to a mimic of oxidative stress created using H2O2 (SANTOKU CHEMICAL INDUSTRIES Co., Ltd., Tokyo, Japan) and a mimic of inflammatory stress created using lipopolysaccharide (LPS) (Wako Pure Chemical Industries, Ltd., Osaka, Osaka, Japan) as an in vitro cerebral infarction model.24,30–33) NG108-15 cells were seeded into 60-mm culture dishes (Corning Inc., Corning, NY, USA) and cultured in DMEM with high-glucose (Sigma-Aldrich Co.), 10% FBS (Thermo Fisher Scientific), penicillin (100 units/mL), streptomycin (100 μg/mL: both from Sigma-Aldrich Co.), and HAT supplement (Thermo Fisher Scientific). The culture dishes were incubated at 37°C in a humidified atmosphere with 5% CO2. Cell survival rates after stress exposure for 3 or 24 h were analyzed and compared with those of the control group. NG108-15 cells were cultured in fresh medium for 48 h, after which the medium was changed to fresh growth medium with 500 μM H2O2 or 200 ng/mL LPS in the stressed groups, as shown in Fig. 2A. After exposure to stress for 3 or 24 h, cells were harvested, centrifuged, and suspended in PBS (n = 10). In the control group, the medium was changed to only fresh growth medium and cells were harvested, centrifuged, and suspended in PBS after 3 or 24 h (n = 10). Trypan blue staining was used to measure cell survival rates in a counting chamber.
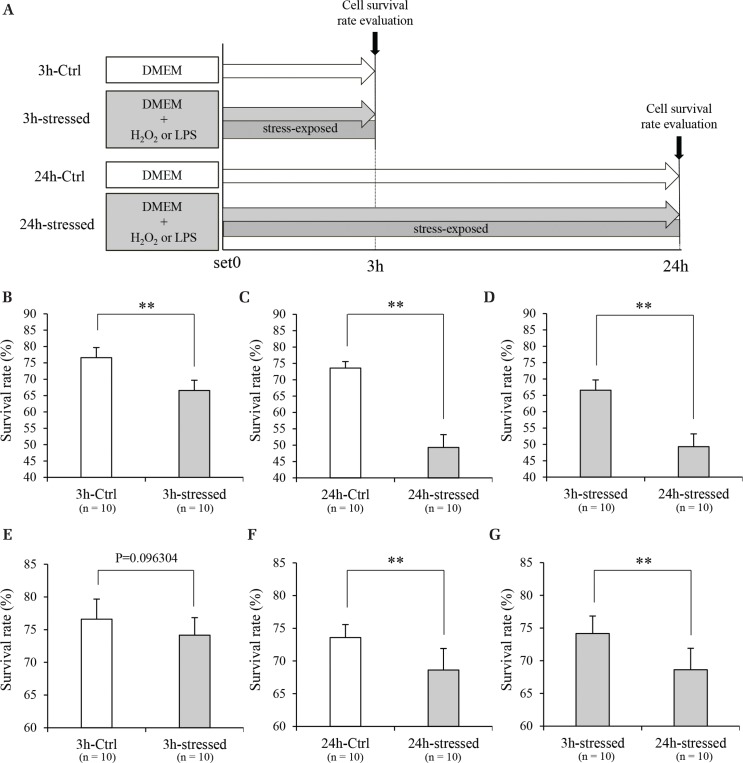
Fig. 2.
Oxidative or inflammatory stress exposure of NG108-15 cells. Schedule of stress exposure experiment using NG108-15 cells (A). The survival rate of oxidative stress-exposed NG108-15 cells at 3 h (B) (n = 10) or 24 h (C) (n = 10). Comparison of the survival rate in NG108-15 cells depending on oxidative stress exposure time (D) (n = 10). The survival rate of inflammatory stress-exposed NG108-15 cells at 3 h (E) (n = 10) or 24 h (F) (n = 10). Comparison of the survival rate in NG108-15 cells depending on inflammatory stress exposure time (G) (n = 10). **P <0.01. DMEM: Dulbecco’s modified Eagle’s medium, LPS: lipopolysaccharide.
Preparation of hiMSCs- and hcMSCs-conditioned medium
To analyze the neuroprotective effect of hiMSCs and hcMSCs after ischemic damage in an in vitro study, hiMSCs and hcMSCs were seeded on 90-mm culture dishes (Corning Inc.) and cultured in growth medium. When the cells reached 80% confluence, the medium was changed to fresh growth medium without FBS. After 24 h, culture supernatants were collected as conditioned medium (CM) and stored at −80°C after 0.2-μm filtration.
Neuroprotective effect of hiMSCs-CM or hcMSCs-CM on stress-exposed NG108-15 cells
The neuroprotective effect of hiMSCs- or hcMSCs-CM was examined based on the protocol shown in Fig. 3A. After the exposure of NG108-15 cells to oxidative or inflammatory stress for 3 or 24 h, the medium was changed to fresh growth medium (absence of hMSCs culture and without FBS) (Ctrl), hiMSCs-CM, or hcMSCs-CM (with 500 μM H2O2 or 200 ng/mL LPS). After culturing with hiMSCs- or hcMSCs-CM for 24 h, cells were harvested, centrifuged, and suspended in PBS (n = 10). Trypan blue staining was used to measure cell survival rates in the counting chamber.
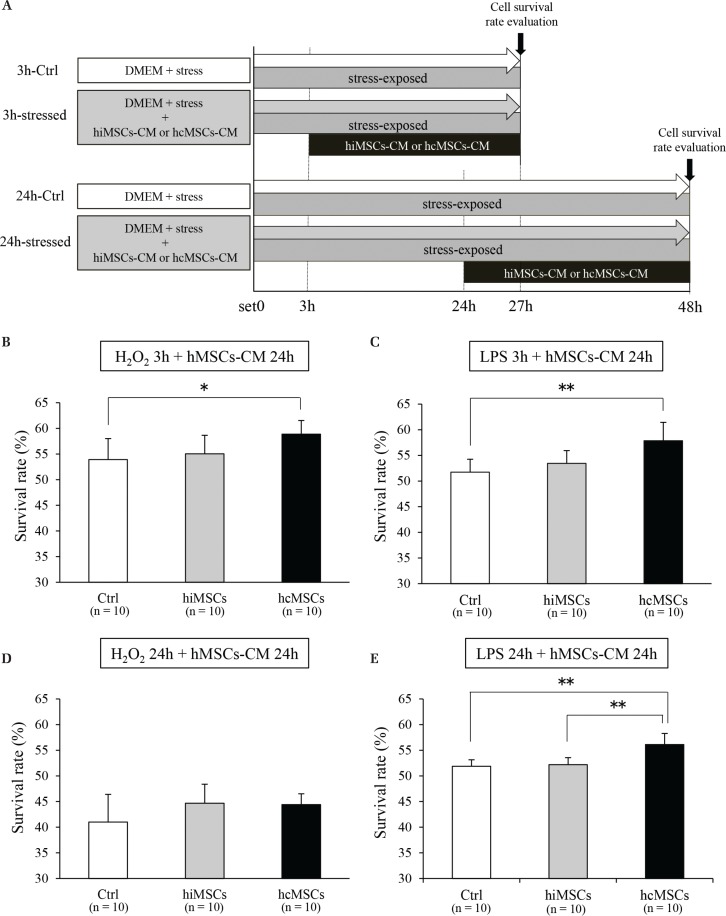
Fig. 3.
The effects of human mesenchymal stem cells-conditioned medium (hMSCs-CM) on survival rate of stress-exposed NG108-15 cells. Schedule of the NG108-15 cell experiment using hMSCs-CM (A). The effect of hMSCs-CM against 3 h of oxidative stress (B) (n = 10), 3 h of inflammatory stress (C) (n = 10), 24 h of oxidative stress (D) (n = 10), and 24 h of inflammatory stress (E) (n = 10). *P <0.05, **P <0.01. DMEM: Dulbecco’s modified Eagle’s medium, LPS: lipopolysaccharide.
Statistical analysis
Modified neurological severity score was analyzed using repeated-measures two-way analysis of variance with Bonferroni’s test. The Mann–Whitney U test was used to analyze mNSS of the hcMSCs transplantation group on days 14 and 28, and survival rates of stress-exposed NG108-15 cells. To analyze the survival rate of oxidative or inflammatory stress-exposed NG108-15 cells cultured with hiMSCs- or hcMSCs-CM, the Kruskal–Wallis test with the modified Tukey’s test was applied. For all analyses, a P-value <0.05 was considered significant. JSTAT software (Sato, Japan) was used to perform statistical analyses.
Results
Neurological functional recovery in MCAO model rats
To compare the transplantation effects of hiMSCs and hcMSCs in a stroke model rat, neurological functional recovery was assessed using mNSS. When MSCs were transplanted 24 h after MCAO, there were no significant differences among the three groups at all observation times (Fig. 1D). When MSCs were transplanted 3 h after MCAO, mNSS in the hcMSCs group was significantly better than in the Ctrl group on days 2–4, 7, 14, 21, and 28, and significantly better than in the hiMSCs group on days 2, 4, and 7 (Fig. 1E). In the hiMSCs group, there were no significant differences in mNSS compared with that in the Ctrl group. In addition, the mNSS of the hcMSCs group (transplantation at 3 h after MCAO) was significantly improved than that of the hcMSCs group (transplantation at 24 h after MCAO) on days 14 and 28 (Figs. 1F and 1G).
The morphology of hiMSCs and hcMSCs
The cell morphology of both hMSCs showed a spindle shape, with no characteristic difference being exhibited between hiMSCs and hcMSCs (Fig. 4A).
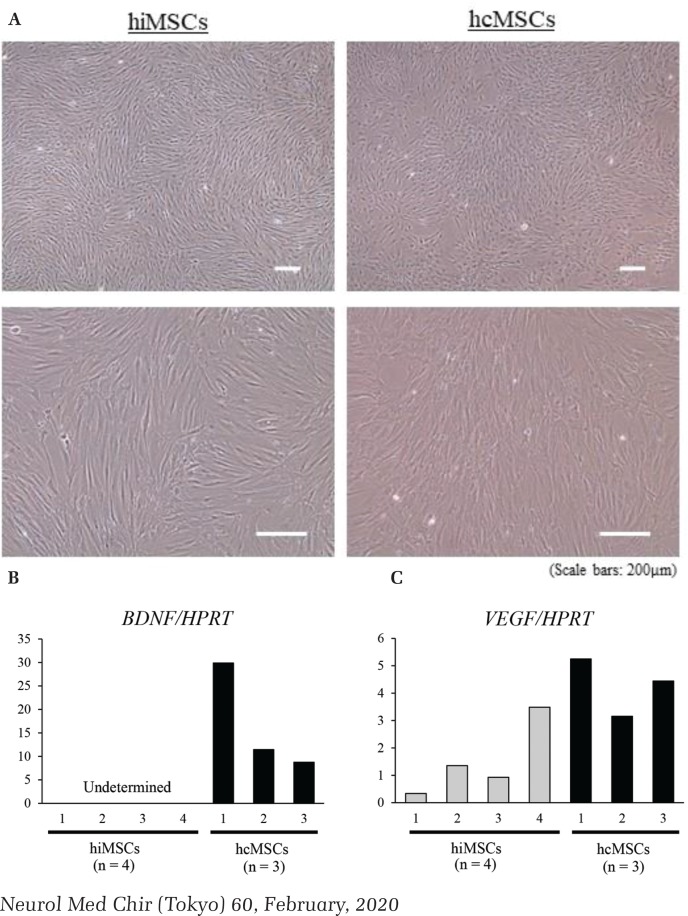
Fig. 4.
The morphology and gene expression of both human mesenchymal stem cells (hMSCs). Both hMSCs showed a spindle shape, with no characteristic difference being exhibited between human iliac bone-derived mesenchymal stem cells (hiMSCs) and human cranial bone-derived mesenchymal stem cells (hcMSCs) (A). Gene expression of brain-derived neurotrophic factor (BDNF) (B), and vascular endothelial growth factor (VEGF) (C) (hiMSCs: n = 4, hcMSCs: n = 3). HPRT: hypoxanthine phosphoribosyltransferase.
Gene expression analysis of hiMSCs and hcMSCs
We used real-time PCR to analyze the neurotrophic factor-associated gene expression. The BDNF expression was not determined in all hiMSCs lines by our analysis, but was determined in all hcMSCs lines (Fig. 4B). The VEGF expression of hcMSCs showed a higher tendency than that of hiMSCs (Fig. 4C).
Survival rates of stress-exposed NG108-15 cells
The survival rate of oxidative stress-exposed NG108-15 cells was significantly lower in both the 3 and 24 h stress groups than in the Ctrl group (Figs. 2B and 2C). The survival rate of NG108-15 cells upon exposure to 24 h of oxidative stress was significantly lower than that for the group with 3 h of exposure (Fig. 2D). The survival rate of inflammatory stress-exposed NG108-15 cells in the 3 h stress group was not significantly different but that of the 24 h stress group was significantly lower than in the Ctrl group (Figs. 2E and 2F). The survival rate of inflammatory stress-exposed NG108-15 cells was significantly lower in the 24 h stress-exposed group than in the 3 h stress-exposed group (Fig. 2G).
Survival rate of oxidative or inflammatory stress-exposed NG108-15 cells cultured with hiMSCs-CM or hcMSCs-CM
The survival rate of NG108-15 cells cultured with hcMSCs-CM after exposure to oxidative or inflammatory stress for 3 h was significantly higher than in the Ctrl group (Figs. 3B and 3C). The survival rate of NG108-15 cells cultured in hcMSCs-CM after exposure to stress for 24 h was not significantly higher under oxidative stress (Fig. 3D), but was significantly higher under inflammatory stress, compared with that of the hiMSCs group or the Ctrl group (Fig. 3E). The survival rate of NG108-15 cells cultured in hiMSCs-CM after 3 and 24 h of exposure to oxidative or inflammatory stress was not significantly higher than that of the Ctrl group (Figs. 3B–3E).
Discussion
The present study indicates that hcMSCs express higher levels of neurotrophic factors (BDNF and VEGF) than hiMSCs. Intravenous transplantation of hcMSCs in ischemic stroke model rats 3 h after MCAO enhanced neurological functional recovery than that in the hiMSCs and control groups. In an in vitro study, although hiMSCs-CM did not increase the survival rate of NG108-15 cells after exposure to stress for 3 or 24 h, hcMSCs-CM improved the survival rate of NG108-15 cells after 3 h of exposure to oxidative or inflammatory stress. In the present study, early transplantation of hcMSCs in ischemic brain injury improved functional recovery via neurotrophic factors, when compared with hiMSCs transplantation. This is the first report demonstrating the functional recovery effect after ischemic stroke by hcMSCs transplantation.
Human cranial bones, such as frontal bone, sphenoidal bone, and temporal bone originate from the neural crest, similar to teeth.34) We reported previously that hcMSCs expressed neural crest markers (SLUG, SNAIL, and P75) at significantly higher levels than hiMSCs.23) It has been reported that DPSCs express neural crest-associated genes.35) In addition, with regard to neurotrophic factors, Abiko et al.24) have observed that rat cranial bone-derived MSCs (rcMSCs) exhibit higher mRNA expression levels of neurotrophic factors, such as Bdnf and nerve growth factor (Ngf), than rat bone marrow-derived MSCs (rbMSCs). Moreover, Sakai et al.20) have revealed that the expression levels of human BDNF, glial cell line-derived neurotrophic factor (GDNF), and ciliary neurotrophic factor (CTNF) in DPSCs was higher than those in MSCs harvested from human iliac bone marrow. Our study revealed that hcMSCs expressed higher levels of neurotrophic factors than hiMSCs. The results suggest that hcMSCs originate from the neural crest, which may lead to the high levels of expression of neurotrophic factors.
The mechanism behind the therapeutic effect of MSC transplantation in CNS diseases, including ischemia is commonly accepted to involve neuroprotection, angiogenesis, neurogenesis, synaptogenesis, and immunomodulation by the secretion of several neurotrophic factors released from MSCs.1,3–5,8,12–15) Chen et al.36) presented that hiMSCs cultured in supernatant derived from ischemic brain extracts can induce the production of BDNF, VEGF, NGF, and hepatocyte growth factor (HGF). In rat cerebral ischemic models, BDNF,3,37) VEGF,8,38,39) GDNF,4,40) NGF,41,42) HGF,39) and placental growth factor5) have also been described to have beneficial effects on cerebral ischemia. It has been reported that the transplantation of rcMSCs in ischemic stroke model rats improved functional recovery compared with rbMSCs transplantation and that the expression of Bdnf and Ngf in rcMSCs was higher than in rbMSCs.24) DPSCs were found to express abundant neurotrophic factors compared with hiMSCs20) and DPSCs transplantation in model rats of ischemic stroke and spinal cord injury promotes functional recovery.20–22) Our study revealed that the transplantation of hcMSCs in rat stroke models improved functional recovery and the expression of BDNF and VEGF in hcMSCs was higher than in hiMSCs. BDNF decreases the local levels of pro-inflammatory cytokines, such as TNF-α, and increases the local levels of anti-inflammatory cytokines, such as IL-10, in addition to the DNA-binding activity of NF-κB;43) it also promotes angiogenesis and neurogenesis in the cerebral ischemic area.22,44) VEGF is also an important neurotrophic factor for the improvement of functional recovery after cerebral ischemia because it stimulates the proliferation of neural progenitors in the non-ischemic region, promotes neuroprotection and neurogenesis in ischemic lesions, and induces angiogenesis in the ischemic penumbra.45,46) In the present study, the higher expression of neurotrophic factors in hcMSCs may lead to excellent functional recovery from ischemic cerebral damage. We consider that, in ischemic stroke, hcMSCs can be a more useful source in transplantation therapy than other MSCs.
In the present study, the effect on functional improvement could not be confirmed by the transplantation of hMSCs at 24 h after MCAO. However, the transplantation of hcMSCs at 3 h after MCAO improved functional recovery. In an in vitro study, the survival rate of NG108-15 cells rapidly decreased as oxidative or inflammatory stress exposure time increased, and hcMSCs-CM improved the survival rate of NG108-15 cells after 3 h of exposure to oxidative or inflammatory stress. Garcia et al.47) confirmed that neuronal necrosis progresses rapidly at 6 h or more after ischemic damage, as revealed by histological analysis, the same as in our in vitro study. Some previous reports presented that intravenous transplantation of MSCs in ischemic stroke model rats reduced apoptosis in the penumbral zone of the lesion.1,15,48) It was suggested that the transplantation of hcMSCs abundantly secreting neurotrophic factors before nerve cell necrosis progresses may rescue the residual nerve cells and result in good functional recovery after cerebral infarction. Although the intravenous transplantation of rcMSCs at 24 h after MCAO in ischemic stroke model rats has been reported to improve functional recovery,24) the transplantation of hcMSCs did not result in functional recovery in our study. Thus, it is necessary to determine the differences in the characteristics of MSCs depending on the species in addition to the differences in transplantation effects between allogeneic and xenogeneic transplantations. Further investigations on the effect of transplanting hcMSCs in patients with cerebral infarction are required.
Cell therapy employs either autograft or allograft. With respect to immunological factors, autograft is considered to be better than allograft. However, at present, hcMSCs require at least 5 weeks to secure a sufficient number of cells for transplantation; therefore, while performing cell therapy for cerebral infarction with autograft, administration is forced in the chronic phase. As a first step, we are planning a clinical study in which patients with severe cerebral infarction who require decompressive craniotomy are administered autologous hcMSCs cultured from their free bone flap in the chronic phase. After this clinical study, we aim to perform allograft so that cell therapy can be conducted as soon as possible after cerebral infarction as a final goal.
In this study and previous reports, we found that cranial bone-derived MSCs secrete more abundant neurotrophic factors than iliac bone-derived MSCs; thus, they have a higher neuroprotective effect and improved function recovery after cerebral infarction.24) Therefore, we consider cranial bone marrow to be more useful than iliac bone marrow as a source of cell therapy for cerebral infarction. Although the collection of skull bone marrow is more invasive than the collection of iliac bone marrow in a clinical setting, initially, we are considering to administrate hcMSCs which are cultured using the free bone flap after decompressive craniectomy to the patients with extensive cerebral infarction including middle cerebral artery perfusion area who need decompressive craniectomy to rescue their lives according to the recommendation by the guideline.49) Thus, patients do not have to undergo any additional invasive procedure to obtain hcMSCs from the cranial bone marrow. In the future, we believe that this could be clinically applied to patients other than those with severe cerebral infarction by investigating methods for collecting cranial bone marrow in a minimally invasive manner, such as by stereotactic procedures, and for efficiently establishing and culturing hcMSCs.
Conclusion
Our results provide novel evidence that the transplantation of hcMSCs in stroke model rats in an early phase improves functional recovery compared with hiMSCs transplantation, via a neuroprotective effect through the strong expression of neurotrophic factors.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI grant number 16K10724) and TWOCELLS Co., Ltd., Hiroshima, Hiroshima, Japan.
Footnotes
Conflicts of Interest Disclosure
All authors have no conflict of interest.
References
- 1).Li Y, Chen J, Chen XG, et al. : Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59: 514–523, 2002 [DOI] [PubMed] [Google Scholar]
- 2).Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS: Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol 183: 355–366, 2003 [DOI] [PubMed] [Google Scholar]
- 3).Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD: I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136: 161–169, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD: Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84: 1495–1504, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Liu H, Honmou O, Harada K, et al. : Neuroprotection by PIGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 129: 2734–2745, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD: Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma 24: 508–520, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Sasaki M, Honmou O, Kocsis JD: A rat middle cerebral artery occlusion model and intravenous cellular delivery. Methods Mol Biol 549: 187–195, 2009 [DOI] [PubMed] [Google Scholar]
- 8).Toyama K, Honmou O, Harada K, et al. : Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol 216: 47–55, 2009 [DOI] [PubMed] [Google Scholar]
- 9).Sasaki M, Honmou O, Radtke C, Kocsis JD: Development of a middle cerebral artery occlusion model in the nonhuman primate and a safety study of i.v. infusion of human mesenchymal stem cells. PLoS One 6: e26577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Bang OY, Lee JS, Lee PH, Lee G: Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57: 874–882, 2005 [DOI] [PubMed] [Google Scholar]
- 11).Honmou O, Onodera R, Sasaki M, Waxman SG, Kocsis JD: Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med 18: 292–297, 2012 [DOI] [PubMed] [Google Scholar]
- 12).Chopp M, Li Y: Treatment of neural injury with marrow stromal cells. Lancet Neurol 1: 92–100, 2002 [DOI] [PubMed] [Google Scholar]
- 13).Chen J, Li Y, Zhang R, et al. : Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res 1005: 21–28, 2004 [DOI] [PubMed] [Google Scholar]
- 14).Bunnell BA, Betancourt AM, Sullivan DE: New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther 1: 34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Gutiérrez-Fernández M, Rodríguez-Frutos B, Alvarez-Grech J, et al. : Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 175: 394–405, 2011 [DOI] [PubMed] [Google Scholar]
- 16).Young HE, Steele TA, Bray RA, et al. : Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec 264: 51–62, 2001 [DOI] [PubMed] [Google Scholar]
- 17).Zuk PA, Zhu M, Ashjian P, et al. : Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13: 4279–4295, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. : Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22: 1338–1345, 2004 [DOI] [PubMed] [Google Scholar]
- 19).Kern S, Eichler H, Stoeve J, Klüter H, Bieback K: Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24: 1294–1301, 2006 [DOI] [PubMed] [Google Scholar]
- 20).Sakai K, Yamamoto A, Matsubara K, et al. : Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122: 80–90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Song M, Lee JH, Bae J, Bu Y, Kim EC: Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. Cell Transplant 26: 1001–1016, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Zhang X, Zhou Y, Li H, et al. : Intravenous administration of DPSCs and BDNF improves neurological performance in rats with focal cerebral ischemia. Int J Mol Med 41: 3185–3194, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Shinagawa K, Mitsuhara T, Okazaki T, et al. : The characteristics of human cranial bone marrow mesenchymal stem cells. Neurosci Lett 606: 161–166, 2015 [DOI] [PubMed] [Google Scholar]
- 24).Abiko M, Mitsuhara T, Okazaki T, et al. : Rat cranial bone-derived mesenchymal stem cell transplantation promotes functional recovery in ischemic stroke model rats. Stem Cells Dev 27: 1053–1061, 2018 [DOI] [PubMed] [Google Scholar]
- 25).Ishizaka S, Horie N, Satoh K, Fukuda Y, Nishida N, Nagata I: Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 44: 720–726, 2013 [DOI] [PubMed] [Google Scholar]
- 26).Kawabori M, Kuroda S, Ito M, et al. : Timing and cell dose determine therapeutic effects of bone marrow stromal cell transplantation in rat model of cerebral infarct. Neuropathology 33: 140–148, 2013 [DOI] [PubMed] [Google Scholar]
- 27).Toyoshima A, Yasuhara T, Kameda M, et al. : Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One 10: e0127302, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Koizumi J, Yoshida Y, Nakazawa T, Ooneda G: Experimental studies of ischemic brain edema. 1: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke 8: 1–8, 1986 [Google Scholar]
- 29).Longa EZ, Weinstein PR, Carlson S, Cummins R: Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91, 1989 [DOI] [PubMed] [Google Scholar]
- 30).Neirinckx V, Agirman G, Coste C, et al. : Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther 6: 211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Pomiès P, Blaquière M, Maury J, Mercier J, Gouzi F, Hayot M: Involvement of the FoxO1/MuRF1/Atrogin-1 signaling pathway in the oxidative stress-induced atrophy of cultured chronic obstructive pulmonary disease myotubes. PLoS One 11: e0160092, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Imura T, Tomiyasu M, Otsuru N, et al. : Hypoxic preconditioning increases the neuroprotective effects of mesenchymal stem cells in a rat model of spinal cord injury. J Stem Cell Res Ther 7: 375, 2017 [Google Scholar]
- 33).Otsuka T, Imura T, Nakagawa K, et al. : Simulated microgravity culture enhances the neuroprotective effects of human cranial bone-derived mesenchymal stem cells in traumatic brain injury. Stem Cells Dev 27: 1287–1297, 2018 [DOI] [PubMed] [Google Scholar]
- 34).Lee YH, Saint-Jeannet JP: Sox9 function in craniofacial development and disease. Genesis 49: 200–208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Martens W, Wolfs E, Struys T, Politis C, Bronckaers A, Lambrichts I: Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs 196: 490–500, 2012 [DOI] [PubMed] [Google Scholar]
- 36).Chen X, Li Y, Wang L, et al. : Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 22: 275–279, 2002 [DOI] [PubMed] [Google Scholar]
- 37).Huang W, Mo X, Qin C, Zheng J, Liang Z, Zhang C: Transplantation of differentiated bone marrow stromal cells promotes motor functional recovery in rats with stroke. Neurol Res 35: 320–328, 2013 [DOI] [PubMed] [Google Scholar]
- 38).Miki Y, Nonoguchi N, Ikeda N, Coffin RS, Kuroiwa T, Miyatake S: Vascular endothelial growth factor gene-transferred bone marrow stromal cells engineered with a herpes simplex virus type 1 vector can improve neurological deficits and reduce infarction volume in rat brain ischemia. Neurosurgery 61: 586–594; discussion 594–595, 2007 [DOI] [PubMed] [Google Scholar]
- 39).Ikegame Y, Yamashita K, Hayashi S, et al. : Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13: 675–685, 2011 [DOI] [PubMed] [Google Scholar]
- 40).Zhang WR, Hayashi T, Iwai M, et al. : Time dependent amelioration against ischemic brain damage by glial cell line-derived neurotrophic factor after transient middle cerebral artery occlusion in rat. Brain Res 903: 253–256, 2001 [DOI] [PubMed] [Google Scholar]
- 41).Luk YO, Chen WY, Wong WJ, et al. : Treatment of focal cerebral ischemia with liposomal nerve growth factor. Drug Deliv 11: 319–324, 2004 [DOI] [PubMed] [Google Scholar]
- 42).Zhu W, Cheng S, Xu G, et al. : Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug Deliv 18: 338–343, 2011 [DOI] [PubMed] [Google Scholar]
- 43).Jiang Y, Wei N, Zhu J, et al. : Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediators Inflamm 2010: 372423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Schäbitz WR, Steigleder T, Cooper-Kuhn CM, et al. : Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 38: 2165–2172, 2007 [DOI] [PubMed] [Google Scholar]
- 45).Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA: Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 99: 11946–11950, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Sun Y, Jin K, Xie L, et al. : VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111: 1843–1851, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Garcia JH, Liu KF, Ho KL: Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke 26: 636–642; discussion 643, 1995 [DOI] [PubMed] [Google Scholar]
- 48).Chen J, Li Y, Katakowski M, et al. : Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res 73: 778–786, 2003 [DOI] [PubMed] [Google Scholar]
- 49).Ogawa A, Izumi S, Katayama Y, Kayama T, Suzuki N: The Joint Committee on Guidelines for the Management of Stroke - Japanese Guideline for the Management of Stroke 2015. Kyowa Kikaku, Tokyo, 2015, 288–291 (Japanese) [Google Scholar]